A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies
Abstract
1. Introduction
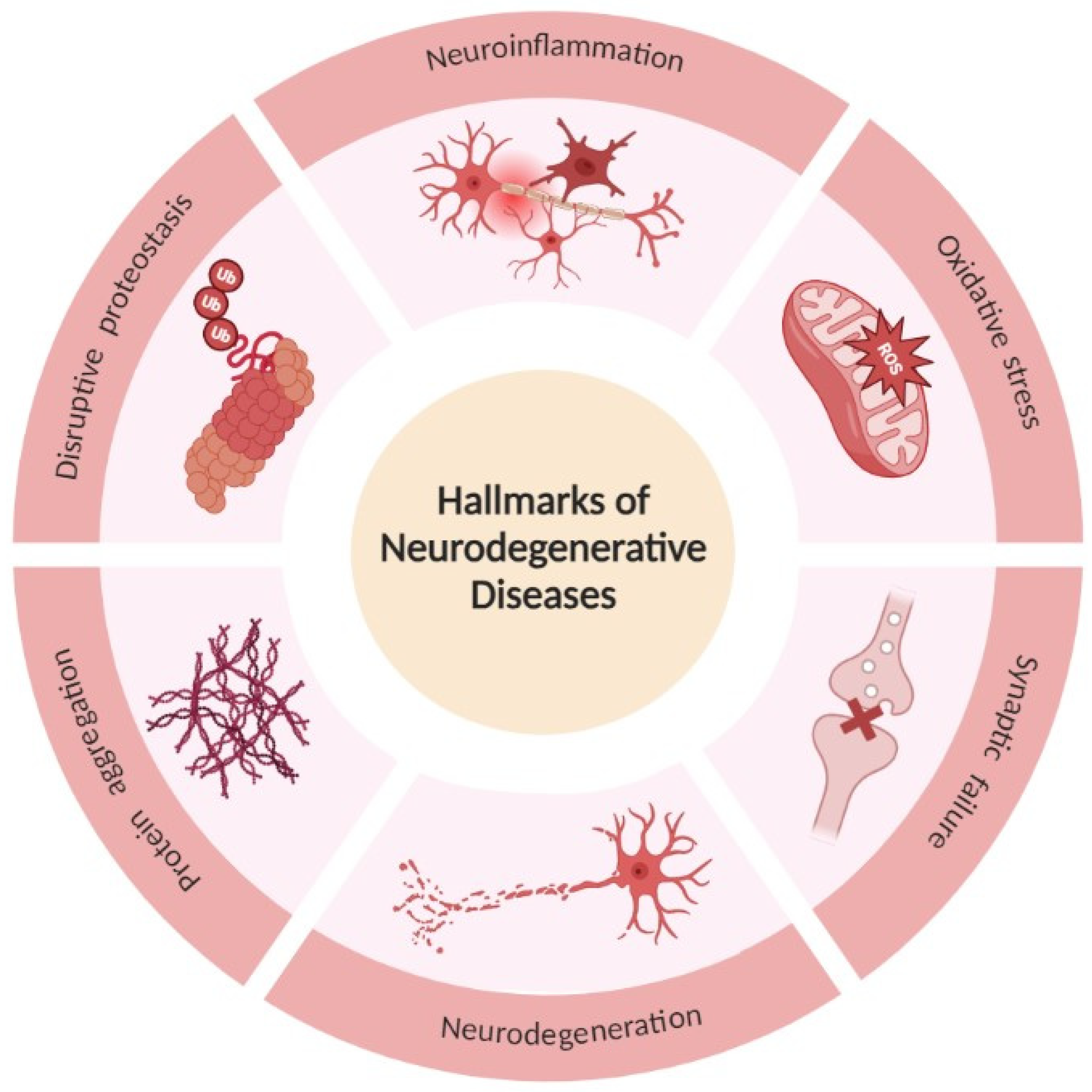
2. Neurodegenerative Diseases
- Tau protein—microtubule-associated protein—encoded by the microtubule-associated protein tau (MAPT) gene. Tau is substantially expressed in the cytoplasm of neurons and plays an important role primarily in the stabilization and assembly of axonal microtubules and also in a variety of physiological processes, which include axonal transport, signal transmission between neurons, neurogenesis, myelination, motor function, neuronal excitability, glucose metabolism, iron homeostasis, and DNA protection [15,16].
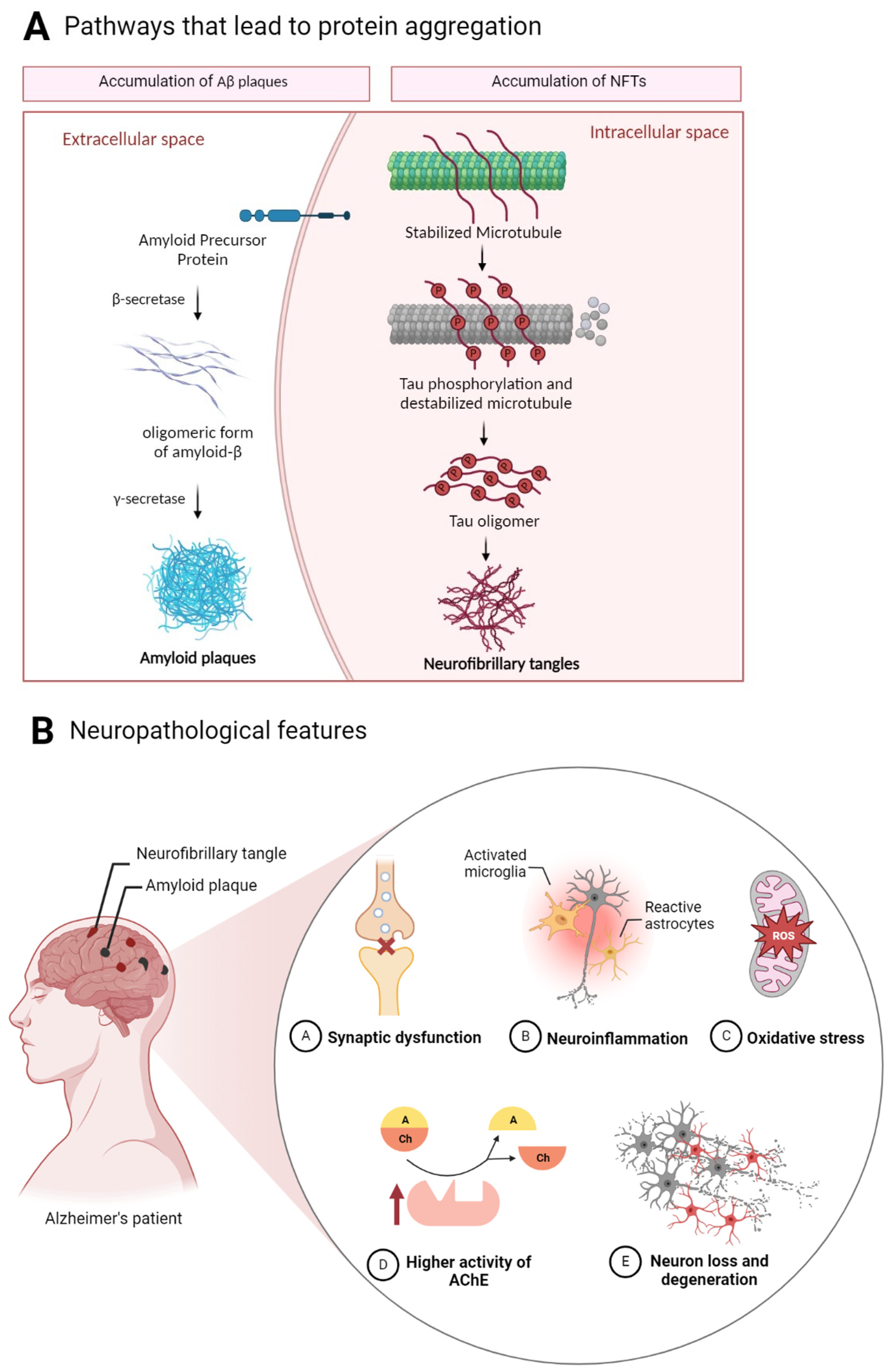
- Aβ—derives from the amyloid precursor protein (APP) and aggregates into amyloid plaques with Aβ polypeptides 40 and 42 amino acids long [17].
- Prion—another protein present in neurodegenerative diseases is the prion protein (PrP) encoded by the PRNP gene. In prion diseases, the prion protein misfolds, propagates, and aggregates rapidly, being responsible for spreading neurodegeneration between cells, and, consequently, brain regions [14].
- α-synuclein—this is a 140-amino-acid protein highly expressed in the brain, encoded by the α-synuclein (SCNA) gene [12].
Alzheimer’s Disease
3. Natural Compounds for AD Treatment
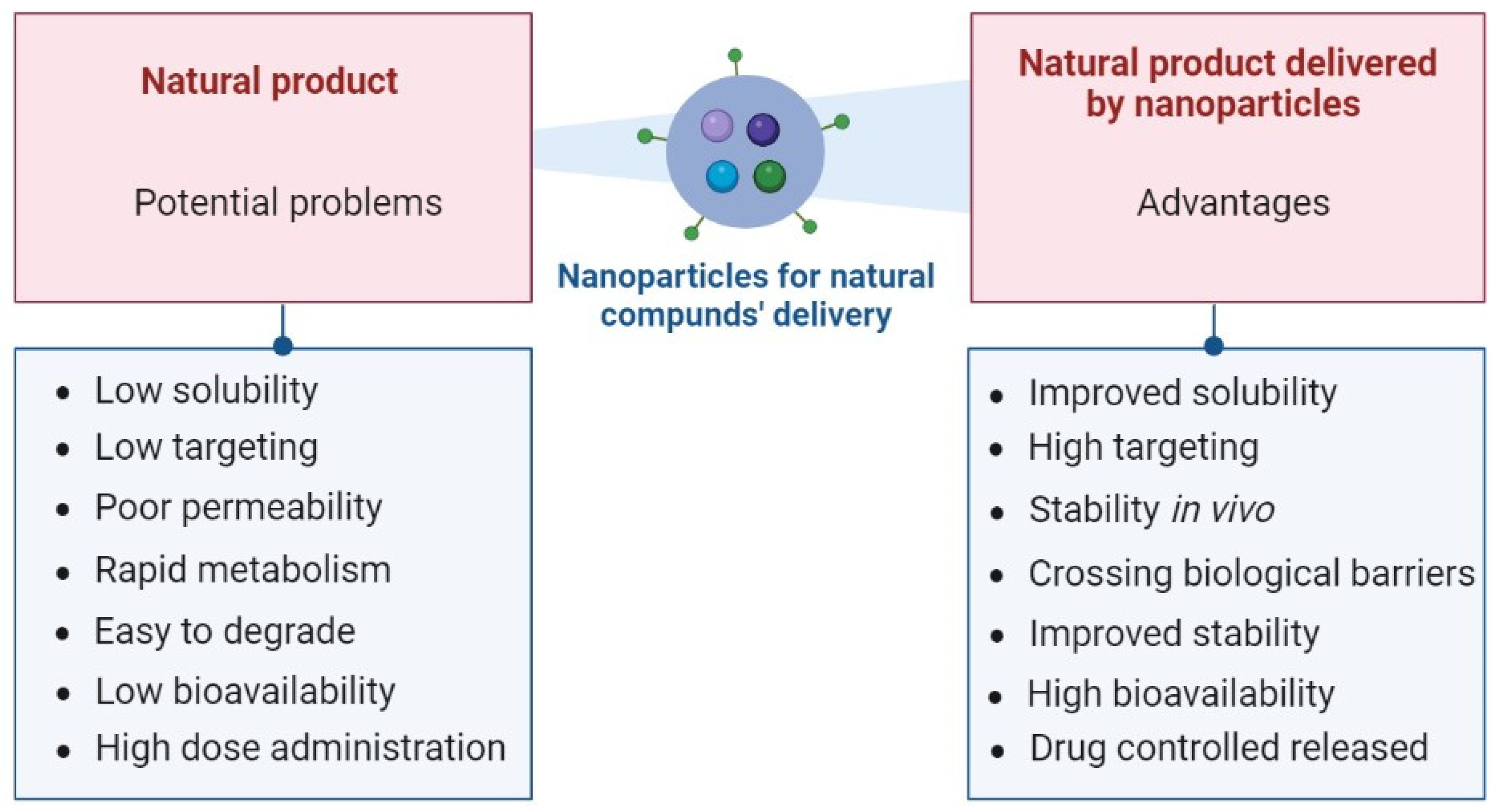
Overcoming Limitations of Natural Compounds with Delivery Systems
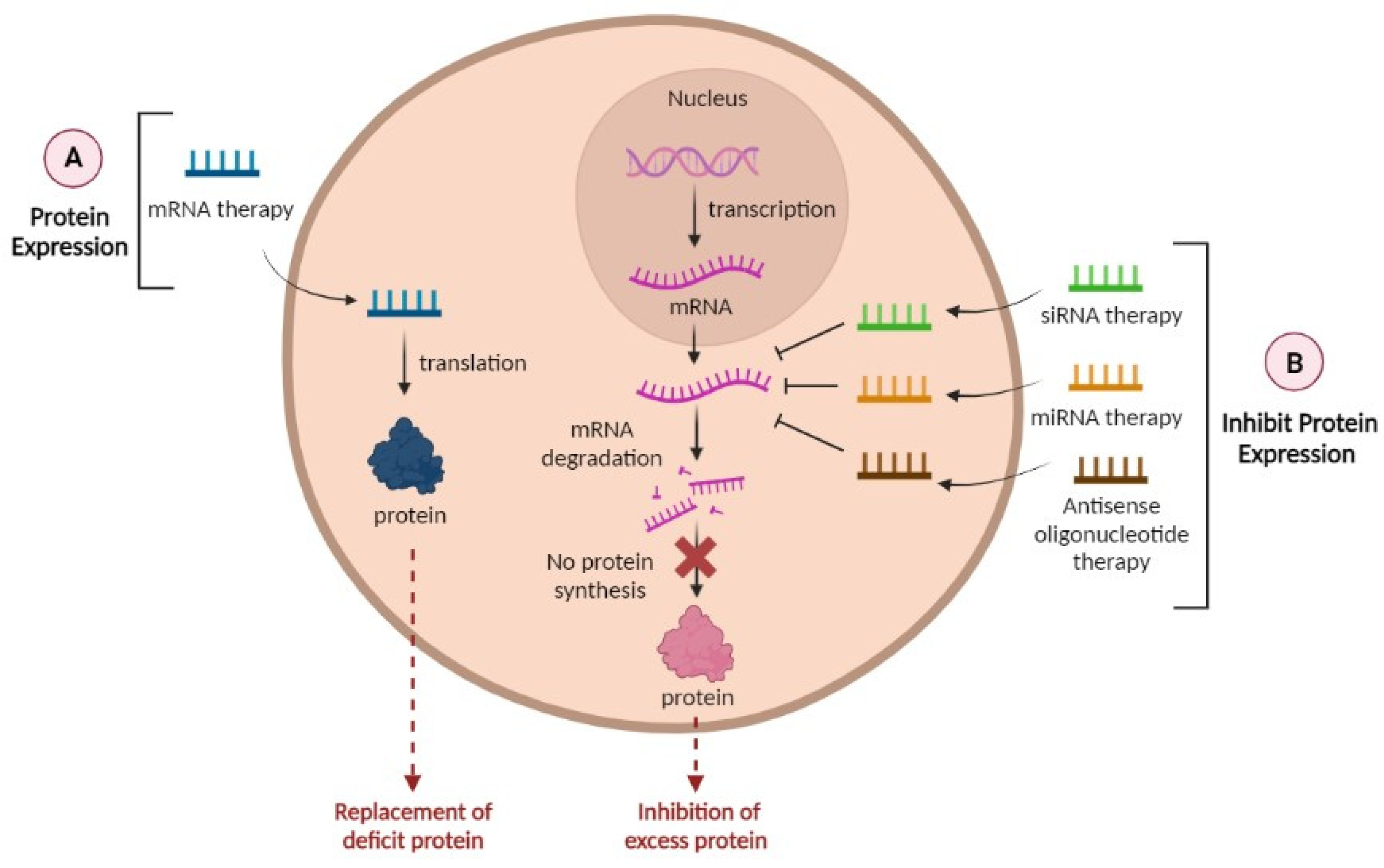
4. RNAs as a Promising Tool in the Treatment of AD
- miRNA
- siRNA
- mRNA
| Role in AD | References | |
|---|---|---|
| Types of miRNA | ||
| miR-101 | Significantly reduced the expression of a reporter under control of APP 3′-UTR in HeLa cells. | [95] |
| miR-106b | Overexpression of miR-106b inhibited Aβ1-42-induced tau phosphorylation at Tyr18 in SH-SY5Y cells stably expressing Tau. | [96] |
| miR-137 | miR-137 inhibited increased expression levels of p-tau induced by Aβ1-42 in SH-SY5Y and inhibited the hyperphosphorylation of Tau protein in a transgenic mouse model of AD. | [97] |
| miR-219 | In a Drosophila model that produces human Tau, reduction of miR-219 exacerbated Tau toxicity, while overexpression of miR-219 partially annulled toxic effects. | [98] |
| miR-17 | miR-17 inhibits elevated miR-17 in adult AD (5xFAD) mice microglia improves Aβ degradation. | [99] |
| miR-20b-5p | Treatment with miR-20b-5p reduced APP mRNA and protein levels in cultured human neuronal cells. | [100] |
| miR-29c | Over-expression of miR-29c in SH-SY5Y, HEK-293T cell lines and miR-29c in transgenic mice downregulated BACE1 protein levels. | [101] |
| miR-298 | miR-298 is a repressor of APP, BACE1, and the two primary forms of Aβ (Aβ40 and Aβ42) in a primary human cell culture model. Thus, miR-298 significantly reduced levels of ~55 and 50 kDa forms of the Tau protein without significant alterations of total Tau or other forms. | [102] |
| miR-485-5p | miR-485-5p overexpression facilitated the learning and memory capabilities of APP/PS1 mice and promoted pericyte viability and prohibited pericyte apoptosis in this model. | [103] |
| miR-9-5p | miR-9-5p overexpression inhibited Aβ25-35-induced mitochondrial dysfunction, cell apoptosis, and oxidative stress by regulating GSK-3β expression in HT22 cells. | [104] |
| miR-132 | miR-132 inhibited hippocampal iNOS expression and oxidative stress by inhibiting MAPK1 expression to improve the cognitive function of rats with AD. | [105] |
| miR-153 | Using miR-153 transgenic mouse model, was verified that miR-153 downregulated the expression of APP and APLP2 protein in vivo. | [106] |
| Targeted gene silencing by siRNA | ||
| Tau | siRNA against MAPT can effectively suppress tau expression in vitro and in vivo without a specific delivery agent. | [107] |
| BACE1 | Polymeric siRNA nanomedicine targeting BACE1 in APP/PS1 transgenic AD mouse model can efficiently penetrate the BBB via glycemia-controlled glucose transporter-1–mediated transport, ensuring that siRNAs decrease BACE1 expression. | [94] |
| Presenilin1 (PS1) | Downregulation of PS1 and Aβ42 in IMR32 cells transfected with siRNA against PS1 was verified. | [108] |
| APP | Infusion of siRNAs that down-regulated mouse APP protein levels into the ventricular system for 2 weeks down-regulated APP mRNA in mouse brain. | [109] |
| Proteins encoded by mRNA | ||
| mRNA encoding neprilysin | Neprilysin plays a major role in the clearance of Aβ in the brain. New mRNA therapeutic strategy utilizing mRNA encoding the mouse neprilysin protein has been shown to decrease Aβ deposition and prevent pathogenic changes in the brain. | [110] |
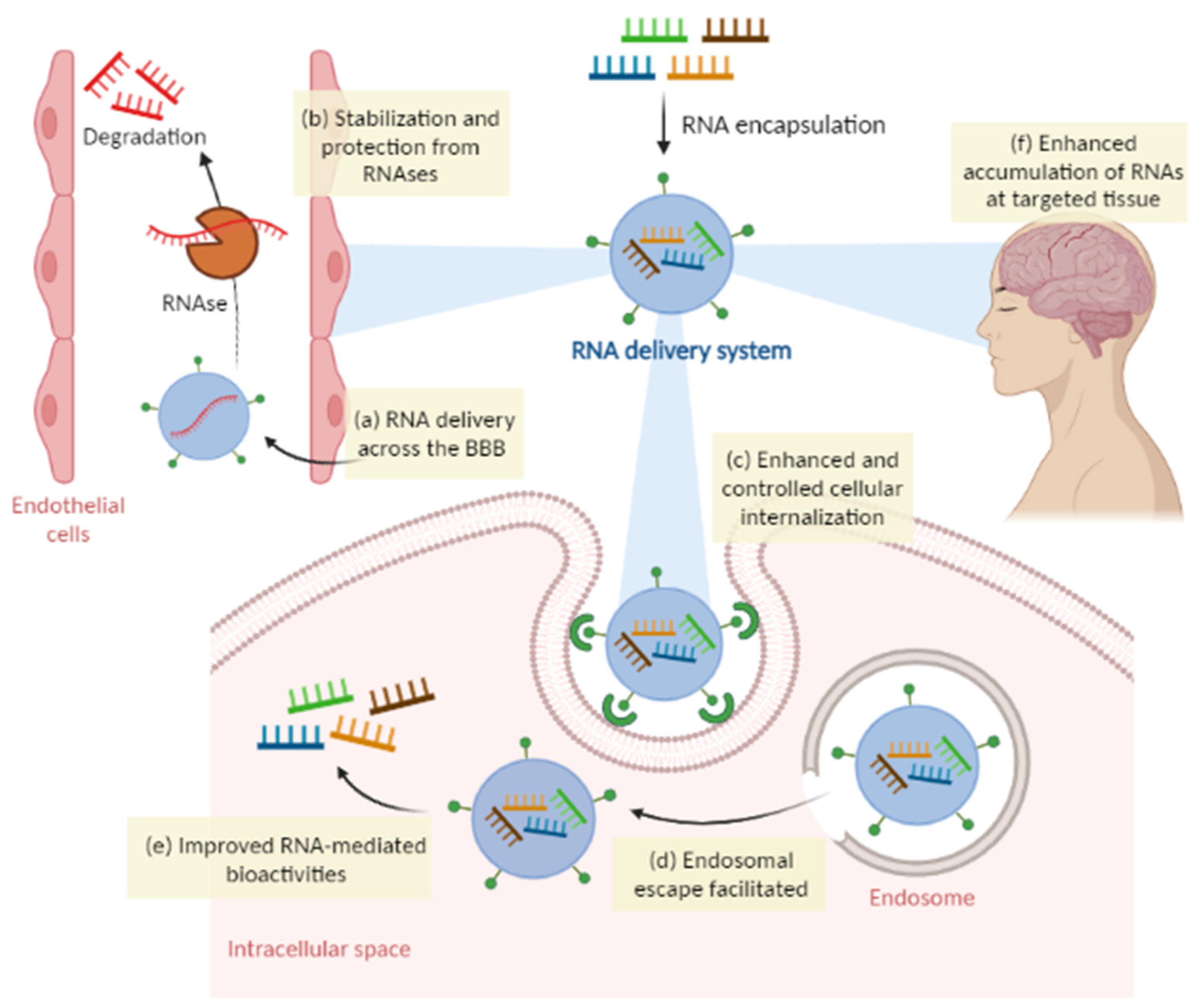
Overcoming Limitations of RNA Therapies with Delivery Systems
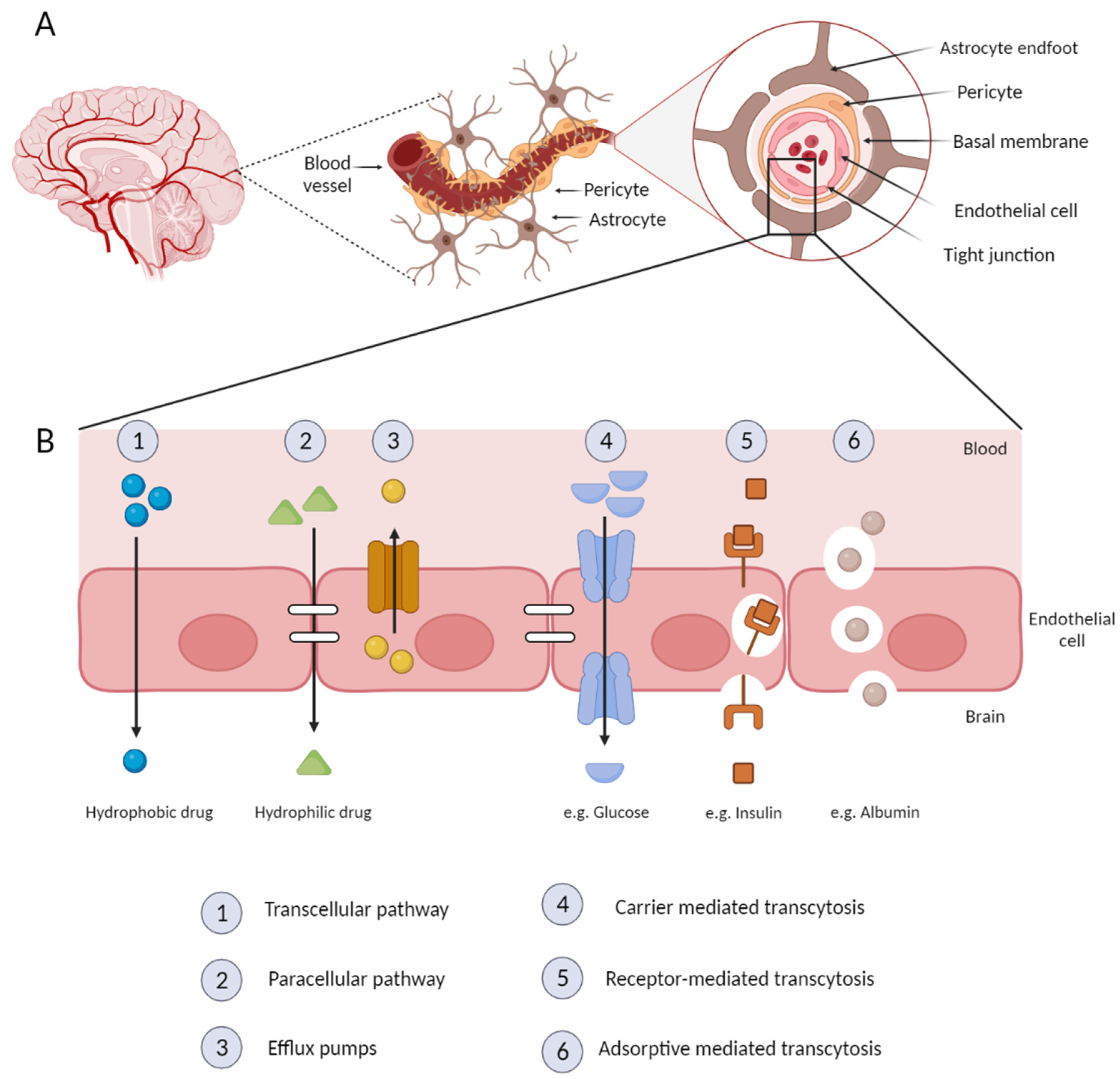
5. Nanoparticles and the BBB
5.1. Exosomes
5.2. Liposomes
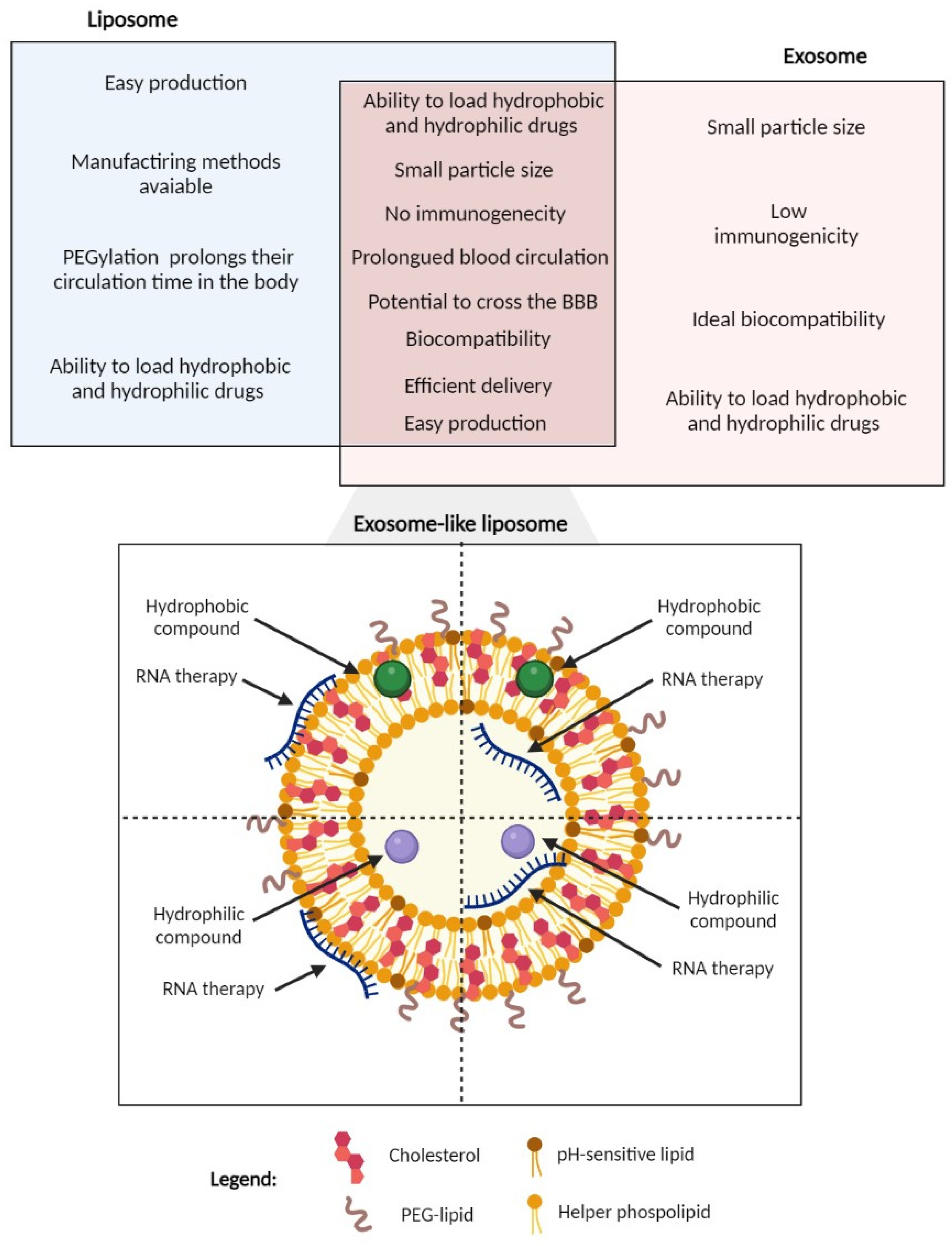
5.3. Exosome-like Liposomes as a Novel Strategy
- Dendrimers: Dendrimers are highly branched, characterized by defined molecular weights and specific encapsulation properties. This type of delivery system is composed of symmetrical polymeric macromolecules with a large number of reactive surface groups, with three distinctive architectural components: An interior core, an interior layer consisting of repeating units radially attached to the inner core, and functional end groups on the outside layer. Because of these unique features, dendrimers can cross-impair the BBB and target astrocytes and microglia after systemic administration in animal models [139].
- Polymeric nanoparticles: Polymeric nanoparticles can be produced from synthetic or natural polymers. However, to be applied in brain drug delivery, these nanoparticles need to be biodegradable and biocompatible. PBCA, PLA, and PLGA nanoparticles are nanoparticles able to cross the BBB. These nanocarriers possess controlled drug release, targeting efficiency, and can avoid phagocytosis by the reticuloendothelial system, thus improving the concentration of drugs in the brain [140].
- Gold nanoparticles: Nanoparticles (mostly < 10 nm in size) composed of a gold core and with covalently or non-covalently attached surface ligands. Multiple in vivo studies on rodents have shown that low amounts of this delivery system were able to cross the BBB. However, greater amounts of the administered dose were found in the liver and in the blood [8]. Additionally, Sela et al. proved that gold nanoparticles could penetrate the BBB of rats without the use of an external field or surface modification and were found to be distributed uniformly in both the hypothalamus and hippocampus indicating there is no selective binding in these regions of the brain [141].
- Carbon quantum dots: This delivery system retains a polymeric core structure and various functional groups on the surface, facilitating their conjugation with drug molecules for specific delivery. This is a carrier with several efficient features for BBB crossing such as excellent biocompatibility and low toxicity due to the lack of metal elements, small size, and photoluminescence, which can be utilized to track the penetration of CDs through the BBB [117].
6. Conclusions
7. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloomingdale, P.; Karelina, T.; Ramakrishnan, V.; Bakshi, S.; Véronneau-Veilleux, F.; Moye, M.; Sekiguchi, K.; Meno-Tetang, G.; Mohan, A.; Maithreye, R.; et al. Hallmarks of neurodegenerative disease: A systems pharmacology perspective. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1399–1429. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lee, L.K.C.; Peng, B.; Choi, C.H.J.; Tong, W.Y.; Voelcker, N.H. Delivering the Promise of Gene Therapy with Nanomedicines in Treating Central Nervous System Diseases. Adv. Sci. 2022, 9, 2201740. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Antimisiaris, S.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.L.; Kovacs, G.G. Current Concepts of Mixed Pathologies in Neurodegenerative Diseases. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2022, 50, 329–345. [Google Scholar] [CrossRef]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666. [Google Scholar] [CrossRef]
- Liang, S.Y.; Wang, Z.T.; Tan, L.; Yu, J.T. Tau Toxicity in Neurodegeneration. Mol. Neurobiol. 2022, 59, 3617–3634. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J. Amyloid-β: A double agent in Alzheimer’s disease? Biomed. Pharmacother. 2021, 139, 111575. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, S.-K.; Zhang, Y.; Rostami, A.; Kenkare, A.; Casella, G.; Yuan, Z.-Q.; Li, X. Role of extracellular vesicles in neurodegenerative diseases. Prog. Neurobiol. 2021, 201, 102022. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wei, M.; Yang, X.; Xu, Y.; Gao, S.; Ren, K. Synaptic Secretion and Beyond: Targeting Synapse and Neurotransmitters to Treat Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 9176923. [Google Scholar] [CrossRef]
- Van Den Berge, N.; Ulusoy, A. Animal models of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 163, 105599. [Google Scholar] [CrossRef]
- Sun, X.; Song, J.; Huang, H.; Chen, H.; Qian, K. Modeling hallmark pathology using motor neurons derived from the family and sporadic amyotrophic lateral sclerosis patient-specific iPS cells. Stem Cell Res. Ther. 2018, 9, 315. [Google Scholar] [CrossRef]
- Xiong, L.; McCoy, M.; Komuro, H.; West, X.Z.; Yakubenko, V.; Gao, D.; Dudiki, T.; Milo, A.; Chen, J.; Podrez, E.A.; et al. Inflammation-dependent oxidative stress metabolites as a hallmark of amyotrophic lateral sclerosis. Free. Radic. Biol. Med. 2022, 178, 125–133. [Google Scholar] [CrossRef]
- Mehler, M.F.; Petronglo, J.R.; Arteaga-Bracho, E.E.; Gulinello, M.E.; Winchester, M.L.; Pichamoorthy, N.; Young, S.K.; DeJesus, C.D.; Ishtiaq, H.; Gokhan, S.; et al. Loss-of-Huntingtin in Medial and Lateral Ganglionic Lineages Differentially Disrupts Regional Interneuron and Projection Neuron Subtypes and Promotes Huntington’s Disease-Associated Behavioral, Cellular, and Pathological Hallmarks. J. Neurosci. 2019, 39, 1892–1909. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef] [PubMed]
- Machiela, E.; Southwell, A.L. Biological Aging and the Cellular Pathogenesis of Huntington’s Disease. J. Huntington’s Dis. 2020, 9, 115–128. [Google Scholar] [CrossRef]
- Gallego Villarejo, L.; Bachmann, L.; Marks, D.; Brachthäuser, M.; Geidies, A.; Müller, T. Role of Intracellular Amyloid β as Pathway Modulator, Biomarker, and Therapy Target. Int. J. Mol. Sci. 2022, 23, 4656. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Mobley, W.C. Alzheimer Disease Pathogenesis: Insights from Molecular and Cellular Biology Studies of Oligomeric Aβ and Tau Species. Front. Neurosci. 2019, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Q.; Chen, S.; Xu, C. Functions of amyloid precursor protein in metabolic diseases. Metabolism 2021, 115, 154454. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Ji, S. Secretases Related to Amyloid Precursor Protein Processing. Membranes 2021, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, D.; Lee, T.H. Phosphorylation Signaling in APP Processing in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Beera, A.M.; Seethamraju, S.M.; Nori, L.P. Alzheimer’s Disease: Perspective on Therapeutic Options and Recent Hallmarks in Clinical Research. Int. J. Pharm. Res. Allied Sci. 2021, 10, 110–120. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, M.; Yin, X.; Chen, K.; Hu, Z.; Zhou, Q.; Cao, X.; Chen, Z.; Liu, D. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl. Neurodegener. 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Liu, F. Pathological Changes of Tau Related to Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 931–944. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Ma, L.; Wei, Y.; Li, H. Possible Mechanisms of Tau Spread and Toxicity in Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 707268. [Google Scholar] [CrossRef]
- Fleeman, R.M.; Proctor, E.A. Astrocytic Propagation of Tau in the Context of Alzheimer’s Disease. Front. Cell Neurosci. 2021, 15, 645233. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- DeVos, S.L.; Corjuc, B.T.; Oakley, D.H.; Nobuhara, C.K.; Bannon, R.N.; Chase, A.; Commins, C.; Gonzalez, J.A.; Dooley, P.M.; Frosch, M.P.; et al. Synaptic Tau Seeding Precedes Tau Pathology in Human Alzheimer’s Disease Brain. Front. Neurosci. 2018, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Riscado, M.; Baptista, B.; Sousa, F. New RNA-Based Breakthroughs in Alzheimer’s Disease Diagnosis and Therapeutics. Pharmaceutics 2021, 13, 1397. [Google Scholar]
- Plascencia-Villa, G.; Perry, G. Neuropathologic Changes Provide Insights into Key Mechanisms of Alzheimer Disease and Related Dementia. Am. J. Pathol. 2022, 192, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 117957352110291. [Google Scholar] [CrossRef]
- Bennett, C.F.; Kordasiewicz, H.B.; Cleveland, D.W. Antisense Drugs Make Sense for Neurological Diseases. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 831–852. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Valis, M.; Kuca, K.; Zhang, B.; Hort, J. MicroRNAs in Alzheimer’s Disease: Diagnostic Markers or Therapeutic Agents? Front. Pharmacol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Noori, T.; Dehpour, A.R.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. Role of natural products for the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 898, 173974. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Ryu, I.S.; Ryu, J.H.; Cho, H.J. miRNAs as Therapeutic Tools in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13012. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Andrade, S.; Loureiro, J.A.; do Carmo Pereira, M. Nanotechnology to improve the Alzheimer’s disease therapy with natural compounds. Drug Deliv. Transl. Res. 2020, 10, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Albratty, M. An update on the novel and approved drugs for Alzheimer disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Said, M.M.; Rabo, M.M.A. Neuroprotective effects of eugenol against aluminiuminduced toxicity in the rat brain. Arch. Ind. Hyg. Toxicol. 2017, 68, 27–37. [Google Scholar] [CrossRef]
- Casares, N.; Alfaro, M.; Cuadrado-Tejedor, M.; Lasarte-Cia, A.; Navarro, F.; Vivas, I.; Espelosin, M.; Cartas-Cejudo, P.; Fernández-Irigoyen, J.; Santamaría, E.; et al. Improvement of cognitive function in wild-type and Alzheimer’s disease mouse models by the immunomodulatory properties of menthol inhalation or by depletion of T regulatory cells. Front. Immunol. 2023, 14, 1130044. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; Moreira, L.K.d.S.; Neri, H.F.d.S.; da Silva, C.R.B.; Pruccoli, L.; dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, J.; Liu, J.; Wu, J.; Chen, L. Ginkgo biloba preparation prevents and treats senile dementia by inhibiting neuro-inflammatory responses. Trop. J. Pharm. Res. 2019, 17, 1961. [Google Scholar] [CrossRef]
- Islam, F.; Nafady, M.H.; Islam, R.; Saha, S.; Rashid, S.; Akter, A.; Or-Rashid, H.; Akhtar, M.F.; Perveen, A.; Ashraf, G.M.; et al. Resveratrol and neuroprotection: An insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol. Neurobiol. 2022, 59, 4384–4404. [Google Scholar] [CrossRef] [PubMed]
- Villegas, C.; Perez, R.; Petiz, L.L.; Glaser, T.; Ulrich, H.; Paz, C. Ginkgolides and Huperzine A for complementary treatment of Alzheimer’s disease. IUBMB Life 2022, 74, 763–779. [Google Scholar] [CrossRef]
- Dubey, T.; Chinnathambi, S. Brahmi (Bacopa monnieri): An ayurvedic herb against the Alzheimer’s disease. Arch. Biochem. Biophys. 2019, 676, 108153. [Google Scholar] [CrossRef]
- Snow, A.D.; Castillo, G.M.; Nguyen, B.P.; Choi, P.Y.; Cummings, J.A.; Cam, J.; Hu, Q.; Lake, T.; Pan, W.; Kastin, A.J.; et al. The Amazon rain forest plant Uncaria tomentosa (cat’s claw) and its specific proanthocyanidin constituents are potent inhibitors and reducers of both brain plaques and tangles. Sci. Rep. 2019, 9, 561. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.J.; Mittal, K.; Katare, D.P. Protective Effects of Quercetin in Zebrafish Model of Alzheimer’s Disease. Asian J. Pharm. 2018, 12, S660. [Google Scholar]
- Leiteritz, A.; Dilberger, B.; Wenzel, U.; Fitzenberger, E. Betaine reduces β-amyloid-induced paralysis through activation of cystathionine-β-synthase in an Alzheimer model of Caenorhabditis elegans. Genes Nutr. 2018, 13, 21. [Google Scholar] [CrossRef]
- Finley, J.W.; Gao, S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ramassamy, C. In vitro screening of neuroprotective activity of Indian medicinal plant Withania somnifera. J. Nutr. Sci. 2017, 6, e54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Bae, H.; Kim, M.-J.; Choi, S.J.; Cho, H.Y.; Hwang, H.-J.; Kim, Y.J.; Lim, S.T.; Kim, E.K.; Kim, H.K.; et al. Inhibitory Effect of Poncirus trifoliate on Acetylcholinesterase and Attenuating Activity against Trimethyltin-Induced Learning and Memory Impairment. Biosci. Biotechnol. Biochem. 2009, 73, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Bihaqi, S.W.; Sharma, M.; Singh, A.P.; Tiwari, M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J. Ethnopharmacol. 2009, 124, 409–415. [Google Scholar] [CrossRef]
- Azimi, A.; Ghaffari, S.M.; Riazi, G.H.; Arab, S.S.; Tavakol, M.M.; Pooyan, S. α-Cyperone of Cyperus rotundus is an effective candidate for reduction of inflammation by destabilization of microtubule fibers in brain. J. Ethnopharmacol. 2016, 194, 219–227. [Google Scholar] [CrossRef]
- Rivera, D.S.; Lindsay, C.; Codocedo, J.F.; Morel, I.; Pinto, C.; Cisternas, P.; Bozinovic, F.; Inestrosa, N.C. Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol. Aging 2016, 46, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, Y.; Zhang, Y.; Chen, J.; Gao, J.; Zhang, T.; Shang, X.; Zhang, X. Baicalein Ameliorates Aβ-Induced Memory Deficits and Neuronal Atrophy via Inhibition of PDE2 and PDE4. Front. Pharmacol. 2021, 12, 794458. [Google Scholar] [CrossRef]
- Celik Topkara, K.; Kilinc, E.; Cetinkaya, A.; Saylan, A.; Demir, S. Therapeutic effects of carvacrol on beta-amyloid-induced impairments in in vitro and in vivo models of Alzheimer’s disease. Eur. J. Neurosci. 2022, 56, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.; Jung, S.W.; Lee, Y.W.; Kim, Y.H. Protective Effects of Decursin and Decursinol Angelate against Amyloid β-Protein-Induced Oxidative Stress in the PC12 Cell Line: The Role of Nrf2 and Antioxidant Enzymes. Biosci. Biotechnol. Biochem. 2011, 75, 434–442. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Xu, F.; Ding, H. Study on the neuroprotective effects of Genistein on Alzheimer’s disease. Brain Behav. 2021, 11, e02100. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.S.; Yu, Y.C.; Wu, C.H.; Lin, J.Y. Protective Effects of Wogonin against Alzheimer’s Disease by Inhibition of Amyloidogenic Pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 3545169. [Google Scholar] [CrossRef]
- Xu, P.-X.; Wang, S.-W.; Yu, X.-L.; Su, Y.-J.; Wang, T.; Zhou, W.-W.; Zhang, H.; Wang, Y.-J.; Liu, R.-T. Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Aβ oligomer level and attenuating oxidative stress and neuroinflammation. Behav. Brain Res. 2014, 264, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Kang, S.; Park, S. Protection against Alzheimer’s disease by luteolin: Role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors 2021, 47, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Asiatic Acid Nullified Aluminium Toxicity in In Vitro Model of Alzheimer’s Disease. Available online: https://www.imrpress.com/journal/FBE/10/2/10.2741/E823 (accessed on 30 May 2023).
- Li, Z.; Zhao, T.; Li, J.; Yu, Q.; Feng, Y.; Xie, Y.; Sun, P. Nanomedicine Based on Natural Products: Improving Clinical Application Potential. J. Nanomater. 2022, 2022, 3066613. [Google Scholar] [CrossRef]
- Woon, C.K.; Hui, W.K.; Abas, R.; Haron, M.H.; Das, S.; Lin, T.S. Natural Product-based Nanomedicine: Recent Advances and Issues for the Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2022, 20, 1498–1518. [Google Scholar] [CrossRef]
- Yu, A.M.; Jian, C.; Yu, A.H.; Tu, M.J. RNA therapy: Are we using the right molecules? Pharmacol. Ther. 2019, 196, 91–104. [Google Scholar] [CrossRef]
- Shin, H.; Park, S.-J.; Yim, Y.; Kim, J.; Choi, C.; Won, C.; Min, D.-H. Recent Advances in RNA Therapeutics and RNA Delivery Systems Based on Nanoparticles. Adv. Ther. 2018, 1, 1800065. [Google Scholar] [CrossRef]
- Zogg, H.; Singh, R.; Ro, S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23, 2736. [Google Scholar] [CrossRef] [PubMed]
- Mollocana-Lara, E.C.; Ni, M.; Agathos, S.N.; Gonzales-Zubiate, F.A. The infinite possibilities of RNA therapeutics. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab063. [Google Scholar] [CrossRef]
- DeLong, R. Ushering in a new era of RNA-based therapies. Commun. Biol. 2021, 4, 577. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K. RNA therapy: Rich history, various applications and unlimited future prospects. Exp. Mol. Med. 2022, 54, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K. RNA Therapy: Current Status and Future Potential. Chonnam Med. J. 2020, 56, 87. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022, 19, 176–190. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, I.; Wang, K. Recent Advances in RNA Therapy and Its Carriers to Treat the Single-Gene Neurological Disorders. Biomedicines 2022, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Walgrave, H.; Zhou, L.; De Strooper, B.; Salta, E. The promise of microRNA-based therapies in Alzheimer’s disease: Challenges and perspectives. Mol. Neurodegener. 2021, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Kreth, S.; Hübner, M.; Hinske, L.C. MicroRNAs as Clinical Biomarkers and Therapeutic Tools in Perioperative Medicine. Obstet. Anesth. Dig. 2018, 126, 670–681. [Google Scholar] [CrossRef]
- Martier, R.; Konstantinova, P. Gene Therapy for Neurodegenerative Diseases: Slowing down the Ticking Clock. Front. Neurosci. 2020, 14, 580179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, F.; Liu, Y.; Zheng, M.; Wang, Y.; Zhang, D.; Anraku, Y.; Zou, Y.; Li, J.; Wu, H.; et al. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 2020, 6, eabc7031. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Lahiri, D.K. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem. Biophys. Res. Commun. 2011, 404, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, J.; Lu, G. miR-106b inhibits tau phosphorylation at Tyr18 by targeting Fyn in a model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2016, 478, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xu, B.; Chen, J.; Sui, Y.; Ren, L.; Li, J.; Zhang, H.; Guo, L.; Sun, X. Micro-RNA-137 Inhibits Tau Hyperphosphorylation in Alzheimer’s Disease and Targets the CACNA1C Gene in Transgenic Mice and Human Neuroblastoma SH-SY5Y Cells. Med. Sci. Monit. 2018, 24, 5635–5644. [Google Scholar] [CrossRef]
- Santa-Maria, I.; Alaniz, M.E.; Renwick, N.; Cela, C.; Fulga, T.A.; Van Vactor, D.; Tuschl, T.; Clark, L.N.; Shelanski, M.L.; McCabe, B.D.; et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Investig. 2015, 125, 681–686. [Google Scholar] [CrossRef]
- Estfanous, S.; Daily, K.P.; Eltobgy, M.; Deems, N.P.; Anne, M.N.K.; Krause, K.; Badr, A.; Hamilton, K.; Carafice, C.; Hegazi, A.; et al. Elevated Expression of MiR-17 in Microglia of Alzheimer’s Disease Patients Abrogates Autophagy-Mediated Amyloid-β Degradation. Front. Immunol. 2021, 12, 705581. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chopra, N.; Nho, K.; Maloney, B.; Obukhov, A.G.; Nelson, P.T.; Counts, S.E.; Lahiri, D.K. Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Mol. Psychiatry 2022, 27, 1256–1273. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, H.; Dong, W.; Quan, X.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Qin, C. miR-29c regulates BACE1 protein expression. Brain Res. 2011, 1395, 108–115. [Google Scholar] [CrossRef]
- Chopra, N.; Wang, R.; Maloney, B.; Nho, K.; Beck, J.S.; Pourshafie, N.; Niculescu, A.; Saykin, A.J.; Rinaldi, C.; Counts, S.E.; et al. MicroRNA-298 reduces levels of human amyloid-β precursor protein (APP), β-site APP-converting enzyme 1 (BACE1) and specific tau protein moieties. Mol. Psychiatry 2021, 26, 5636–5657. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Su, C.; Zhang, W.; Wan, Q. miR-485-5p alleviates Alzheimer’s disease progression by targeting PACS1. Transl. Neurosci. 2021, 12, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zuo, X.; Han, J.; Dai, Q.; Xu, H.; Liu, Y.; Cui, S. MiR-9-5p inhibits mitochondrial damage and oxidative stress in AD cell models by targeting GSK-3β. Biosci. Biotechnol. Biochem. 2020, 84, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, J.; Sun, X.; Ma, G.; Luo, G.; Miao, Z.; Song, L. miR-132 improves the cognitive function of rats with Alzheimer’s disease by inhibiting the MAPK1 signal pathway. Exp. Ther. Med. 2020, 20, 159. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, H.; Xu, Y.; Huang, L.; Ma, C.; Deng, W.; Liu, Y.; Qin, C. MicroRNA-153 negatively regulates the expression of amyloid precursor protein and amyloid precursor-like protein 2. Brain Res. 2012, 1455, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Rosler, T.W.; Carlsson, T.; Andrade, A.; Fiala, O.; Hollerhage, M.; Oertel, W.H.; Goedert, M.; Aigner, A.; Hoglinger, G.U. Tau Silencing by siRNA in the P301S Mouse Model of Tauopathy. Curr. Gene Ther. 2014, 14, 343–351. [Google Scholar] [CrossRef]
- Kandimalla, R.J.; Wani, W.Y.; Bk, B.; Gill, K.D. siRNA against presenilin 1 (PS1) down regulates amyloid b42 production in IMR-32 cells. J. Biomed. Sci. 2012, 19, 2. [Google Scholar] [PubMed]
- Senechal, Y.; Kelly, P.H.; Cryan, J.F.; Natt, F.; Dev, K.K. Amyloid precursor protein knockdown by siRNA impairs spontaneous alternation in adult mice: In vivo knockdown of APP by RNAi. J. Neurochem. 2007, 102, 1928–1940. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Perche, F.; Ikegami, M.; Uchida, S.; Kataoka, K.; Itaka, K. Messenger RNA-based therapeutics for brain diseases: An animal study for augmenting clearance of beta-amyloid by intracerebral administration of neprilysin mRNA loaded in polyplex nanomicelles. J. Control. Release 2016, 235, 268–275. [Google Scholar] [CrossRef]
- Lim, S.A.; Cox, A.; Tung, M.; Chung, E.J. Clinical progress of nanomedicine-based RNA therapies. Bioact. Mater. 2022, 12, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, A.; Purvinsh, L.; Brodskaia, A.; Vasin, A. Exosomes as Natural Nanocarriers for RNA-Based Therapy and Prophylaxis. Nanomaterials 2022, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, M.; Zivko, C.; Demetzos, C.; Mahairaki, V. Lipid-based nanoparticles and RNA as innovative neuro-therapeutics. Front. Pharmacol. 2022, 13, 900610. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Dias-Teixeira, M.; Delerue-Matos, C.; Grosso, C. Critical Review of Lipid-Based Nanoparticles as Carriers of Neuroprotective Drugs and Extracts. Nanomaterials 2021, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Bors, L.; Erdő, F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of Current Strategies for Delivering Alzheimer’s Disease Drugs across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Woo, H.; Kim, S.R. Addressing Blood–Brain Barrier Impairment in Alzheimer’s Disease. Biomedicines 2022, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, M.K.; Yen, T.-L.; Jan, J.-S.; Tang, R.-D.; Wang, J.-Y.; Taliyan, R.; Yang, C.-H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef] [PubMed]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Synthesis and Potential Applications of Lipid Nanoparticles in Medicine. Materials 2022, 15, 682. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Abriz, A.E.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Vakili, B.; Nezafat, N. Exosome-based vaccines and their position in next generation vaccines. Int. Immunopharmacol. 2022, 113, 109265. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Malih, S.; Eini, M.; Jafari, R.; Gholipourmalekabadi, M.; Sadeghizadeh, M.; Samadikuchaksaraei, A. Improvement, scaling-up, and downstream analysis of exosome production. Crit. Rev. Biotechnol. 2020, 40, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Su, H.; Li, J.; Lyon, C.; Tang, W.; Wan, M.; Hu, T.Y. Clinical applications of exosome membrane proteins. Precis. Clin. Med. 2020, 3, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Zhang, N.; He, F.; Li, T.; Chen, J.; Jiang, L.; Ouyang, X.-P.; Zuo, L. Role of Exosomes in Brain Diseases. Front. Cell Neurosci. 2021, 15, 743353. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Feng, R.; Patil, S.; Zhao, X.; Miao, Z.; Qian, A. RNA Therapeutics—Research and Clinical Advancements. Front. Mol. Biosci. 2021, 8, 710738. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Andronescu, E. Lipid Nanoparticles and Liposomes for Bone Diseases Treatment. Biomedicines 2022, 10, 3158. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Younis, M.A.; Alsowinea, A.F.; Abdallah, E.M.; Abdel-Bakky, M.S.; Al-Subaiyel, A.; Hassan, Y.A.; Tawfeek, H.M. Lipid nanoparticles technology in vaccines: Shaping the future of prophylactic medicine. Colloids Surf. B Biointerfaces 2023, 222, 113111. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Gamarra, L. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Gauro, R.; Nandave, M.; Jain, V.K.; Jain, K. Advances in dendrimer-mediated targeted drug delivery to the brain. J. Nanoparticle Res. 2021, 23, 76. [Google Scholar] [CrossRef]
- Teleanu, D.; Chircov, C.; Grumezescu, A.; Volceanov, A.; Teleanu, R. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Sela, H.; Cohen, H.; Elia, P.; Zach, R.; Karpas, Z.; Zeiri, Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J. Nanobiotechnol. 2015, 13, 71. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef] [PubMed]
- Schiffelers, R.; Kooijmans, S.; Vader, P.; Dommelen, V.; Solinge, V. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar] [CrossRef]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef]
- Zha, Y.; Lin, T.; Li, Y.; Zhang, X.; Wang, Z.; Li, Z.; Ye, Y.; Wang, B.; Zhang, S.; Wang, J. Exosome-mimetics as an engineered gene-activated matrix induces in-situ vascularized osteogenesis. Biomaterials 2020, 247, 119985. [Google Scholar] [CrossRef] [PubMed]
- Severic, M.; Ma, G.; Pereira, S.G.T.; Ruiz, A.; Cheung, C.C.; Al-Jamal, W.T. Genetically-engineered anti-PSMA exosome mimetics targeting advanced prostate cancer in vitro and in vivo. J. Control. Release 2021, 330, 101–110. [Google Scholar] [CrossRef]
- Lee, C.-S.; Fan, J.; Hwang, H.S.; Kim, S.; Chen, C.; Kang, M.; Aghaloo, T.; James, A.W.; Lee, M. Bone-Targeting Exosome Mimetics Engineered by Bioorthogonal Surface Functionalization for Bone Tissue Engineering. Nano Lett. 2023, 23, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lee, C.-S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of Small RNA-Modulated Exosome Mimetics for Bone Regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, M.; Jin, L.; Guo, P.; Zhang, Z.; Zhanghuang, C.; Tan, X.; Mi, T.; Liu, J.; Wu, X.; et al. Exosome mimetics derived from bone marrow mesenchymal stem cells deliver doxorubicin to osteosarcoma in vitro and in vivo. Drug Deliv. 2022, 29, 3291–3303. [Google Scholar] [CrossRef]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Li, Y.-J.; Wang, J.; Hu, X.-B.; Huang, S.; Luo, S.; Xiang, D.-X. Multifunctional exosome-mimetics for targeted anti-glioblastoma therapy by manipulating protein corona. J. Nanobiotechnol. 2021, 19, 405. [Google Scholar] [CrossRef]
- Yan, Y.; Du, C.; Duan, X.; Yao, X.; Wan, J.; Jiang, Z.; Qin, Z.; Li, W.; Pan, L.; Gu, Z.; et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm. Sin. B 2022, 12, 939–951. [Google Scholar] [CrossRef]
| Natural Compound | Role in AD | References |
|---|---|---|
| Eugenol | Rats were fed aluminum, a neurotoxic metal that leads to oxidative brain injury and enhanced lipid peroxidation, disruption of neurotrophic, cholinergic, and serotonergic functions, and induce apoptosis with ultimate neuronal and astrocyte damages. A neuroprotective role of eugenol against the aluminum effects was verified through its antioxidant, antiapoptotic potential and its neurotrophic properties. | [51] |
| Menthol | Menthol inhalation by mice (1 week per month, for 6 months) prevented cognitive impairment in the APP/PS1 mouse model of Alzheimer’s. | [52] |
| Chrysin | Chrysin showed the ability to act as a membrane shield against early oxidative events mediated by O2˙- and other ROS that contribute to neuronal death triggered by AlCl3 exposure, showing chrysin’s neuroprotective action. | [53] |
| Rosmarinic acid | Suppresses Aβ accumulation in mice. | [54] |
| Ginkgo biloba | Ginkgo biloba improves microcirculation, inhibits the expression of inflammatory factors, and reduces inflammatory damage to neurons, thereby improving the spatial exploration memory of dementia model rats. | [55] |
| Resveratrol | Multiple studies demonstrated that resveratrol has neuroprotective, anti-inflammatory, and antioxidant characteristics and the ability to minimize Aβ peptide aggregation and toxicity in the hippocampus of Alzheimer’s patients, stimulating neurogenesis and inhibiting hippocampal degeneration. Furthermore, resveratrol’s antioxidant effect promotes neuronal development by activating the silent information regulator-1, which can protect against the detrimental effects of oxidative stress. | [56] |
| Huperzine A | Huperzine A is natural, potent, highly specific reversible inhibitor of acetylcholinesterase, with the ability to cross the BBB. | [57] |
| Brahmi | The neuroprotective properties of Brahmi include the reduction of ROS and neuroinflammation, the inhibition of the aggregation of Aβ and the improvement of cognitive and learning behavior. | [58] |
| Uncaria tomentosa | Inhibits plaques and tangles formation. | [59] |
| Berberine | Berberine has antioxidant activity and promotes AChE and monoamine oxidase inhibition. Berberine has been shown to improve memory, lower Aβ and APP concentration, and diminish Aβ plaque accumulation. | [60] |
| Quercetin | Behavioral and biochemical tests confirm that quercetin promotes the reduction in oxidative stress and increased cognition in zebrafish AD models induced with aluminum chloride. | [61] |
| Betaine | Betaine has been shown to decrease homocysteine levels and Aβ toxicity in Caenorhabditis elegans AD model. | [62] |
| Curcumin | Curcumin is known to be a potent antioxidant, anti-inflammatory and anti-amyloidogenic compound, that plays a beneficial role in treating AD through several mechanisms. Curcumin can promote a significant reduction of Aβ oligomers and fibril formation. | [46] |
| Crocin | Crocin, the main constituent of Crocus sativus L., has a multifunctional role in protecting brain cells, modulating aggregation of Aβ and Tau proteins, attenuating cognitive and memory impairments, and improving oxidative stress. | [63] |
| Withania somnifera | Withania somnifera extract can protect against Aβ peptide- and acrolein-induced toxicity. Treatment with this extract significantly protected against Aβ and acrolein, in various cell survival assays with the human neuroblastoma cell line SK-N-SH, significantly reduced the generation of ROS and was demonstrated to be a potent inhibitor of AChE activity. | [64] |
| Poncirus trifoliate | The extract of Poncirus trifoliate is a naturally occurring AChE inhibitor. It showed a 47.31% inhibitory effect on the activity of acetylcholine. | [65] |
| Convolvulus pluricaulis | Convolvulus pluricaulis prevented aluminum-induced neurotoxicity in rat cerebral cortex. | [66] |
| α-Cyperone | α-Cyperone binds and interacts with tubulin, being capable of destabilizing microtubule polymerization. The effect of this interaction could result in reduction of inflammation. | [67] |
| Andrographolide | Andrographolide has beneficial effects in the recovery of spatial memory and learning performance, recovery of synaptic basal transmission, partial or complete protection of certain synaptic proteins and shows a specific neuroprotective effect, that includes the reduction of phosphorylated Tau and Aβ aggregate maturation, in aged degus. | [68] |
| Apigenin | Apigenin has been shown to have anti-inflammatory and neuroprotective properties in a number of cell and animal models. This compound is also able to protect human induced pluripotent stem cell-derived AD neurons via multiple pathways, by reducing the frequency of spontaneous Ca2+ signals and significantly reducing caspase-3/7 mediated apoptosis. | [69] |
| Baicalein | Baicalein has antioxidant and anti-inflammatory effects. | [70] |
| Carvacrol | Carvacrol possesses anti-AChE, antioxidant, and neuroprotective properties. This compound alleviated Aβ-induced deficits by reducing cellular neurotoxicity and oxidative stress in the SH-SY5Y cell line, and by reducing oxidative stress and memory impairment in a rat model of AD. | [71] |
| Decursin/Decursinol angelate | Decursin and decursinol angelate increase cellular resistance to Aβ-induced oxidative injury in PC12 cells. | [72] |
| Genistein | In vivo studies have shown that genistein improves brain function, antagonizes the toxicity of Aβ and has neuroprotective effects. | [73] |
| Wogonin | Wogonin has various neuroprotective and neurotrophic activities, such as inducing neurite outgrowth. | [74] |
| Rutin | Rutin is antioxidant, anti-inflammatory, and has the capacity of reducing Aβ oligomer activities. | [75] |
| Luteolin | Luteolin has the capacity to cross the BBB and can inhibit β- and γ-secretase to decrease Aβ. It can also reduce neuroinflammation and attenuate the phosphorylation of Tau. | [76] |
| Linalool | A linalool-treated mice model of AD showed improved learning and spatial memory. This compound reverses the histopathological hallmarks of AD and restores cognitive and emotional functions via an anti-inflammatory effect. | [77] |
| Asiatic acid | Pre-treatment with Asiatic Acid enhanced cell viability, attenuated rotenone-induced ROS, mitochondrial membrane dysfunction and apoptosis regulating AKT/GSK-3β signaling pathway, after aluminum maltolate neurotoxicity induction in SH-SY5Y neuroblastoma cells. | [78] |
| Exosome-like Liposomes Applications | References |
|---|---|
| Exosome mimetics-mediated gene-activated matrix encapsulating the plasmid of vascular endothelial growth factor (VEGF) was able to sustainably deliver VEGF gene and significantly enhance the vascularized osteogenesis in vivo. | [145] |
| PSMA-exosome mimetics showed increased cellular internalization in PSMA-positive PC cell lines (LNCaP and C4-2B) and higher tumor targeting was observed in solid C4-2B tumors, following intravenous administration, confirming their targeting ability in vivo. | [146] |
| Exosome mimetics are reported for bone targeting involving the introduction of hydroxyapatite-binding moieties through bioorthogonal functionalization. Bone-binding ability of the engineered exosome mimetics is verified with hydroxyapatite-coated scaffolds and an ex vivo bone-binding assay. | [147] |
| Administration of mesenchymal stem cells-exosome mimetics in conjunction with an injectable chitosan hydrogel into mouse nonhealing calvarial defects demonstrated robust bone regeneration. | [148] |
| Bone marrow mesenchymal stem cells were sequentially extruded to generate exosome-mimetic to encapsulate doxorubicin to treat osteosarcoma. The results showed that demonstrated significantly more potent tumor inhibition activity and fewer side effects than free doxorubicin. | [149] |
| In vitro, chemotherapeutic drug-loaded exosome-mimetics induced TNF-R-stimulated endothelial cell death in a dose-dependent manner. In vivo, experiments in mice showed that the chemotherapeutic drug-loaded exosome-mimetics traffic to tumor tissue and reduce tumor growth without the adverse effects observed with equipotent free drug. | [150] |
| Multifunctional exosomes-mimetics decorated with angiopep-2 (Ang-EM) incorporating Docetaxel, for enhancing glioblastoma drug delivery by manipulating protein corona, Ang-EM showed enhanced BBB penetration ability and targeting ability to the gioblastoma. Ang-EM-mediated delivery increased the concentration of docetaxel in the tumor area. | [151] |
| A designed lung-targeting liposomal nanovesicle carrying miR-29a-3p that mimics the exosomes, significantly down-regulated collagen I secretion by lung fibroblasts in vivo, thus alleviating the establishment of a pro-metastatic environment for circulating lung tumor cells. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.; Lopes, I.; Gomes, A.C. A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies. Molecules 2023, 28, 6015. https://doi.org/10.3390/molecules28166015
Ribeiro J, Lopes I, Gomes AC. A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies. Molecules. 2023; 28(16):6015. https://doi.org/10.3390/molecules28166015
Chicago/Turabian StyleRibeiro, Joana, Ivo Lopes, and Andreia Castro Gomes. 2023. "A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies" Molecules 28, no. 16: 6015. https://doi.org/10.3390/molecules28166015
APA StyleRibeiro, J., Lopes, I., & Gomes, A. C. (2023). A New Perspective for the Treatment of Alzheimer’s Disease: Exosome-like Liposomes to Deliver Natural Compounds and RNA Therapies. Molecules, 28(16), 6015. https://doi.org/10.3390/molecules28166015






