1. Introduction
The central nervous system (CNS) is regarded as the processing center of the human body and the most vital part of the human body as a whole [
1]. Besides being responsible for learning and memory, the CNS facilitates ongoing repairs to sustain active function and regulate the body’s physiological processes [
2]. The balance between excitatory and inhibitory neurotransmitters is crucial in various physiological processes. GABA (γ-aminobutyric acid) and glutamate are essential to maintaining this balance. Gamma-aminobutyric acid (GABA) functions as the principal inhibitory neurotransmitter inside the central nervous system (CNS), counteracting the excitatory actions exerted by glutamate, which serves as the major excitatory neurotransmitter. Collectively, these mechanisms contribute to regulating neuronal activity and managing synaptic transmission, promoting optimal functioning of the central nervous system (CNS) by preventing excessive excitement that might potentially result in developing neurological diseases. A comprehensive grasp of the underlying functional association between GABA and Glutamate is crucial in neuropharmacology, particularly concerning drugs such as benzodiazepines and 2,3-benzodiazepines (2,3-BDZ), which exert their effects via modulating these neurotransmitters [
3].
The brain is subjected to several systems that control and maintain many activities as a delicate organ. However, the exact mechanisms that protect it from defects may also produce disorders due to impaired functions, resulting in various neurological conditions. As a complex and vulnerable organ, the brain depends on several defensive systems to sustain its optimum functioning and safeguard itself against possible damage. The protective mechanisms include the blood-brain barrier, immunological reactions, and the brain’s capacity for neural plasticity. Although essential for maintaining brain health, these systems may lead to the development of neurological diseases when they malfunction. The blood-brain barrier is a protective mechanism that restricts the entry of detrimental chemicals into the brain. However, this physiological barrier may impede the efficient administration of drugs intended to address neurological disorders. The immune system, which is intricately constructed to safeguard the body against infectious agents, has the potential to malfunction and result in autoimmune illnesses that specifically affect the brain. Neural plasticity, an essential mechanism for acquiring knowledge and adjusting to new circumstances, may lead to maladaptive alterations contributing to chronic pain and mobility difficulties.
Furthermore, abnormalities in neurotransmitters and the influence of hereditary factors have been identified as potential contributors to neurological disorders, including Parkinson’s disease, Alzheimer’s disease, and epilepsy. Neurodevelopmental diseases such as autism spectrum disorder may arise due to abnormalities during crucial developmental periods. In addition, oxidative stress and excitotoxicity have been implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease and traumatic brain trauma. Gaining a comprehensive understanding of these systems and their possible dysfunctions is crucial to progress in developing therapies for neurological illnesses and safeguarding the overall health of the brain [
4,
5,
6]. Neurodegenerative diseases are marked by the slow loss of groups of sensitive neurons to certain stimuli. This differs from the loss of specific neurons caused by physiological or damaging disorders. Neuron-related diseases include neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s, as well as neuro-traumatic diseases such as stroke, traumatic brain injury, spinal cord injury, and neuropsychiatric problems such as depression, autism, and hyperactivity disorders [
5]. Due to the complexity of the CNS, several factors are connected with the etiology of neurological disorders [
6]. Almost every neurological condition affects synapse function in the brain, either directly or indirectly. However, each condition affects the neuronal synapses in distinct ways, impairing synaptic transmission and/or plasticity [
7].
The majority of synapses present in the nervous system are classified as chemical synapses, wherein the release of a neurotransmitter from the axon of the presynaptic neuron facilitates the process of synaptic transmission. This neurotransmitter subsequently diffuses across a narrow synaptic cleft and interacts with receptors located on the dendrites of the postsynaptic neuron [
8]. Glutamatergic neurotransmission underlies most of the fast synaptic neurotransmission in the CNS [
8]. Glutamatergic synaptic transmission is regulated by three primary subtypes of ionotropic receptors, one of which is the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor (AMPAR) [
9]. AMPARs are tetrameric, cation-permeable ionotropic glutamate receptors found across the brain. The number of AMPARs in a given synapse ranges from tens to hundreds, corresponding with synaptic strength in mature synapses. The efficacy of synaptic connections between nerve cells can be strengthened or deteriorated depending on the number of AMPA receptors at the synapse [
10,
11,
12]. It has been established that abnormalities in AMPA receptor trafficking and synaptic assembly processes are associated with cognitive decline and various neurological disorders [
13,
14].
Functional AMPA receptors are homo- or hetero-oligomeric assemblies comprising combinations of four subunits, GluA1, GluA2, GluA3, and GluA4 [
15]. The GluA2 subunit confers calcium impermeability to natural AMPA receptor channels. The scarcity of GluA2-containing AMPA receptors inside motor nerve cells results in heightened calcium ion permeability, elevating susceptibility to excitotoxicity after AMPAR activation. The GluA2 subunit is subjected to an RNA editing mechanism that converts glutamine to arginine. While most AMPA receptors in the brain are Ca
2+-impermeable, they have an edited GluA2 subunit [
16,
17]. Each subunit has a large extracellular amino-terminal domain (ATD) that aids with the receptors’ assembly, trafficking, and modulation. It also has a ligand-binding domain (LBD) formed by two segments, S1 and S2, that are important for binding and activation gating, a transmembrane domain (TMD) that forms the membrane-spanning ion channel, and a cytoplasmic carboxy-terminal domain that helps with receptor localization and regulation [
18,
19,
20]. The ATD, LBD, and TMD impact the tetrameric assembly of AMPARs [
21]. Each subunit comprises three transmembrane helices (M1, M3, and M4) and a central pore-like helix (M2). The S1–M1 polypeptide segment leads from the LBD in an extended conformation until it reaches the TMD. The M4 helix is located outside the ion channel domain and is linked to the LBD’s S2 segment through two helix twists and a briefly extended polypeptide region [
22].
Currently, evidence indicates that several chemical groups can engage with the AMPA receptor at allosteric modulatory sites, resulting in alterations in the gating mechanism of the receptor, including processes such as activation, deactivation, and desensitization. Multiple allosteric modulators, such as 2,3-BDZ, have been extensively researched for their effects on the processes of desensitization and deactivation [
9,
23,
24,
25]. The area of 2,3-BDZ compounds has seen notable advancements, resulting in new compounds with distinct and exceptional characteristics. In addition, these substances have undergone assessment for their anxiolytic-like and antidepressant-like properties and their potential for causing memory impairment and toxicity in Swiss mice [
26,
27]. BDZs are a category of psychoactive substances that have been widely acknowledged for their impact on the central nervous system (CNS), namely in the regulation of GABA (γ-aminobutyric acid) and glutamate, two essential neurotransmitters responsible for maintaining brain activity equilibrium. However, GABA is the principal inhibitory neurotransmitter, and glutamate is the major excitatory complement [
28]. BDZs have a facilitatory influence on the action of GABA, resulting in a net inhibitory impact on neuronal activity. Modifying physiological processes, such as drowsiness, muscular relaxation, anti-anxiety, and anticonvulsant actions, is paramount. The field of neuropharmacology emphasizes the study of 2,3-BDZs, which represent a distinct subclass within the broader category of benzodiazepines. These chemicals interact with GABA and glutamate receptors, including N-methyl-D-aspartate (NMDA) and AMPA receptors. The capacity to regulate both excitatory and inhibitory neurotransmission endows them with the capability to impact diverse neurological processes, therefore offering prospective therapeutic prospects for the treatment of numerous neurological illnesses. The distinctive capacity of 2,3-BDZs to modulate AMPA receptors sets them apart from other benzodiazepines. The selective engagement of these interactions can potentially mitigate excitotoxicity and seizure activity, presenting a potentially revolutionary treatment approach for a range of neurological illnesses that require the delicate equilibrium of excitatory and inhibitory neurotransmission.
In addition, recent advancements in understanding the composition and operation of 2,3-BDZs with AMPA receptors have laid the groundwork for creating innovative molecules that selectively interact with these receptors [
29]. Early research revealed that binding sites for negative allosteric modulators (NAMs) were situated in the LBD-TMD linker region and that residues in the linkers joining the S1-M1 and S2-M4 areas affected NAM inhibitory activity [
30,
31]. The implications of binding to S1-M1 (Lys 506–Gly 513) and S2-M4 (Gly 774–Ser 788) linkers on gating characteristics suggest that these linkers undergo a shift during channel gating. The proposal suggests that the movement in question is a crucial step in converting agonist binding to channel opening and that the hindrance of this movement plays a pivotal role in the molecular mechanism of NAM inhibition. Upon binding to S1-M1 and S2-M4, these compounds stabilize the linker regions, thereby impeding the generation of a conformational change sufficient for the agonist contact to facilitate channel opening [
22,
31]. Investigating how these ligands alter channel gating may provide further information about receptor-function mechanisms. We used electrophysiological recordings to investigate how four novel 2,3-BDZ derivatives (2,3-BDZ-1-2,3-BDZ-4) (
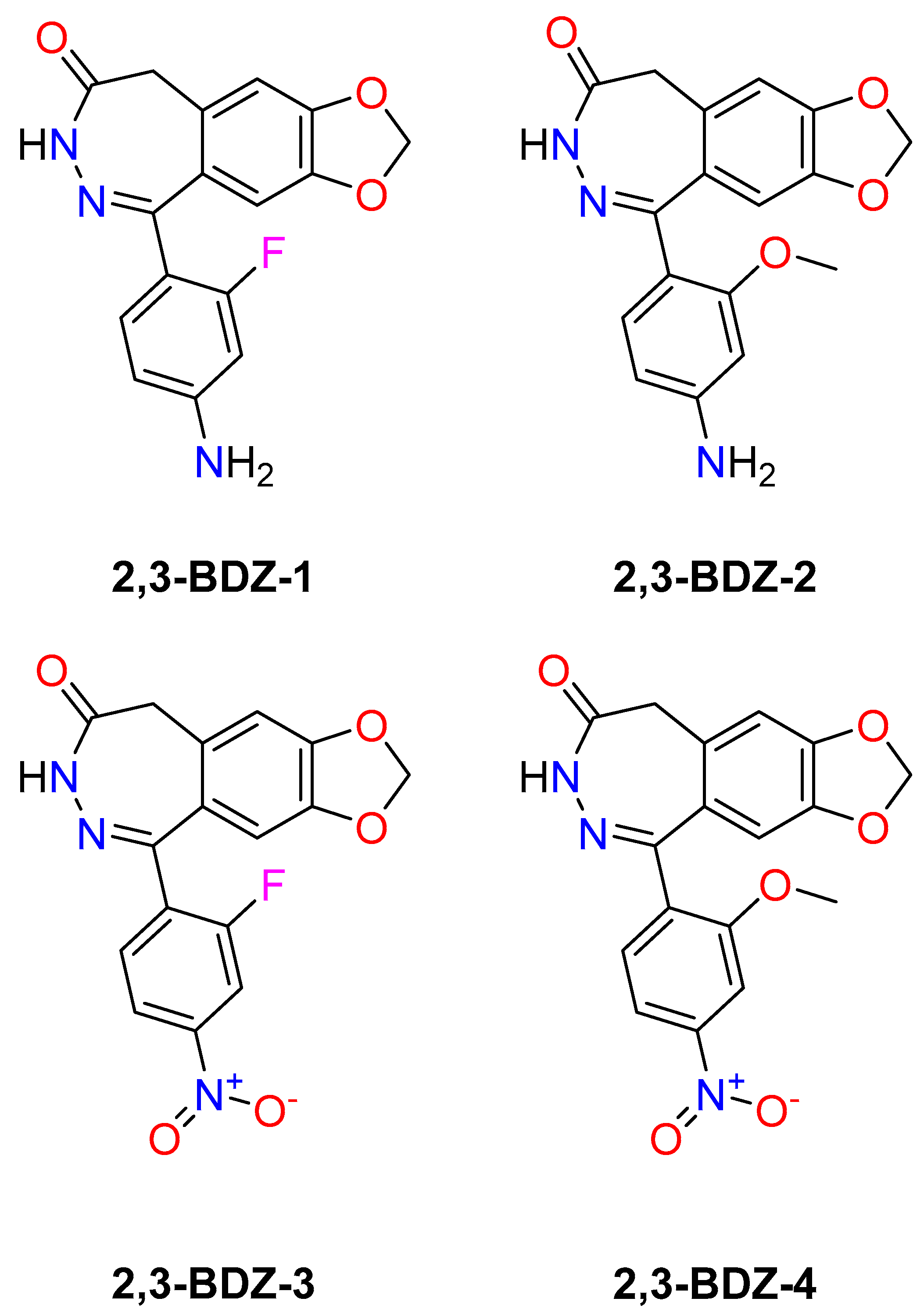
Figure 1) bind and impact AMPA receptors.
During the 1990s, many research groups started chemical and pharmacological studies to learn more about the structure-activity relationship of benzodiazepines [
32], a group of compounds that selectively block AMPA receptors without competing with them, and to find new derivatives with better pharmacological properties. The GYKI 52466 prototype, belonging to the family of allosteric modulators, exhibits anticonvulsant properties across various seizure models. Despite the comprehensive exploration and lucid explication of structure-activity relationship investigations, the pharmacological characteristics of the most favorable derivatives necessitate significant enhancement [
33]. GYKI-52466 and GYKI-53405 are gold standards in AMPA receptor-mediated neuronal activity research. A few hundred analogs have resulted in a thorough knowledge of replacing various side chains in the fundamental chemical structure of 2,3-BDZ [
32]. Previously, our lab investigated two sets of novel 2,3-BDZ derivatives to better understand the structure-activity relationship and how modifications in the side chains may impact the compounds’ ability to inhibit the AMPAR subunits. In our initial study on 2,3-BDZ compounds, we directed our attention toward particular attributes: eliminating the amine group from the phenyl ring and introducing a halogen group at either the meta or ortho position of the phenyl ring. Our observations revealed that the presence of an amino group is not essential for inhibition when an electron-withdrawing group is positioned at the meta site. In our subsequent investigation concerning 2,3-BDZ derivatives, we examined the impact of incorporating an electron-withdrawing group, specifically Cl or Br, and the significance of the phenyl ring.
Additionally, we assessed the potential cytotoxic effects of compounds
4b and
4e. Our findings suggest that these additions significantly contribute to the inhibition of AMPA receptors. Furthermore, compounds
4b and
4e exhibited promising cytotoxicity against diverse cancer cell lines. As mentioned, the information enhances comprehension about AMPARs, presents alternative medication candidates of 2,3-BDZ distinct from standard derivatives, and potentially functions as neuroprotective agents [
25,
34]. However, it is uncertain how much chemical structural changes affect the selectivity of newly synthesized drugs for AMPA receptors. The research will be enhanced by investigating homomeric and heteromeric AMPA receptor subunits.
Given the intricate nature of AMPA receptor activity and the possible therapeutic implications associated with 2,3-BDZa, this work aims to examine their interconnection comprehensively. The primary aims of this study are to create new 2,3-BDZ derivatives through synthesis methods and analyze their chemical properties. Additionally, the study seeks to evaluate the effects of these newly synthesized derivatives on AMPA receptor channels using electrophysiological recordings. Furthermore, the study aims to investigate the potential of these derivatives as therapeutic agents for neurological disorders involving excitotoxicity and neurotransmission imbalances. The hypothesis in this study suggests that the new compounds of 2,3-BDZs would exhibit notable interactions with the AMPA receptors, affecting their gated characteristics. This, in turn, may provide prospective avenues for treatments for diverse neurological disorders. The primary objective of this study is to provide significant contributions to understanding the interactions between 2,3-BDZs and AMPA receptors, enhancing our knowledge of their prospective uses in neuropharmacology.
3. Discussion
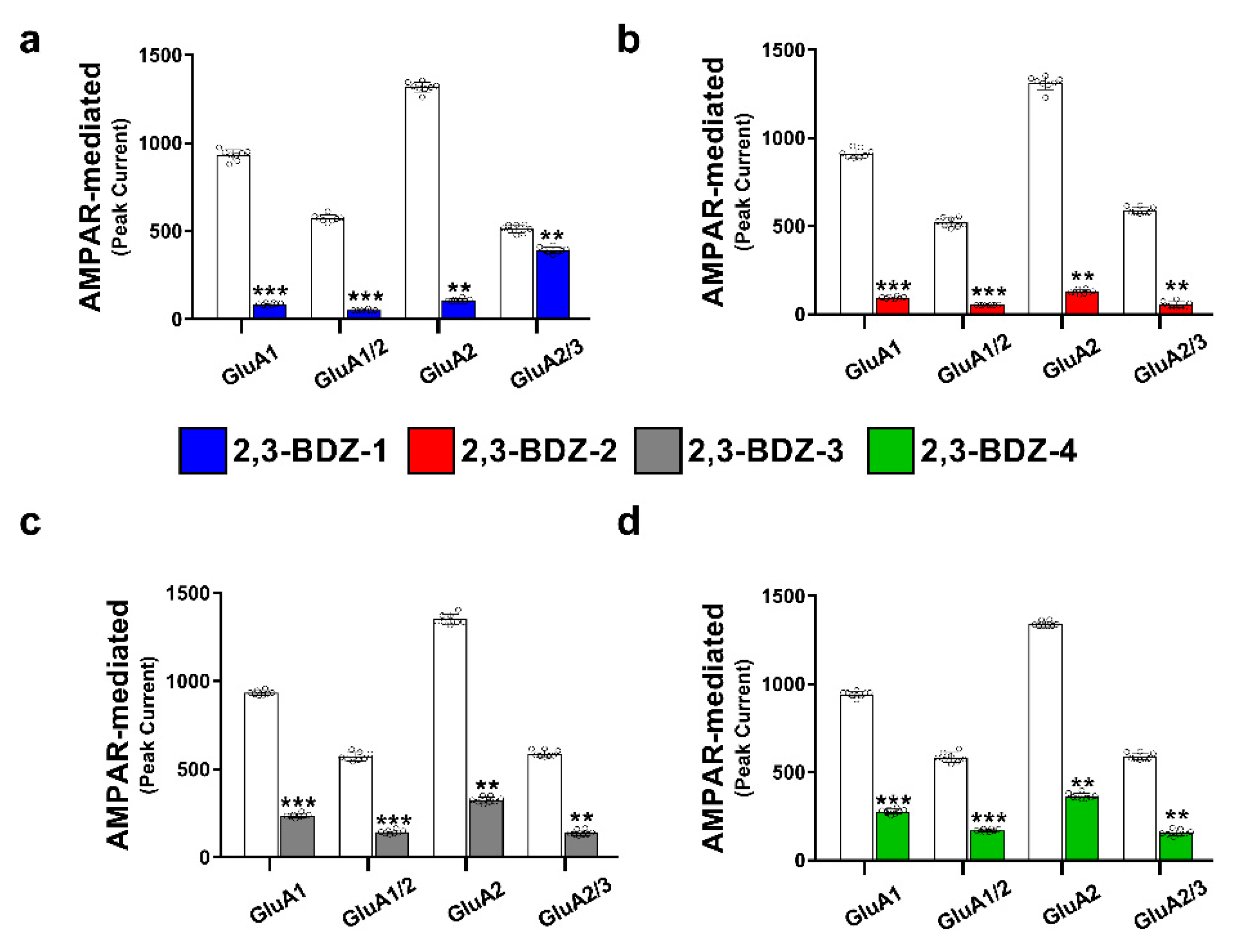
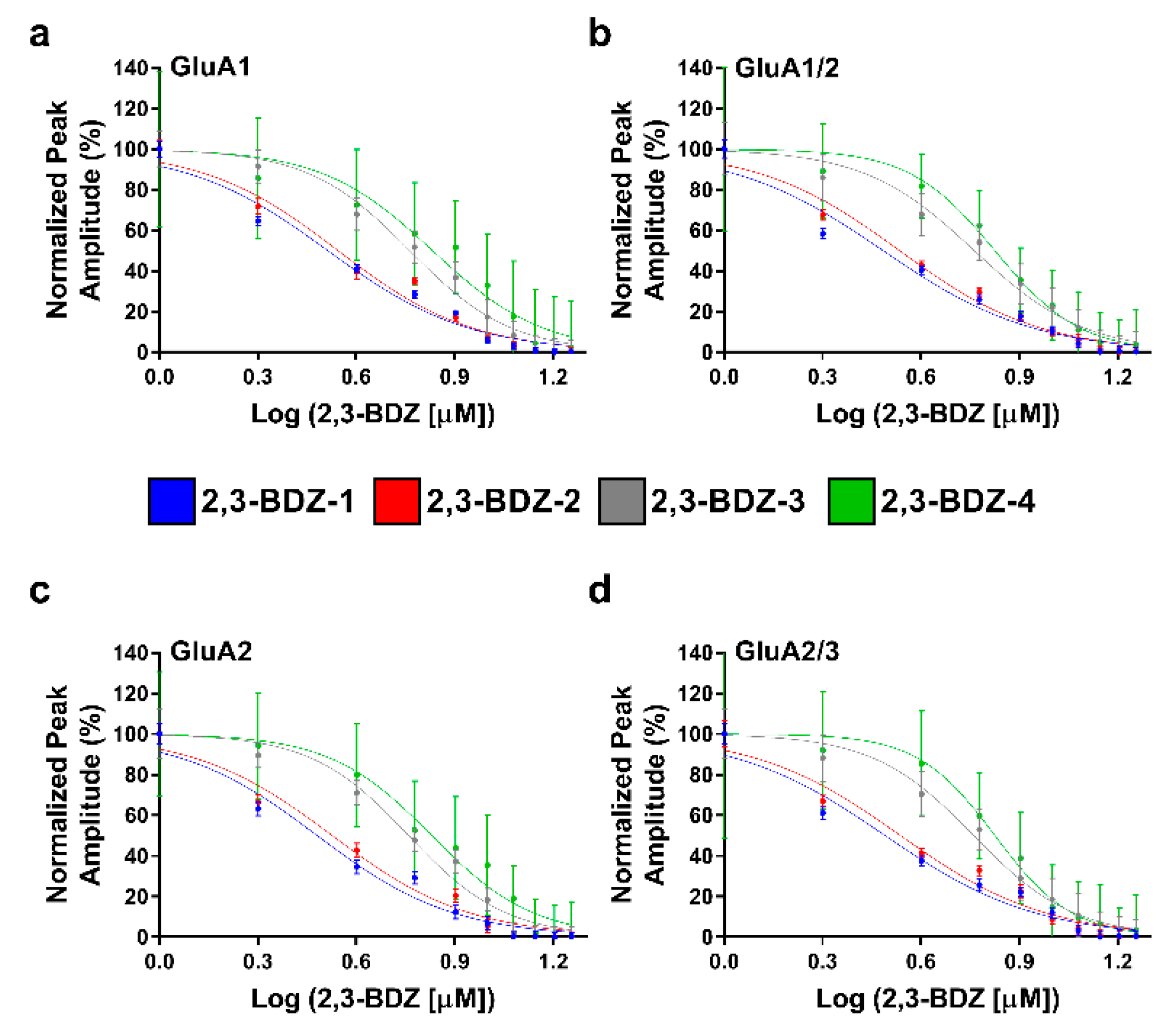
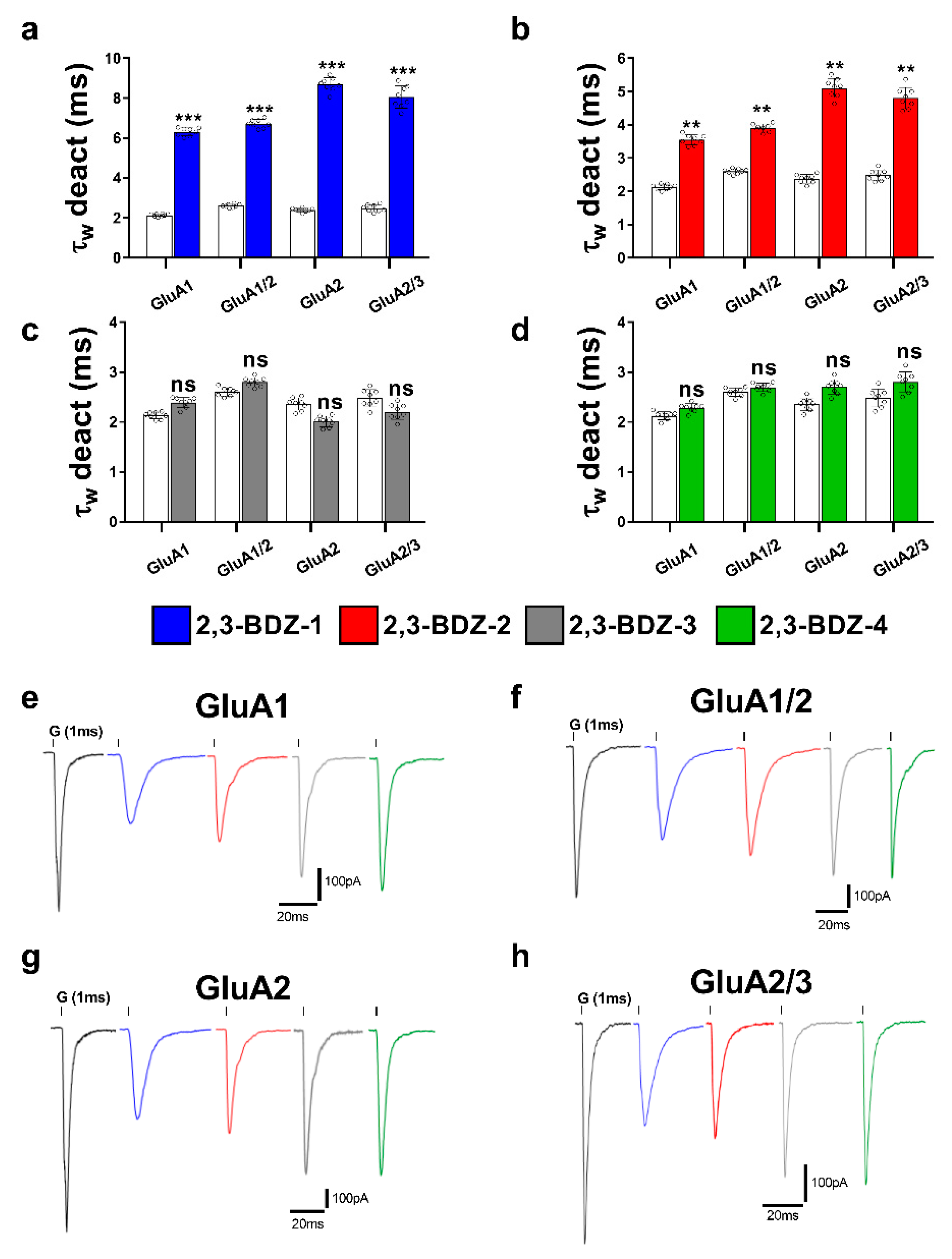
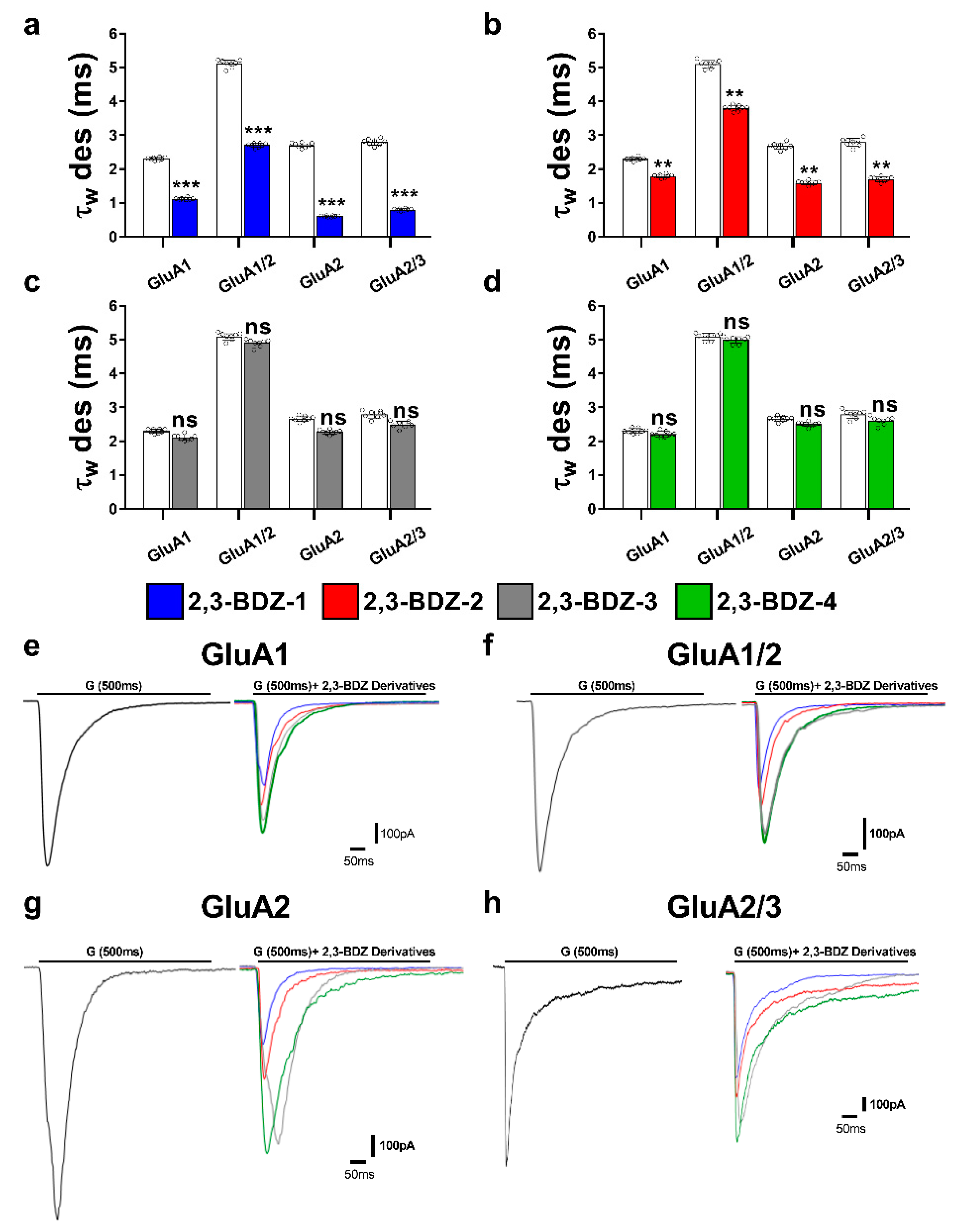
The primary objective of this research endeavor was to investigate the impact of four novel derivatives of 2,3-BDZs, namely 2,3-BDZ-1 through 2,3-BDZ-4, on specific components of the AMPA receptor. This work aimed to investigate the inhibitory effects of these compounds and their rates of desensitization and deactivation. The main result of our investigation is that the 2,3-BDZ compounds have inhibitory characteristics on both homomeric and heteromeric AMPAR subunits. All four compounds exhibited inhibitory effects on AMPAR currents, with 2,3-BDZ-1 and 2,3-BDZ-2 displaying the highest effectiveness in suppressing AMPAR activity. The results of this research highlight the potential of these chemicals as prospective candidates for the modification of AMPA receptor activity.
Furthermore, it was noted that the 2,3-BDZ compounds had a greater effect on the GluA2 subunit compared to the other subunits. Both 2,3-BDZ-1 and 2,3-BDZ-2 had an increased inhibitory impact on the GluA2 subunit, indicating that GluA2 potentially assumes a pivotal role as a target for the mechanism of action of these drugs. The results of our study indicate that the effective manipulation of 2,3-BDZ compounds in modulating the GluA2 subunit can significantly impact the regulation of AMPA receptors. The comprehension of the relative potencies of diverse derivatives on distinct AMPAR subunits is particularly significant in clarifying the structural and functional variances among these subunits. It offers a valuable understanding of synaptic plasticity and excitotoxicity mechanisms. The post-transcriptional editing process occurs at a specific site known as the Q/R site of GluA2, with a precise location at position 607. The M2 segment is observed to extend into the pore, resulting in the non-rectifying nature of the channel and its impermeability to calcium. This highlights the crucial involvement of GluA2 in the mechanism, as mentioned earlier [
36].
Different AMPAR subunits have distinct structural and functional features, and targeting various subunits allows for more extensive AMPAR activity control. In the case of chronic pain, targeting both GluA2/3 and GluA1/2 has been more successful than targeting GluA2 solely in lowering pain sensation and avoiding the development of central sensitization [
37]. Targeting GluA2-containing AMPARs alone may not be sufficient to avoid excitotoxicity and neuronal injury while targeting additional AMPAR subunits such as GluA2/3 complexes is more beneficial [
38]. It is noteworthy because 2,3-BDZ compounds might operate on GluA2 homomers, reducing their unusual conductance property when desensitized [
39].
The inhibitory effects of 2,3-BDZ-1 and 2,3-BDZ-2 are substantially stronger than those of 2,3-BDZ-3 and 2,3-BDZ-4, according to the current study results. The structural difference between the first two compounds (2,3-BDZ-1 and 2,3-BDZ-2) and the final two compounds (2,3-BDZ-3 and 2,3-BDZ-4) is that the nitro group in 2,3-BDZ-1 and 2,3-BDZ-2 is reduced to an amino group positioned on the phenyl ring in the para position. While the nitro group works as a hydrogen bond acceptor, the ability of the amino group to establish hydrogen bonds with the carbonyl groups of the receptor may explain the difference in inhibitory action between the two sets of molecules. Moreover, the amino group at the para position of the phenyl ring may bind to the amino acids in the AMPA receptor structure more strongly than the nitro group.
Furthermore, 2,3-BDZ-1 inhibited AMPA receptor subunits the most significantly. One probable reason is that the amino group of the phenyl ring is in the para position, while an electron-drawing group (fluorine) is in the ortho position. For the reason that fluorine has the greatest electronegativity of any element, its group has the largest electron-withdrawing potential. Fluorine-containing organic compounds have been shown to alter intermolecular interactions in ligand-protein complexes and to create hydrogen bonds with the receptor. Furthermore, fluorine has the greatest affinity for glycine, which is one of the amino acids found at the S1-M1 (Lys 506-Gly 513) and S2-M4 (Gly 774-Ser 788) linkers, where NAMs often bind AMPAR [
40,
41]. The observed inhibitory properties of 2,3-BDZ derivatives on AMPA receptor subunits can be attributed to distinct interactions between these compounds and the structural components of the receptor. Identifying functional groups such as amino and nitro groups in the 2,3-BDZ compounds indicates the possibility of establishing hydrogen bonding interactions with specific amino acid residues located within the subunits of the AMPA receptor. This interaction can potentially enhance the stability of the complex formed between the ligand and the receptor.
Moreover, the benzene rings present in the compounds have the potential to participate in π-π interactions with aromatic residues located in the receptor. This interaction mechanism significantly contributes to the binding affinity observed between the compounds and the receptor. The 2,3-BDZ compounds may experience electrostatic interactions with charged groups in the receptor’s binding pocket. These interactions can potentially stabilize the ligand and receptor complex by engaging with oppositely charged residues. Van der Waals, specifically London dispersion forces, may contribute to molecular binding.
Furthermore, there may be domain-specific interactions where various 2,3-BDZ compounds selectively target specific regions, such as the ligand-binding domain or amino-terminal domain, thereby influencing the functioning of the receptor. The specific characteristics of these interactions may exhibit variability contingent upon the subunit composition of the receptor complex. By using advanced computational methods and doing mutagenesis studies, we might be able to learn more about the specific binding mechanisms and essential amino acid residues that are key to these interactions [
25,
29].
Our prior investigation examined the impact of two distinct 2,3-BDZ derivatives on AMPAR subunits. Remarkably, we discovered that the presence of an amino group on the phenyl ring was not essential for inhibition. Instead, we found that an electron-drawing group could effectively replace the amino group in mediating inhibition. Moreover, our findings demonstrated that 2,3-BDZs containing an electron-withdrawing group in the meta position exhibited greater efficacy in inhibiting AMPAR than those with an electron-withdrawing group in the ortho position [
34]. Following this, we conducted subsequent experiments involving five additional 2,3-BDZ derivatives. These experiments’ results confirmed that adding an electron-withdrawing group, such as Cl or Br, to the phenyl ring significantly enhanced the compounds’ ability to inhibit AMPAR currents [
25]. These additional findings further support the structural requirements for AMPAR inhibition by 2,3-BDZ derivatives and highlight the potential significance of electron-withdrawing groups in improving their inhibitory effects. Such insights could be valuable in advancing future drug development efforts related to AMPAR modulation. Replacing the amino group with electron-drawing groups in 2,3-BDZ derivatives may lead to enhanced inhibition of AMPA receptors through specific mechanisms. Firstly, these electron-drawing groups can augment the phenyl ring’s electron-withdrawing ability, thereby increasing the compound’s propensity to interact with the receptor’s active site more effectively.
Additionally, introducing electron-drawing groups can enhance the compound’s lipophilicity, making it more likely to traverse the blood-brain barrier and reach the brain tissue, facilitating interactions with AMPA receptors at the target site. However, while these explanations offer potential reasons for the observed effects, a comprehensive understanding of the underlying mechanisms requires further investigation. Detailed studies elucidating the specific interactions between the compounds and the receptor’s binding site will provide valuable insights into the structure-activity relationship and the potential therapeutic applications of these novel 2,3-BDZ derivatives.
The diverse range of chemical compounds in the 2,3-BDZ family has attracted significant interest, specifically tofisopam (commercially known as Grandaxin), girisopam, and nerisopam. Tofisopam exhibits distinctive anxiolytic properties that set it apart from conventional benzodiazepines. It interacts with specific AMPA receptor sites instead of the GABA receptors that sedative benzodiazepines typically target, giving it this distinct advantage. Furthermore, the anxiolytic effects of girisopam, while not yet fully understood, may potentially involve the modulation of AMPA receptors. This suggests the possibility of exploring new therapeutic approaches for anxiety disorders. Although less extensively studied, Nerisopam presents an additional prospective contender for AMPA receptor interaction, owing to its chemical lineage within the 2,3-BDZ family. Within the framework of our research, which extensively examined the effects of 2,3-BDZ derivatives such as 2,3-BDZ-1 to 2,3-BDZ-4 on AMPARs, a coherent and persuasive narrative arises regarding the wider scope of the 2,3-BDZ family in modulating these receptors. In particular, a comprehensive analysis of the structural distinctions among these compounds, compared to tofisopam and similar substances, could yield valuable insights into the therapeutic subtleties observed within this group. This thorough investigation has the potential to enhance our current understanding, providing valuable insights for future studies and advancements in the treatment of neurological disorders associated with excessive AMPA receptor activation [
27,
42].
The present study offers valuable strengths and insights into the effects of 2,3-BDZ compounds on AMPA receptor subunits, encompassing both homomeric and heteromeric subunits. This investigation sheds light on the inhibitory mechanisms of these compounds on AMPA receptors, providing potential avenues for developing effective treatments for neurological disorders associated with AMPA receptor overstimulation. Notably, the study identifies that 2,3-BDZ-1 and 2,3-BDZ-2 uniquely affect the desensitization rates of AMPARs, representing a novel discovery for an AMPAR inhibitor.
However, future research must address certain limitations to interpret the findings comprehensively. Firstly, the specific binding sites of the 2,3-BDZs on the AMPAR subunits remain limited, necessitating further research to understand the precise interaction between these compounds and the receptor. Secondly, the mechanisms by which the 2,3-BDZs alter the deactivation and desensitization rates of AMPARs are yet to be fully elucidated, requiring further investigation to unravel the underlying molecular processes. Several additional variables, such as voltage dependence, ionic flux, and experimental conditions, must be meticulously regulated to ensure the accuracy of electrophysiological data. While the study’s use of recombinant AMPA receptor subunits expressed in human embryonic kidney cells enables precise electrophysiological recordings and exploration of specific subunit combinations, it fails to replicate the brain’s cellular environment’s complexity fully. Future research should investigate the impact of 2,3-BDZs on AMPA receptor complexes in an in vivo setting to understand their effects comprehensively.
Moreover, addressing the constraints of in vitro experimentation and exploring the potential clinical uses of 2,3-BDZs as AMPA receptor antagonists for neurological disorders associated with excessive receptor stimulation are essential for future research endeavors. Independent replication of the study is crucial to validating the observed effects and establishing the robustness of the findings. To further our understanding, exploring the specific binding sites and interactions between 2,3-BDZ compounds and AMPA receptor subunits is imperative, offering deeper insights into the molecular mechanisms of inhibition. Additionally, investigating the broader physiological consequences of these compounds in neurological disorders and synaptic function is vital to assess potential off-target effects and overall impacts on neuronal excitability, synaptic plasticity, and cognitive function in vivo. By addressing these limitations, future research can effectively advance the therapeutic potential of 2,3-BDZ compounds in treating neurological disorders.
The 2,3-BDZ family holds substantial therapeutic potential and promising applications in treating neurological disorders. The results of this study lay the groundwork for future research and refinement of 2,3-BDZ derivatives to enhance their specificity, effectiveness, and safety characteristics. By refining the chemical structure, scientists can develop compounds with enhanced specificity towards specific subunits of the AMPA receptor, enabling precise interventions within specific brain regions or circuits. Additionally, investigating how 2,3-BDZ and other allosteric modulators work together may lead to new combinations that synergistically improve therapeutic outcomes. Due to the many ways AMPA receptors are involved in synaptic plasticity, memory consolidation, and excitotoxicity, more research is needed to see if 2,3-BDZ could treat neurological disorders caused by abnormal AMPA receptor activity. These compounds are promising therapeutic agents in conditions such as epilepsy, traumatic brain injury, and stroke, where abnormal AMPA receptor activity contributes to neuronal damage.
Moreover, it is critical to explore their potential as analgesics for managing chronic pain, especially in cases with central sensitization and heightened pain perception. Advancements in drug delivery technologies present an opportunity to develop precise delivery systems for 2,3-BDZ. Such systems can enhance brain penetration while minimizing adverse effects on peripheral tissues, optimizing the therapeutic range, and reducing potential adverse reactions. Safety considerations are paramount, and thorough assessments and preclinical research are essential to uncovering any side effects and long-term consequences of prolonged use. Understanding the impact of 2,3-BDZ on other neurotransmitter systems and brain functions is vital to avoiding unintended effects. Translational research that leads to clinical trials is needed to determine if 2,3-BDZ derivatives are safe and effective for people with neurological disorders. Implementing a methodical approach that considers patient subgroups and individual variability is crucial to identifying positive responders and optimizing treatment strategies. Despite the therapeutic potential of these compounds, several important considerations need resolution. Achieving desired levels of selectivity is a significant challenge due to the widespread distribution of AMPA receptors in the brain and their involvement in various physiological processes. Selectively targeting specific subunits may reduce unintended effects and enhance therapeutic efficacy. Furthermore, it is important to figure out how 2,3-BDZ affects synaptic plasticity, learning, and memory since these receptors are so important to these functions. Striking a balance between inhibition and excitotoxicity is essential to preserving proper brain function and preventing detrimental consequences. To optimize treatment outcomes, the permeability of these compounds across the blood-brain barrier (BBB) needs consideration. Precise drug delivery to the brain while minimizing systemic exposure and peripheral side effects is critical. Long-term safety testing is important because long-term use of AMPA receptor antagonists may cause neuroadaptations that change how receptors work, synapses work, and neural circuits work. Diligent patient monitoring is essential for detecting adverse effects and assessing the likelihood of tolerance development. Finally, acknowledging individual variability in response, including genetic variations and comorbidities, underscores the importance of personalized treatment strategies in clinical settings. Addressing these considerations will advance the therapeutic potential of 2,3-BDZ, paving the way for more effective and targeted interventions in neurological disorders [
43,
44,
45].