Propolis-Based Nanostructured Lipid Carriers for α-Mangostin Delivery: Formulation, Characterization, and In Vitro Antioxidant Activity Evaluation
Abstract
1. Introduction
2. Results
2.1. Propolis Extraction
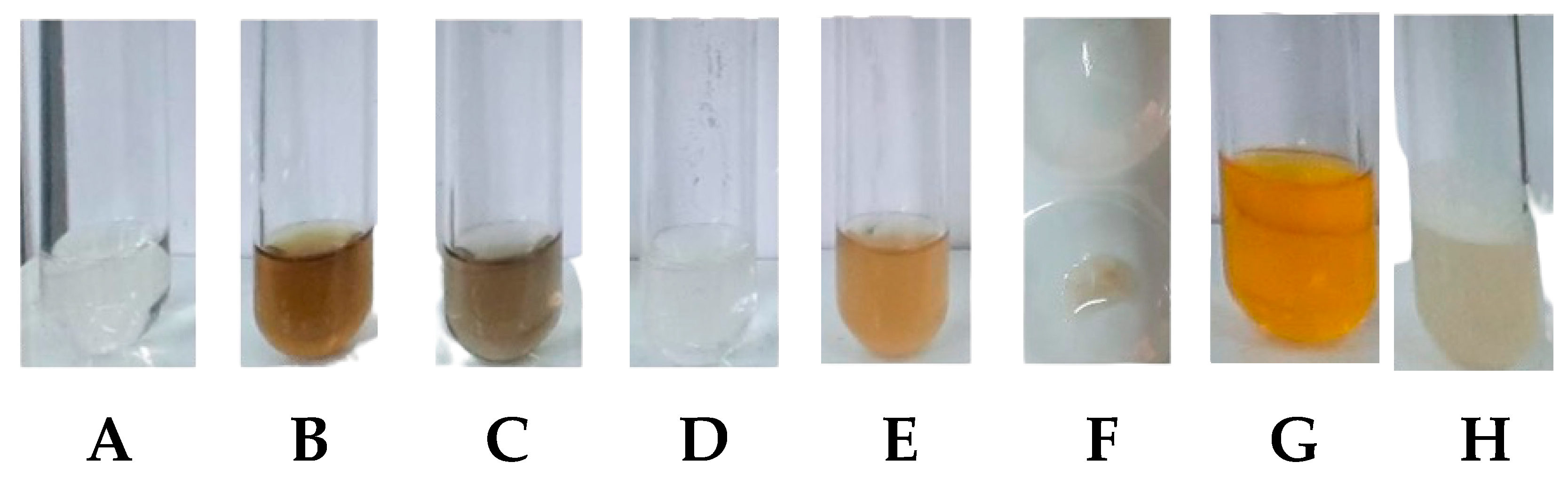
2.1.1. Phytochemical Screening of Propolis Extract
2.1.2. Measurement of Total Flavonoid Levels of Propolis Extract
2.1.3. Measurement of Total Phenol Levels of Propolis Extract
2.2. Characterization of Propolis-Based NLC Loaded with α-Mangostin
2.2.1. Particle Size Analysis (PSA), Zeta Potential (ZP), and Polydispersity Index (PI)
2.2.2. Transmission Electron Microscopy (TEM) Imaging
2.2.3. Entrapment Efficiency
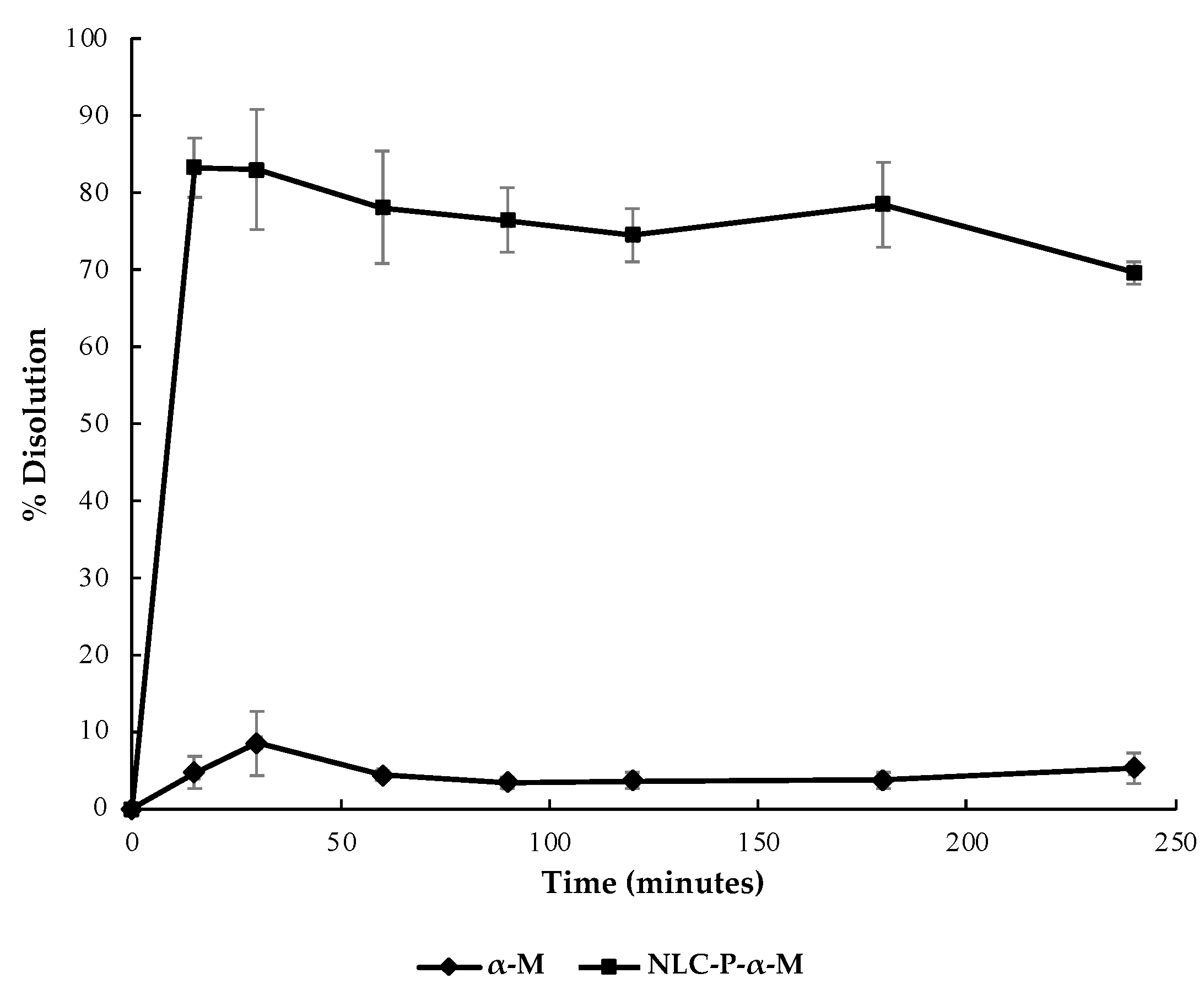
2.2.4. Dissolution Test
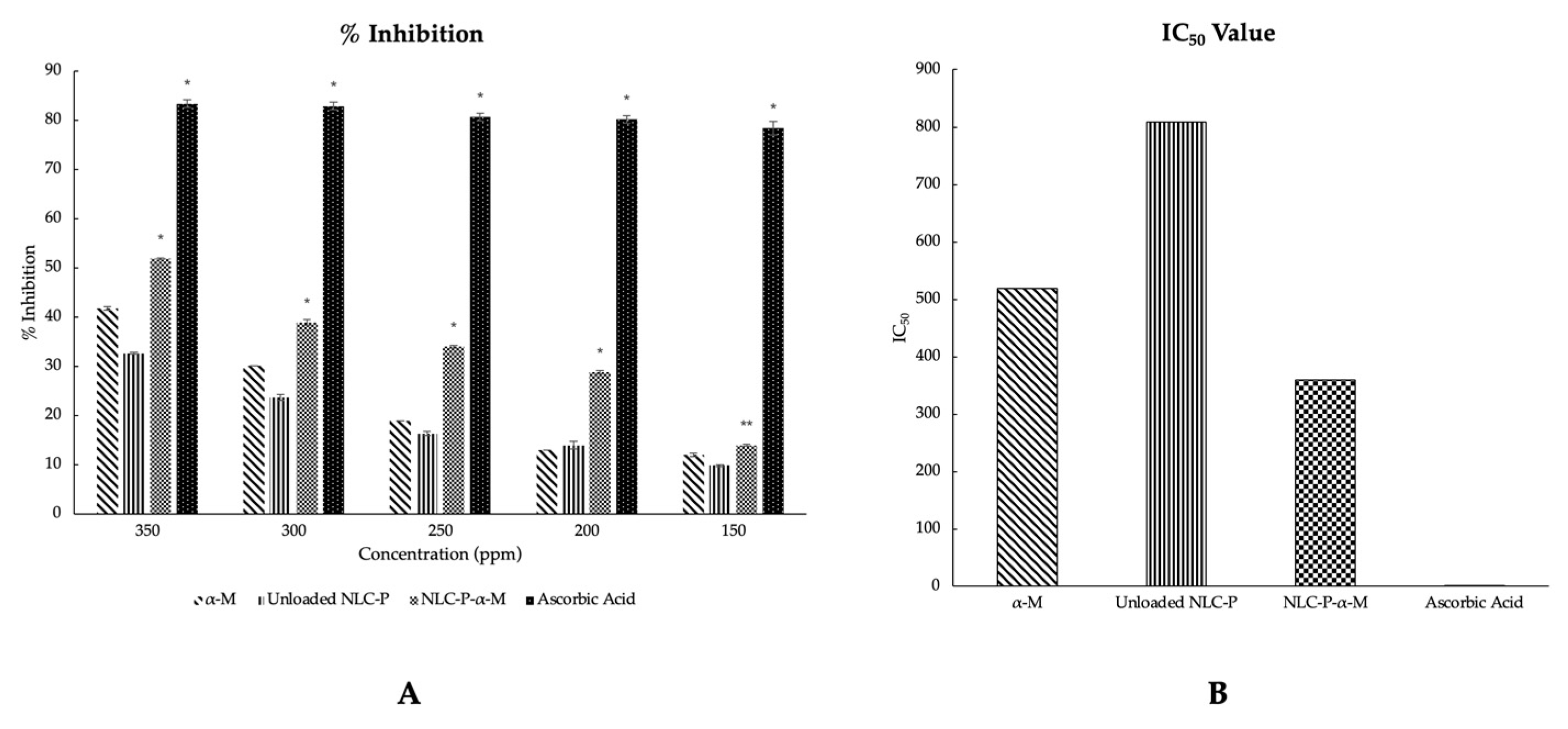
2.3. In Vitro Antioxidant Activity Assay of Propolis-Based NLC Formulations
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
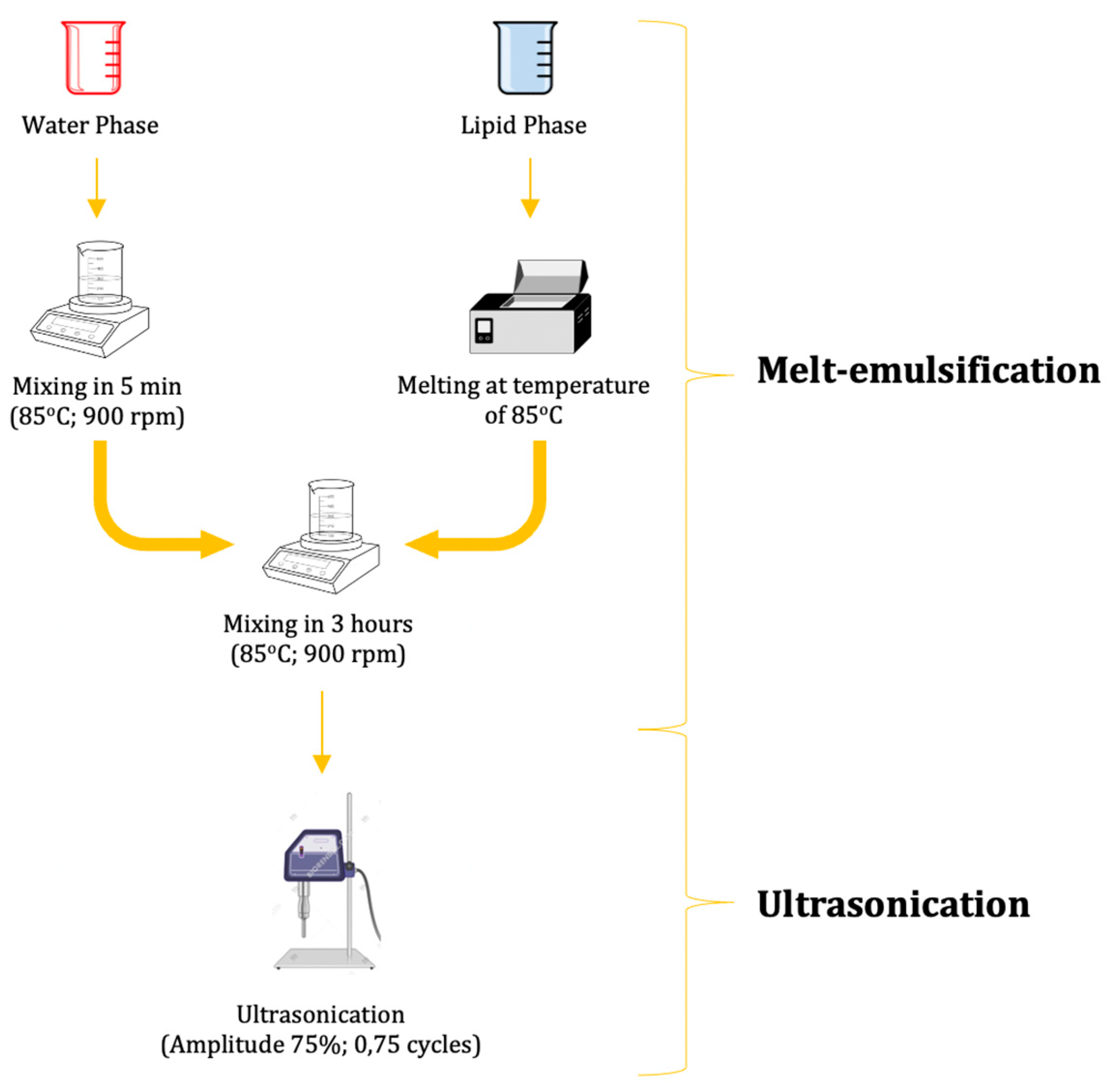
4.2.1. Propolis-Based Nanostructured Lipid Carrier (NLC) of α-Mangostin Preparation
Propolis Extraction
Phytochemical Screening of Propolis Extract
- Phenols: three drops of an alcohol solution containing ferric chloride (FeCl3 5%) were mixed with the sample. After agitating the mixture, the tube was examined for any color changes or the formation of a significant amount of dark-colored precipitate. The presence of phenols was indicated by hues that ranged from blue to red [42].
- Tannins: the sample was subjected to the addition of three drops of an alcohol-based solution containing ferric chloride (FeCl3 1%). The resulting mixture was then observed for the formation of a precipitate, specifically noting its color. If a bluish dark–green precipitate was observed, it indicated the presence of tannins in the EEP sample [42].
- Flavonoids: carried out by two methods, including alkaline reagent test and Shinod’s test.
- Alkaline reagent test: two to three drops of NaOH 10% were introduced into 2 mL of the extract. Initially, a deep yellow color emerged as a result. This color change indicated the presence of flavonoids in the extract [43].
- Shinod’s test: the extract was combined with ten drops of concentrated hydrochloric acid (HCl) and a piece of magnesium. As a result, a deep pink color developed, which served as an indication of the presence of flavonoids in the mixture [43].
- Triterpenoids and steroids: the dried residue underwent three extractions with 2 mL of chloroform, and the resulting solution was filtered into a test tube using a cotton-covered funnel with anhydrous sodium sulfate. After filtration, acetic anhydride (1 mL) was added and mixed, followed by the addition of concentrated sulfuric acid (three drops) with agitation. The test tube was then observed for color changes: evanescent blue to permanent green for free steroids, and a range of brown to red for free pentacyclic triterpenoids [42].
- Saponins: the insoluble residue from chloroform was dissolved in 8 mL of distilled water and filtered into a test tube. After vigorous agitation for three minutes, the test tube was checked for the formation of persistent foam indicating the presence of saponin glycosides (saponins) [42].
- Alkaloids: the sample was added by 1 mL of Dragendorff’s reagent. An orange–red precipitate indicates the presence of alkaloids [42].
Measurement of Total Flavonoid Levels of Propolis Extract
Measurement of Total Phenol Levels of Propolis Extract
Propolis-Based Nanostructured Lipid Carrier (NLC-P) Preparation
α-Mangostin-Loaded Propolis-Based NLC (NLC-P-α-M) Preparation
4.2.2. Characterization of α-Mangostin-Loaded Propolis-based NLC
Particle Size Analysis (PSA), Zeta Potential (ZP), and Polydispersity Index (PI)
Transmission Electron Microscopy (TEM) Imaging
Entrapment Efficiency
Dissolution Test
4.2.3. In Vitro Antioxidant Activity Assay of Propolis-Based NLC Formulations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References
- Pomalingo, D.R.; Suhandi, C.; Megantara, S.; Muchtaridi, M. The Optimization of α-Mangostin as a New Drug Candidate through Molecular Docking and Dynamic Simulations. Rasayan J. Chem. 2021, 14, 698–704. [Google Scholar] [CrossRef]
- Megantara, S.; Wathoni, N.; Mohammed, A.F.A.; Suhandi, C.; Ishmatullah, M.H.; Putri, M.F.F.D. In Silico Study: Combination of α-Mangostin and Chitosan Conjugated with Trastuzumab against Human Epidermal Growth Factor Receptor 2. Polymers 2022, 14, 2747. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and Pharmacological Properties of α-Mangostin from the Mangosteen Fruit: A Review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, G.; Shen, Y. The Naturally Occurring Xanthone α-Mangostin Induces ROS-Mediated Cytotoxicity in Non-Small Scale Lung Cancer Cells. Saudi J. Biol. Sci. 2018, 25, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverrí, J.; Reyes-Fermín, L.M.; Nolasco-Amaya, E.G.; Orozco-Ibarra, M.; Medina-Campos, O.N.; González-Cuahutencos, O.; Rivero-Cruz, I.; Mata, R. ROS Scavenging Capacity and Neuroprotective Effect of α-Mangostin against 3-Nitropropionic Acid in Cerebellar Granule Neurons. Exp. Toxicol. Pathol. 2009, 61, 491–501. [Google Scholar] [CrossRef]
- Martínez, A.; Galano, A.; Vargas, R. Free Radical Scavenger Properties of α-Mangostin: Thermodynamics and Kinetics of HAT and RAF Mechanisms. J. Phys. Chem. B 2011, 115, 12591–12598. [Google Scholar] [CrossRef]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Functional Beverage of Garcinia Mangostana (Mangosteen) Enhances Plasma Antioxidant Capacity in Healthy Adults. Food Sci. Nutr. 2015, 3, 32–38. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Aisha, A.F.A.; Ismail, Z.; Abu-salah, K.M.; Majid, A.M.S.A. Solid Dispersions of α-Mangostin Improve Its Aqueous Solubility through Self-Assembly of Nanomicelles. J. Pharm. Sci. 2012, 101, 815–825. [Google Scholar] [CrossRef]
- Sodalee, K.; Sapsuphan, P.; Wongsirikul, R.; Puttipipatkhachorn, S. Preparation and Evaluation of Alpha-Mangostin Solid Self-Emulsifying Drug Delivery System. Asian J. Pharm. Sci. 2016, 11, 225–226. [Google Scholar] [CrossRef][Green Version]
- Verma, R.K.; Yu, W.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. α-Mangostin-Encapsulated PLGA Nanoparticles Inhibit Pancreatic Carcinogenesis by Targeting Cancer Stem Cells in Human, and Transgenic (KrasG12D, and KrasG12D/Tp53R270H) Mice. Sci. Rep. 2016, 6, 32743. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Brunner, I.; Han, A.R.; Hamburger, M.; Kinghorn, A.D.; Frye, R.; Butterweck, V. Pharmacokinetics of α-Mangostin in Rats after Intravenous and Oral Application. Mol. Nutr. Food Res. 2011, 55 (Suppl. S1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Ashkar, A.; Sosnik, A.; Davidovich-Pinhas, M. Structured Edible Lipid-Based Particle Systems for Oral Drug-Delivery. Biotechnol. Adv. 2022, 54, 107789. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured Lipid Carriers: An Emerging Platform for Improving Oral Bioavailability of Lipophilic Drugs. Int. J. Pharm. Investig. 2015, 5, 182–191. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured Lipid Matrices for Improved Microencapsulation of Drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured Lipid Carriers (NLC) in Cosmetic Dermal Products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Muti, L.; D’ambrosio, M.; Grifoni, L.; Bergonzi, M.C.; Luceri, C.; Bilia, A.R. Nanostructured Lipid Carriers Can Enhance Oral Absorption of Khellin, a Natural Pleiotropic Molecule. Molecules 2021, 26, 7657. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, O.M.; Badawy, M.E.I.; Noreldin, A.E.; Abou-Ahmed, H.M.; El-Kammar, M.H.; Elkhenany, H.A. Comparative Evaluation of Propolis Nanostructured Lipid Carriers and Its Crude Extract for Antioxidants, Antimicrobial Activity, and Skin Regeneration Potential. BMC Complement. Med. Ther. 2022, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules. 2014, 19, 78. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Contieri, L.S.; de Souza Mesquita, L.M.; Sanches, V.L.; Viganó, J.; Martinez, J.; da Cunha, D.T.; Rostagno, M.A. Standardization Proposal to Quality Control of Propolis Extracts Commercialized in Brazil: A Fingerprinting Methodology Using a UHPLC-PDA-MS/MS Approach. Food Res. Int. 2022, 161, 111846. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 697. [Google Scholar] [CrossRef]
- Mahbubul, I.M. Preparation, Characterization, Properties and Application of Nanofluid; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Suhandi, C.; Alfathonah, S.S.; Hasanah, A.N. Potency of Xanthone Derivatives from Garcinia Mangostana L. for COVID-19 Treatment through Angiotensin-Converting Enzyme 2 and Main Protease Blockade: A Computational Study. Molecules 2023, 28, 5187. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-Mangostin: Anti-Inflammatory and Antioxidant Effects on Established Collagen-Induced Arthritis in DBA/1J Mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef]
- Wathoni, N.; Rusdin, A.; Motoyama, K.; Joni, I.M.; Lesmana, R.; Muchtaridi, M. Nanoparticle Drug Delivery Systems for α-Mangostin. Nanotechnol. Sci. Appl. 2020, 13, 23–36. [Google Scholar] [CrossRef]
- Samprasit, W.; Vasarach, C.; Opanasopit, P.; Sriamornsak, P.; Chamsai, B. Topical Nanostructured Lipid Carriers of Alpha-Mangostin and Resveratrol for Synergistic Antioxidant Activity. Pharm. Nanotechnol. 2022, 10, 220–231. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different Extraction Methods of Biologically Active Components from Propolis: A Preliminary Study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef]
- Dutra, R.P.; De Barros Abreu, B.V.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona Fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Üner, M. Preparation, Characterization and Physico-Chemical Properties of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Their Benefits as Colloidal Drug Carrier Systems. Pharmazie 2006, 61, 375–386. [Google Scholar]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and Characterization of Novel Nanostructured Lipid Carriers Made from Beeswax, Propolis Wax and Pomegranate Seed Oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Cao, V.D.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Thi, T.T.H. Soy Lecithin-Derived Liposomal Delivery Systems: Surface Modification and Current Applications. Int. J. Mol. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, C.; Festag, M.; Kishore, R.S.K.; Roethlisberger, D.; Schmitt, G. Pediatric Safety of Polysorbates in Drug Formulations. Children 2020, 7, 1. [Google Scholar] [CrossRef]
- Bertoni, S.; Albertini, B.; Ronowicz-Pilarczyk, J.; Calonghi, N.; Passerini, N. Solvent-Free Fabrication of Biphasic Lipid-Based Microparticles with Tunable Structure. Pharmaceutics 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Christiansen, A.; Backensfeld, T.; Kühn, S.; Weitschies, W. Stability of the Non-Ionic Surfactant Polysorbate 80 Investigated by HPLC-MS and Charged Aerosol Detector. Pharmazie 2011, 66, 666–671. [Google Scholar] [CrossRef]
- Vutti, N.B.; Gudhanti, S.K.R.; Alavala, R.R.; Desu, P.K.; Budha, R.R.; Govada, K.B.; Arja, D.P.; Ch, N. Additives Screening for the Formulation of Solid Lipid Nanoparticles of Alpha-Mangostin. J. Res. Pharm. 2023, 27, 794–810. [Google Scholar] [CrossRef]
- Jug, M.; Karas, O.; Kosalec, I. The Influence of Extraction Parameters on Antimicrobial Activity of Propolis Extracts. Nat. Prod. Commun. 2017, 12, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.O.; Hong, I.; Han, S. Extraction Properties of Propolis with Ethanol Concentration. J. Apic. 2015, 30, 211. [Google Scholar] [CrossRef]
- de Mendonça, I.C.G.; de Moraes Porto, I.C.C.; Nascimento, T.G.; de Souza, N.S.; Oliveira, J.M.S.; Arruda, R.E.S.; Mousinho, K.C.; Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian Red Propolis: Phytochemical Screening, Antioxidant Activity and Effect against Cancer Cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Kancherla, N.; Dhakshinamoothi, A.; Chitra, K.; Komaram, R.B. Preliminary Analysis of Phytoconstituents and Evaluation of Anthelminthic Property of Cayratia Auriculata (In Vitro). Maedica 2019, 14, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Syed Salleh, S.N.A.; Mohd Hanapiah, N.A.; Ahmad, H.; Wan Johari, W.L.; Osman, N.H.; Mamat, M.R. Determination of Total Phenolics, Flavonoids, and Antioxidant Activity and GC-MS Analysis of Malaysian Stingless Bee Propolis Water Extracts. Scientifica 2021, 2021, 3789351. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An Appraisal of Eighteen Commonly Consumed Edible Plants as Functional Food Based on Their Antioxidant and Starch Hydrolase Inhibitory Activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Neamatallah, T.; Alshehri, S.; Mujtaba, M.A.; Riadi, Y.; Radhakrishnan, A.K.; Khalilullah, H.; Gupta, M.; Akhter, M.H. Development, Characterization, and Evaluation of α-Mangostin-Loaded Polymeric Nanoparticle Gel for Topical Therapy in Skin Cancer. Gels 2021, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, P.; Patil, A.; Taskar, P.; Ashour, E.; Majumdar, S. Curcumin-Loaded Nanostructured Lipid Carriers for Ocular Drug Delivery: Design Optimization and Characterization. J. Drug Deliv. Sci. Technol. 2018, 47, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Zheng, Q.; Feng, X.; Tan, W.; Feng, K.; Liu, Y.; Hu, W. Preparation of Solid Lipid Nanoparticles of Cinnamaldehyde and Determination of Sustained Release Capacity. Nanomaterials 2022, 12, 4460. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-Loaded Nanostructured Lipid Carriers (NLCs) for Nasal Administration: Design, Characterization, and in Vivo Study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Raheem, I.A.A.; Razek, A.A.; Elgendy, A.A.; Saleh, N.M.; Shaaban, M.I.; El-Hady, F.K.A. Design, Evaluation and Antimicrobial Activity of Egyptian Propolis-Loaded Nanoparticles: Intrinsic Role as a Novel and Naturally Based Root Canal Nanosealer. Int. J. Nanomed. 2019, 14, 8379–8398. [Google Scholar] [CrossRef]
- Ganesan, S.; Alagarasan, J.K.; Sonaimuthu, M.; Aruchamy, K.; Alkallas, F.H.; Ben Gouider Trabelsi, A.; Kusmartsev, F.V.; Polisetti, V.; Lee, M.; Lo, H.M. Preparation and Characterization of Salsalate-Loaded Chitosan Nanoparticles: In Vitro Release and Antibacterial and Antibiofilm Activity. Mar. Drugs 2022, 20, 733. [Google Scholar] [CrossRef]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical Analysis, Antioxidant, Cytotoxic and Antimicrobial Properties of Propolis from Different Geographic Regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]

| Parameter | Result | Requirement |
|---|---|---|
| Yield (%) | 48.24 | ≥11 |
| Flavonoid total (% w/w) | 0.39 | ≥0.25 |
| Phenolic total (% w/w) | 2.43 | ≥0.50 |
| Secondary Metabolite(s) | Reagent(s) | Result * |
|---|---|---|
| Phenolics | FeCl3 5% | (+) |
| Tannins | FeCl3 1% | (+) |
| Flavonoids | Concentrated HCl + Mg | (−) |
| NaOH 10% | (+) | |
| Triterpenoids | Concentrated H2SO4 + acetic anhydride | (+) |
| Steroids | (−) | |
| Saponins | Agitated then heated | (+) |
| Alkaloids | Dragendorff | (−) |
| Preparation | PSA (nm) | ZP (mV) | PI | EE (%) |
|---|---|---|---|---|
| NLC-P | 72.7 ± 1.082 | −5.100 ± 0.954 | 0.211 ± 0.028 | N/A |
| NLC-P-α-M | 80.3 ± 1.015 | −2.767 ± 0.874 | 0.231 ± 0.028 | 87.972 ± 0.246 |
| Component | Unloaded Propolis-Based NLC | α-Mangostin-Loaded Propolis-Based NLC | |
|---|---|---|---|
| Lipid Phase | Propolis Extract | 0.9 g | 0.9 g |
| Lecithin | 0.24 g | 0.24 g | |
| α-Mangostin * | - | 0.01 g | |
| Water Phase | Polysorbate 80 (Tween-80®) | 2.06 g | 2.06 g |
| Phosphate Buffer pH 7.4 | add 20 mL | add 20 mL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhandi, C.; Wilar, G.; Lesmana, R.; Zulhendri, F.; Suharyani, I.; Hasan, N.; Wathoni, N. Propolis-Based Nanostructured Lipid Carriers for α-Mangostin Delivery: Formulation, Characterization, and In Vitro Antioxidant Activity Evaluation. Molecules 2023, 28, 6057. https://doi.org/10.3390/molecules28166057
Suhandi C, Wilar G, Lesmana R, Zulhendri F, Suharyani I, Hasan N, Wathoni N. Propolis-Based Nanostructured Lipid Carriers for α-Mangostin Delivery: Formulation, Characterization, and In Vitro Antioxidant Activity Evaluation. Molecules. 2023; 28(16):6057. https://doi.org/10.3390/molecules28166057
Chicago/Turabian StyleSuhandi, Cecep, Gofarana Wilar, Ronny Lesmana, Felix Zulhendri, Ine Suharyani, Nurhasni Hasan, and Nasrul Wathoni. 2023. "Propolis-Based Nanostructured Lipid Carriers for α-Mangostin Delivery: Formulation, Characterization, and In Vitro Antioxidant Activity Evaluation" Molecules 28, no. 16: 6057. https://doi.org/10.3390/molecules28166057
APA StyleSuhandi, C., Wilar, G., Lesmana, R., Zulhendri, F., Suharyani, I., Hasan, N., & Wathoni, N. (2023). Propolis-Based Nanostructured Lipid Carriers for α-Mangostin Delivery: Formulation, Characterization, and In Vitro Antioxidant Activity Evaluation. Molecules, 28(16), 6057. https://doi.org/10.3390/molecules28166057








