Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136
Abstract
1. Introduction
2. Results
2.1. NaF Decreased ATP Level in CCL136 Muscle Cell Line
2.2. NaF Induced Glycogen Mobilisation in CCL136 Muscle Cell Line
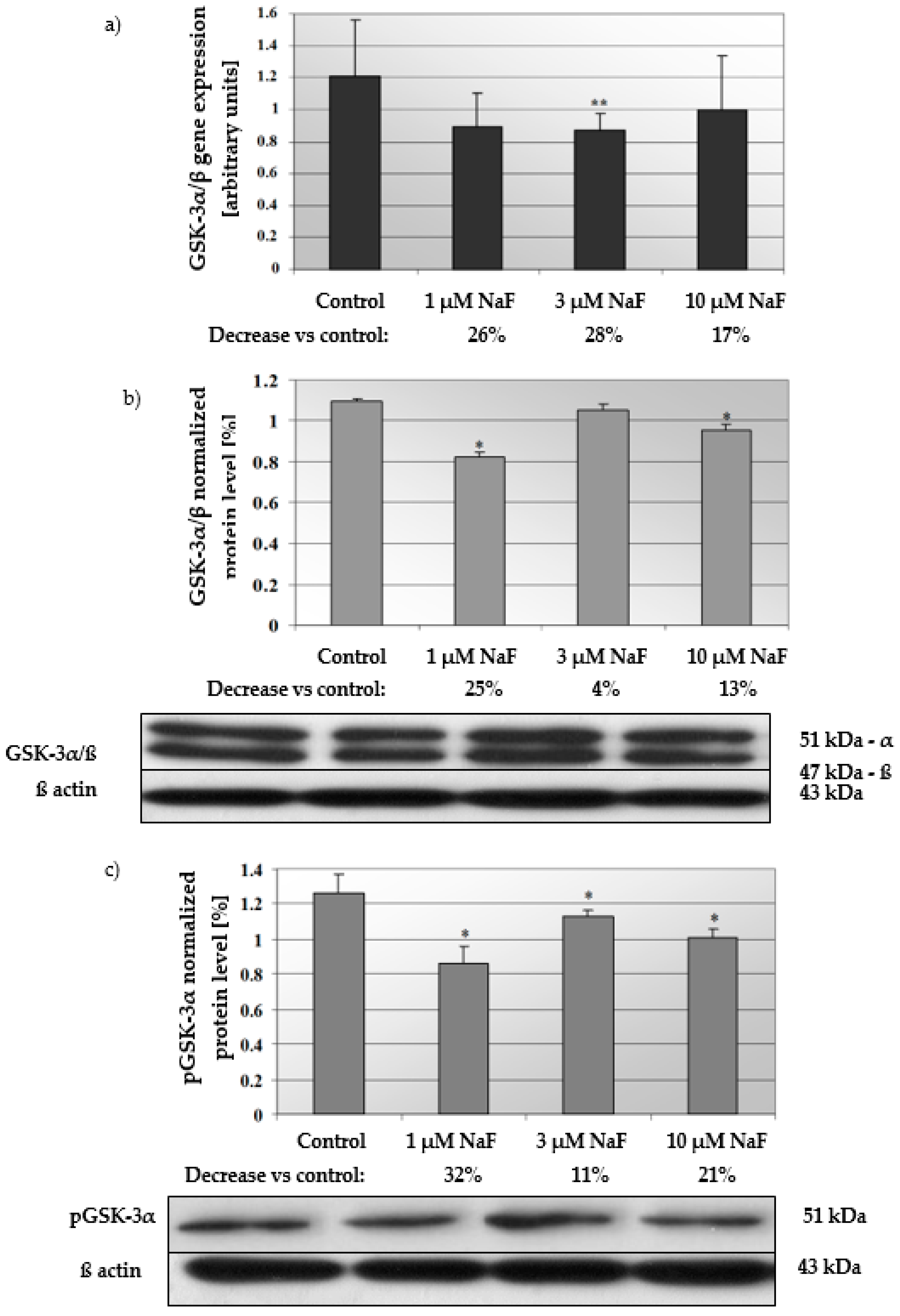
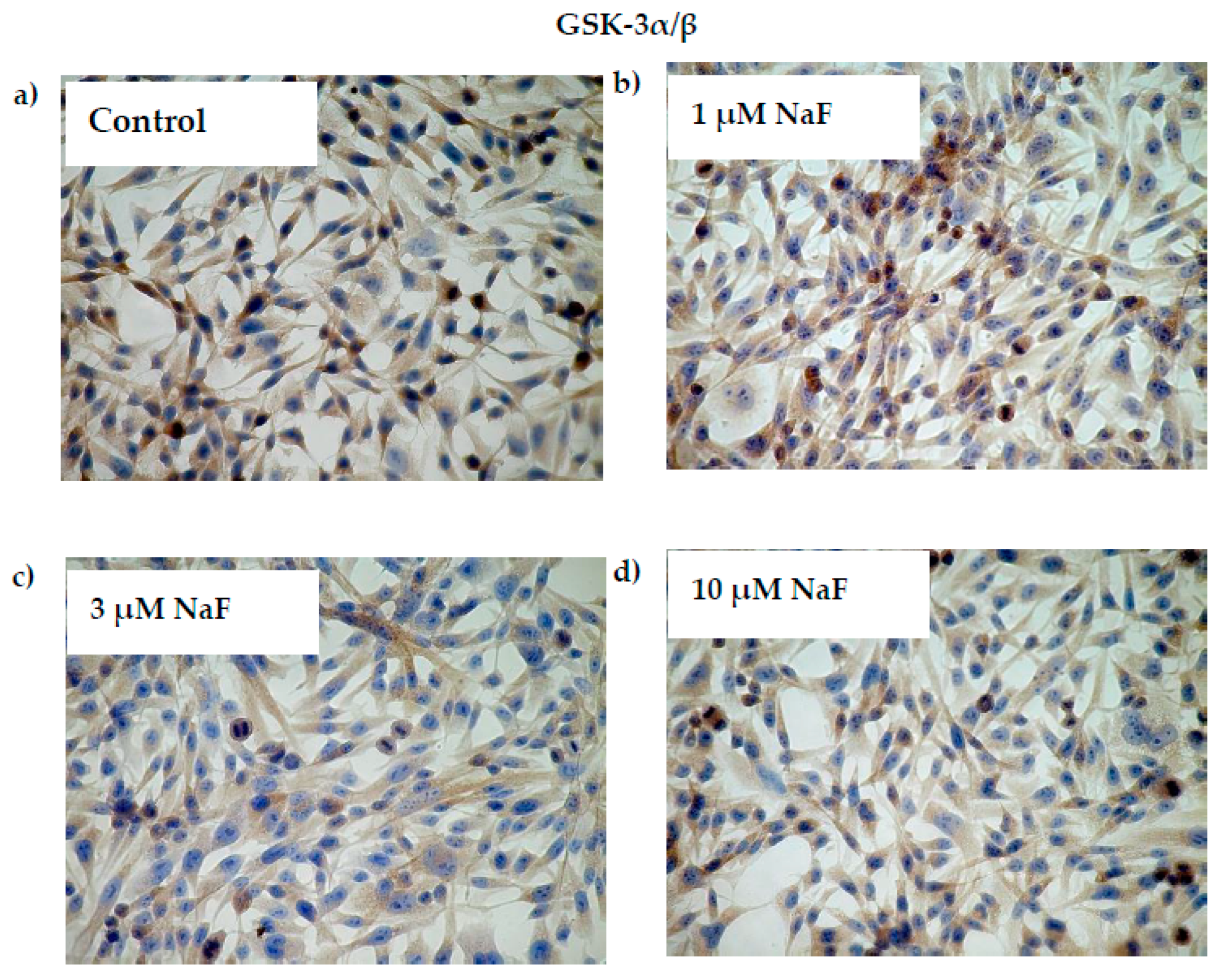
2.3. NaF Induce Depletion in Gene Expression of GSK-3α/β as Well as Level of Active GSK-3α/β and Its Phosphorylated Form (pGSK-3α) in CCL136 Muscle Cell Line
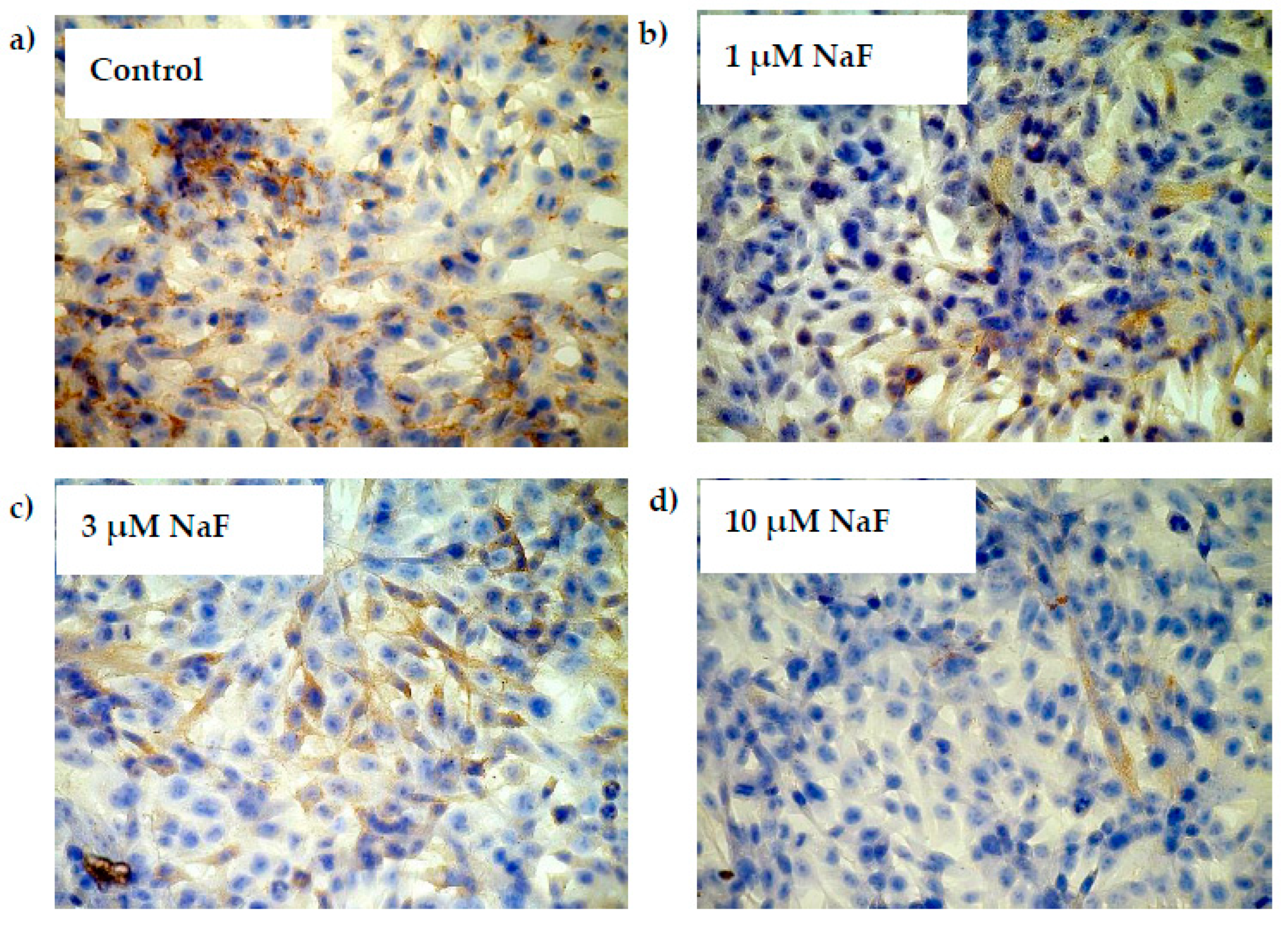
2.4. NaF Decreased Gene Expression and Protein Level of GYS-1 Enzyme
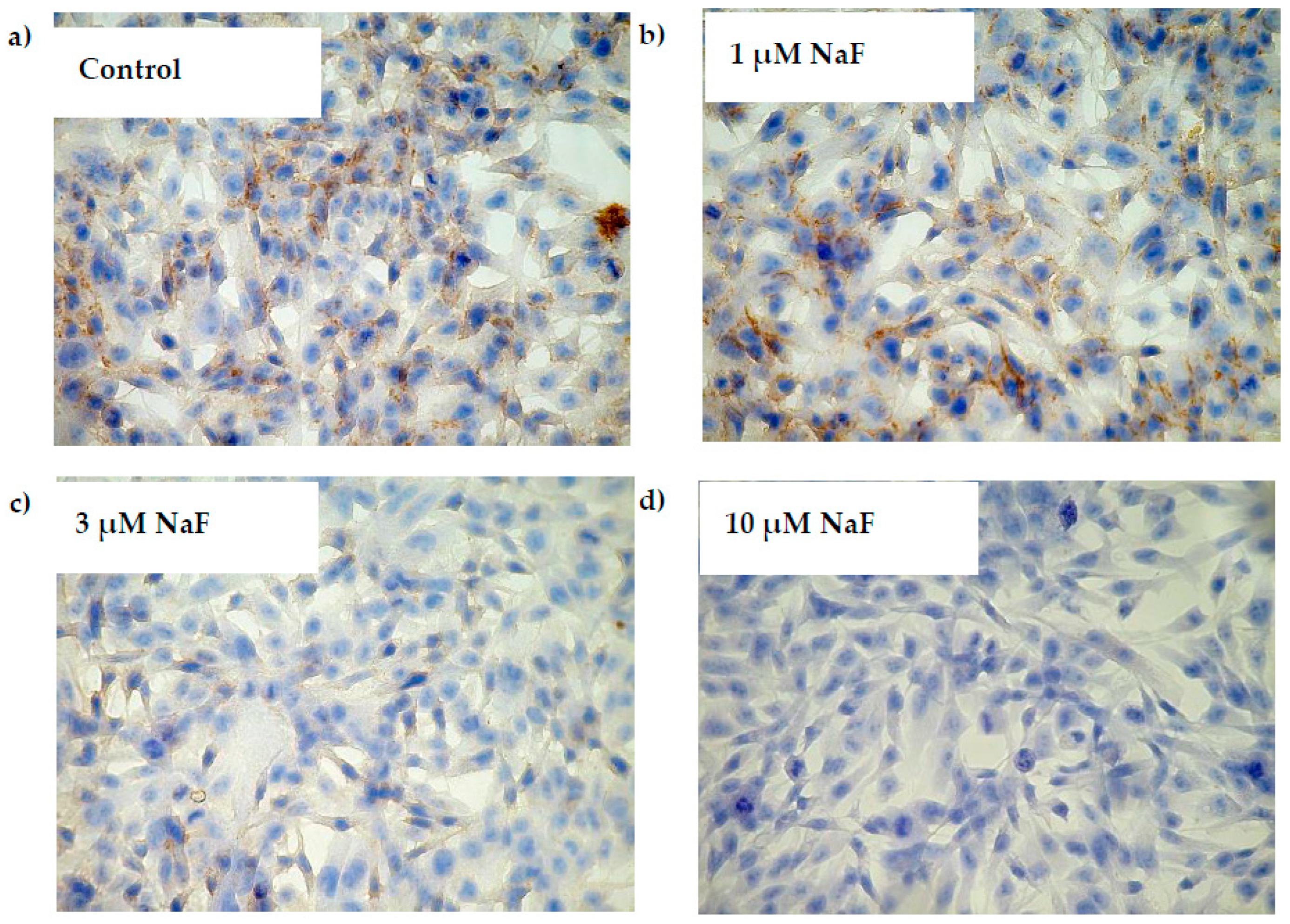
2.5. NaF Decreased Gene Expression and Protein Level of PYGM Enzyme
3. Discussion
4. Materials and Methods
4.1. Cell Culture Preparation
4.2. Fluorimetric Assay: Intracellular Glycogen Accumulation—Quantitative Analysis
4.3. Fluorescent Microscopy: Imaging of Glycogen Accumulation Using PAS (Periodic Acid Schiff) Method
4.4. High Performance Liquid Chromatography: Intracellular ATP Level—Quantitative Analysis
4.5. Real-Time PCR Analysis—Glycogen-Related Enzymes Genes Expression Level
4.5.1. RNA Extraction and cDNA Synthesis
4.5.2. Quantification of Gene Expression Using Real-Time PCR
4.6. Western Blotting Analysis—Glycogen-Related Enzymes Protein Level
4.7. Immunohistochemistry
4.8. Determination of Protein Concentration
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| ATP | adenosine triphosphate |
| BCA | bicinchoninic acid |
| Ca-ATPase | calcium dependent transmembrane ATPase |
| CCL136 | muscle cell line |
| DMEM | Dulbecco’s Modified Eagle’s high glucose Medium |
| FBS | fetal bovine serum |
| GSK-3α/ß | glycogen synthase kinase 3 |
| GSK-3α/β | glycogen synthase kinase, |
| GYS | glycogen synthase |
| GYS-1 | muscle isoform of glycogen synthase, |
| HPLC | High Performance Liquid Chromatography |
| Na/K-ATPase | sodium/potasium pump, an electrogenic transmembrane ATPase |
| NaF | sodium fluoride |
| PBS | phosphate-buffered saline |
| Pgsk | 3α–glycogen synthase kinase, phosphorylated form |
| PhK | phosphorylase kinase |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | cAMP-dependent protein kinase A |
| PKB/Akt | protein kinase B |
| pPYGM | muscle isoform of glycogen phosphorylase, phosphorylated form |
| PYGM | glycogen phosphorylase |
| PYGM | muscle isoform of glycogen phosphorylase |
| ROS | reactive oxygen species |
References
- Friden, J.; Seger, J.; Ekblom, B. Topographical localization of muscle glycogen: An ultrahistochemical study in the human vastus lateralis. Acta Physiol. Scand. 1989, 135, 381–391. [Google Scholar] [CrossRef]
- Marchand, I.; Chorneyko, K.; Tarnopolsky, M.; Hamilton, S.; Shearer, J.; Potvin, J.; Graham, T.E. Quantification of subcellular glycogen in resting human muscle: Granule size, number, and location. J. Appl. Physiol. 2002, 93, 1598–1607. [Google Scholar] [CrossRef]
- Graham, T.E.; Yuan, Z.; Hill, A.K.; Wilson, R.J. The regulation of muscle glycogen: The granule and its proteins. Acta Physiol. 2010, 199, 489–498. [Google Scholar] [CrossRef]
- Graham, T.E. Glycogen: An overview of possible regulatory roles of the proteins associated with the granule. Appl. Physiol. Nutr. Metab. 2009, 34, 488–492. [Google Scholar] [CrossRef]
- Yeaman, S.J.; Armstrong, J.L.; Bonavaud, S.M.; Poinasamy, D.; Pickersgill, L.; Halse, R. Regulation of glycogen synthesis in human muscle cells. Biochem. Soc. Trans. 2001, 29 Pt 4, 537–541. [Google Scholar] [CrossRef]
- Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Gąssowska, M.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Chibowska, K.; Goschorska, M.; Lubkowska, A.; Chlubek, D. Glycogen metabolism in brain and neurons—Astrocytes metabolic cooperation can be altered by pre- and neonatal lead (Pb) exposure. Toxicology 2017, 390, 146–158. [Google Scholar] [CrossRef]
- Jensen, T.E.; Richter, E.A. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 2012, 590 Pt 5, 1069–1076. [Google Scholar] [CrossRef]
- Philp, A.; Hargreaves, M.; Baar, K. More than a store: Regulatory roles for glycogen in skeletal muscle adaptation to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1343–E1351. [Google Scholar] [CrossRef]
- Roach, P.J. Glycogen and its metabolism. Curr. Mol. Med. 2002, 2, 101–120. [Google Scholar] [CrossRef]
- Poureslami, H.R.; Khazaeli, P.; Noori, G.R. Fluoride in food and water consumed in Koohbanan (Kuh-E Banan), Iran. Fluoride 2008, 41, 216–219. [Google Scholar]
- World Health Organization. Fluorine and fluorides. In World Health Organization. Environmental Health Criteria 36; International Programme on Chemical Safety; WHO: Geneva, Switzerland, 1984. [Google Scholar]
- Kalyanalakshmi, P.; Vijayabhaskar, M.; Dhananjaya Naidu, M. Lipid peroxidation and antioxidant enzyme status of adult males with skeletal fluorosis in Andhra Pradesh, India. Fluoride 2007, 40, 42–45. [Google Scholar]
- Gutowska, I.; Baranowska-Bosiacka, I.; Siennicka, A.; Telesiński, A.; Stańczyk-Dunaj, M.; Wesołowska, T.; Gąssowska, M.; Kłos, P.; Zakrzewska, H.; Machaliński, B.; et al. Activation of phospholipase A(2) by low levels of fluoride in THP1 macrophages via altered Ca(2+) and cAMP concentration. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 99–105. [Google Scholar] [CrossRef]
- Park, S.; Ajtai, K.; Burghardt, T.P. Inhibition of myosin ATPase by metal fluoride complexes. Biochim. Biophys. Acta 1999, 1430, 127–140. [Google Scholar] [CrossRef]
- Vani, M.L.; Reddy, K.P. Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride 2000, 33, 17–26. [Google Scholar]
- Shashi, A. Fluoride and adrenal gland function in rabbits. Fluoride 2003, 36, 241–251. [Google Scholar]
- Kumar, A.; Swami, V.P. Sodium fluoride induce alterations in glycogen metabolism in freshwater catfish, Clarias batrachus (Linn.). Int. J. Fish. Aquat. Stud. 2022, 10, 101–104. [Google Scholar] [CrossRef]
- McGown, E.L.; Suttie, J.W. Mechanism of fluoride-induced hyperglycemia in the rat. Toxicol. Appl. Pharmacol. 1977, 40, 83–90. [Google Scholar] [CrossRef]
- Waldbott, G.L.; Burgstahler, A.W.; McKinney, H.L. Fluoridation: The Great Dilemma; Coronado Press: Lawrence, Kansas, 1978. [Google Scholar]
- Susheela, A.K. A Treatise on Fluorosis; Fluorosis Research and Rural Development Foundation: Delhi, India, 2001. [Google Scholar]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 1, 119241. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Sharma, D.K.; Damodaran, T. Fluoride in the environment and its metabolism in humans. Rev. Environ. Contam. Toxicol. 2011, 211, 121–142. [Google Scholar] [CrossRef]
- Gupta, A.R.; Dey, S.; Swarup, D.; Saini, M. Effects of excessive fluoride ingestion on collagen protein and expression of type I collagen gene in skeletal muscles of rats. Fluoride 2013, 46, 149–155. [Google Scholar]
- Nagendra, A.H.; Najar, M.A.; Bose, B.; Shenoy, P.S. High concentration of sodium fluoride in drinking water induces hypertrophy versus atrophy in mouse skeletal muscle via modulation of sarcomeric proteins. J. Hazard. Mater. 2022, 432, 128654. [Google Scholar] [CrossRef]
- Shashi, A.; Rana, N. Effect of Fluoride on the Expression of type I Collagen Gene (COL1A1) in Skeletal Muscle of Rats. Asian J. Biol. Sci. 2016, 5, 62–65. [Google Scholar]
- Shenoy, P.S.; Sen, U.; Kapoor, S.; Ranade, A.V.; Chowdhury, C.R.; Bose, B. Sodium fluoride induced skeletal muscle changes: Degradation of proteins and signaling mechanism. Environ. Pollut. 2019, 244, 534–548. [Google Scholar] [CrossRef]
- He, L.-F.; Chen, J.-G. DNA damage, apoptosis and cell cycle changes induced by fluoride in rat oral mucosal cells and hepatocytes. World J. Gastroenterol. 2006, 12, 1144–1148. [Google Scholar] [CrossRef]
- Nagendra, A.H.; Bose, B.; Shenoy, P.S. Recent advances in cellular effects of fluoride: An update on its signalling pathway and targeted therapeutic approaches. Mol. Biol. Rep. 2021, 48, 5661–5673. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J. Muscle glycogen and cell function—Location, location, location. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S4), 34–40. [Google Scholar] [CrossRef]
- Yan, X.; Yang, X.; Hao, X.; Ren, Q.; Gao, J.; Wang, Y.; Chang, N.; Qiu, Y.; Song, G. Sodium Fluoride Induces Apoptosis in H9c2 Cardiomyocytes by Altering Mitochondrial Membrane Potential and Intracellular ROS Level. Biol. Trace Elem. Res. 2015, 166, 210–215. [Google Scholar] [CrossRef]
- Wei, M.; Ye, Y.; Ali, M.M.; Chamba, Y.; Tang, J.; Shang, P. Effect of Fluoride on Cytotoxicity Involved in Mitochondrial Dysfunction: A Review of Mechanism. Front. Vet. Sci. 2022, 9, 850771. [Google Scholar] [CrossRef]
- Strunecka, A.; Strunecky, O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl. Sci. 2020, 10, 7100. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Yujra, V.Q.; da Silva, V.H.P.; Claudio, S.R.; Estadella, D.; de Barros Viana, M.; Oshima, C.T.F. Putative mechanisms of genotoxicity induced by fluoride: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2017, 24, 15254–15259. [Google Scholar] [CrossRef]
- Aulestia, F.J.; Groeling, J.; Bomfim, G.H.S.; Costiniti, V.; Manikandan, V.; Chaloemtoem, A.; Concepcion, A.R.; Li, Y.; Wagner, L.E., 2nd; Idaghdour, Y.; et al. Fluoride exposure alters Ca2+ signaling and mitochondrial function in enamel cells. Sci. Signal. 2020, 13, eaay0086. [Google Scholar] [CrossRef]
- Gutowska, I.; Baranowska-Bosiacka, I.; Baśkiewicz, M.; Millo, B.; Siennicka, A.; Marchlewicz, M.; Wiszniewska, B.; Machaliński, B.; Stachowska, E. Fluoride as a pro-inflammatory factor and inhibitor of ATP bioavailability in differentiated human THP1 monocytic cells. Toxicol. Lett. 2010, 196, 74–79. [Google Scholar] [CrossRef]
- Monick, M.M.; Powers, L.S.; Barrett, C.W.; Hinde, S.; Ashare, A.; Groskreutz, D.J.; Nyunoya, T.; Coleman, M.; Spitz, D.R.; Hunninghake, G.W. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J. Immunol. 2000, 180, 7485–7496. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, W.; Xue, X.; Zhang, Y.; Niu, R.; Li, X.; Li, B.; Wang, X.; Wang, J. Fluoride decreased the sperm ATP of mice through inhabiting mitochondrial respiration. Chemosphere 2016, 144, 1012–1017. [Google Scholar] [CrossRef]
- Suska, M. The effect of sodium fluoride on the adenine nucleotide pool in erythrocytes of Wistar rats. Int. J. Occup. Med. Environ. Health 2001, 14, 369–373. [Google Scholar]
- Agalakova, N.I.; Gusev, G.P. Fluoride induces oxidative stress and ATP depletion in the rat erythrocytes in vitro. Environ. Toxicol. Pharmacol. 2012, 34, 334–337. [Google Scholar] [CrossRef]
- Zhao, W.P.; Wang, H.W.; Liu, J.; Zhang, Z.H.; Zhu, S.Q.; Zhou, B.H. Mitochondrial respiratory chain complex abnormal expressions and fusion disorder are involved in fluoride-induced mitochondrial dysfunction in ovarian granulosa cells. Chemosphere 2019, 215, 619–625. [Google Scholar] [CrossRef]
- Suzuki, M.; Bandoski, C.; Bartlett, J.D. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic. Biol. Med. 2015, 89, 369–378. [Google Scholar] [CrossRef]
- Barnes, M.; Gibson, L.M.; Stephenson, D.G. Increased muscle glycogen content is associated with increased capacity to respond to T-system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflug. Arch. 2001, 442, 101–106. [Google Scholar] [CrossRef]
- Chin, E.R.; Allen, D.G. Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J. Physiol. 1997, 498 Pt 1, 17–29. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J.; Saltin, B.; Holmberg, H.C. Role of glycogen availability in sarcoplasmic reticulum Ca2+ kinetics in human skeletal muscle. J. Physiol. 2011, 589 Pt 3, 711–725. [Google Scholar] [CrossRef]
- Nielsen, J.; Schrøder, H.D.; Rix, C.G.; Ortenblad, N. Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J. Physiol. 2009, 587 Pt 14, 3679–3690. [Google Scholar] [CrossRef]
- Stephenson, D.G. Molecular cogs in machina carnis. Clin. Exp. Pharmacol. Physiol. 1996, 23, 898–907. [Google Scholar] [CrossRef]
- Gejl, K.D.; Hvid, L.G.; Frandsen, U.; Jensen, K.; Sahlin, K.; Ørtenblad, N. Muscle glycogen content modifies SR Ca2+ release rate in elite endurance athletes. Med. Sci. Sports Exerc. 2014, 46, 496–505. [Google Scholar] [CrossRef]
- Wang, Y.Z. The cartilage damage of fluorosis. Fluoride 1995, 28, 39. [Google Scholar]
- Ream, L.J.; Principato, R. Glycogen accumulation in the parathyroid gland of the rat after fluoride ingestion. Cell Tissue Res. 1981, 220, 125–130. [Google Scholar] [CrossRef]
- Dost, F.N.; Knaus, R.M.; Johnson, D.E.; Wang, C.H. Fluoride impairment of glucose utilization: Nature of effect in rats during and after continuous NaF infusion. Toxicol. Appl. Pharmacol. 1977, 41, 451–458. [Google Scholar] [CrossRef]
- Vasant, R.A.; Narasimhacharya, A.V. Ameliorative effect of tamarind leaf on fluoride-induced metabolic alterations. Environ. Health Prev. Med. 2012, 17, 484–493. [Google Scholar] [CrossRef]
- Shashi, A.; Grewal, A.; Bhardwaj, M. Toxicological effect of fluoride on the rat testicular glycogen. Asian J. Microbiol. Biotechnol. Environ. Sci. 2009, 11, 643–646. [Google Scholar]
- Iwase, T. Studies on the glycogen and phosphorylase variations in myocardium, skeletal muscle, liver in experimental fluorosis. Part I Influence of fluorine on glycogen. Shikoku Acta Med. 1958, 12, 616–623. [Google Scholar]
- Singh, J.; Thapar, S.P. Changes in glycogen content in some tissues during fluorosis—An experimental study on rabbits. Fluoride 1988, 21, 82–86. [Google Scholar]
- Kale, M.D. Impact of Sodium Fluoride (NaF) on Protein and Lipid Concentration of Fresh Water Fishes Labeo rohita. Int. J. Creat. Res. Thoughts 2020, 8, 2544–2549. [Google Scholar]
- Zebrowski, E.J.; Suttie, J.W. Glucose Oxidation and Glycogen Metabolism in Fluoride-fed Rats. J. Nutr. 1966, 88, 267–272. [Google Scholar] [CrossRef]
- Souza, D.N.; Mendes, F.M.; Nogueira, F.N.; Simões, A.; Nicolau, J. Lithium Induces Glycogen Accumulation in Salivary Glands of the Rat. Biol. Trace Elem. Res. 2016, 169, 271–278. [Google Scholar] [CrossRef]
- Jensen, J.; Lai, Y.C. Regulation of muscle glycogen synthase phosphorylation and kintetic properties by insulin, exercise, adrenaline and role in insulin resisntance. Arch. Pysiol. Biochem. 2009, 115, 13–21. [Google Scholar] [CrossRef]
- Prats, C.; Helge, J.W.; Nordby, P.; Qvortrup, K.; Ploug, T.; Dela, F.; Wojtaszewski, J.F. Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization. J. Biol. Chem. 2009, 284, 15692–15700. [Google Scholar] [CrossRef]
- Hardy, T.A.; Roach, P.J. Control of yeast glycogen synthase-2 by COOH-terminal phosphorylation. J. Biol. Chem. 1993, 268, 23799–23805. [Google Scholar] [CrossRef]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef]
- De Wulf, H.; Hers, H.G. The interconversion of liver glycogen synthetase a and b in vitro. Eur. J. Biochem. 1968, 6, 552–557. [Google Scholar] [CrossRef]
- Force, T.; Woodgett, J.R. Unique and overlapping functions of GSK-3 isoforms in cell differentiation and proliferation and cardiovascula development. J. Biol. Chem. 2009, 284, 9643–9647. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Dokken, B.B. Role of glycogen synthase kinase-3 in insulin resistance and type 2 diabetes. Curr. Drug Targets 2006, 7, 1435–1441. [Google Scholar] [CrossRef]
- Stambolic, V.; Woodgett, J.R. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem. J. 1994, 303 Pt 3, 701–704. [Google Scholar] [CrossRef]
- Wang, Q.M.; Fiol, C.J.; DePaoli-Roach, A.A.; Roach, P.J. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 1994, 269, 14566–14574. [Google Scholar] [CrossRef]
- Murai, H.; Okazaki, M.; Kikuchi, A. Tyrosine dephosphorylation of glycogen synthase kinase-3 is involved in its extracellular signal-dependent inactivation. FEBS Lett. 1996, 392, 153–160. [Google Scholar] [CrossRef]
- Fang, X.; Yu, S.X.; Lu, Y.; Bast, R.C., Jr.; Woodgett, J.R.; Mills, G.B. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl. Acad. Sci. USA 2000, 97, 11960–11965. [Google Scholar] [CrossRef]
- Adamek, E.; Pawłowska-Góral, K.; Bober, K. In vitro and in vivo effects of fluoride ions on enzyme activity. Ann. Acad. Med. Stetin. 2005, 51, 69–85. [Google Scholar]
- Shen, L.; Feng, C.; Xia, S.; Wei, Y.; Zhang, H.; Zhao, D.; Yao, F.; Liu, X.; Zhao, Y.; Zhang, H. Progressive Research in the Molecular Mechanisms of Chronic Fluorosis. In Environmental Chemistry and Recent Pollution Control Approaches; Saldarriaga-Noreña, H., Murillo-Tovar, M.A., Farooq, R., Dongre, R., Riaz, S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Fan, B.; Yu, Y.; Zhang, Y. PI3K-Akt1 expression and its significance in liver tissues with chronic fluorosis. Int. J. Clin. Exp. Pathol. 2015, 8, 1226–1236. [Google Scholar]
- Pan, L.; Shi, X.; Liu, S.; Guo, X.; Zhao, M.; Cai, R.; Sun, G. Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway. Toxicol. Lett. 2014, 225, 34–42. [Google Scholar] [CrossRef]
- Greenberg, C.C.; Jurczak, M.J.; Danos, A.M.; Brady, M.J. Glycogen branches out: New perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1–E8. [Google Scholar] [CrossRef]
- Houdijk, A.P.; Van Leeuwen, P.A.; Adolfs, M.J.; Bonta, I.L. GTP-related difference in cyclic AMP production between resident and inflammatory human peritoneal macrophages. Int. J. Tissue React. 1991, 13, 279–285. [Google Scholar]
- Shahed, A.; Allman, D.W. Stimulation of adenylate cyclase activity by Na2PO3F and NaF in intact rat hepatocytes and plasma membrane fractions. Fluoride 1984, 17, 210–217. [Google Scholar]
- Kornegay, D.; Pennigton, S. A review of the effect of fluoride ion on adenyl cyclase. Fluoride 1973, 6, 19–32. [Google Scholar]
- Shashi, A.; Bhardwaj, M. Study on blood biochemical diagnostic indices for hepatic function biomarkers in endemic skeletal fluorosis. Biol. Trace Elem. Res. 2011, 143, 803–814. [Google Scholar] [CrossRef]
- Pan, Y.; Lü, P.; Yin, L.; Chen, K.; He, Y. Effect of fluoride on the proteomic profile of the hippocampus in rats. Z. Naturforsch. C J. Biosci. 2015, 70, 151–157. [Google Scholar] [CrossRef]
- Strunecka, A.; Patocka, J.; Blaylock, L.R.; Chinoy, J.N. Fluoride Interactions: From Molecules to Disease. Curr. Signal Transduct. Ther. 2007, 2, 190–213. [Google Scholar] [CrossRef]
- Opydo-Szymaczek, J.; Borysewicz-Lewicka, M. Variations in concentration of fluoride in blood plasma of pregnant women and their possible consequences for amelogenesis in a fetus. Homo 2006, 57, 295–307. [Google Scholar] [CrossRef]
- Zawierta, J.; Dąbkowska, E.; Jakubowska, K.; Olszewska, M.; Noceń, I. The fluorine, iron, copper, zinc and selenium contents in plasma of healthy people living in Szczecin and its vicinity. Met. Fluor. 1998, 200–205. [Google Scholar]
- Tabatabaei Shafiei, M.; Carvajal Gonczi, C.M.; Rahman, M.S.; East, A.; François, J.; Darlington, P.J. Detecting glycogen in peripheral blood mononuclear cells with periodic acid schiff staining. J. Vis. Exp. 2014, 2014, 52199. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutowska, I.; Maruszewska, A.; Skórka-Majewicz, M.; Kempińska-Podhorodecka, A.; Kolasa, A.; Wszołek, A.; Baranowska-Bosiacka, I.; Żwierełło, W. Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136. Molecules 2023, 28, 6065. https://doi.org/10.3390/molecules28166065
Gutowska I, Maruszewska A, Skórka-Majewicz M, Kempińska-Podhorodecka A, Kolasa A, Wszołek A, Baranowska-Bosiacka I, Żwierełło W. Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136. Molecules. 2023; 28(16):6065. https://doi.org/10.3390/molecules28166065
Chicago/Turabian StyleGutowska, Izabela, Agnieszka Maruszewska, Marta Skórka-Majewicz, Agnieszka Kempińska-Podhorodecka, Agnieszka Kolasa, Agata Wszołek, Irena Baranowska-Bosiacka, and Wojciech Żwierełło. 2023. "Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136" Molecules 28, no. 16: 6065. https://doi.org/10.3390/molecules28166065
APA StyleGutowska, I., Maruszewska, A., Skórka-Majewicz, M., Kempińska-Podhorodecka, A., Kolasa, A., Wszołek, A., Baranowska-Bosiacka, I., & Żwierełło, W. (2023). Fluoride as a Potential Repressor of Glycogen Metabolism in Skeletal Muscle Cell Line CCL136. Molecules, 28(16), 6065. https://doi.org/10.3390/molecules28166065






