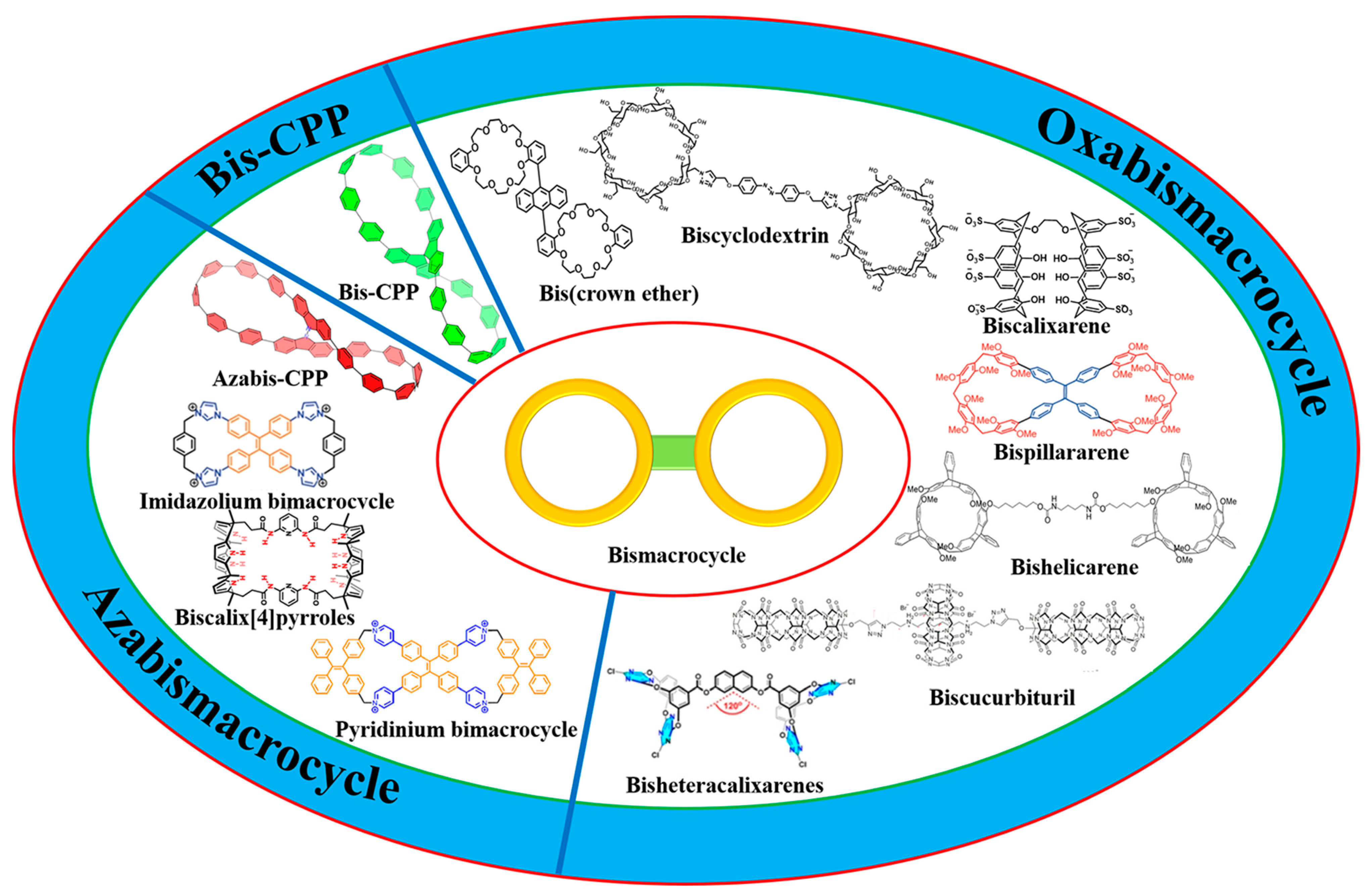
Bismacrocycle: Structures and Applications
Abstract
1. Introduction
2. Oxabismacrocycle
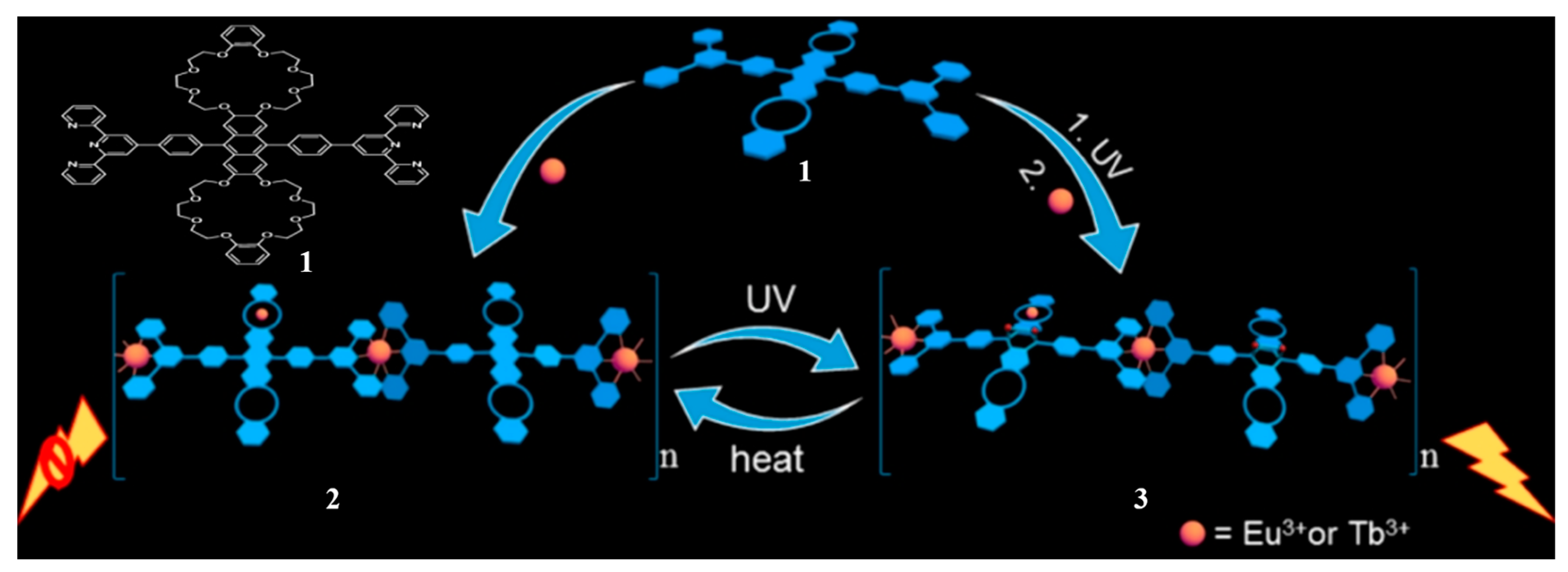
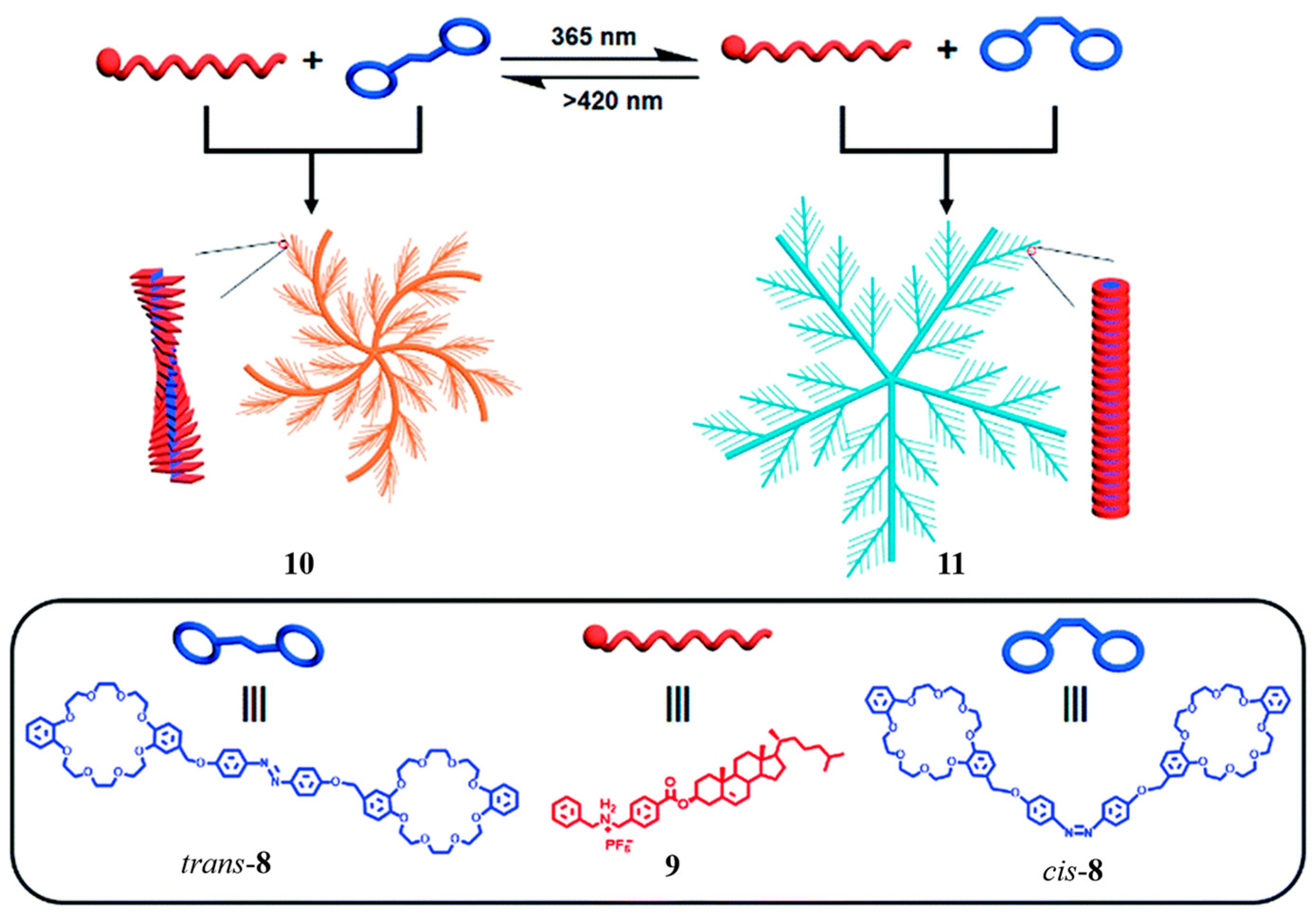
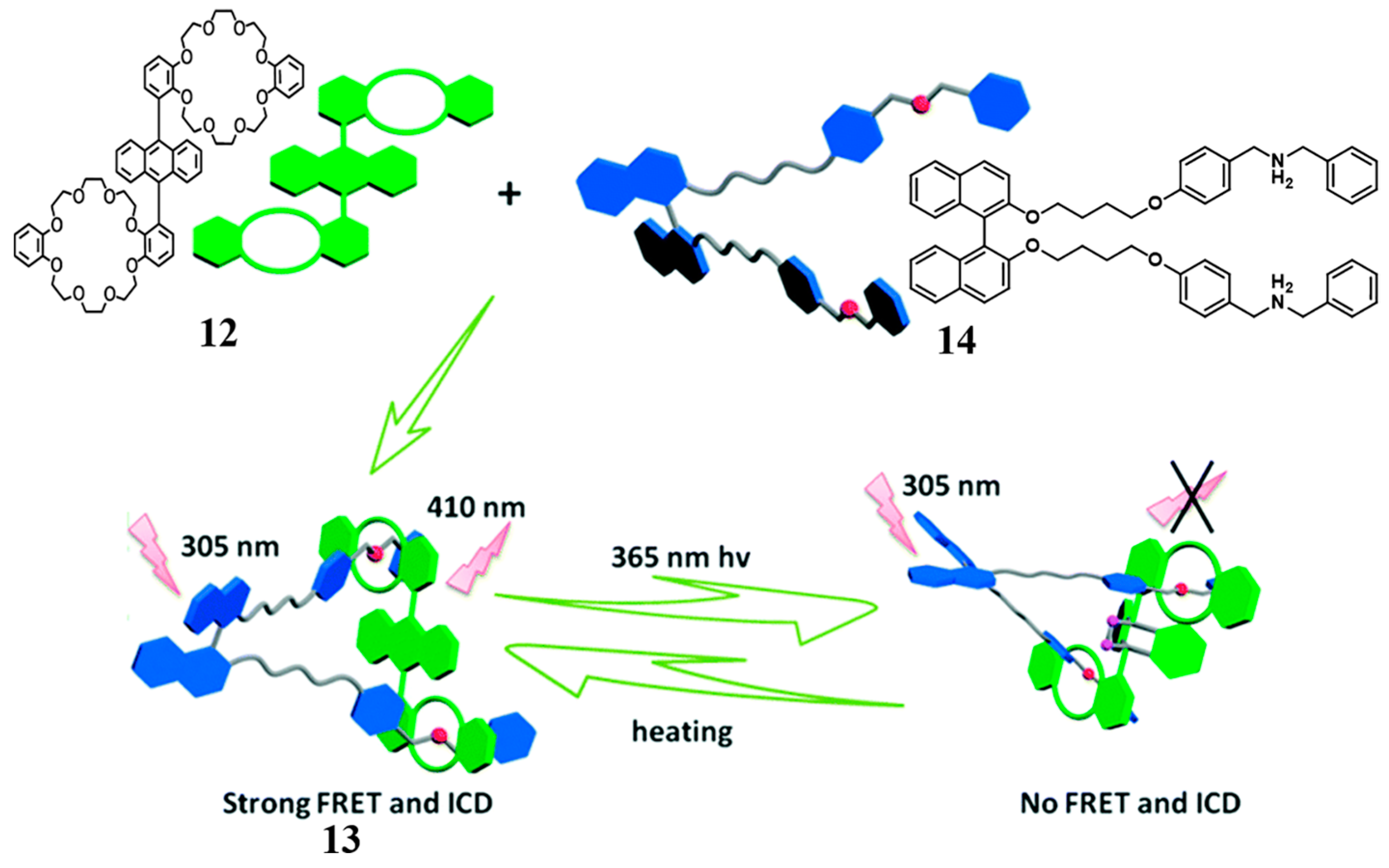
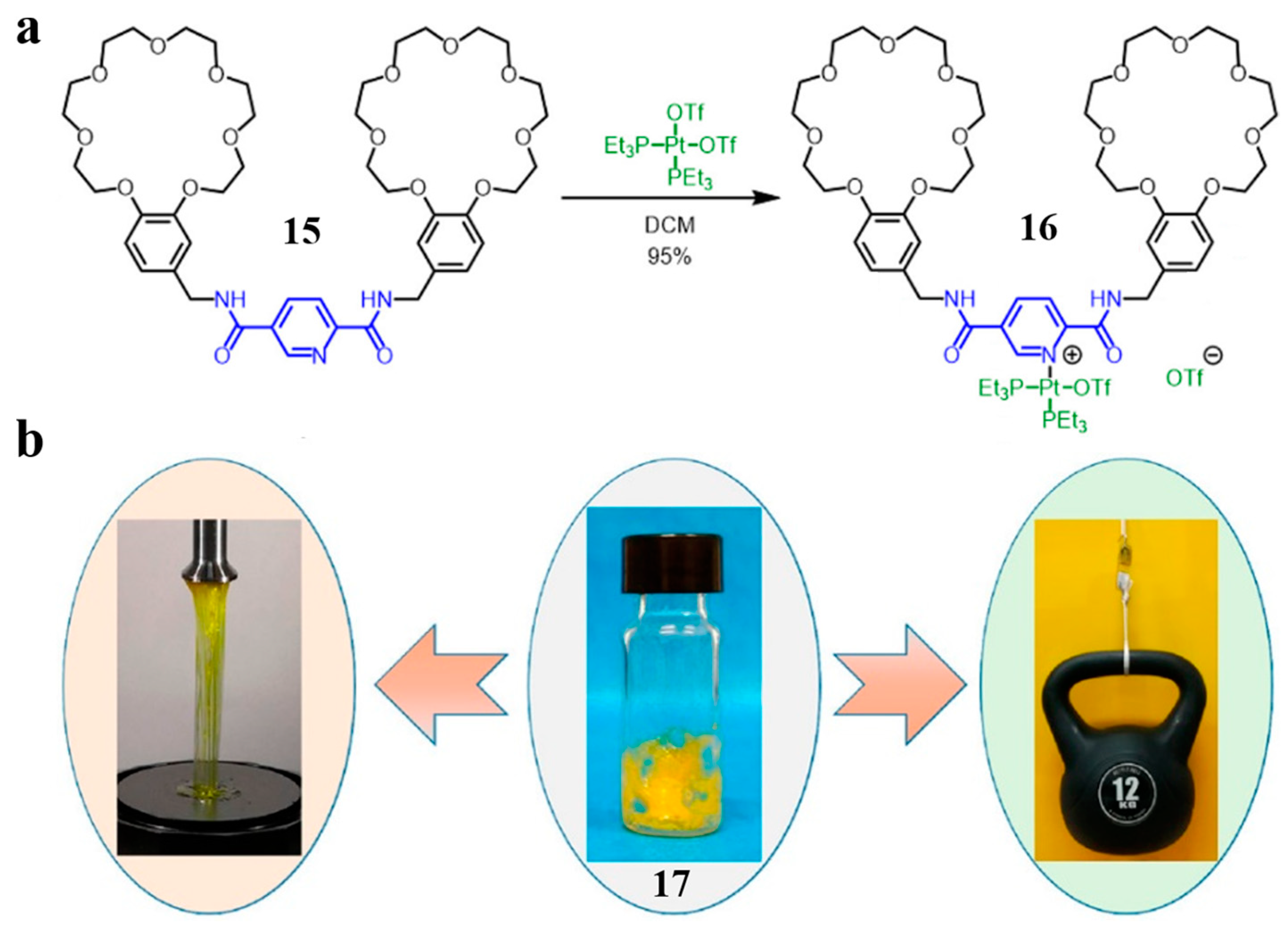
2.1. Bis(crown ether)
2.2. Biscyclodextrin
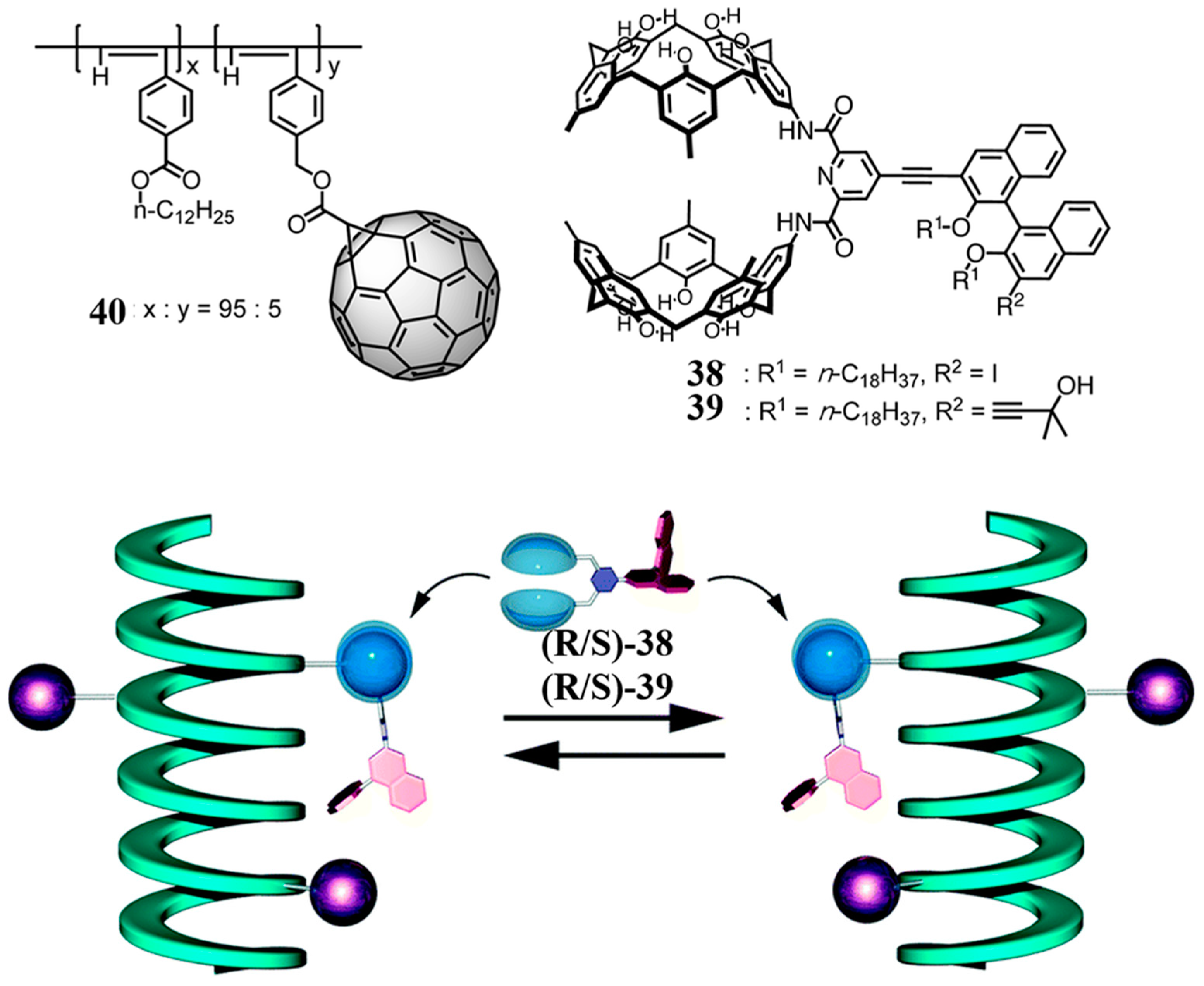
2.3. Biscalixarene
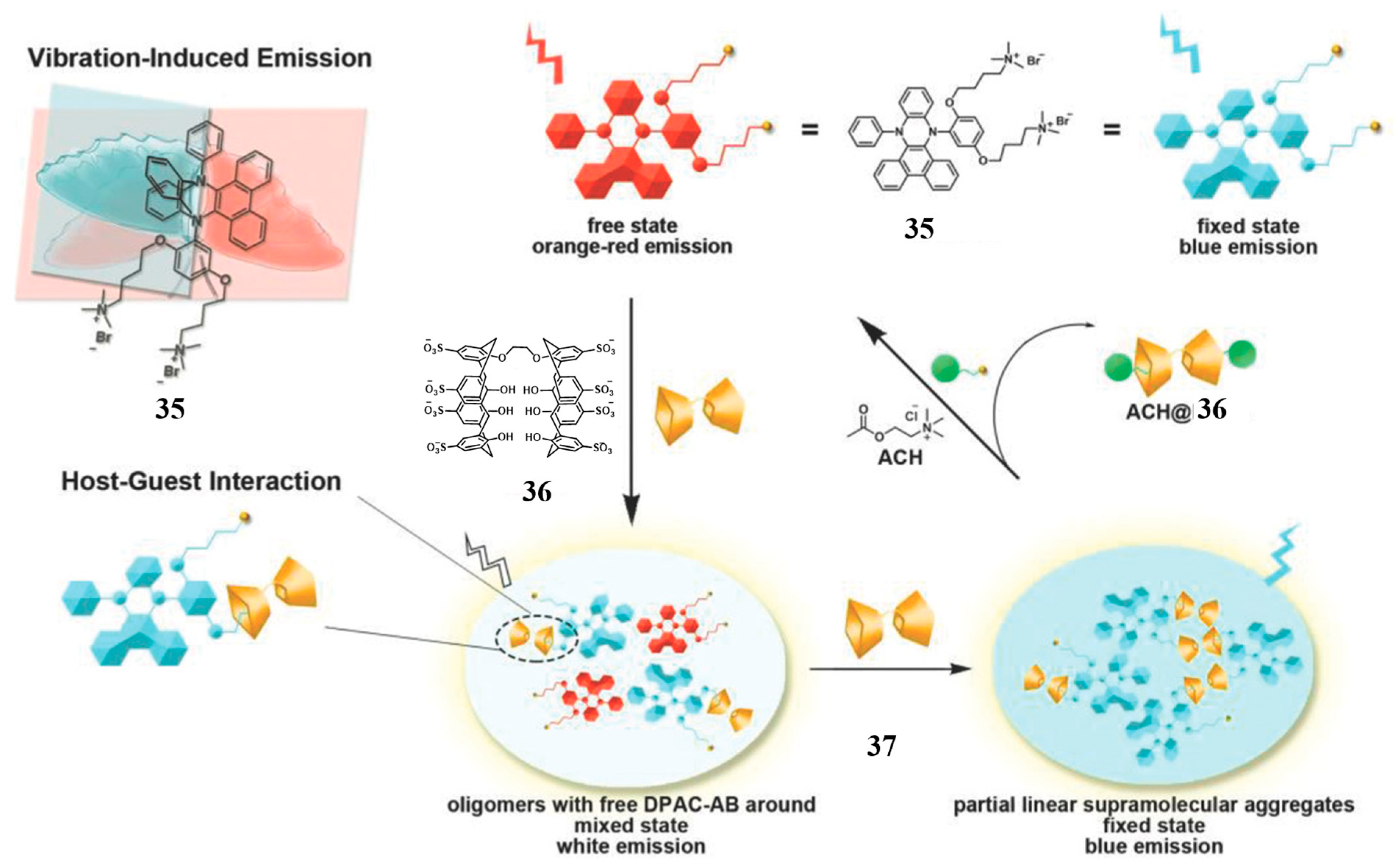
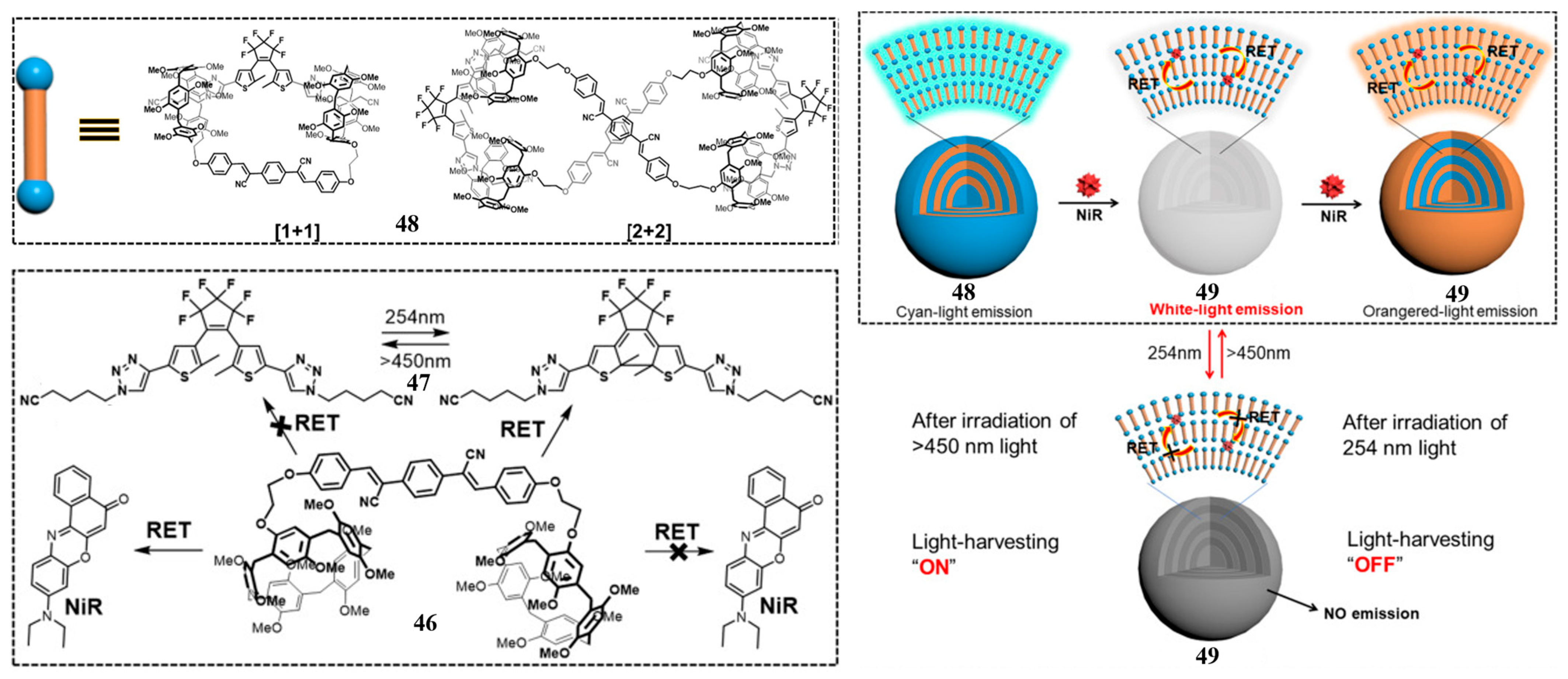
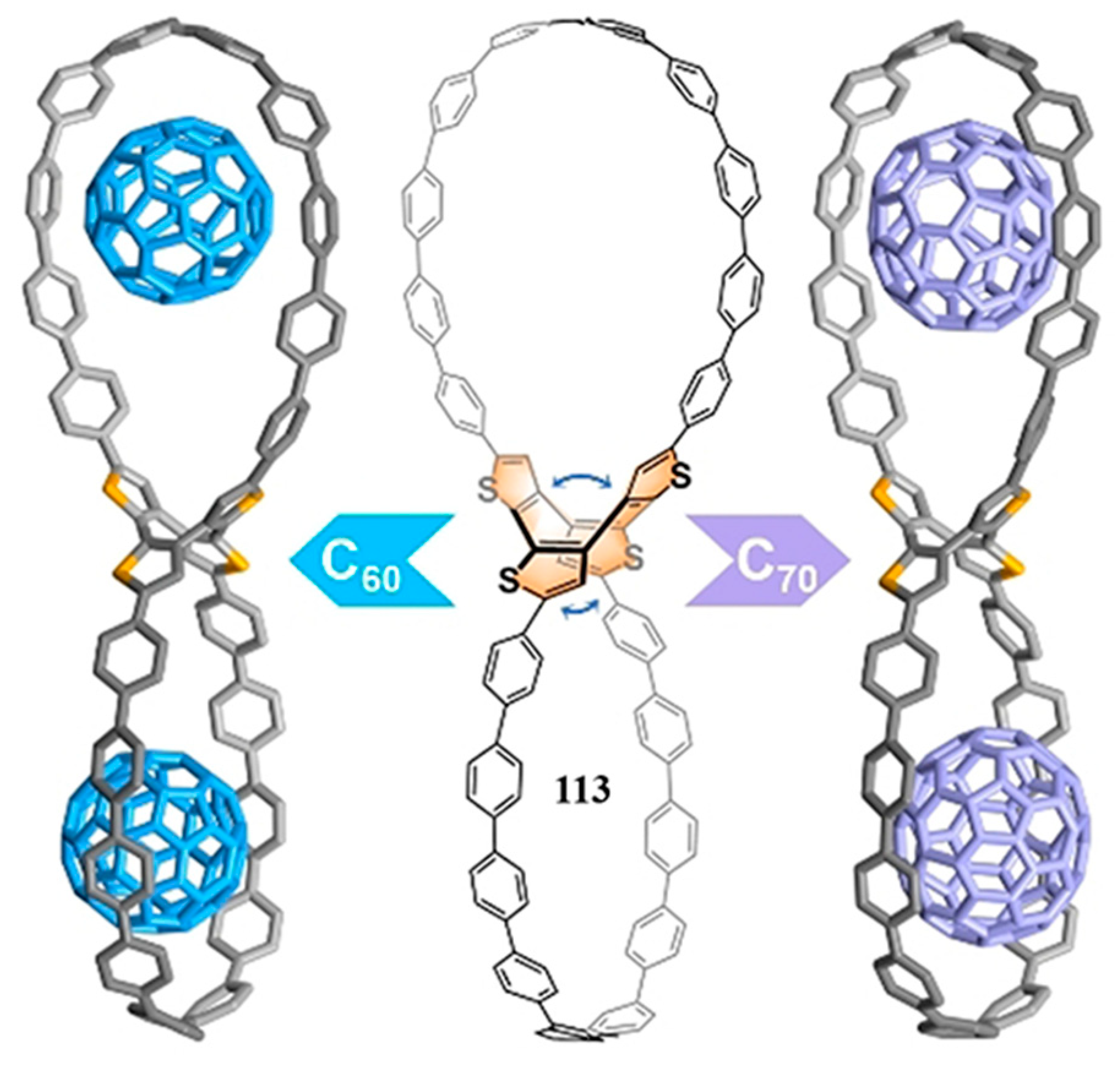
2.4. Bispillararene
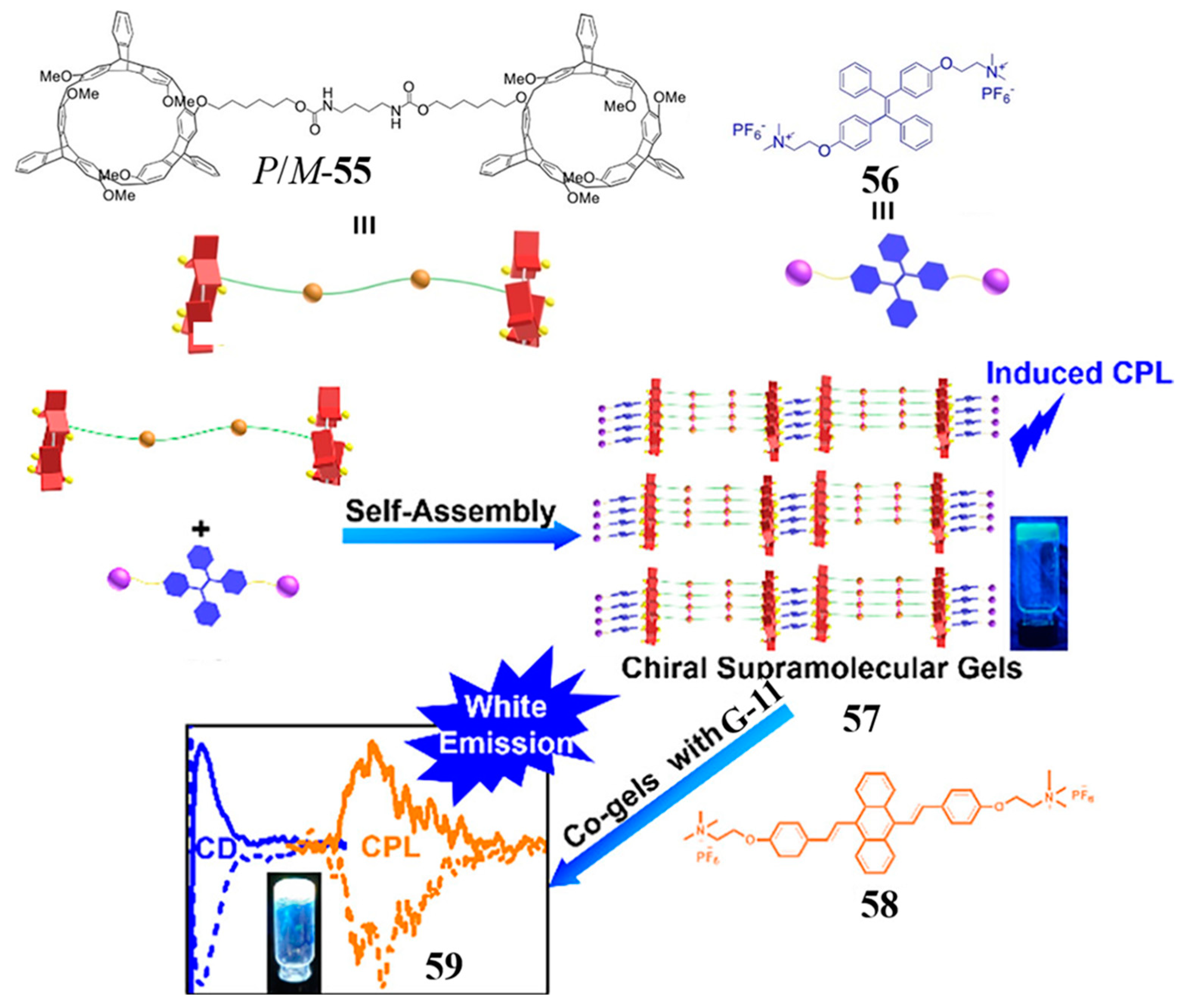
2.5. Bishelicarene
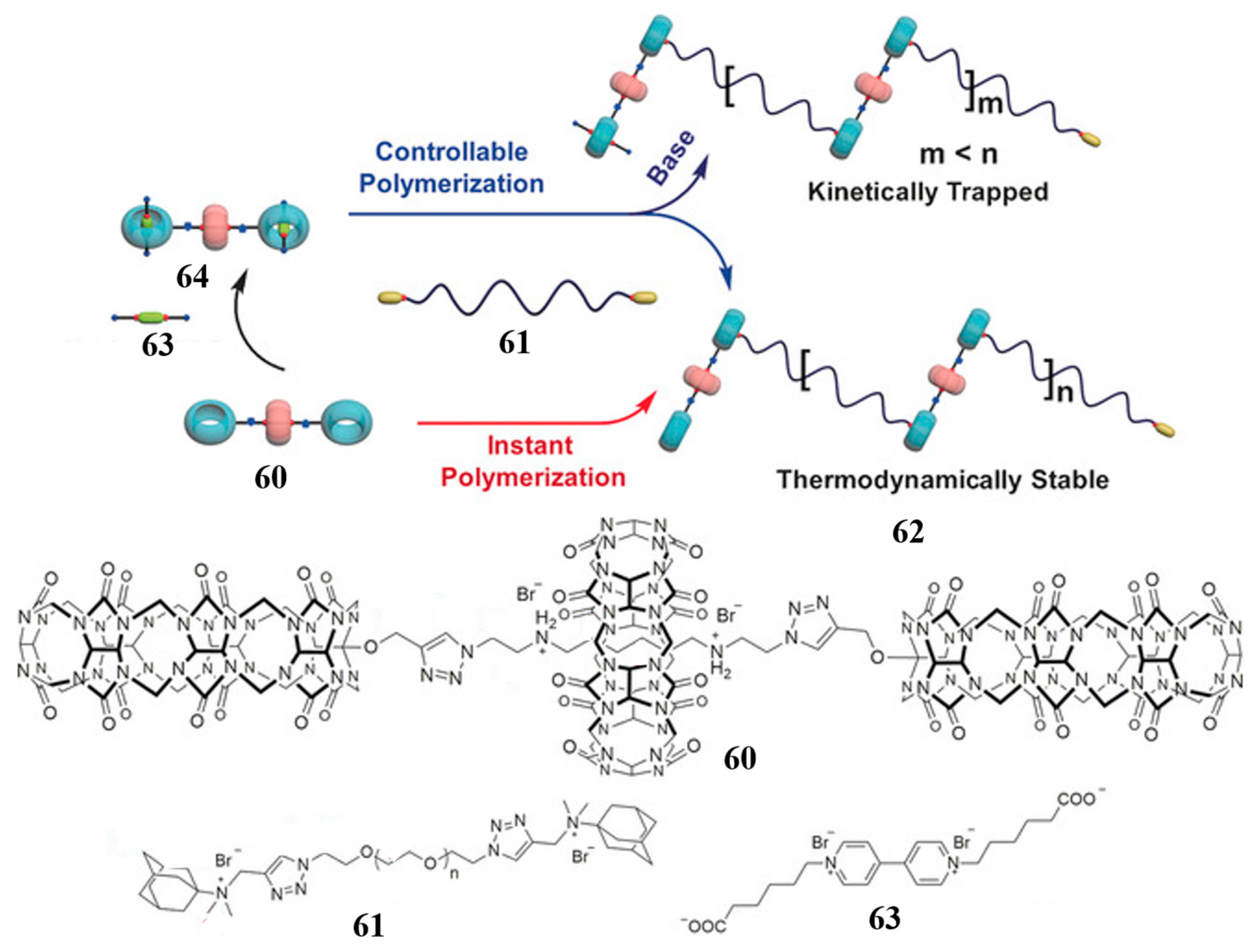
2.6. Biscucurbituril
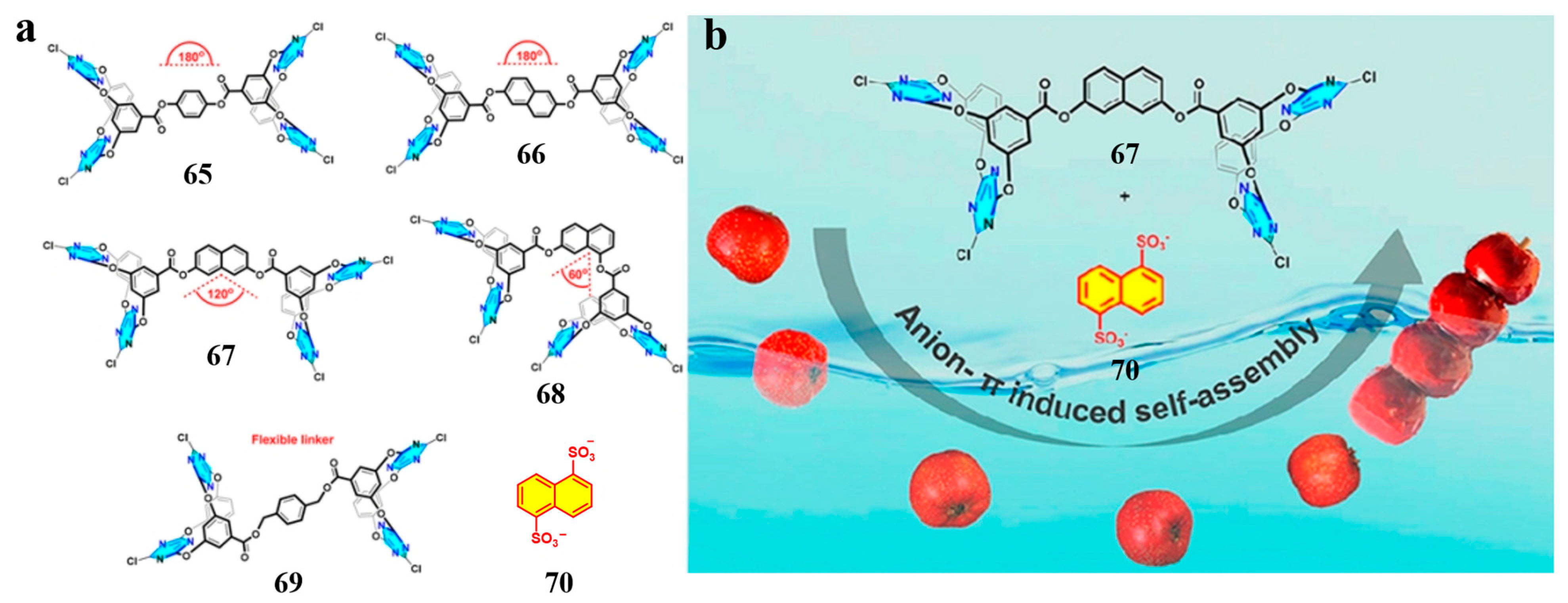
2.7. Bisheteracalixarenes
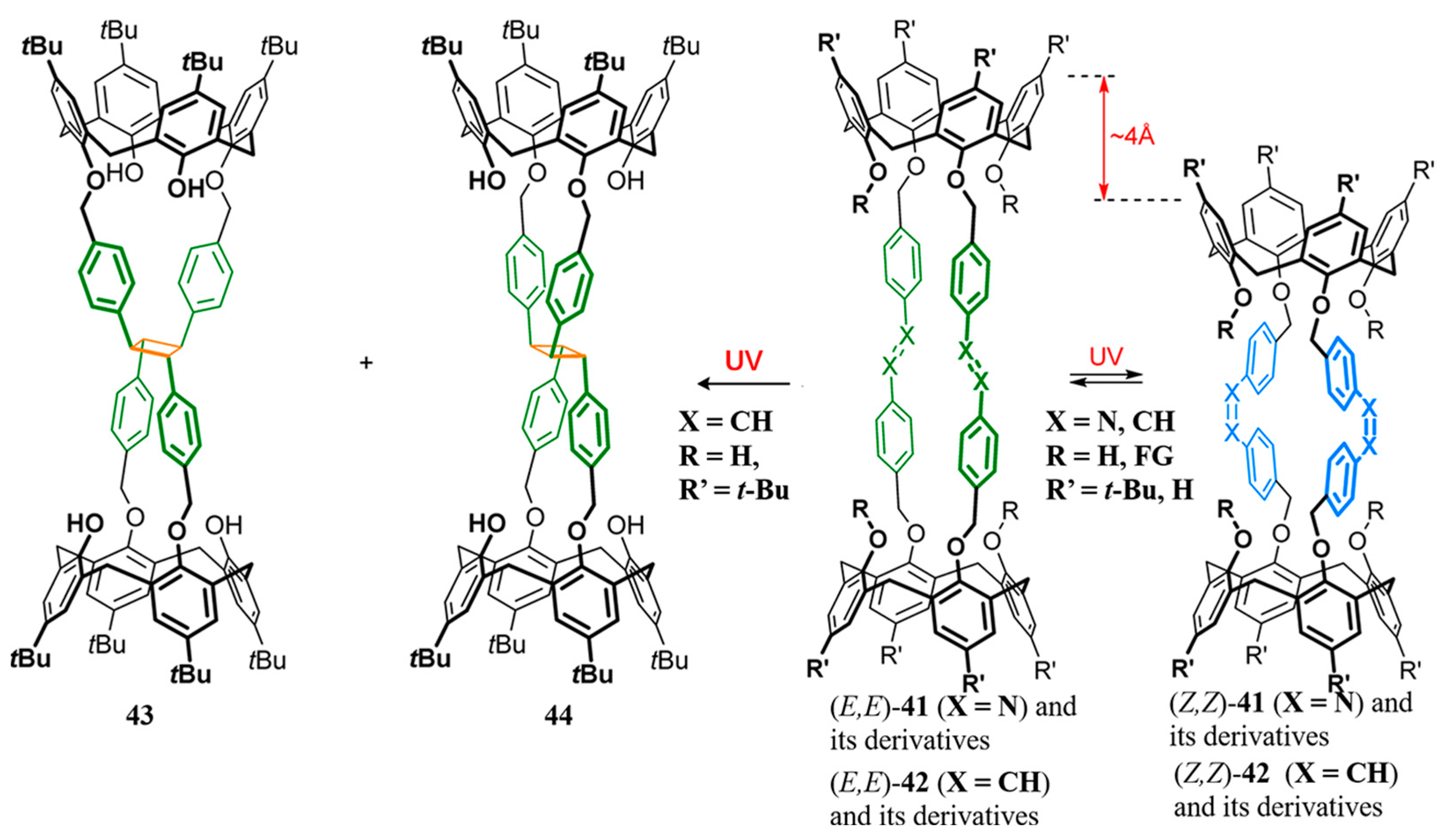
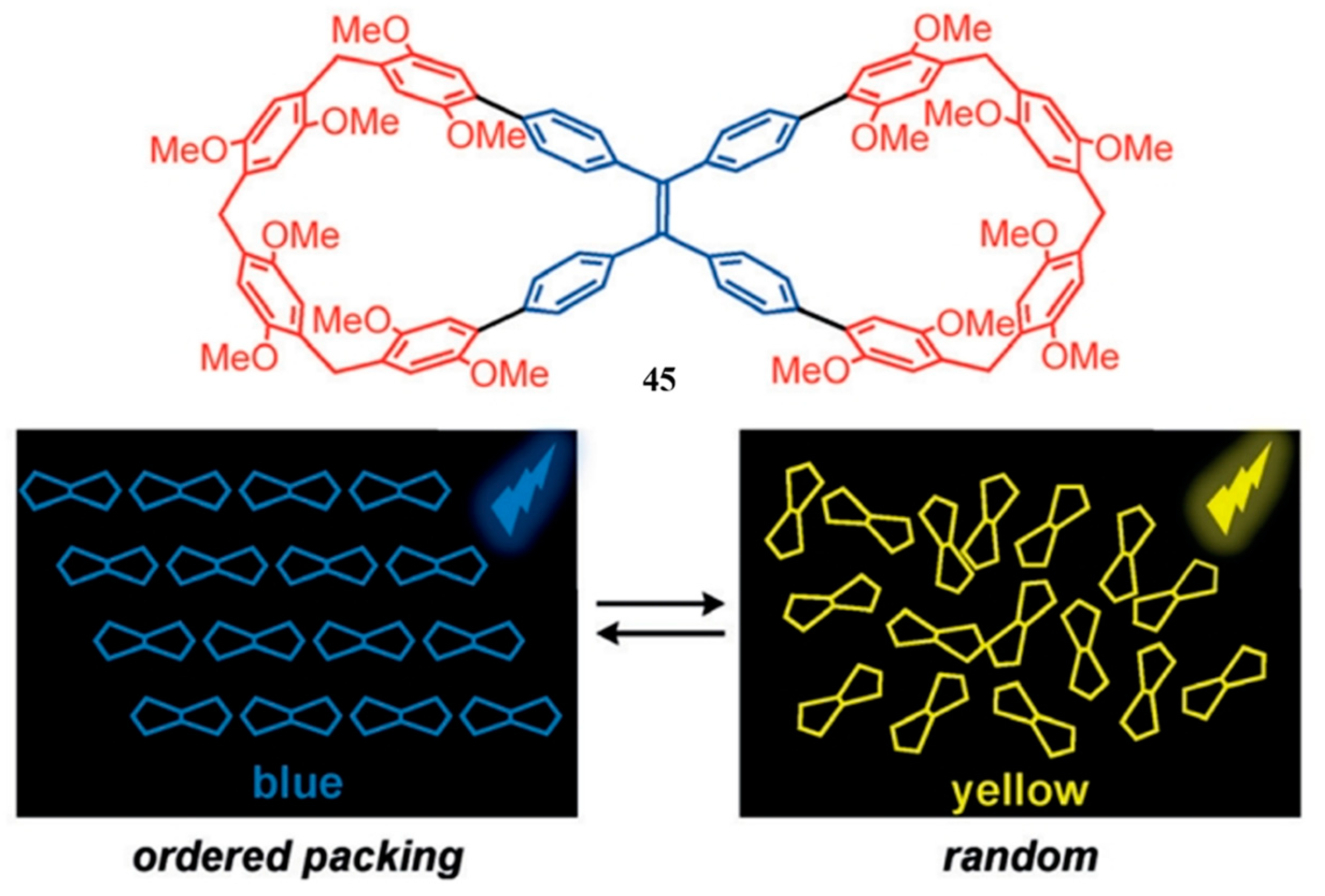
2.8. Other Oxabismacrocycle
3. Azabismacrocycle
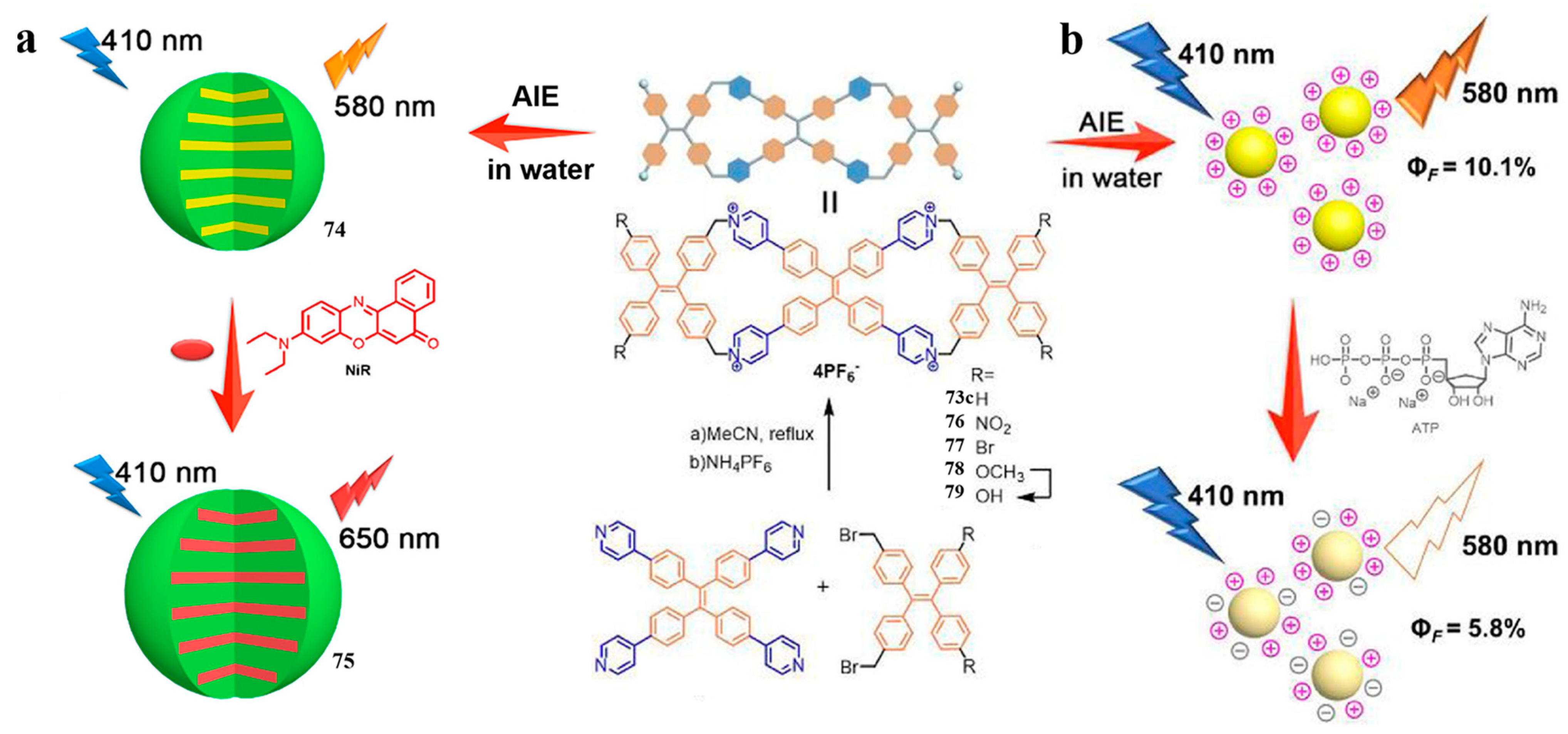
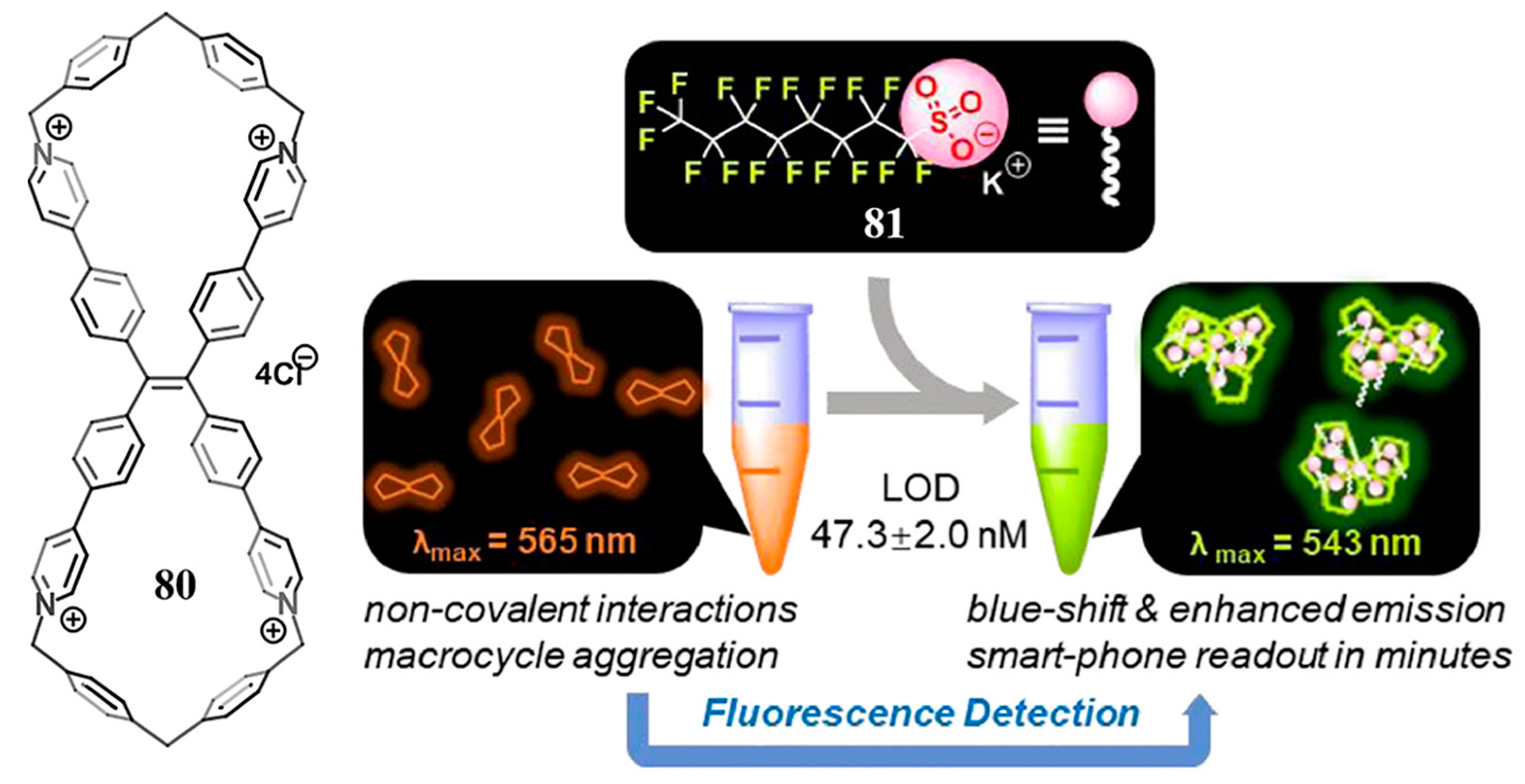
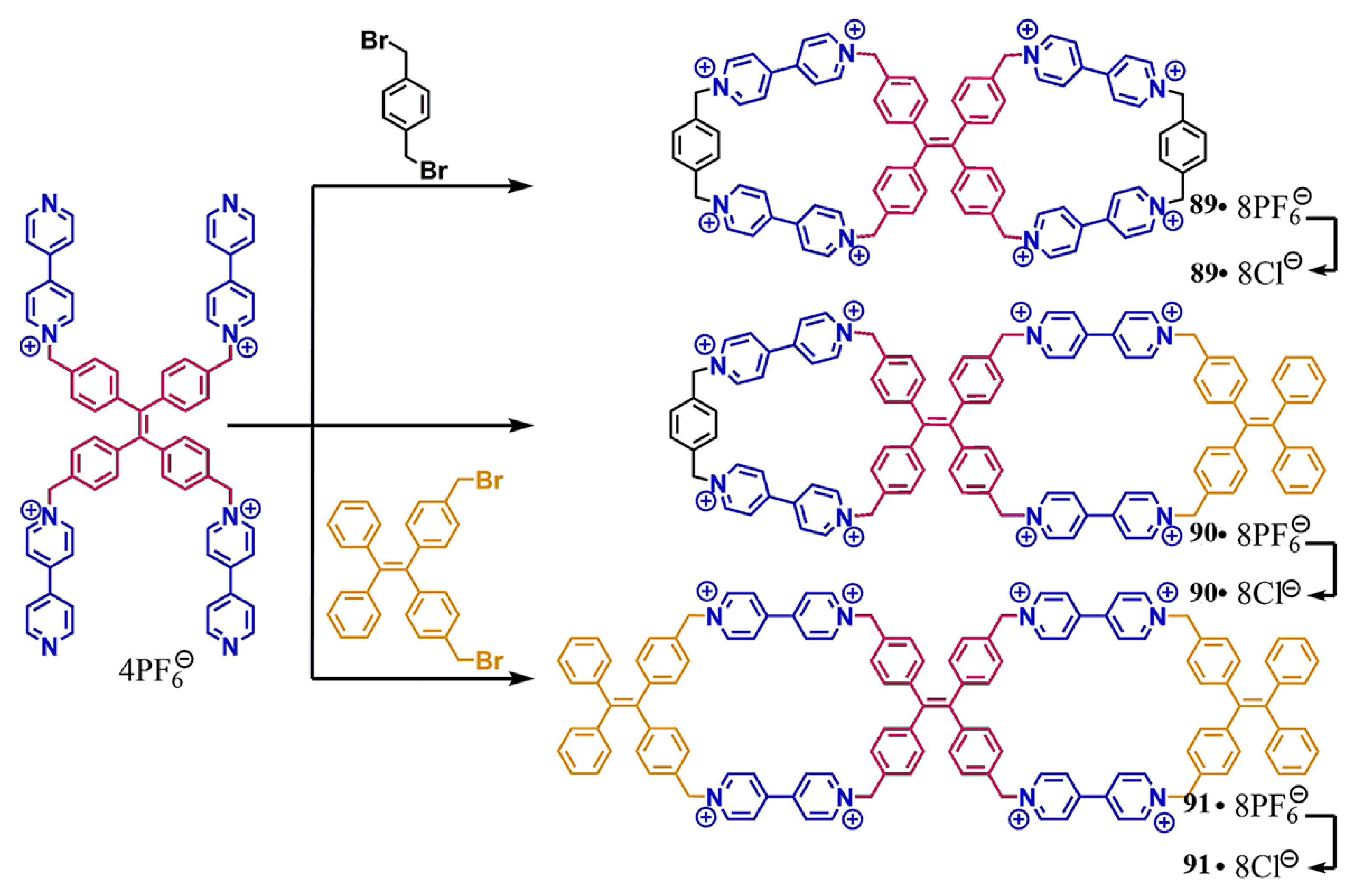
3.1. Pyridinium Bismacrocycle
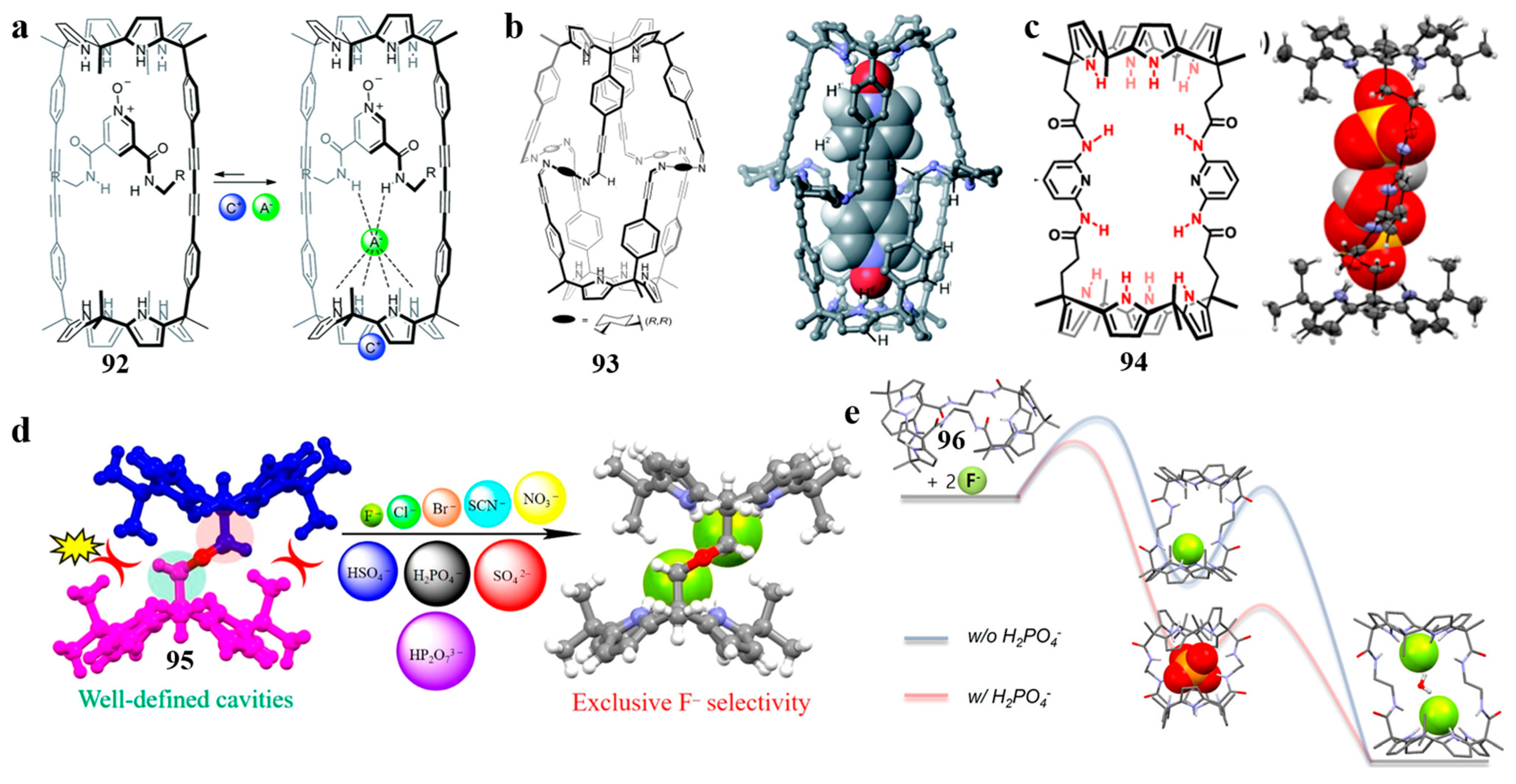
3.2. Biscalix[4]pyrroles
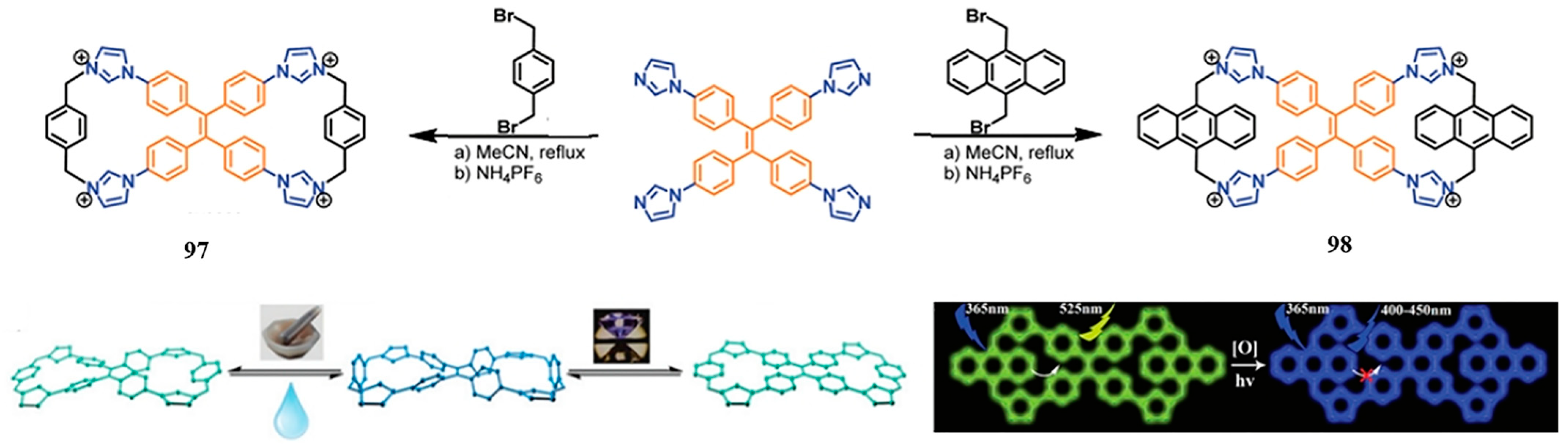
3.3. Imidazolium Bismacrocycle
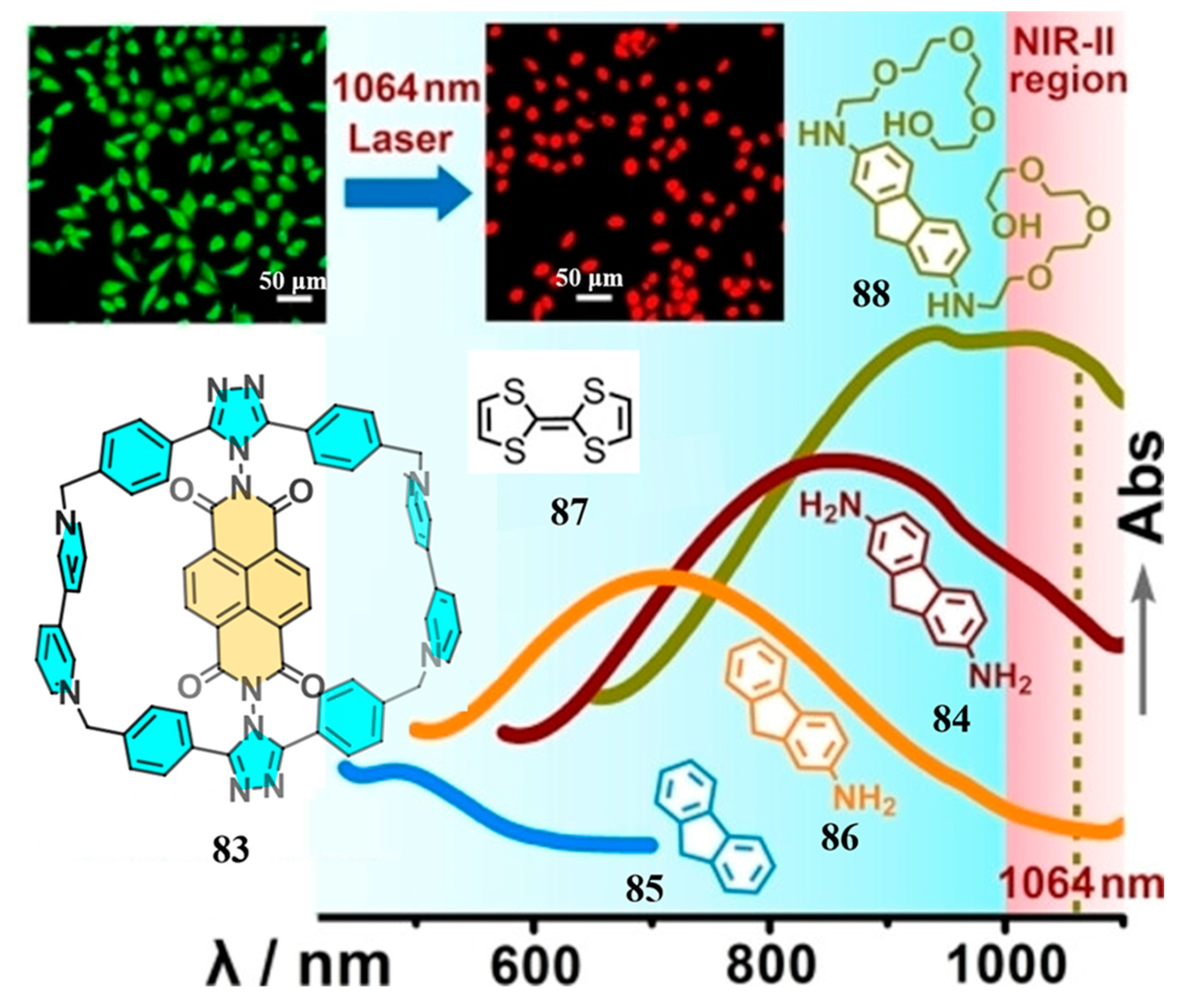
3.4. Azabiscycloparaphenylene
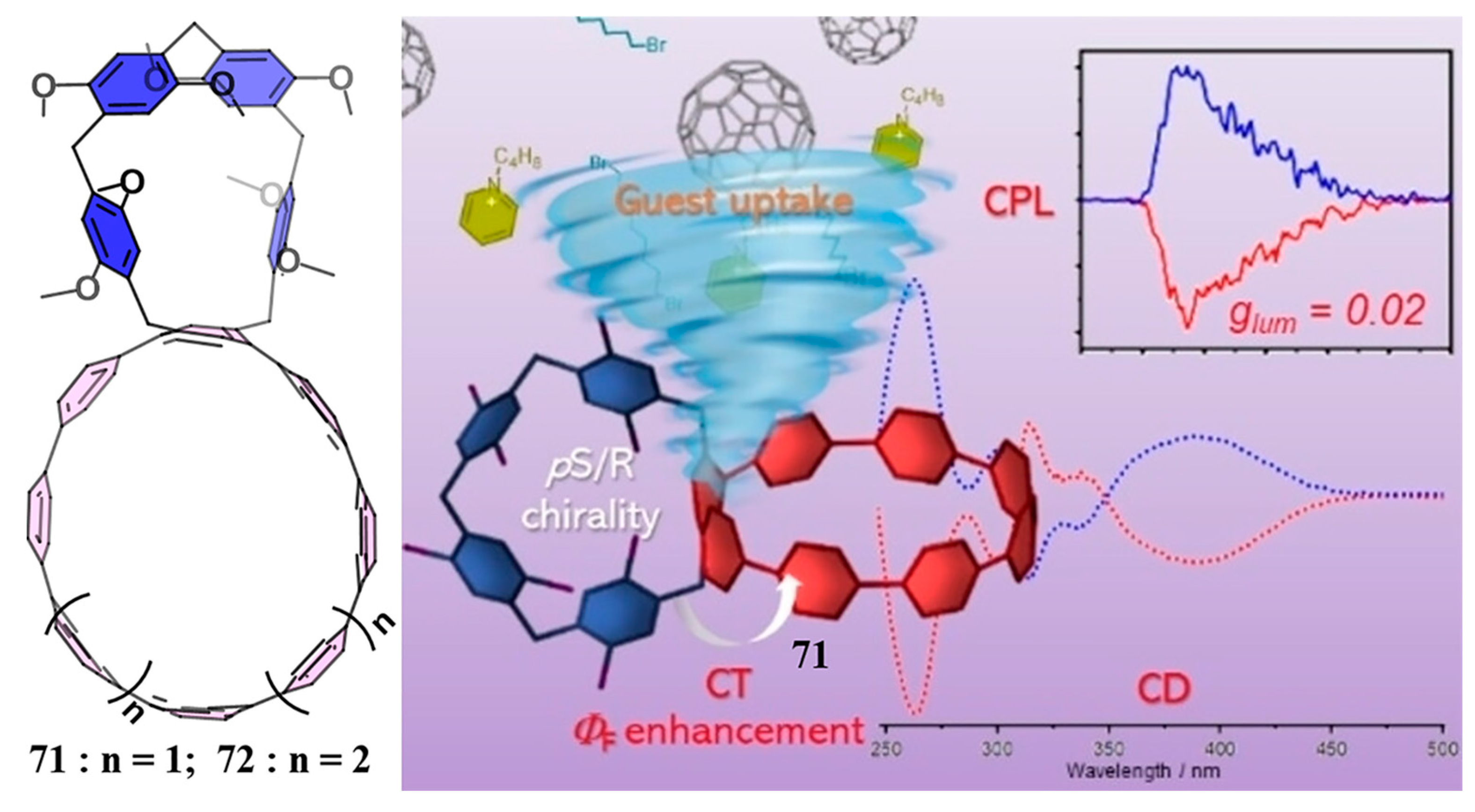
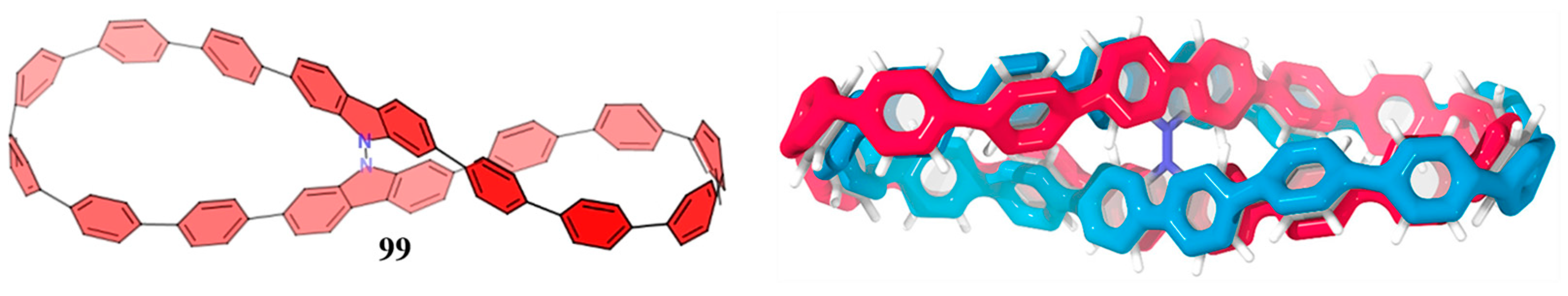
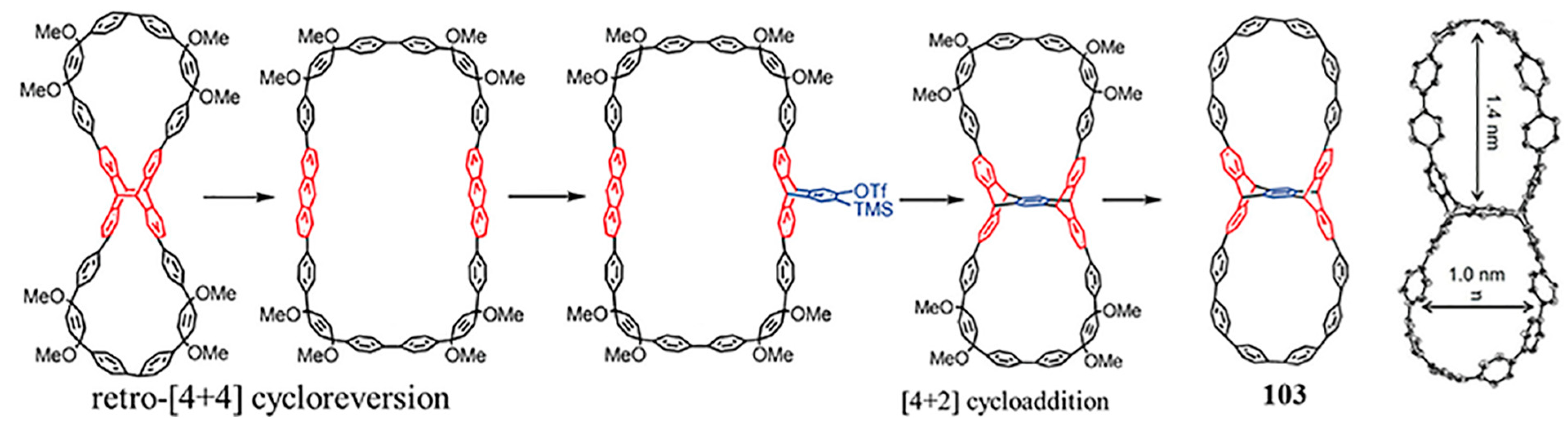
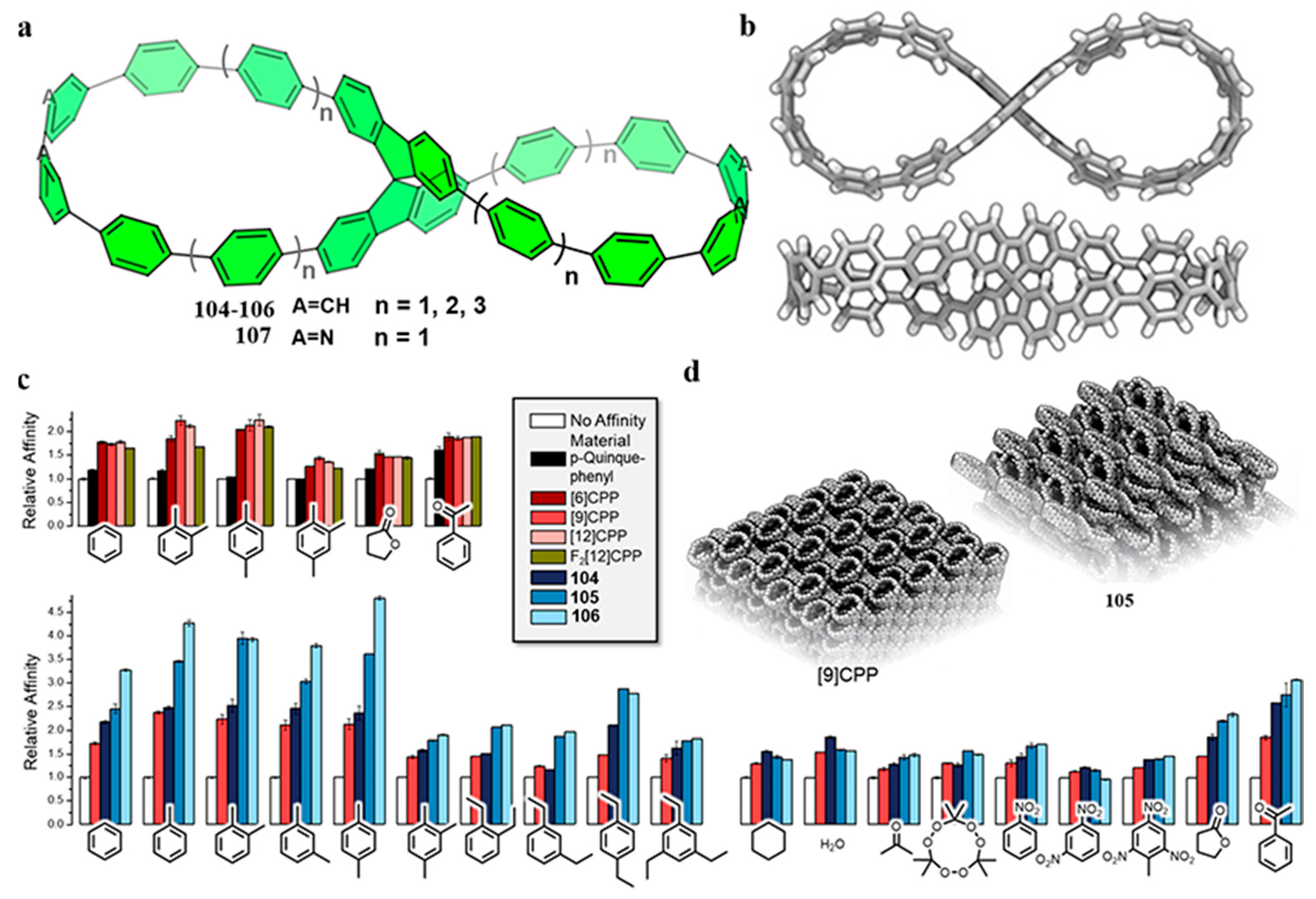
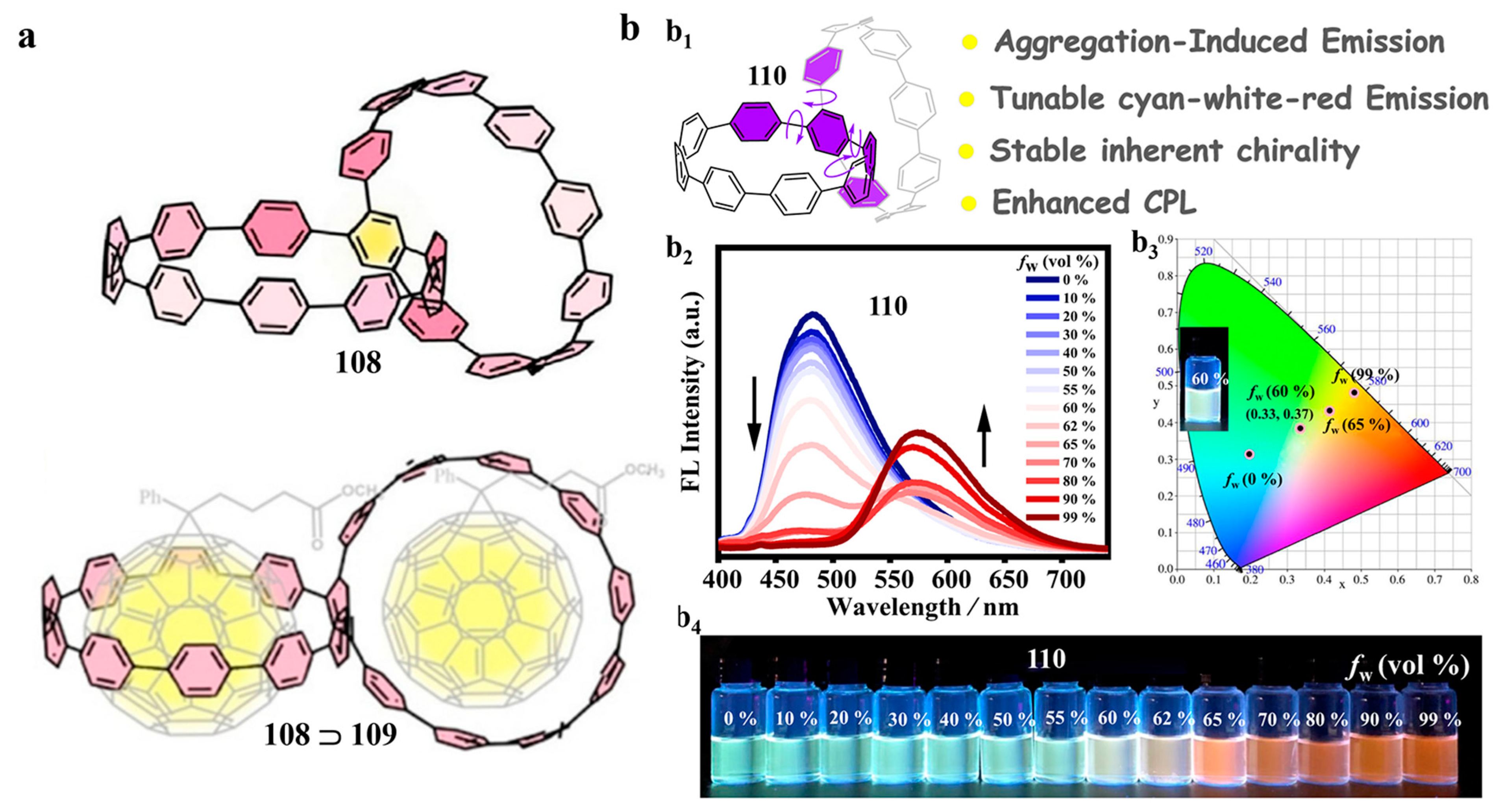
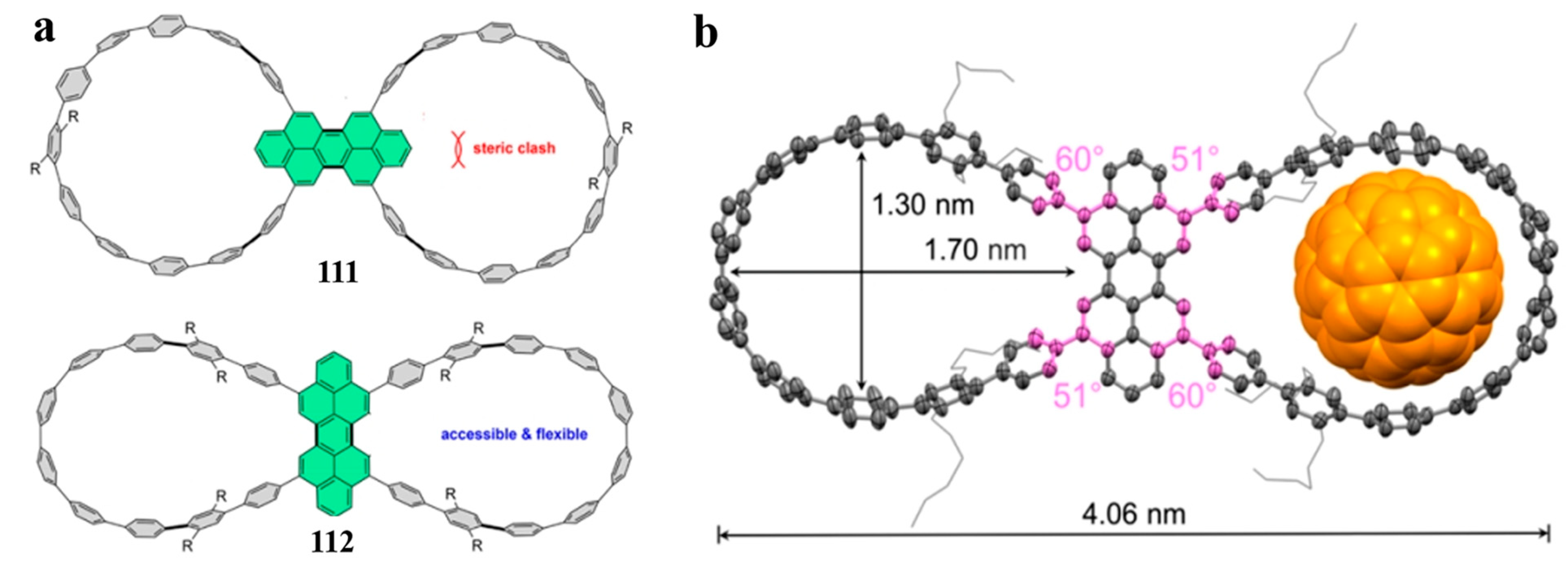
4. Biscycloparaphenylene (bis-CPP)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qi, Z.; Qin, Y.; Wang, J.; Zhao, M.; Yu, Z.; Xu, Q.; Nie, H.; Yan, Q.; Ge, Y. The aqueous supramolecular chemistry of crown ethers. Front. Chem. 2023, 11, 1119240. [Google Scholar] [CrossRef] [PubMed]
- Gokel, M.R.; McKeever, M.; Meisel, J.W.; Negin, S.; Patel, M.B.; Yin, S.; Gokel, G.W. Crown ethers having side arms: A diverse and versatile supramolecular chemistry. J. Coord. Chem. 2021, 74, 14–39. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
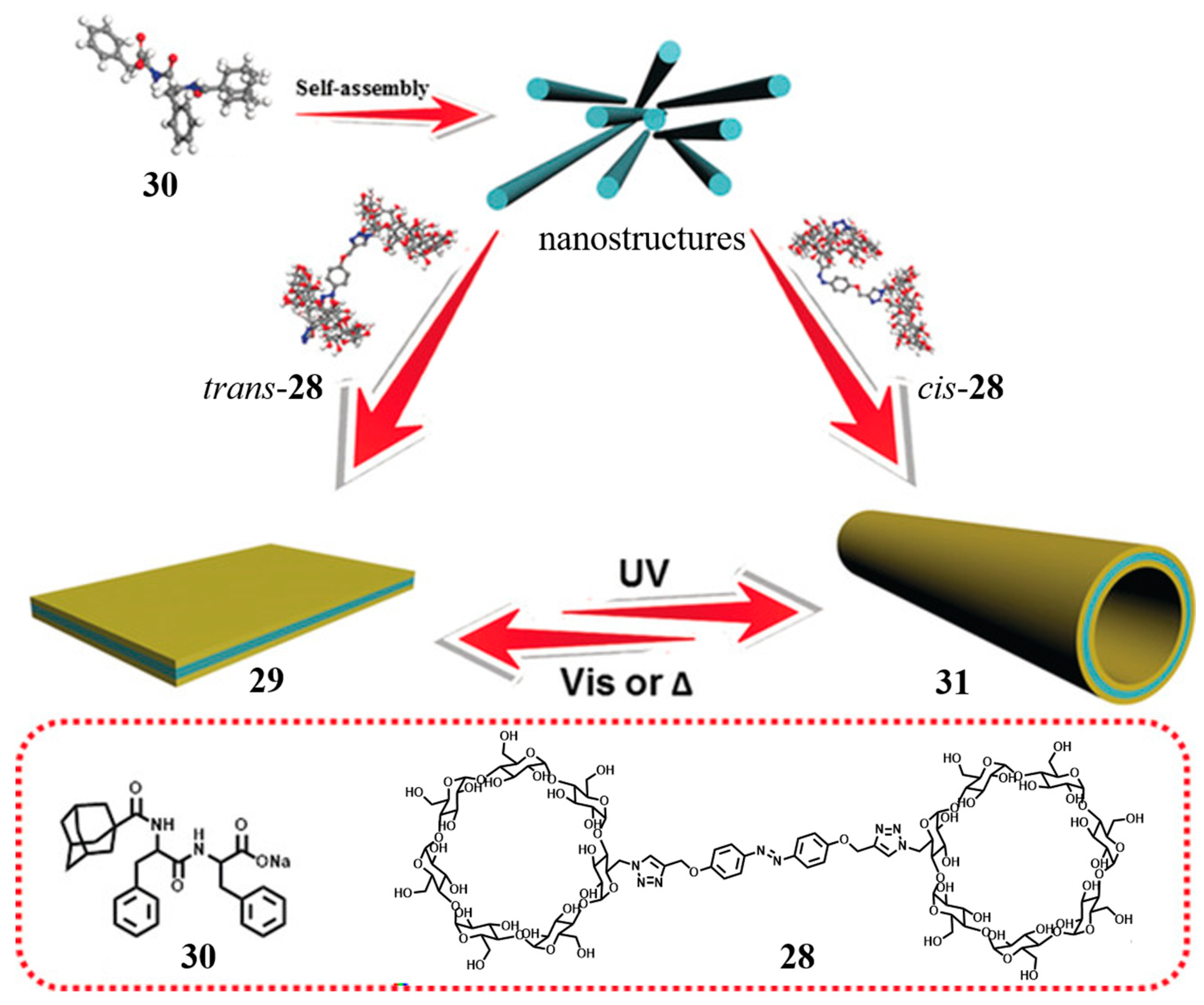
- Hu, W.; Ye, B.; Yu, G.; Huang, F.; Mao, Z.; Ding, Y.; Wang, W. Recent Development of Supramolecular Cancer Theranostics Based on Cyclodextrins: A Review. Molecules 2023, 28, 3441. [Google Scholar] [CrossRef] [PubMed]
- Healy, B.; Yu, T.; da Silva Alves, D.C.; Okeke, C.; Breslin, C.B. Cyclodextrins as Supramolecular Recognition Systems: Applications in the Fabrication of Electrochemical Sensors. Materials 2021, 14, 1668. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Hu, X.-Y.; Guo, D.-S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Yang, K.; Chen, X.; Wang, R. Cucurbituril-Based Supramolecular Polymers for Biomedical Applications. Angew. Chem. Int. Ed. 2022, 61, e2022067. [Google Scholar]
- Liu, Y.-H.; Zhang, Y.-M.; Yu, H.-J.; Liu, Y. Cucurbituril-Based Biomacromolecular Assemblies. Angew. Chem. Int. Ed. 2021, 60, 3870–3880. [Google Scholar] [CrossRef]
- Shen, Y.-J.; Zhu, K.-L.; Liang, J.-Q.; Sun, X.; Gong, H.-Y. Carbon-rich macrocycles and carbon nanoribbons as unique optical materials. J. Mater. Chem. C 2023, 11, 4267–4287. [Google Scholar] [CrossRef]
- Guo, G.-H.; Qiu, Y.; Wang, M.-X.; Stoddart, J.F. Aromatic hydrocarbon belts. Nat. Chem. 2021, 13, 402–419. [Google Scholar] [CrossRef]
- Leonhardt, E.J.; Jasti, R. Emerging applications of carbon nanohoops. Nat. Rev. Chem. 2019, 3, 672–686. [Google Scholar] [CrossRef]
- Crowley, P.B. Protein−Calixarene Complexation: From Recognition to Assembly. Acc. Chem. Res. 2022, 55, 2019–2032. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef]
- Yu, G.; Jie, K.; Huang, F. Supramolecular Amphiphiles Based on Host–Guest Molecular Recognition Motifs. Chem. Rev. 2015, 115, 7240–7303. [Google Scholar] [CrossRef]
- Wu, J.-R.; Wu, G.; Li, D.; Yang, Y.-W. Macrocycle-Based Crystalline Supramolecular Assemblies Built with Intermolecular Charge-Transfer Interactions. Angew. Chem. Int. Ed. 2023, 135, e202218142. [Google Scholar] [CrossRef]
- Nie, H.; Wei, Z.; Ni, X.-L.; Liu, Y. Assembly and Applications of Macrocyclic-Confinement-Derived Supramolecular Organic Luminescent Emissions from Cucurbiturils. Chem. Rev. 2022, 122, 9032–9077. [Google Scholar] [CrossRef]
- Chi, X.; Tian, J.; Luo, D.; Gong, H.-Y.; Huang, F.; Sessler, J.L. “Texas-Sized” Molecular Boxes: From Chemistry to Applications. Molecules 2021, 26, 2426. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artifificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed]
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.; Giuseppone, N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Faour, L.; Carmona-Vargas, C.C.; Giuseppone, N. From Molecular Machines to Stimuli-Responsive Materials. Adv. Mater. 2020, 32, 1906036. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ovalle, M.; Seale, J.S.W.; Lee, C.K.; Kim, D.J.; Astumian, R.D.; Stoddart, J.F. Molecular Pumps and Motors. J. Am. Chem. Soc. 2021, 143, 5569–5591. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhu, W.; Fang, S.; Jie, K.; Huang, F. Reimplementing Guest Shape Sorting of Nonporous Adaptive Crystals via Substituent-Size-Dependent Solid-Vapor Postsynthetic Modification. Angew. Chem. Int. Ed. 2022, 61, e202211780. [Google Scholar]
- Liang, Y.; Li, E.; Wang, K.; Guan, Z.-J.; He, H.-H.; Zhang, L.; Zhou, H.-C.; Huang, F.; Fang, Y. Organo-macrocycle-containing hierarchical metal–organic frameworks and cages: Design, structures, and applications. Chem. Soc. Rev. 2022, 51, 8378–8405. [Google Scholar] [CrossRef]
- Ji, X.; Ahmed, M.; Long, L.; Khashab, N.M.; Huang, F.; Sessler, J.L. Adhesive supramolecular polymeric materials constructed from macrocycle-based host–guest interactions. Chem. Soc. Rev. 2019, 48, 2682–2697. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jie, K.; Zhao, R.; Huang, F. Supramolecular-Macrocycle-Based Crystalline Organic Materials. Adv. Mater. 2019, 32, 1904824. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, R.; Zhao, Y. Macrocycle-based metal-organic frameworks. Coord. Chem Rev. 2015, 292, 74–90. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, X.; Li, X.; Zhang, Z.; Liu, Y. Recent Advances in Supramolecular-Macrocycle-Based Nanomaterials in Cancer Treatment. Molecules 2023, 28, 1241. [Google Scholar] [CrossRef]
- Shan, P.H.; Hu, J.H.; Liu, M.; Tao, Z.; Xiao, X.; Redshaw, C. Progress in host-guest macrocycle/pesticide research: Recognition, detection, release and application. Coord. Chem Rev. 2022, 467, 214580. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Guo, D.-S.; Liu, Y. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Zhao, T.; Sessler, J.L.; He, Q. Bis–Calix[4]pyrroles: Preparation, structure, complexation properties and beyond. Coord. Chem. Rev. 2020, 425, 213528. [Google Scholar] [CrossRef]
- Peng, H.-Q.; Zhu, W.; Guo, W.-J.; Li, Q.; Ma, S.; Bucher, C.; Liu, B.; Ji, X.; Huang, F.; Sessler, J.L. Supramolecular polymers: Recent advances based on the types of underlying interactions. Prog. Polym. Sci. 2023, 137, 101635. [Google Scholar] [CrossRef]
- Bourgoin, M.; Wong, K.H.; Hui, J.Y.; Smid, J. Interactions of macrobicyclic polyethers with ions and ion pairs of picrate salts. J. Am. Chem. Soc. 1975, 97, 3462–3467. [Google Scholar] [CrossRef]
- An, H.; Bradshaw, J.S.; Izatt, R.M.; Yan, Z. Bis- and Oligo(benzocrown ether)s. Chem. Rev. 1994, 94, 939–991. [Google Scholar] [CrossRef]
- Li, S.; Lu, H.-Y.; Shen, Y.; Chen, C.-F. A Stimulus-Response and Self-Healing Supramolecular Polymer Gel Based on Host–Guest Interactions. Macromol. Chem. Phys. 2013, 214, 1596–1601. [Google Scholar] [CrossRef]
- Yan, X.; Xu, D.; Chen, J.; Zhang, M.; Hu, B.; Yu, Y.; Huang, F. A self-healing supramolecular polymer gel with stimuli-responsiveness constructed by crown ether based molecular recognition. Polym. Chem. 2013, 4, 3312–3322. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.-Y.; Zhang, Z.-Y.; Liu, Y. Tunable Luminescent Lanthanide Supramolecular Assembly Based on Photoreaction of Anthracene. J. Am. Chem. Soc. 2017, 139, 7168–7171. [Google Scholar] [CrossRef]
- Wang, W.; Xing, H. A novel supramolecular polymer network based on a catenane-type crosslinker. Polym. Chem. 2018, 9, 2087–2091. [Google Scholar] [CrossRef]
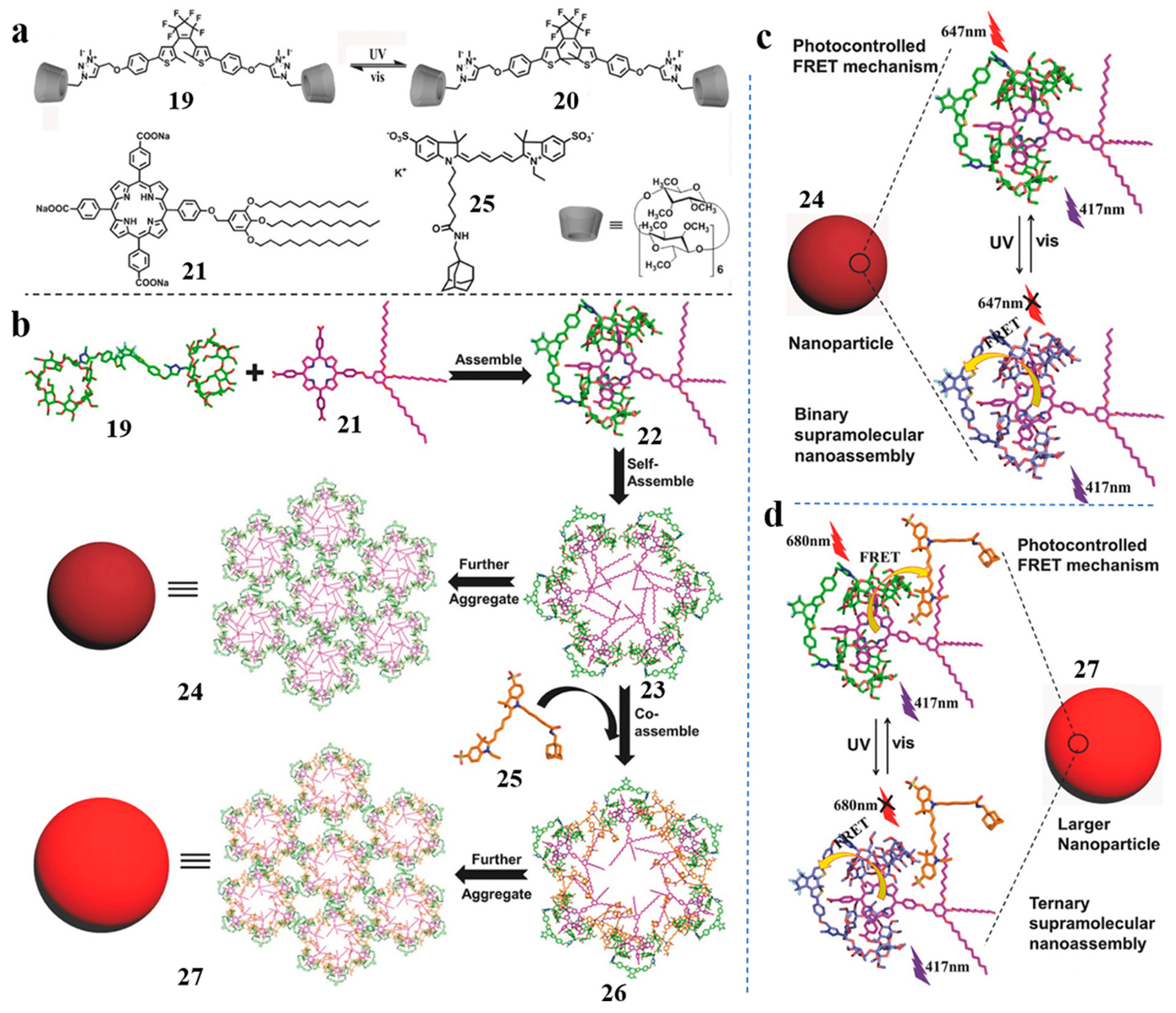
- Wang, H.-J.; Zhang, H.-Y.; Wu, H.; Dai, X.-Y.; Li, P.-Y.; Liu, Y. Photocontrolled morphological conversion and chiral transfer of a snowflake-like supramolecular assembly based on azobenzene-bridged bis(dibenzo-24-crown-8) and a cholesterol derivative. Chem. Commun. 2019, 55, 4499–4502. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-G.; Zhang, H.-Y.; Zhang, H.-Y.; Liu, Y. Photo-controlled chirality transfer and FRET effects based on pseudo[3]rotaxane. Chem. Commun. 2019, 55, 13462–13465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, T.; Duan, A.; Dong, S.; Zhao, W.; Stang, P.J. Formation of a Supramolecular Polymeric Adhesive via Water–Participant Hydrogen Bond Formation. J. Am. Chem. Soc. 2019, 141, 8058–8063. [Google Scholar] [CrossRef] [PubMed]
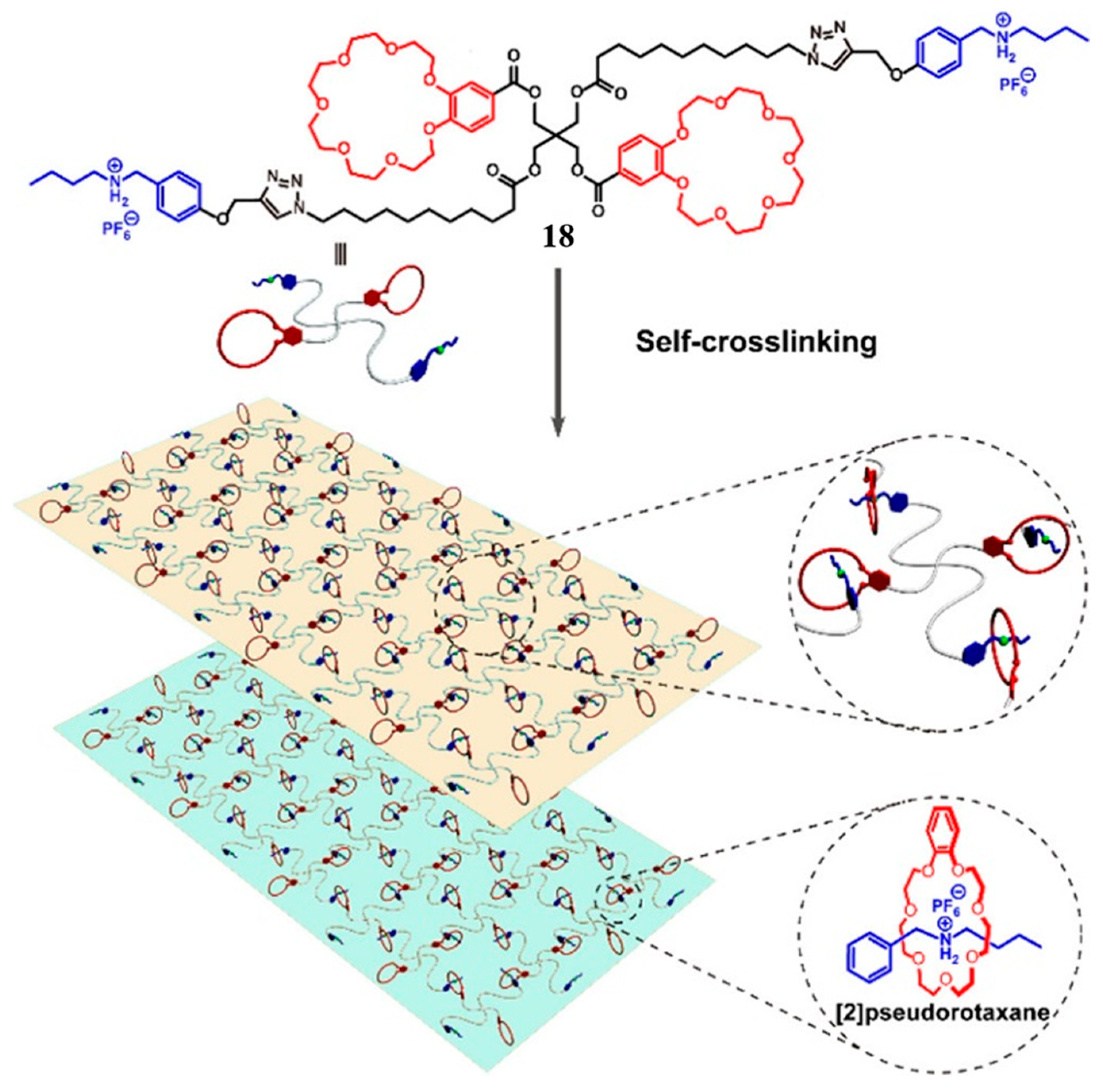
- Wang, L.; Cheng, L.; Li, G.; Liu, K.; Zhang, Z.; Li, P.; Dong, S.; Yu, W.; Huang, F.; Yan, X. A Self-Cross-Linking Supramolecular Polymer Network Enabled by Crown-Ether-Based Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 2051–2058. [Google Scholar] [CrossRef]
- Kuad, P.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. External Stimulus-Responsive Supramolecular Structures Formed by a Stilbene Cyclodextrin Dimer. J. Am. Chem. Soc. 2007, 129, 12630–12631. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Su, Y.; Yu, S.; Zhou, Y.; Lu, Y.; Zhu, X. A redox-responsive cationic supramolecular polymer constructed from small molecules as a promising gene vector. Chem. Commun. 2013, 49, 9845–9847. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Q.; Zhang, Y.-M.; Chen, Y.; Liu, Y. A Supramolecular Tubular Nanoreactor. Chem. Eur. J. 2014, 20, 8566–8570. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.R.; Guo, D.-S.; Jiang, B.-P. Supramolecular Assembly of Perylene Bisimide with β-Cyclodextrin Grafts as a Solid-State Fluorescence Sensor for Vapor Detection. Adv. Funct. Mater. 2009, 19, 2230–2235. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.-M.; Xu, X.; Zhang, L.; Yu, Q.; Zhao, Q.; Zhao, C.; Liu, Y. Controllable Photoluminescence Behaviors of Amphiphilic Porphyrin Supramolecular Assembly Mediated by Cyclodextrins. Adv. Opt. Mater. 2017, 5, 1700770. [Google Scholar] [CrossRef]
- Sun, H.-L.; Chen, Y.; Han, X.; Liu, Y. Tunable Supramolecular Assembly and Photoswitchable Conversion of Cyclodextrin/Diphenylalanine-Based 1D and 2D Nanostructures. Angew. Chem. Int. Ed. 2017, 56, 7062–7065. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, Y.; Yu, J.; Li, J.-J.; Liu, Y. Enhanced DNA Binding and Photocleavage Abilities of β-Cyclodextrin Appended Ru(II) Complex through Supramolecular Strategy. Bioconjugate Chem. 2018, 29, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Rebek, J. Host–guest chemistry of calixarene capsules. Chem. Commun. 2000, 637–643. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Liu, Y.; Zhou, H.; Chen, W.; Sun, G.; Su, J.; Ma, X.; Tian, H. Tunable Photoluminescence Including White-Light Emission Based on Noncovalent Interaction-Locked N,N′-Disubstituted Dihydrodibenzo[a,c]phenazines. Adv. Opt. Mater. 2018, 6, 1800074. [Google Scholar] [CrossRef]
- Hirao, T.; Iwabe, Y.; Hisano, N.; Haino, T. Helicity of a polyacetylene directed by molecular recognition of biscalixarene and fullerene. Chem. Commun. 2020, 56, 6672–6675. [Google Scholar] [CrossRef] [PubMed]
- Lentin, I.; Gorbunov, A.; Bezzubov, S.; Nosova, V.; Cheshkov, D.; Kovalev, V.; Vatsouro, I. Shrinkable/stretchable bis(calix[4]arenes) comprising photoreactive azobenzene or stilbene linkers. Org. Chem. Front. 2023, 10, 1470–1484. [Google Scholar] [CrossRef]
- Lei, S.-N.; Xiao, H.; Zeng, Y.; Tung, C.-H.; Wu, L.-Z.; Cong, H. BowtieArene: A Dual Macrocycle Exhibiting Stimuli-Responsive Fluorescence. Angew. Chem. Int. Ed. 2020, 59, 10059–10065. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, H.; Xu, X.; Zhou, Q.; Dai, X.; Fan, L.; Mao, P.; Liu, Y. Supramolecular photoswitch with white-light emission based on bridged bis(pillar[5]arene)s. Mater. Today Chem. 2021, 22, 100628. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R.; Song, Z.; Zhang, K.; Tian, X.; Pangannaya, S.; Zuo, M.; Hu, X.-Y. Dimeric Pillar[5]arene as a Novel Fluorescent Host for Controllable Fabrication of Supramolecular Assemblies and Their Photocatalytic Applications. Adv. Sci. 2023, 10, 2206897. [Google Scholar] [CrossRef]
- Guo, Y.; Han, Y.; Du, X.-S.; Chen, C.-F. Chiral Bishelic[6]arene-Based Supramolecular Gels with Circularly Polarized Luminescence Property. ACS Appl. Polym. Mater. 2022, 4, 3473–3481. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.; Wu, H.; Xu, J.-F.; Zhang, X. Supramolecular Polymerization Controlled through Kinetic Trapping. Angew. Chem. Int. Ed. 2017, 56, 16575–16578. [Google Scholar] [CrossRef]
- Tuo, D.-H.; Liu, W.; Wang, X.-Y.; Wang, X.-D.; Ao, Y.-F.; Wang, Q.-Q.; Li, Z.-Y.; Wang, D.-X. Toward Anion−π Interactions Directed Self-Assembly with Predesigned Dual Macrocyclic Receptors and Dianions. J. Am. Chem. Soc. 2019, 141, 1118–1125. [Google Scholar] [CrossRef]
- Xu, Y.; Steudel, F.; Leung, M.-Y.; Xia, B.; von Delius, M.; Yam, V.W.-W. [n]Cycloparaphenylene-Pillar[5]arene Bismacrocycles: Their Circularly Polarized Luminescence and Multiple Guest Recognition Properties. Angew. Chem. Int. Ed. 2023, 62, e202302978. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Chen, H.; Stoddart, J.F. The story of the little blue box: A tribute to Siegfried Hünig. Angew. Chem. Int. Ed. 2023, 62, e202211387. [Google Scholar]
- Li, Y.; Dong, Y.; Cheng, L.; Qin, C.; Nian, H.; Zhang, H.; Yu, Y.; Cao, L. Aggregation-Induced Emission and Light-Harvesting Function of Tetraphenylethene-Based Tetracationic Dicyclophane. J. Am. Chem. Soc. 2019, 141, 8412–8415. [Google Scholar] [CrossRef]
- Qin, C.; Li, Y.; Li, Q.; Yan, C.; Cao, L. Aggregation-induced emission and self-assembly of functional tetraphenylethene-based tetracationic dicyclophanes for selective detection of ATP in water. Chin. Chem. Lett. 2021, 32, 3531–3534. [Google Scholar] [CrossRef]
- Lei, S.-N.; Cong, H. Fluorescence detection of perfluorooctane sulfonate in water employing a tetraphenylethylene-derived dual macrocycle BowtieCyclophane. Chin. Chem. Lett. 2022, 33, 1493–1496. [Google Scholar] [CrossRef]
- Yang, F.; Li, R.; Wei, W.; Ding, X.; Xu, Z.; Wang, P.; Wang, G.; Xu, Y.; Fu, H.; Zhao, Y. Water-Soluble Doubly-Strapped Isolated Perylene Diimide Chromophore. Angew. Chem. Int. Ed. 2022, 61, e202202491. [Google Scholar]
- Li, R.; Yang, F.; Zhang, L.; Li, M.; Wang, G.; Wang, W.; Xu, Y.; Wei, W. Manipulating Host-Guest Charge Transfer of a Water-Soluble Double-Cavity Cyclophane for NIR-II Photothermal Therapy. Angew. Chem. Int. Ed. 2023, 62, e202301267. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Z.; Cheng, L.; Nian, H.; Yao, N.; Zhao, Y.; Liu, K.; Cao, L. Tetraphenylethene-linked octacationic dicyclophanes with enhanced recognition of NADH over NAD+ in water. Dyes Pigments 2023, 216, 111364. [Google Scholar] [CrossRef]
- Kim, D.S.; Sessler, J.F. Calix[4]pyrroles: Versatile molecular containers with ion transport, recognition, and molecular switching functions. Chem. Soc. Rev. 2015, 44, 532–546. [Google Scholar] [CrossRef]
- Romero, J.R.; Aragay, G.; Ballester, P. Ion-pair recognition by a neutral [2]rotaxane based on a bis-calix[4]pyrrole cyclic component. Chem. Sci. 2017, 8, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Galán, A.; Escudero-Adán, E.C.; Ballester, P. Template-directed self-assembly of dynamic covalent capsules with polar interiors. Chem. Sci. 2017, 8, 7746–7750. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Kelliher, M.; Bähring, S.; Lynch, V.M.; Sessler, J.L. A Bis-calix[4]pyrrole Enzyme Mimic That Constrains Two Oxoanions in Close Proximity. J. Am. Chem. Soc. 2017, 139, 7140–7143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Chen, F.; Zhao, T.; Li, A.; Xu, G.; Sessler, J.L.; He, Q. Selective Inclusion of Fluoride within the Cavity of a Two-Wall Bis-calix[4]pyrrole. Org. Lett. 2020, 22, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Hay, B.P.; Lynch, V.M.; Li, H.; Sessler, J.L.; Kim, S.K. Calix[4]pyrrole-Based Molecular Capsule: Dihydrogen Phosphate Promoted 1:2 Fluoride Anion Complexation. J. Am. Chem. Soc. 2022, 144, 16996–17009. [Google Scholar] [CrossRef]
- Nian, H.; Li, A.; Li, Y.; Cheng, L.; Wang, L.; Xu, W.; Cao, L. Tetraphenylethene-based tetracationic dicyclophanes: Synthesis, mechanochromic luminescence, and photochemical reactions. Chem. Commun. 2020, 56, 3195–3198. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kondratowicz, M.; Lis, T.; Chmielewski, P.J.; Cybińska, J.; Zafra, J.L.; Casado, J.; Vives, T.; Crassous, J.; Favereau, L.; et al. Lemniscular [16]Cycloparaphenylene: A Radially Conjugated Figure-Eight Aromatic Molecule. J. Am. Chem. Soc. 2019, 141, 7421–7427. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z.; Deng, H.; Zhou, Z.; Dang, Y.; Sun, Z. Dimeric Cycloparaphenylenes with a Rigid Aromatic Linker. Angew. Chem. Int. Ed. 2021, 60, 7649–7653. [Google Scholar] [CrossRef]
- Xu, W.; Yang, X.-D.; Fan, X.-B.; Wang, X.; Tung, C.-H.; Wu, L.-Z.; Cong, H. Synthesis and Characterization of a Pentiptycene-Derived Dual Oligoparaphenylene Nanohoop. Angew. Chem. Int. Ed. 2019, 58, 3943–3947. [Google Scholar] [CrossRef]
- Huang, Z.-A.; Chen, C.; Yang, X.-D.; Fan, X.-B.; Zhou, W.; Tung, C.-H.; Wu, L.-Z.; Cong, H. Synthesis of Oligoparaphenylene-Derived Nanohoops Employing an Anthracene Photodimerization–Cycloreversion Strategy. J. Am. Chem. Soc. 2016, 138, 11144–11147. [Google Scholar] [CrossRef]
- Schaub, T.A.; Prantl, E.A.; Kohn, J.; Bursch, M.; Marshall, C.R.; Leonhardt, E.J.; Lovell, T.C.; Zakharov, L.N.; Brozek, C.K.; Waldvogel, S.R.; et al. Exploration of the Solid-State Sorption Properties of Shape-Persistent Macrocyclic Nanocarbons as Bulk Materials and Small Aggregates. J. Am. Chem. Soc. 2020, 142, 8763–8775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, H.; Zhuang, G.; Wang, S.; Wang, J.; Yang, S.; Shao, X.; Du, P. A Highly Strained All-Phenylene Conjoined Bismacrocycle. Angew. Chem. Int. Ed. 2021, 60, 17368–17372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, H.; Zhuang, G.; Yang, S.; Du, P. An unexpected dual-emissive luminogen with tunable aggregation-induced emission and enhanced chiroptical property. Nat. Commun. 2022, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Blacque, O.; Sato, S.; Juríček, M. Cycloparaphenylene–Phenalenyl Radical and Its Dimeric Double Nanohoop. Angew. Chem. Int. Ed. 2021, 60, 13529–13535. [Google Scholar] [CrossRef]
- Yang, Y.; Huangfu, S.; Sato, S.; Juríček, M. Cycloparaphenylene Double Nanohoop: Structure, Lamellar Packing, and Encapsulation of C60 in the Solid State. Org. Lett. 2021, 23, 7943–7948. [Google Scholar] [CrossRef]
- Cong, H. A Conjugated Figure-of-Eight Oligoparaphenylene Nanohoop with Adaptive Cavities Derived from Cyclooctatetrathiophene Core. Angew. Chem. Int. Ed. 2022, 61, e202113334. [Google Scholar]
- Xu, H.; Guan, D.; Ma, L. The bio-inspired heterogeneous single-cluster catalyst Ni100–Fe4S4 for enhanced electrochemical CO2 reduction to CH4. Nanoscale 2023, 15, 2756–2766. [Google Scholar] [CrossRef]
- Xiao, W.; Kiran, G.K.; Yoo, K.; Kim, J.-H.; Xu, H. The Dual-Site Adsorption and High Redox Activity Enabled by Hybrid Organic-Inorganic Vanadyl Ethylene Glycolate for High-Rate and Long-Durability Lithium–Sulfur Batteries. Small 2023, 19, 2206750. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D. Exceptional Anisotropic Noncovalent Interactions in Ultrathin Nanorods: The Terminal σ-Hole. ACS Appl. Mater. Interfaces 2022, 14, 51190–51199. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, H.; Xu, W.; Peng, B.; Zhao, C.; Xie, M.; Lv, X.; Gao, Y.; Hu, K.; Fang, Y.; et al. Quasi-Topological Intercalation Mechanism of Bi0.67NbS2 Enabling 100 C Fast-Charging for Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300790. [Google Scholar] [CrossRef]





| Bismacrocycle | Sub-Classification | Representative Structure | Applications |
|---|---|---|---|
| Oxabismacrocycle | Bis(crown ether) | 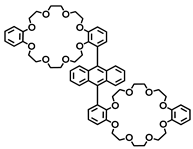 | Self-assembly, Supramolecular polymer, Luminescent material, chiral luminescent material. |
| Biscyclodextrin | 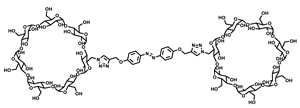 | Self-assembly, Supramolecular polymer, Luminescent material, chiral luminescent material, anticancer active materials. | |
| Biscalixarene |  | Reversible self-assembly, supramolecular polymer. | |
| Bispillararene | 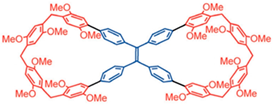 | (Stimulus-responsive) luminescent material | |
| Bishelicarene | 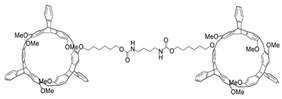 | CPL-active supramolecular gels | |
| Biscucurbituril | 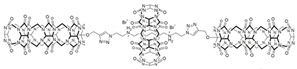 | Controlled supramolecular polymer | |
| Bisheteracalixarenes | 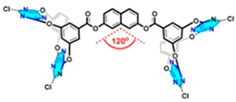 | anion-π interaction-directed self-assembly | |
| Other oxabismacrocycle | 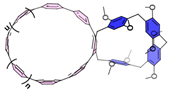 | Chiral luminescent material | |
| Azabismacrocycle | Pyridinium bismacrocycle |  | Highly efficient luminescent material, multicomponent assemblies, AIE fluorescent nanomaterial, pollutant detection. |
| Biscalix[4]pyrroles | 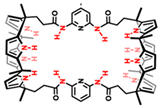 | Two/multi-component self-assembly, anion recognition. | |
| Imidazolium bismacrocycle |  | Mechanochromic and photochromic luminescence compounds. | |
| Azabiscycloparaphenylene |  | Complex chiral compounds, conformationally interchangeable fluorescent compounds. | |
| Biscycloparaphenylene | Biscycloparaphenylene | 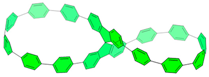 | Complex chiral (luminescent) compounds, VOC adsorbent materials, self-assembly, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-L.; Yu, S.-Q.; Huang, X.-H.; Gong, H.-Y. Bismacrocycle: Structures and Applications. Molecules 2023, 28, 6043. https://doi.org/10.3390/molecules28166043
Chen X-L, Yu S-Q, Huang X-H, Gong H-Y. Bismacrocycle: Structures and Applications. Molecules. 2023; 28(16):6043. https://doi.org/10.3390/molecules28166043
Chicago/Turabian StyleChen, Xu-Lang, Si-Qian Yu, Xiao-Huan Huang, and Han-Yuan Gong. 2023. "Bismacrocycle: Structures and Applications" Molecules 28, no. 16: 6043. https://doi.org/10.3390/molecules28166043
APA StyleChen, X.-L., Yu, S.-Q., Huang, X.-H., & Gong, H.-Y. (2023). Bismacrocycle: Structures and Applications. Molecules, 28(16), 6043. https://doi.org/10.3390/molecules28166043







