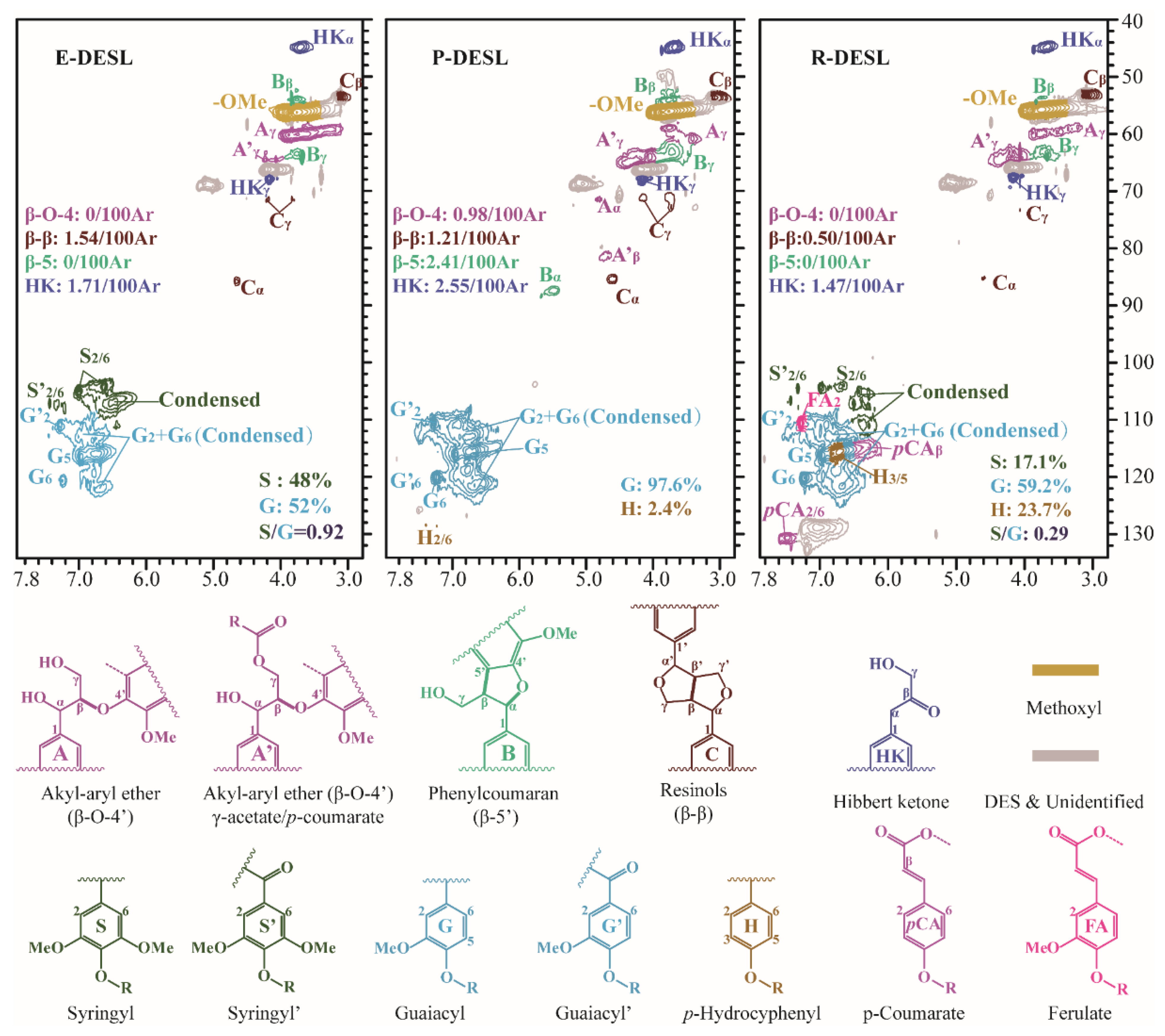
2.3. The Thermochemical Depolymerization Analysis of Lignin Isolated through the Use of Deep Eutectic Solvent
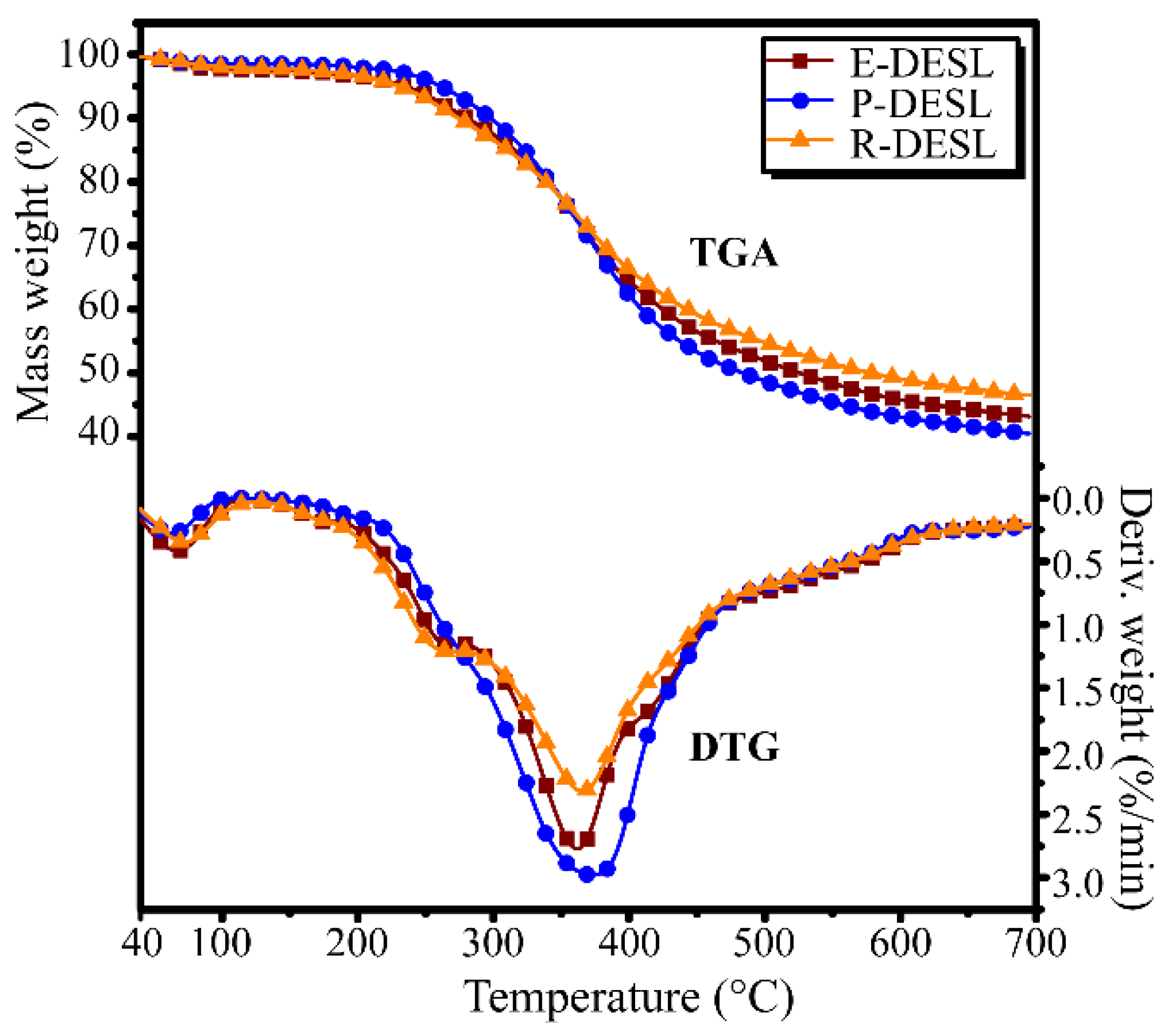
Thermogravimetric analysis (TGA) was applied for the determination of the thermal degradation properties of the DESLs, and the TGA and derivative thermogravimetic analysis (DTG) curves of the DESLs were obtained. As shown in
Figure 2, negligible moisture volatilized between 40 and 120 °C according to the appearance of a small degradation peak at this temperature range. The peaks between 200 and 300 °C appeared in the E-DESL and R-DESL curves, but not in the P-DESL DTG curves, which indicated that the residual carbohydrates remained in small amounts in the E-DESL and R-DESL but were completely removed in the P-DESL [
35,
36].
The main pyrolysis of the DESLs occurred at temperatures between 300 to 600 °C, at which the DESLs were degraded into low-molecular compounds and were released in gaseous form due to the bonds breaking and functional group reforming reactions. It was interesting to find that compared with the E-DESL and R-DESL, the maximum degradation rate (MDR) of the P-DESL was the highest, and the temperature at which the MDR (Tmax) of the P-DESL occurred was higher than that of the E-DESL and R-DESL. This suggested that compared with the E-DESL and R-DESL, the P-DESL had higher thermal stability, which resulted in the P-DESL producing less pyrolysis coke. Compared with the E-DESL, the R-DESL had a higher MDR and a lower Tmax. This suggested that the R-DESL with a more complex composition of basic structural units released less volatile matter during pyrolysis.
To further investigate the relationship between the structure of lignin isolated from different types of biomass feedstocks via DES and the distributions of pyrolysis products, fast pyrolysis experiments for the DESLs at 400 °C, 550 °C, and 700 °C were carried out, and about 42 of the products were retrieved and sorted via GC/MS, as shown in the
Supplementary Materials. Since the products from lignin pyrolysis were mainly composed of aromatic compounds and the types of pyrolysis products were closely related to the lignin structural units, the pyrolysis products of the DESLs were categorized into guaiacyl type (G)-phenols,
p-hydroxyphenyl type (H)-phenols, syringyl type (S)-phenols, catechol type (C)-phenols, monomeric aromatic hydrocarbons (MAHs), and others (
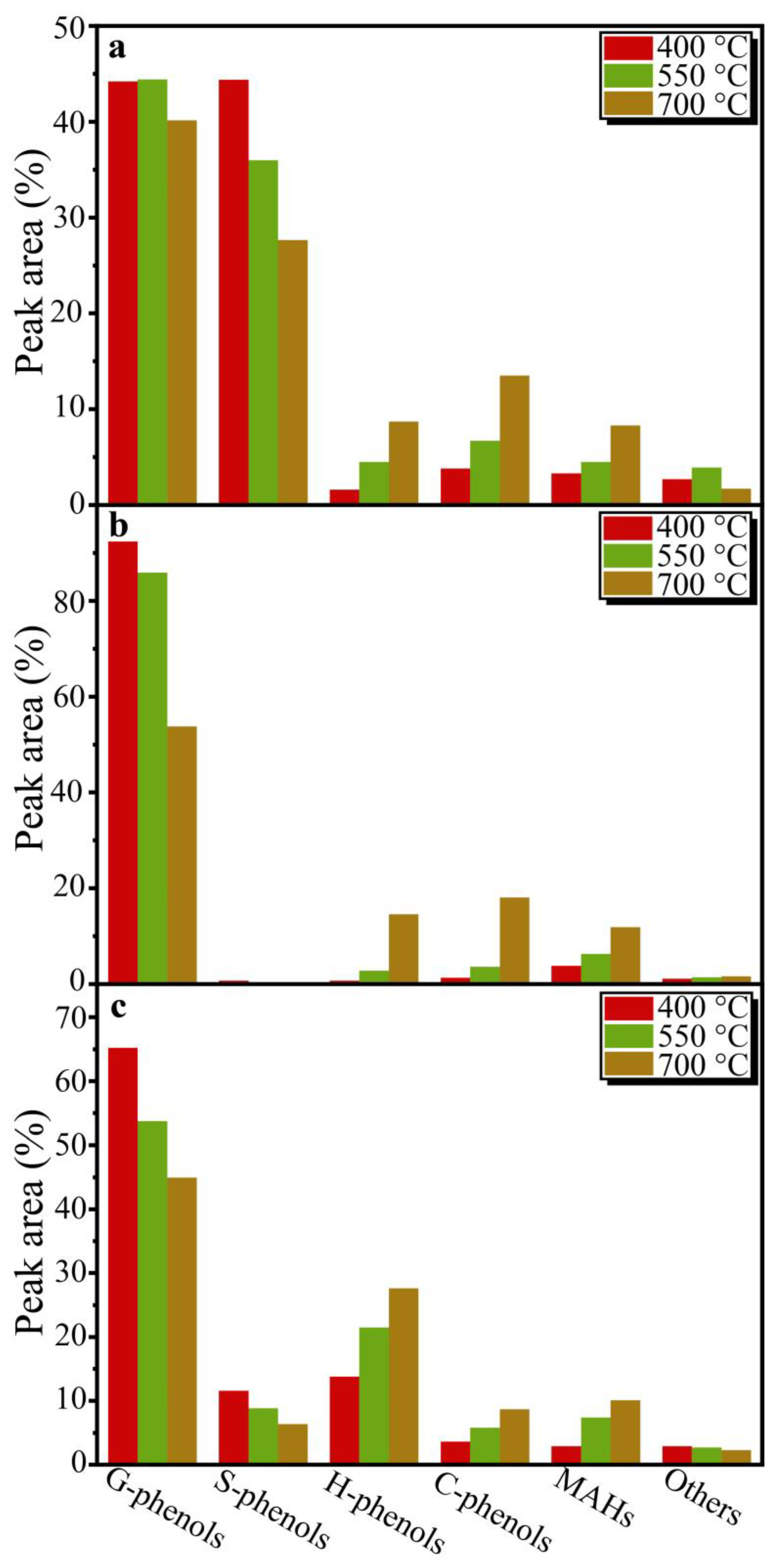
Figure 3). In addition, the distribution of the main DESL pyrolysis products at different temperatures is shown in
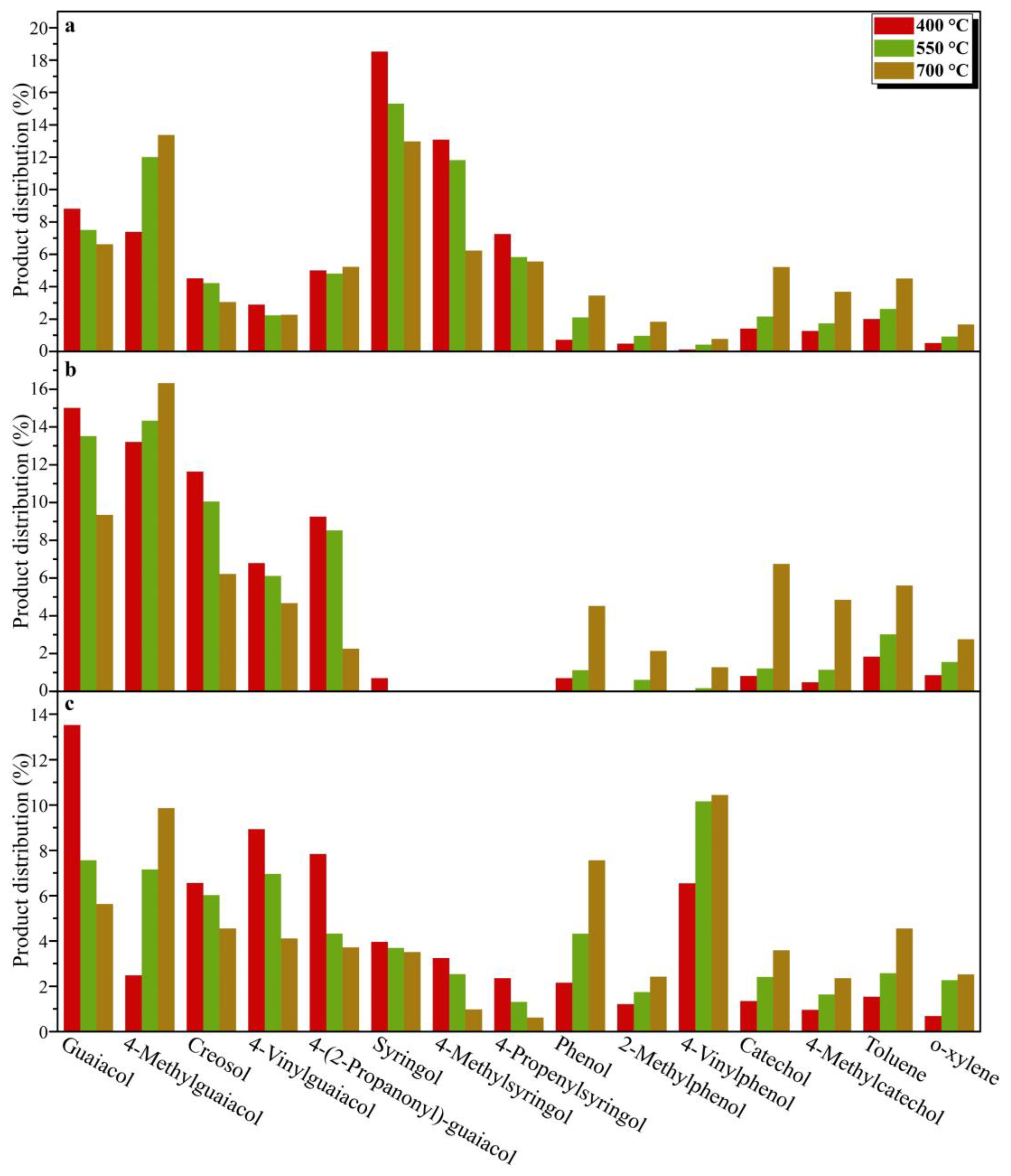
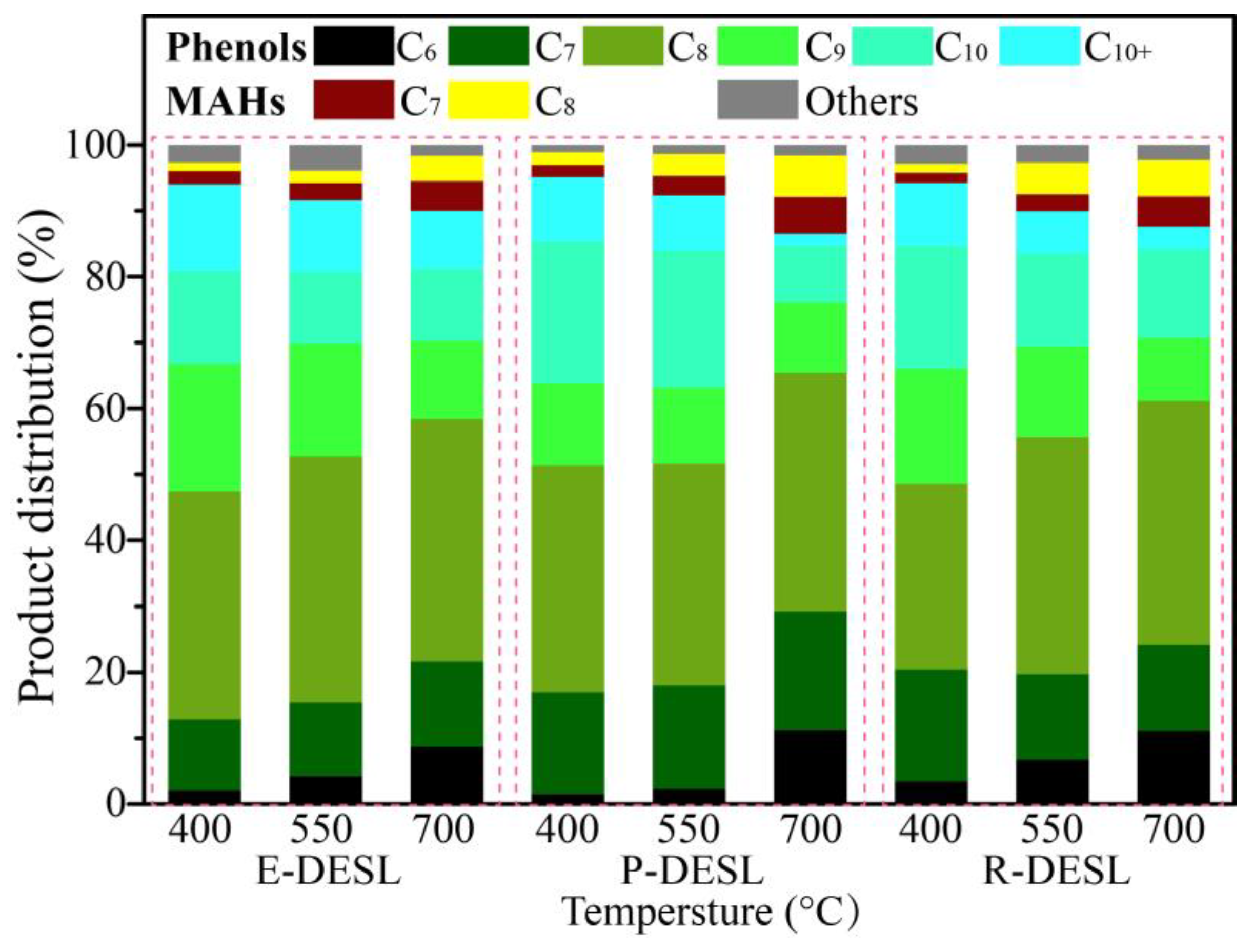
Figure 4. The carbon number distribution of pyrolysis products derived from the three DESLs is shown in
Figure 5. The carbon number distribution of the longest side-chain of products from DESL pyrolysis under different temperatures is also shown in
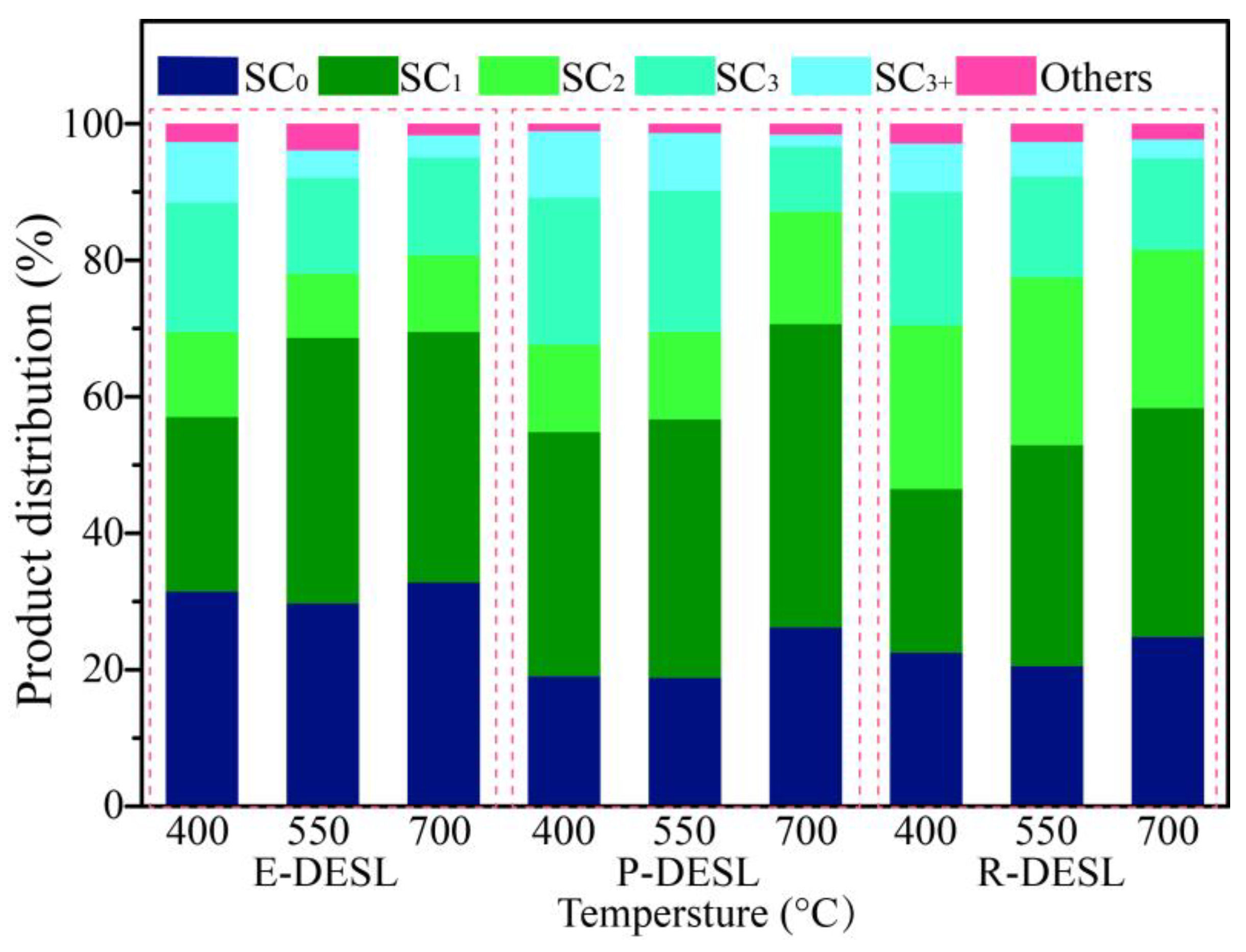
Figure 6.
After the identification of products from DESL pyrolysis at 400 °C, the G-phenols and S-phenols were the main components of E-DESL pyrolysis products (
Figure 3a), G-phenols were the dominant component of P-DESL pyrolysis products (
Figure 3b), and in addition to G-phenols, H-phenols also played a significant role in the R-DESL pyrolysis products, as shown in
Figure 3c, which is mainly due to the thermal fracture of the linkages between the basic structural units of DESLs with poor thermal stability at 400 °C based on the TGA results. It is worth noting that the 4-(2-propanonyl)-guaiacol as the main pyrolysis product of DESLs was produced in large quantities at the pyrolysis temperature of 400 °C, and the distribution of 4-(2-propanonyl)-guaiacol in the three DESLs’ pyrolysis products was consistent with the HK content shown in the 2D HSQC NMR results.
With the pyrolysis temperature gradually increased, more products are released during lignin pyrolysis because of free radical recombination and side-chain breakage [
37]. As shown in
Figure 3a and
Figure 4a–c, compared with G-phenols, the content of S-phenols including syringol, 4-methylsyringol, 4-acetylsyringol, and 4-propenylsyringol was greatly affected by the pyrolysis temperature. This indicated that the S-type structural unit in the E-DESL was thermally unstable and easily degraded by heating during the pyrolysis process. The H-phenols, such as phenol, 2-methylphenol, and 4-vinylphenol, and the C-phenols, including 4-methylcatechol and catechol, were gradually released due to the drastic demethoxylation and demethylation as the pyrolysis temperature increased [
38]. The yield of MAHs, such as
o-xylene and toluene, was also significantly increased due to the occurrence of a deep deoxidation reaction under the pyrolysis condition of 700 °C [
39]. As shown in
Figure 3b,c, compared to the E-DESL, the yield of G-phenols from P-DESL and R-DESL pyrolysis was obviously affected by the pyrolysis temperature. However, the formation of H-phenols, C-phenols, and MAHs from P-DESL and R-DESL pyrolysis was positively correlated with the pyrolysis temperature. The formation of S-phenols from R-DESL pyrolysis was similar to that from E-DESL pyrolysis.
As shown in
Figure 5, the pyrolysis products with a carbon number more than C
9 were produced in large quantities at the pyrolysis temperature of 400 °C, and their content showed a gradually decreasing trend due to the degradation of G-phenols, S-phenols, and pyrolysis products containing long side-chains with the increase in pyrolysis temperature. At the same time, the pyrolysis products without -OCH
3 and with short side-chains, such as H-phenols, C-phenols, and MAHs, gradually increased due to the demethoxylation reaction, and the side-chain fracture reaction intensified with the increase in pyrolysis temperature [
40], resulting in the carbon number distribution of pyrolysis products gradually concentrating in the range of C
6–C
8. At the pyrolysis temperature of 700 °C, the content of pyrolysis products with C
6 was significantly increased compared with the content of that from pyrolysis at 400 °C, which resulted from the massive release of phenol and catechol, as shown in
Figure 4a–c and
Figure 5. The effect of pyrolysis on the lignin side-chain was also further investigated, and it can be seen in
Figure 4a and
Figure 6 that due to the rapid release of syringol and guaiacol from E-DESL pyrolysis at 400 °C, the products with the carbon number distribution of the longest side-chain (SC) of 0 were significantly greater than those from P-DESL and R-DESL pyrolysis. Since the rapid release of phenol mainly occurred in the process of high-temperature pyrolysis, the content of pyrolysis products with SC
0 reached a peak at 700 °C pyrolysis. It was intriguing to find that although the G-phenols and S-phenols gradually decreased with the increase in pyrolysis temperature, 4-methylguaiacol with SC
1 belonging to the G-phenols showed a positive correlation with pyrolysis temperature. In addition, the content of catechol derived from the degradation of the G-type structural unit was also positively correlated with the pyrolysis temperature (
Figure 4a–c). These results indicated that the C
α–C
β of the G-type structural unit in the DESLs was easily broken via heating during pyrolysis, and therefore, the 4-methylguaiacol and 4-methylcatechol with strong thermal stability were formed. This was also the main reason as to why the pyrolysis products with SC
1 increased with the pyrolysis temperature increasing. The trend of pyrolysis products with SC
1 changing with the temperature was more significant in the distribution of products from the pyrolysis of the P-DESL consisting mainly of G-type structural units, as shown in
Figure 6. Different from the E-DESL and P-DESL, the content of products with SC
2 from E-DESL pyrolysis significantly increased. This result was mainly attributed to the release of a large amount of 4-vinylphenol directly derived from the H-type structural unit with SC
2 during E-DESL pyrolysis.
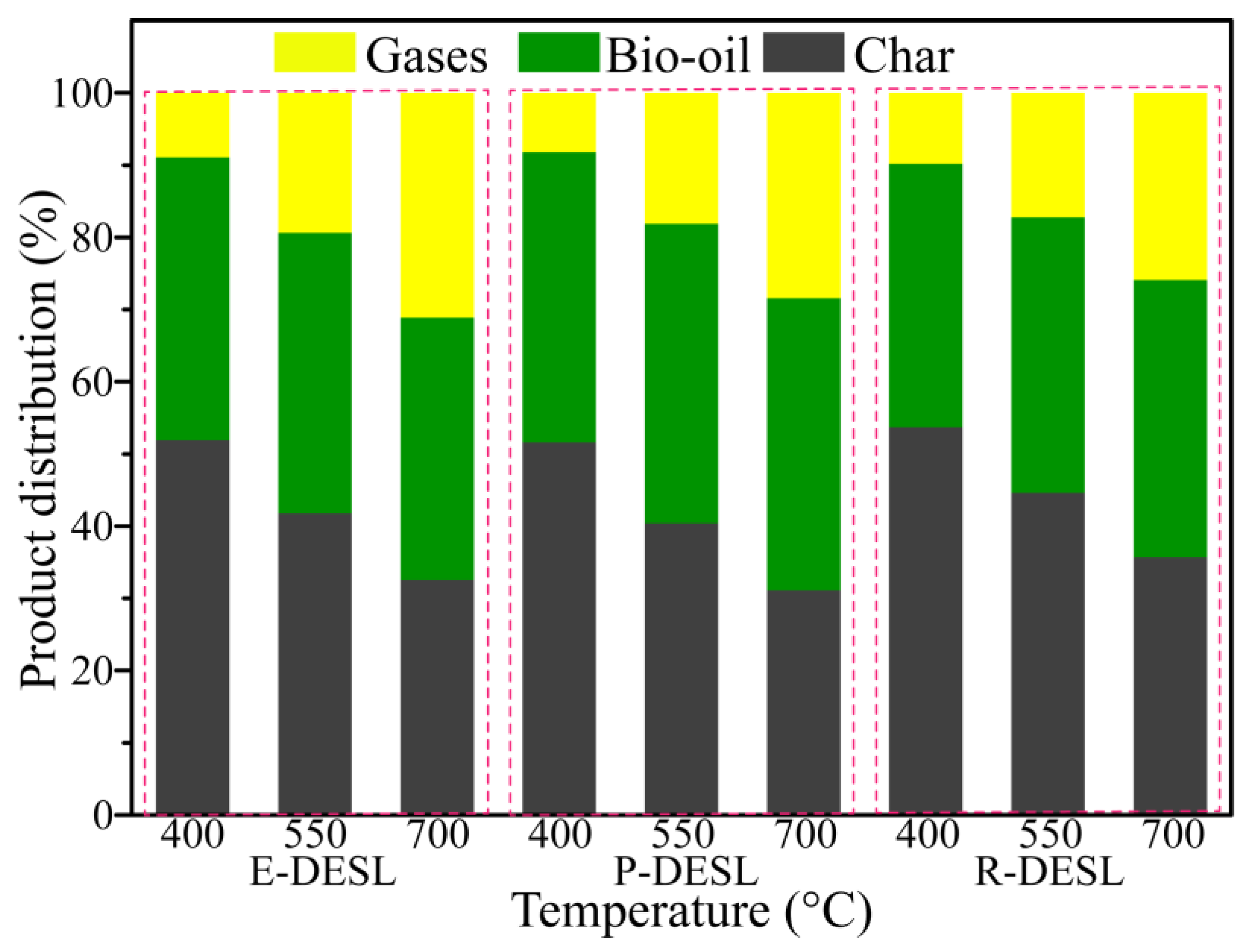
The distributions of three-phase products from DESL pyrolysis at different temperatures were also investigated. As shown in
Figure 7, with the pyrolysis temperature increasing, the content of char gradually decreased, and the content of the gases had a gradually increasing trend due to the breakage of side-chains and oxygen-containing bonds between the basic structural units of the DESLs. This means that the yield of bio-oil derived from P-DESL pyrolysis was greater than that from the E-DESL and R-DESL. In addition, the yield of char derived from the P-DESL was lower than that from the other DESLs. This was probably attributed to several reasons: in comparison with the E-DESL and R-DESL, the P-DESL had lower content of condensed structures (
Table 6); a small number of β-O-4 bonds still existed in the P-DESL; and the higher H/C
eff was another reason for the char decrease in P-DESL pyrolysis [
41,
42]. It can also be seen in
Figure 7 that the content of pyrolysis gases released mainly through the degradation of the lignin side-chain and the removal of methoxy occupied the highest proportion in the E-DESL but the lowest proportion in the R-DESL when the pyrolysis temperature was above 550 °C. This might be due to the existence of a considerable content of S-type units to provide a large amount of methoxy for the E-DESL (
Table 6).