The Synthesis and Polymer-Reinforced Mechanical Properties of SiO2 Aerogels: A Review
Abstract
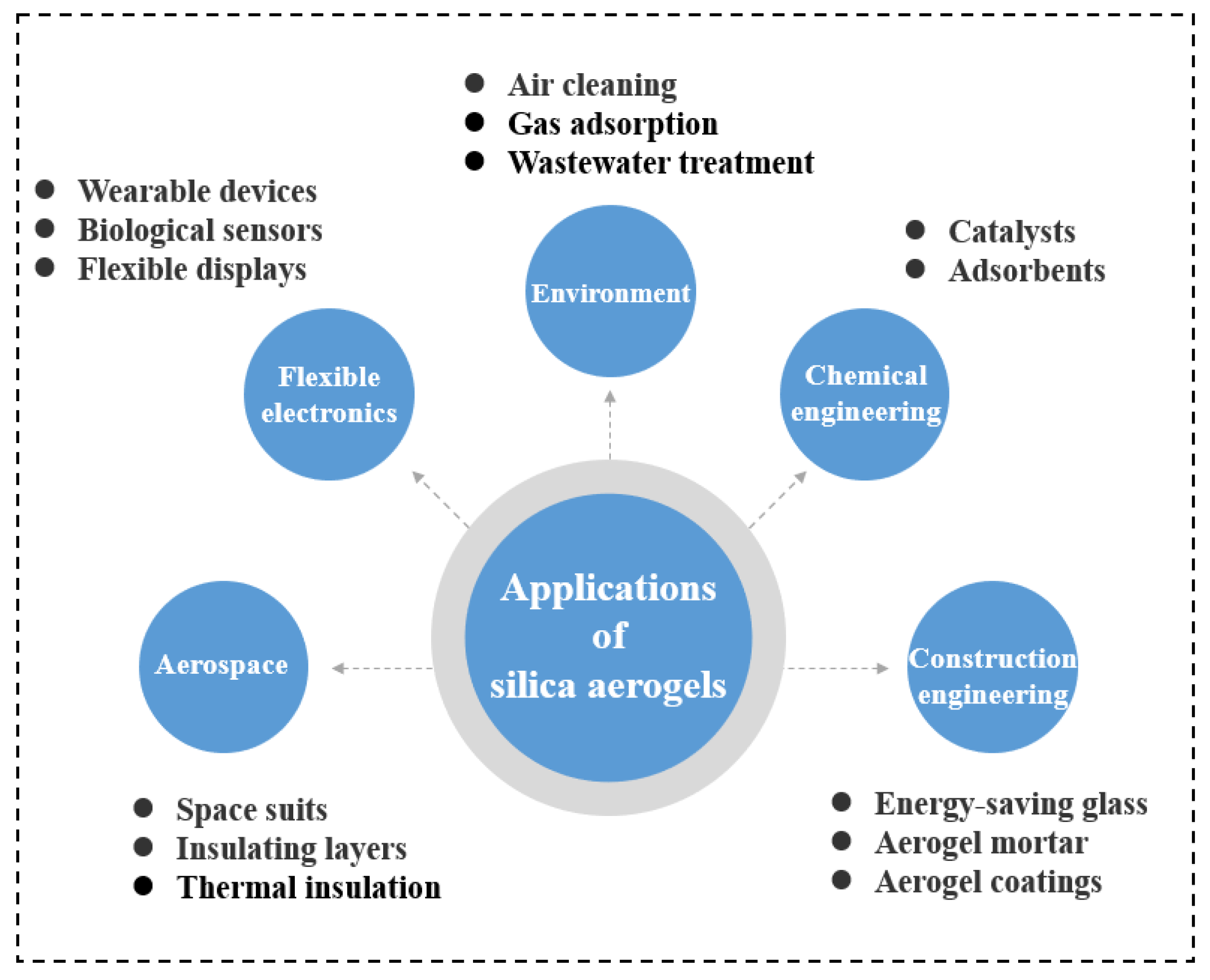
1. Introduction
2. Synthesis of Silica Aerogel
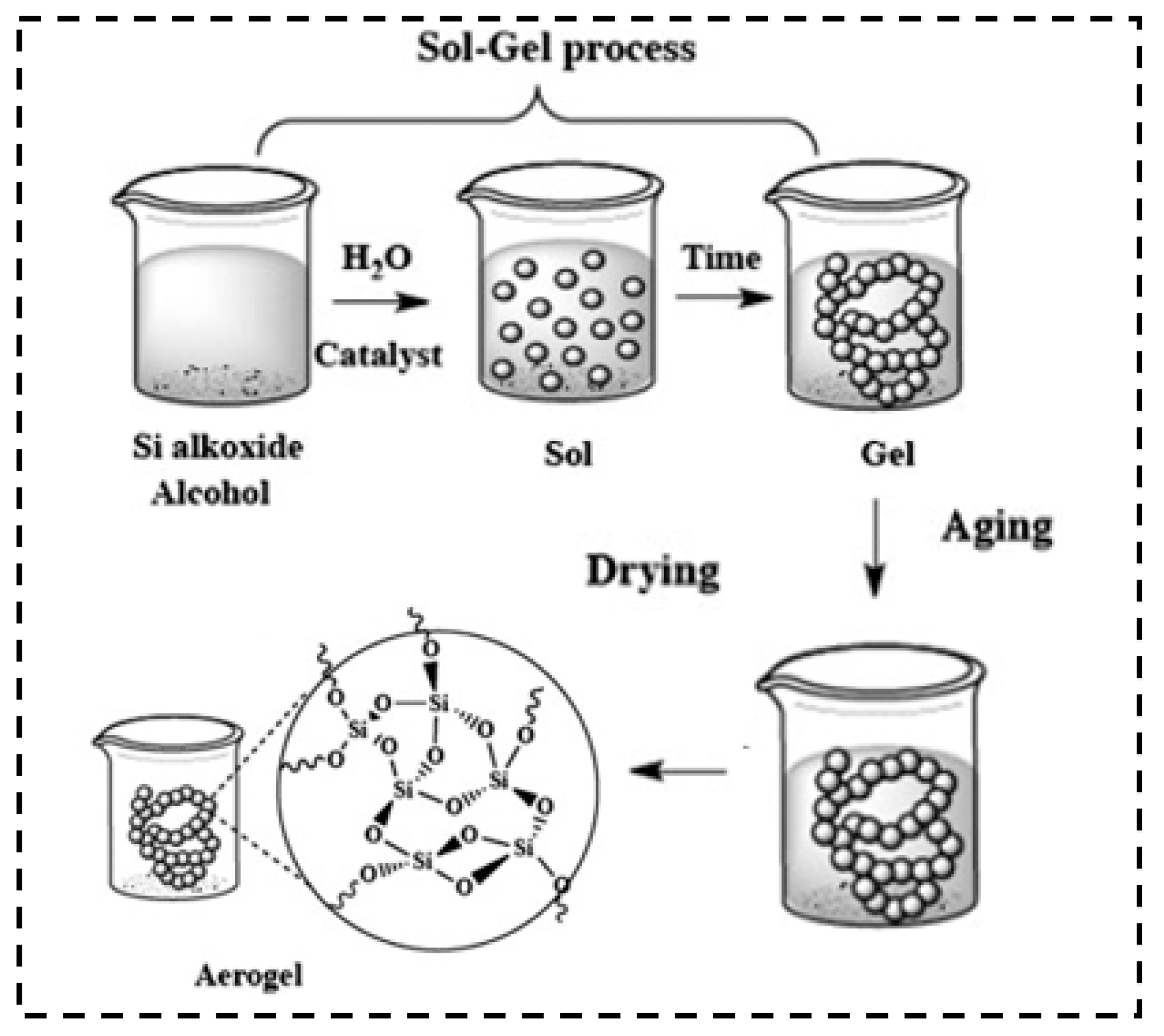
2.1. Sol–Gel Chemistry
| Silica Precursor | Chemical Formula | Abbreviation | Physical Properties | Mechanical Properties | Thermal Properties | Ref. |
|---|---|---|---|---|---|---|
| Tetraethylorthosilicate | Si (OC2H5)4 | TEOS | / | G modulus: 10.7 MPa | / | [79] |
| Tetramethylorthosilicate | Si (OCH3)4 | TMOS | Skeletal densities: 2.2 g/cm3 | / | / | [80] |
| Trimethylchlorosilane | Si (CH3)3Cl | TMCS | Surface area: 914.4 m2/g; porosity: 96.16% | / | / | [81] |
| Methyltrimethoxysilane | Si (OCH3)3CH3 | MTMS | Shrinkage: 3.5% | / | / | [82] |
| Methyltriethoxysilane | Si (OC2H5)3CH3 | MTES | Density: 0.1 g/cm3; porosity: 95.5% | Unrecoverable strain loss: 10% | Thermal conductivity: 0.038 W/m·K | [83] |
| Aminopropyltrimethoxysilane | H2N (CH2)3Si(OCH3)3 | APTMS | / | / | Young’s modulus: 14 MPa | [84] |
| Aminopropyltriethoxysilane | H2N (CH2)3Si(OC2H5)3 | APTES | Surface area: 150.9 m2/g | Young’s modulus: 18 MPa | Thermal conductivity: 0.037 W/m·K | [85] |
| Propyltriethoxysilane | C9H22O3Si | PTES | Density: 0.172 g/cm3; porosity: >90% | Elastic module: 0.35 MPa | / | [86] |
| Vinyltrimethoxysilane | H2C=CHSi(OCH3)3 | VTMS | / | Elongation at break: 40~50% | Thermal conductivity: 0.06 W/m·K | [87] |
| Vinyltriethoxysilane | C8H18O3Si | VTES | Surface area: 321 m2/g | Compressive stress: 0.571 MPa | Thermal conductivity: 0.024 W/m·K | [88] |
| 3-glycidoxypropyltrimethoxysilane | C9H20O5Si | GPTMS | / | / | Thermal conductivity: 0.032 W/m·K | [89] |
| Bis [3-(triethoxysilyl)propyl]disulfide | C18H42O6S2Si2 | BTSPD | Density: 0.21 g/cm3; porosity: 85.5% | Young’s modulus: 2.1 MPa | / | [90] |
| 1,6-bis(trimethoxysilyl)hexane | C12H30O6Si2 | BTMSH | / | Strain: 50% | / | [91] |
| Bis(trimethoxysilylpropyl)amine | C12H31NO6Si2 | BTMSPA | Density: 0.308 g/cm3; porosity: 78%; surface area: 325 m2/g | Shrink: 11%, compression Modulus: 15 MPa | / | [92] |
| Dimethyldiethoxysilane | C6H16O2Si | DMDES | Density: 0.082 g/cm3; surface area: 162.1 m2/g; porosity: 94.2% | / | Maximum degradation rate: 150 °C | [93] |
2.2. Aging
2.3. Drying
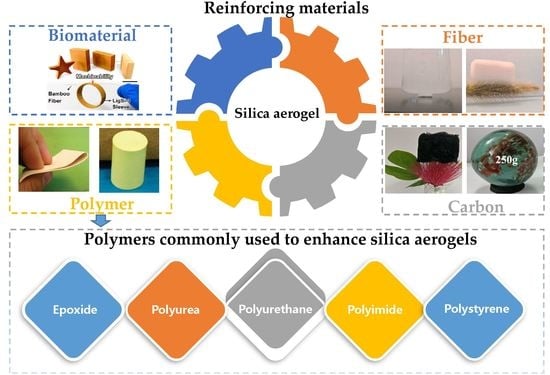
3. Polymer-Modified Silica Aerogel Composites
3.1. Epoxide
3.2. Polyurea
3.3. Polyurethane
3.4. Polyimide
3.5. Polystyrene
4. Conclusions
5. Outlook and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Cheng, X.; He, S.; Huang, D.; Bi, H.; Yang, H. Preparation of ambient pressure dried MTMS/TEOS co-precursor silica aerogel by adjusting NH4OH concentration. Mater. Lett. 2014, 129, 12–15. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A special material or a new state of matter: A review and reconsideration of the aerogel. Materials. 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.J.; Zhao, D.; Lee, G.; Liu, J. Strong, machinable carbon aerogels for high performance supercapacitors. Adv. Funct. Mater. 2016, 26, 4976–4983. [Google Scholar] [CrossRef]
- Hrubesh, L.W.; Coronado, P.R.; Satcher, J.H. Solvent removal from water with hydrophobic aerogels. J. Non-Cryst. Solids 2001, 285, 328–332. [Google Scholar] [CrossRef]
- Su, L.; Niu, M.; Lu, D.; Cai, Z.; Li, M.; Wang, H. A review on the emerging resilient and multifunctional ceramic aerogels. J. Mater. Sci. Technol. 2021, 75, 1–13. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, P.; Song, L.; Li, J.; Ji, B.; Li, J.; Chen, L. Polyethylene glycol supported by phosphorylated polyvinyl alcohol/graphene aerogel as a high thermal stability phase change material. Compos. Part B Eng. 2019, 179, 107545. [Google Scholar] [CrossRef]
- Yan, M.; Pan, Y.; Cheng, X.; Zhang, Z.; Deng, Y.; Lun, Z.; Gong, L.; Gao, M.; Zhang, H. “Robust–Soft” anisotropic nanofibrillated cellulose aerogels with superior mechanical, flame-retardant, and thermal insulating properties. ACS Appl. Mater. Interfaces 2021, 13, 27458–27470. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J.; Riffat, S.B. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sustain. Energy Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Wa, L.; Fengyun, L.; Fanlu, Z.; Mengjing, C.; Qiang, C.; Jue, H.; Weijun, Z.; Mingwei, M. Preparation of silica aerogels using CTAB/SDS as template and their efficient adsorption. Appl. Surf. Sci. 2015, 353, 1031–1036. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef]
- Long, D.; Chen, Q.; Qiao, W.; Zhan, L.; Liang, X.; Ling, L. Three-dimensional mesoporous carbon aerogels: Ideal catalyst supports for enhanced H2S oxidation. Chem. Commun. 2009, 3898–3900. [Google Scholar] [CrossRef]
- Lee, J.K.; Gould, G.L.; Rhine, W. Polyurea based aerogel for a high performance thermal insulation material. J. Sol-Gel Sci. Technol. 2009, 49, 209–220. [Google Scholar] [CrossRef]
- Hou, X.; Mao, Y.; Zhang, R.; Fang, D. Super-flexible polyimide nanofiber cross-linked polyimide aerogel membranes for high efficient flexible thermal protection. Chem. Eng. J. 2021, 417, 129341. [Google Scholar] [CrossRef]
- Merillas, B.; Villafañe, F.; Rodríguez-Pérez, M.Á. Improving the insulating capacity of polyurethane foams through polyurethane aerogel inclusion: From insulation to superinsulation. Nanomaterials 2022, 12, 2232. [Google Scholar] [CrossRef]
- Kulkarni, A.; Jana, S.C. Surfactant-free syndiotactic polystyrene aerogel foams via pickering emulsion. Polymer 2021, 212, 123125. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Shen, J. Metal oxide aerogels for high-temperature applications. J. Sol-Gel Sci. Technol. 2023, 106, 360–380. [Google Scholar] [CrossRef]
- Liao, J.; Liu, P.; Xie, Y.; Zhang, Y. Metal oxide aerogels: Preparation and application for the uranium removal from aqueous solution. Sci. Total Environ. 2021, 768, 144212. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wen, D. Recent advances of noble metal aerogels in biosensing. View 2021, 2, 20200124. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Paninho, A.B.; Mustapa, A.N.; Mahmudov, K.T.; Pombeiro, A.J.L.; Guedes Da Silva, M.F.C.; Bermejo, M.D.; Martín, Á.; Cocero, M.J.; Nunes, A.V.M. A bio-based alginate aerogel as an ionic liquid support for the efficient synthesis of cyclic carbonates from CO2 and epoxides. Catalysts 2021, 11, 872. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, M.; Silvennoinen, R.J.; Houbenov, N.; Nykänen, A.; Ruokolainen, J.; Sainio, J.; Pore, V.; Kemell, M.; Ankerfors, M.; Lindström, T.; et al. Photoswitchable superabsorbency based on nanocellulose aerogels. Adv. Funct. Mater. 2011, 21, 510–517. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Chan, C.H.; Zakaria, S.; Kaco, H.; Chook, S.W.; Chin, S.X.; Noor, A.A.M. Bifunctional graphene oxide–cellulose nanofibril aerogel loaded with Fe(iii) for the removal of cationic dye via simultaneous adsorption and fenton oxidation. Rsc Adv. 2016, 6, 19819–19825. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Ren, J.; Feng, J.; Wang, L.; Chen, G.; Zhou, Z.; Li, Q. High specific surface area hybrid silica aerogel containing POSS. Microporous Mesoporous Mater. 2021, 310, 110456. [Google Scholar] [CrossRef]
- Schultz, J.M.; Jensen, K.I.; Kristiansen, F.H. Super insulating aerogel glazing. Sol. Energy Mater. Sol. Cells 2005, 89, 275–285. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica aerogel: Synthesis and applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Worsley, M.A.; Maboudian, R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded-Aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Y.; Hu, Z.; Li, L.; Fan, X. Preparation and characterization of granular silica aerogel/polyisocyanurate rigid foam composites. Constr. Build. Mater. 2015, 93, 309–316. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Bheekhun, N.; Abu Talib, A.R.; Hassan, M.R. Aerogels in aerospace: An overview. Adv. Mater. Sci. Eng. 2013, 2013, 406065. [Google Scholar] [CrossRef]
- Jones, S.M. Aerogel: Space exploration applications. J. Sol-Gel Sci. Technol. 2006, 40, 351–357. [Google Scholar] [CrossRef]
- Ochoa, M.; Durães, L.; Beja, A.M.; Portugal, A. Study of the suitability of silica based xerogels synthesized using ethyltrimethoxysilane and/or methyltrimethoxysilane precursors for aerospace applications. J. Sol-Gel Sci. Technol. 2012, 61, 151–160. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moghaddas, J. Synthesis, adsorption and regeneration of nanoporous silica aerogel and silica aerogel-activated carbon composites. Chem. Eng. Res. Des. 2015, 94, 475–484. [Google Scholar] [CrossRef]
- Perdigoto, M.L.N.; Martins, R.C.; Rocha, N.; Quina, M.J.; Gando-Ferreira, L.; Patrício, R.; Durães, L. Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J. Colloid Interface Sci. 2012, 380, 134–140. [Google Scholar] [CrossRef]
- He, S.; Du, C.; Sheng, H.; He, C.; Liu, X.; Jin, X.; Chen, Q.; Tian, F. Ultrasensitive and self-powered multiparameter pressure–temperature–humidity sensor based on ultra-flexible conductive silica aerogel. Gels 2023, 9, 162. [Google Scholar] [CrossRef]
- Feng, X.; Wang, L.; Yao, X.; Dong, H.; Wang, X.; Wang, Y. Trace water/amino-modified silica aerogel catalytic system in the one-pot sequential reaction of benzaldehyde dimethyl acetal and nitromethane. Catal. Commun. 2017, 90, 106–110. [Google Scholar] [CrossRef]
- Ward, D.A.; Ko, E.I. Cheminform abstract: Preparing catalytic materials by the sol-gel method. Cheminform 1995, 26, 421–433. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Shang, L.; Lyu, Y.; Han, W. Microstructure and thermal insulation property of silica composite aerogel. Materials 2019, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Madyan, O.A.; Fan, M.; Feo, L.; Hui, D. Physical properties of clay aerogel composites: An overview. Compos. Part B Eng. 2016, 102, 29–37. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.-M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Bertino, M.F.; Hund, J.F.; Zhang, G.; Sotiriou-Leventis, C.; Tokuhiro, A.T.; Leventis, N. Room temperature synthesis of noble metal clusters in the mesopores of mechanically strong silica-polymer aerogel composites. J. Sol-Gel Sci. Technol. 2004, 30, 43–48. [Google Scholar] [CrossRef]
- Ma, H.-S.; Roberts, A.P.; Prévost, J.-H.; Jullien, R.; Scherer, G.W. Mechanicalstructure-propertyrelationshipofaerogels. J. Non-Cryst. Solids 2000, 277, 127–141. [Google Scholar] [CrossRef]
- Meti, P.; Mahadik, D.B.; Lee, K.-Y.; Wang, Q.; Kanamori, K.; Gong, Y.-D.; Park, H.-H. Overview of organic–inorganic hybrid silica aerogels: Progress and perspectives. Mater. Des. 2022, 222, 111091. [Google Scholar] [CrossRef]
- Parmar, K.R.; Dora, D.T.K.; Pant, K.K.; Roy, S. An ultra-light flexible aerogel-based on methane derived CNTs as a reinforcing agent in silica-CMC matrix for efficient oil adsorption. J. Hazard. Mater. 2019, 375, 206–215. [Google Scholar] [CrossRef]
- Yi, Z.; Zhang, X.; Yan, L.; Huyan, X.; Zhang, T.; Liu, S.; Guo, A.; Liu, J.; Hou, F. Super-insulated, flexible, and high resilient mullite fiber reinforced silica aerogel composites by interfacial modification with nanoscale mullite whisker. Compos. Part B Eng. 2022, 230, 109549. [Google Scholar] [CrossRef]
- Guo, H.; Meador, M.A.B.; Mccorkle, L.; Quade, D.J.; Guo, J.; Hamilton, B.; Cakmak, M.; Sprowl, G. Polyimide aerogels cross-linked through amine functionalized polyoligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2011, 3, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Ou, R.; Hao, X.; Deng, Q.; Liu, Z.; Sun, L.; Zhang, C.; Guo, C.; Bai, X.; Wang, Q. Water-induced self-assembly and in situ mineralization within plant phenolic glycol-gel toward ultrastrong and multifunctional thermal insulating aerogels. Acs Nano 2022, 16, 9062–9076. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Keefer, K.D.; Schaefer, D.W.; Ashley, C.S. Sol-gel transition in simple silicates. J. Non-Cryst. Solids 1982, 48, 47–64. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Man, Y.; Peng, Z.; Zhang, X.; Luo, X. A three-dimensional mullite-whisker network ceramic with ultra-light weight and high-strength prepared by the foam-gelcasting method. J. Asian Ceram. Soc. 2020, 8, 387–395. [Google Scholar] [CrossRef]
- Borzęcka, N.H.; Nowak, B.; Gac, J.M.; Głaz, T.; Bojarska, M. Kinetics of mtms-based aerogel formation by the sol-gel method—experimental results and theoretical description. J. Non-Cryst. Solids 2020, 547, 120310. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Gurav, J.L. Effect of protic solvents on the physical properties of the ambient pressure dried hydrophobic silica aerogels using sodium silicate precursor. J. Porous Mater. 2008, 15, 507–512. [Google Scholar] [CrossRef]
- Xia, B.; Wang, Z.; Gou, L.; Zhang, M.; Guo, M. Porous mullite ceramics with enhanced compressive strength from fly ash-based ceramic microspheres: Facile synthesis, structure, and performance. Ceram. Int. 2022, 48, 10472–10479. [Google Scholar] [CrossRef]
- Rao, A.V.; Kulkarni, M.M.; Amalnerkar, D.P.; Seth, T. Superhydrophobic silica aerogels based on methyltrimethoxysilane precursor. J. Non-Cryst. Solids 2003, 330, 187–195. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels-promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Zarzycki, J. Past and present of sol-gel science and technology. J. Sol-Gel Sci. Technol. 1997, 8, 17–22. [Google Scholar] [CrossRef]
- Alié, C.; Ferauche, F.; Pirard, R.; Lecloux, A.J.; Pirard, J.-P. Preparation of low-density xerogels by incorporation of additives during synthesis. J. Non-Cryst. Solids 2001, 289, 88–96. [Google Scholar] [CrossRef]
- Wu, G.; Wang, J.; Shen, J.; Yang, T.; Zhang, Q.; Zhou, B.; Deng, Z.; Bin, F.; Zhou, D.; Zhang, F. Properties of sol-gel derived scratch-resistant nano-porous silica films by a mixed atmosphere treatment. J. Non-Cryst. Solids 2000, 275, 169–174. [Google Scholar] [CrossRef]
- Venkateswara Rao, A.; Bhagat, S.D. Synthesis and physical properties of TEOS-based silica aerogels prepared by two step (acid–base) sol–gel process. Solid State Sci. 2004, 6, 945–952. [Google Scholar] [CrossRef]
- Kirkbir, F.; Murata, H.; Meyers, D.; Chaudhuri, S.R.; Sarkar, A. Drying and sintering of sol-gel derived large SiO2 monoliths. J. Sol-Gel Sci. Technol. 1996, 6, 203–217. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Applications of pore-expanded mesoporous silicas. 3. Triamine silane grafting for enhanced co2 adsorption. Ind. Eng. Chem. Res. 2006, 45, 3248–3255. [Google Scholar] [CrossRef]
- Araki, S.; Doi, H.; Sano, Y.; Tanaka, S.; Miyake, Y. Preparation and CO2 adsorption properties of aminopropyl-functionalized mesoporous silica microspheres. J. Colloid Interface Sci. 2009, 339, 382–389. [Google Scholar] [CrossRef]
- Lee, C.J.; Kim, G.S.; Hyun, S.H. Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 2002, 37, 2237–2241. [Google Scholar] [CrossRef]
- Siouffi, A.-M. Silica gel-based monoliths prepared by the sol-gel method: Facts and figures. J. Chromatogr. A 2003, 1000, 801–818. [Google Scholar] [CrossRef]
- Li, Q.; Afeworki, M.; Callen, N.M.; Colby, R.J.; Gopinadhan, M.; Nines Kochersperger, M.L.; Peterson, B.K.; Sansone, M.; Weston, S.C.; Calabro, D.C. Template-free self-assembly of mesoporous organosilicas. Chem. Mater. 2018, 30, 2218–2228. [Google Scholar] [CrossRef]
- Nakanishi, K.; Minakuchi, H.; Soga, N.; Tanaka, N. Double pore silica gel monolith applied to liquid chromatography. J. Sol-Gel Sci. Technol. 1997, 8, 547–552. [Google Scholar] [CrossRef]
- Tamon, H.; Kitamura, T.; Okazaki, M. Preparation of silica aerogel from TEOS. J. Colloid Interface Sci. 1998, 197, 353–359. [Google Scholar] [CrossRef]
- Wong, J.C.H.; Kaymak, H.; Brunner, S.; Koebel, M.M. Mechanical properties of monolithic silica aerogels made from polyethoxydisiloxanes. Microporous Mesoporous Mater. 2014, 183, 23–29. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Tousley, M.E.; Shonkwiler, B.; Mccorkle, L.; Scheiman, D.A.; Palczer, A. Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Latthe, S.S.; Hirashima, H.; Rao, A.V. Studies on rheological properties of methyltriethoxysilane (MTES) based flexible superhydrophobic silica aerogels. Microporous Mesoporous Mater. 2009, 117, 617–626. [Google Scholar] [CrossRef]
- Bhagat, S.D.; Rao, A.V. Surface chemical modification of TEOS based silica aerogels synthesized by two step (acid–base) sol–gel process. Appl. Surf. Sci. 2006, 252, 4289–4297. [Google Scholar] [CrossRef]
- Zu, G.; Kanamori, K.; Shimizu, T.; Zhu, Y.; Maeno, A.; Kaji, H.; Nakanishi, K.; Shen, J. Versatile double-cross-linking approach to transparent, machinable, supercompressible, highly bendable aerogel thermal superinsulators. Chem. Mater. 2018, 30, 2759–2770. [Google Scholar] [CrossRef]
- Hæreid, S.; Dahle, M.; Lima, S.; Einarsrud, M.-A. Preparation and properties of monolithic silica xerogels from TEOS–based alcogels aged in silane solutions. J. Non-Cryst. Solids 1995, 186, 96–103. [Google Scholar] [CrossRef]
- Tajiri, K.; Igarashi, K.; Nishio, T. Effects of supercritical drying media on structure and properties of silica aerogel. J. Non-Cryst. Solids 1995, 186, 83–87. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Mai, N.T.; Reddy, V.R.M.; Jung, J.H.; Truong, N.T.N. Synthesis of silica aerogel particles and its application to thermal insulation paint. Korean J. Chem. Eng. 2020, 37, 1803–1809. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, L.; Pan, H.; Shen, Y.; Wang, L.; Xie, M. Eco-friendly approach for preparation of water-based superhydrophobic silica aerogels via ambient pressure drying. Mater. Res. Express 2021, 8, 015021. [Google Scholar] [CrossRef]
- Shao, Z.; He, X.; Cheng, X.; Zhang, Y. A simple facile preparation of methyltriethoxysilane based flexible silica aerogel monoliths. Mater. Lett. 2017, 204, 93–96. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Malfait, W.J.; Sadeghpour, A.; Girão, A.V.; Silva, R.F.; Durães, L. Influence of 1D and 2D carbon nanostructures in silica-based aerogels. Carbon 2021, 180, 146–162. [Google Scholar] [CrossRef]
- Wu, X.; Man, J.; Liu, S.; Huang, S.; Lu, J.; Tai, J.; Zhong, Y.; Shen, X.; Cui, S.; Chen, X. Isocyanate-crosslinked silica aerogel monolith with low thermal conductivity and much enhanced mechanical properties: Fabrication and analysis of forming mechanisms. Ceram. Int. 2021, 47, 26668–26677. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Li, J.; Wu, Z.; Yang, C. Silica nanowires reinforced self-hydrophobic silica aerogel derived from crosslinking of propyltriethoxysilane and tetraethoxysilane. J. Sol-Gel Sci. Technol. 2017, 83, 545–554. [Google Scholar] [CrossRef]
- Matias, T.; Varino, C.; de Sousa, H.C.; Braga, M.E.M.; Portugal, A.; Coelho, J.F.J.; Durães, L. Novel flexible, hybrid aerogels with vinyl- and methyltrimethoxysilane in the underlying silica structure. J. Mater. Sci. 2016, 51, 6781–6792. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, H.; Li, X.; Ding, H.; Ji, H. Hyperelastic and hydrophobic silica aerogels with enhanced compressive strength by using VTES/MTMS as precursors. J. Non-Cryst. Solids 2019, 525, 119677. [Google Scholar] [CrossRef]
- He, S.; Sun, G.; Cheng, X.; Dai, H.; Chen, X. Nanoporous SiO2 grafted aramid fibers with low thermal conductivity. Compos. Sci. Technol. 2017, 146, 91–98. [Google Scholar] [CrossRef]
- Guo, H.; Nguyen, B.N.; Mccorkle, L.S.; Shonkwiler, B.; Meador, M.A.B. Elastic low density aerogels derived from bis[3-(triethoxysilyl)propyl]disulfide, tetramethylorthosilicate and vinyltrimethoxysilane via a two-step process. J. Mater. Chem. 2009, 19, 9054–9062. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Weber, A.S.; Hindi, A.; Naumenko, M.; Mccorkle, L.; Quade, D.; Vivod, S.L.; Gould, G.L.; White, S.; Deshpande, K. Structure-property relationships in porous 3d nanostructures: Epoxy-cross-linked silica aerogels produced using ethanol as the solvent. ACS Appl. Mater. Interfaces 2009, 1, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Polymer reinforced silica aerogels: Effects of dimethyldiethoxysilane and bis(trimethoxysilylpropyl) amine as silane precursors. J. Mater. Chem. A 2013, 1, 6642–6652. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Q.; Li, X.; Wang, L.; Nie, C. Facile preparation of a phenyl-reinforced flexible silica aerogel with excellent thermal stability and fire resistance. Mater. Chem. Front. 2021, 5, 4214–4224. [Google Scholar] [CrossRef]
- Estella, J.; Echeverría, J.C.; Laguna, M.; Garrido, J.J. Effects of aging and drying conditions on the structural and textural properties of silica gels. Microporous Mesoporous Mater. 2007, 102, 274–282. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Yun, S.; Wie, J.J.; Zhang, X.; Dresselhaus, M.S.; Kong, J.; Park, H.S. Cartilage-inspired superelastic ultradurable graphene aerogels prepared by the selective gluing of intersheet joints. Nanoscale 2016, 8, 12900–12909. [Google Scholar] [CrossRef]
- Einarsrud, M.-A.; Haereid, S. Preparation of transparent, monolithic silica xerogels with low density. J. Sol-Gel Sci. Technol. 1994, 2, 903–906. [Google Scholar] [CrossRef]
- Omranpour, H.; Motahari, S. Effects of processing conditions on silica aerogel during aging: Role of solvent, time and temperature. J. Non-Cryst. Solids 2013, 379, 7–11. [Google Scholar] [CrossRef]
- He, F.; Zhao, H.; Qu, X.; Zhang, C.; Qiu, W. Modified aging process for silica aerogel. J. Mater. Process. Technol. 2009, 209, 1621–1626. [Google Scholar] [CrossRef]
- Rashid, A.B.; Shishir, S.I.; Mahfuz, M.A.; Hossain, M.T.; Hoque, M.E. Silica aerogel: Synthesis, characterization, applications, and recent advancements. Part. Part. Syst. Charact. 2023, 40, 2200186. [Google Scholar] [CrossRef]
- Wu, G.-P.; Yang, J.; Wang, D.; Xu, R.; Amine, K.; Lu, C.-X. A novel route for preparing mesoporous carbon aerogels using inorganic templates under ambient drying. Mater. Lett. 2014, 115, 1–4. [Google Scholar] [CrossRef]
- Leventis, N.; Palczer, A.; Mccorkle, L.; Zhang, G.; Sotiriou-Leventis, C. Nanoengineered silica-polymer composite aerogels with no need for supercritical fluid drying. J. Sol-Gel Sci. Technol. 2005, 35, 99–105. [Google Scholar] [CrossRef]
- Chen, H.-B.; Hollinger, E.; Wang, Y.-Z.; Schiraldi, D.A. Facile fabrication of poly(vinyl alcohol) gels and derivative aerogels. Polymer 2014, 55, 380–384. [Google Scholar] [CrossRef]
- Chen, H.-B.; Liu, B.; Huang, W.; Wang, J.-S.; Zeng, G.; Wu, W.-H.; Schiraldi, D.A. Fabrication and properties of irradiation-cross-linked poly(vinyl alcohol)/clay aerogel composites. ACS Appl. Mater. Interfaces 2014, 6, 16227–16236. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, T. Preparation of silica aerogel from rice hull ash by supercritical carbon dioxide drying. J. Supercrit. Fluids 2005, 35, 91–94. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Development of mechanically strong ambient pressure dried silica aerogels with optimized properties. J. Phys. Chem. C 2015, 119, 7689–7703. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/carbon-based hybrid aerogels: Preparation, properties and applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, J.; Lü, X.; Jiang, D. Synthesis and characterization of superhydrophobic silica and silica/titania aerogels by sol–gel method at ambient pressure. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 97–101. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Barełkowski, M.; Niemier, S.; Jakubowska, P. Synthesis and characterisation of silica aerogel/carbon microfibers nanocomposites dried in supercritical and ambient pressure conditions. J. Sol-Gel Sci. Technol. 2015, 76, 227–232. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of silica aerogel by supercritical drying method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Alemán, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; Mccorkle, L. Polyimide aerogels with amide cross-links: A low cost alternative for mechanically strong polymer aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef]
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, M. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. Part B Eng. 2011, 42, 856–873. [Google Scholar] [CrossRef]
- Boday, D.J.; Stover, R.J.; Muriithi, B.; Keller, M.W.; Wertz, J.T.; Defriend Obrey, K.A.; Loy, D.A. Strong, low-density nanocomposites by chemical vapor deposition and polymerization of cyanoacrylates on aminated silica aerogels. ACS Appl. Mater. Interfaces 2009, 1, 1364–1369. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhao, Y.; Zhang, X. Superhydrophobic polyimide aerogels via conformal coating strategy with excellent underwater performances. J. Appl. Polym. Sci. 2020, 137, 48849. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, J.-U.; Lee, H.; Kim, S.Y.; Kim, S.H.; Kim, J.; Jung, Y.C.; Yang, B.J. Thermal management in polymer composites: A review of physical and structural parameters. Adv. Eng. Mater. 2018, 20, 1800204. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Jung, H.-N.; Han, W.; Cho, H.H.; Park, H.-H. Flexible, elastic, and superhydrophobic silica-polymer composite aerogels by high internal phase emulsion process. Compos. Sci. Technol. 2017, 147, 45–51. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. A new trend for development of mechanically robust hybrid silica aerogels. Mater. Lett. 2016, 179, 206–209. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Lu, S.-Y.; Chang, Y.-C. Transparent, hydrophobic composite aerogels with high mechanical strength and low high-temperature thermal conductivities. J. Phys. Chem. B 2008, 112, 11881–11886. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Vivod, S.L.; Mccorkle, L.; Quade, D.; Sullivan, R.M.; Ghosn, L.J.; Clark, N.; Capadona, L.A. Reinforcing polymer cross-linked aerogels with carbon nanofibers. J. Mater. Chem. 2008, 18, 1843–1852. [Google Scholar] [CrossRef]
- Kim, M.; Eo, K.; Lim, H.J.; Kwon, Y.K. Low shrinkage, mechanically strong polyimide hybrid aerogels containing hollow mesoporous silica nanospheres. Compos. Sci. Technol. 2018, 165, 355–361. [Google Scholar] [CrossRef]
- Novak, B.M.; Auerbach, D.; Verrier, C. Low-density, mutually interpenetrating organic-inorganic composite materials via supercritical drying techniques. Chem. Mater. 1994, 6, 282–286. [Google Scholar] [CrossRef]
- Leventis, N.; Sadekar, A.; Chandrasekaran, N.; Sotiriou-Leventis, C. Click synthesis of monolithic silicon carbide aerogels from polyacrylonitrile-coated 3d silica networks. Chem. Mater. 2010, 22, 2790–2803. [Google Scholar] [CrossRef]
- Guise, M.T.; Hosticka, B.; Earp, B.C.; Norris, P.M. An experimental investigation of aerosol collection utilizing packed beds of silica aerogel microspheres. J. Non-Cryst. Solids 2001, 285, 317–322. [Google Scholar] [CrossRef]
- Boday, D.J.; Keng, P.Y.; Muriithi, B.; Pyun, J.; Loy, D.A. Mechanically reinforced silica aerogel nanocomposites via surface initiated atom transfer radical polymerizations. J. Mater. Chem. 2010, 20, 6863–6865. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Capadona, L.A.; Mccorkle, L.; Papadopoulos, D.S.; Leventis, N. Structure-property relationships in porous 3d nanostructures as a function of preparation conditions: isocyanate cross-linked silica aerogels. Chem. Mater. 2007, 19, 2247–2260. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring mechanical properties of aerogels for aerospace applications. ACS Appl. Mater. Interfaces 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Rezaei, S.; Jalali, A.; Zolali, A.M.; Alshrah, M.; Karamikamkar, S.; Park, C.B. Robust, ultra-insulative and transparent polyethylene-based hybrid silica aerogel with a novel non-particulate structure. J. Colloid Interface Sci. 2019, 548, 206–216. [Google Scholar] [CrossRef]
- Lenarda, M.; Chessa, G.; Moretti, E.; Polizzi, S.; Storaro, L.; Talon, A. Toward the preparation of a nanocomposite material through surface initiated controlled/“living” radical polymerization of styrene inside the channels of mcm-41 silica. J. Mater. Sci. 2006, 41, 6305–6312. [Google Scholar] [CrossRef]
- Rezaei, S.; Zolali, A.M.; Jalali, A.; Park, C.B. Novel and simple design of nanostructured, super-insulative and flexible hybrid silica aerogel with a new macromolecular polyether-based precursor. J. Colloid Interface Sci. 2020, 561, 890–901. [Google Scholar] [CrossRef]
- Salimian, S.; Malfait, W.J.; Zadhoush, A.; Talebi, Z.; Naeimirad, M. Fabrication and evaluation of silica aerogel-epoxy nanocomposites: Fracture and toughening mechanisms. Theor. Appl. Fract. Mech. 2018, 97, 156–164. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Talebi, Z.; Fischer, B.; Winiger, P.; Winnefeld, F.; Zhao, S.; Barbezat, M.; Koebel, M.M.; Malfait, W.J. Silica aerogel-epoxy nanocomposites: Understanding epoxy reinforcement in terms of aerogel surface chemistry and epoxy–silica interface compatibility. ACS Appl. Nano Mater. 2018, 1, 4179–4189. [Google Scholar] [CrossRef]
- Albooyeh, A.; Bayat, M.; Rafieian, P.; Dadrasi, A.; Khatibi, M.M. Silica aerogel/epoxy nanocomposites: Mechanical, vibrational, and morphological properties. J. Appl. Polym. Sci. 2020, 137, 49338. [Google Scholar] [CrossRef]
- Domènech, B.; Mata, I.; Molins, E. Tuning the structure and the mechanical properties of epoxy-silica sol-gel hybrid materials. RSC Adv. 2016, 6, 10736–10742. [Google Scholar] [CrossRef]
- Çok, S.S.; Ünal, H.Y.; Koç, F.; Pekbey, Y.; Gizli, N. Ionic liquid functionalized silica aerogels as reinforcing agents for epoxy nanocomposites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2445–2458. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Su, D.; Ji, H.; Qiao, Y. High-strength mullite fibers reinforced ZrO2–SiO2 aerogels fabricated by rapid gel method. J. Mater. Sci. 2015, 50, 7488–7494. [Google Scholar] [CrossRef]
- Selver, E.; Öztaş, B.; Uçar, M.; Uçar, N.; Baydoğan, M.; Altay, P.; Geygel, B. Mechanical and thermal properties of glass/epoxy composites filled with silica aerogels. Plast. Rubber Compos. 2021, 50, 371–383. [Google Scholar] [CrossRef]
- Churu, G.; Zupančič, B.; Mohite, D.; Wisner, C.; Luo, H.; Emri, I.; Sotiriou-Leventis, C.; Leventis, N.; Lu, H. Synthesis and mechanical characterization of mechanically strong, polyurea-crosslinked, ordered mesoporous silica aerogels. J. Sol-Gel Sci. Technol. 2015, 75, 98–123. [Google Scholar] [CrossRef]
- Capadona, L.A.; Meador, M.A.B.; Alunni, A.; Fabrizio, E.F.; Vassilaras, P.; Leventis, N. Flexible, low-density polymer crosslinked silica aerogels. Polymer 2006, 47, 5754–5761. [Google Scholar] [CrossRef]
- Yang, H.; Kong, X.; Zhang, Y.; Wu, C.; Cao, E. Mechanical properties of polymer-modified silica aerogels dried under ambient pressure. J. Non-Cryst. Solids 2011, 357, 3447–3453. [Google Scholar] [CrossRef]
- Verdolotti, L.; Oliviero, M.; Lavorgna, M.; Santillo, C.; Tallia, F.; Iannace, S.; Chen, S.; Jones, J.R. “Aerogel-like” polysiloxane-polyurethane hybrid foams with enhanced mechanical and thermal-insulating properties. Compos. Sci. Technol. 2021, 213, 108917. [Google Scholar] [CrossRef]
- Cho, J.; Jang, H.G.; Kim, S.Y.; Yang, B. Flexible and coatable insulating silica aerogel/polyurethane composites via soft segment control. Compos. Sci. Technol. 2019, 171, 244–251. [Google Scholar] [CrossRef]
- Duan, Y.; Jana, S.C.; Lama, B.; Espe, M.P. Reinforcement of silica aerogels using silane-end-capped polyurethanes. Langmuir 2013, 29, 6156–6165. [Google Scholar] [CrossRef] [PubMed]
- Merillas, B.; Lamy-Mendes, A.; Villafañe, F.; Durães, L.; Rodríguez-Pérez, M.Á. Silica-based aerogel composites reinforced with reticulated polyurethane foams: Thermal and mechanical properties. Gels 2022, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yang, Y.; Xue, T.; Chao, G.; Fan, W.; Liu, T. Highly flexible and compressible polyimide/silica aerogels with integrated double network for thermal insulation and fire-retardancy. J. Mater. Sci. Technol. 2022, 105, 194–202. [Google Scholar] [CrossRef]
- Kantor, Z.; Wu, T.; Zeng, Z.; Gaan, S.; Lehner, S.; Jovic, M.; Bonnin, A.; Pan, Z.; Mazrouei-Sebdani, Z.; Opris, D.M.; et al. Heterogeneous silica-polyimide aerogel-in-aerogel nanocomposites. Chem. Eng. J. 2022, 443, 136401. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, Z.; Chen, G.; Li, K. Preparation of tetraethoxysilane-based silica aerogels with polyimide cross-linking from 3, 3′, 4, 4′-biphenyltetracarboxylic dianhydride and 4, 4′-oxydianiline. J. Sol-Gel Sci. Technol. 2018, 85, 506–513. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, Z.; Chen, G.; Li, K.; Zhao, S.; Su, G. Preparation and characterization of glass fiber/polyimide/SiO2 composite aerogels with high specific surface area. J. Mater. Sci. 2018, 53, 12885–12893. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, X.; Li, C.; You, B.; Sun, G. Co-gel strategy for preparing hierarchically porous silica/polyimide nanocomposite aerogel with thermal insulation and flame retardancy. J. Mater. Chem. A 2020, 8, 9701–9712. [Google Scholar] [CrossRef]
- Faysal Ilhan, U.; Fabrizio, E.F.; Mccorkle, L.; Scheiman, D.A.; Dass, A.; Palczer, A.; Meador, M.B.; Johnston, J.C.; Leventis, N. Hydrophobic monolithic aerogels by nanocasting polystyrene on amine-modified silica. J. Mater. Chem. 2006, 16, 3046–3054. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. Synthesis of mechanically reinforced silica aerogels via surface-initiated reversible addition-fragmentation chain transfer (raft) polymerization. J. Mater. Chem. A 2015, 3, 1594–1600. [Google Scholar] [CrossRef]
- Defriend, K.A.; Espinoza, B.; Patterson, B. Templating silica aerogel with polystyrene to improve their mechanical properties. Fusion Sci. Technol. 2007, 51, 693–700. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Zhang, G.; Vassilaras, P.; Johnston, J.C.; Leventis, N. Cross-linking amine-modified silica aerogels with epoxies: Mechanically strong lightweight porous materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef]
- Fu, B.; Luo, H.; Wang, F.; Churu, G.; Chu, K.T.; Hanan, J.C.; Sotiriou-Leventis, C.; Leventis, N.; Lu, H. Simulation of the microstructural evolution of a polymer crosslinked templated silica aerogel under high-strain-rate compression. J. Non-Cryst. Solids 2011, 357, 2063–2074. [Google Scholar] [CrossRef]
- Li, M.-E.; Wang, S.-X.; Han, L.-X.; Yuan, W.-J.; Cheng, J.-B.; Zhang, A.-N.; Zhao, H.-B.; Wang, Y.-Z. Hierarchically porous sio2/polyurethane foam composites towards excellent thermal insulating, flame-retardant and smoke-suppressant performances. J. Hazard. Mater. 2019, 375, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cimavilla-Román, P.; Pérez-Tamarit, S.; Santiago-Calvo, M.; Rodríguez-Pérez, M.Á. Influence of silica aerogel particles on the foaming process and cellular structure of rigid polyurethane foams. Eur. Polym. J. 2020, 135, 109884. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhao, Y.; Zhang, X. Template-free self-assembly of fluorine-free hydrophobic polyimide aerogels with lotus or petal effect. ACS Appl. Mater. Interfaces 2018, 10, 16901–16910. [Google Scholar] [CrossRef]
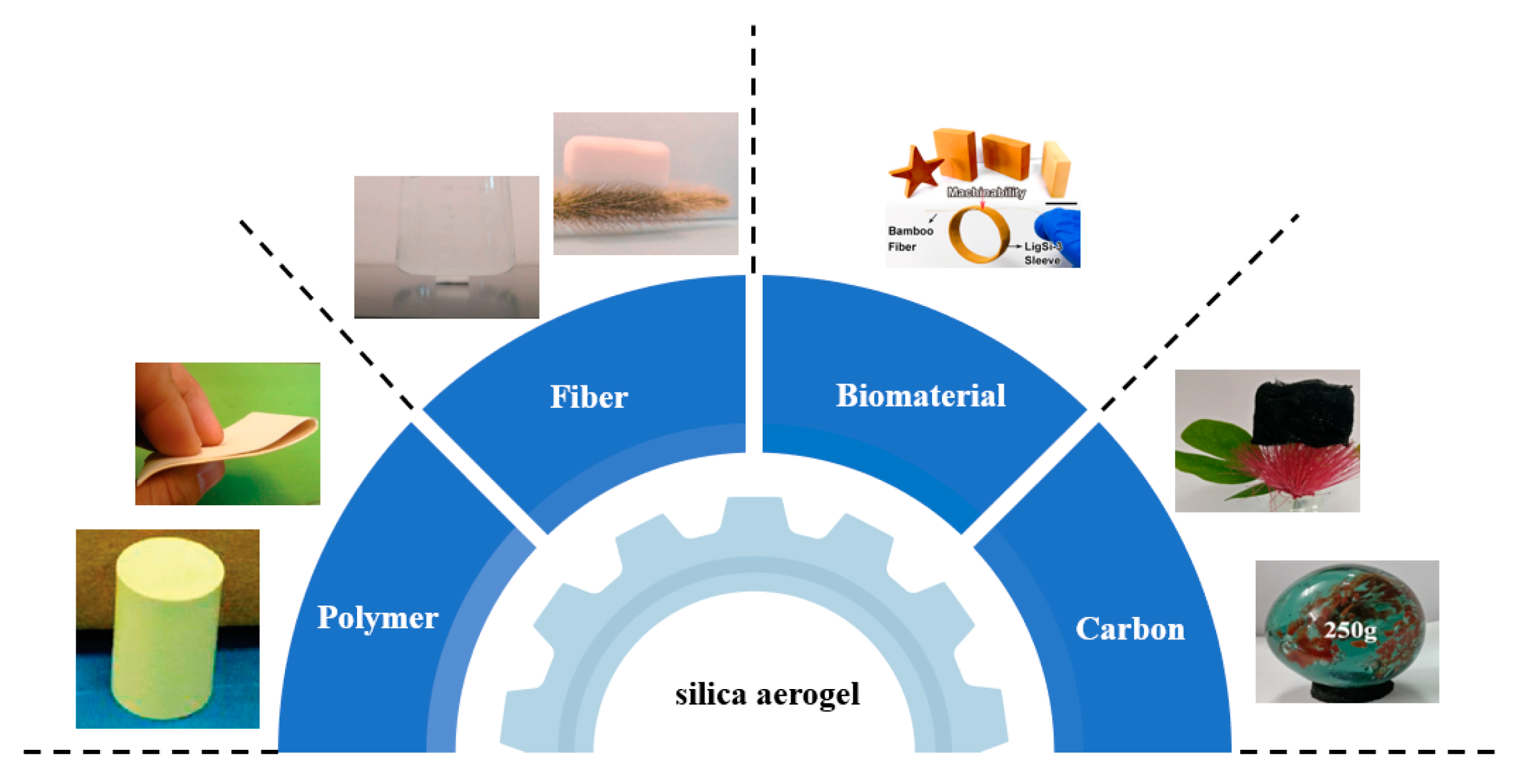

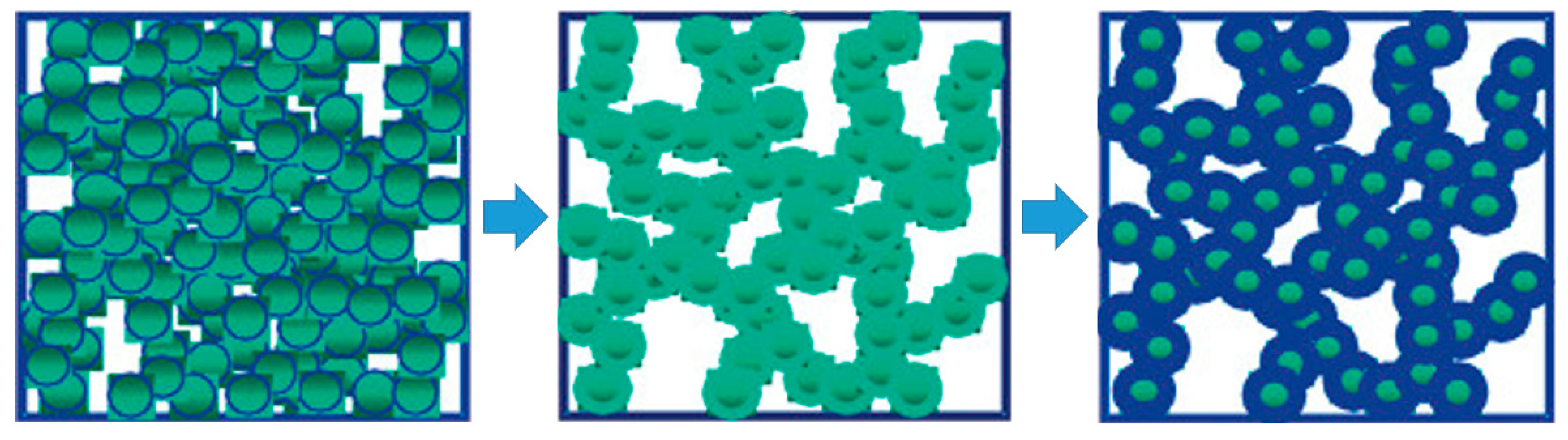
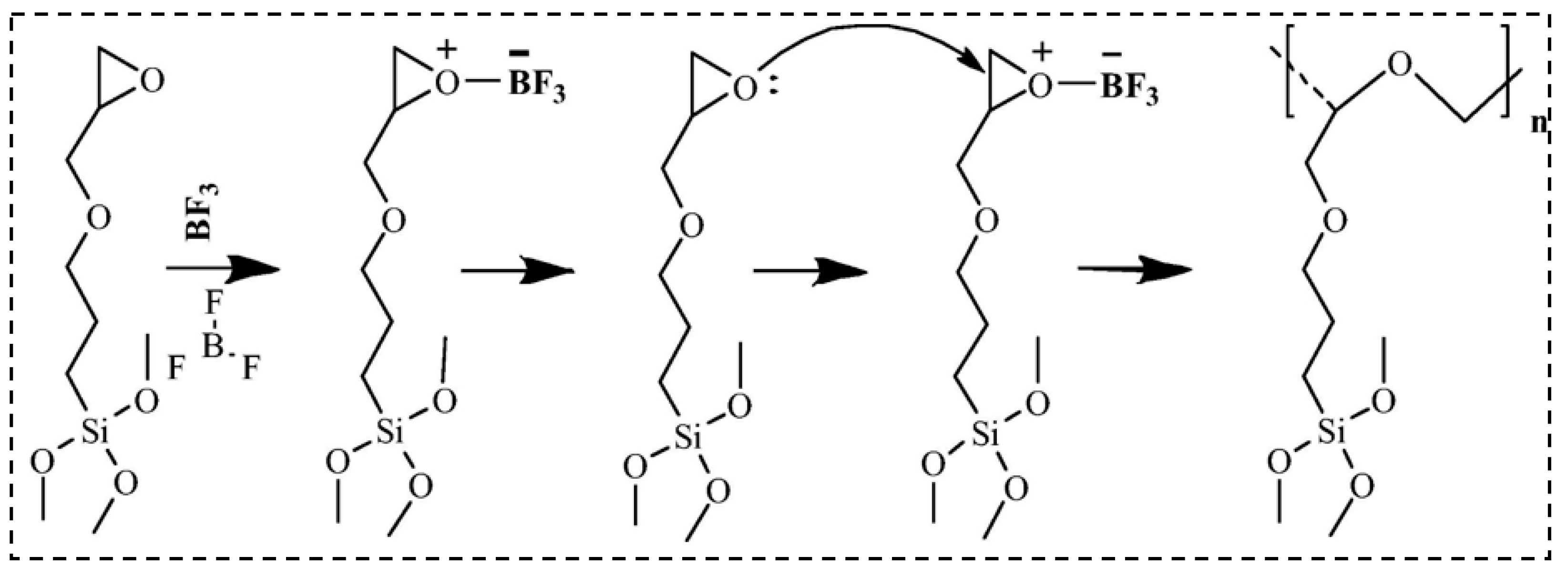
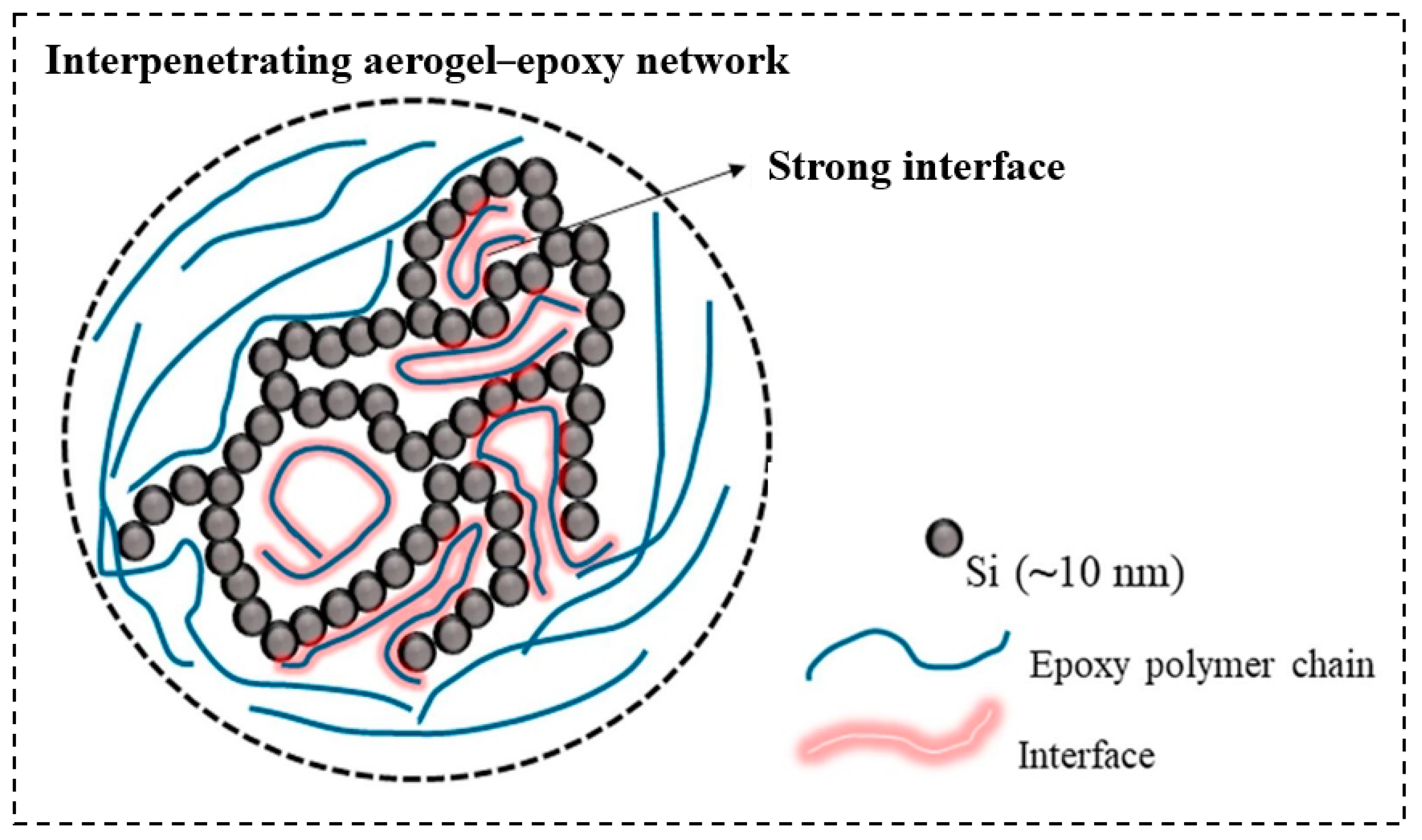
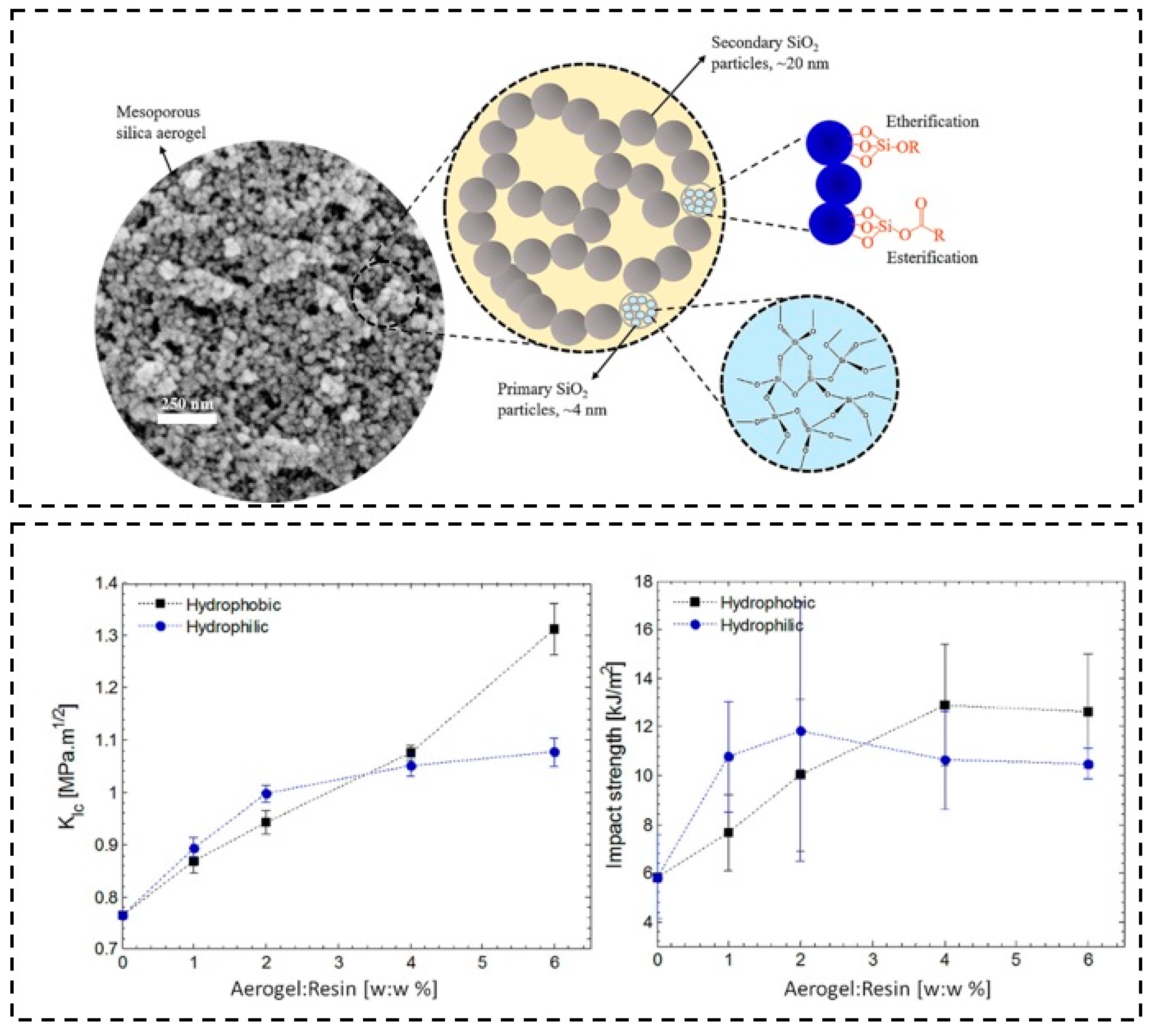
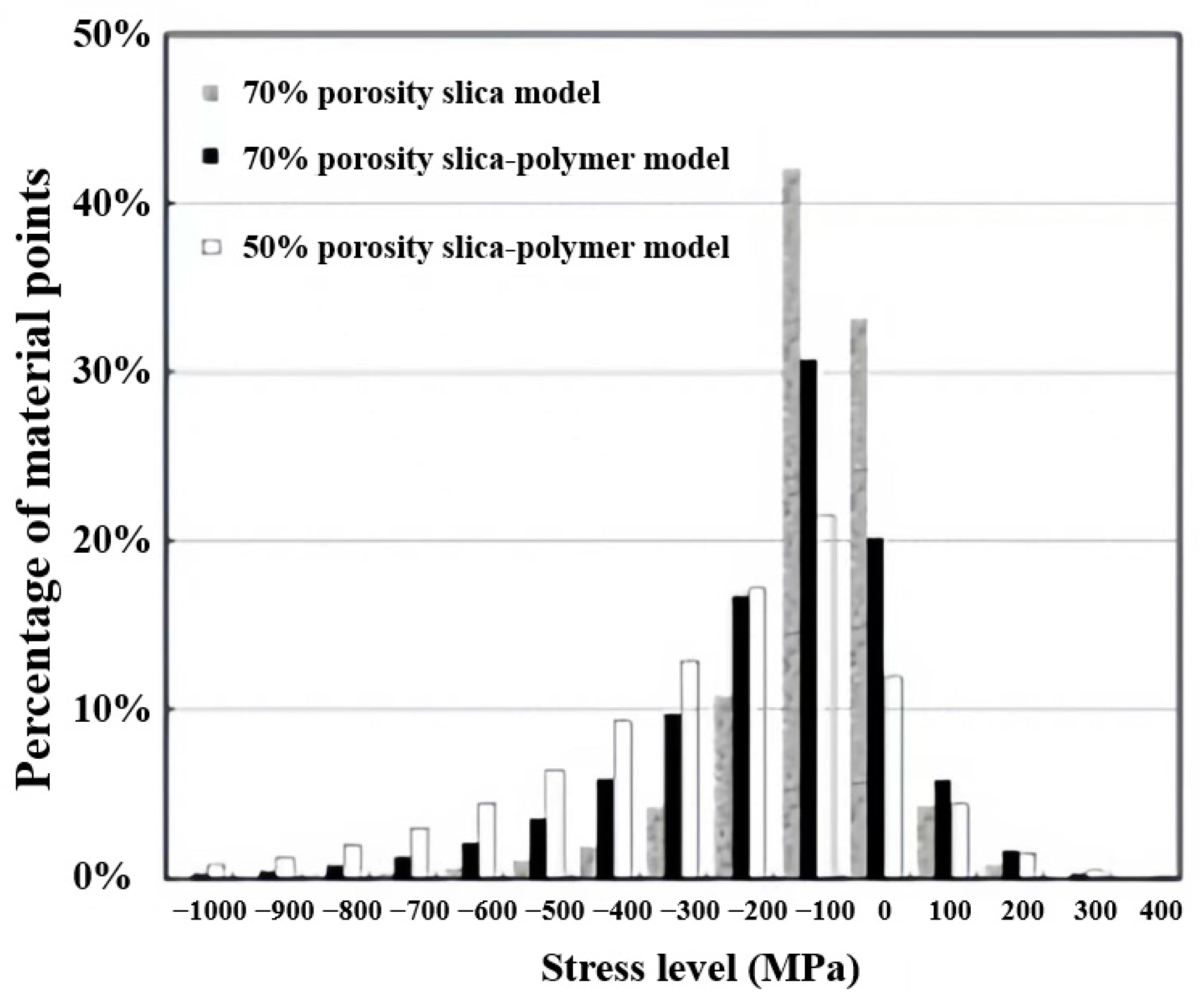
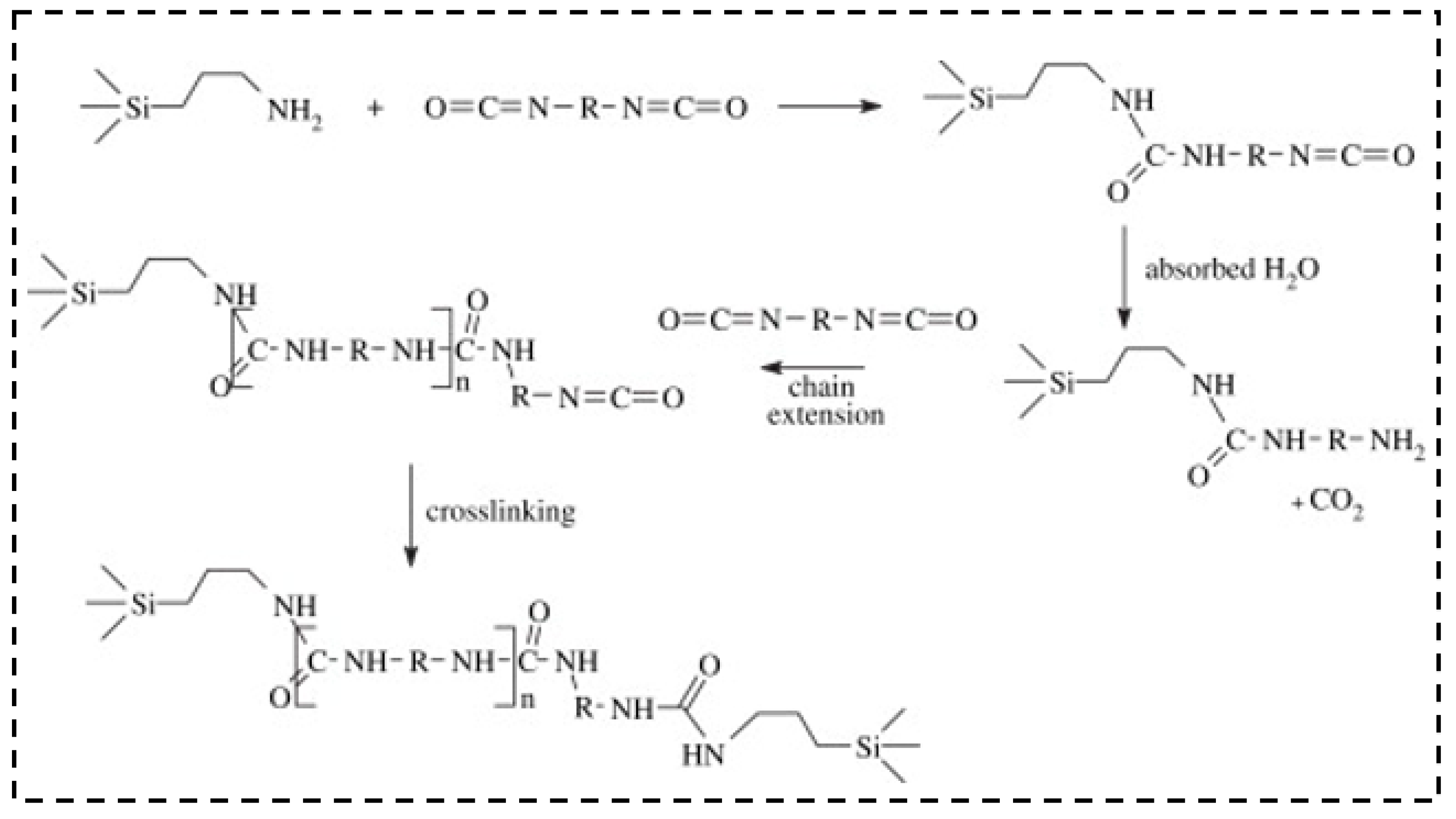
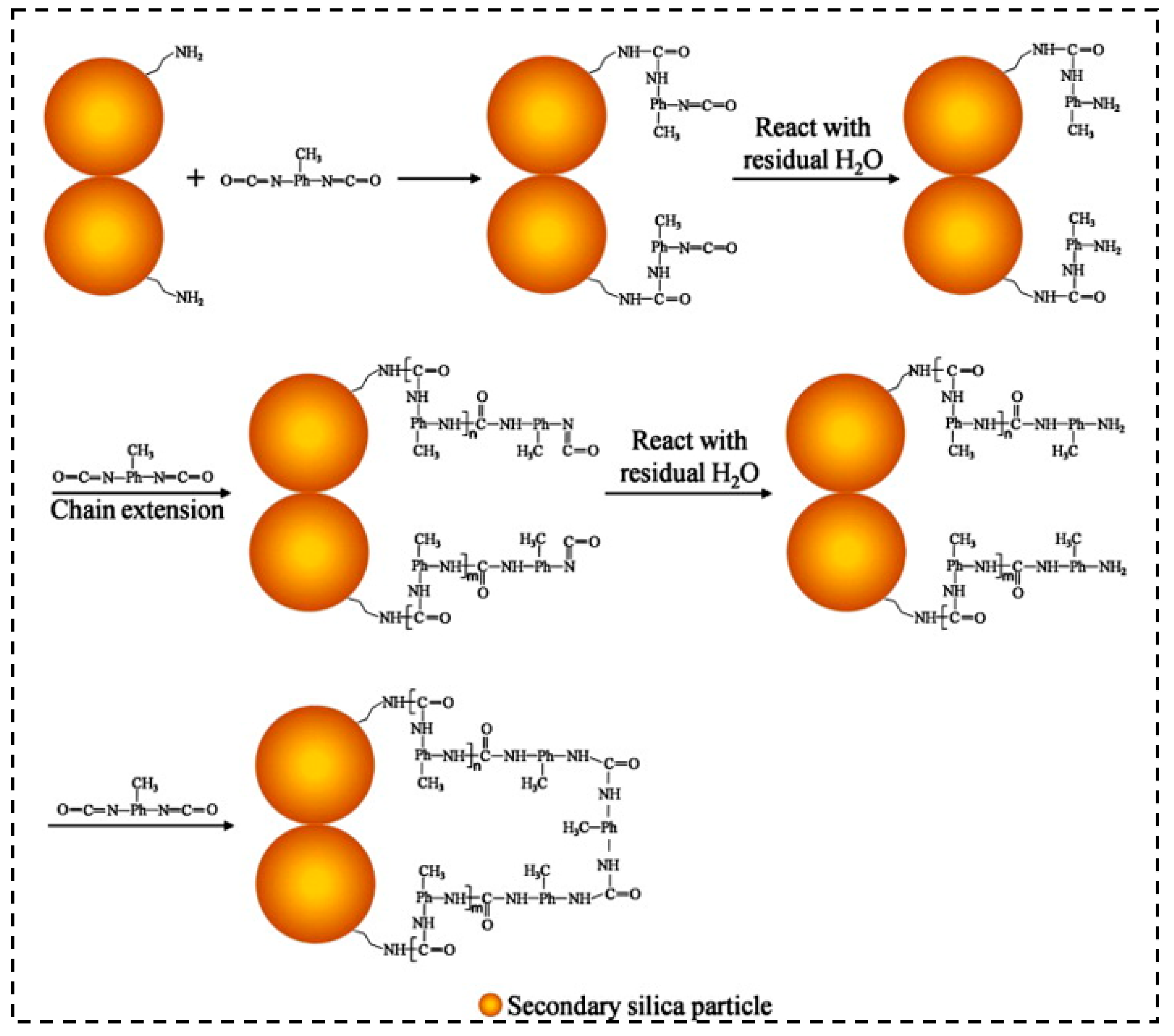
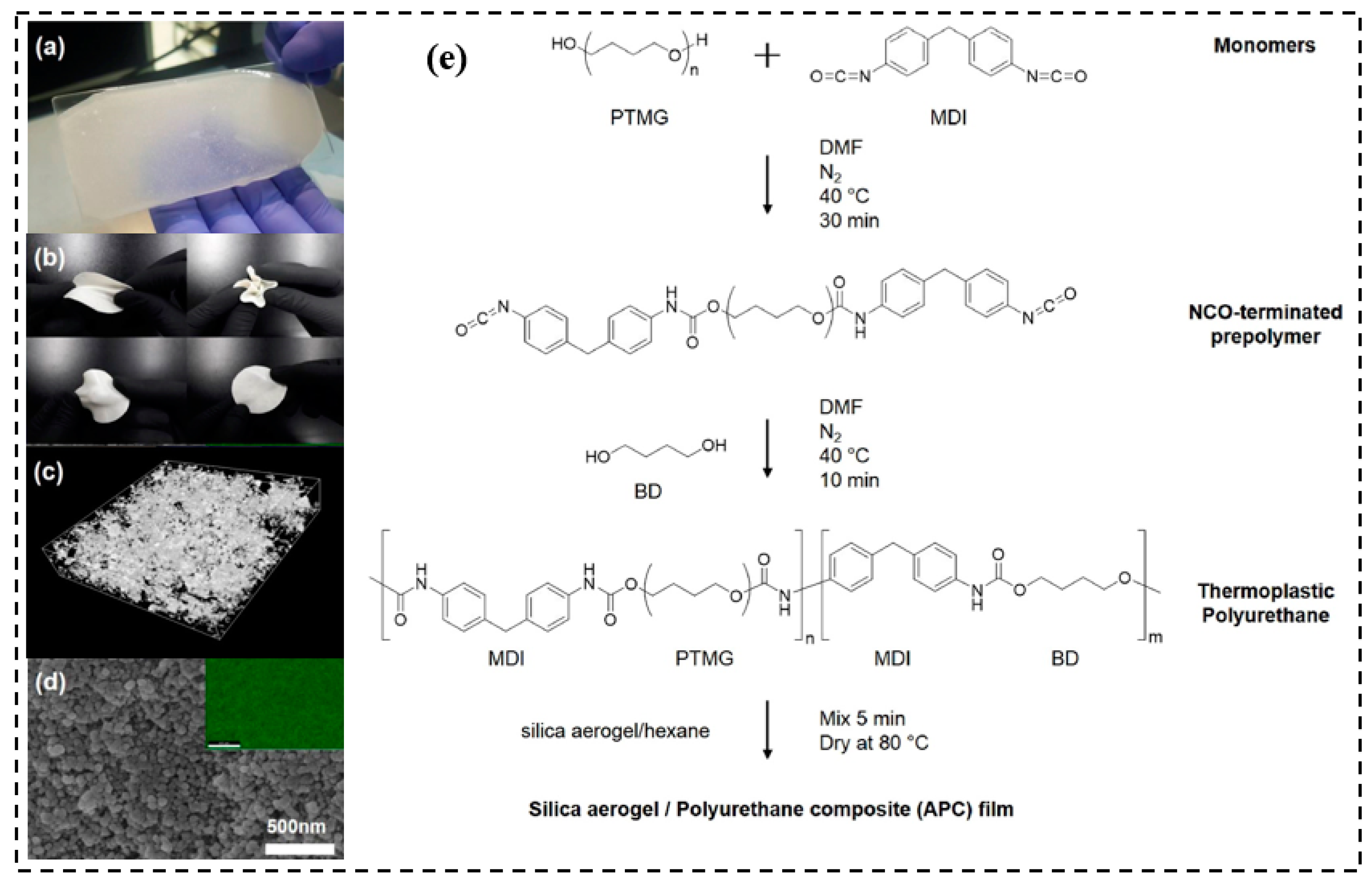
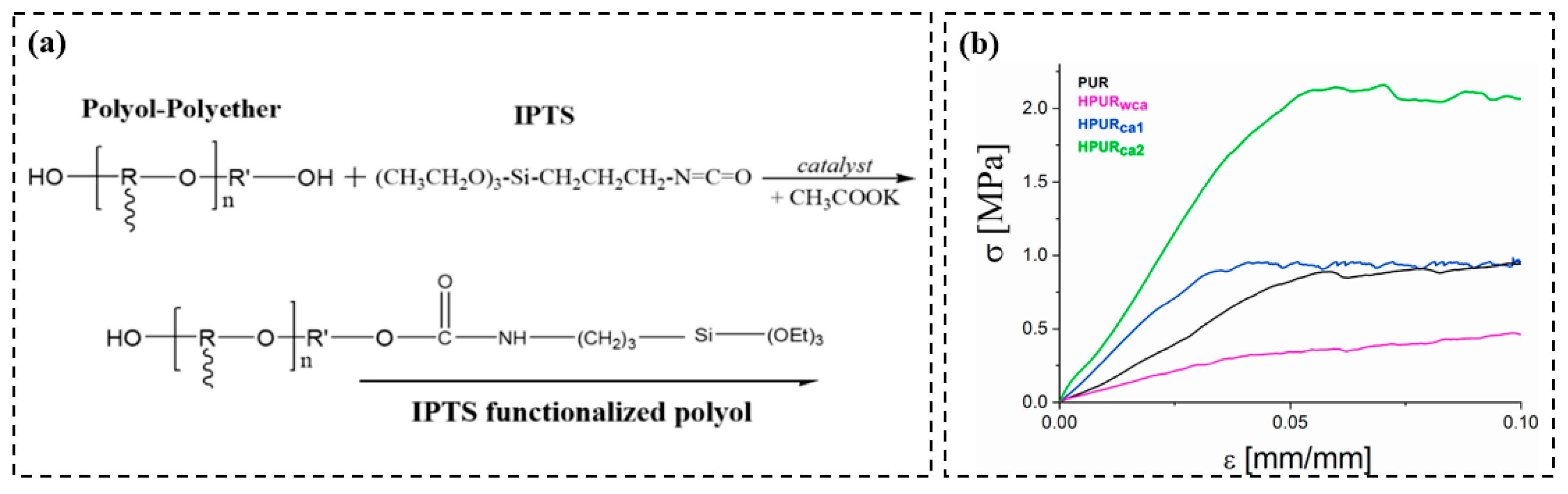
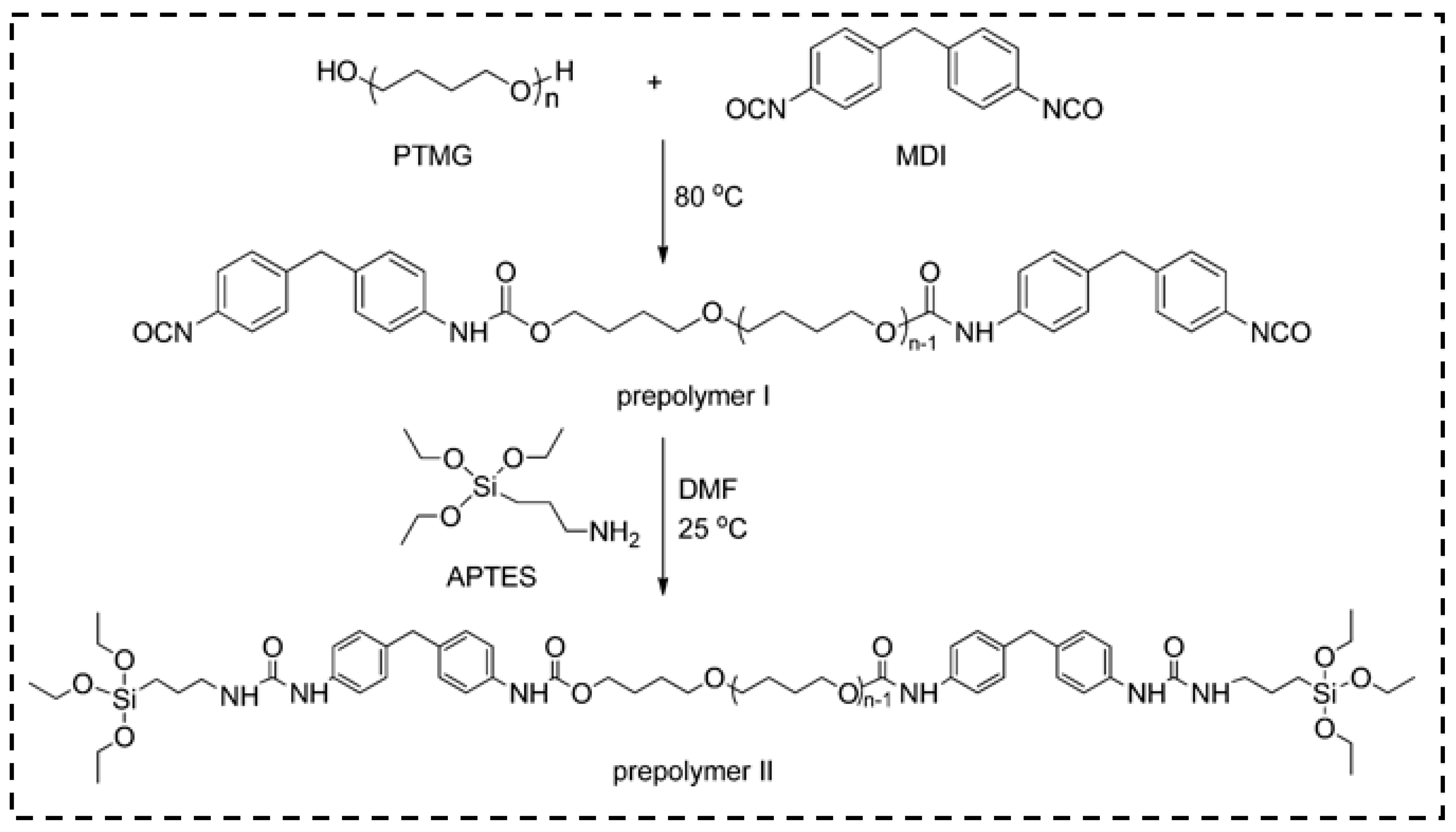

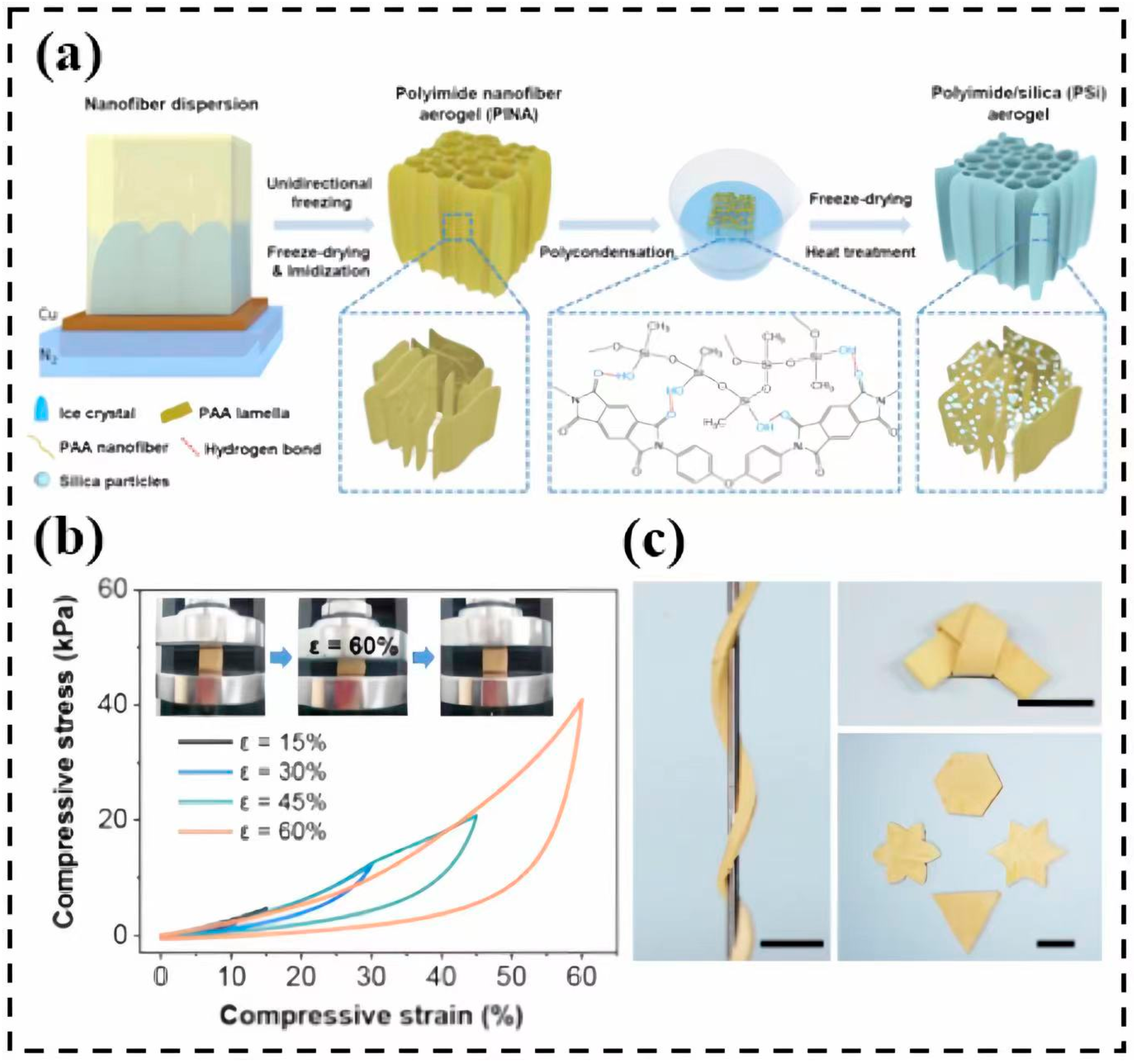
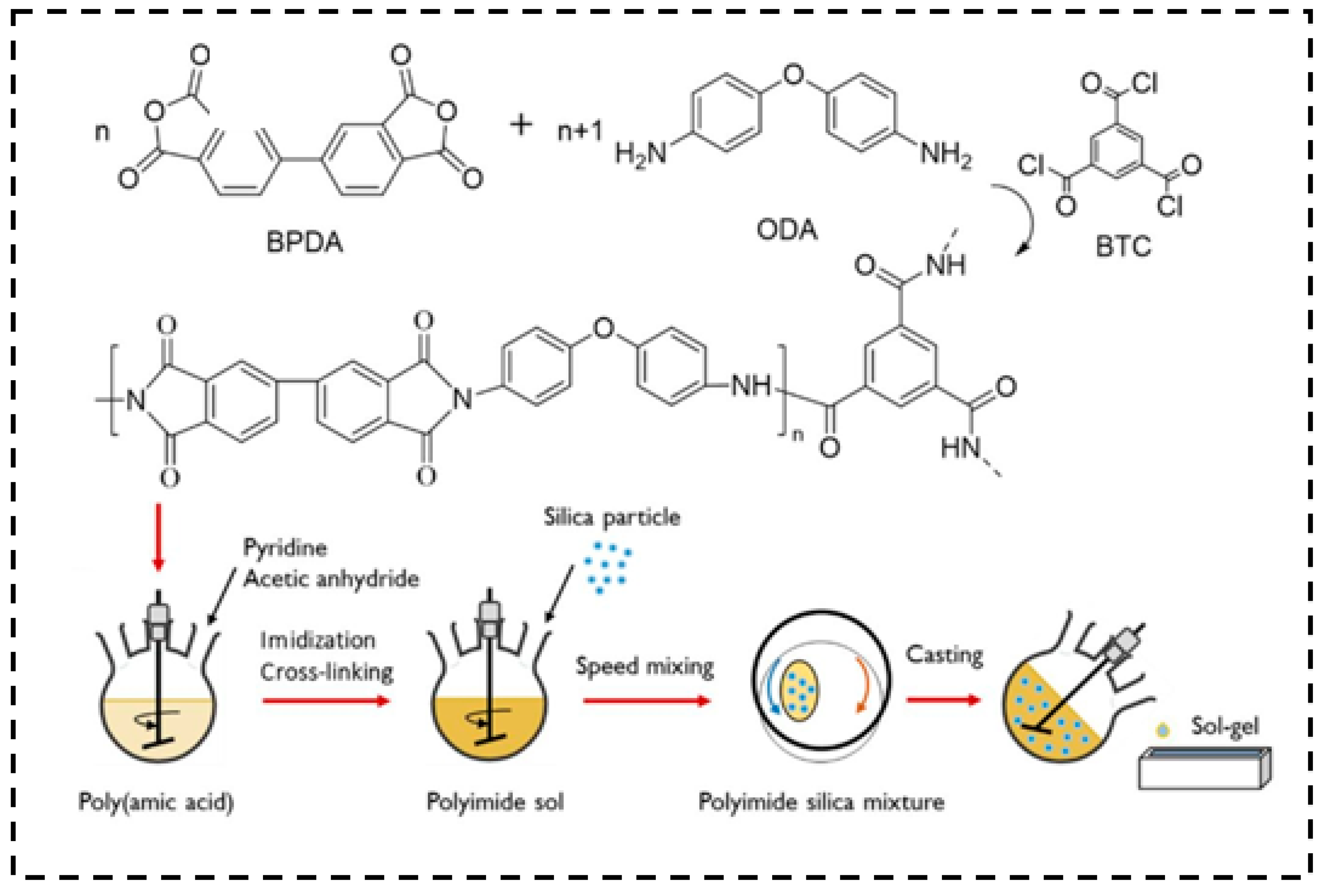
| Precursor Formulation | Polymer Matrix | Enhanced Properties | Ref |
|---|---|---|---|
| GPTMS/VTMS | Epoxide | ➢ Elastic deformation: 3~5% | [128] |
| / | Epoxide | ➢ Elastic modulus: 35%, tensile strength: 62%, toughness: 126% | [129] |
| / | Epoxide | ➢ (Hydrophobic aerogel) contact angles: 107°, fracture toughness: improved by up to ∼70%, impact strength: improved by up to ∼120% | [130] |
| / | Epoxide | ➢ Elastic modulus: 3770 ± 71 MPa, stress at yield point: 43.2 ± 1.8 MPa, strain at yield point: 1.24 ± 0.03 MPa, ultimate tensile strength: 51.0 ± 2.1 MPa, strain at break point: 3.3 ± 0.3%, toughness: 1.29 ± 0.08 J/m3 | [131] |
| TEOS/APTES | Epoxide | ➢ Strain: 80% (18 N) | [132] |
| TEOS/APTES | Epoxide | ➢ Tensile strength: 45.05± 4.56 MPa, modulus of elasticity: 4363.88± 209.57 MPa, break strain: 1.19 ± 0.17% | [133] |
| TEOS | Epoxide | ➢ Density: 0.419 g/cm3, porosity: 89%, compressive strength: 0.438 MPa, thermal conductivity: 0.0273 W/m·K | [134] |
| / | Epoxide | ➢ (Warp direction) strength: 464.3 MPa, modulus: 1.76 GPa, (weft direction) strength: 410.2 MPa, modulus: 1.68 GPa | [135] |
| TMOS | Polyurea | ➢ Shrinkage: 14.6 ± 0.7%, bulk density: 0.594 ± 0.026 g/cm3, skeletal density: 1.290 ± 0.003 g/cm3, porosity: 54% | [136] |
| TEOS/APTES | Polyurea | ➢ Bulk density: 0.046 g/cm3, flexural modulus: 0.14 MPa | [137] |
| TEOS/APTES | Polyurea | ➢ Linear shrinkage: 15.73%, bulk density: 0.392 g/m3, average elastic modulus: 14.57 MPa | [138] |
| TEOS /MTEOS | Polyurethane | ➢ Density: 0.190 ± 0.006 g/m3, yield strength: 2.15 ± 0.04 MPa, Young’s modulus: 50 ± 0.09 MPa | [139] |
| / | Polyurethane | ➢ Heat resistance index: 193.6%, char yield: 31.6%, bulk density: 0.580 g/mL | [140] |
| TEOS/APTES | Polyurethane | ➢ BET surface area: 242.9 m2/g, BJH desorption average pore diameter: 10.8 nm | [141] |
| TEOS | Polyurethane | ➢ Density: 117.68 kg/m3, porosity: 92.3%, linear shrinkage: −8.38%, thermal conductivity: 0.014 ± 0.00033 W/m·K | [142] |
| MTMS | Polyimide | ➢ Compressive strain: 50%, thermal conductivity: 0.0212 W/m·K | [143] |
| / | Polyimide | ➢ Surface area: 609 m2/g, thermal conductivity: 0.017.5 W/m·K | [144] |
| TEOS/APTES | Polyimide | ➢ Compressive strength: 3.82 MPa, Young’s modulus: 44.16 MPa | [145] |
| TEOS/APTES | Polyimide | ➢ Density: 0.145 g/cm3, strain: 9%, strength: 0.29 MPa, Young’s modulus: 3.22 MPa | [146] |
| TEOS | Polyimide | ➢ Compressive modulus:1.96 MPa, thermal conductivity: 0.0311~0.0585 W/m·K | [147] |
| TMOS/APTES | Polystyrene | ➢ Density: 0.41~0.77 g/cm3, surface area: 213~393 m2/g, thermal conductivity: 0.041 W/m·K, contact angles: 120° | [148] |
| TMOS | Polystyrene | ➢ Density: 0.13~0.17 g/cm3, surface area: 350~780 m2/g, thermal conductivity: 0.03~0.04 W/m·K | [149] |
| MTMS/VTMS/TMOS | Polystyrene | ➢ Bulk density: 163.1 ± 11.7 kg/cm3, porosity: 88%, surface area: 227 m2/g, thermal conductivity: 0.072 ± 0.001 W/m·K, Young’s modulus: 91 kPa, compression strength: 68 kPa | [87] |
| TMOS | Polystyrene | ➢ Modulus: 3 MPa | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, W.; Chen, L.; Kong, Q.; Li, L.; Chen, M.; Jiang, J.; Li, W.; Shi, F.; Xu, Z. The Synthesis and Polymer-Reinforced Mechanical Properties of SiO2 Aerogels: A Review. Molecules 2023, 28, 5534. https://doi.org/10.3390/molecules28145534
Zhan W, Chen L, Kong Q, Li L, Chen M, Jiang J, Li W, Shi F, Xu Z. The Synthesis and Polymer-Reinforced Mechanical Properties of SiO2 Aerogels: A Review. Molecules. 2023; 28(14):5534. https://doi.org/10.3390/molecules28145534
Chicago/Turabian StyleZhan, Wang, Le Chen, Qinghong Kong, Lixia Li, Mingyi Chen, Juncheng Jiang, Weixi Li, Fan Shi, and Zhiyuan Xu. 2023. "The Synthesis and Polymer-Reinforced Mechanical Properties of SiO2 Aerogels: A Review" Molecules 28, no. 14: 5534. https://doi.org/10.3390/molecules28145534
APA StyleZhan, W., Chen, L., Kong, Q., Li, L., Chen, M., Jiang, J., Li, W., Shi, F., & Xu, Z. (2023). The Synthesis and Polymer-Reinforced Mechanical Properties of SiO2 Aerogels: A Review. Molecules, 28(14), 5534. https://doi.org/10.3390/molecules28145534