Abstract
Vibriosis and parasitic leech infestations cause the death of various farmed fish, such as groupers, hybrid groupers, sea bass, etc., in Malaysia and other Southeast Asian countries. In the absence of natural control agents, aquaculture operators rely on toxic chemicals to control Vibrio infections and parasitic leeches, which can have a negative impact on the environment and health. In the present study, we investigated the antivibrio and antiparasitic activities of the aqueous extract of giant sword fern (GSF) (Nephrolepis biserrata, Nephrolepidaceae, locally known as “Paku Pedang”) against four Vibrio spp. and the parasitic leech Zeylanicobdella arugamensis, as well as its metabolic composition using the ultra-high-performance liquid chromatography–high-resolution mass spectrometry system (UHPLC-HRMS). The data show that the aqueous extract of GSF at a concentration of 100 mg/mL exhibits potent bactericidal activity against V. parahaemolyticus with a zone of inhibition of 19.5 mm. In addition, the extract showed dose-dependent activity against leeches, resulting in the complete killing of the parasitic leeches within a short period of 11–43 min when tested at concentrations ranging from 100 to 25 mg/mL. The UHPLC-HRMS analysis detected 118 metabolites in the aqueous extract of GSF. Flavonoids were the primary metabolites, followed by phenolic, aromatic, fatty acyl, terpenoid, vitamin and steroidal compounds. Notably, several of these metabolites possess antibacterial and antiparasitic properties, including cinnamaldehyde, cinnamic acid, apigenin, quercetin, cynaroside, luteolin, naringenin, wogonin, 6-gingerol, nicotinamide, abscisic acid, daidzein, salvianolic acid B, etc. Overall, our study shows the significant antibacterial and antiparasitic potential of the GSF aqueous extract, which demonstrates the presence of valuable secondary metabolites. Consequently, the aqueous extract is a promising natural alternative for the effective control of Vibrio infections and the treatment of parasitic leeches in aquaculture systems.
1. Introduction
Vibriosis is a bacterial disease that poses a significant threat to fish and shellfish in aquaculture systems. It is primarily caused by several species of Vibrio bacteria, including V. parahaemolyticus, V. alginolyticus, V. anguillarum and V. harveyi. Of these, V. parahaemolyticus stands out as one of the most dominant pathogens. These Vibrio bacteria are capable of infecting a wide range of fish species, such as sea bass, grouper, etc. [1,2]. Outbreaks of vibriosis in aquaculture farms are triggered by a combination of factors, including changes in the physicochemical properties of the water and overpopulation [3]. In addition to vibriosis, the aquaculture industry also has to deal with the problem of parasite infestation. A well-known example is the parasitic leech Zeylanicobdella arugamensis de Silva, 1963 (Hirudinea, Piscicolidae) (Figure 1), which thrives in tropical and subtropical regions along the coasts of several countries, including Malaysia, Thailand, Indonesia, Brunei Darussalam, Singapore, India, Iran, Sri Lanka, the Philippines, Australia and Japan [4,5,6,7]. Leong & Wong (1988) were the first to describe the infestations of unidentified parasitic leeches in Malaysian groupers cultured in floating cages, with a prevalence of 0.4 percent [8]. However, Z. arugamensis has also been regularly isolated in other marine-farmed fish [4,6,9,10], in addition to other parasites [11,12,13]. The parasitic leech has affected various farmed fish, including groupers, hybrid groupers, orange-spotted groupers, sea bass, etc. [6,9,10]. It attaches itself mainly in large numbers to the pectoral, ventral, anal and caudal fins, as well as to the skin folds under the lower jaw, around the eyes and in the mouth areas. Infected fish often show frayed fins, hemorrhages and larger wounds at the feeding and attachment site of the parasite [9]. Infected fish experience rapid mortality and the parasitic leeches are also thought to contribute to Vibriosis [5,9,14]. The mortality caused by Vibrio infections and these parasitic leeches has caused considerable damage to the aquaculture industry and resulted in significant economic losses [2,15].

Figure 1.
Parasitic leech Zeylanicobdella arugamensis.
In the aquaculture industry, the use of toxic chemicals is a common practice to control Vibriosis and prevent parasite infestations [2,16,17]. However, it is important to recognize that the use of these pollutants has serious negative impacts on fish, humans and the ecosystem as a whole [17,18]. It is therefore important to prioritize the development of biological control agents that effectively control Vibriosis and manage parasitic leeches.
Giant sword fern (GSF), known as Nephrolepis biserrata, is a medicinal plant belonging to the Nephrolepidaceae family and is locally known as “Paku Pedang”. The plant holds considerable potential as a source of metabolites with antiparasitic properties. In our previous studies, we investigated the antiparasitic potential of the methanol extract and fractions of the plant [6,19,20,21]. Building on this preliminary work, our current study aims to explore the antimicrobial potential of the aqueous extract of GSF and to identify several novel antibacterial and antiparasitic metabolites using an ultra-high-performance liquid chromatography–high-resolution mass spectrometry system (UHPLC-HRMS).
2. Results
2.1. Antivibrio Activity
The aqueous extract of GSF at a concentration of 100 mg/mL (0.5 mL) exhibited antivibrio activity against V. parahaemolyticus only compared to other Vibrio spp., including V. alginolyticus, V. anguillarum and V. harveyi (Table 1).

Table 1.
Antivibrio activity of aqueous extract of GSF.
2.2. Antiparasitic Activity
Table 2 shows the antiparasitic activity of the aqueous extract of GSF. Complete parasite mortality was noticed after exposure to the various concentrations of the aqueous extracts. Higher concentrations of the aqueous extract (100 mg/mL) resulted in parasitic leeches’ mortality in a shorter time compared to medium (50 mg/mL) and low concentrations (25 mg/mL). On the other hand, no mortality was recorded in the control group treated with seawater only.

Table 2.
Time till death and percentage of all parasitic leeches at different concentrations of GSF aqueous extract.
2.3. Physio-Chemical Parameters
Table 3 lists physio-chemical parameters, such as temperature, pH, salinity and dissolved oxygen. All the metrics are constant, except the pH values of the plant extracts, which differ slightly from the control groups.

Table 3.
Water quality parameters of the solutions of the normal control and treatment groups.
2.4. Metabolites Detected in the Aqueous Extract of GSF
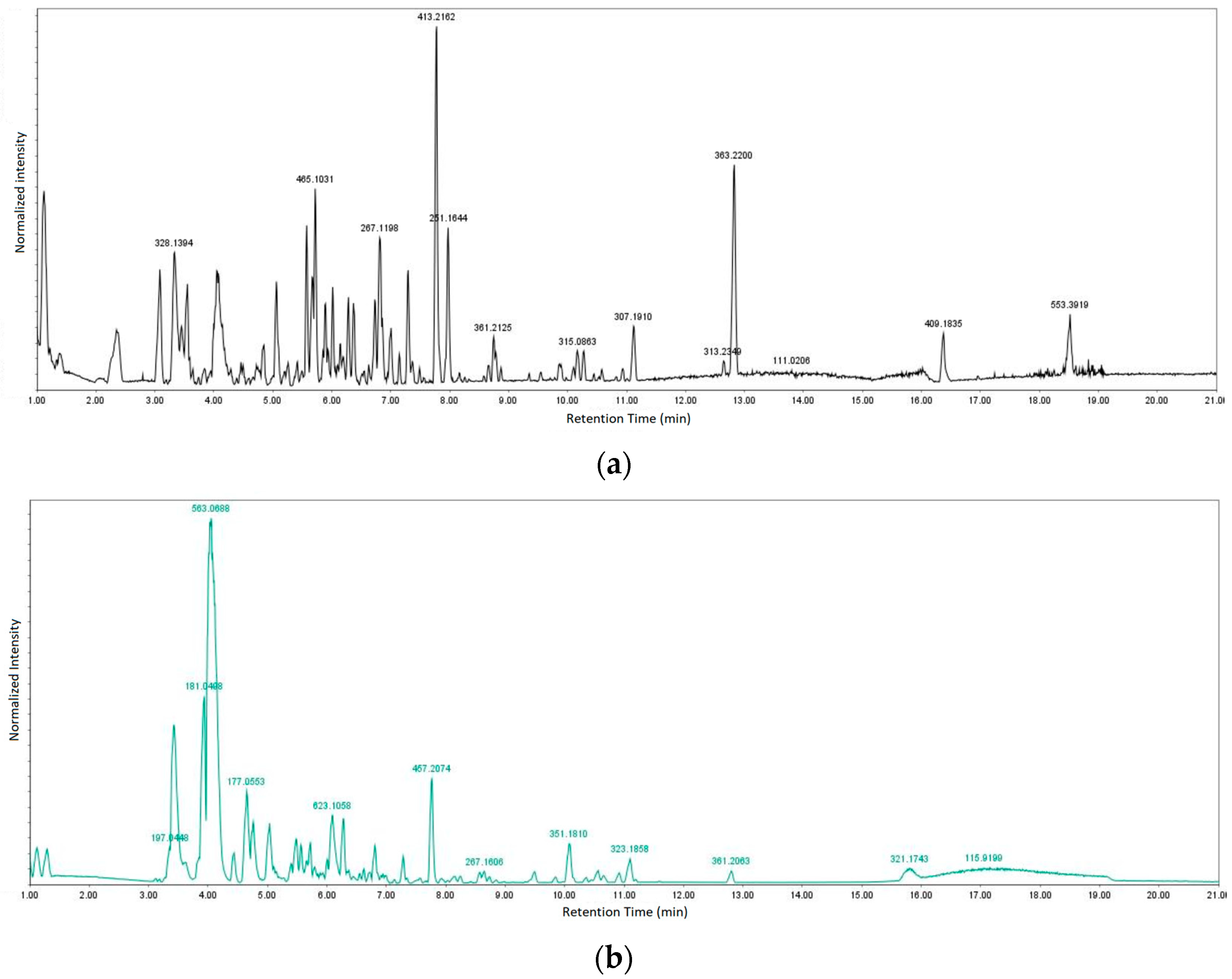
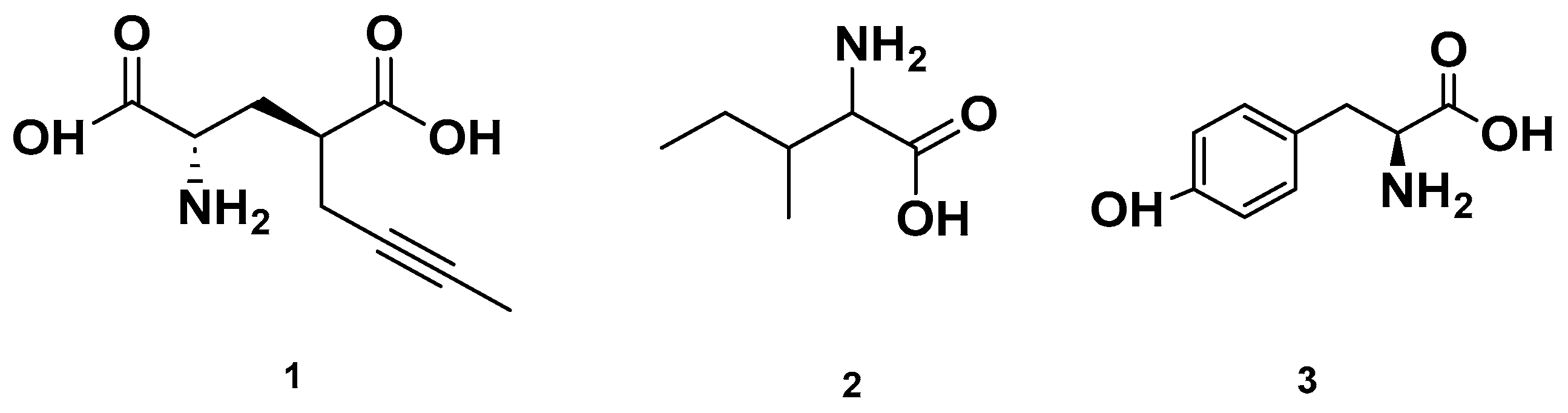
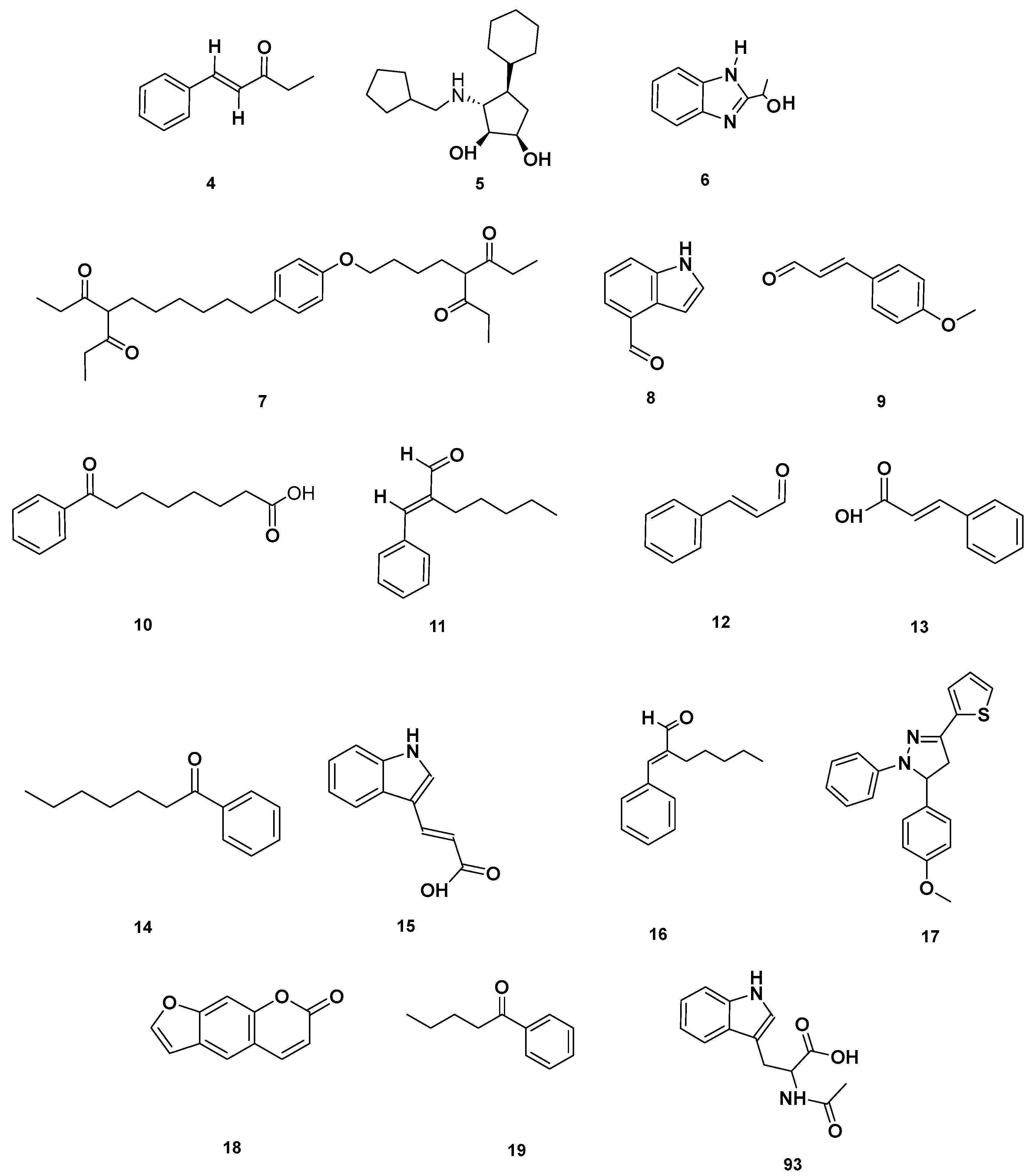
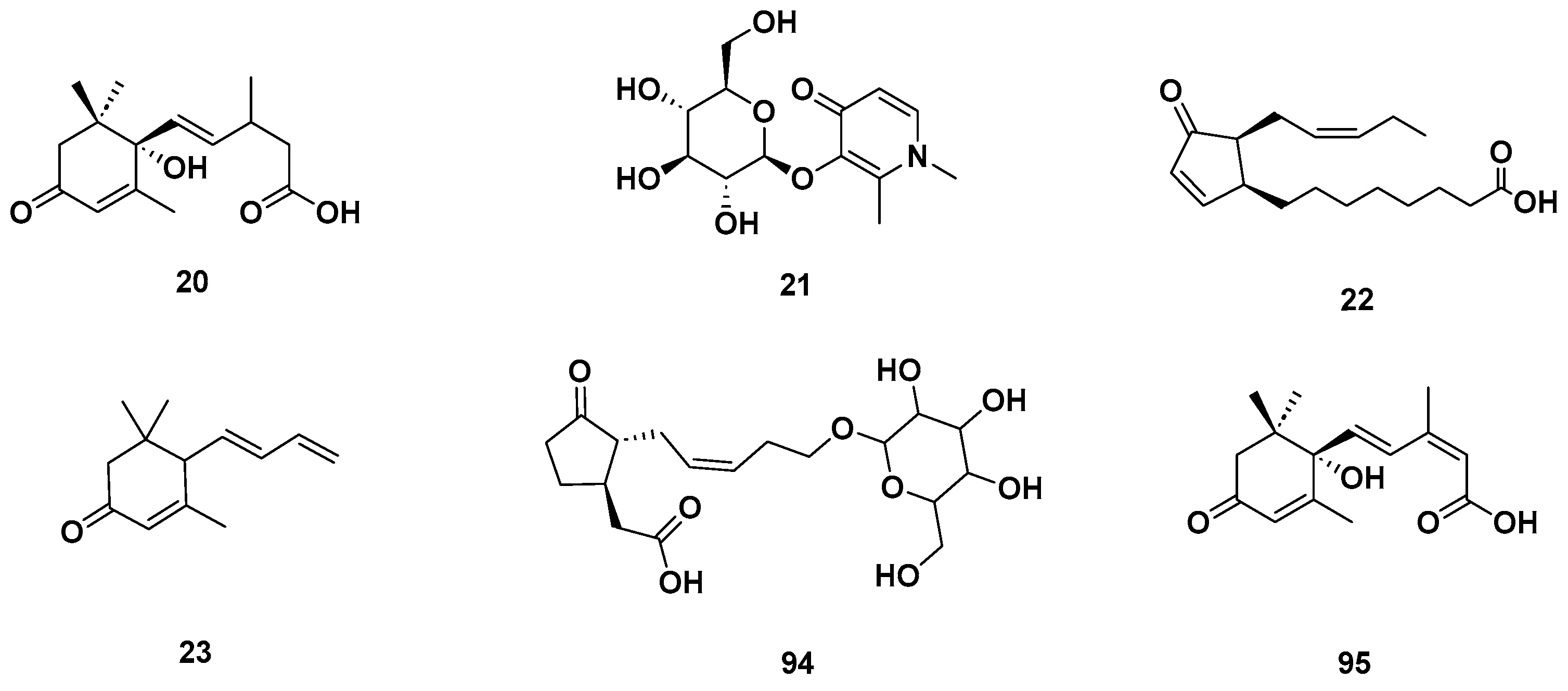
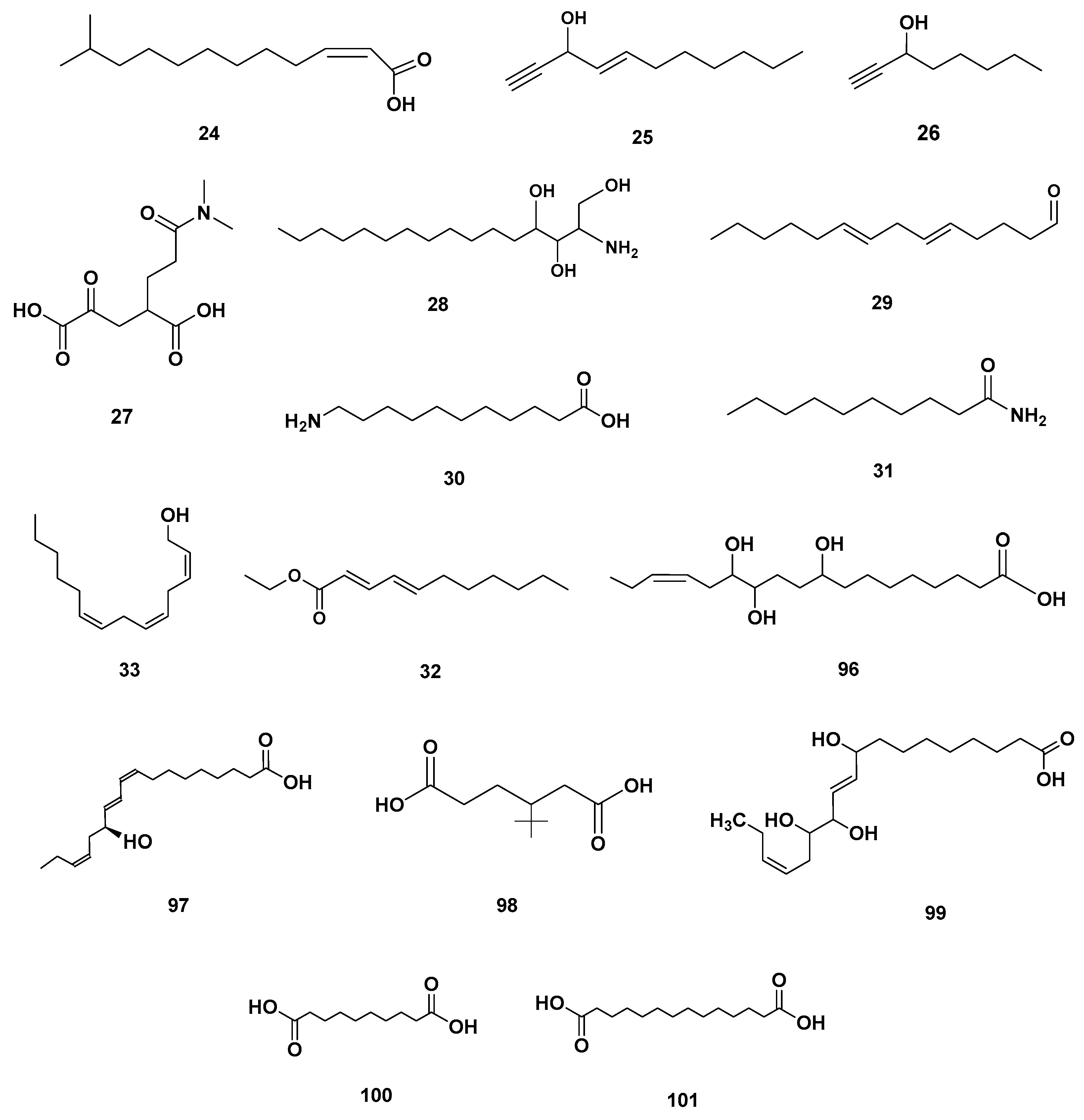
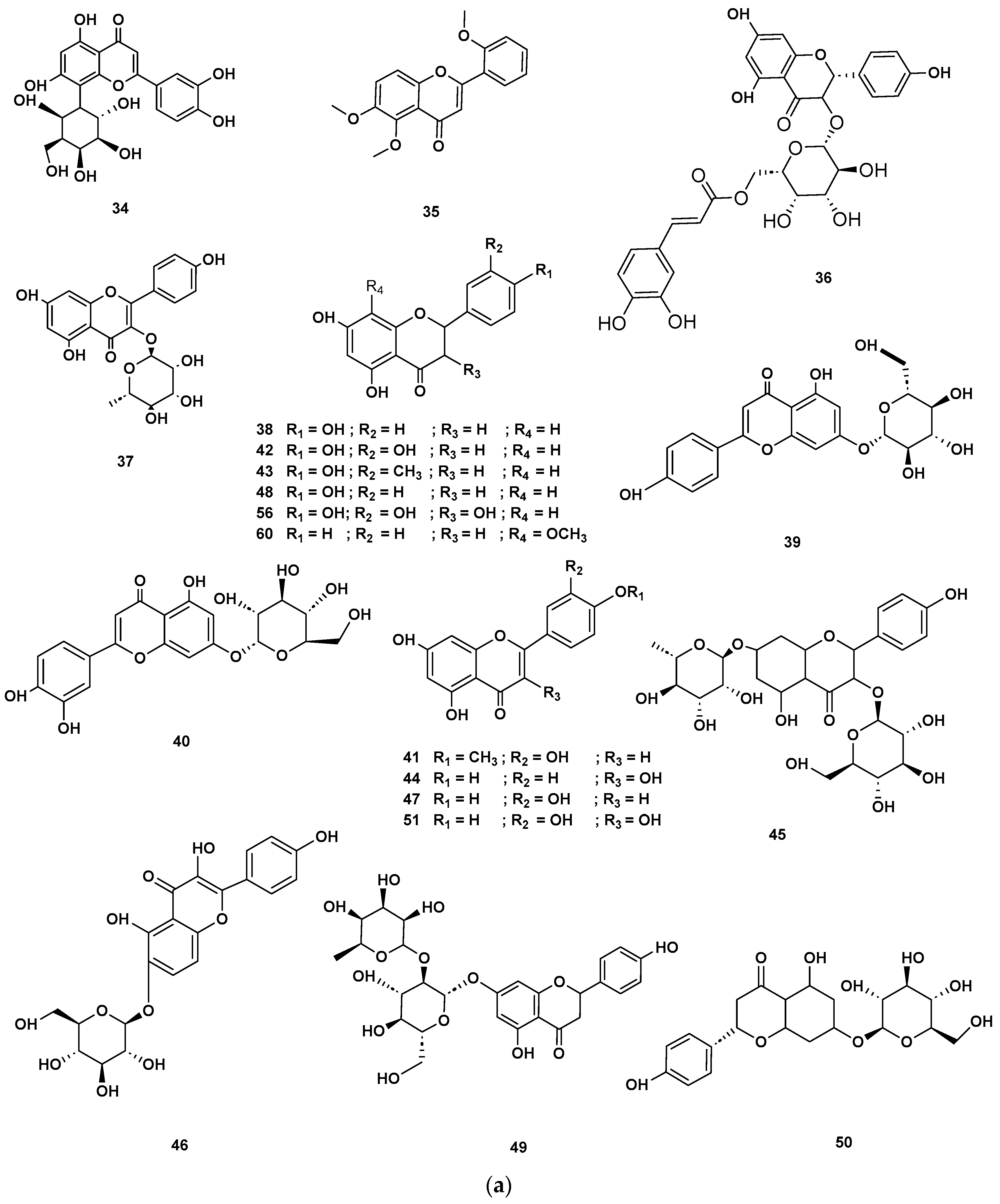
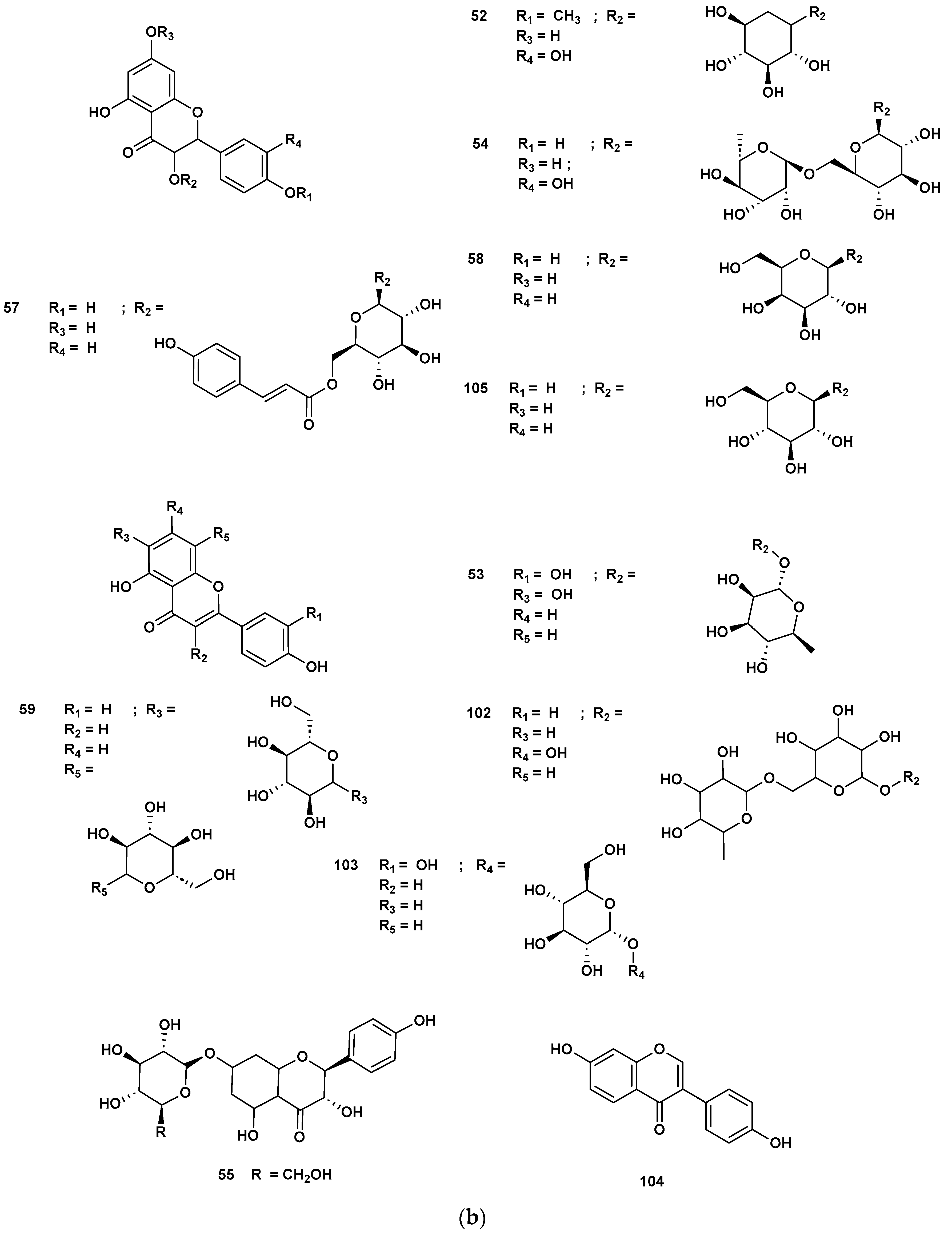
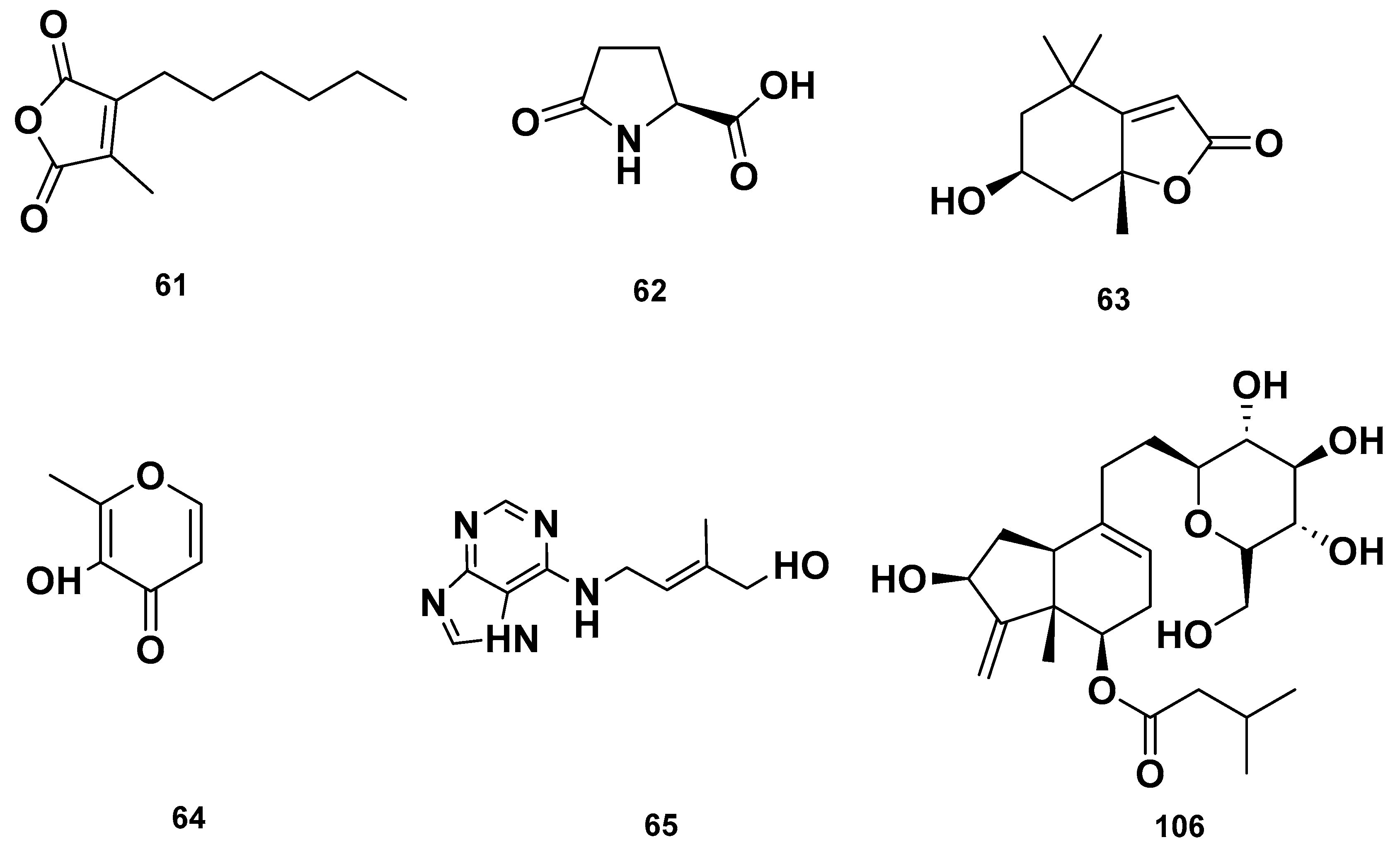
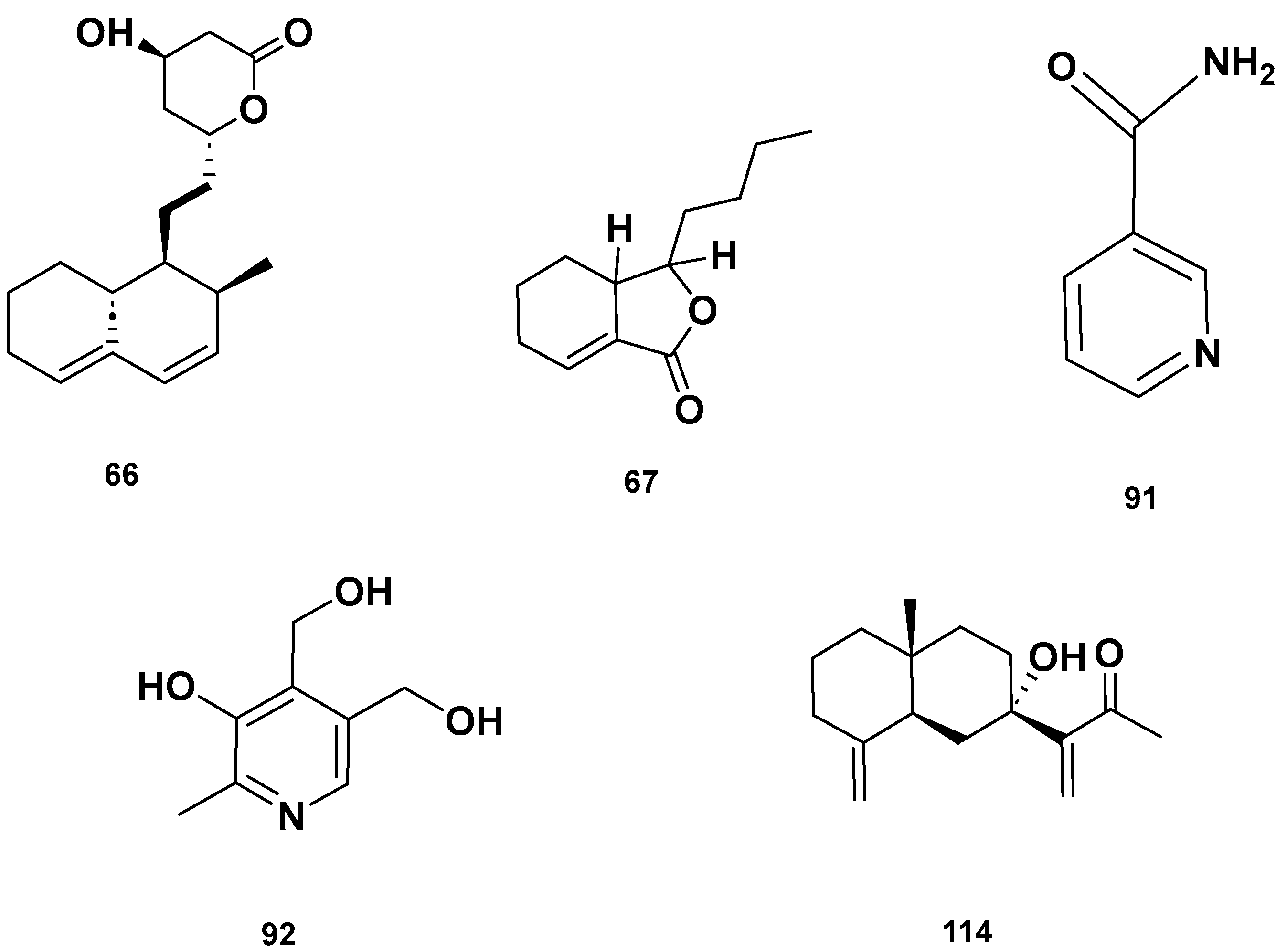
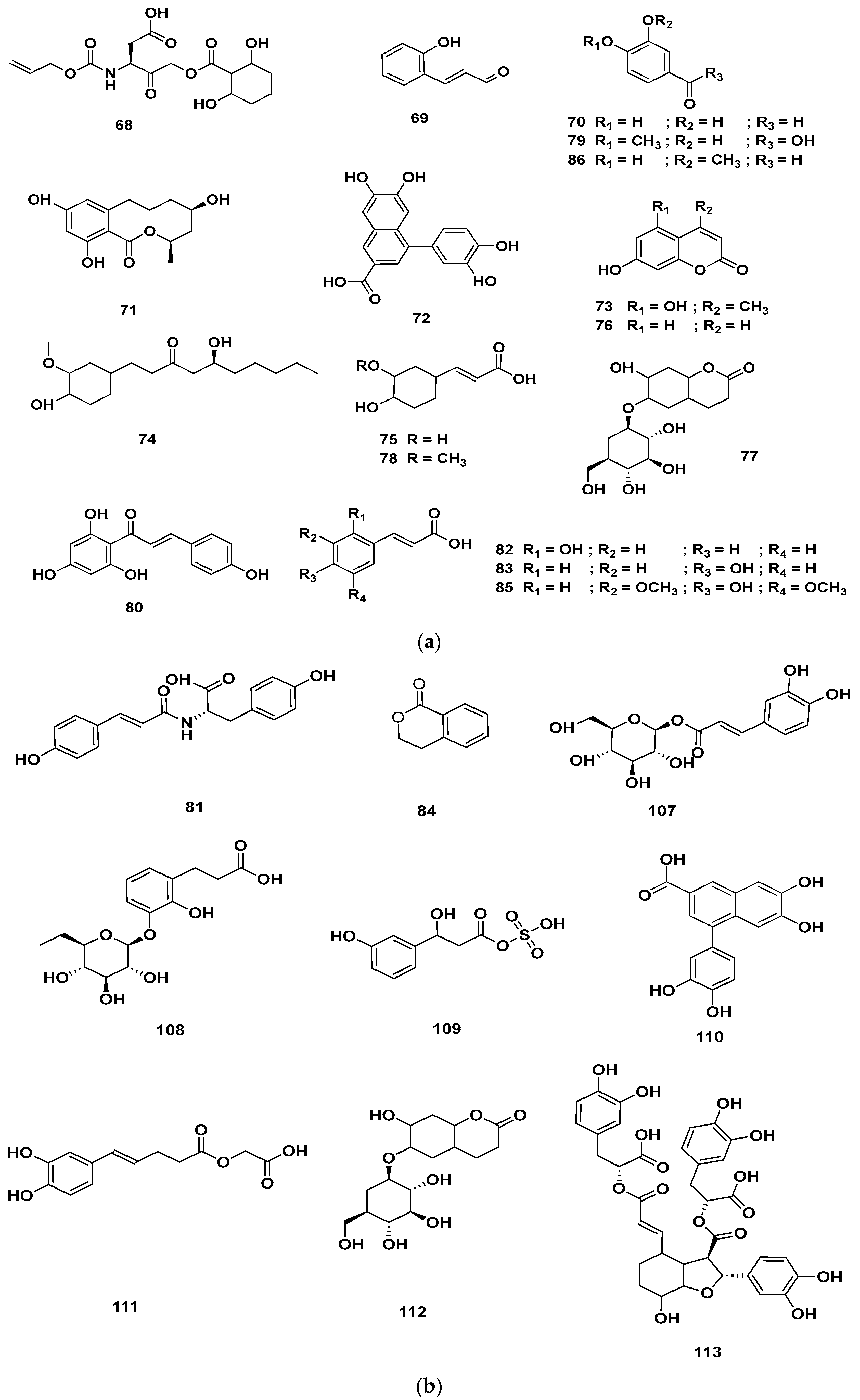
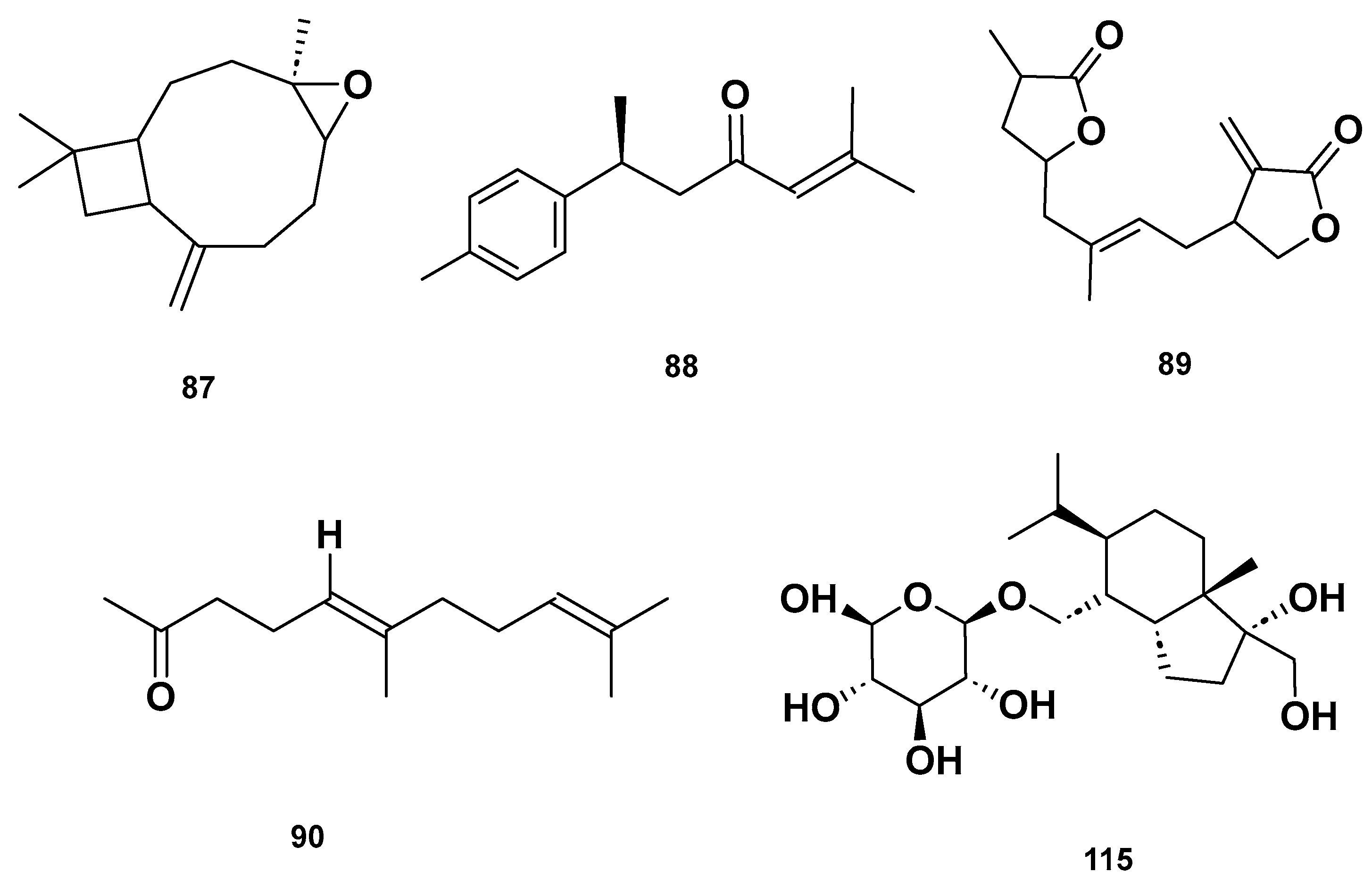
Table 4 and Table 5 list the names, molecular formulas and classes of metabolites identified in the aqueous extract of GSF using ultra-high-performance liquid chromatography–high-resolution mass spectrometry system (UHPLC-HRMS). The chromatograms for positive and negative modes of data acquisition are shown in Figure 2. Overall, 118 metabolites were effectively matched, with amino acids accounting for 3, aromatics for 18, cyclic ketones for 6, fatty acyl for 16, flavonoids for 32, heterocyclic for 6, lactone for 2, phenolics for 26, polycyclic for 1, steroid for 1, terpenoid for 5 and vitamin B2 for 2. The chemical structures of the metabolites are exhibited in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11 according to their respective chemical groups.

Table 4.
Metabolite profiles in aqueous extract of GSF analyzed via positive mode UHPLC-HRMS.

Table 5.
Secondary metabolite profiles in aqueous extract of GSF analyzed via negative mode UHPLC-HRMS.
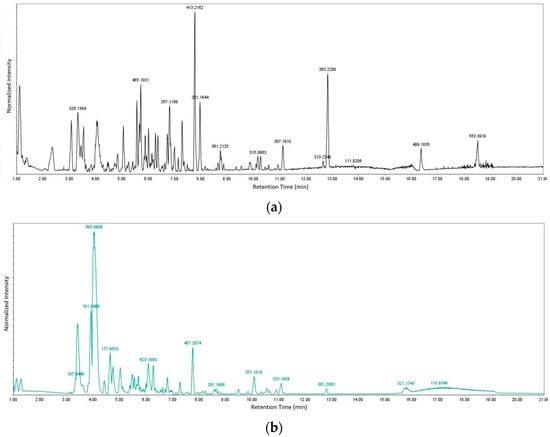
Figure 2.
Chromatograms for (a) positive and (b) negative modes of data acquisition.
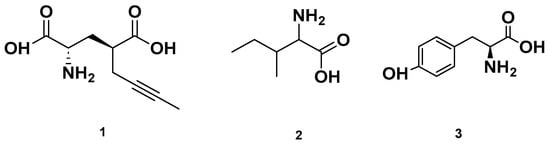
Figure 3.
Chemical structures of amino acids detected in GSF.
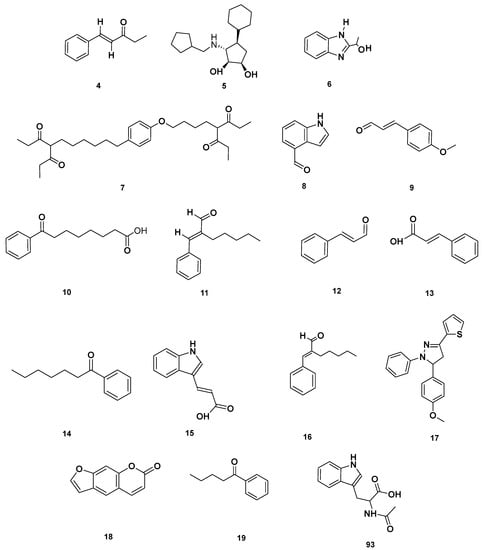
Figure 4.
Aromatic compounds identified in GSF extracts.
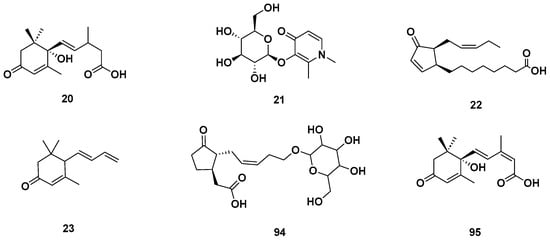
Figure 5.
Cyclic ketones identified in GSF extracts.
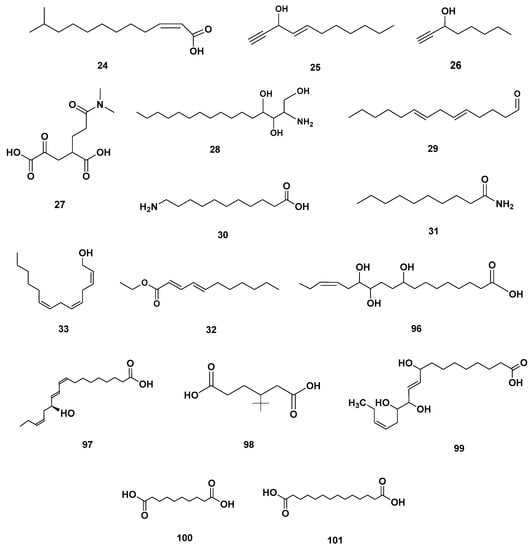
Figure 6.
Fatty acyl identified in GSF extracts.
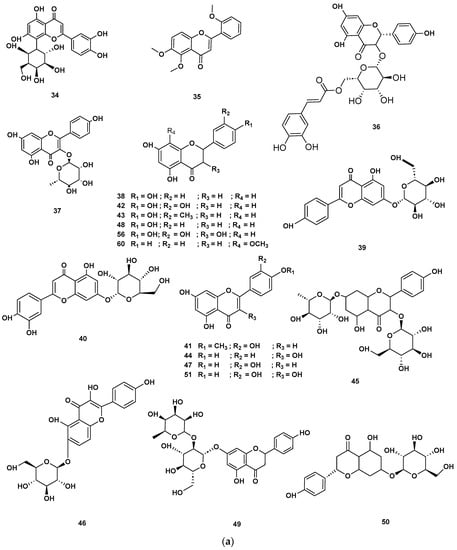
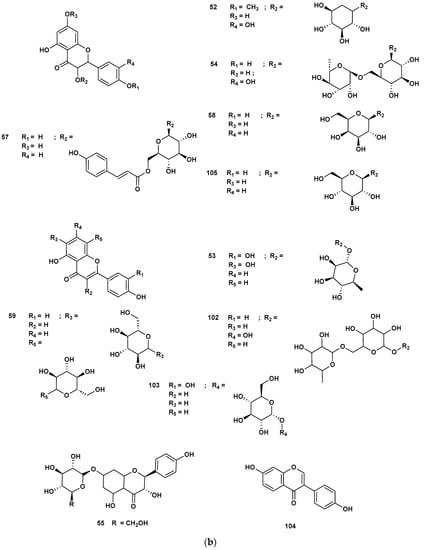
Figure 7.
(a) Chemical structures of flavonoids identified in GSF extracts. (b) Chemical structures of additional flavonoids identified in GSF extracts.
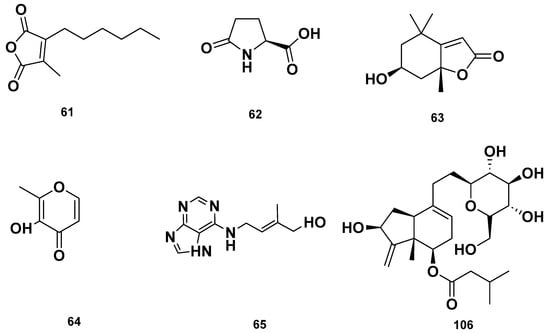
Figure 8.
Heterocyclic compounds detected in GSF extracts.
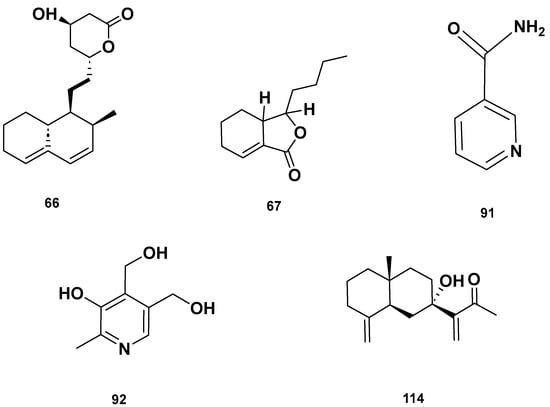
Figure 9.
Lactone (67–68), Vitamin B (92–93) and polycyclic (114) metabolites in the GSF extracts.
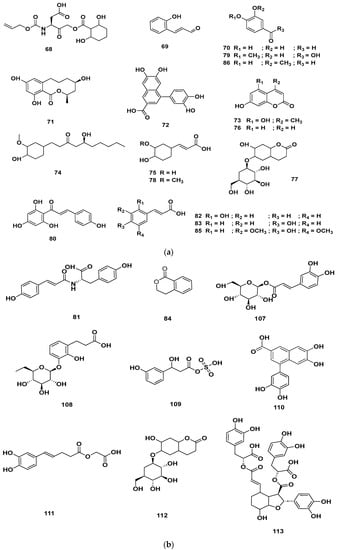
Figure 10.
(a) Chemical structures of phenolics identified in GSF extracts. (b) Chemical structures of additional phenolics identified in GSF extracts.
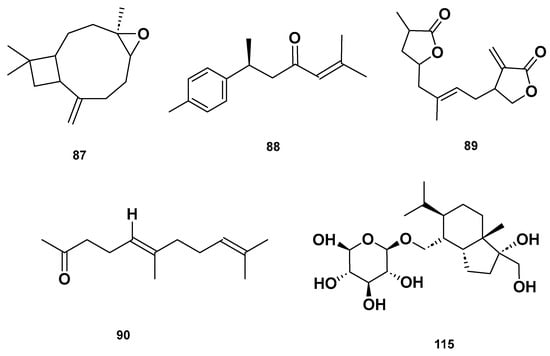
Figure 11.
Chemical structures of terpenoids in the GSF extracts.
3. Discussion
Utilizing natural control agents to effectively combat bacterial infections and manage parasitic infestations is a viable and good alternative due to the existence of several beneficial metabolites [23]. In the current study, we aimed to test the aqueous extract of GSF against Vibrio spp., which is known to cause secondary infection in the infested host fish [5,9,14]. Our results displayed that the aqueous extract of GSF exhibited significant inhibition of V. parahaemolyticus at a 100 mg/mL concentration; however, no inhibition was noticed against V. alginolyticus, V. anguillarum and V. harveyi. This finding is consistent with previous research that has reported the antivibrio property of the methanol and chloroform extracts from the same plant against V. parahaemolyticus [24]. However, in the current study, the aqueous extract of GSF demonstrated enhanced antivibrio activity against V. parahaemolyticus, as evidenced by a larger diameter of the zone of inhibition measuring 19.5 mm. In comparison, the methanol and chloroform extracts have only displayed zone of inhibition measuring 8 and 6 mm, respectively [24]. Our results displayed that the aqueous extract of GSF possesses better antivibrio potential against V. parahaemolyticus compared to the previously examined methanol and chloroform extracts.
The aqueous extract of GSF also displayed antiparasitic potential and caused the complete death of the parasitic leech, Z. arugamensis, in a dose-dependent manner. At doses ranging from 100 to 25 mg/mL, all the parasitic leeches were killed in 11–43 min, whereas the methanol extract and fraction 3 at concentrations of 100 and 2.5 mg/mL of the same plant killed the leeches in an average time of 4 and 1.9 min, respectively [6,21]. It appears, based on our previously published data that the methanol extract of the plant caused parasitic leeches to die faster than the aqueous extract. On the other hand, the aqueous extract of GSF revealed different metabolites compared to the methanol extract and fractions. The methanol and aqueous extracts of the neem plant have been reported to have antiparasitic activity against Z. arugamensis. The extract, at a concentration of 100 mg/mL, has resulted in the mortality of parasitic leeches within a time limit of 9 and 6 min, respectively. The methanol leaves extract of Dillenia suffruticosa at concentrations of 100 mg/mL has resulted in the complete mortality of parasitic leeches in 14 [25]. The aqueous extracts of some of the tropical plants, such as Melastoma malabathricum, Piper betle, Tetracera indica and Etlingera coccinea, have been tested in vitro for potential anti-leech Z. arugamensis activity. The anti-leech activity has been determined by exposing Z. arugamensis to 20 μL of plant extract (500 mg/mL) for 5 min in a 24-well plate. The effects of aqueous extracts of all the mentioned plants on the leeches were very rapid, causing death in less than 5 min [26]. The published data indicated that plant extracts can be applied as a natural control against parasitic leeches. In addition, plant extracts have also been reported to have antiparasitic activity against other classes of parasitic leeches, which include Allium sativum against the leech Limnatis nilotica. The methanol extract of A. sativum at concentrations of 600 µg/mL resulted in the death of leeches in 68 min [27]. The anti-leech effects of methanol extracts of Cassia alata, Costus afer, Ficus sur and Platostoma africanum have been reported against the parasitic leech Hirudo medicinalis [28].
The UHPLC-HRMS analysis displayed the metabolic composition of the aqueous extract of GSF. This analysis enabled us to identify several classes of metabolites, including flavonoids, phenolics, fatty acyls, etc. Notably, flavonoids and phenolics were found to be the dominant metabolites in the extracts. These classes of metabolites have been identified in the extract of other plant species and have been associated with antioxidant, antimicrobial, anti-inflammatory and antiparasitic potential [29,30,31,32,33,34,35]. However, we have not detected any peptides or large lipids in the aqueous extract of our sample, which might be attributed to the aqueous extract’s lower affinity for peptides and lipids in comparison to methanol extraction. In contrast, our analysis of the aqueous extract of GSF revealed more metabolites compared to our previous examination of the methanol extract [6]. This demonstrates that the extraction efficiency of GSF with the aqueous solution is higher than that of methanol, an enhancement likely due to the boiling effect. Additionally, our profiling was specifically targeted within the 100–1500 m/z range.
Metabolites, such as abscisic acid [36], daidzein [37], quercetin-3β-D-glucoside [38] and salvianolic acid B [39,40], have been reported to possess antibacterial properties. Among the amino acids identified in the aqueous extracts, isoleucine and tyrosin were prominent. These amino acids play an important role as antioxidant agents [30,31]. Additionally, aromatic compounds, such as cinnamaldehyde and cinnamic acid, have been found to possess antiparasitic activity against Dactylogyrus intermedius monogenean parasites [29]. Flavonoid compounds, including apigenin and quercetin, have been found to possess antiparasitic efficacy against Leishmania tropica amastigotes, Cryptosporidium parvum and Encephalitozoon intestinalis [32,33]. Cynaroside has been found to possess antiparasitic properties against the protozoan Leishmania donovani [41], while eriodictyol and kaempferol have shown antiparasitic activity against Plasmodium falciparum and P. berghei [42,43]. Luteolin has been reported to display antiparasitic activity against L. donovani [44], and naringenin has shown promise in combating Eimeria spp. Parasite [45]. Wogonin has also exhibited antiparasitic activity against Schistosoma mansoni [46]. Furthermore, phenolic compounds, like 6-gingerol in combination with amphotericin B, have been found to have antiparasitic potential against Leishmania infection [47]. Nicotinamide, a type of vitamin B, has demonstrated antiparasitic activities against P. falciparum, Trypanosoma cruzi.
The presence of these antibacterial and antiparasitic metabolites in the aqueous extract of GSF suggests that they may be responsible for the inhibition of Vibrio spp. and the elimination of parasitic leeches. However, it is essential to conduct further research to isolate these antibacterial and antiparasitic metabolites and access their efficacy in their pure form against the Vibrio spp. and parasitic leeches.
Furthermore, our research also revealed that the aqueous extract of GSF contained more diverse metabolites compared to the previously reported methanol extract of the same plants, particularly in terms of flavonoid and phenolic metabolites [6].
4. Materials and Methods
4.1. Sample Collection and Extraction
The aerial parts of the plant were taken from Universiti Malaysia Sabah. The plant was recognized and a voucher specimen was deposited at Universiti Malaysia Sabah, Kota Kinabalu. The leaves of the plant were cleaned with distilled water and oven dried at 37 °C. A high-capacity mill was used to grind the dried plant separately. In total, 100 g of the dry plant powder was boiled with distilled water in a ratio of 1:10 for 10 min on a stir plate. The decoctions were drawn off and allowed to cool at room temperature for 1 h. The extracts were filtered through a strainer to remove coarse residues, and the filtrate was then filtered again using Whatman No. 1 filter paper. The filtrate was lyophilized using a freeze dryer after being held at −80 °C for 24 h.
4.2. Vibrio Strains and Stock Preparation
The antivibrio activities of the extract were tested against four different species of Gram-negative bacteria of the genus Vibrio, including V. alginolyticus (ATCC17749), V. anguillarum (ATCC19264), V. harveyi (ATCC35084) and V. parahaemolyticus (ATCC17802). The bacterial stock was obtained from the Fish Disease Laboratory, Borneo Marine Research Institute, Universiti Malaysia Sabah. The bacterial strains were thawed and cultured. Approximately 50 µL of the bacteria were cultured in 5 mL tryptone soy broth (TSB) containing 2% sodium chloride and incubated for 24 h at 25 °C in a shaking incubator.
4.3. Antivibrio Activity
The antivibrio activity of the extract was evaluated using the disc diffusion method with slight modifications [48]. Tryptone soy agar plates were used in a biosafety chamber to simulate aseptic conditions and prevent contamination. The agar plates were divided into two sections to test the same extract at the same concentration. Whatman filter discs (8 mm diameter) were inserted into the nutrient agar plates using a sterile cork borer (5 mm), and the bacteria were spread on the solid plates using a sterile swab moistened with the bacterial suspension. Then 100 μL of the aqueous extract (100 mg/mL) was added to the discs prepared in the inoculated plates. As a positive control, filter paper discs containing oxytetracycline (10 µg in 2 mL DMSO) were utilized. The plates were incubated at 37 °C for 24 h, and the zone of inhibition, if any, around the wells was measured in mm.
4.4. Parasitic Leech Collection
The parasitic leeches, Z. arugamensis, ranging in length from 1 to 1.5 cm, were obtained from the aquaculture facilities of Borneo Marine Research Institute, Universiti Malaysia Sabah. A hybrid grouper (Epinephelus fuscoguttatus × E. lanceolatus) (20–350 g) infested with parasitic leeches were placed in a small tank containing seawater from the cage, and leeches were removed individually by hand. The separated leeches were placed into a container containing filtered seawater.
4.5. Antiparasitic Activity
Healthy and adult parasitic leeches were selected and divided into 5 groups. Each group was provided with 6 parasitic leeches in a Petri dish.
Group 1 was treated with seawater only (negative control).
Group 2 was treated with 0.25% formalin solution (positive control).
Group 3 was treated with 25, mg/mL of the aqueous extract of GSF.
Group 4 was treated with 50, mg/mL of the aqueous extract of GSF.
Group 5 was treated with 100, mg/mL of the aqueous extract of GSF, respectively.
The aqueous extract was dissolved in filtered seawater and applied to the parasitic leeches in a Petri dish. During the experiment, inactivity, mortality time and percentage of the parasite leeches were recorded [21].
4.6. Liquid Chromatography
Liquid chromatography (LC) was performed using a Dionex UltiMate 3000 UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a Thermo Syncronis C18 column (2.1 mm × 100 mm × 1.7 μm; Thermo Fisher Scientific, Waltham, MA, USA), which was maintained at 55 °C and a flow rate of 450 µL/min during analysis. The mobile phases consisted of solvent A (water added with 0.1% formic acid) and solvent B (acetonitrile added with 0.1% formic acid). The gradient elution program was started at 0.5% of solvent B for 1 min, then from 0.5% to 99.5% of solvent B for 15 min and held for 4 min. The columns were then conditioned for 2 min before the next injection. The injection volume was set to 2 µL.
4.7. Data Acquisition
MS and MS/MS data were acquired using the Thermo Scientific Q Exactive HF Orbitrap mass spectrometry system (Thermo Fisher Scientific, Waltham, MA, USA), which is equipped with a heated electrospray ionization (HESI) probe. Data acquisition was set between an m/z of 100–1000 for MS and 200–1000 for MS /MS. The resolutions of the MS and MS/MS data were acquired at 60k and 15k, respectively. Positive and negative HESI were both used at 3.5 kV and 3.0 kV, respectively. Ion source conditions were set as follows: capillary temperature of 320 °C, sheath gas flow rate of 50, the aux gas flow rate of 18, sweep gas flow rate of 0 and aux gas heater temperature of 300 °C. Calibrations were performed with Pierce LTQ ESI Positive Calibrations solution and Pierce LTQ ESI Negative Calibrations solution (Thermo Fisher Scientific, Waltham, MA, USA) before analyzing the samples.
4.8. Data Analysis
The recorded data were processed and analyzed using Thermo Scientific Compound Discoverer 3.0 software (Thermo Fisher Scientific, Waltham, MA, USA) with the default settings for compound identification. Briefly, the default workflow includes background subtraction with blank data, retention time alignment, feature detection, elemental composition determination, library matching and fragment ion search (FISh) scoring. Compound identification was primarily based on matching MS/MS data with the mzCloud and mzVault databases. Mismatched signals were attempted with the ChemSpider database using MS data and supported with a FISh scoring of above 50.
4.9. Statistical Analysis
The IBM SPSS Statistics 25 Window package (IBM, Armonk, NY, US) was used to analyze the data. Significant differences between groups were determined using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. The means and standard error of the means (S.E.) were used to present all results. The p-values of less than 0.05 were considered significant.
5. Conclusions
The results of the current study are very promising, highlighting the potential of the aqueous extract of giant sword fern (GSF) in inhibiting Vibrio spp., especially V. parahemlyticus, and eliminating parasitic leeches. Analysis using the ultra-high-performance liquid chromatography–high-resolution mass spectrometry system revealed the presence of 118 compounds, with flavonoid metabolites accounting for the majority, followed by phenolic, aromatic, fatty acyl, terpenoid, vitamin and steroidal metabolites. Cinnamaldehyde, cinnamic acid, apigenin, quercetin, cynaroside, luteolin, naringenin, wogonin, 6-gingerol, nicotinamide, abscisic acid, daidzein, querce-tin-3β-d-glucoside, salvianolic acid B and others are examples of metabolites with antibacterial and antiparasitic activity. The results suggest that the aqueous extract of GSF can be used as a natural alternative to control Vibrio infections and to treat parasitic leeches in aquaculture systems.
Author Contributions
B.A.V.M.: investigation; methodology, validation, visualization, review and editing. K.P., D.K.C., T.M., M.T.M.L. and E.J.J.: data curation, review and editing. J.K.T.: data curation, formal analysis. Y.S.Y.: data curation, formal analysis, review and editing. W.S.C.: review and editing. O.B. and S.S.: review and editing and funding acquisition. M.D.S.: investigation, methodology, data curation, formal analysis, writing original draft, review and editing, funding acquisition and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research work has been supported by the Universiti Malaysia Sabah Project: SLB2232 to MDS. Russian Scientific Foundation: grant No. 22-16-00044 to OB and SS. UMS Project SDN0073-2019 to BAVM and Ministry of Higher Education, the Fundamental Research Grant Scheme (FRGS) 2022, FRGS/1/2022/WAB05/UMS/02/1 to WSC, FRG0568-1/2022 to WSC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Souza Valente, C.; Wan, A.H.L. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebr. Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Amal, M.N.A.; Yasin, I.S.M.; Zamri Saad, M.; Nasruddin, N.S.; Al-saari, N.; Mino, S.; Sawabe, T. Vibriosis in cultured marine fishes: A review. Aquaculture 2019, 512, 734289. [Google Scholar] [CrossRef]
- Kumar, P.; Thirunavukkarasu, A.R.; Subburaj, R.; Thiagarajan, G. Concept of stress and its mitigation in aquaculture. In Advances in Marine and Brackishwater Aquaculture; Springer: New Delhi, India, 2015; pp. 95–100. ISBN 9788132222712. [Google Scholar]
- Nagasawa, K.; Uyeno, D. Zeylanicobdella arugamensis (Hirudinida, Piscicolidae), a leech infesting brackish-water fishes, new to Japan. Biogeography 2009, 11, 125–130. [Google Scholar]
- Ravi, R.; Shariman Yahaya, Z. Zeylanicobdella arugamensis, the marine leech from cultured crimson snapper (Lutjanus erythropterus), Jerejak Island, Penang, Malaysia. Asian Pac. J. Trop. Biomed. 2017, 7, 473–477. [Google Scholar] [CrossRef]
- Shah, M.D.; Venmathi Maran, B.A.; Haron, F.K.; Ransangan, J.; Ching, F.F.; Shaleh, S.R.M.; Shapawi, R.; Yong, Y.S.; Ohtsuka, S. Antiparasitic potential of Nephrolepis biserrata methanol extract against the parasitic leech Zeylanicobdella arugamensis (Hirudinea) and LC-QTOF analysis. Sci. Rep. 2020, 10, 22091. [Google Scholar] [CrossRef]
- Azmey, S.; Taruna, M.; Taha, H.; Arai, T. Prevalence and infestation intensity of a piscicolid leech, Zeylanicobdella arugamensis on cultured hybrid grouper in Brunei Darussalam. Vet. Parasitol. Reg. Stud. Reports 2020, 20, 100398. [Google Scholar] [CrossRef]
- Leong, T.S.; Wong, S.Y. A comparative study of the parasite fauna of wild and cultured grouper (Epinephelus malabaricus Bloch et Schneider) in Malaysia. Aquaculture 1988, 68, 203–207. [Google Scholar] [CrossRef]
- Cruz-Lacierda, E.R.; Toledo, J.D.; Tan-Fermin, J.D.; Burreson, E.M. Marine leech (Zeylanicobdella arugamensis) infestation in cultured orange-spotted grouper, Epinephelus coioides. Aquaculture 2000, 185, 191–196. [Google Scholar] [CrossRef]
- Kua, B.C.; Azmi, M.A.; Hamid, N.K.A. Life cycle of the marine leech (Zeylanicobdella arugamensis) isolated from sea bass (Lates calcarifer) under laboratory conditions. Aquaculture 2010, 302, 153–157. [Google Scholar] [CrossRef]
- Venmathi Maran, B.A.; Moon, S.Y.; Oh, S.Y.; Soh, H.Y.; Myoung, J.G. Redescription of two Pennellids (Copepoda, Siphonostomatoida) from Korea with a key to species of Peniculus von Nordmann, 1832. Zookeys 2012, 243, 1–14. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Lin, C.L.; Ho, J.S.; Ohtsuka, S.; Venmathi Maran, B.A.; Justine, J. Lou A revision of the family Dissonidae Kurtz, 1924 (Copepoda: Siphonostomatoida). Syst. Parasitol. 2008, 70, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Rajkumar, M.; Sun, J.; Parida, A.; Maran, B.A.V. Integrated biological control of water hyacinths, Eichhornia crassipes by a novel combination of grass carp, Ctenopharyngodon idella (Valenciennes, 1844), and the weevil, Neochetina spp. Chinese J. Oceanol. Limnol. 2011, 29, 162–166. [Google Scholar] [CrossRef]
- Rimmer, M.A.; Glamuzina, B. A review of grouper (Family Serranidae: Subfamily Epinephelinae) aquaculture from a sustainability science perspective. Rev. Aquac. 2019, 11, 58–87. [Google Scholar] [CrossRef]
- Shinn, A.A.P.; Pratoomyot, J.; Bron, J.E.; Paladini, G.G.; Brooker, E.; Brooker, A.J. Economic Impacts of Aquatic Parasites on Global Finfish Production; Global Seafood Alliance: Portsmouth, NH, USA, 2015; pp. 82–84. [Google Scholar]
- Mohamed, S.; Nagaraj, G.; Chua, F.H.C.; Wang, Y.G. The use of chemicals in aquaculture in Malaysia and Singapore. In Use of Chemicals in Aquaculture in Asia: Proceedings of the Meeting on the Use of Chemicals in Aquaculture in Asia, Tigbauan, Iloilo, Philippines, 20–22 May 1996; Arthur, J.R., Lavilla-Pitogo, C.R., Subasinghe, R.P., Eds.; Southeast Asian Fisheries Development Center: Tigbauan, Iloilo, Philippines, 2000; pp. 127–141. [Google Scholar]
- Leal, J.F.; Neves, M.G.P.M.S.; Santos, E.B.H.; Esteves, V.I. Use of formalin in intensive aquaculture: Properties, application and effects on fish and water quality. Rev. Aquac. 2018, 10, 281–295. [Google Scholar] [CrossRef]
- Pitten, F.A.; Kramer, A.; Herrmann, K.; Bremer, J.; Koch, S. Formaldehyde neurotoxicity in animal experiments. Pathol. Res. Pract. 2000, 196, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Langeland, K.A. Natural Area Weeds: Distinguishing Native and Non-native “Boston Ferns” and “Sword Ferns”. 2014. Available online: https://edis.ifas.ufl.edu/publication/AG120 (accessed on 7 March 2023).
- Venmathi Maran, B.A.; Iqbal, M.; Gangadaran, P.; Ahn, B.-C.; Rao, P.V.; Shah, M.D. Hepatoprotective potential of Malaysian medicinal plants: A review on phytochemicals, oxidative Stress, and antioxidant mechanisms. Molecules 2022, 27, 1533. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Tani, K.; Yong, Y.S.; Ching, F.F.; Shaleh, S.R.M.; Vairappan, C.S.; Venmathi Maran, B.A. Antiparasitic potential of chromatographic fractions of Nephrolepis biserrata and liquid chromatography-quadrupole time-of-flight-mass spectrometry analysis. Molecules 2021, 26, 499. [Google Scholar] [CrossRef]
- Murphy, R.C. Tandem Mass Spectrometry of Lipids; Royal Society of Chemistry: London, UK, 2014; ISBN 978-1-84973-827-9. [Google Scholar]
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. Phytotherapy as an alternative for treating fish disease. J. Vet. Pharmacol. Ther. 2015, 38, 417–428. [Google Scholar] [CrossRef]
- Maulianawati, D.; Suharni, S. Antibacterial activity of Nephrolepis biserrata extract against Aeromonas hydrophila and Vibrio parahaemolyticus. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012010. [Google Scholar] [CrossRef]
- Shah, M.D.; Venmathi Maran, B.A.; Iqbal, M.; Ching, F.F.; Mohamad Lal, M.T.; Binti Othman, R.; Shapawi, R. Antiparasitic activity of the medicinal plant Dillenia suffruticosa against the marine leech Zeylanicobdella arugamensis (Hirudinea) and its phytochemical composition. Aquac. Res. 2020, 51, 215–221. [Google Scholar] [CrossRef]
- Wan Norhana, M.N.; Kua, B.C.; Liyana, R. Evaluation of selected plant extracts for in vitro anti-marine leech (Zeylanicobdella arugamensis) activity. Trop. Biomed. 2021, 38, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Abbasi, J.; Mohsenzadegan, A.; Sadeghian, S.; Ahangaran, M.G. Allium sativum L.: The anti-immature leech (Limnatis nilotica) activity compared to Niclosomide. Comp. Clin. Path. 2013, 22, 165–168. [Google Scholar] [CrossRef][Green Version]
- Gbe-Emi Ke, D.; Amawulu, E. Effect of methanolic extract of some selected plants on the mortality of leech (Hirudo medicinalis). Ecologia 2021, 12, 1–6. [Google Scholar] [CrossRef]
- Ling, F.; Jiang, C.; Liu, G.; Li, M.; Wang, G. Anthelmintic efficacy of cinnamaldehyde and cinnamic acid from cortex cinnamon essential oil against Dactylogyrus intermedius. Parasitology 2015, 142, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Cheng, L.; Wang, D.; Qin, G.; Zhang, X.; Zhen, Y.; Wang, T.; Sun, Z. Protective mechanism of leucine and isoleucine against H2O2-induced oxidative damage in bovine mammary epithelial cells. Oxid. Med. Cell. Longev. 2022, 2022, 4013575. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, B.; Behl, C. Cytoprotective antioxidant function of tyrosine and tryptophan residues in transmembrane proteins. Eur. J. Biochem. 2000, 267, 5687–5692. [Google Scholar] [CrossRef]
- Naddaf, N.; Haddad, S. Apigenin effect against Leishmania tropica amastigotes in vitro. J. Parasit. Dis. 2020, 44, 574–578. [Google Scholar] [CrossRef]
- Mead, J.R.; McNair, N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef]
- Soares, M.B.P.; Silva, C.V.; Bastos, T.M.; Guimarães, E.T.; Figueira, C.P.; Smirlis, D.; Azevedo, W.F. Anti-Trypanosoma cruzi activity of nicotinamide. Acta Trop. 2012, 122, 224–229. [Google Scholar] [CrossRef]
- Sereno, D.; Monte Alegre, A.; Silvestre, R.; Vergnes, B.; Ouaissi, A. In vitro antileishmanial activity of nicotinamide. Antimicrob. Agents Chemother. 2005, 49, 808–812. [Google Scholar] [CrossRef]
- Kim, S.G.; Hong, I.P.; Woo, S.O.; Jang, H.R.; Pak, S.C.; Han, S.M. Isolation of abscisic acid from Korean acacia honey with anti-Helicobacter pylori activity. Pharmacogn. Mag. 2017, 13, S170–S173. [Google Scholar] [CrossRef]
- Parida, S.; Singh, T.U.; Thangamalai, R.; Narasimha Reddy, C.E.; Panigrahi, M.; Kandasamy, K.; Singh, V.; Mishra, S.K. Daidzein pretreatment improves survival in mouse model of sepsis. J. Surg. Res. 2015, 197, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Szwedowicz, U.; Szewczyk, A.; Gołab, K.; Choromańska, A. Evaluation of wound healing activity of salvianolic acid b on in vitro experimental model. Int. J. Mol. Sci. 2021, 22, 7728. [Google Scholar] [CrossRef]
- Huttunen, S.; Toivanen, M.; Liu, C.; Tikkanen-Kaukanen, C. Novel anti-infective potential of salvianolic acid B against human serious pathogen Neisseria meningitidis. BMC Res. Notes 2016, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Rahman, F.; Ali, R.; Alouffi, A.S.; Akand, S.K.; Alshehri, B.M.; Alshammari, F.A.; Alam, A.; Alaidarous, M.A.; Banawas, S.; et al. Cynaroside inhibits Leishmania donovani UDP-galactopyranose mutase and induces reactive oxygen species to exert antileishmanial response. Biosci. Rep. 2021, 41, BSR20203857. [Google Scholar] [CrossRef]
- van Baren, C.; Martino, V.; Di Leo Lira, P.; Anao, I.; Houghton, P.; Debenedetti, S.; Croft, S. Triterpenic Acids and Flavonoids from Satureja parvifolia. Evaluation of their Antiprotozoal Activity. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 189–192. [Google Scholar] [CrossRef]
- Somsak, V.; Damkaew, A.; Onrak, P. Antimalarial Activity of Kaempferol and Its Combination with Chloroquine in Plasmodium berghei Infection in Mice. J. Pathog. 2018, 2018, 3912090. [Google Scholar] [CrossRef]
- Mittra, B.; Saha, A.; Chowdhury, A.R.; Pal, C.; Mandal, S.; Mukhopadhyay, S.; Bandyopadhyay, S.; Majumder, H.K. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol. Med. 2000, 6, 527–541. [Google Scholar] [CrossRef]
- Pérez-Fonseca, A.; Alcala-Canto, Y.; Salem, A.Z.M.; Alberti-Navarro, A.B. Anticoccidial efficacy of naringenin and a grapefruit peel extract in growing lambs naturally-infected with Eimeria spp. Vet. Parasitol. 2016, 232, 58–65. [Google Scholar] [CrossRef]
- Pekkle Lam, H.Y.; Hung, M.-Y.; Cheng, P.-C.; Peng, S.-Y. Use of wogonin as a cooperative drug with praziquantel to better combat schistosomiasis. J. Microbiol. Immunol. Infect. 2022, 55, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Keyhani, A.; Sharifi, I.; Salarkia, E.; Khosravi, A.; Tavakoli Oliaee, R.; Babaei, Z.; Ghasemi Nejad Almani, P.; Hassanzadeh, S.; Kheirandish, R.; Mostafavi, M.; et al. In vitro and in vivo therapeutic potentials of 6-gingerol in combination with amphotericin B for treatment of Leishmania major infection: Powerful synergistic and multifunctional effects. Int. Immunopharmacol. 2021, 101, 108274. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).