Synthesis and Applications of Hybrid Polymer Networks Based on Renewable Natural Macromolecules
Abstract
1. Introduction
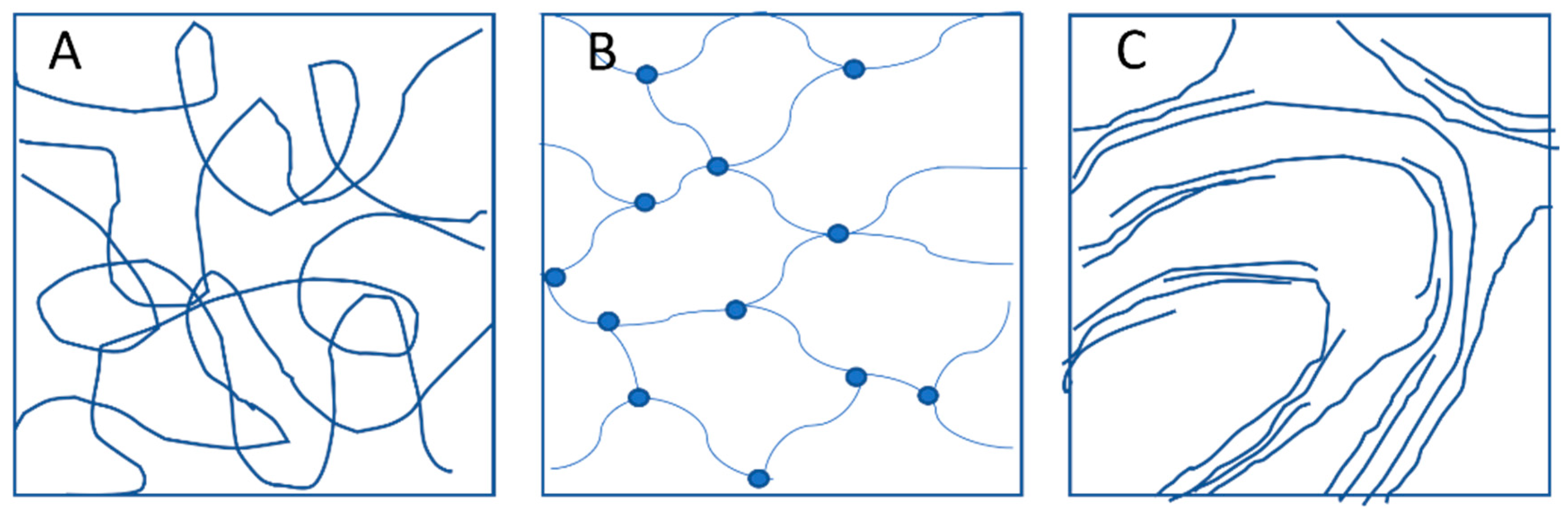
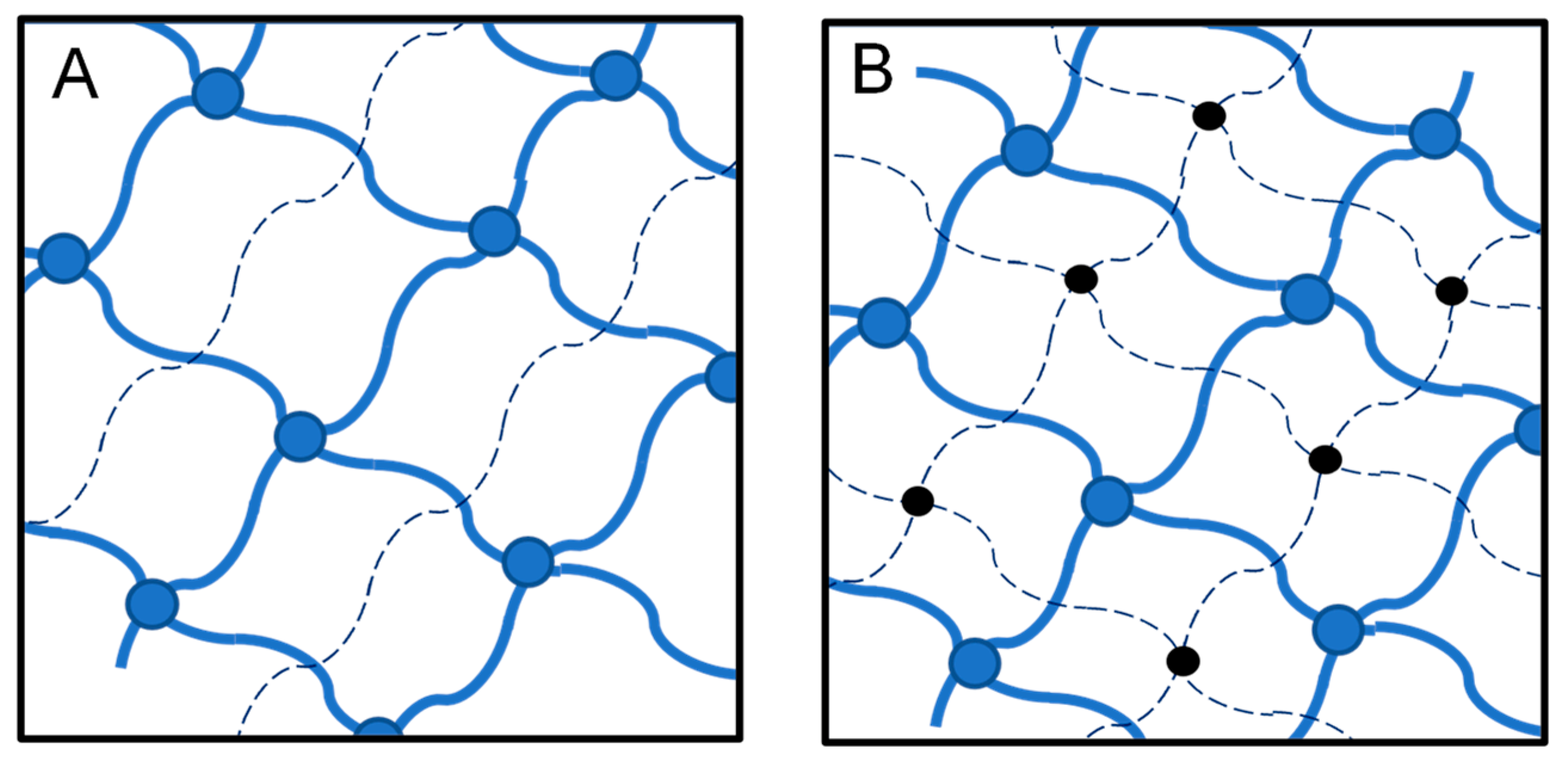
2. Networks: Classification and Applications
3. Cellulose-Based Networks
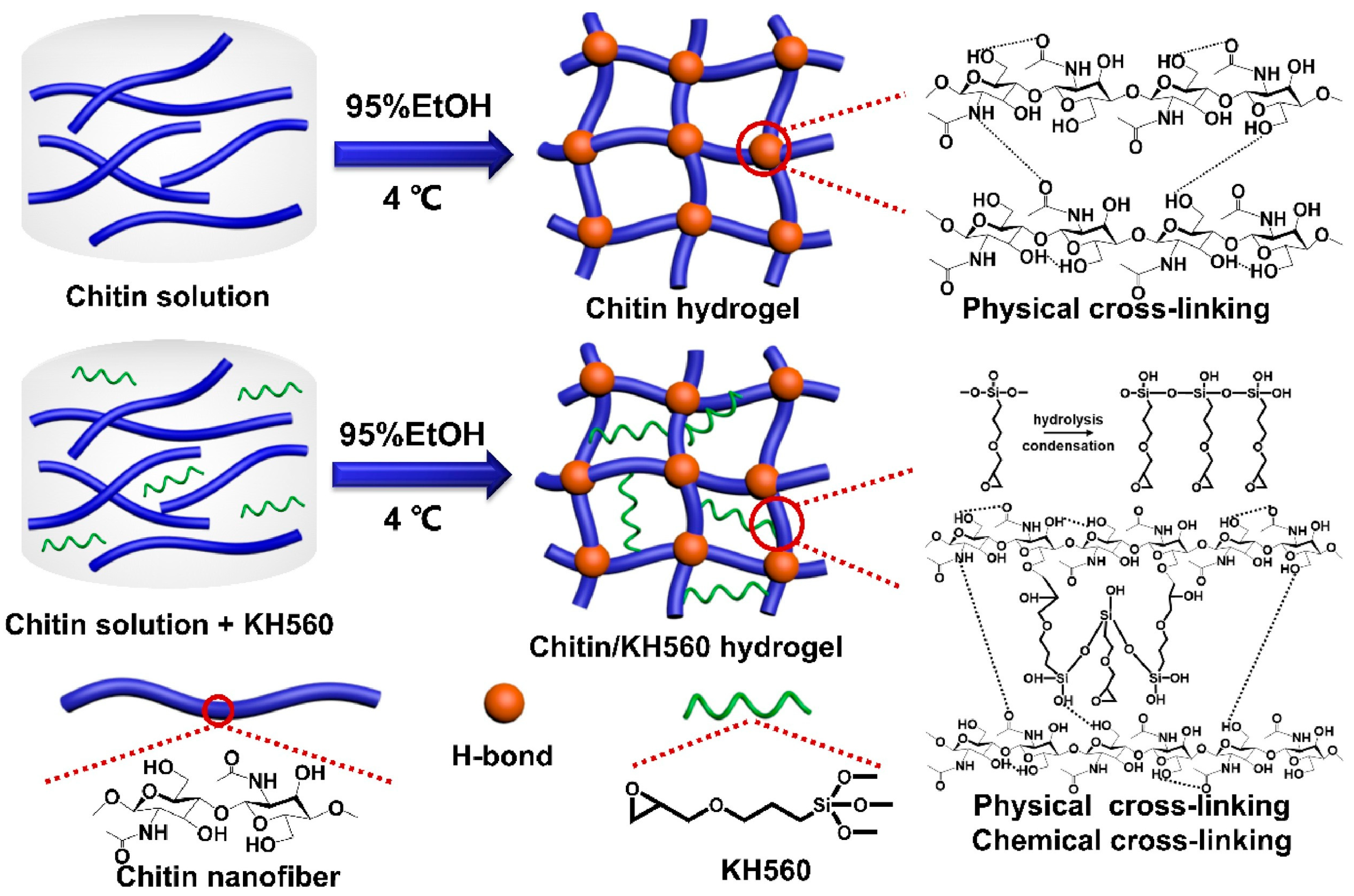
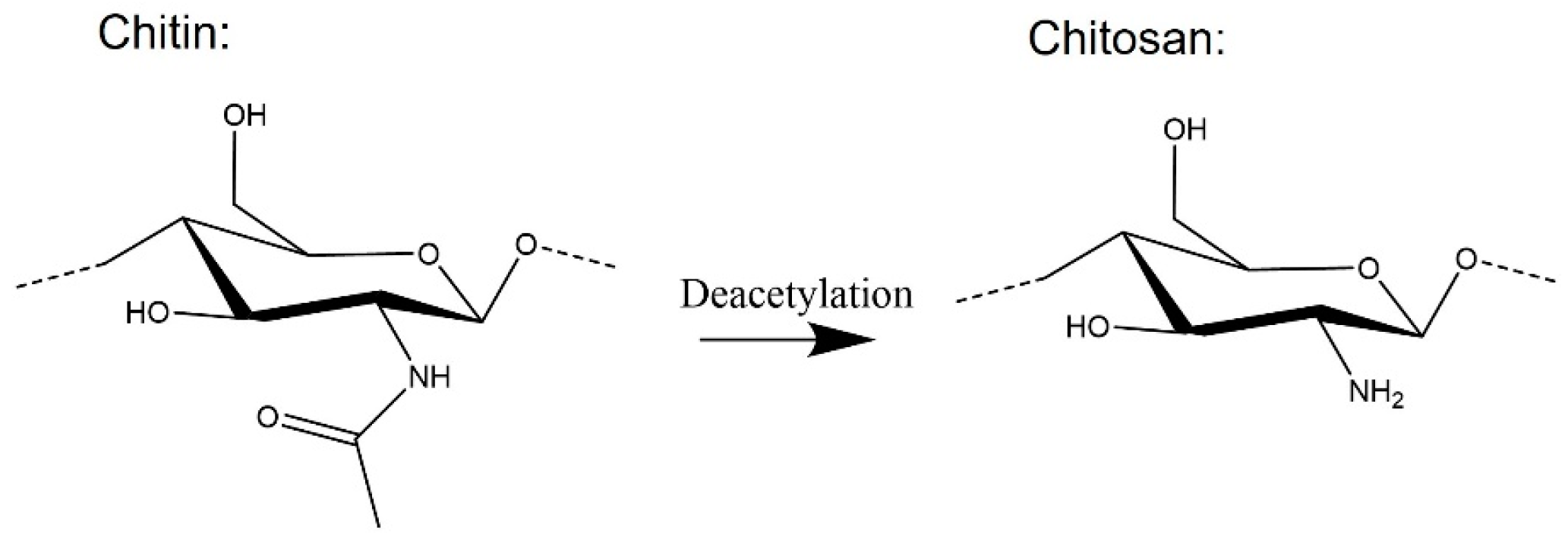
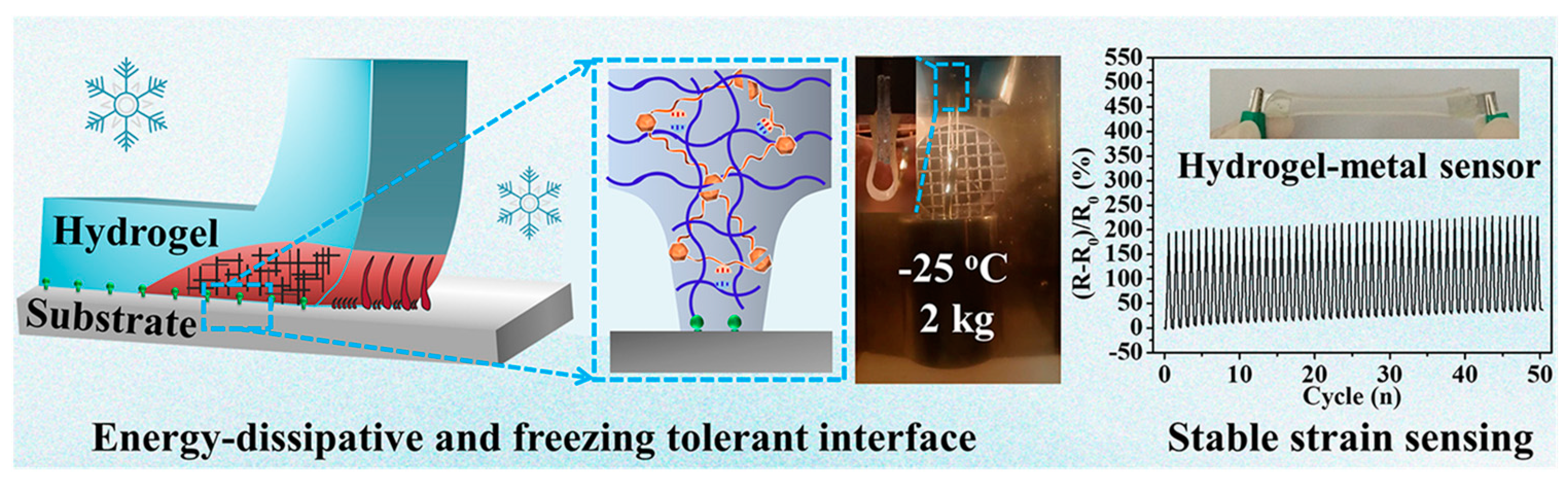
4. Chitin-Containing Networks
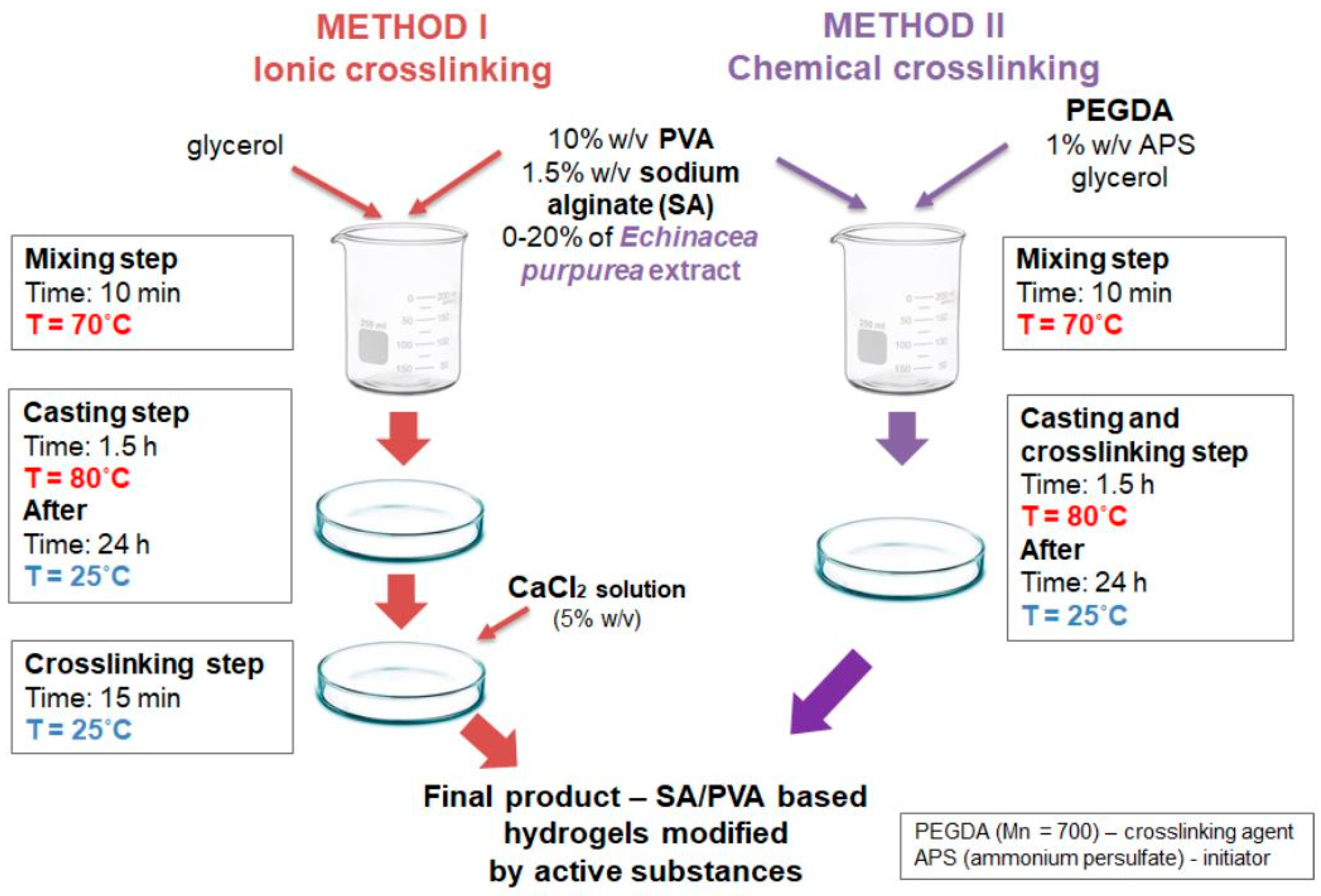
5. Alginic Acid Networks
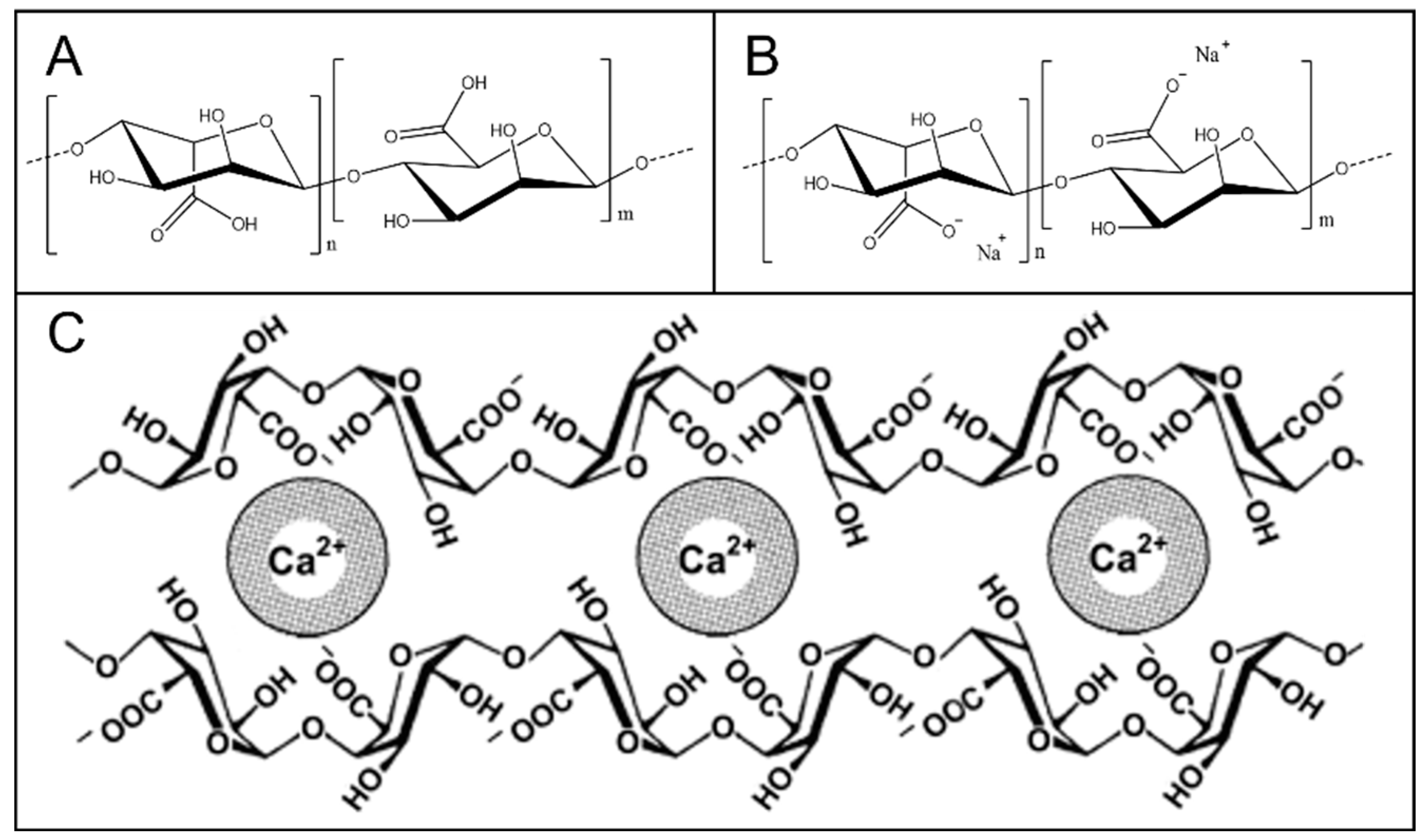
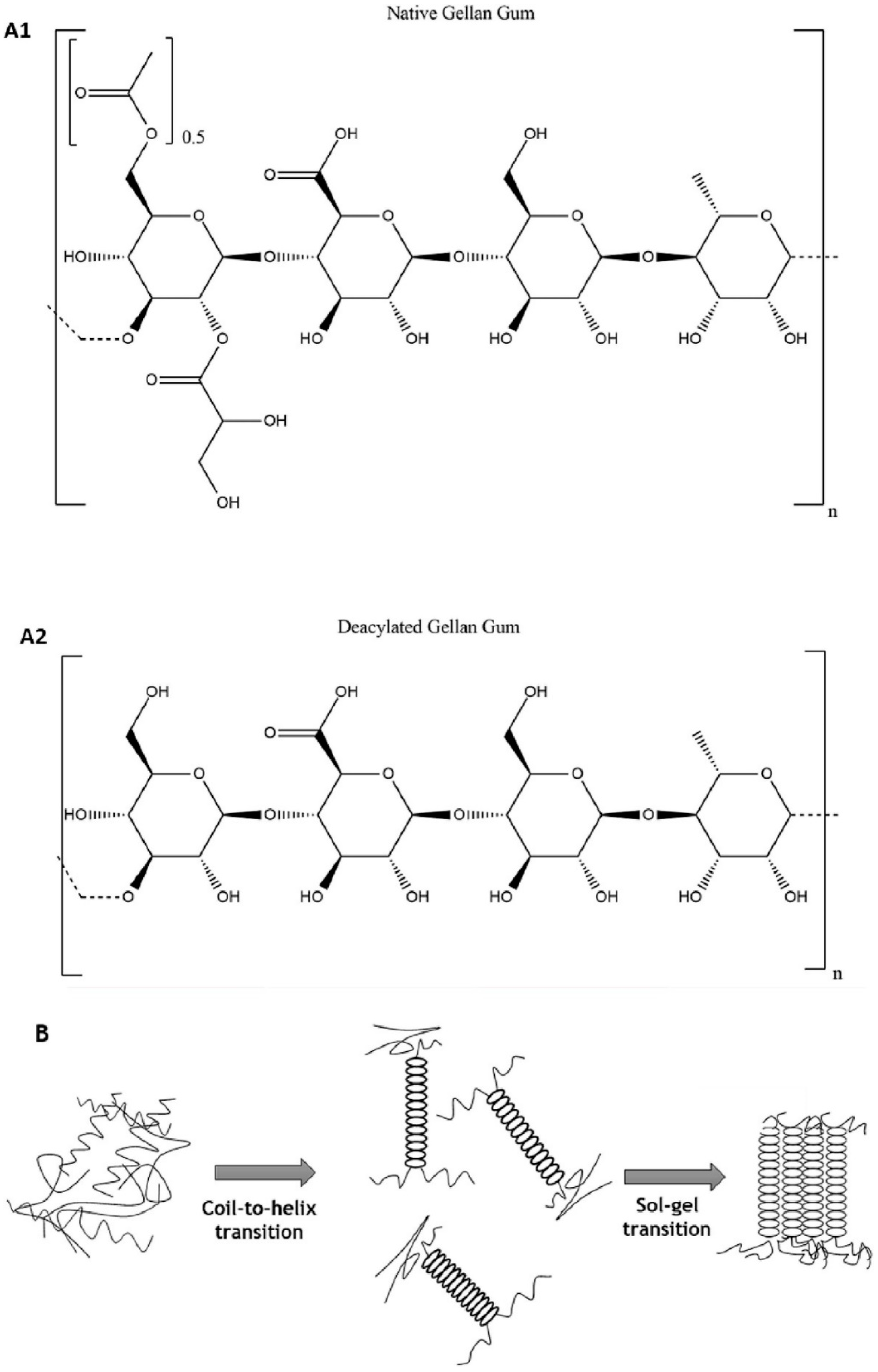
6. Gellan Gum-Derived Networks
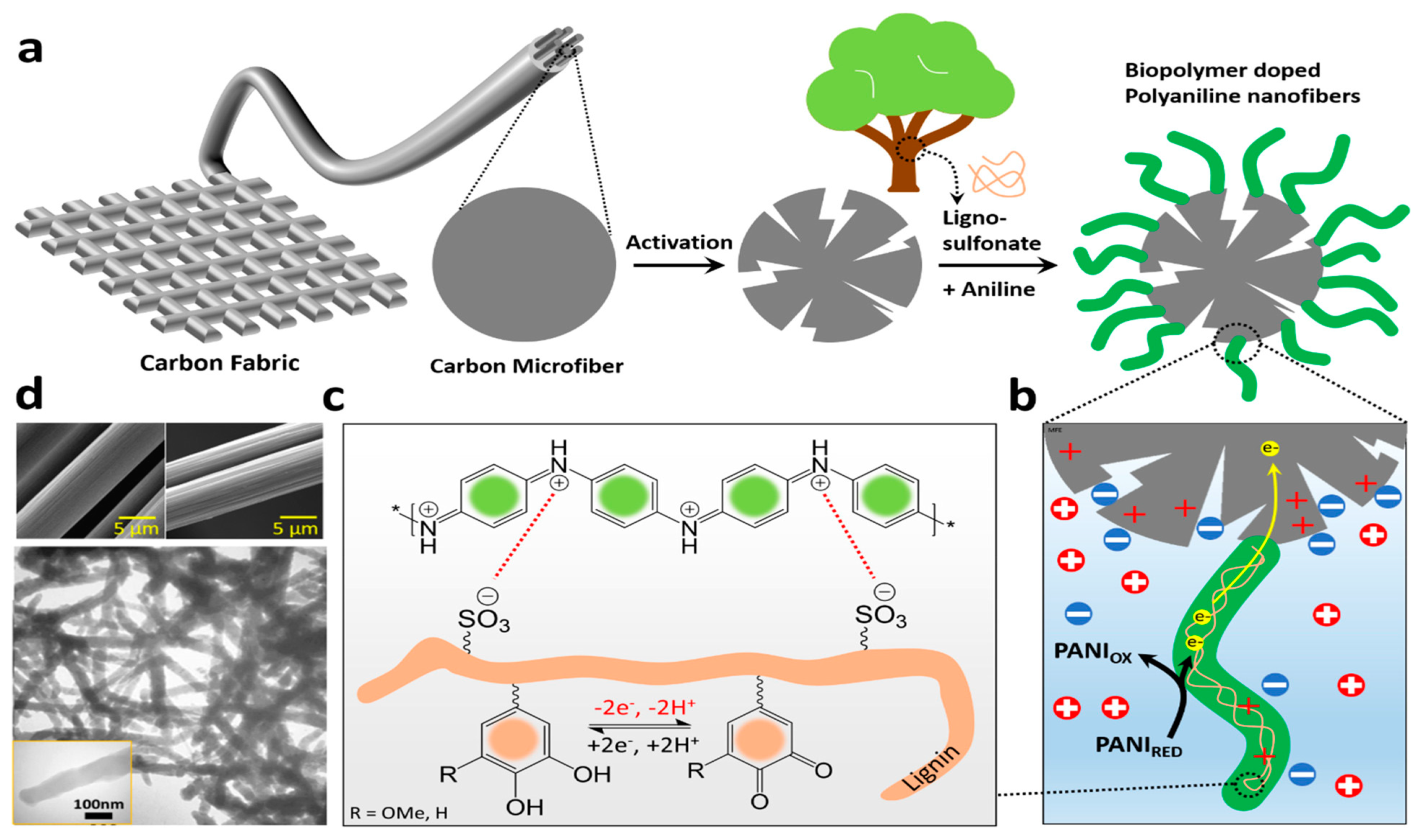
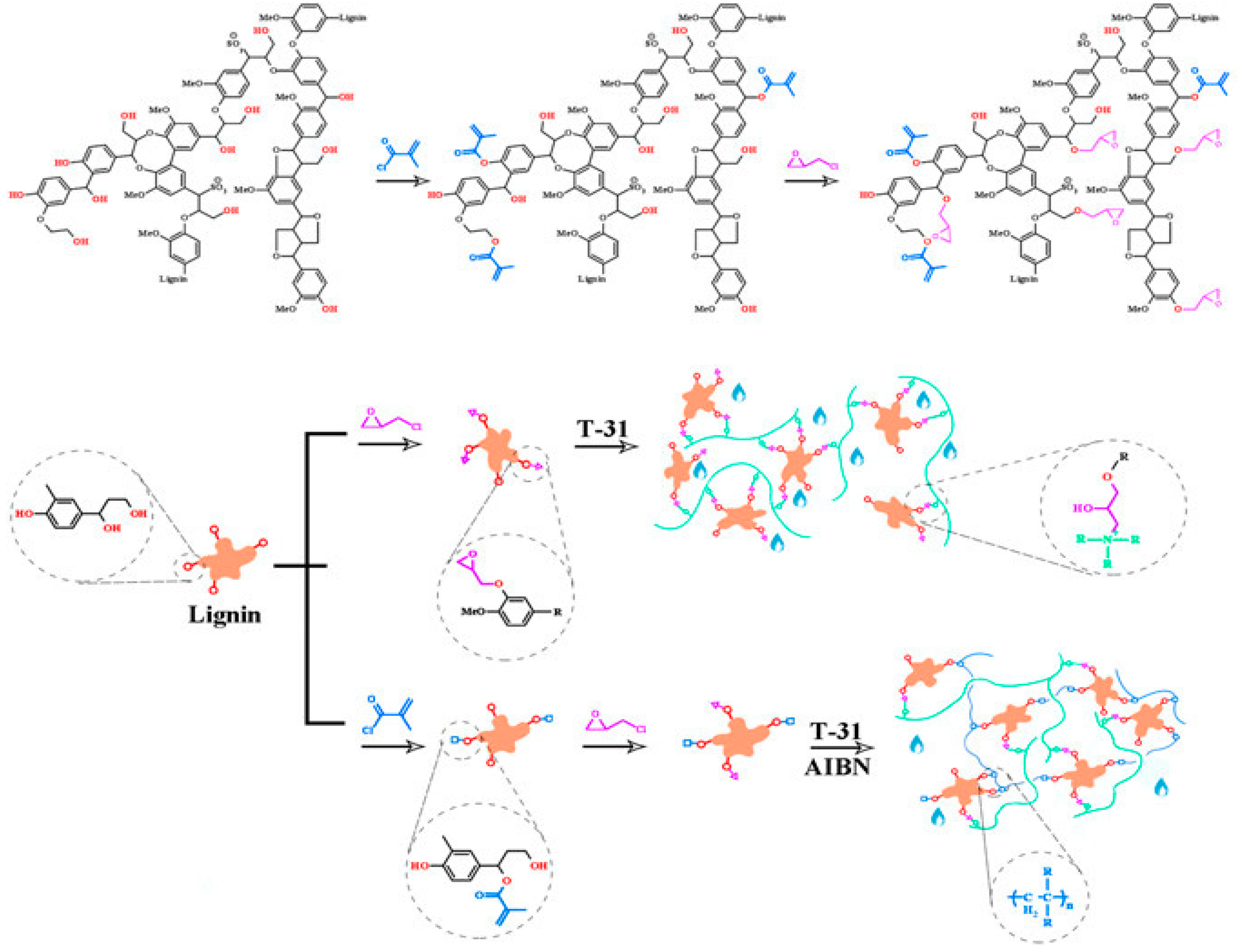
7. Lignin-Based Networks
8. Other Hybrid Networks
Advantages of Hybrid Networks and Remaining Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early pre-Hispanic use of indigo blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef]
- Beker, C.; Benecke, N.; Grabundžija, A.; Küchelmann, H.-C.; Pollock, S.; Schier, W.; Schoch, C.; Schrakamp, I.; Schütt, B.; Schumacher, M. The Textile Revolution. Research into the Origin and Spread of Wool Production. eTopoi J. Anc. Stud. 2016, 6, 102–151. [Google Scholar]
- Schönbein, C.F. Notitz über eine Veränderung der Pflanzenfaser und einiger andern organischen Substanzen. In Bericht Über Die Verhandlungen der Naturforschenden Gesellschaft in Basel; 1846; Volume 7, pp. 26–27. Available online: https://books.google.com/books?id=TSVJAAAAcAAJ&pg=PA26 (accessed on 13 July 2023).
- Schönbein, C.F. Ueber Schiesswolle. In Bericht über die Verhandlungen der Naturforschenden Gesellschaft in Basel; 1846; Volume 7, p. 27. Available online: https://books.google.com/books?id=TSVJAAAAcAAJ&pg=PA27 (accessed on 13 July 2023).
- Hyatt, J.W. Address of Acceptance. Ind. Eng. Chem. 1914, 6, 158–161. [Google Scholar] [CrossRef]
- Porta, R. Anthropocene, the plastic age and future perspectives. FEBS Open Bio 2021, 11, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, E.A.; Cullen, J.M. Toward a sustainable materials system. Science 2018, 360, 1396–1398. [Google Scholar] [CrossRef]
- Mori, R. Replacing all petroleum-based chemical products with natural biomass-based chemical products: A tutorial review. RSC Sustain. 2023, 1, 179–212. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Kwak, M.; Herrmann, A. Nucleic Acid/Organic Polymer Hybrid Materials: Synthesis, Superstructures, and Applications. Angew. Chem. 2010, 49, 8574–8587. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of Polymer Hydrogel Composites and Their Applications. J. Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Djabourov, M.; Leblond, L.; Papon, P. Gelation of aqueous gelatin solutions. II. Rheology of the sol-gel transition. J. Phys. 1988, 49, 333–343. [Google Scholar] [CrossRef]
- Quinn, F.X.; Hatakeyama, T.; Yoshida, H.; Takahashi, M.; Hatakeyama, H. The Conformational Properties of Gellan Gum Hydrogels. Polym. Gels Net. 1993, 1, 93–114. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk Bræk, G.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.I. Gelatinization and rheological properties of starch. Starch-Stärke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels. A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- DeRossi, D.; Kajiwara, K.; Osada, Y.; Yamauchi, A. Polymer gels. In Fundamentals and Biomedical Applications; Springer: New York, NY, USA, 1991; p. 354. [Google Scholar] [CrossRef]
- Bialik-Was, K.; Królicka, E.; Malina, D. Impact of the Type of Crosslinking Agents on the Properties of Modified Sodium Alginate/Poly(vinyl Alcohol) Hydrogels. Molecules 2021, 26, 2381. [Google Scholar] [CrossRef]
- Kim, Y.J.; Min, J. Property modulation of the alginate-based hydrogel via semi-interpenetrating polymer network (semi-IPN) with poly(vinyl alcohol). Int. J. Biol. Macromol. 2021, 193, 1068–1077. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed. In Gold Book; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Shivashankar, M.; Mandal, B.K. A Review on Interpenetrating Polymer Network. Int. J. Pharm. Pharm. Sci. 2012, 4, 1–7. [Google Scholar]
- Zeng, M.; Fang, Z. Preparation of sub-micrometer porous membrane from chitosan/polyethylene glycol semi-IPN. J. Membr. Sci. 2004, 245, 95–102. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Comstock, M.J. Sound and Vibration Damping with Polymers. In Sound and Vibration Damping with Polymers; Corsaro, R.D., Sperling, L.H., Eds.; ACS Symposium Series; American Chemical Society: Washington DC, USA, 1991; pp. 1–4. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Gellert, E.; Burchill, P.; Challis, K. Review of advanced composite structures for naval ships and submarines. Compos. Str. 2001, 53, 21–42. [Google Scholar] [CrossRef]
- Moradi, G.; Nassiri, P.; Ershad-Langroudi, A.; Monazzam, M.R. Preparation of Sound Absorption Material Based on Interpenetrating Polymer Network (PU/PMMA IPN). Health Scope 2019, 8. [Google Scholar] [CrossRef]
- Culin, J. Interpenetrating polymer network composites containing polyurethanes designed for vibration damping. Polimery/Polymers 2016, 61, 159–165. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, T.; Yang, C.; Liu, Y.; Leng, J. Preparation and characterization of shape memory composite foams with interpenetrating polymer networks. Smart Mat. Struct. 2006, 25, 035002. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Visage, C.L.; Tassin, J.F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-Based Interpenetrating Polymer Network (IPN) Hydrogels for Cartilage Applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Khan, A.; Nagpal, K.; Gupta, M. Interpenetrating polymer network as a pioneer drug delivery system: A review. Polym. Bull. 2020, 77, 5027–5050. [Google Scholar] [CrossRef]
- Yang, C.; Xue, R.; Zhang, Q.; Yang, S.; Liu, P.; Chen, L.; Wang, K.; Zhang, X.; Wei, Y. Nanoclay cross-linked semi-IPN silk sericin/poly(NIPAm/LMSH) nanocomposite hydrogel: An outstanding antibacterial wound dressing. Mater. Sci. Eng. 2017, 81, 303–313. [Google Scholar] [CrossRef]
- Vallittu, P.K. Interpenetrating Polymer Networks (IPNs) in Dental Polymers and Composites. J. Adhes. Sci. Technol. 2009, 23, 961–972. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.; Zhou, L.; Chen, S. Mechanical properties, water-swelling behavior, and morphology of water-swellable rubber prepared using crosslinked sodium polyacrylate. J. Appl. Polym. Sci. 2006, 102, 1489–1496. [Google Scholar] [CrossRef]
- Gan, P.G.; Sam, S.T.; bin Abdullah, M.F.; Omar, M.F. Thermal properties of nanocellulose-reinforced composites: A review. J. Appl. Polym. Sci. 2020, 137, 48544. [Google Scholar] [CrossRef]
- Saxena, I.M.; Broun, R.M. Cellulose Biosynthesis: Current Views and Evolving Concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
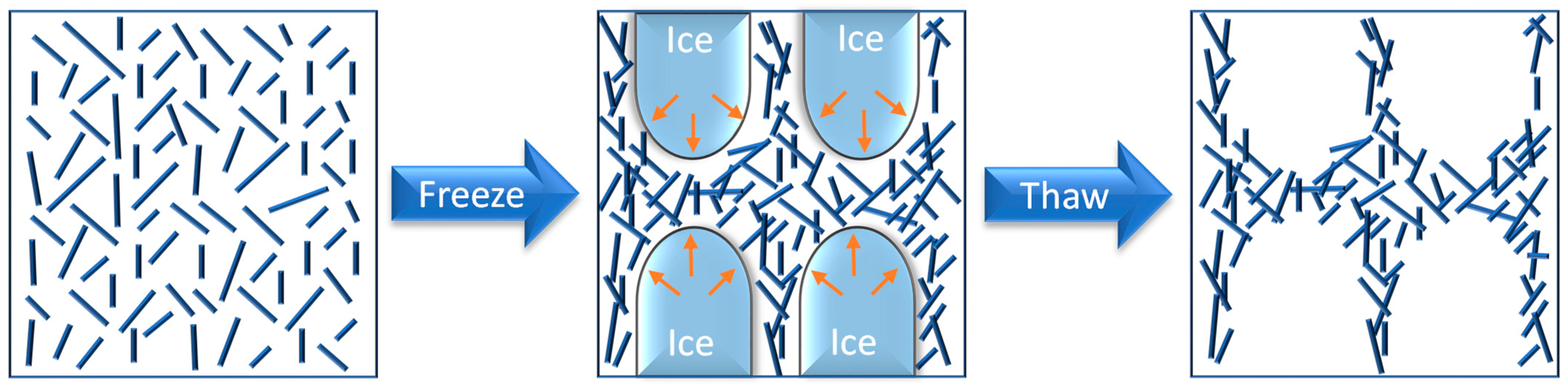
- Lewis, L.; Hatzikiriakos, S.G.; Hamad, W.Y.; Maclachlan, M.J. Freeze-Thaw Gelation of Cellulose Nanocrystals. ACS Macro Lett. 2019, 8, 486–491. [Google Scholar] [CrossRef]
- Wang, M.; Jia, X.; Liu, W.; Lin, X. Water insoluble and flexible transparent film based on carboxymethyl cellulose. Carbohyd. Pol. 2021, 255, 117353. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Nankawa, T.; Yunoki, S.; Sugita, T.; Nakagawa, H.; Yamada, T. Eco-friendly Carboxymethyl Cellulose Nanofiber Hydrogels Prepared via Freeze Cross-Linking and Their Applications. ACS Appl. Polym. Mater. 2020, 2, 5482–5491. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef]
- Geng, H. A one-step approach to make cellulose-based hydrogels of various transparency and swelling degrees. Carbohydr. Polym. 2018, 186, 208–216. [Google Scholar] [CrossRef]
- Seera, S.D.K.; Kundu, D.; Banerjee, T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: Freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 2020, 27, 6521–6535. [Google Scholar] [CrossRef]
- Gomri, C.; Cretin, M.; Semsarilar, M. Recent progress on chemical modification of cellulose nanocrystal (CNC) and its application in nanocomposite films and membranes—A comprehensive review. Carbohydr. Polym. 2022, 294, 119790. [Google Scholar] [CrossRef]
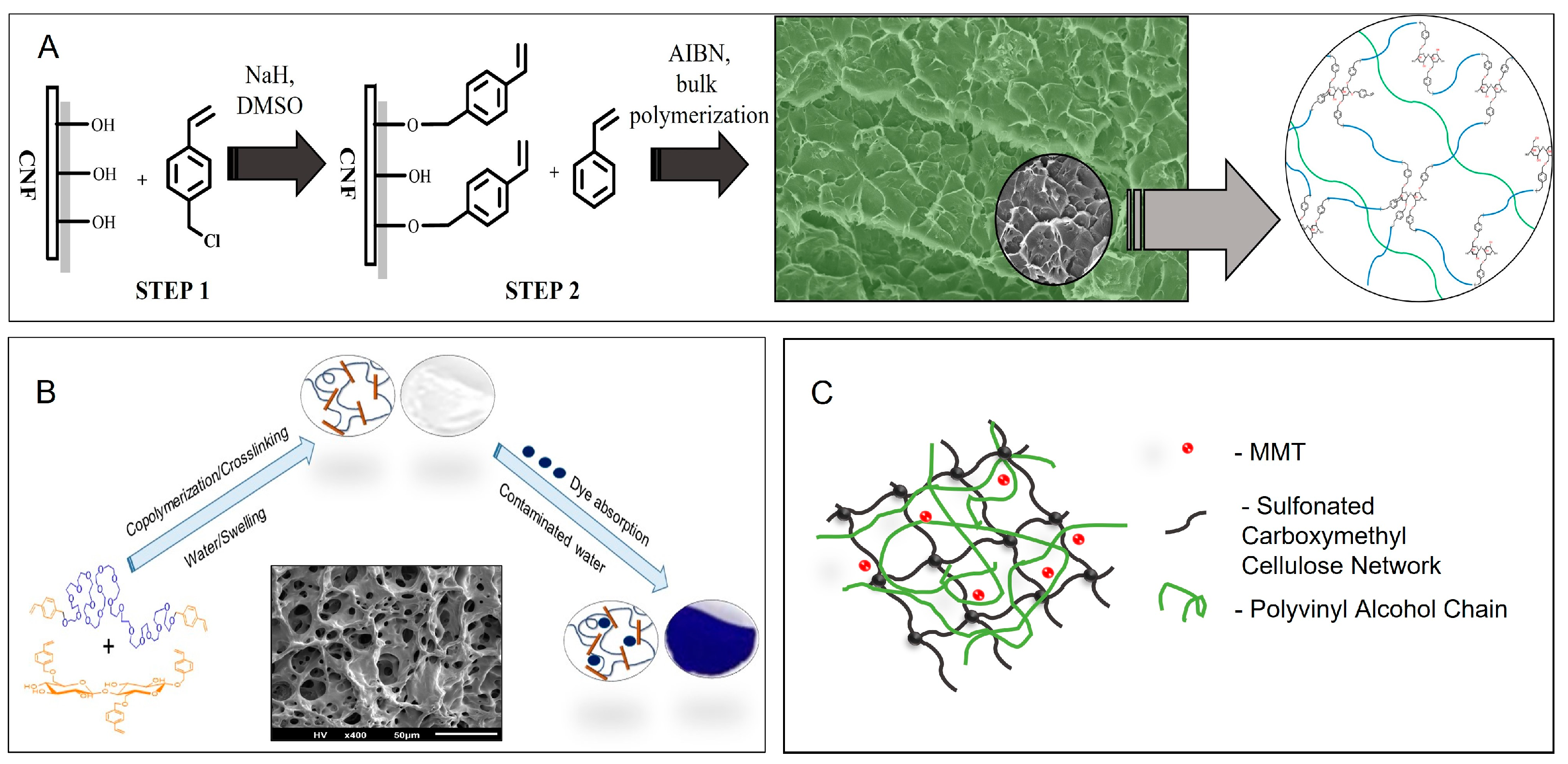
- Getya, D.; Gitsov, I. Reactive Cellu-mers—A Novel Approach to Improved Cellulose/Polymer Composites. Polymers 2022, 14, 1670. [Google Scholar] [CrossRef]
- Getya, D.; Lucas, A.; Gitsov, I. Composite Hydrogels Based on Poly(Ethylene Glycol) and Cellulose Macromonomers as Fortified Materials for Environmental Cleanup and Clean Water Safeguarding. Int. J. Mol. Sci. 2023, 24, 7558. [Google Scholar] [CrossRef]
- Olad, A.; Aghayan, J.; Gharekhani, H.; Akbarzadeh, M. Carboxymethyl cellulose-based semi-IPN hydrogel nanocomposite with improved physicochemical and mechanical properties. J. Polym. Res. 2022, 29, 460. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Li, S.; Wang, G.; Guo, T.; Han, H. Facile fabrication of semi-IPN hydrogel adsorbent based on quaternary cellulose via amino-anhydride click reaction in water. Int. J. Biol. Macromol. 2022, 207, 622–634. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, D.; Lu, Y.; Chen, J.; Li, H.; Xie, J.; Xu, Y.; Yuan, H.; Liu, X.; Zhu, X.; et al. A graphene oxide modified cellulose nanocrystal/PNIPAAm IPN hydrogel for the adsorption of Congo red and methylene blue. New J. Chem. 2021, 45, 16679–16688. [Google Scholar] [CrossRef]
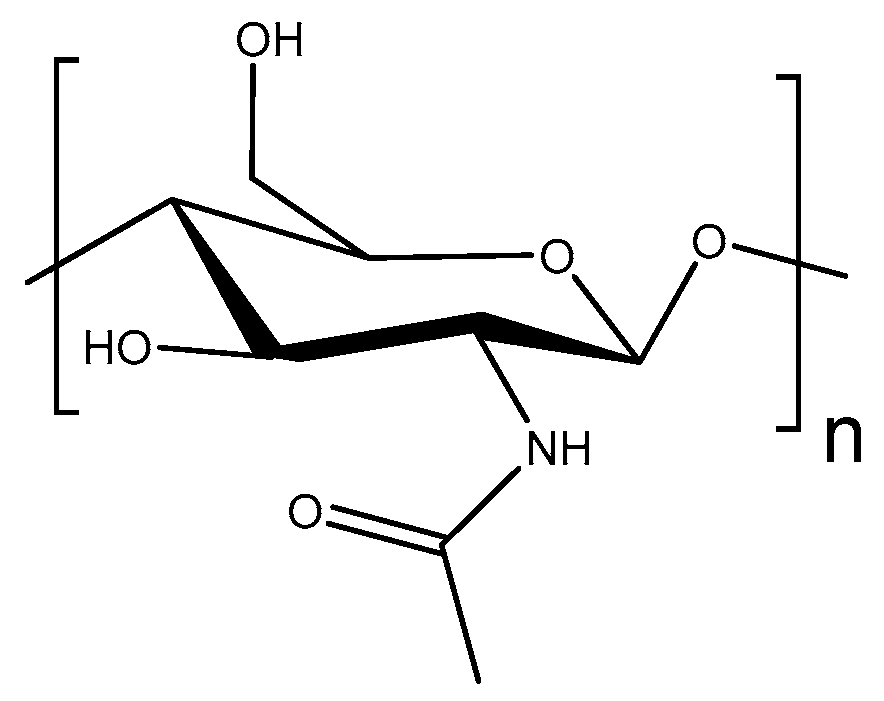
- Lee, S.; Hao, L.T.; Park, J.; Oh, D.X.; Hwang, D.S. Nanochitin and Nanochitosan: Chitin Nanostructure Engineering with Multiscale Properties for Biomedical and Environmental Applications. Adv. Mater. 2023, 35, 2203325. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, M.; Vassalini, I. Green Routes for the Development of Chitin/Chitosan. Sustain. Chem. 2020, 1, 325–344. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Berton, P.; Rogers, R.D. Advances in Functional Chitin Materials: A Review. ACS Sustain. Chem. Eng. 2019, 7, 6444–6457. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, L.; Duan, B. Polyphenol-mediated chitin self-assembly for constructing a fully naturally resourced hydrogel with high strength and toughness. Mater. Horiz. 2021, 8, 2503–2512. [Google Scholar] [CrossRef]
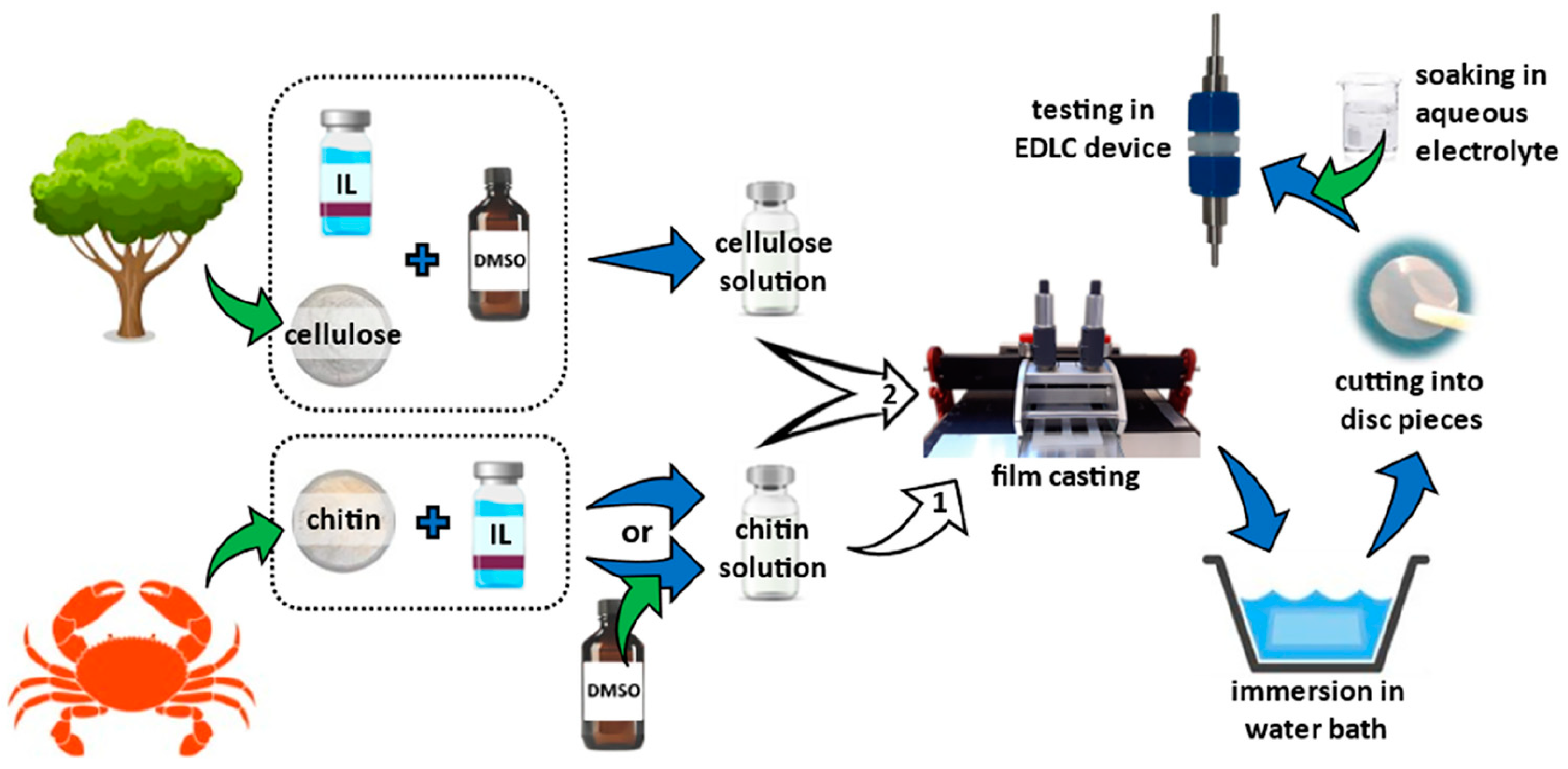
- Kasprzak, D.; Galiński, M. Chitin and chitin-cellulose composite hydrogels prepared by ionic liquid-based process as the novel electrolytes for electrochemical capacitors. J. Solid State Electrochem. 2021, 25, 2549–2563. [Google Scholar] [CrossRef]
- Liao, J.; Hou, B.; Huang, H. Preparation, properties and drug controlled release of chitin-based hydrogels: An updated review. Carbohydr. Polym. 2022, 283, 119177. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Bioinspired hydrogels: Quinone crosslinking reaction for chitin nanofibers with enhanced mechanical strength via surface deacetylation. Carbohydr. Polym. 2019, 207, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wu, S.; Ye, Q. Fabrication and characterization of biodegradable KH560 crosslinked chitin hydrogels with high toughness and good biocompatibility. Carbohydr. Polym. 2021, 259, 117707. [Google Scholar] [CrossRef]
- Bi, B.; Ma, M.; Lv, S.; Zhuo, R.; Jiang, X. In-situ forming thermosensitive hydroxypropyl chitin-based hydrogel crosslinked by Diels-Alder reaction for three dimensional cell culture. Carbohydr. Polym. 2019, 212, 368–377. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Xiao, C. Self-healable and pH-sensitive high-strength water-soluble chitosan/chemically cross-linked polyvinyl alcohol semi-IPN hydrogel. Int. J. Biol. Macromol. 2019, 138, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hong, L. Preparation of chitin composite hydrogel for dye-contaminated water treatment. Pol. J. Environ. Stud. 2022, 31, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sirajudheen, P.; Poovathumkuzhi, N.C.; Vigneshwaran, S.; Chelaveettil, B.M.; Meenakshi, S. Applications of chitin and chitosan-based biomaterials for the adsorptive removal of textile dyes from water—A comprehensive review. Carbohydr. Polym. 2021, 273, 118604. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L.; Zhang, H.; Luo, J.; Gao, X. Green Fabrication of Chitin/Chitosan Composite Hydrogels and Their Potential Applications. Macromol. Biosci. 2021, 21, 2000389. [Google Scholar] [CrossRef]
- Thirupathi, K.; Raorane, C.J.; Ramkumar, V.; Ulagesan, S.; Santhamoorthy, M.; Raj, V.; Krishnakumar, G.S.; Phan, T.T.V.; Kim, S.C. Update on Chitosan-Based Hydrogels: Preparation, Characterization, and Its Antimicrobial and Antibiofilm Applications. Gels 2023, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, M.; Peng, J.; Wang, X.; Liu, Y.; Wang, W.; Wu, D. Robust, anti-freezing and conductive bonding of chitosan-based double-network hydrogels for stable-performance flexible electronic. Carbohydr. Polym. 2023, 276, 118753. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Rong, Z.; Zhu, K.; Wu, Y. Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int. J. Biol. Macromol. 2022, 222, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Y.; Song, T.; Zhang, B.; Li, D.; Xiao, Y.; Zhanga, X. A chitosan-based multifunctional hydrogel containing in situ rapidly bioreduced silver nanoparticles for accelerating infected wound healing. J. Mater. Chem. B 2022, 10, 2135–2147. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling growth of egg-box structure during Calcium-mediated molecular assembly of alginate. J. Colloid Interface Sci. 2023, 634, 747–756. [Google Scholar] [CrossRef]
- Yin, L.; Fei, L.; Tang, C.; Yin, C. Synthesis, characterization, mechanical properties and biocompatibility of interpenetrating polymer network–superporous hydrogel containing sodium alginate. Polym. Int. 2007, 56, 1563–1571. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Gossett, C.; Camirand, W.; Robertson, G.H. Ionomeric Films of Alginic Acid. J. Food Sci. 2006, 64, 61–63. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Mukhopadhyay, P.; Pramanik, N.; Kundu, P.P. Effect of polyethylene glycol on bis(2-hydroxyethyl) terephthalate-based polyurethane/alginate Ph-sensitive blend for oral protein delivery. Adv. Pol. Technol. 2016, 35, 21525. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, É.; de Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growth. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef]
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and molecular design criteria for 3D printable hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter. 2019, 15, 7852–7862. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Sreedhar, V.; Mutalik, S.; Setty, C.M.; Sa, B. Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int. J. Biol. Macromol. 2010, 47, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Toxicological Profile for Glutaraldehyde. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp208-c2.pdf (accessed on 4 April 2023).
- Santos, N.T.; Das, G.; Moraes, L.F.; da Silva, M.G.C.; Vieira, M.G.A. Recovery of gold through adsorption onto sericin and alginate particles chemically crosslinked by proanthocyanidins. J. Clean. Prod. 2020, 253, 119925. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Wang, X.; Zong, L.; Kang, Y.; Wang, A. Fast and highly efficient adsorption removal of toxic Pb(Ⅱ) by a reusable porous semi-IPN hydrogel based on alginate and poly(vinyl alcohol). Front. Chem. 2021, 9, 662482. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Kadri, R.; Elkhoury, K.; Ben Messaoud, G.; Kahn, C.; Tamayol, A.; Mano, J.F.; Arab-Tehrany, E.; Sánchez-González, L. Physicochemical Interactions in Nanofunctionalized Alginate/GelMA IPN Hydrogels. Nanomaterials 2021, 11, 2256. [Google Scholar] [CrossRef]
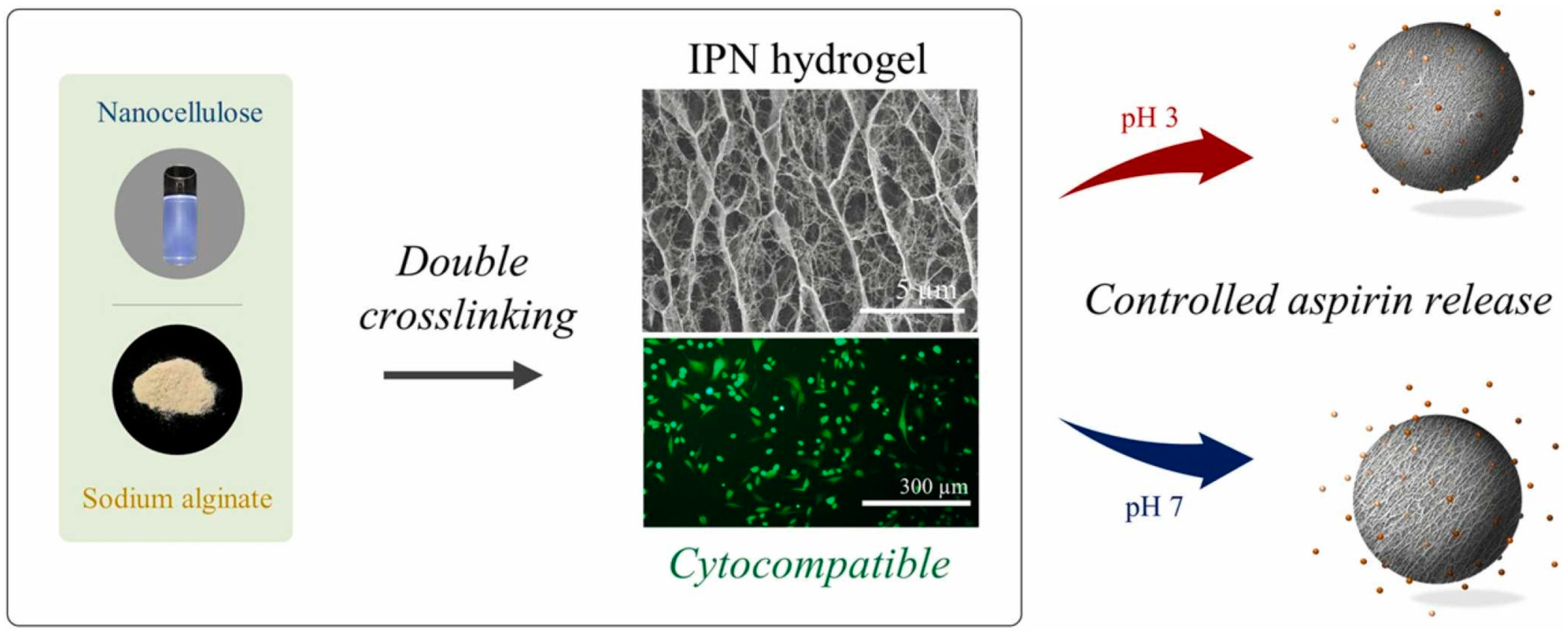
- Ma, H.; Zhao, J.; Liu, Y.; Liu, L.; Yu, J.; Fan, Y. Controlled delivery of aspirin from nanocellulose-sodium alginate interpenetrating network hydrogels. Ind. Crops Prod. 2023, 192, 116081. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Shojarazavi, N.; Mashayekhan, S.; Pazooki, H.; Mohsenifard, S.; Baniasadi, H. Alginate/cartilage extracellular matrix-based injectable interpenetrating polymer network hydrogel for cartilage tissue engineering. J. Biomater. Appl. 2021, 36, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Ansson, P.; Lindberg, B. Structural studies of gellan gum, an extracellular polysaccharide elaborated by pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Gomes, D.; Batista-Silva, J.P.; Sousa, A.; Passarinha, L.A. Progress and opportunities in Gellan gum-based materials: A review of preparation, characterization and emerging applications. Carbohydr. Pol. 2023, 311, 120782. [Google Scholar] [CrossRef]
- Bemiller, J.N. Structure-Property Relationships of Water-Soluble Polysaccharides. J. Appl. Glycosci. 1996, 43, 377–384. [Google Scholar]
- Title 21-Food and Drugs. Chapter I-Food and Drug Administration. Department of Health and Human Services. Subchapter B -Food for Human Consumption. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.665 (accessed on 4 April 2023).
- Vilela, C.A.; Correia, C.; da Silva Morais, A.; Santos, T.C.; Gertrudes, A.C.; Moreira, E.S.; Frias, A.M.; Learmonth, D.A.; Oliveira, P.; Oliveira, J.M. In vitro and in vivo performance of methacrylated gellan gum hydrogel formulations for cartilage repair. J. Biomed. Mater. Res. A 2018, 106, 1987–1996. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Navarro, G.; Di Meo, C.; Matricardi, P.; Torchilin, V. Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: A multi-drug delivery system for a combination therapy in cancer treatment. Eur. J. Pharm. Biopharm. 2014, 87, 208–216. [Google Scholar] [CrossRef]
- Kanyuck, K.M.; Mills, T.B.; Norton, I.T.; Norton-Welch, A.B. Swelling of high acyl gellan gum hydrogel: Characterization of network strengthening and slower release. Carbohydr. Polym. 2021, 259, 117758. [Google Scholar] [CrossRef]
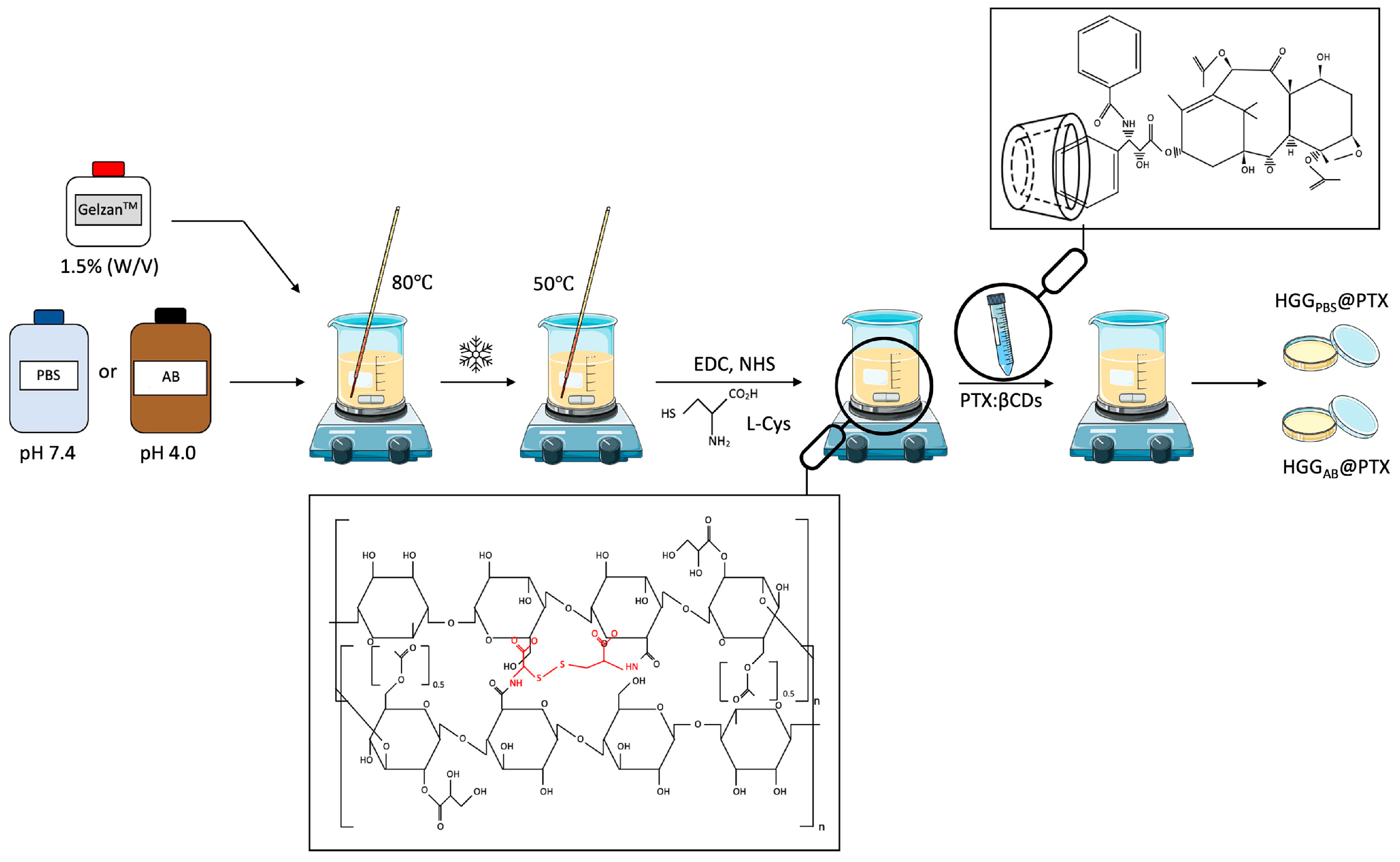
- Nieto, C.; Vega, M.A.; Rodríguez, V.; Pérez-Esteban, P.; Martín del Valle, E.M. Biodegradable gellan gum hydrogels loaded with paclitaxel for HER2+ breast cancer local therapy. Carbohydr. Polym. 2022, 294, 119732. [Google Scholar] [CrossRef]
- Kunwar, P.; Andrada, B.L.; Poudel, A.; Xiong, Z.; Aryal, U.; Geffert, Z.J.; Poudel, S.; Fougnier, D.; Gitsov, I.; Soman, P. Printing Double-Network Tough Hydrogels Using Temperature-Controlled Projection Stereolithography (TOPS). ACS Appl. Mater. Interf. 2023, 15, 30780–30792. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Ioniță, M. The current state of the art in gellan-based printing inks in tissue engineering. Carbohydr. Polym. 2023, 309, 120676. [Google Scholar] [CrossRef]
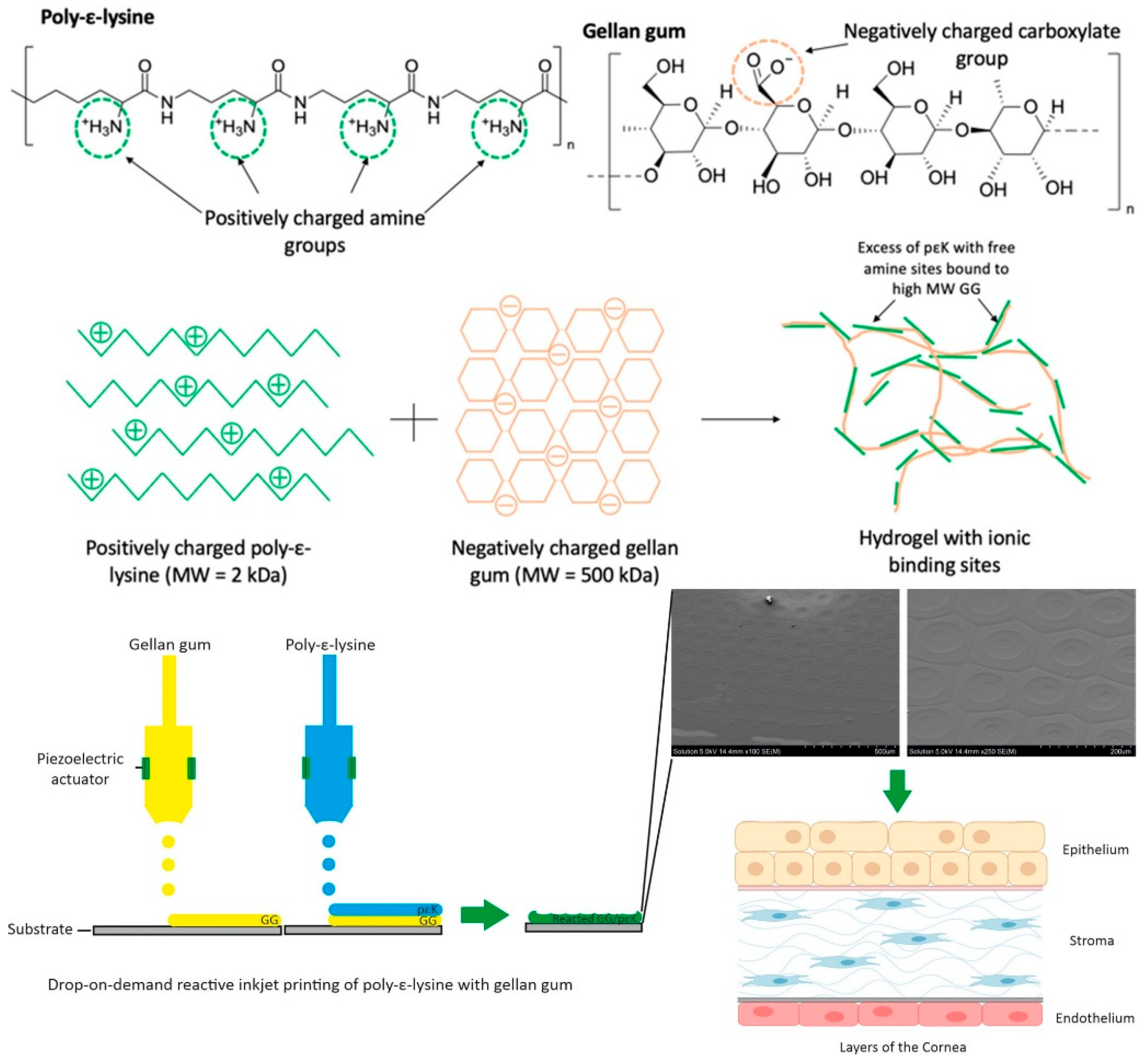
- Duffy, G.L.; Liang, H.; Williams, R.L.; Wellings, D.A.; Black, K. 3D reactive inkjet printing of poly-ɛ-lysine/gellan gum hydrogels for potential corneal constructs. Mater. Sci. Eng. C 2021, 131, 112476. [Google Scholar] [CrossRef] [PubMed]
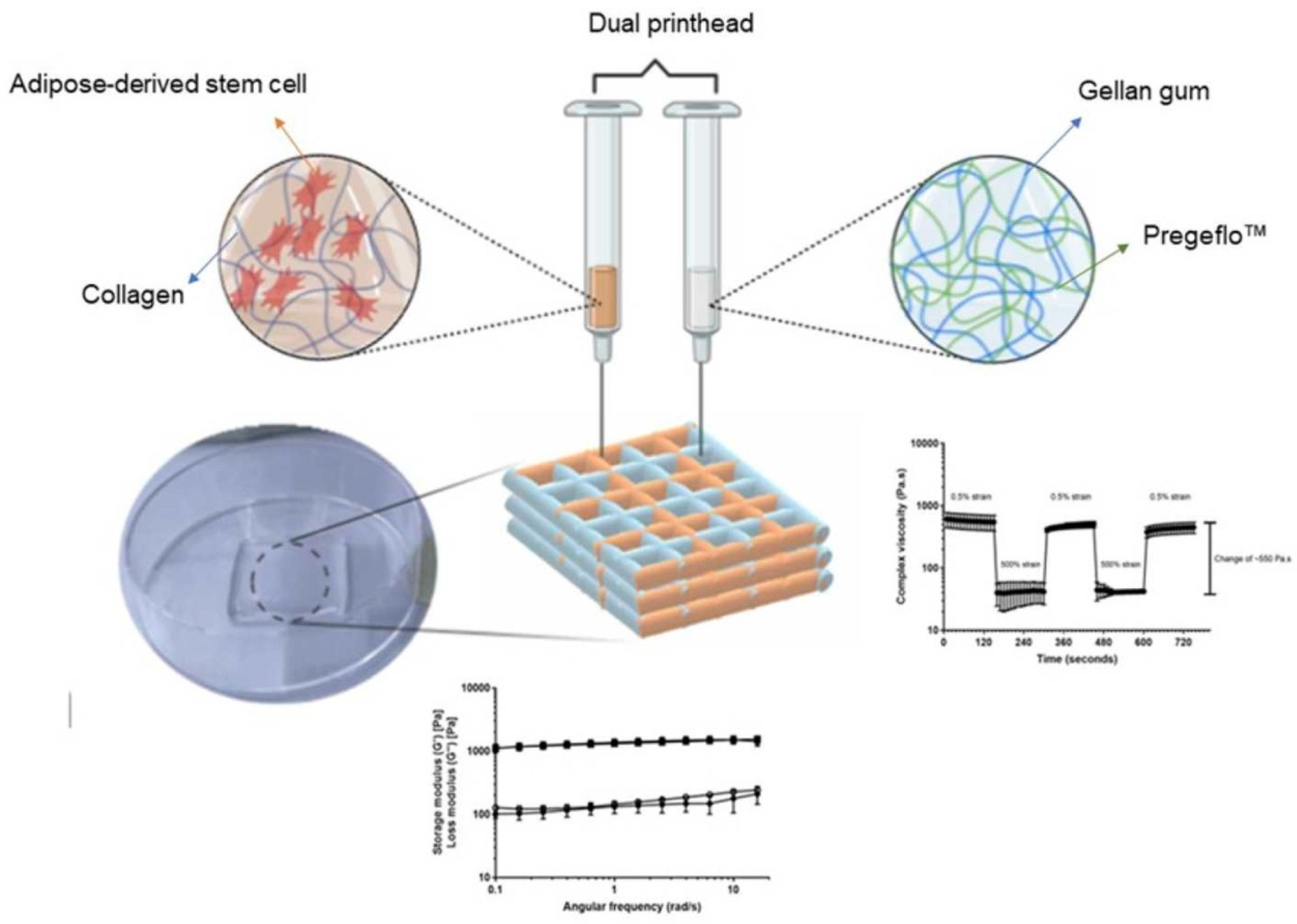
- Ng, J.Y.; Yu, P.; Murali, D.M.; Liu, Y.S.; Gokhale, R.; Ee, P.L.R. The influence of pregelatinized starch on the rheology of a gellan gum-collagen IPN hydrogel for 3D bioprinting. Chem. Eng. Res. Des. 2023, 192, 477–486. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Elkony, A.M.; El-Bahy, S.M. Methylene blue uptake by gum arabic/acrylic amide/3-allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt semi-IPN hydrogel. Int. J. Biol. Macromol. 2021, 186, 268–277. [Google Scholar] [CrossRef]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Cheng, L.; Maiti, A. The utilization of agro-biomass/byproducts for effective bio-removal of dyes from dyeing wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104901. [Google Scholar] [CrossRef]
- Karthika, J.S.; Vishalakshi, B. Novel stimuli responsive gellan gum-graft-poly (DMAEMA) hydrogel as adsorbent for anionic dye. Int. J. Biol. Macromol. 2015, 81, 648–655. [Google Scholar] [CrossRef]
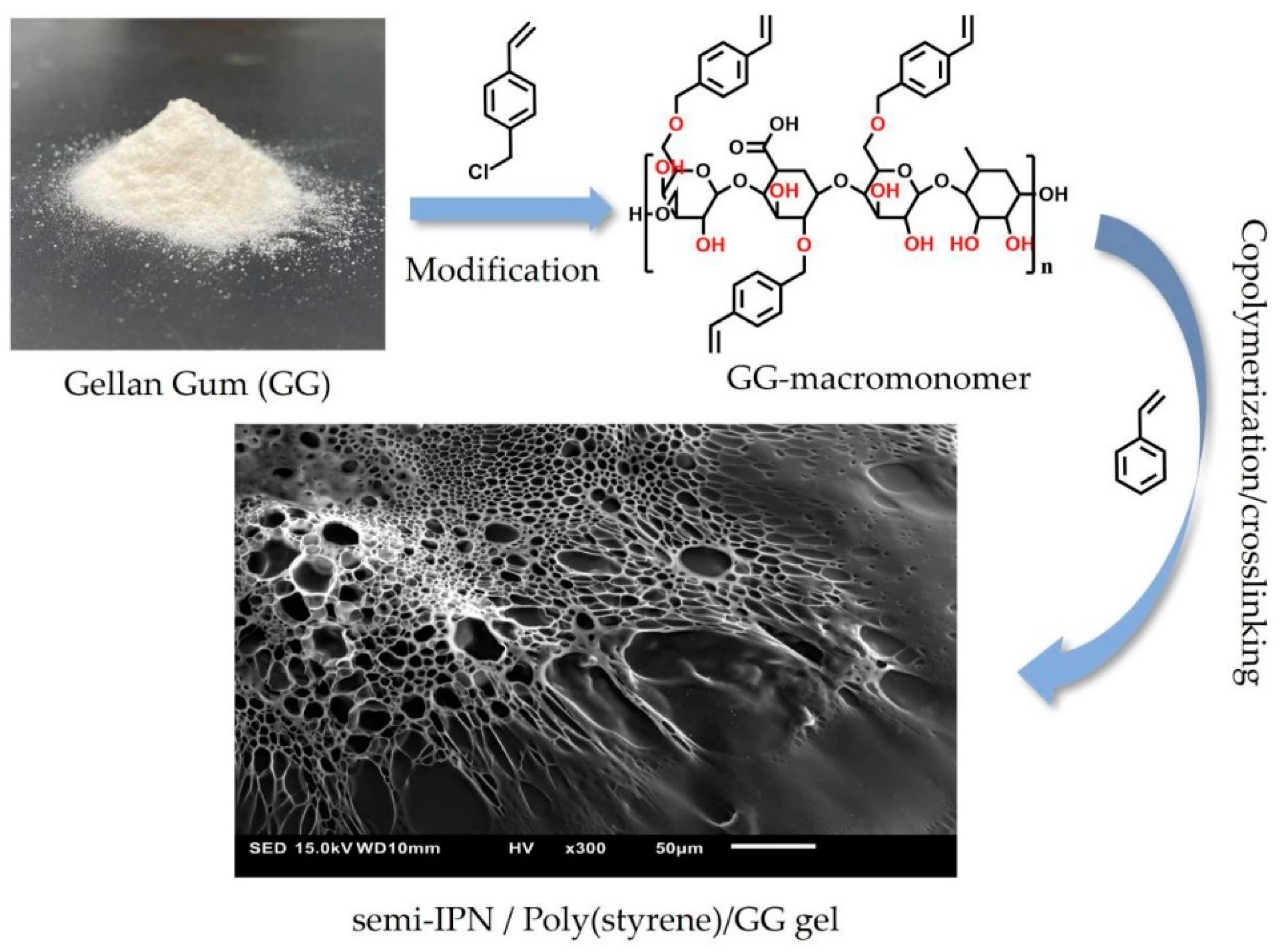
- Getya, D.; Gitsov, I. Stronger together. Poly(styrene) gels reinforced by soft gellan gum. Gels 2022, 8, 607. [Google Scholar] [CrossRef]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
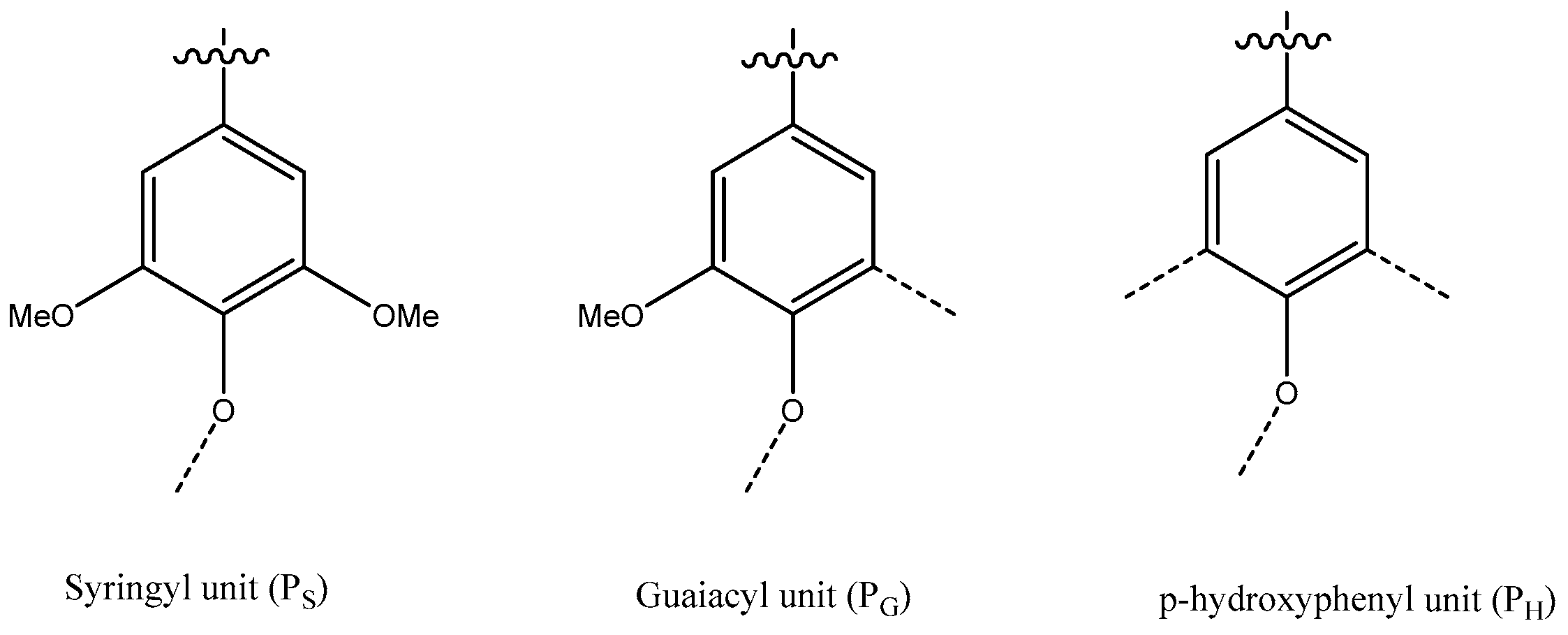
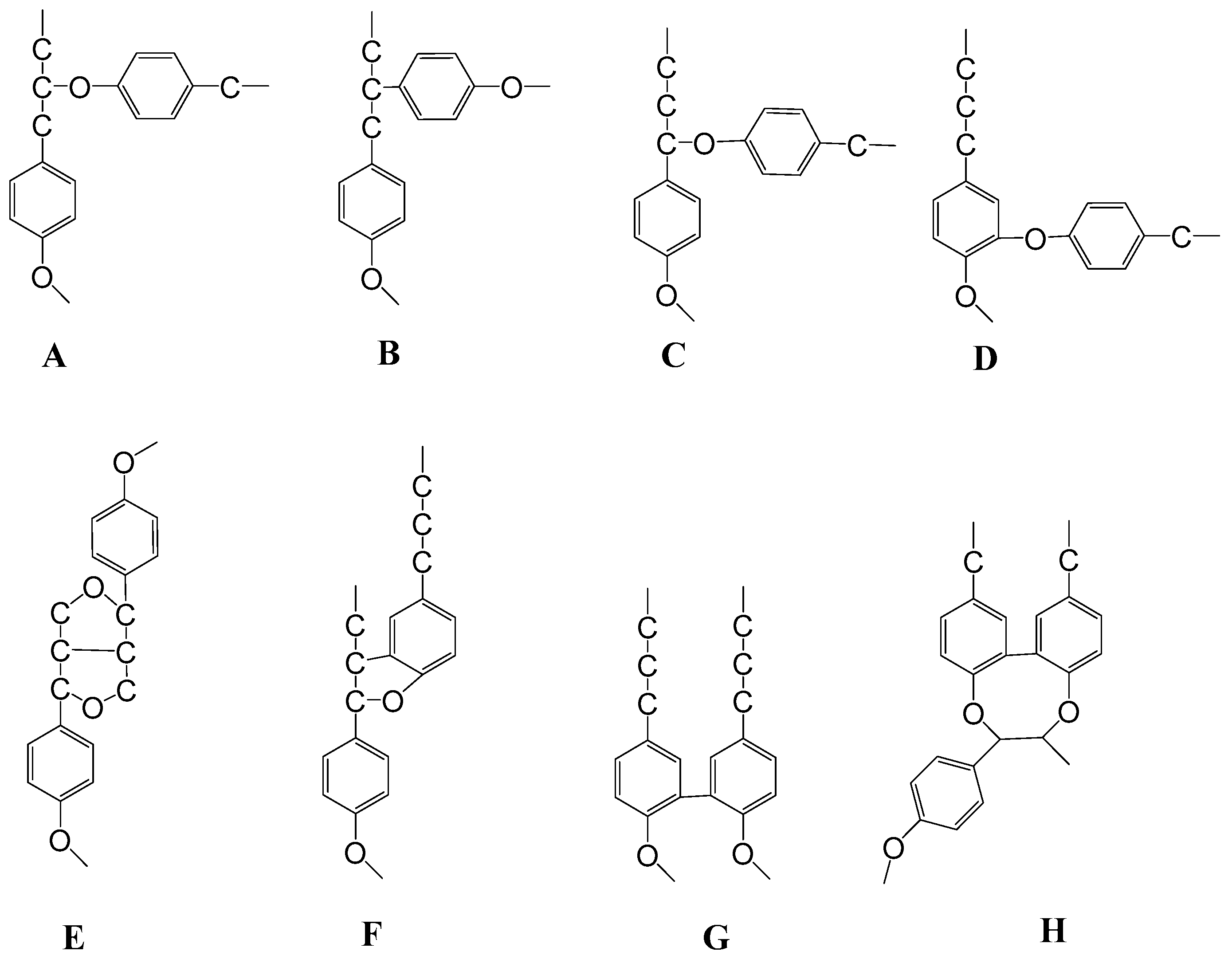
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Christensen, C.S.L.; Rasmussen, S.K. Lignin Biosynthesis and Structure. Agronomy 2019, 9, 256. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, P.G.; Lu, F.; Kim, H.; Schatz, P.; Boerjan, W. Lignins: Natural polymer from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Li, S.; Park, S.; Sherman, B.D.; Yoo, C.G.; Leem, G. Photoelectrochemical approaches for the conversion of lignin at room temperature. Chem. Commun. 2022, 59, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, H.C.; Chu, C.; Li, S.; Yoo, K.; Wijethunga, U.K.; Zheng, W.; Yoo, C.G.; Sherman, B.D.; Lee, J.-J.; et al. Solar Energy Driven C-C Bond Cleavage of Lignin with a D–π–A Organic Dye-Sensitized Photoanode. Sustain. Energy Fuels 2023, 7, 2339–2348. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Gujjala, L.K.S.; Kim, J.; Won, W. Technical lignin to hydrogels: An Eclectic review on suitability, synthesis, applications, challenges and future prospects. J. Clean. Prod. 2022, 363, 132585. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, R.; Chahal, G.K. Synthesis of lignin-based hydrogels and their applications in agriculture: A review. Chem. Papers 2021, 75, 4465–4478. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, C.; Zhaozhe, Y.; Wu, G.; Liu, G.; Kong, Z. Recent advances in lignin-based porous materials for pollutants removal from wastewater. Int. J. Biol. Macromol. 2021, 187, 880–891. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is lignin from being a biomedical material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive hydrogels based on industrial lignin: Opportunities and challenges. Polymers 2022, 14, 3739. [Google Scholar] [CrossRef]
- Dianat, N.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Chang, X.; Kaner, R.B.; Mousavi, M.F. Polyaniline-lignin interpenetrating network for supercapacitive energy storage. Nano Lett. 2021, 21, 9485–9493. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Soledad Peresin, M.; Tamminen, T.; Rodríguez, A.; Larrañeta, E.; Jääskeläinen, A. Lignin-based hydrogels with “super-swelling” capacities for dye removal. Int. J. Biol. Macromol. 2018, 115, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zhang, H.; Chen, T.; Sun, G.; Han, Y.; Li, J. Double-interpenetrating-network lignin-based epoxy resin adhesives for resistance to extreme environment. Biomacromolecules 2022, 23, 779–788. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Yang, H.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications. Pharmaceutics 2023, 15, 1323. [Google Scholar] [CrossRef]
- Adedeji, O.E.; Choi, J.Y.; Park, G.E.; Kang, H.J.; Aminu, M.O.; Min, J.H.; Chinma, C.E.; Moon, K.D.; Jung, Y.H. Formulation and characterization of an interpenetrating network hydrogel of locust bean gum and cellulose microfibrils for 3D printing. Innov. Food Sci. Emerg. Technol. 2022, 80, 103086. [Google Scholar] [CrossRef]
- He, M.; Wang, Z.; Cao, Y.; Zhao, Y.; Duan, B.; Chen, Y.; Xu, M.; Zhang, L. Construction of Chitin/PVA Composite Hydrogels with Jellyfish Gel-Like Structure and Their Biocompatibility. Biomacromolecules 2014, 15, 3358–3365. [Google Scholar] [CrossRef]
- Balitaan, J.N.I.; Hsiao, C.D.; Yeh, J.M.; Santiago, K.S. Innovation inspired by nature: Biocompatible self-healing injectable hydrogels based on modified-β-chitin for wound healing. Int. J. Biol. Macromol. 2020, 162, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D printed gellan gum/graphene oxide scaffold for tumor therapy and bone reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Jana, S.; Pramanik, R.; Kumar, A.; Kumar, K. Gellan gum (GG)-based IPN microbeads for sustained drug release. J. Drug Deliv. Sci. Technol. 2022, 69, 103034. [Google Scholar] [CrossRef]
- Lameirinhas, N.S.; Teixeira, M.C.; Carvalho, J.P.F.; Valente, B.F.A.; Pinto, R.J.B.; Oliveira, H.; Luís, J.L.; Pires, L.; Oliveira, J.M.; Vilela, C.; et al. Nanofibrillated cellulose/gellan gum hydrogel-based bioinks for 3D bioprinting of skin cells. Int. J. Biol. Macromol. 2023, 229, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Puglia, D.; Gracia, L. Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl arginate. Food Packag. Shelf Life 2023, 36, 101050. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Jiang, Z.; Sakai, E.; Qiu, J.; Zhu, P. Highly temperature resistant cellulose nanofiber/polyvinyl alcohol hydrogel using aldehyde cellulose nanofiber as cross-linker. Cellulose 2019, 26, 5291–5303. [Google Scholar] [CrossRef]
- He, X.; Zeng, L.; Cheng, X.; Yang, C.; Chen, J.; Chen, H.; Ni, H.; Bai, Y.; Yu, W.; Zhao, K.; et al. Shape memory composite hydrogel based on sodium alginate dual crosslinked network with carboxymethyl cellulose. Eur. Polym. J. 2021, 156, 110592. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and Processable Hydrogels Based on Lignin and Hydrophilic Polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Rico-García, D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Hernández-Olmos, S.L.; Guerrero-Ramírez, G.L.; Vilas-Vilela, J.L. Lignin-based hydrogels: Synthesis and applications. Polymers 2020, 12, 81. [Google Scholar] [CrossRef]
- Kulkarni, V.; Butte, K.; Rathod, S. Natural Polymers—A Comprehensive Review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Yaşayan, G.; Alarçin, E.; Bal-Öztürk, A.; Avci-Adali, M. Natural polymers for wound dressing applications. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 74. [Google Scholar] [CrossRef]
- Nagalakshmaiah, M.; Afrin, S.; Malladi, R.P.; Elkoun, S.; Robert, M.; Ansari, M.A.; Svedberg, A.; Karim, Z. Biocomposites: Present trends and challenges for the future. Green Compos. Automot. Appl. 2018, 9, 197–215. [Google Scholar] [CrossRef]
- Environment Policy: General Principles and Basic Framework. Available online: https://www.europarl.europa.eu/factsheets/en/sheet/71/environment-policy-general-principles-and-basic-framework (accessed on 24 July 2023).
- Chen, S.; Zhang, Z.L.; Song, F.; Wang, X.L.; Wang, Y.Z. Rapid Synthesis of Polymer-Grafted Cellulose Nanofiber Nanocomposite via Surface-Initiated Cu(0)-Mediated Reversible Deactivation Radical Polymerization. Macromolecules 2021, 54, 7409–7420. [Google Scholar] [CrossRef]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Littunen, K.; Hippi, U.; Johansson, L.S.; Österberg, M.; Tammelin, T.; Laine, J.; Seppälä, J. Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr. Polym. 2011, 84, 1039–1047. [Google Scholar] [CrossRef]
- Yu, L.; Dean, K.; Li, L. Polymer Blends and Composites from Renewable Resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Recent Developments in Chemical Modificationand Characterization of Natural Fiber-ReinforcedComposites. Polym. Compos. 2008, 29, 119–237. [Google Scholar] [CrossRef]
- Cheng, H.N.; Neiss, T.G. Solution NMR Spectroscopy of Food Polysaccharides. Polym. Rev. 2012, 52, 81–114. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Nie, S.; Xie, M.; Li, S. MALDI mass spectrometry in food carbohydrates analysis: A review of recent researches. Food Chem. 2023, 399, 133968. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Chu, C.K.; Joseph, A.J.; Limjoco, M.D.; Yang, J.; Bose, S.; Thapa, L.S.; Langer, R.; Anderson, D.G. Chemical Tuning of Fibers Drawn from Extensible Hyaluronic Acid Networks. J. Am. Chem. Soc. 2020, 142, 19715–19721. [Google Scholar] [CrossRef]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, S.; Huemmerich, D.; Hermanson, K.D.; Scheibel, T.; Bausch, A.R. Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl. Phys. A 2006, 82, 261–264. [Google Scholar] [CrossRef]
- McGill, M.; Grant, J.M.; Kaplan, D.L. Enzyme-Mediated Conjugation of Peptides to Silk Fibroin for Facile Hydrogel Functionalization. Ann. Biomed. Eng. 2020, 48, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.; Chen, Z.; Li, Y.; Li, J. Facile and green preparation of diverse arabinoxylan hydrogels from wheat bran by combining subcritical water and enzymatic crosslinking. Carbohydr. Polym. 2020, 241, 116317. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Amoli, M.S.; Huysecom, A.-S.; Amorim, P.A.; Agten, H.; Geris, L.; Bloemen, V. A tunable gelatin-hyaluronan dialdehyde/methacryloyl gelatin interpenetrating polymer network hydrogel for additive tissue manufacturing. Biomed. Mater. 2022, 17, 045027. [Google Scholar] [CrossRef]






| Natural Precursor | Potential Use | Synthesis Method | Reference |
|---|---|---|---|
| Cellulose and locust bean gum | 3D printing | Suspensions of locust bean gum and cellulose microfibers are mixed and homogenized. Prepared hydrogel is inserted into Luer Lock syringes and refrigerated for complete gelation. Inks are printed using a 3D bioprinter and stored at ambient temperature. | [124] |
| Chitin | Potential tissue engineering | Chitin is dissolved in an 8% NaOH/6% urea solution using the freezing/thawing method and then blended with sodium alginate solution, attapulgite powder, and epichlorohydrin. | [125] |
| Chitin and alginate | Wound healing | Self-healing hydrogels are prepared by cross-linking acrylamide-modified β-chitin (Am-β-Chn) with alginate dialdehyde (AD). Chitin solution (2%) is prepared, and a fixed volume of various AD concentrations are added to yield pre-hydrogel mixtures. Solutions are left to gel completely. | [126] |
| Gellan gum (GG) | Tumor therapy and bone reconstruction | GG is dissolved in deionized water at 80 °C. Graphene oxide is dispersed in deionized water using ultrasonication. Bio-inks are formed by mixing GG and GO aqueous solutions in different concentrations. Prepared GG/GO 3D scaffolds are then soaked in CaCl2 solution. Final hydrogels are obtained after freeze-drying. | [127] |
| Gellan gum | Sustained drug release | Spherical diclofenac sodium-loaded carboxymethyl tamarind gum-GG IPN microbeads are prepared using AlCl3 as an ionic cross-linker. Aqueous GG and CTG solutions are mixed, and the diclofenac sodium is added. The homogeneous drug–polymer mixtures are added dropwise into 3% w/v AlCl3 solution, resulting in the formation of rigid spherical microbeads. | [128] |
| Gellan gum and cellulose | 3D bioprinting of skin cells | Hydrogel formulations are prepared by mixing GG water solution and a suspension of nano-fibrillated cellulose. Hydrogels are fully cross-linked in 30 min by immersion in a CaCl2 1% (w/v) bath for mechanical characterization. | [129] |
| Polylactic acid, chitosan, cellulose nanocrystals, and ethyl lauryl alginate | Antibacterial food packaging material | An antibacterial food packaging material with a trilayer structure is obtained through the combination of extrusion, coaxial electrospinning, and coating techniques. | [130] |
| Cellulose nanofiber/polyvinyl alcohol with aldehyde cellulose nanofiber as cross-linker | Highly temperature-resistant hydrogels | Cellulose nanofibers and polyvinyl alcohol are dispersed into water and stirred. HNO3 catalyst is added to the mixture, and the hydrogel precursor is transferred into a sealed transparent mold. Hydrogel is placed in an oven for 24 h. | [131] |
| Sodium alginate and carboxymethyl cellulose | Material with excellent water-induced shape memory properties | A dual-crosslinked gel is formed by sodium alginate reaction with glutaraldehyde (GA) and Ca2+. Carboxymethyl cellulose (CMC) is introduced into the cross-linked network. | [132] |
| Lignin and polyurethane | Tough, thin, printable hydrogels | Lignin is used as a toughening component for polyether-based polyurethane (HPU) hydrogels. Various concentrations (0, 0.5, 1, and 2.5 wt%) are prepared from lignin stock solutions and added to HPU at pH 7–8. Samples are cast and dried at 60 °C for 3 days. | [133] |
| Lignin, poly(ethylene glycol) methyl ether methacrylate (PEGMA)-grafted lignin | Mechanically responsive and self-healing hydrogels | Hyperbranched copolymers are prepared by atom transfer radical polymerization to form supramolecular hydrogels with a very low critical gelation concentration of 1 wt% in the presence of α-cyclodextrin. | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getya, D.; Gitsov, I. Synthesis and Applications of Hybrid Polymer Networks Based on Renewable Natural Macromolecules. Molecules 2023, 28, 6030. https://doi.org/10.3390/molecules28166030
Getya D, Gitsov I. Synthesis and Applications of Hybrid Polymer Networks Based on Renewable Natural Macromolecules. Molecules. 2023; 28(16):6030. https://doi.org/10.3390/molecules28166030
Chicago/Turabian StyleGetya, Dariya, and Ivan Gitsov. 2023. "Synthesis and Applications of Hybrid Polymer Networks Based on Renewable Natural Macromolecules" Molecules 28, no. 16: 6030. https://doi.org/10.3390/molecules28166030
APA StyleGetya, D., & Gitsov, I. (2023). Synthesis and Applications of Hybrid Polymer Networks Based on Renewable Natural Macromolecules. Molecules, 28(16), 6030. https://doi.org/10.3390/molecules28166030







