Molecular and Cellular Mechanisms of Action of Cannabidiol
Abstract
1. Introduction
1.1. CBD as a Therapeutic Option
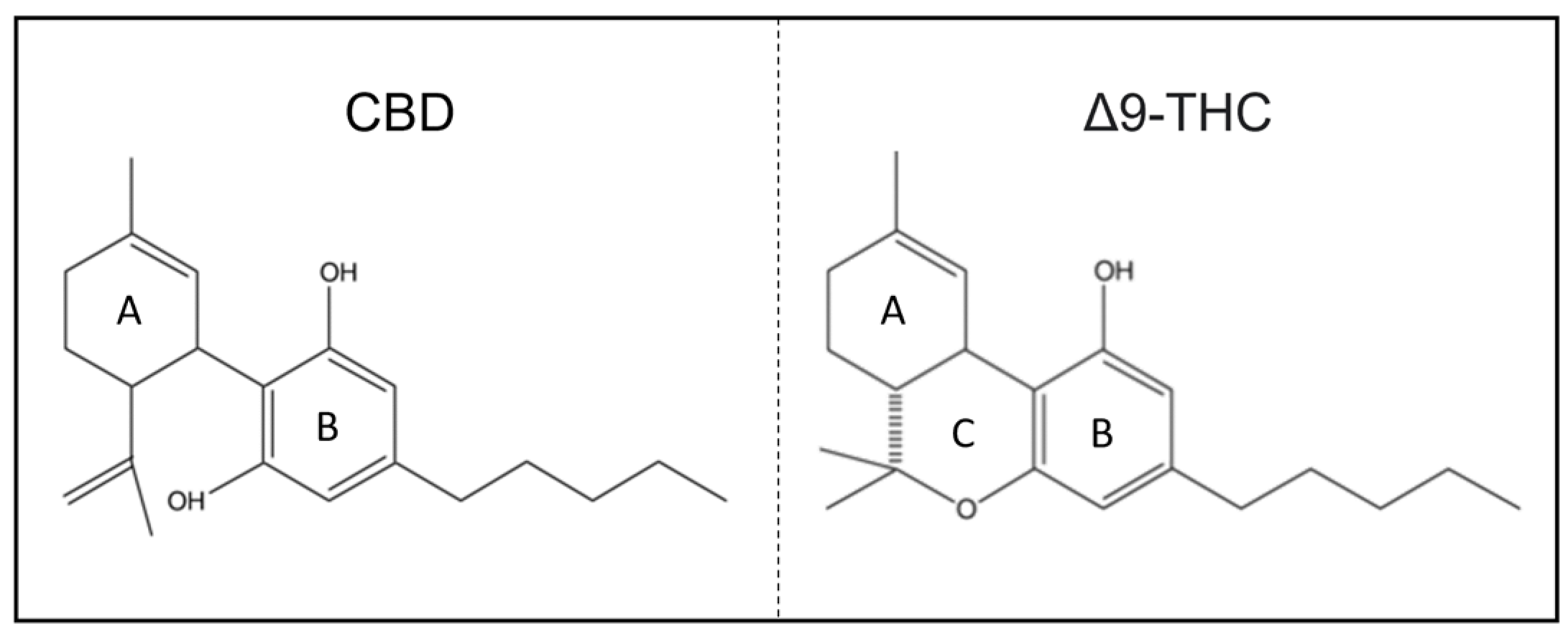
1.2. Chemical Structure
2. Pharmacokinetics
Safety and Adverse Effects
3. The Endocannabinoid System
3.1. Cannabinoid Receptors and Endocannabinoids
3.2. CBD and the Cannabinoid Receptors
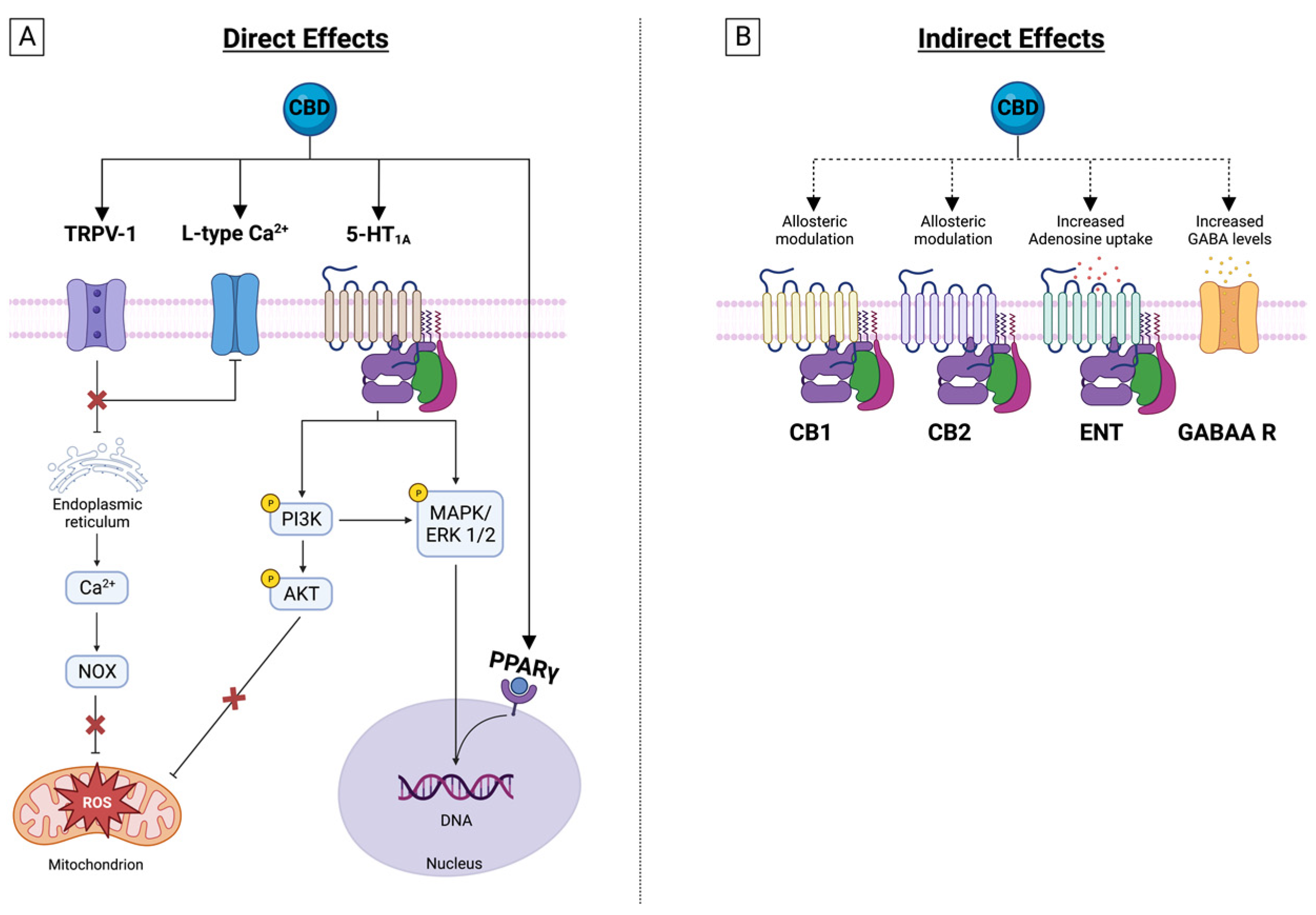
4. CBD Effects on Membrane Receptors
4.1. Transient Receptor Potential Cation Channels (TRP)
4.2. Serotonin Receptor 1 A
4.3. GABAA Receptors
4.4. Nuclear Peroxisome Proliferator-Activated Receptors (PPAR)
4.5. Adenosine Receptors
5. CBD’s Effect on Inflammatory Signaling
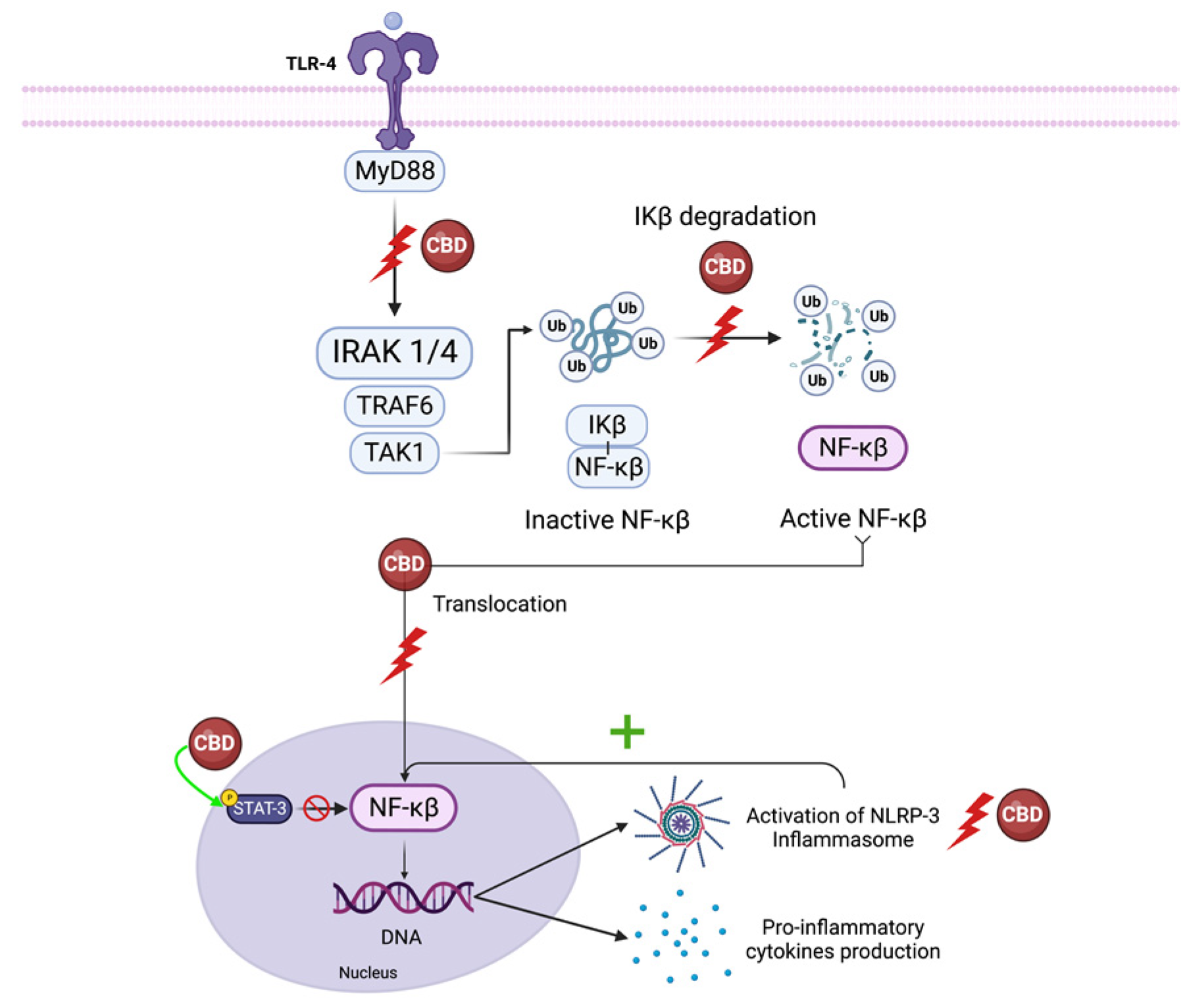
5.1. NF-κB and Interferon Beta
5.1.1. NF-κB
5.1.2. IFN-β
5.2. NLRP3 Inflammasome
5.3. IFN-γ
5.4. TNF-α
5.5. Oxidative Damage
6. CBD Modulates Inflammatory Cell Functions
6.1. Neutrophil Activation
6.2. Effects on Lymphocytes
7. CBD Affects the Fibrotic Response
8. CBD Regulates Apoptosis
9. CBD Effect on Ion Channels
9.1. Calcium Channels
9.2. Sodium Channels
9.3. Potassium Channels
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. Available online: https://www.sciencedirect.com/science/article/pii/S003194221500134X (accessed on 31 January 2023). [CrossRef]
- Rock, E.M.; Parker, L.A. Constituents of Cannabis sativa. In Cannabinoids and Neuropsychiatric Disorders; Murillo-Rodriguez, E., Pandi-Perumal, S.R., Monti, J.M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–13. ISBN 978-3-030-57369-0. [Google Scholar] [CrossRef]
- Subramaniam, V.N.; Menezes, A.R.; DeSchutter, A.; Lavie, C.J. The Cardiovascular Effects of Marijuana: Are the Potential Adverse Effects Worth the High? Mo. Med. 2019, 116, 146–153. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6461323/ (accessed on 24 May 2023). [PubMed]
- Viana, M.B.; de Aquino, P.E.A.; Estadella, D.; Ribeiro, D.A.; Viana, G.S.B. Cannabis sativa and Cannabidiol: A Therapeutic Strategy for the Treatment of Neurodegenerative Diseases? Med. Cannabis Cannabinoids 2022, 5, 207–219. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9710321/ (accessed on 2 January 2023). [CrossRef] [PubMed]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. Available online: https://pubs.acs.org/doi/abs/10.1021/ja01858a058 (accessed on 31 January 2023). [CrossRef]
- Mechoulam, R.; Parker, L.A.; Gallily, R. Cannabidiol: An Overview of Some Pharmacological Aspects. J. Clin. Pharmacol. 2002, 42, 11S–19S. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/j.1552-4604.2002.tb05998.x (accessed on 2 January 2023). [CrossRef] [PubMed]
- Mechoulam, R.; Shvo, Y. Hashish—I: The structure of Cannabidiol. Tetrahedron 1963, 19, 2073–2078. Available online: https://www.sciencedirect.com/science/article/pii/004040206385022X (accessed on 18 January 2023). [CrossRef] [PubMed]
- Orrin Devinsky, J.; Cross, H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, M.D.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Maguire, R.F.; Wilkinson, D.J.; England, T.J.; O’Sullivan, S.E. The Pharmacological Effects of Plant-Derived versus Synthetic Cannabidiol in Human Cell Lines. Med. Cannabis Cannabinoids 2021, 4, 86–96. Available online: https://www.karger.com/Article/FullText/517120 (accessed on 31 January 2023). [CrossRef]
- Jung, B.; Lee, J.K.; Kim, J.; Kang, E.K.; Han, S.Y.; Lee, H.-Y.; Choi, I.S. Synthetic Strategies for (-)-Cannabidiol and Its Structural Analogs. Chem. Asian J. 2019, 14, 3749–3762. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. Available online: https://www.mdpi.com/2076-3921/9/1/21 (accessed on 1 March 2023). [CrossRef]
- Jones, P.G.; Falvello, L.; Kennard, O.; Sheldrick, G.M.; Mechoulam, R. Cannabidiol. Acta Crystallogr. Sect. B 1977, 33, 3211–3214. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1107/S0567740877010577 (accessed on 1 March 2023). [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6177698/ (accessed on 1 March 2023). [CrossRef]
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and drug metabolizing enzymes: Potential for drug-drug interactions and implications for drug safety and efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef]
- Knaub, K.; Sartorius, T.; Dharsono, T.; Wacker, R.; Wilhelm, M.; Schön, C. A Novel Self-Emulsifying Drug Delivery System (SEDDS) Based on VESIsorb® Formulation Technology Improving the Oral Bioavailability of Cannabidiol in Healthy Subjects. Molecules 2019, 24, 2967. Available online: https://www.mdpi.com/1420-3049/24/16/2967 (accessed on 31 January 2023). [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.; Lee, J.B.; Mistry, J.; Sivak, O.; Wasan, K.M.; Hennig, I.M.; Barrett, D.A.; Constantinescu, C.S.; Fischer, P.M.; et al. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis-based medicines. Am. J. Transl. Res. 2016, 8, 3448–3459. [Google Scholar] [PubMed]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.P.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806. Available online: https://www.nature.com/articles/s41386-020-0667-2 (accessed on 1 February 2023). [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7052834/ (accessed on 2 January 2023). [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. Available online: http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pr.110.003004 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. Available online: https://www.science.org/doi/full/10.1126/science.1115740 (accessed on 15 February 2023). [CrossRef] [PubMed]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. Available online: https://www.sciencedirect.com/science/article/pii/S0006899305016239 (accessed on 15 February 2023). [CrossRef]
- Juan-Picó, P.; Fuentes, E.; Javier Bermúdez-Silva, F.; Javier Díaz-Molina, F.; Ripoll, C.; Rodríguez de Fonseca, F.; Nadal, A. Cannabinoid receptors regulate Ca2+ signals and insulin secretion in pancreatic β-cell. Cell Calcium 2006, 39, 155–162. Available online: https://www.sciencedirect.com/science/article/pii/S0143416005002010 (accessed on 15 February 2023). [CrossRef] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. Available online: https://pharmrev.aspetjournals.org/content/58/3/389 (accessed on 15 February 2023). [CrossRef]
- Karsak, M.; Cohen-Solal, M.; Freudenberg, J.; Ostertag, A.; Morieux, C.; Kornak, U.; Essig, J.; Erxlebe, E.; Bab, I.; Kubisch, C.; et al. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005, 14, 3389–3396. [Google Scholar] [CrossRef]
- Idris, A.I.; van’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. Available online: https://www.nature.com/articles/nm1255 (accessed on 15 February 2023). [CrossRef] [PubMed]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fat. Acids PLEFA 2002, 66, 101–121. Available online: https://www.sciencedirect.com/science/article/pii/S0952327801903412 (accessed on 15 February 2023). [CrossRef]
- Ross, R.A. Allosterism and cannabinoid CB1 receptors: The shape of things to come. Trends Pharmacol. Sci. 2007, 28, 567–572. Available online: https://www.sciencedirect.com/science/article/pii/S0165614707002313 (accessed on 15 February 2023). [CrossRef]
- Iversen, L.L. Medical uses of marijuana? Nature 1993, 365, 12–13. Available online: http://www.nature.com/articles/365012a0 (accessed on 13 January 2023). [CrossRef] [PubMed]
- Hillard, C.J.; Jarrahian, A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem. Phys. Lipids 2000, 108, 123–134. Available online: https://www.sciencedirect.com/science/article/pii/S0009308400001912 (accessed on 15 February 2023). [CrossRef]
- Piomelli, D.; Beltramo, M.; Glasnapp, S.; Lin, S.Y.; Goutopoulos, A.; Xie, X.-Q.; Makriyannis, A. Structural determinants for recognition and translocation by the anandamide transporter. Proc. Natl. Acad. Sci. USA 1999, 96, 5802–5807. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC21941/ (accessed on 15 February 2023). [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. Available online: https://www.mdpi.com/1422-0067/21/14/5064 (accessed on 4 August 2023). [CrossRef] [PubMed]
- Petitet, F.; Jeantaud, B.; Reibaud, M.; Imperato, A.; Dubroeucq, M.-C. Complex pharmacology of natural cannabivoids: Evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998, 63, PL1–PL6. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0024320598002380 (accessed on 15 February 2023). [CrossRef]
- Thomas, B.F.; Gilliam, A.F.; Burch, D.F.; Roche, M.J.; Seltzman, H.H. Comparative Receptor Binding Analyses of Cannabinoid Agonists and Antagonists. J. Pharmacol. Exp. Ther. 1998, 285, 285–292. Available online: https://jpet.aspetjournals.org/content/285/1/285 (accessed on 15 February 2023).
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramirez-Rodriguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1038/sj.bjp.0707133 (accessed on 18 January 2023). [CrossRef]
- Szallasi, A.; Di Marzo, V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000, 23, 491–497. Available online: https://www.sciencedirect.com/science/article/pii/S0166223600016301 (accessed on 16 February 2023). [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1476-5381.2010.01166.x (accessed on 16 February 2023). [CrossRef] [PubMed]
- Petrocellis, L.D.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Marzo, V.D. Plant-Derived Cannabinoids Modulate the Activity of Transient Receptor Potential Channels of Ankyrin Type-1 and Melastatin Type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. Available online: https://jpet.aspetjournals.org/content/325/3/1007 (accessed on 16 February 2023). [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K. Vanilloid Receptor-1 Is Essential for Inflammatory Thermal Hyperalgesia. Nature 2000, 405, 183–187. Available online: https://www.nature.com/articles/35012076 (accessed on 16 February 2023). [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1038/sj.bjp.0704327 (accessed on 16 February 2023). [CrossRef]
- Watanabe, K.; Kayano, Y.; Matsunaga, T.; Yamamoto, I.; Yoshimura, H. Inhibition of Anandamide Amidase Activity in Mouse Brain Microsomes by Cannabinoids. Biol. Pharm. Bull. 1996, 19, 1109–1111. [Google Scholar] [CrossRef]
- Campos, A.C.; Guimarães, F.S. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology 2008, 199, 223–230. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. Available online: https://www.sciencedirect.com/science/article/pii/S1043661816311392 (accessed on 23 January 2023). [CrossRef]
- Ruffolo, G.; Gaeta, A.; Cannata, B.; Pinzaglia, C.; Aronica, E.; Morano, A.; Cifelli, P.; Palma, E. GABAergic Neurotransmission in Human Tissues Is Modulated by Cannabidiol. Life 2022, 12, 2042. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9786817/ (accessed on 31 May 2023). [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. Available online: https://www.sciencedirect.com/science/article/pii/009286749490006X (accessed on 20 February 2023). [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. Available online: https://www.nature.com/articles/nm1025 (accessed on 20 February 2023). [CrossRef] [PubMed]
- Széles, L.; Töröcsik, D.; Nagy, L. PPARgamma in immunity and inflammation: Cell types and diseases. Biochim. Biophys. Acta 2007, 1771, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H.; Nonaka, T.; Kishimoto, T.; Komoriya, K.; Tsuji, K.; Nakahata, T. Peroxisome proliferator-activated receptors are expressed in human cultured mast cells: A possible role of these receptors in negative regulation of mast cell activation. Eur. J. Immunol. 2000, 30, 3363–3370. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1521-4141%282000012%2930%3A12%3C3363%3A%3AAID-IMMU3363%3E3.0.CO%3B2-B (accessed on 17 February 2023). [CrossRef] [PubMed]
- Castrillo, A.; Díaz-Guerra, M.J.M.; Hortelano, S.; Martín-Sanz, P.; Boscá, L. Inhibition of IκB Kinase and IκB Phosphorylation by 15-Deoxy-Δ12,14-Prostaglandin J2 in Activated Murine Macrophages. Mol. Cell. Biol. 2000, 20, 1692–1698. Available online: https://journals.asm.org/doi/full/10.1128/MCB.20.5.1692-1698.2000 (accessed on 17 February 2023). [CrossRef]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 2000, 403, 103–108. Available online: https://www.nature.com/articles/47520 (accessed on 17 February 2023). [CrossRef]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the Cardiovascular System. Antioxid. Redox Signal. 2009, 11, 1415–1452. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2737093/, (accessed on 26 January 2023). [CrossRef]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1038/sj.bjp.0707423 (accessed on 17 February 2023). [CrossRef]
- Stanley, C.P.; Hind, W.H.; O’Sullivan, S.E. Is the cardiovascular system a therapeutic target for cannabidiol? Br. J. Clin. Pharmacol. 2013, 75, 313–322. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3579247/ (accessed on 26 January 2023). [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. Available online: https://www.sciencedirect.com/science/article/pii/S0014299909002003 (accessed on 18 January 2023). [CrossRef]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef]
- Bolognini, D.; Costa, B.; Maione, S.; Comelli, F.; Marini, P.; Di Marzo, V.; Parolaro, D.; Ross, R.A.; Gauson, L.A.; Cascio, M.G.; et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol. 2010, 160, 677–687. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1476-5381.2010.00756.x (accessed on 17 February 2023). [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/bph.13497 (accessed on 17 February 2023). [CrossRef] [PubMed]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol protects an in vitro model of the blood–brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br. J. Pharmacol. 2016, 173, 815–825. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/bph.13368 (accessed on 17 February 2023). [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol Reduces Aβ-Induced Neuroinflammation and Promotes Hippocampal Neurogenesis through PPARγ Involvement. PLoS ONE 2011, 6, e28668. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0028668 (accessed on 17 February 2023). [CrossRef] [PubMed]
- Haskó, G.; Cronstein, B.N. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2018.00113 (accessed on 20 February 2023). [CrossRef]
- Chan, E.S.L.; Cronstein, B.N. Adenosine in fibrosis. Mod. Rheumatol. 2010, 20, 114–122. [Google Scholar] [CrossRef]
- Sajjadi, F.G.; Takabayashi, K.; Foster, A.C.; Domingo, R.C.; Firestein, G.S. Inhibition of TNF-alpha expression by adenosine: Role of A3 adenosine receptors. J. Immunol. 1996, 156, 3435–3442. [Google Scholar] [CrossRef]
- Wei, W.; Du, C.; Lv, J.; Zhao, G.; Li, Z.; Wu, Z.; Haskó, G.; Xie, X. Blocking A2B Adenosine Receptor Alleviates Pathogenesis of Experimental Autoimmune Encephalomyelitis via Inhibition of IL-6 Production and Th17 Differentiation. J. Immunol. 2013, 190, 138–146. [Google Scholar] [CrossRef]
- Haskó, G.; Kuhel, D.G.; Chen, J.-F.; Schwarzschild, M.A.; Deitch, E.A.; Mabley, J.G.; Marton, A.; Szabó, C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000, 14, 2065–2074. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1096/fj.99-0508com (accessed on 20 February 2023). [CrossRef]
- Le Moine, O.; Stordeur, P.; Schandené, L.; Marchant, A.; de Groote, D.; Goldman, M.; Devière, J. Adenosine enhances IL-10 secretion by human monocytes. J. Immunol. 1996, 156, 4408–4414. [Google Scholar] [CrossRef]
- Sunda, F.; Arowolo, A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J. 2020, 34, 14083–14092. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1096/fj.202000975R (accessed on 20 February 2023). [CrossRef]
- Gonca, E.; Darıcı, F. The Effect of Cannabidiol on Ischemia/Reperfusion-Induced Ventricular Arrhythmias: The Role of Adenosine A1 Receptors. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Vitoretti, L.B.; Mariano-Souza, D.P.; Quinteiro-Filho, W.M.; Akamine, A.T.; Almeida, V.I.; Quevedo, J.; Dal-Pizzol, F.; et al. Cannabidiol, a non-psychotropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. Eur. J. Pharmacol. 2012, 678, 78–85. Available online: https://www.sciencedirect.com/science/article/pii/S0014299912000052 (accessed on 30 June 2023). [CrossRef] [PubMed]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. Available online: https://www.sciencedirect.com/science/article/pii/S096999610900309X (accessed on 30 June 2023). [CrossRef] [PubMed]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 2006, 103, 7895–7900. Available online: https://www.pnas.org/doi/abs/10.1073/pnas.0511232103 (accessed on 26 January 2023). [CrossRef] [PubMed]
- Liou, G.I.; Auchampach, J.A.; Hillard, C.J.; Zhu, G.; Yousufzai, B.; Mian, S.; Khan, S.; Khalifa, Y. Mediation of Cannabidiol Anti-inflammation in the Retina by Equilibrative Nucleoside Transporter and A2A Adenosine Receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5526–5531. [Google Scholar] [CrossRef]
- Pandolfo, P.; Silveirinha, V.; dos Santos-Rodrigues, A.; Venance, L.; Ledent, C.; Takahashi, R.N.; Cunha, R.A.; Köfalvi, A. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur. J. Pharmacol. 2011, 655, 38–45. Available online: https://www.sciencedirect.com/science/article/pii/S0014299911000537 (accessed on 30 June 2023). [CrossRef]
- Viczjan, G.; Szilagyi, A.; Takacs, B.; Ovari, I.; Szekeres, R.; Tarjanyi, V.; Erdei, T.; Teleki, V.; Zsuga, J.; Szilvassy, Z.; et al. The effect of a long-term treatment with cannabidiol-rich hemp extract oil on the adenosinergic system of the zucker diabetic fatty (ZDF) rat atrium. Front. Pharmacol. 2022, 13, 1043275. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9797669/ (accessed on 30 June 2023). [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. Available online: https://www.sciencedirect.com/science/article/pii/S0969996113001939 (accessed on 20 February 2023). [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-activated NF-κB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2804319/ (accessed on 2 January 2023). [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. Available online: https://www.nature.com/articles/sigtrans201723 (accessed on 22 May 2023). [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. ImmunoTargets Ther. 2020, 9, 131–140. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7445536/ (accessed on 20 February 2023). [CrossRef] [PubMed]
- Rothwarf, D.M.; Karin, M. The NF-κB Activation Pathway: A Paradigm in Information Transfer from Membrane to Nucleus. Sci. STKE 1999, 1999, re1. Available online: https://www.science.org/doi/full/10.1126/stke.1999.5.re1 (accessed on 20 February 2023). [CrossRef] [PubMed]
- Davis, C.N.; Mann, E.; Behrens, M.M.; Gaidarova, S.; Rebek, M.; Rebek, J.; Bartfai, T. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl. Acad. Sci. USA 2006, 103, 2953–2958. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1413805/ (accessed on 20 February 2023). [CrossRef]
- Martinez-Naya, N.; Nirar, A.H.; Kim, M.M.; Mauro, A.G.; Mezzaroma, E.; Narayan, P.; Hamer, A.; Bolton, J.; Abbate, A.; Toldo, S. Protective effects of cannabidiol in a mouse model of acute pericarditis. Circ. Res. 2022, 131, e169–e190. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. Toll-like Receptors and Type I Interferons. J. Biol. Chem. 2007, 282, 15319–15323. Available online: https://www.jbc.org/article/S0021-9258(20)87404-3/abstract (accessed on 9 June 2023). [CrossRef]
- Fitzpatrick, J.-M.; Hackett, B.; Costelloe, L.; Hind, W.; Downer, E.J. Botanically-Derived Δ9-Tetrahydrocannabinol and Cannabidiol, and Their 1:1 Combination, Modulate Toll-like Receptor 3 and 4 Signalling in Immune Cells from People with Multiple Sclerosis. Molecules 2022, 27, 1763. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018, 15, 203–214. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.-Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxid. Med. Cell. Longev. 2017, 2017, 9743280. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell. Physiol. 2019, 234, 21224–21234. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jcp.28728 (accessed on 20 February 2023). [CrossRef] [PubMed]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7, 559. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2016.00559 (accessed on 20 February 2023). [CrossRef]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory Effect of Cannabidiol on the Activation of NLRP3 Inflammasome Is Associated with Its Modulation of the P2X7 Receptor in Human Monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Front. Immunol. 2021, 11, 613613. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.613613 (accessed on 20 February 2023). [CrossRef] [PubMed]
- Bhat, M.Y.; Solanki, H.S.; Advani, J.; Khan, A.A.; Keshava Prasad, T.S.; Gowda, H.; Thiyagarajan, S.; Chatterjee, A. Comprehensive network map of interferon gamma signaling. J. Cell Commun. Signal. 2018, 12, 745–751. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6235777/ (accessed on 21 February 2023). [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1189/jlb.0603252 (accessed on 21 February 2023). [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef]
- Weiss, L.; Zeira, M.; Reich, S.; Slavin, S.; Raz, I.; Mechoulam, R.; Gallily, R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 2008, 54, 244–249. Available online: https://www.sciencedirect.com/science/article/pii/S0028390807001888 (accessed on 31 January 2023). [CrossRef]
- Kaplan, B.L.F.; Springs, A.E.B.; Kaminski, N.E. The Profile of Immune Modulation by Cannabidiol (CBD) Involves Deregulation of Nuclear Factor of Activated T Cells (NFAT). Biochem. Pharmacol. 2008, 76, 726–737. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2748879/ (accessed on 6 June 2023). [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. Available online: https://www.sciencedirect.com/science/article/pii/S016561470900128X (accessed on 10 February 2023). [CrossRef]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanuš, L.O. Cannabidiol—Recent Advances. Chem. Biodivers. 2007, 4, 1678–1692. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/cbdv.200790147 (accessed on 2 January 2023). [CrossRef] [PubMed]
- Yu, L.; Zeng, L.; Zhang, Z.; Zhu, G.; Xu, Z.; Xia, J.; Weng, J.; Li, J.; Pathak, J.L. Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Biomolecules 2023, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. Available online: https://www.nature.com/articles/s41598-017-10924-8 (accessed on 22 February 2023). [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. Available online: https://www.jacc.org/doi/abs/10.1016/j.jacc.2010.07.033 (accessed on 18 January 2023). [CrossRef] [PubMed]
- Zhang, J.; Luo, Z.; Zhang, Z.; Zhao, M.; Tong, C.; Cong, P.; Mao, S.; Zhao, Y.; Hou, M.; Piao, Y.; et al. Protective effect and mechanism of cannabidiol on myocardial injury in exhaustive exercise training mice. Chem. Biol. Interact. 2022, 365, 110079. Available online: https://www.sciencedirect.com/science/article/pii/S0009279722002848 (accessed on 21 February 2023). [CrossRef] [PubMed]
- Fouda, M.A.; Fathy Mohamed, Y.; Fernandez, R.; Ruben, P.C. Anti-inflammatory effects of cannabidiol against lipopolysaccharides in cardiac sodium channels. Br. J. Pharmacol. 2022, 179, 5259–5272. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/bph.15936 (accessed on 21 February 2023). [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. Available online: https://www.liebertpub.com/doi/full/10.1089/ars.2013.5447 (accessed on 9 February 2023). [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal 2021, 5, NS20200080. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8385185/ (accessed on 9 February 2023). [CrossRef]
- Hong, S.-W.; Shin, J.-S.; Lee, Y.-M.; Kim, D.-G.; Lee, S.-Y.; Yoon, D.H.; Jung, S.-Y.; Hwang, J.J.; Lee, S.-J.; Cho, D.-H.; et al. p34SEI-1 inhibits ROS-induced cell death through suppression of ASK1. Cancer Biol. Ther. 2011, 12, 421–426. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive Oxygen Species in Cancer: A Dance with the Devil. Cancer Cell 2015, 27, 156–157. Available online: https://www.sciencedirect.com/science/article/pii/S1535610815000227 (accessed on 9 February 2023). [CrossRef]
- Levy, D.; Zochodne, D.W. Local nitric oxide synthase activity in a model of neuropathic pain. Eur. J. Neurosci. 1998, 10, 1846–1855. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1460-9568.1998.00186.x (accessed on 9 February 2023). [CrossRef] [PubMed]
- Sharpe, M.A.; Robb, S.J.; Clark, J.B. Nitric oxide and Fenton/Haber–Weiss chemistry: Nitric oxide is a potent antioxidant at physiological concentrations. J. Neurochem. 2003, 87, 386–394. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1046/j.1471-4159.2003.02001.x (accessed on 9 February 2023). [CrossRef] [PubMed]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/eji.200940168 (accessed on 9 February 2023). [CrossRef] [PubMed]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2016, 2016, e2183026. Available online: https://www.hindawi.com/journals/omcl/2016/2183026/ (accessed on 9 February 2023). [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Borges, R.S.; Batista, J.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B.F. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663–12674. Available online: https://www.mdpi.com/1420-3049/18/10/12663 (accessed on 9 February 2023). [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. Available online: https://www.pnas.org/doi/full/10.1073/pnas.95.14.8268 (accessed on 9 February 2023). [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. Available online: https://journals.physiology.org/doi/full/10.1152/ajpheart.00236.2007 (accessed on 18 January 2023). [CrossRef]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol Attenuates Cisplatin-Induced Nephrotoxicity by Decreasing Oxidative/Nitrosative Stress, Inflammation, and Cell Death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. Available online: https://jpet.aspetjournals.org/content/328/3/708 (accessed on 1 March 2023). [CrossRef]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. Available online: https://www.sciencedirect.com/science/article/pii/S001429990601257X (accessed on 8 February 2023). [CrossRef]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. Available online: https://www.sciencedirect.com/science/article/pii/S221323171630338X (accessed on 9 February 2023). [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. Available online: https://www.mdpi.com/2073-4409/8/8/827 (accessed on 9 February 2023). [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0061462 (accessed on 1 March 2023).
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int. Immunopharmacol. 2009, 9, 216–222. Available online: https://www.sciencedirect.com/science/article/pii/S1567576908003469 (accessed on 22 February 2023). [CrossRef] [PubMed]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Δ8THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res. 2018, 3, 11–20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5812319/ (accessed on 22 February 2023). [CrossRef]
- Mabou Tagne, A.; Marino, F.; Legnaro, M.; Luini, A.; Pacchetti, B.; Cosentino, M. A Novel Standardized Cannabis sativa L. Extract and Its Constituent Cannabidiol Inhibit Human Polymorphonuclear Leukocyte Functions. Int. J. Mol. Sci. 2019, 20, 1833. Available online: https://www.mdpi.com/1422-0067/20/8/1833 (accessed on 9 February 2023). [CrossRef]
- Gómez, C.T.; Lairion, F.; Repetto, M.; Ettcheto, M.; Merelli, A.; Lazarowski, A.; Auzmendi, J. Cannabidiol (CBD) Alters the Functionality of Neutrophils (PMN). Implications in the Refractory Epilepsy Treatment. Pharmaceuticals 2021, 14, 220. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8001508/ (accessed on 22 February 2023). [CrossRef] [PubMed]
- Williams, M.R.; Azcutia, V.; Newton, G.; Alcaide, P.; Luscinskas, F.W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011, 32, 461–469. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Fujiwara, M. Therapeutic Potential of Non-Psychotropic Cannabidiol in Ischemic Stroke. Pharmaceuticals 2010, 3, 2197–2212. Available online: https://www.mdpi.com/1424-8247/3/7/2197 (accessed on 7 February 2023). [CrossRef] [PubMed]
- Ma, Y.; Yabluchanskiy, A.; Iyer, R.P.; Cannon, P.L.; Flynn, E.R.; Jung, M.; Henry, J.; Cates, C.A.; Deleon-Pennell, K.Y.; Lindsey, M.L. Temporal neutrophil polarization following myocardial infarction. Cardiovasc. Res. 2016, 110, 51–61. Available online: https://academic.oup.com/cardiovascres/article/110/1/51/2463216 (accessed on 22 February 2023).
- Baban, B.; Hoda, N.; Malik, A.; Khodadadi, H.; Simmerman, E.; Vaibhav, K.; Mozaffari, M.S. Impact of cannabidiol treatment on regulatory T-17 cells and neutrophil polarization in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2018, 315, F1149–F1158. Available online: https://journals.physiology.org/doi/full/10.1152/ajprenal.00112.2018 (accessed on 22 February 2023). [CrossRef]
- Cosentino, M.; Legnaro, M.; Luini, A.; Ferrari, M.; Sodergren, M.; Pacchetti, B.; Marino, F. Effect of Cannabidiol on Cyclooxygenase Type 1 and 2 Expression and Function in Human Neutrophils. Cannabis Cannabinoid Res. 2022. Available online: https://www.liebertpub.com/doi/10.1089/can.2022.0008 (accessed on 22 February 2023).
- Durst, R.; Danenberg, H.; Gallily, R.; Mechoulam, R.; Meir, K.; Grad, E.; Beeri, R.; Pugatsch, T.; Tarsish, E.; Lotan, C. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3602–H3607. Available online: https://journals.physiology.org/doi/full/10.1152/ajpheart.00098.2007 (accessed on 18 January 2023). [PubMed]
- Wu, H.-Y.; Chu, R.-M.; Wang, C.-C.; Lee, C.-Y.; Lin, S.-H.; Jan, T.-R. Cannabidiol-induced apoptosis in primary lymphocytes is associated with oxidative stress-dependent activation of caspase-8. Toxicol. Appl. Pharmacol. 2008, 226, 260–270. Available online: https://www.sciencedirect.com/science/article/pii/S0041008X0700419X (accessed on 22 February 2023). [CrossRef] [PubMed]
- Li, H.; Kong, W.; Chambers, C.R.; Yu, D.; Ganea, D.; Tuma, R.F.; Ward, S.J. The non-psychoactive phytocannabinoid cannabidiol (CBD) attenuates pro-inflammatory mediators, T cell infiltration, and thermal sensitivity following spinal cord injury in mice. Cell. Immunol. 2018, 329, 1–9. Available online: https://www.sciencedirect.com/science/article/pii/S0008874918300911 (accessed on 24 February 2023). [CrossRef]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. Available online: https://www.sciencedirect.com/science/article/pii/S0891584911000116 (accessed on 23 February 2023). [CrossRef]
- Ignatowska-Jankowska, B.; Jankowski, M.; Glac, W.; Swiergel, A.H. Cannabidiol-induced lymphopenia does not involve NKT and NK cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. S3), 99–103. [Google Scholar]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J. Neuroinflammation 2016, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids Decrease the Th17 Inflammatory Autoimmune Phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. Available online: https://link.springer.com/article/10.1007/s11481-013-9493-1 (accessed on 24 February 2023). [CrossRef]
- Zhou, L.; Ivanov, I.I.; Spolski, R.; Min, R.; Shenderov, K.; Egawa, T.; Levy, D.E.; Leonard, W.J.; Littman, D.R. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 2007, 8, 967–974. Available online: https://www.nature.com/articles/ni1488 (accessed on 24 February 2023). [CrossRef]
- del Río, C.; Ruiz-Pino, F.; Prados, M.E.; Fiebich, B.L.; Tena-Sempere, M.; Muñoz, E. Cannabidiol markedly alleviates skin and liver fibrosis. Front. Pharmacol. 2022, 13, 981817. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9627610/ (accessed on 23 February 2023). [CrossRef] [PubMed]
- Diaz, J.A.; Booth, A.J.; Lu, G.; Wood, S.C.; Pinsky, D.J.; Bishop, D.K. Critical Role for IL-6 in Hypertrophy and Fibrosis in Chronic Cardiac Allograft Rejection. Am. J. Transplant. 2009, 9, 1773–1783. Available online: https://www.sciencedirect.com/science/article/pii/S1600613522270993 (accessed on 23 February 2023). [CrossRef] [PubMed]
- Blyszczuk, P.; Kania, G. Myeloid Differentiation Factor-88/Interleukin-1 Signaling Controls Cardiac Fibrosis and Heart Failure Progression in Inflammatory Dilated Cardiomyopathy. Circ. Res. 2009, 105, 912–920. Available online: https://www.ahajournals.org/doi/full/10.1161/CIRCRESAHA.109.199802 (accessed on 23 February 2023). [CrossRef] [PubMed]
- Blyszczuk, P.; Berthonneche, C.; Behnke, S.; Glönkler, M.; Moch, H.; Pedrazzini, T.; Lüscher, T.F.; Eriksson, U.; Kania, G. Nitric oxide synthase 2 is required for conversion of pro-fibrogenic inflammatory CD133+ progenitors into F4/80+ macrophages in experimental autoimmune myocarditis. Cardiovasc. Res. 2013, 97, 219–229. [Google Scholar] [CrossRef] [PubMed]
- De Ternay, J.; Naassila, M.; Nourredine, M.; Louvet, A.; Bailly, F.; Sescousse, G.; Maurage, P.; Cottencin, O.; Carrieri, P.M.; Rolland, B. Therapeutic Prospects of Cannabidiol for Alcohol Use Disorder and Alcohol-Related Damages on the Liver and the Brain. Front. Pharmacol. 2019, 10, 627. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2019.00627 (accessed on 23 February 2023). [CrossRef] [PubMed]
- Lee, W.S.; Erdelyi, K.; Matyas, C.; Mukhopadhyay, P.; Varga, Z.V.; Liaudet, L.; Haskú, G.; Čiháková, D.; Mechoulam, R.; Pacher, P. Cannabidiol Limits T Cell–Mediated Chronic Autoimmune Myocarditis: Implications to Autoimmune Disorders and Organ Transplantation. Mol. Med. 2016, 22, 136–146. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5004721/ (accessed on 18 January 2023). [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. Available online: https://www.mdpi.com/1422-0067/20/17/4133 (accessed on 10 February 2023). [CrossRef]
- Sreevalsan, S. Induction of Apoptosis by Cannabinoids in Prostate and Colon Cancer Cells Is Phosphatase Dependent. Anticancer Res. 2011, 31, 3799–3807. Available online: https://ar.iiarjournals.org/content/31/11/3799 (accessed on 10 February 2023).
- Lee, C.-Y.; Wey, S.-P.; Liao, M.-H.; Hsu, W.-L.; Wu, H.-Y.; Jan, T.-R. A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int. Immunopharmacol. 2008, 8, 732–740. Available online: https://www.sciencedirect.com/science/article/pii/S1567576908000234 (accessed on 10 February 2023). [CrossRef]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Mol. Pharmacol. 2006, 70, 897–908. Available online: https://pubmed.ncbi.nlm.nih.gov/16754784/ (accessed on 10 February 2023). [CrossRef]
- Gross, C.; Ramirez, D.A.; McGrath, S.; Gustafson, D.L. Cannabidiol Induces Apoptosis and Perturbs Mitochondrial Function in Human and Canine Glioma Cells. Front. Pharmacol. 2021, 12, 725136. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2021.725136 (accessed on 10 February 2023). [PubMed]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast 2018, 41, 34–41. Available online: https://www.sciencedirect.com/science/article/pii/S0960977618301218 (accessed on 10 February 2023). [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2125.2012.04298.x (accessed on 6 February 2023). [CrossRef] [PubMed]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 779. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6791884/ (accessed on 10 February 2023).
- Wu, H.-Y.; Huang, C.-H.; Lin, Y.-H.; Wang, C.-C.; Jan, T.-R. Cannabidiol induced apoptosis in human monocytes through mitochondrial permeability transition pore-mediated ROS production. Free Radic. Biol. Med. 2018, 124, 311–318. Available online: https://www.sciencedirect.com/science/article/pii/S0891584918311134 (accessed on 10 February 2023).
- Feng, Y.; Chen, F.; Yin, T.; Xia, Q.; Liu, Y.; Huang, G.; Zhang, J.; Oyen, R.; Ni, Y. Pharmacologic Effects of Cannabidiol on Acute Reperfused Myocardial Infarction in Rabbits: Evaluated with 3.0T Cardiac Magnetic Resonance Imaging and Histopathology. J. Cardiovasc. Pharmacol. 2015, 66, 354–363. Available online: http://journals.lww.com/00005344-201510000-00005 (accessed on 2 January 2023). [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. Available online: https://www.sciencedirect.com/science/article/pii/S0891584911001262 (accessed on 8 February 2023). [CrossRef]
- Wu, H.Y.; Chang, A.C.; Wang, C.C.; Kuo, F.H.; Lee, C.Y.; Liu, D.Z.; Jan, T.R. Cannabidiol induced a contrasting pro-apoptotic effect between freshly isolated and precultured human monocytes. Toxicol. Appl. Pharmacol. 2010, 246, 141–147. Available online: https://www.sciencedirect.com/science/article/pii/S0041008X10001675 (accessed on 31 May 2023). [CrossRef]
- Wade, D.T.; Collin, C.; Stott, C.; Duncombe, P. Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult. Scler. 2010, 16, 707–714. Available online: https://journals.sagepub.com/doi/epdf/10.1177/1352458510367462 (accessed on 31 May 2023).
- Patti, F.; Messina, S.; Solaro, C.; Amato, M.P.; Bergamaschi, R.; Bonavita, S.; Bossio, R.B.; Morra, V.B.; Costantino, G.F.; Cavalla, P.; et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J. Neurol. Neurosurg. Psychiatry 2016, 87, 944–951. Available online: https://jnnp.bmj.com/content/87/9/944 (accessed on 31 May 2023). [CrossRef] [PubMed]
- Grant, A.O. Cardiac Ion Channels. Circ. Arrhythm. Electrophysiol. 2009, 2, 185–194. Available online: https://www.ahajournals.org/doi/full/10.1161/CIRCEP.108.789081 (accessed on 7 February 2023). [CrossRef] [PubMed]
- Drysdale, A.J.; Ryan, D.; Pertwee, R.G.; Platt, B. Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 2006, 50, 621–631. Available online: https://www.sciencedirect.com/science/article/pii/S0028390805003941 (accessed on 19 January 2023).
- Ali, R.M.; Al Kury, L.T.; Yang, K.-H.S.; Qureshi, A.; Rajesh, M.; Galadari, S.; Shuba, Y.M.; Howarth, F.C.; Oz, M. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium 2015, 57, 290–299. Available online: https://www.sciencedirect.com/science/article/pii/S0143416015000330 (accessed on 18 January 2023). [CrossRef]
- Le Marois, M.; Ballet, V.; Sanson, C.; Maizières, M.-A.; Carriot, T.; Chantoiseau, C.; Partiseti, M.; Bohme, G.A. Cannabidiol inhibits multiple cardiac ion channels and shortens ventricular action potential duration in vitro. Eur. J. Pharmacol. 2020, 886, 173542. Available online: https://www.sciencedirect.com/science/article/pii/S0014299920306348 (accessed on 2 January 2023). [CrossRef] [PubMed]
- Isaev, D.; Shabbir, W.; Dinc, E.Y.; Lorke, D.E.; Petroianu, G.; Oz, M. Cannabidiol Inhibits Multiple Ion Channels in Rabbit Ventricular Cardiomyocytes. Front. Pharmacol. 2022, 13, 821758. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8850628/ (accessed on 2 January 2023). [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of Recombinant Human T-type Calcium Channels by Δ9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. Available online: https://www.jbc.org/article/S0021-9258(20)46150-2/abstract (accessed on 23 January 2023). [CrossRef]
- Walsh, S.K.; Hepburn, C.Y.; Kane, K.A.; Wainwright, C.L. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br. J. Pharmacol. 2010, 160, 1234–1242. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2936031/ (accessed on 2 January 2023). [CrossRef]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. 2018, 293, 16546–16558. Available online: https://www.jbc.org/article/S0021-9258(20)33222-1/abstract (accessed on 23 January 2023). [CrossRef]
- Robertson-Gray, O.J.; Walsh, S.K.; Ryberg, E.; Jönsson-Rylander, A.C.; Lipina, C.; Wainwright, C.L. L-α-Lysophosphatidylinositol (LPI) aggravates myocardial ischemia/reperfusion injury via a GPR55/ROCK-dependent pathway. Pharmacol. Res. Perspect. 2019, 7, e00487. Available online: https://rgu-repository.worktribe.com/output/244288/l-lysophosphatidylinositol-lpi-aggravates-myocardial-ischemiareperfusion-injury-via-a-gpr55rock-dependent-pathway (accessed on 7 February 2023). [CrossRef]
- Nathan, S.; Gabelli, S.B.; Yoder, J.B.; Srinivasan, L.; Aldrich, R.W.; Tomaselli, G.F.; Ben-Johny, M.; Amzel, L.M. Structural basis of cytoplasmic NaV1.5 and NaV1.4 regulation. J. Gen. Physiol. 2020, 153, e202012722. [Google Scholar] [CrossRef]
- Ghovanloo, M.-R.; Ruben, P.C. Cannabidiol and Sodium Channel Pharmacology: General Overview, Mechanism, and Clinical Implications. Neuroscientist 2022, 28, 318–334. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9344566/ (accessed on 7 February 2023).
- Loussouarn, G.; Sternberg, D.; Nicole, S.; Marionneau, C.; Le Bouffant, F.; Toumaniantz, G.; Barc, J.; Malak, O.A.; Fressart, V.; Péréon, Y.; et al. Physiological and Pathophysiological Insights of Nav1.4 and Nav1.5 Comparison. Front. Pharmacol. 2016, 6, 314. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4712308/ (accessed on 7 February 2023).
- Topal, L.; Naveed, M.; Orvos, P.; Pászti, B.; Prorok, J.; Bajtel, Á.; Kiss, T.; Csupor-Löffler, B.; Csupor, D.; Baczkó, I.; et al. The electrophysiological effects of cannabidiol on action potentials and transmembrane potassium currents in rabbit and dog cardiac ventricular preparations. Arch. Toxicol. 2021, 95, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Jenny, M.; Santer, E.; Pirich, E.; Schennach, H.; Fuchs, D. Δ9-Tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J. Neuroimmunol. 2009, 207, 75–82. Available online: https://www.sciencedirect.com/science/article/pii/S0165572808004943 (accessed on 7 February 2023). [PubMed]
- Granjeiro, É.M.; Gomes, F.V.; Guimarães, F.S.; Corrêa, F.M.A.; Resstel, L.B.M. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol. Biochem. Behav. 2011, 99, 743–748. Available online: https://www.sciencedirect.com/science/article/pii/S0091305711002310 (accessed on 1 February 2023). [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Naya, N.; Kelly, J.; Corna, G.; Golino, M.; Abbate, A.; Toldo, S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules 2023, 28, 5980. https://doi.org/10.3390/molecules28165980
Martinez Naya N, Kelly J, Corna G, Golino M, Abbate A, Toldo S. Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules. 2023; 28(16):5980. https://doi.org/10.3390/molecules28165980
Chicago/Turabian StyleMartinez Naya, Nadia, Jazmin Kelly, Giuliana Corna, Michele Golino, Antonio Abbate, and Stefano Toldo. 2023. "Molecular and Cellular Mechanisms of Action of Cannabidiol" Molecules 28, no. 16: 5980. https://doi.org/10.3390/molecules28165980
APA StyleMartinez Naya, N., Kelly, J., Corna, G., Golino, M., Abbate, A., & Toldo, S. (2023). Molecular and Cellular Mechanisms of Action of Cannabidiol. Molecules, 28(16), 5980. https://doi.org/10.3390/molecules28165980








