Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping
Abstract
1. Introduction
2. Experimental Procedure
2.1. Starting Materials
2.2. Synthesis of Undoped and RE–Doped CeO2
2.3. Characterization
2.4. Evaluation of OSC
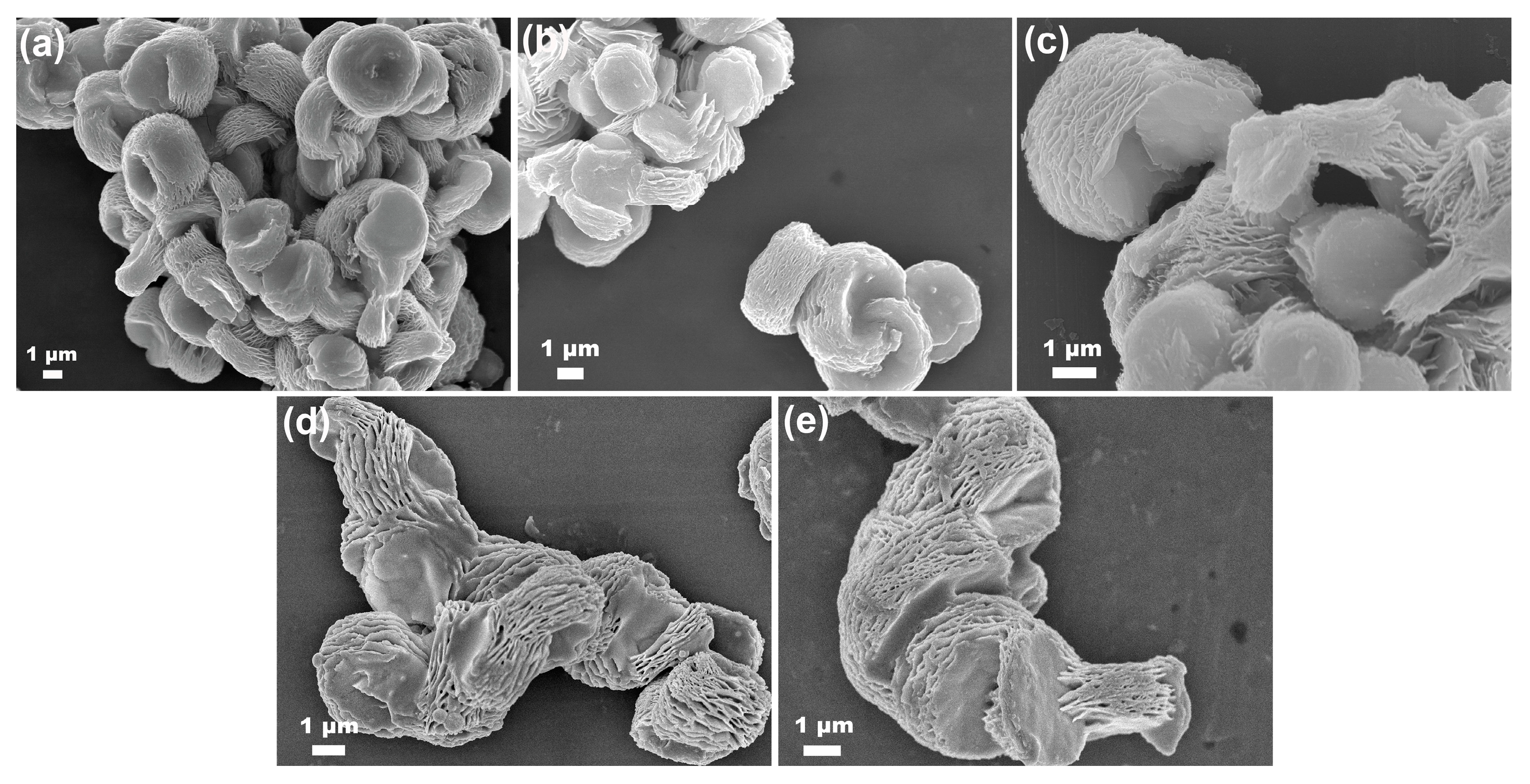
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Arnold, D. Composition-driven structural phase transitions in rare-earth-doped BiFeO3 ceramics: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2015, 62, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ren, Y.; Fu, H.; Zhao, H.; Wang, L.; Meng, X.; Qin, G. Recent developments in rare-earth free wrought magnesium alloys having high strength: A review. J. Alloys Compd. 2016, 663, 321–331. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H.; Luo, Z. A General Method for the Large-Scale Preparation of Multifunctional Magnetic Sm-Fe/Co Nanomaterials. Adv. Funct. Mater. 2023, 33, 2301350. [Google Scholar] [CrossRef]
- Fornasiero, P.; Trovarelli, A. Catalysis by ceria. Catal. Today 2015, 253, 1–2. [Google Scholar] [CrossRef]
- Shcherbakov, A.B.; Ivanov, V.K.; Zholobak, N.M.; Ivanova, O.S.; Krysanov, E.Y.; Baranchikov, A.E.; Spivak, N.Y.; Tretyakov, Y.D. Nanocrystalline ceria based materials-Perspectives for biomedical application. Biophysics 2011, 56, 987–1004. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Yang, J.; Wang, L.; Wang, Y.; Zhan, W.; Guo, Y.; Zhao, Y.; Guo, Y. Understanding the three-way catalytic reaction on Pd/CeO2 by tuning the chemical state of Pd. Appl. Surf. Sci. 2021, 556, 149766. [Google Scholar] [CrossRef]
- Yang, X.; Yang, L.; Lin, S.; Zhou, R. Investigation on properties of Pd/CeO2-ZrO2-Pr2O3 catalysts with different Ce/Zr molar ratios and its application for automotive emission control. J. Hazard. Mater. 2015, 285, 182–189. [Google Scholar] [CrossRef]
- Li, Y.; Kottwitz, M.; Vincent, J.L.; Enright, M.J.; Liu, Z.; Zhang, L.; Huang, J.; Senanayake, S.D.; Yang, W.C.D.; Crozier, P.A.; et al. Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction. Nat. Commun. 2021, 12, 914. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, A.; Xiong, S.; Xiao, X.; Zhou, C.; Pan, Y. The catalytic performance of Ga2O3-CeO2 composite oxides over reverse water gas shift reaction. ChemCatChem. 2022, 14, e202200049. [Google Scholar] [CrossRef]
- Si, R.; Flytzani-Stephanopoulos, M. Shape and Crystal-Plane Effects of Nanoscale Ceria on the Activity of Au-CeO2 Catalysts for the Water-Gas Shift Reaction. Angew. Chem. 2008, 120, 2926–2929. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, C.; Wang, L.; Xu, J.; Zheng, Q.; Pan, L.; Zou, J.; Zhang, X.; Li, G. Boosting hydrogen production from steam reforming of ethanol on nickel by lanthanum doped ceria. Appl. Catal. B-Environ. 2021, 286, 119884. [Google Scholar] [CrossRef]
- Moraes, T.S.; Neto, R.C.R.; Ribeiro, M.C.; Mattos, L.V.; Kourtelesis, M.; Verykios, X.; Noronha, F.B. Effects of Ceria Morphology on Catalytic Performance of Ni/CeO2 Catalysts for Low Temperature Steam Reforming of Ethanol. Top. Catal. 2015, 58, 281–294. [Google Scholar] [CrossRef]
- Kim, J.; Ryou, Y.; Kim, T.H.; Hwang, G.; Bang, J.; Jung, J.; Bang, Y.; Kim, D.H. Highly selective production of syngas (>99%) in the partial oxidation of methane at 480 °C over Pd/CeO2 catalyst promoted by HCl. Appl. Surf. Sci. 2021, 560, 150043. [Google Scholar] [CrossRef]
- Liu, H.; Lei, Q.; Miao, R.; Sun, M.; Qin, C.; Zhang, L.; Ye, G.; Yao, Y.; Huang, B.; Ma, Z. Asymmetric Coordination of Single-atom Co Sites Achieves Efficient Dehydrogenation Catalysis. Adv. Funct. Mater. 2022, 32, 2207408. [Google Scholar] [CrossRef]
- Prasad, D.H.; Ji, H.I.; Kim, H.R.; Son, J.W.; Kim, B.K.; Lee, H.W.; Lee, J.H. Effect of nickel nano-particle sintering on methane reforming activity of Ni-CGO cermet anodes for internal steam reforming SOFCs. Appl. Catal. B-Environ. 2011, 101, 531–539. [Google Scholar] [CrossRef]
- Liu, T.; Yang, R.; Zhang, G.; Wu, W.; Yang, Z.; Lin, R.; Wang, X.; Jiang, Y. Mechanism of selective catalytic reduction of NO with NH3 over CeO2-TiO2: Insight from in-situ DRIFTS and DFT calculations. Appl. Surf. Sci. 2021, 568, 150764. [Google Scholar] [CrossRef]
- Lu, J.; Asahina, S.; Takami, S.; Yoko, A.; Seong, G.; Tomai, T.; Adschiri, T. Interconnected 3D Framework of CeO2 with High Oxygen Storage Capacity: High-Resolution Scanning Electron Microscopic Observation. ACS Appl. Nano Mater. 2020, 3, 2346–2353. [Google Scholar] [CrossRef]
- Li, R.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T. UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater. Chem. Phys. 2022, 75, 39–44. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, S.; Chen, D.; He, C.; Tian, S.; Xiong, Y. Grey Fe-CeO2-σ for boosting photocatalytic ozonation of refractory pollutants: Roles of surface and bulk oxygen vacancies. Appl. Catal. B-Environ. 2021, 286, 119928. [Google Scholar] [CrossRef]
- Trovarelli, A. Structural and oxygen storage/release properties of CeO2-based solid solutions. Comment. Inorg. Chem. 1999, 20, 263–284. [Google Scholar] [CrossRef]
- Herz, R.K. Dynamic behavior of automotive catalysts. 1. Catalyst oxidation and reduction. Ind. Eng. Chem. Res. 1981, 20, 451–457. [Google Scholar] [CrossRef]
- McFarland, E.W.; Metiu, H. Catalysis by doped oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Tan, S.; Wang, D.; Wu, J.; Jia, T.; Liu, Q.; Qi, Y.; Qi, X.; He, P.; Zhou, M. CeO2/BiOIO3 heterojunction with oxygen vacancies and Ce4+/Ce3+ redox centers synergistically enhanced photocatalytic removal heavy metal. Appl. Surf. Sci. 2020, 530, 147116. [Google Scholar] [CrossRef]
- Gayen, A.; Priolkar, K.R.; Sarode, P.R.; Jayaram, V.; Hegde, M.S.; Subbanna, G.N.; Emura, S. Ce1−xRhxO2−δ Solid Solution Formation in Combustion-Synthesized Rh/CeO2 Catalyst Studied by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2004, 16, 2317–2328. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Thambidurai, M.; Dang, C.; Velauthapillai, D. Gadolinium doped CeO2 for efficient oxygen and hydrogen evolution reaction. Fuel 2022, 310, 122319. [Google Scholar] [CrossRef]
- Sawka, A.; Kwatera, A. Low temperature synthesis of Y2O3-doped CeO2 layers using MOCVD. Mat. Sci. Eng. B 2022, 276, 115580. [Google Scholar] [CrossRef]
- Balaguer, M.; Solís, C.; Serra, J.M. Structural-Transport Properties Relationships on Ce1−xLnxO2−δ System (Ln = Gd, La, Tb, Pr, Eu, Er, Yb, Nd) and Effect of Cobalt Addition. J. Phys. Chem. C 2012, 116, 7975–7982. [Google Scholar] [CrossRef]
- Zhang, L.; Spezzati, G.; Muravev, V.; Verheijen, M.A.; Hensen, E. Improved Pd/CeO2 catalysts for low-temperature no reduction: Activation of CeO2 lattice oxygen by Fe doping. ACS Catal. 2021, 11, 5614–5627. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Wu, L.; Zhao, H.; Li, L.; Sun, C.; An, B. Vacancy-engineered CeO2/Co heterostructure anchored on the nitrogen-doped porous carbon nanosheet arrays vertically grown on carbon cloth as an integrated cathode for the oxygen reduction reaction of rechargeable Zn-air battery. J. Mater. Chem. A 2022, 10, 9858–9868. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Liu, W.; Wang, S.; Xie, A.; Liu, X.; Wang, J.; Yang, Y. Facile Preparation of Mn+-Doped (M = Cu, Co, Ni, Mn) Hierarchically Mesoporous CeO2 Nanoparticles with Enhanced Catalytic Activity for CO Oxidation. Eur. J. Inorg. Chem. 2015, 6, 969–976. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Shelly, L.; Rosen, B.A.; Hayun, S. Surface properties of Ca, Ti-doped CeO2 and their influence on the reverse water-gas shift reaction. J. Am. Ceram. Soc. 2021, 104, 2237–2247. [Google Scholar] [CrossRef]
- Carey, J.J.; Nolan, M. Cation doping size effect for methane activation on alkaline earth metal doping of the CeO2 (111) surface. Catal. Sci. Technol. 2016, 6, 3544–3558. [Google Scholar] [CrossRef]
- Murakami, K.; Mizutani, Y.; Sampei, H.; Ishikawa, A.; Sekine, Y. Manipulation of co adsorption over Me1/CeO2 by heterocation doping: Key roles of single-atom adsorption energy. J. Chem. Phys. 2021, 154, 164705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Lin, Y.; Huang, W.; Ding, D.; Zheng, Y.; Chen, M.; Wan, H. Insight into the high efficiency of Cu/CeO2 (110) catalysts for preferential oxidation of CO from hydrogen rich fuel. Appl. Surf. Sci. 2021, 566, 150707. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Wang, Y.; Yin, R.; Yang, W.; Wang, G.; Si, W.; Peng, Y.; Li, J. Interaction mechanism for simultaneous elimination of nitrogen oxides and toluene over the bifunctional CeO2-TiO2 mixed oxide catalyst. Environ. Sci. Technol. 2022, 56, 4467–4476. [Google Scholar] [CrossRef]
- Chen, L.A.; Lu, Y.S.; Lin, Y.T.; Lee, Y.L. Preparation and characterization of cerium-based conversion coating on a Fe50Mn30Co10Cr10 dual-phase high-entropy alloy. Appl. Surf. Sci. 2021, 562, 150200. [Google Scholar] [CrossRef]
- Yang, S.M.; Lee, S.; Jian, J.; Zhang, W.; Lu, P.; Jia, Q.; Wang, H.; Noh, T.T.; Kalinin, S.V.; MacManus-Driscoll, J.L. Strongly enhanced oxygen ion transport through samarium-doped CeO2 nanopillars in nanocomposite films. Nat. Commun. 2015, 6, 8588. [Google Scholar] [CrossRef]
- Tarui, K.; Oomori, T.; Ito, Y.; Yamamoto, T. Origin of room-temperature ferromagnetism in Co-doped CeO2. Phys. B Condens. Matter 2021, 619, 413158. [Google Scholar] [CrossRef]
- Motaung, D.E.; Tshabalala, Z.P.; Makgwane, P.R.; Mahmoud, F.A.; Oosthuizen, D.N.; Cummings, F.R.; Leshabane, N.; Hintsho-Mbita, N.; Li, X.; Ray, S.S.; et al. Multi-functioning of CeO2-SnO2 heterostructure as room temperature ferromagnetism and chemiresistive sensors. J. Alloy. Compd. 2022, 906, 164317. [Google Scholar] [CrossRef]
- Singh, P.; Hegde, M.S. Controlled synthesis of nanocrystalline CeO2 and Ce1−xMxO2−δ (M = Zr, Y, Ti, Pr and Fe) solid solutions by the hydrothermal method: Structure and oxygen storage capacity. J. Solid State Chem. 2008, 181, 3248–3256. [Google Scholar] [CrossRef]
- Ansari, A.A.; Labis, J.; Alam, M.; Ramay, S.M.; Ahmad, N.; Mahmood, A. Physicochemical and Redox Characteristics of Fe Ion-doped CeO2 Nanoparticles. J. Chin. Chem. Soc. 2015, 62, 925–932. [Google Scholar] [CrossRef]
- Si, R.; Zhang, Y.W.; Li, S.J.; Lin, B.X.; Yan, C.H. Urea-Based Hydrothermally Derived Homogeneous Nanostructured Ce1−xZrxO2 (x = 0–0.8) Solid Solutions: A Strong Correlation between Oxygen Storage Capacity and Lattice Strain. J. Phys. Chem. B 2004, 108, 12481–12488. [Google Scholar] [CrossRef]
- Shannon, R.T. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Soni, B.; Makkar, S.; Biswas, S. Effects of surface structure and defect behavior on the magnetic, electrical, and photocatalytic properties of Gd-doped CeO2 nanoparticles synthesized by a simple chemical process. Mater. Charact. 2021, 174, 110990. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Yuan, S.; Liu, S.; Zhang, M.; Ohno, T. Synthesis and photocatalytic performance of yttrium-doped CeO2 with a hollow sphere structure. Catal. Today 2017, 281, 135–143. [Google Scholar] [CrossRef]
- Ke, J.; Xiao, J.W.; Zhu, W.; Liu, H.; Si, R.; Zhang, Y.W.; Yan, C.H. Dopant-Induced Modification of Active Site Structure and Surface Bonding Mode for High-Performance Nanocatalysts: CO Oxidation on Capping-free (110)-oriented CeO2:Ln (Ln = La-Lu) Nanowires. J. Am. Chem. Soc. 2013, 135, 15191–15200. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. 1976, 17, 1686–1698. [Google Scholar] [CrossRef]
- Fernández-García, M.; Martínez-Arias, A.; Guerrero-Ruiz, A.; Conesa, J.C.; Soria, J. Ce-Zr-Ca Ternary Mixed Oxides: Structural Characteristics and Oxygen Handling Properties. J. Catal. 2002, 211, 326–334. [Google Scholar] [CrossRef]
- Dohčević-Mitrović, Z.D.; Grujić-Brojčin, M.; Šćepanović, M.; Popović, Z.V.; Bošković, S.; Matović, B.; Zinkevich, M.; Aldinger, F. Ce1−xY(Nd)xO2−δ nanopowders: Potential materials for intermediate temperature solid oxide fuel cells. J. Phys. Condens. Matter 2006, 18, S2061–S2068. [Google Scholar] [CrossRef]
- Lin, X.M.; Li, L.P.; Li, G.S.; Su, W.H. Transport property and Raman spectra of nanocrystalline solid solutions Ce0.8Nd0.2O2−δ with different particle size. Mater. Chem. Phys. 2001, 69, 236–240. [Google Scholar] [CrossRef]
- Fernández-García, M.; Wang, X.Q.; Belver, C.; Iglesias-Juez, A.; Hanson, J.C.; Rodriguez, J. Ca Doping of Nanosize Ce-Zr and Ce-Tb Solid Solutions: Structural and Electronic Effects. Chem. Mater. 2005, 17, 4181–4193. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and x-ray studies of Ce1−xRExO2−y, where RE= La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.W.; Herman, I.P. Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef]
- Pu, Z.Y.; Lu, J.Q.; Luo, M.F.; Xie, Y.L. Study Of Oxygen Vacancies In Ce0.9Pr0.1O2−δ Solid Solution By In Situ X-Ray Diffraction And In Situ Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 18695–18702. [Google Scholar] [CrossRef]
- Tang, X.L.; Zhang, B.C.; Li, Y.; Xu, Y.D.; Xin, Q.; Shen, W.J. Carbon monoxide oxidation over CuO/CeO2 catalysts. Catal. Today 2004, 93, 191–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Deng, J.; Dai, H.; He, H. Controlled Synthesis, Characterization, and Morphology-Dependent Reducibility of Ceria-Zirconia-Yttria Solid Solutions with Nanorod-like, Microspherical, Microbowknot-like, and Micro-octahedral Shapes. Inorg. Chem. 2009, 48, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Katta, L.; Sudarsanam, P.; Thrimurthulu, G.; Reddy, B.M. Doped nanosized ceria solid solutions for low temperature soot oxidation: Zirconium versus lanthanum promoters. Appl. Catal. B-Environ. 2010, 101, 101–108. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Yang, P.; Song, X.; Zhu, Y.; Ma, Q. Fabrication of electronspun porous CeO2 nanofibers with large surface area for pollutants removal. Ceram. Int. 2016, 42, 14028–14035. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, X.; Wang, F.; Kang, S.; Yin, C.; Li, X. Nanocasting synthesis of chromium doped mesoporous CeO2 with enhanced visible-light photocatalytic CO2 reduction performance. J. Hazard. Mater. 2017, 372, 69–76. [Google Scholar] [CrossRef]
- Zhao, P.S.; Gao, X.M.; Zhu, F.X.; Hu, X.M.; Zhang, L.L. Ultrasonic-assisted Solution-Phase Synthesis and Property Studies of Hierarchical Layer-by-Layer Mesoporous CeO2. Bull. Korean Chem. Soc. 2018, 39, 375–380. [Google Scholar] [CrossRef]
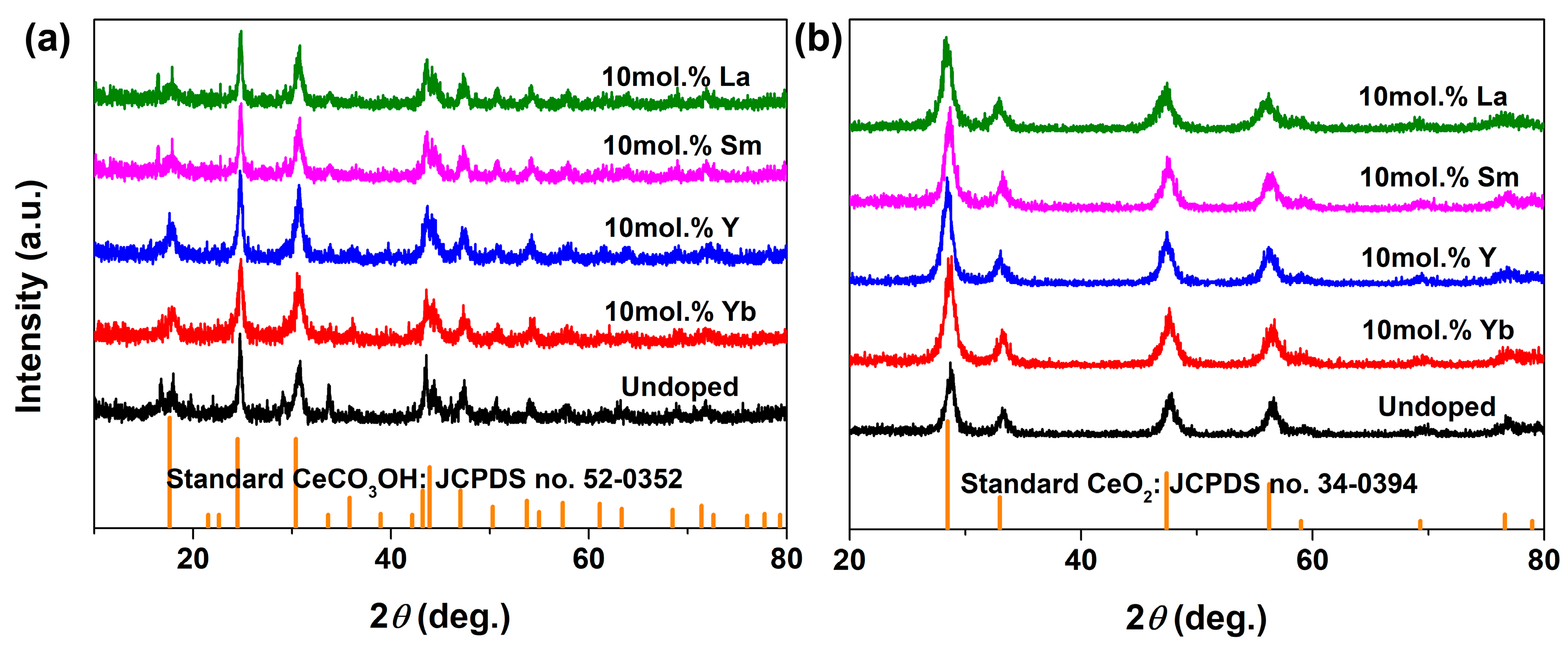
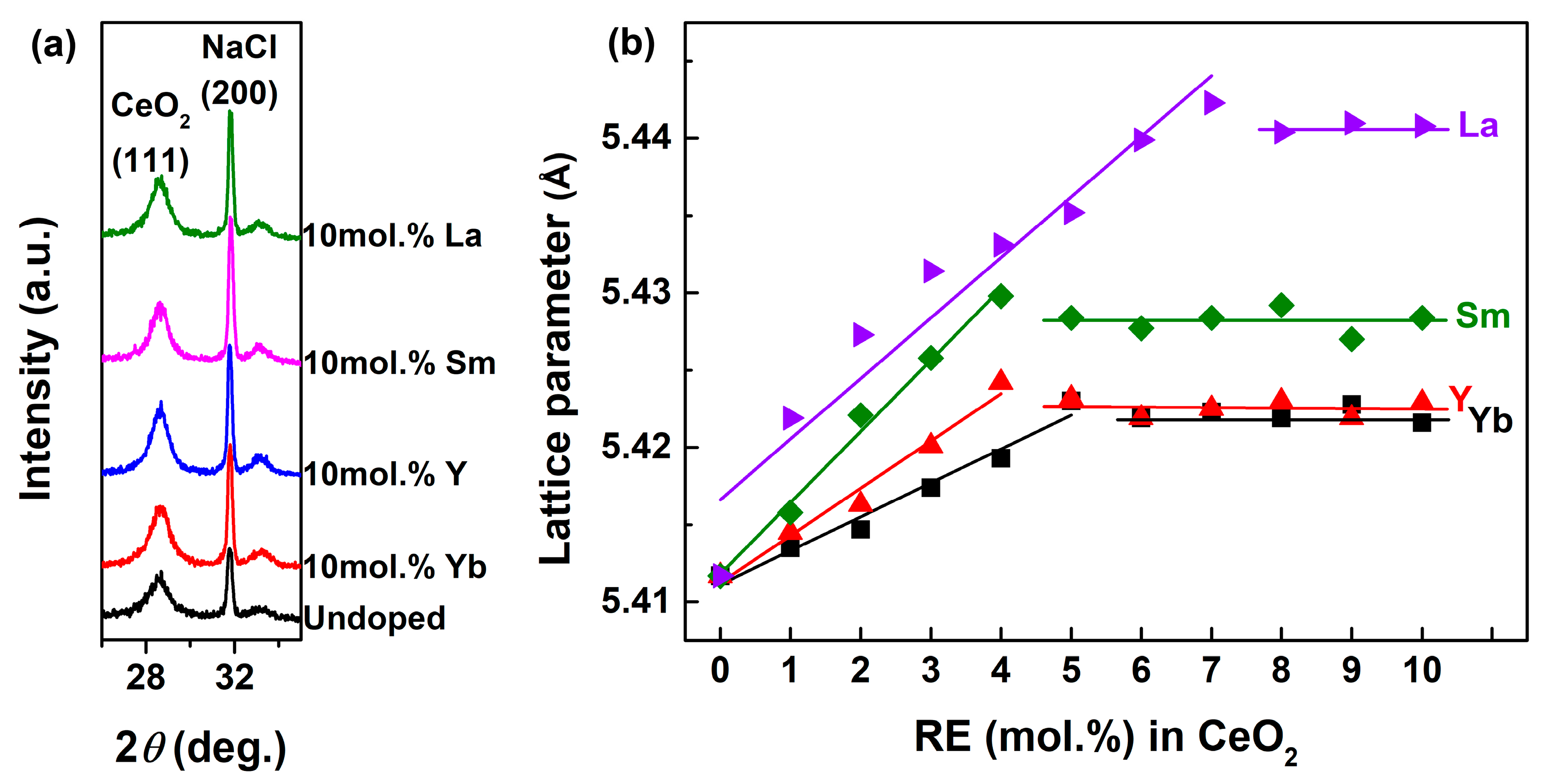

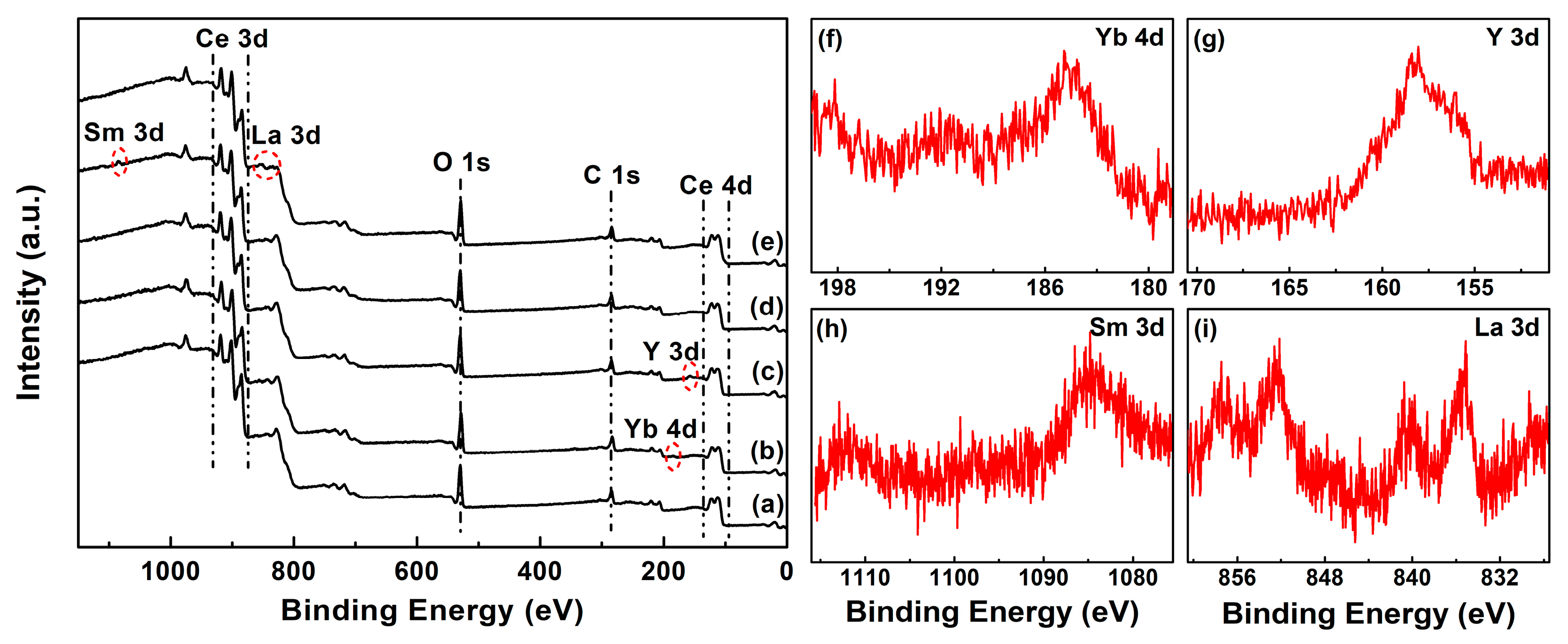
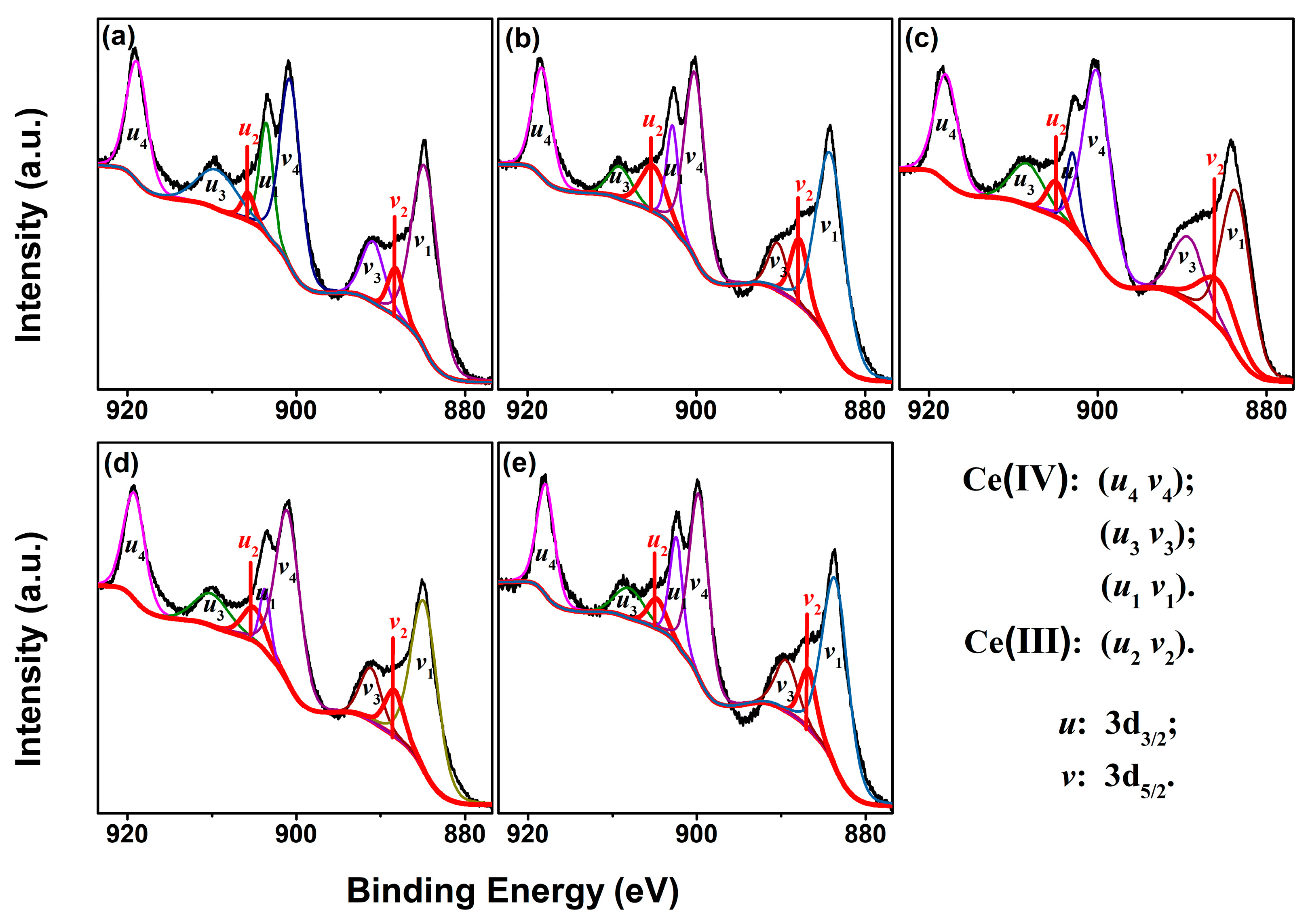
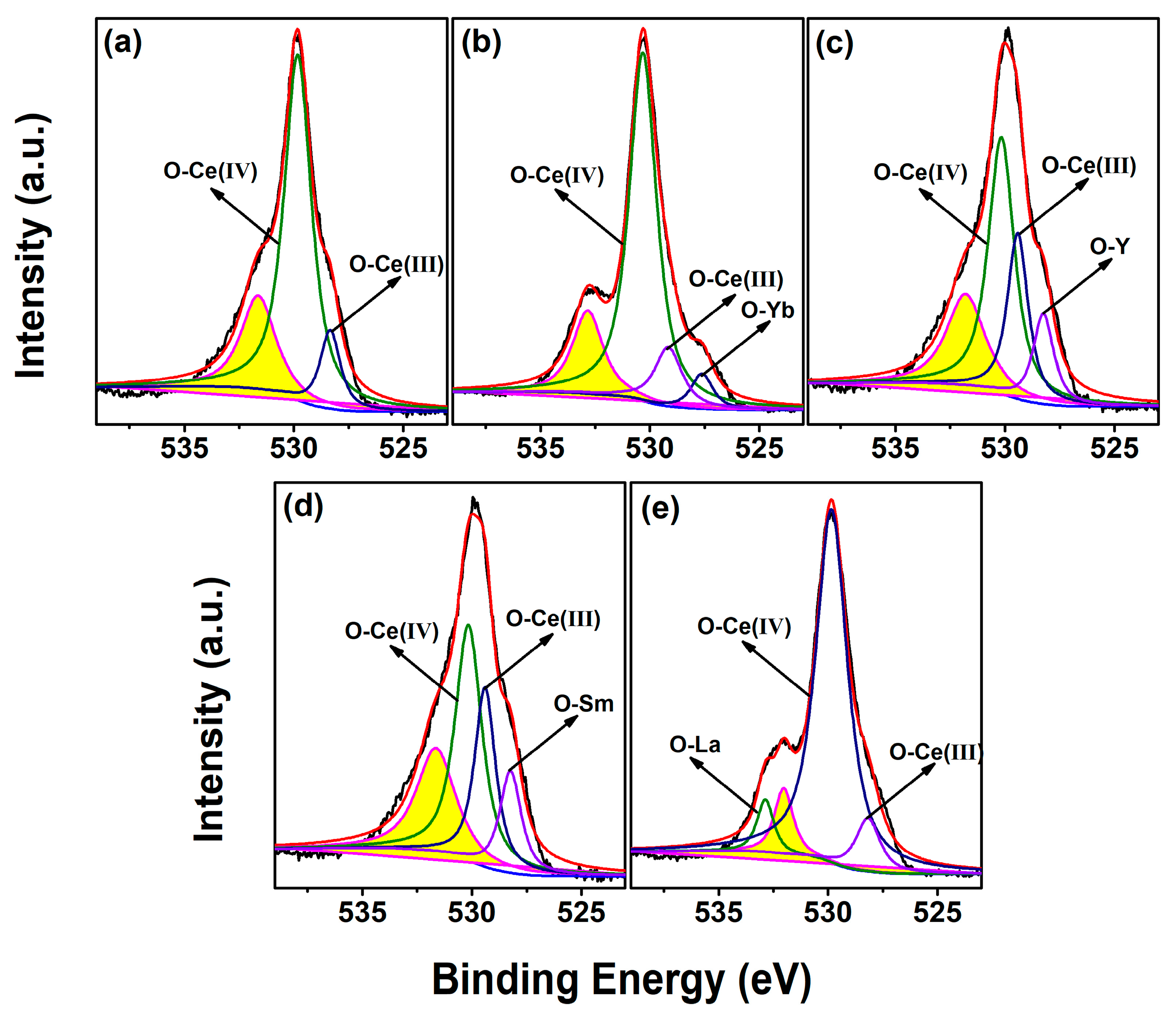
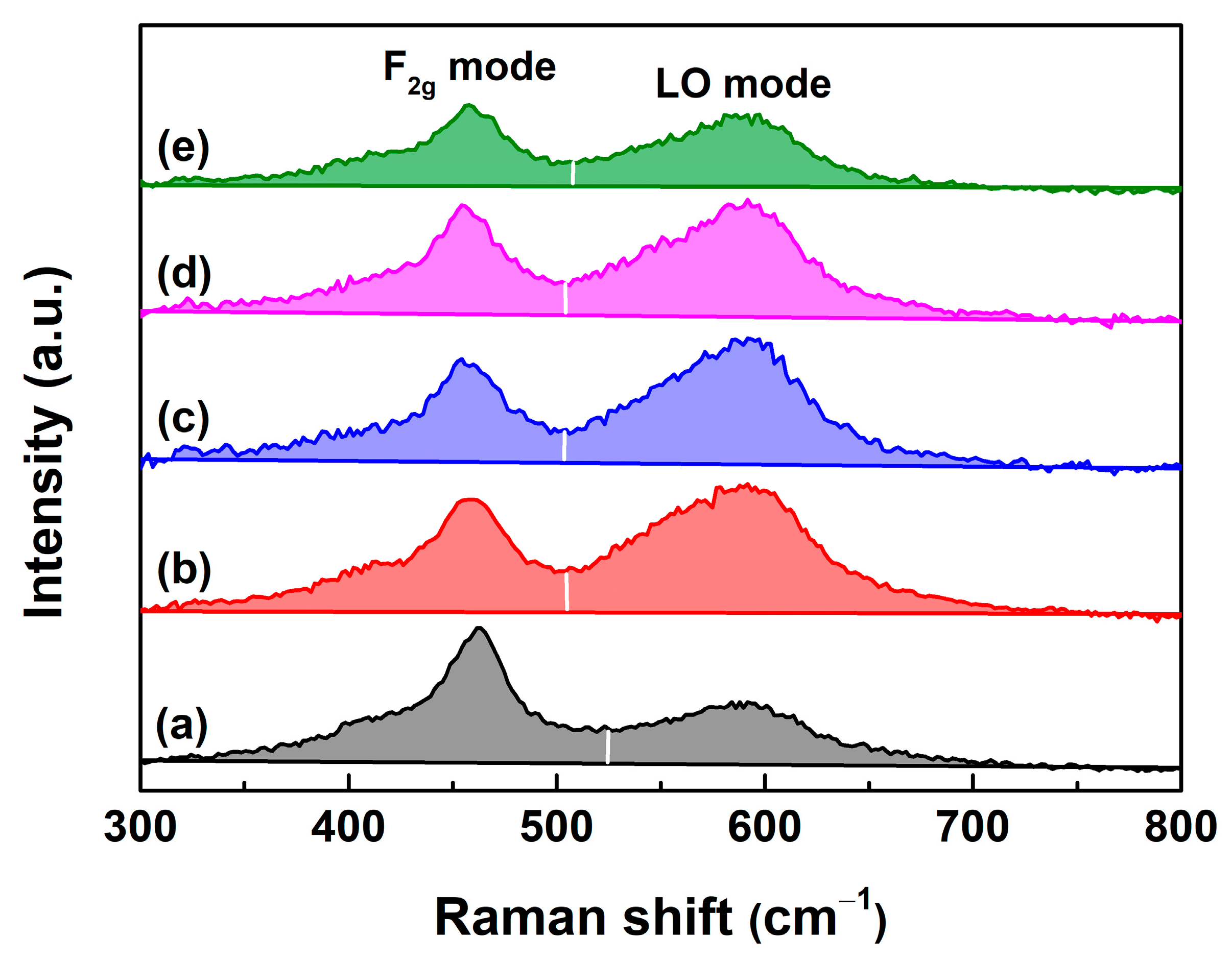
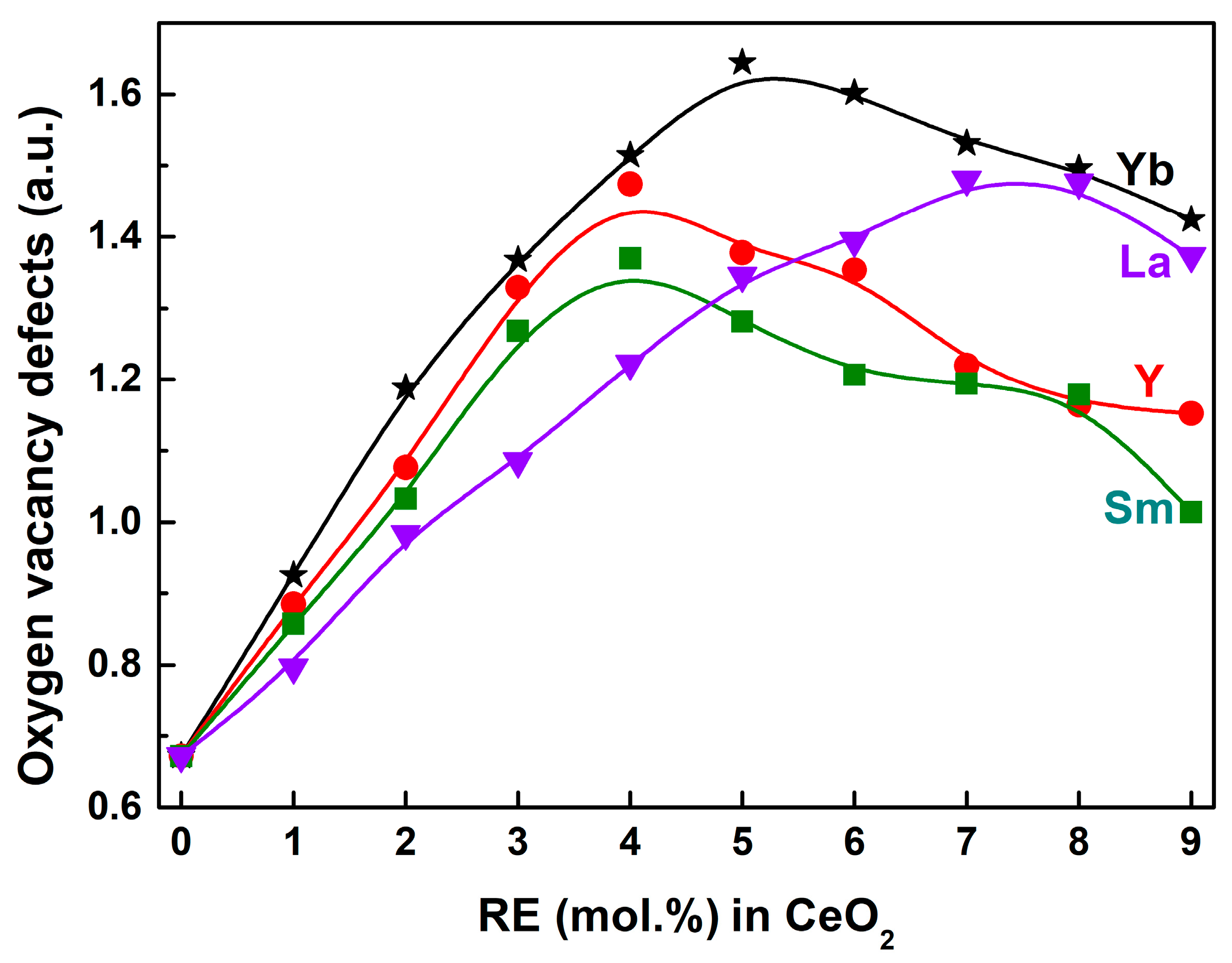
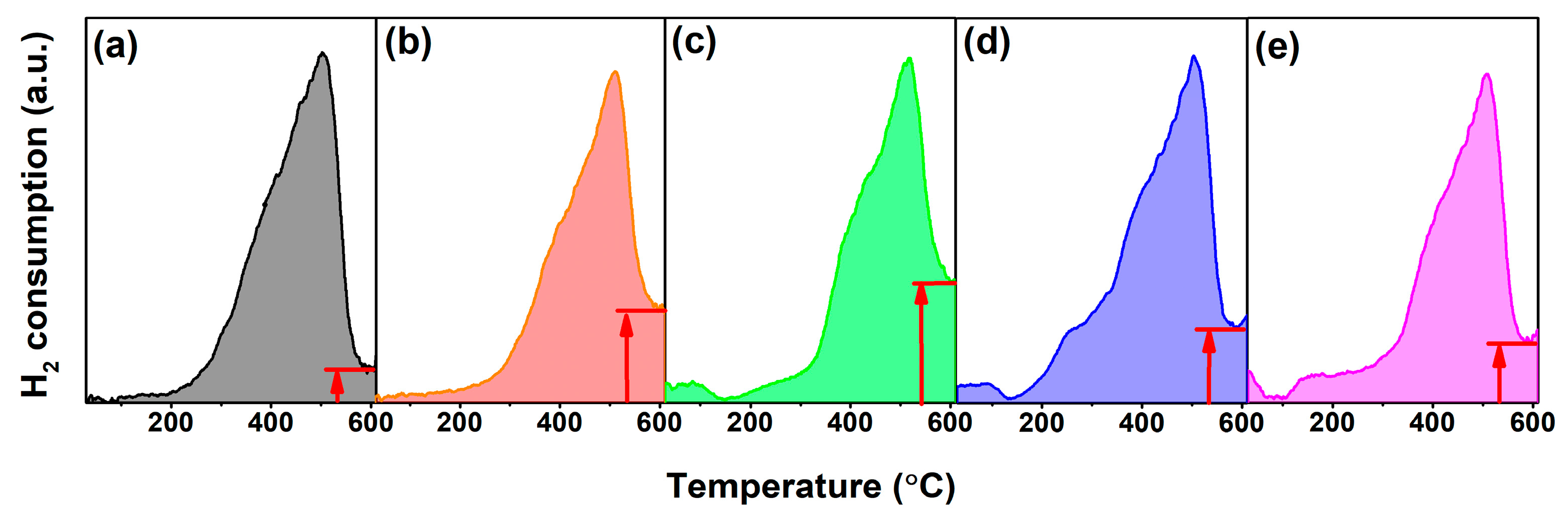


| RE in CeO2 (mol.%) | Yb | Y | Sm | La | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Nominal contents | 2 | 5 | 9 | 2 | 4 | 9 | 2 | 4 | 9 | 2 | 7 | 9 |
| * Practical RE contents | 2.58 | 5.26 | 9.62 | 1.92 | 4.24 | 8.67 | 2.41 | 4.27 | 9.38 | 2.19 | 6.79 | 9.25 |
| Sample | Undoped CeO2 | 4 mol.% RE–Doped CeO2 | ||||
|---|---|---|---|---|---|---|
| Parameter | Yb | Y | Sm | La | ||
| [Ce3+]XPS (%) | 6.54 | 13.78 | 12.60 | 10.94 | 9.78 | |
| [VO]XPS (%) | 13.42 | 30.02 | 26.82 | 26.81 | 17.28 | |
| Specific surface area (m2/g) | 96.0 | 89.7 | 98.1 | 112.6 | 104.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Gao, L.; Hou, Q.; Wu, P.; Zhou, Y.; Ding, Z. Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping. Molecules 2023, 28, 6005. https://doi.org/10.3390/molecules28166005
Xu Y, Gao L, Hou Q, Wu P, Zhou Y, Ding Z. Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping. Molecules. 2023; 28(16):6005. https://doi.org/10.3390/molecules28166005
Chicago/Turabian StyleXu, Yaohui, Liangjuan Gao, Quanhui Hou, Pingkeng Wu, Yunxuan Zhou, and Zhao Ding. 2023. "Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping" Molecules 28, no. 16: 6005. https://doi.org/10.3390/molecules28166005
APA StyleXu, Y., Gao, L., Hou, Q., Wu, P., Zhou, Y., & Ding, Z. (2023). Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping. Molecules, 28(16), 6005. https://doi.org/10.3390/molecules28166005








