Effect of Adding Intermediate Layers on the Interface Bonding Performance of WC-Co Diamond-Coated Cemented Carbide Tool Materials
Abstract
1. Introduction
2. Results and Discussion
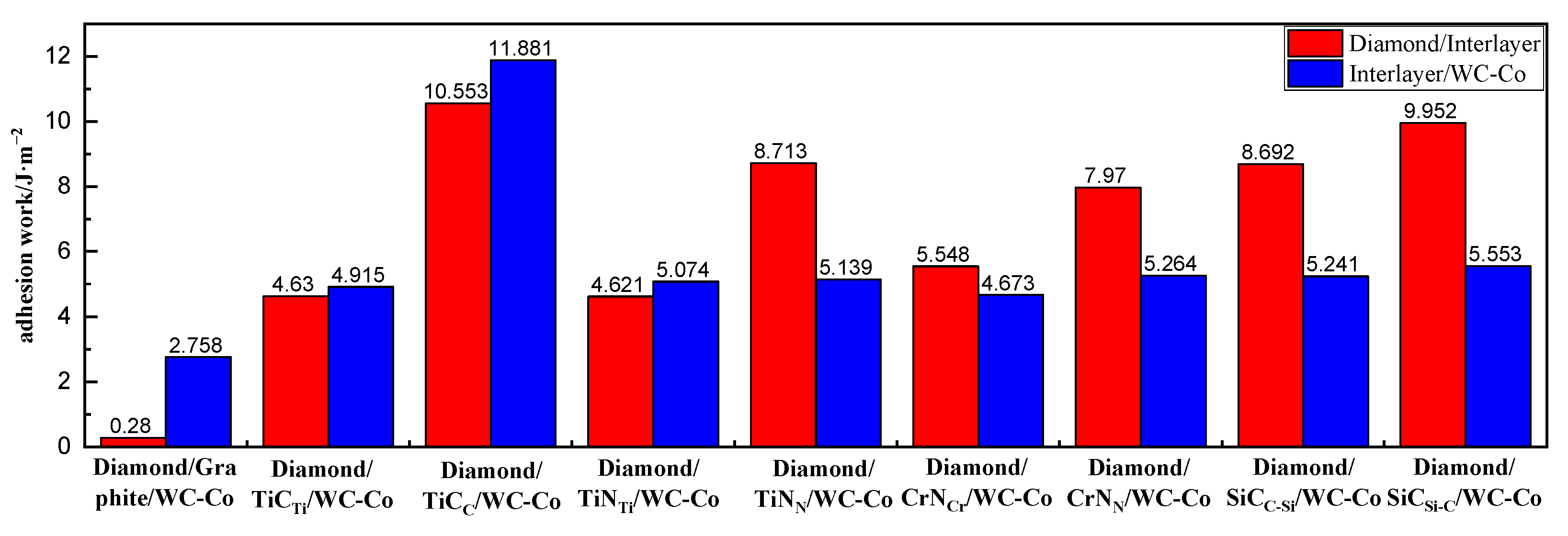
2.1. Interface Adhesion Work
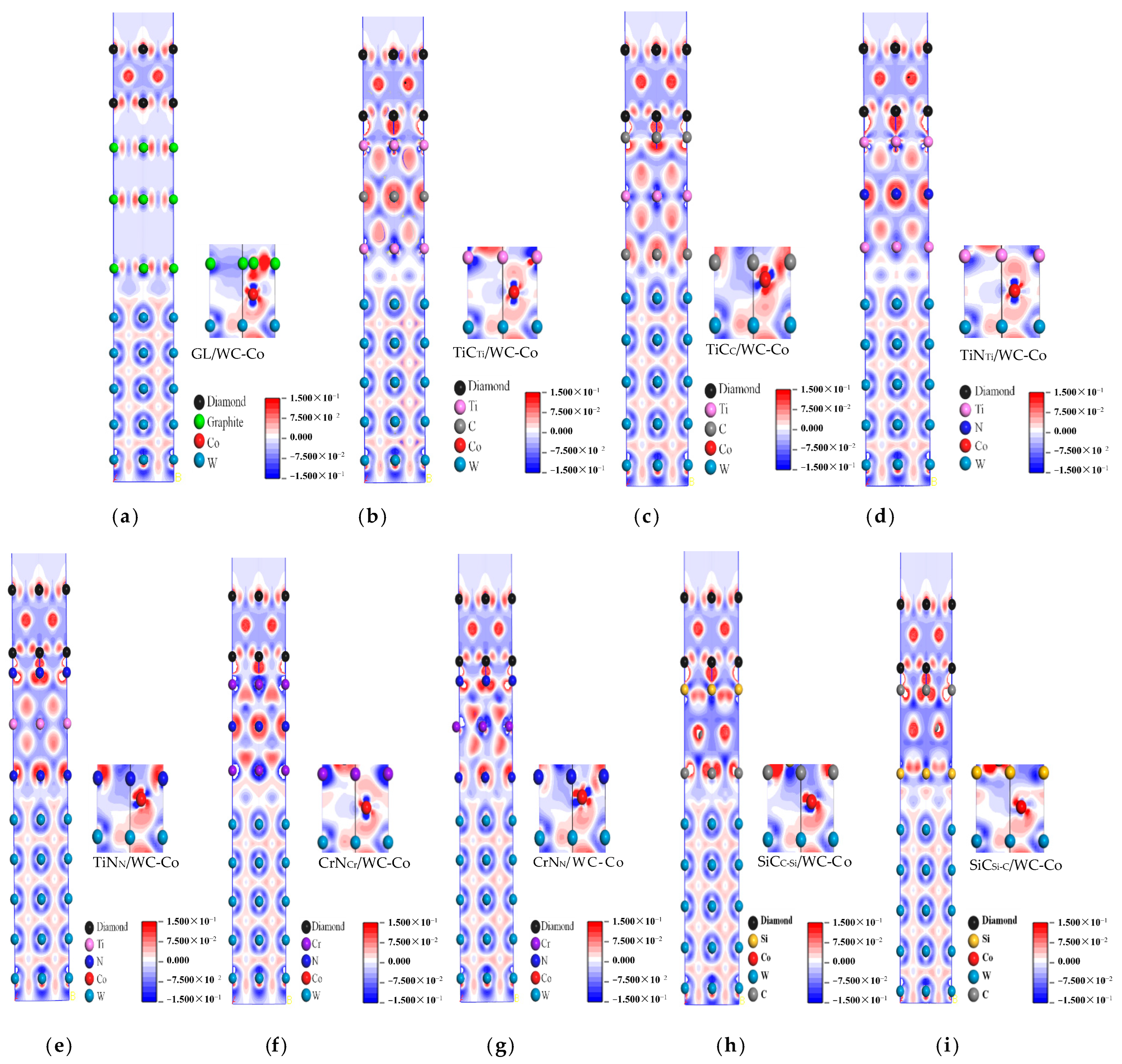
2.2. Charge Density Difference Analysis
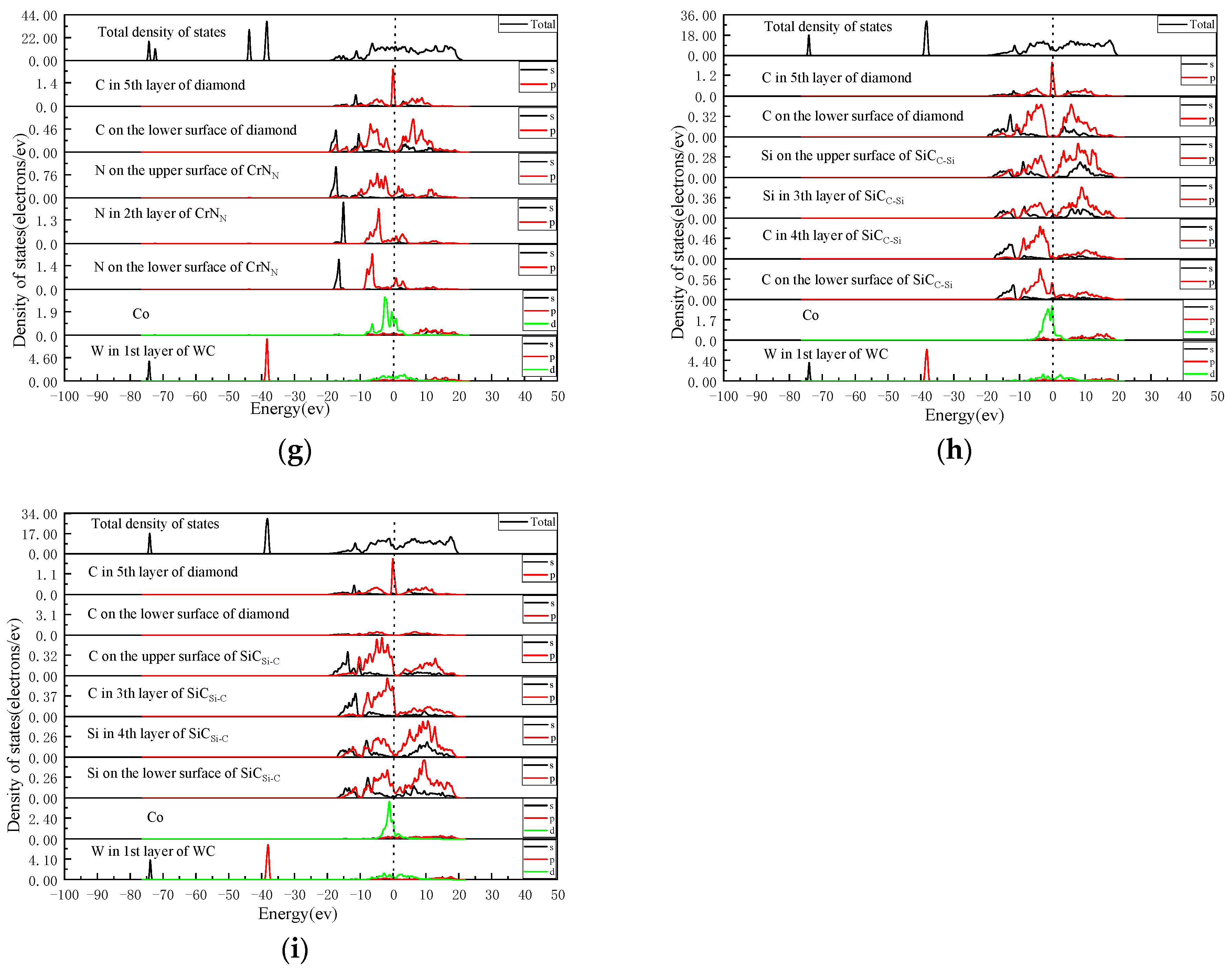
2.3. Analysis of the Density of States
3. Materials and Methods
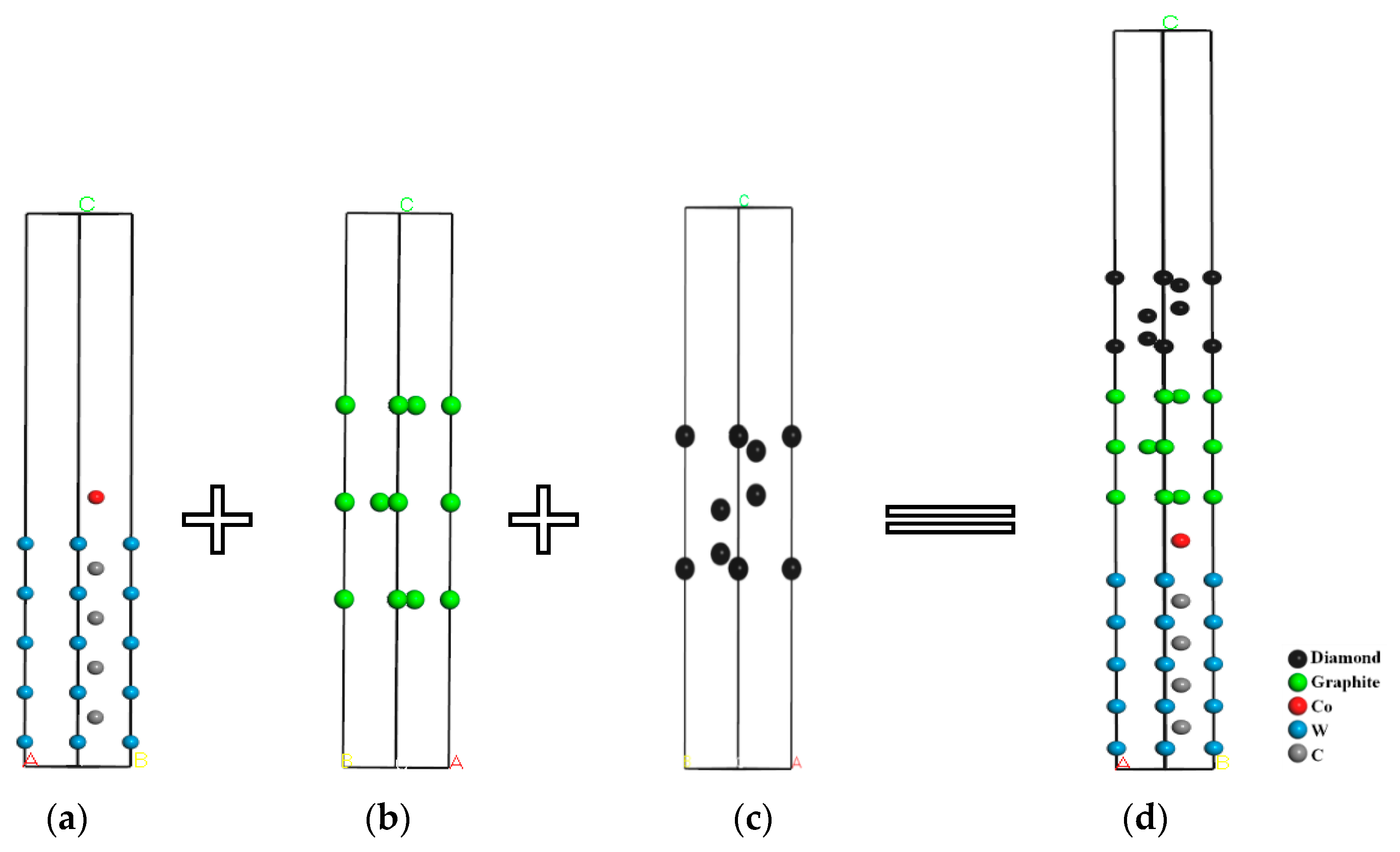
3.1. Material Interface Model Construction Process
3.2. Material Interface Model Optimization
4. Conclusions
- (1)
- The adhesion work analysis of the interface models showed that compared with the interface model with no intermediate layers, the interface adhesion work between the diamond coating (DC) and WC-Co cemented carbide substrate increased significantly after the addition of TiC, TiN, CrN, and SiC intermediate layers; namely, the interface bonding performance of DCCC increased after the addition of the intermediate layer. Among the interface models with the TiC intermediate layer, DC/TiCTi/WC-Co had the lowest adhesion work, with a value of 4.630 J/m2. Among the interface models with the TiN intermediate layer, DC/TiNTi/WC-Co had the lowest adhesion work, with a value of 4.621 J/m2. Among the interface models with the CrN intermediate layer, DC/CrNCr/WC-Co had the lowest adhesion work, with a value of 4.673 J/m2. Among the interface models with the SiC intermediate layer, DC/SiCC-Si/WC-Co had the lowest adhesion work, with a value of 5.241 J/m2. The adhesion work values of the above four interface models were ranked in descending order as DC/SiCC-Si/WC-Co > DC/CrNCr/WC-Co > DC/TiCTi/WC-Co > DC/TiNTi/WC-Co. The improvement effects of four intermediate layers on the interface bonding properties of DCCC were ranked as SiC > CrN > TiC > TiN.
- (2)
- The charge density difference analysis showed that DCCC without intermediate layers had no charge transfer and no bonding between the atoms at the DC/GL interface and at the GL/WC-Co interface. Van der Waals forces combined the atoms at the interface with poor interface bonding performance. After adding the intermediate layers, the electron cloud between atoms at the interface overlapped to form a more stable chemical bond. Thus, the interface bonding performance was improved. The charge distributions of four interface models with a weak bonding effect after adding different intermediate layers were compared and analyzed. It was found that the charge overlap of atoms at the interface of the diamond/SiCC-Si/WC-Co interface model was significant, with the shortest bond length of 1.62 Å. The corresponding interatomic bonding effect at the interface was strong, and the interface bonding performance was the best. The corresponding bond length at the interface of the DC/TiNTi/WC-Co interface model was the longest, namely 4.11 Å. Thus, the corresponding interatomic bonding effect at the interface was weak, indicating the worst effect on improving the interface bonding performance.
- (3)
- The analysis of the density of states revealed that the density of states at the interface in DCCC without intermediate layers was low, and there were no formed resonance peaks. The interaction between atoms was weak. After adding the intermediate layer, resonance peaks were formed between atoms at the interface. The density of states of the atoms in the energy overlap region increased, enhancing the bonding force between the atoms at the interface and improved the interface bonding performance. After comparing and analyzing the density of states of four interface models with weak interfacial atomic forces after adding intermediate layers, it was shown that the energy region of the resonance peak formed by the density of states of the atoms at the interface of the DC/SiCC-Si/WC-Co interface model was the largest (−5–20 eV). The interatomic bonding strength was the strongest, and the interface bonding performance was the best. The energy region of the orbital resonance of the DC/TiNTi/WC-Co interface model was the smallest (−5–16 eV). The bonding between the atoms at the interface was the weakest, with the worst effect on improving the interface bonding performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Almeida, F.A.; Soares, E.; Fernandes, A.J.S.; Sacramento, J.; Silva, R.F.; Oliveira, F.J. Diamond film adhesion onto sub-micrometric WC–Co substrates. Vacuum 2011, 85, 1135–1139. [Google Scholar] [CrossRef]
- Xiang, D.; Guo, Z.; Zhang, L.; Feng, H. Preparation and cutting performance of ultra-smooth CVD composite diamond coated ladder-shape drilling tools. Diam. Relat. Mater. 2018, 81, 54–60. [Google Scholar] [CrossRef]
- Behera, M.; Jena, A.; Pattnaik, S.K.; Padhi, S.; Sarangi, S.K. The effect of transition-metal seeding powder on deposition and growth of diamond synthesized by hot filament chemical vapor deposition processes on cemented carbide substrates and its characterization. Mater. Chem. Phys. 2020, 256, 123638. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kanda, K.; Tsubokawa, T. Reduction of diamond-coated cutting tool wear during graphite cutting. Precis. Eng. 2018, 51, 186–189. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Shen, X.; Sun, F. Tribological behaviors of the diamond films sliding against the T800/X850 CFRP laminates. Wear 2019, 418–419, 191–200. [Google Scholar] [CrossRef]
- Lee, S.-T.; Lin, Z.; Jiang, X. CVD diamond films: Nucleation and growth. Mater. Sci. Eng. R Rep. 1999, 25, 123–154. [Google Scholar] [CrossRef]
- Zheng, Y. Synthesis of High Quality CVD Diamond and Research on Its Application in Graver. Master’s Thesis, Jilin University, Changchun, China, 2010. [Google Scholar]
- Wang, X.-C.; Wang, C.-C.; He, W.-K.; Sun, F.-H. Co evolutions for WC–Co with different Co contents during pretreatment and HFCVD diamond film growth processes. Trans. Nonferrous Met. Soc. China 2018, 28, 469–486. [Google Scholar] [CrossRef]
- Polini, R.; Delogu, M.; Marcheselli, G. Adherent diamond coatings on cemented tungsten carbide substrates with new Fe/Ni/Co binder phase. Thin Solid Films 2006, 494, 133–140. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, W.; Lu, W.; Du, X. Preparation and tribological properties of micro-textured diamond/WSx coatings. Surf. Coat. Technol. 2020, 4, 126369. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, W.; Feng, W.; Du, X.; Zuo, D. Effect of substrate surface texture on adhesion performance of diamond coating. Int. J. Refract. Met. Hard Mater. 2021, 95, 105402. [Google Scholar] [CrossRef]
- Guo, Z.; Deng, F.; Zhang, L.; Hao, C.; Zhao, X.; Xiang, D. Fabrication and tribological properties of textured diamond coatings on WC-Co cemented carbide surfaces. Ceram. Int. 2021, 47, 5423–5431. [Google Scholar] [CrossRef]
- Li, Y.S.; Tang, Y.; Yang, Q.; Shimada, S.; Wei, R.; Lee, K.Y.; Hirose, A. Al-enhanced nucleation and adhesion of diamond films on WC–Co substrates. Int. J. Refract. Met. Hard Mater. 2008, 26, 465–471. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.S.; Yang, Q.; Hirose, A. Deposition and characterization of diamond coatings on WC-Co cutting tools with W/Al interlayer. Diam. Relat. Mater. 2010, 19, 496–499. [Google Scholar] [CrossRef]
- Park, J.-K.; Lee, H.-J.; Lee, W.-S.; Baik, Y.-J. Effect of TiAl-based interlayer on the surface morphology and adhesion of nanocrystalline diamond film deposited on WC–Co substrate by hot filament CVD. Surf. Coat. Technol. 2014, 258, 108–113. [Google Scholar] [CrossRef]
- Cabral, G.; Gabler, J.; Lindner, J.; Gracio, J.; Polini, R. A study of diamond film deposition on WC–Co inserts for graphite machining: Effectiveness of SiC interlayer prepared by HFCVD. Diam. Relat. Mater. 2008, 17, 1008–1014. [Google Scholar] [CrossRef]
- Yang, S.; Lu, Z.; Fan, Z.; Yao, N.; Zhang, B. Preparation of high adhesion diamond film on WC-Co cemented carbide substrate. J. Inorg. Mater. 2005, 1, 235–238. (In Chinese) [Google Scholar]
- Liu, J.; Liu, Y.; Tu, M. Effect of TiC intermediate transition layer on diamond-like carbon deposition on cemented carbide surface. Trans. Met. Heat Treat. 1996, S1, 38–41. (In Chinese) [Google Scholar]
- Chandran, M.; Sammler, F.; Uhlmann, E.; Akhvlediani, R.; Hoffman, A. Wear performance of diamond coated WC-Co tools with a CrN interlayer. Diam. Relat. Mater. 2017, 73, 47–55. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Ding, M.; Liu, Y.; Tang, W.; Zhang, B. Diamond coatings deposited on cemented carbide substrates with SiC as interlayers: Preparation and erosion resistance tests. Diam. Relat. Mater. 2017, 73, 105–113. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Finnis, M.W. The theory of metal–ceramic interfaces. J. Phys. Condens. Matter. 1996, 8, 5811–5836. [Google Scholar] [CrossRef]
- Liu, M.N.; Bian, Y.B.; Zheng, S.J.; Zhu, T.; Chen, Y.G.; Chen, Y.L.; Wang, J.S. Growth and mechanical properties of diamond films on cemented carbide with buffer layers. Thin Solid Films 2015, 584, 165–169. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Xiao, B.; Min, T.; Fan, Z.; Ma, S.; Yi, D. Theoretical study on the electronic properties and stabilities of low-index surfaces of WC polymorphs. Comput. Mater. Sci. 2011, 50, 939–948. [Google Scholar] [CrossRef]
- Liu, J. Preparation of CVD Diamond Coated Tools. Master’s Thesis, Dalian University of Technology, Dalian, China, 2015. [Google Scholar]
- Liu, H.; Gao, C.; Li, X.; Wang, C.; Han, Y.; Zou, G.; Wang, W.; Wen, C. Selective growth of diamond films by nanocrystalline method. J. At. Mol. Phys. 2000, 17, 548–551. (In Chinese) [Google Scholar]
- Wei, Q.; Yu, Z.; Ma, L.; Yang, L.; Liu, W.; Xiao, H. Effect of chemical cobalt removal on diamond film deposited from cemented carbide. Chinses J. Nonferrous Met. 2008, 18, 1070–1081. (In Chinese) [Google Scholar]
- Christensen, M.; Wahnstrom, G. Effects of cobalt intergranular segregation on interface energetics in WC–Co. Acta Mater. 2004, 52, 2199–2207. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, L.; Song, H.; Lu, Z.; Yang, B.; Xiong, J.; Huang, N.; Jiang, X. Improvement in the universality of high-performance CVD diamond coatings on different WC-Co substrates by introducing multilayered diamond/β-SiC composite. Diam. Relat. Mater. 2021, 116, 108369. [Google Scholar] [CrossRef]


| Interface Models | Interface | Eα/eV | Eβ/(eV) | Eα/β/(eV) | Aα/β/(Å2) | Wad/(J/m2) |
|---|---|---|---|---|---|---|
| Diamond/Graphite/WC-Co | Diamond/Graphite | −920.966 | −12,254.485 | −13,175.464 | 7.38 | 0.028 |
| Graphite/WC-Co | −1842.669 | −11,331.522 | −13,175.464 | 2.758 | ||
| Diamond/TiCTi/WC-Co | Diamond/TiCTi | −920.733 | −18,214.304 | −19,137.174 | 4.630 | |
| TiCTi/WC-Co | −7803.415 | −11,331.491 | −19,137.174 | 4.915 | ||
| Diamond/TiCC/WC-Co | Diamond/TiCC | −920.509 | −16,762.721 | −17,688.098 | 10.553 | |
| TiCC/WC-Co | −6351.349 | −11,331.268 | −17,688.098 | 11.881 | ||
| Diamond/TiNTi/WC-Co | Diamond/TiNTi | −920.719 | −18,568.229 | −19,491.080 | 4.621 | |
| TiNTi/WC-Co | −8157.288 | −11,331.451 | −19,491.080 | 5.074 | ||
| Diamond/TiNN/WC-Co | Diamond/TiNN | −920.586 | −17,234.426 | −18,159.032 | 8.713 | |
| TiNN/WC-Co | −6825.187 | −11,331.474 | −18,159.032 | 5.139 | ||
| Diamond/CrNCr/WC-Co | Diamond/CrNCr | −920.696 | −22,017.431 | −22,940.687 | 5.548 | |
| CrNCr/WC-Co | −11,607.038 | −11,331.493 | −22,940.687 | 4.673 | ||
| Diamond/CrNN/WC-Co | Diamond/CrNN | −920.601 | −19,820.510 | −20,744.788 | 7.970 | |
| CrNN/WC-Co | −9410.965 | −11,331.394 | −20,744.788 | 5.264 | ||
| Diamond/SiCC-Si/WC-Co | Diamond/SiCC-Si | −920.699 | −12,117.603 | −13,042.312 | 8.692 | |
| SiCC-Si/WC-Co | −1708.362 | −11,331.532 | −13,042.312 | 5.241 | ||
| Diamond/SiCSi-C/WC-Co | Diamond/SiCSi-C | −920.621 | −12,117.240 | −13,042.452 | 9.952 | |
| SiCSi-C/WC-Co | −1708.763 | −11,331.532 | −13,042.452 | 5.553 |
| Interlayer | Graphite | TiCTi | TiCC | TiNTi | TiNN | CrNCr | CrNN | SiCC-Si | SiCSi-C |
|---|---|---|---|---|---|---|---|---|---|
| Bond length of interface at diamond/interlayer (Å) | 4.05 | 2.14 | 1.45 | 2.15 | 1.59 | 1.97 | 1.69 | 1.62 | 1.51 |
| Bond length of interface at interlayer/WC-Co (Å) | 5.06 | 4.01 | 1.97 | 4.11 | 3.97 | 4.05 | 3.86 | 3.91 | 3.67 |
| Atomic Layer Number | WC(001)W Surface Energy/J·m−2 | TiC(111)Ti Surface Energy/J·m−2 | TiC(111)C Surface Energy/J·m−2 | TiN(111)Ti Surface Energy/ J·m−2 | TiN(111)N Surface Energy/ J·m−2 | CrN(111)Cr Surface Energy/ J·m−2 | CrN(111)N Surface Energy/ J·m−2 |
|---|---|---|---|---|---|---|---|
| 3 | 3.356 | 1.798 | 7.532 | 1.966 | 4.513 | 3.287 | 3.149 |
| 5 | 3.460 | 1.826 | 7.791 | 2.108 | 4.017 | 3.458 | 2.969 |
| 7 | 3.431 | 1.892 | 8.069 | 2.034 | 3.910 | 3.427 | 2.915 |
| 9 | 3.414 | 1.935 | 8.060 | 2.029 | 3.922 | 3.423 | 2.912 |
| 11 | 3.409 | 1.952 | 8.040 | 2.002 | 3.957 | 3.422 | 2.905 |
| Atomic Layer Number | Diamond (111) Surface Energy/ J·m−2 | SiC(111) Surface Energy/ J·m−2 | Atomic Layer Number | Graphite (001) Surface Energy/J·m−2 |
|---|---|---|---|---|
| 4 | 3.356 | 1.798 | 1 | 0.0004 |
| 6 | 3.460 | 1.826 | 2 | 0.0012 |
| 8 | 3.431 | 1.892 | 3 | 0.0026 |
| 10 | 3.414 | 1.935 | 4 | 0.0037 |
| 12 | 3.409 | 1.952 | 5 | 0.0057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Yue, Y.; Lv, H.; Ren, B.; Zhang, Y. Effect of Adding Intermediate Layers on the Interface Bonding Performance of WC-Co Diamond-Coated Cemented Carbide Tool Materials. Molecules 2023, 28, 5958. https://doi.org/10.3390/molecules28165958
Yang J, Yue Y, Lv H, Ren B, Zhang Y. Effect of Adding Intermediate Layers on the Interface Bonding Performance of WC-Co Diamond-Coated Cemented Carbide Tool Materials. Molecules. 2023; 28(16):5958. https://doi.org/10.3390/molecules28165958
Chicago/Turabian StyleYang, Junru, Yanping Yue, Hao Lv, Baofei Ren, and Yuekan Zhang. 2023. "Effect of Adding Intermediate Layers on the Interface Bonding Performance of WC-Co Diamond-Coated Cemented Carbide Tool Materials" Molecules 28, no. 16: 5958. https://doi.org/10.3390/molecules28165958
APA StyleYang, J., Yue, Y., Lv, H., Ren, B., & Zhang, Y. (2023). Effect of Adding Intermediate Layers on the Interface Bonding Performance of WC-Co Diamond-Coated Cemented Carbide Tool Materials. Molecules, 28(16), 5958. https://doi.org/10.3390/molecules28165958






