Advancements in Sustainable Natural Dyes for Textile Applications: A Review
Abstract
1. Introduction
1.1. Synthetic and Natural Dyes: Advantages and Disadvantages
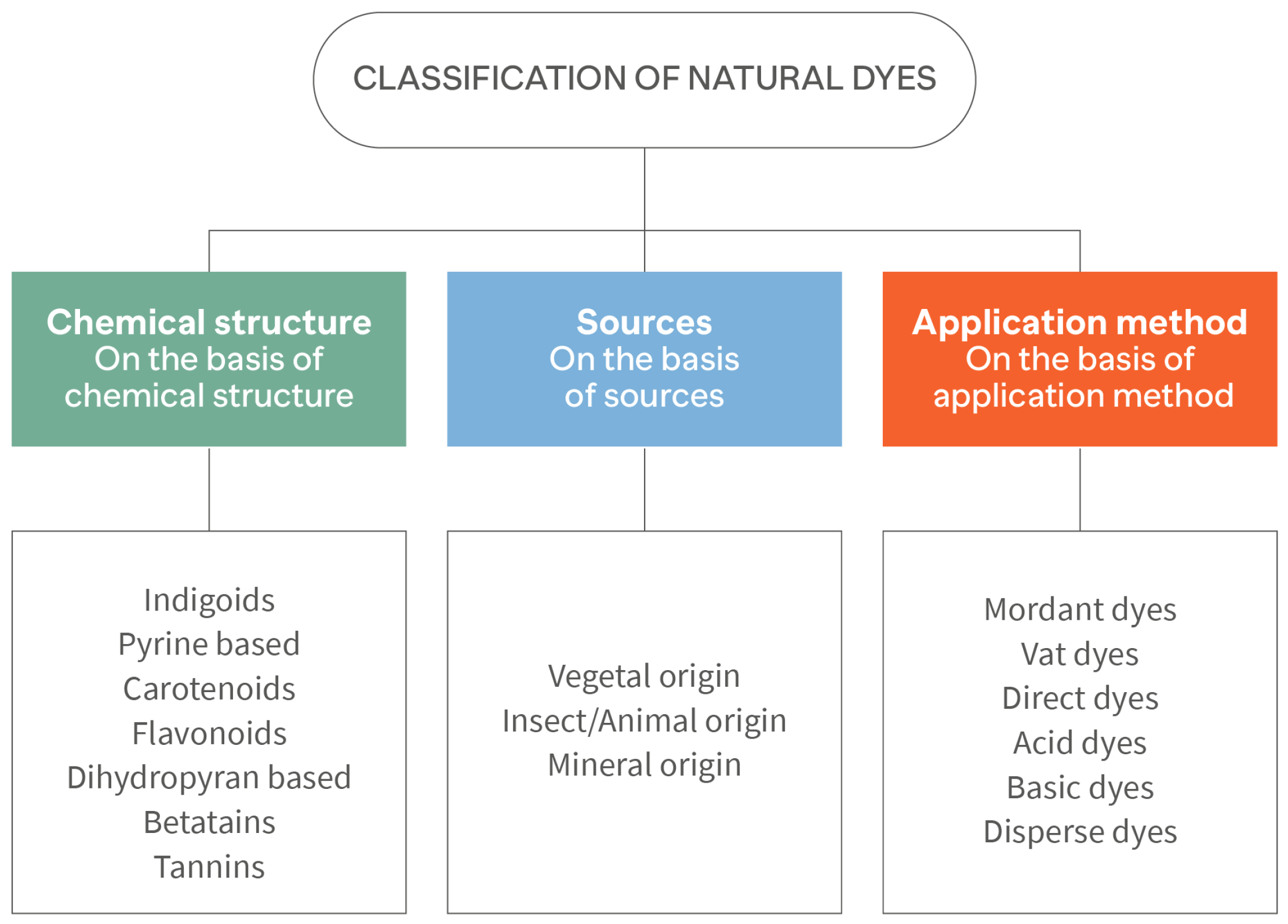
1.2. Natural Dyes Sourcing
2. Advancements in Natural Dyes Extraction
2.1. Aqueous Extraction
2.2. Solvent Extraction
2.3. Alkali or Acid Extraction
2.4. Ultrasound- and Microwave-Assisted Extraction
2.5. Enzymatic Extraction and Fermentation
2.6. Supercritical Fluids
3. Advancements in Mordanting and Substrate Pretreatment
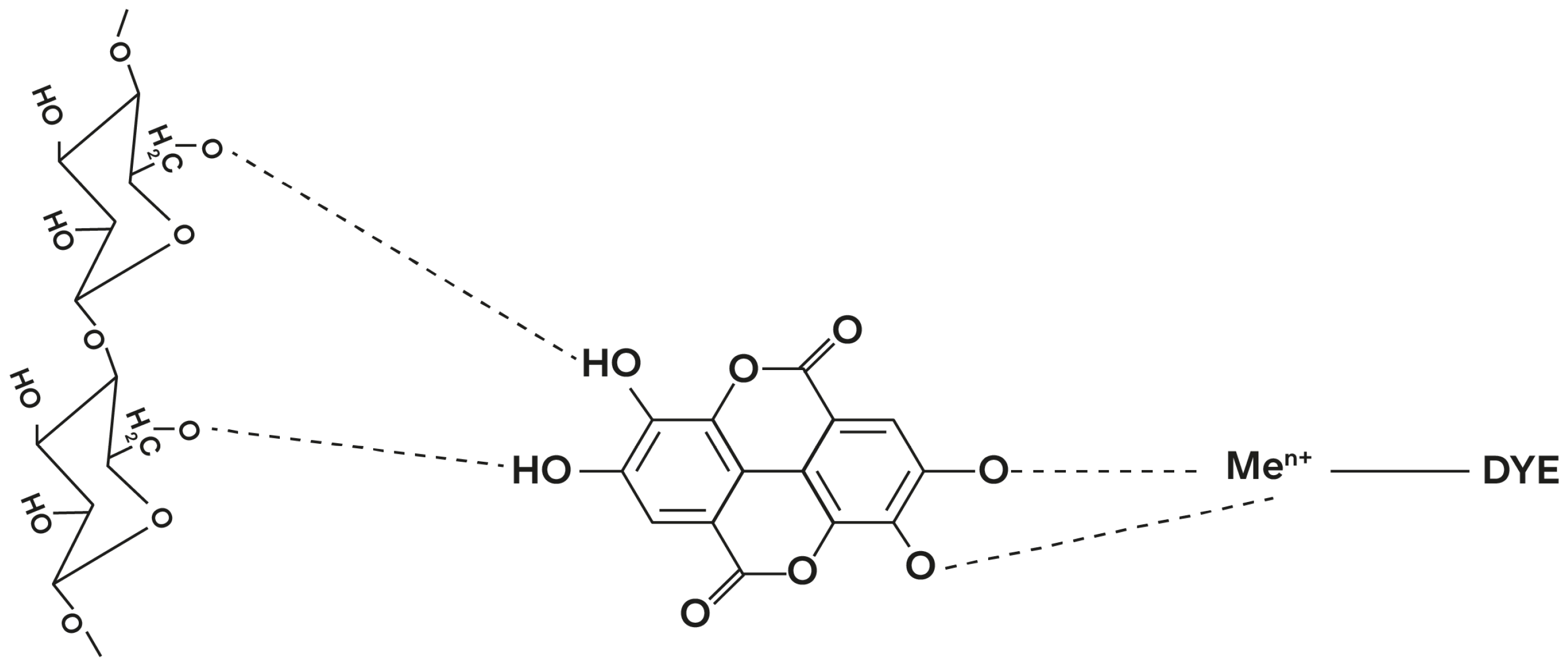
3.1. Mordants and Biomordants
3.2. Enzyme Treatment
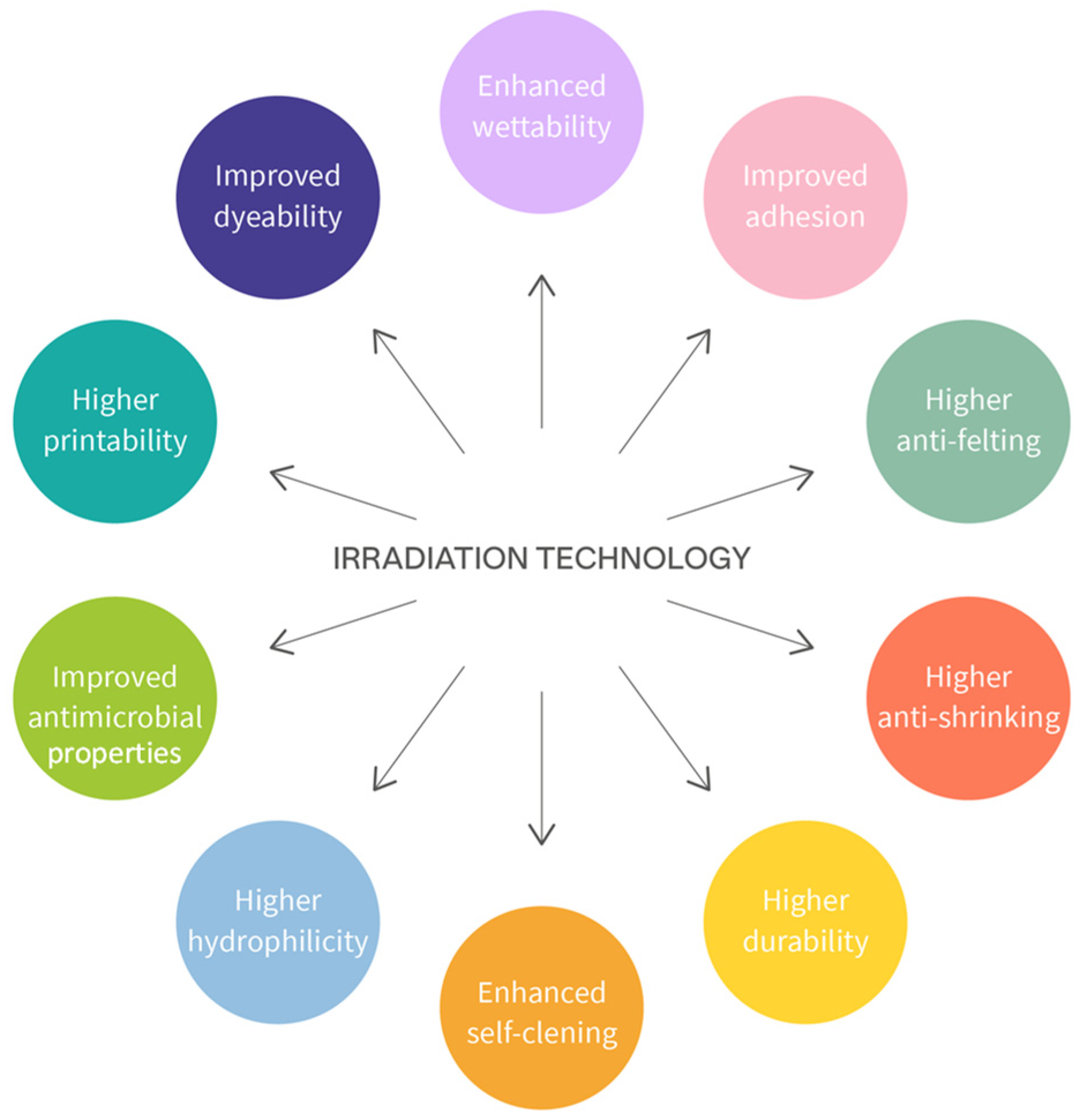
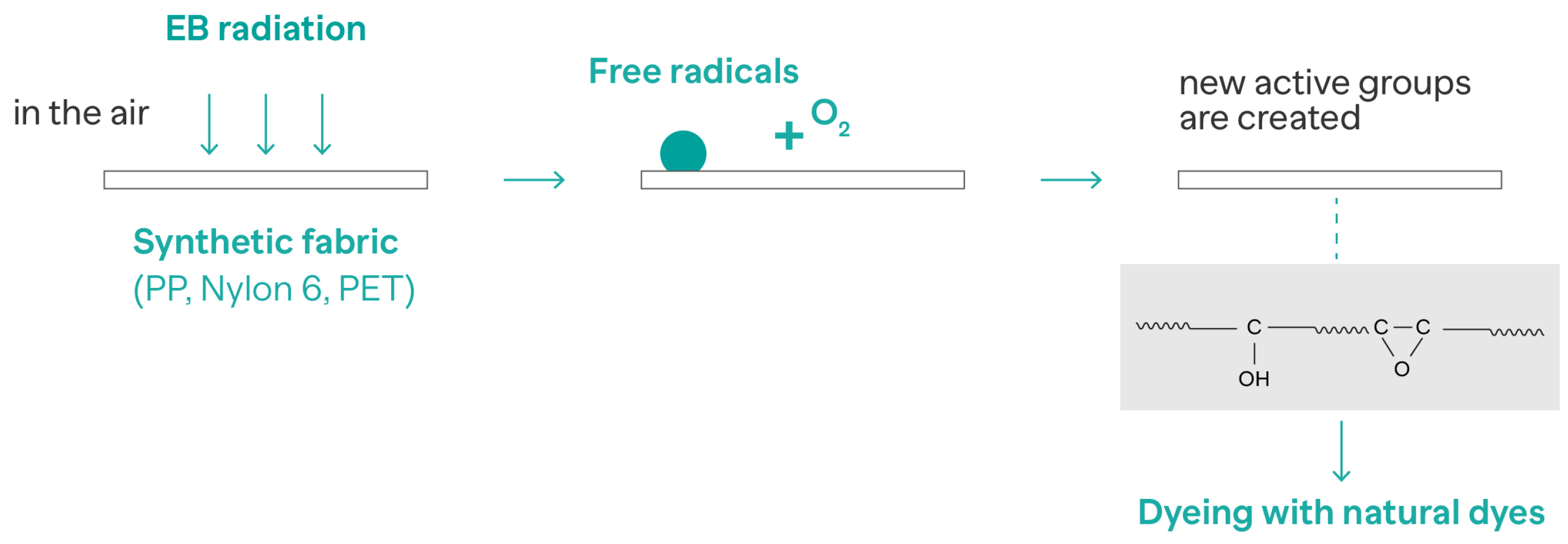
3.3. Irradiation Technologies
4. Advancements in the Dyeing Process
5. Conclusions and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Quantis. Measuring Fashion: Insights from the Environmental Impact of the Global Apparel and Footwear Industries. Full Report and Methodological Considerations. 2018. Available online: https://quantis.com/wp-content/uploads/2019/11/measuringfashion_globalimpactstudy_quantis_2018.pdf (accessed on 24 July 2023).
- Ellen MacArthur Foundation. A New Textiles Economy: Redesigning Fashion’s Future. 2017. Available online: https://ellenmacarthurfoundation.org/a-new-textiles-economy (accessed on 3 June 2023).
- UN Environment Programme (UNEP). Sustainability and Circularity in the Textile Value Chain; Global Stocktaking: Nairobi, Kenya, 2020. [Google Scholar]
- Turley, D.B.; Horne, M.; Blackburn, R.S.; Stott, E.; Laybourn, S.R.; Copeland, J.E.; Harwood, J. The Role and Business Case for Existing and Emerging Fibres in Sustainable Clothing: Final Report to the Department for Environment; Food and Rural Affairs (Defra): London, UK, 2009.
- Sandin, G.; Roos, S.; Peters, G. Environmental Assessment of Swedish Clothing Consumption—Six Garments, Sustainable Futures. 2019. Available online: https://core.ac.uk/download/pdf/270109142.pdf (accessed on 3 June 2023).
- Maxwell, D.M.; Andrew, L.; Ryan, J. The State of the Apparel Sector 2015 Special Report—Water a Report for the Global Leadership Award in Sustainable Apparel; The Sustainable Business Group: London, UK, 2015. [Google Scholar]
- Health and Safety Executive (HSE). Dyes and Chemicals in Textile Finishing: An introduction. Dyeing and Finishing Information Sheet No 1—HSE Information Sheet. 2016. Available online: https://www.hse.gov.uk/textiles/dyes-dyeing.htm (accessed on 4 June 2023).
- Hassaan, M.A.; Nemr, A.E. Health and Environmental Impacts of Dyes: Mini Review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar]
- Bide, M. Sustainable dyeing with synthetic dyes. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Springer: Berlin/Heidelberg, Germany, 2014; pp. 81–107. [Google Scholar]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golinska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- European Commission. Zero Brine, D6.1 Wastewater and Solution Provider Knowledge Models, Correlations and Interlinks, October 2020. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5d4a61977&appId=PPGMS (accessed on 4 June 2023).
- Gürses, A.; Açıkyıldız, M.; Güne¸s, K.; Gürses, M.S. Classification of Dye and Pigments. In Springer Briefs in Molecular Science; Dyes and Pigments; Gürses, A., Açıkyıldız, M., Güne¸s, K., Gürses, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–45. [Google Scholar]
- KEMI; Swedish Chemicals Agency. Hazardous Chemical Substances in Textiles, Proposals for Risk Management Measures; Swedish Chemicals Agency: Cham, Switzerland, 2016.
- European Commission. Final Report, Study on the Link Between Allergic Reactions and Chemicals in Textile Products, 7 January 2013. Available online: https://commission.europa.eu/ (accessed on 6 June 2023).
- Lisi, P.; Stngeni, L. Gruppo Italiano Ricerca Dermatiti da Contatto e Ambientali (GIRDCA) epidemiological survey of contact dermatitis. Am. J. Contact Dermat. 2010, 10, 30–37. [Google Scholar]
- Affat, S.S. Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: A review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Saxena, S.; Raja, A.S.M. Natural dyes: Sources, chemistry, application and sustainability issues. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Springer: Berlin/Heidelberg, Germany, 2014; pp. 37–80. [Google Scholar]
- Salauddin, S.; Mia, R.; Haque, M.A.; Shamim, A.M. Review on extraction and application of natural dyes. Text. Leather Rev. 2021, 4, 218–233. [Google Scholar] [CrossRef]
- Kamboj, A.; Jose, S.; Singh, A. Antimicrobial activity of natural dyes—A comprehensive review. J. Nat. Fibers 2022, 19, 5380–5394. [Google Scholar] [CrossRef]
- Kannahi, M. Vinotha, Antimicrobial activity of Lawsonia inermis leaf extracts against some human pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 342–349. [Google Scholar]
- Iqbal, S.; Ansari, T.N. Extraction and application of natural dyes. In Sustainable Practices in the Textile Industry; Scrivener Publishing LLC.: Beverly, MA, USA, 2021; pp. 1–40. [Google Scholar]
- Hwang, H.J.; Hong, K.H. Effect of pretreatment on Dyeability and functionalities of summer rayon fabrics finished by gallnut extract. Fash. Text. Res. J. 2016, 18, 244–251. [Google Scholar] [CrossRef]
- Baseri, S. Eco-friendly production of anti-UV and antibacterial cotton fabrics via waste products. Cellulose 2020, 27, 10407–10423. [Google Scholar] [CrossRef]
- Hou, X.; Chen, X.; Cheng, Y.; Xu, H.; Chen, L.; Yang, Y. Dyeing and UV-protection properties of water extracts from orange peel. J. Clean. Prod. 2013, 52, 410–419. [Google Scholar] [CrossRef]
- Gupta, V.K. Fundamentals of natural dyes and its application on textile substrates. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Senthilkumar, R.; Vaneshwari, V.; Sathiyavimal, S.; Amsaveni, R.; Kalaiselvi, M.; Malayaman, V. Natural Colors from dyeing plants for textiles. Int. J. Biosci. Nanosci. 2015, 2, 160–174. [Google Scholar]
- Khattab, T.A.; Abdelrahman, M.S.; Rehan, M. Textile dyeing industry: Environmental impacts and remediation. Environ. Sci. Pollut. Res. 2020, 27, 3803–3818. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; Raes, K.; Van Speybroeck, V.; Roosen, M.; De Clerck, K.; De Meester, S. Non-food applications of natural dyes extracted from agro-food residues: A critical review. J. Clean. Prod. 2021, 301, 126920. [Google Scholar] [CrossRef]
- Moussa, I.; Ghezal, I.; Sakli, F. Valorization of Pelargonium graveolens L’Hér. Hydrodistillation Solid Waste as Natural Dye for Wool Fabrics. J. Nat. Fibers 2023, 20, 2156966. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Rather, L.J.; Li, Q. Agricultural waste of Eriobotrya japonica L.(Loquat) seeds and flora leaves as source of natural dye and bio-mordant for coloration and bio-functional finishing of wool textile. Ind. Crops Prod. 2021, 169, 113633. [Google Scholar] [CrossRef]
- Sukemi, P.K.; Srisuwannaket, C.; Niamnont, N.; Mingvanish, W. Dyeing of cotton with the natural dye extracted from waste leaves of green tea. Color. Technol. 2019, 135, 121–126. [Google Scholar] [CrossRef]
- Jose, S.; Pandit, P.; Pandey, R. Chickpea husk—A potential agro waste for coloration and functional finishing of textiles. Ind. Crops Prod. 2019, 142, 111833. [Google Scholar] [CrossRef]
- Ganglberger, E. Environmental aspects and sustainability. In Handbook of Natural Colorants; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 353–366. [Google Scholar]
- Shahidi, S.; Khoshechin, E.; Sharifi, S.D.; Mongkholrattanasit, R. Investigation of the Effect of Various Natural Dyes on UV Protection Properties and Antibacterial Activity of Cotton Fabrics. J. Nat. Fibers 2022, 19, 7213–7228. [Google Scholar] [CrossRef]
- Merdan, N.; Seyda, E.; Mujgan, N.D. Ecological and sustainable natural dyes. In Textiles and Clothing Sustainability: Sustainable Textile Chemical Processes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–41. [Google Scholar]
- Pervaiz, S.; Mughal, T.A.; Khan, F.Z.; Hayat, S.; Aslam, A.; Shah, S.F. Environmental friendly leather dyeing using Tagetes erecta L. (Marigold) waste flowers. Int. J. Biosci. 2017, 10, 382–390. [Google Scholar]
- Al-Alwani, M.A.; Mohamad, A.B.; Kadhum AA, H.; Ludin, N.A. Effect of solvents on extraction and adsorption of natural dyes extracted from Cordyline fruticosa and Hylocereus polyrhizus. Asian J. Chem. 2014, 26, 6285. [Google Scholar] [CrossRef]
- Baaka, N.; Ticha, M.B.; Haddar, W.; Hammami, S.; Mhenni, M.F. Extraction of natural dye from waste wine industry: Optimization survey based on a central composite design method. Fibers Polym. 2015, 16, 38. [Google Scholar] [CrossRef]
- Adeel, S.; Habib, N.; Arif, S.; ur Rehman, F.; Azeem, M.; Batool, F.; Amin, N. Microwave-assisted eco-dyeing of bio mordanted silk fabric using cinnamon bark (Cinnamomum verum) based yellow natural dye. Sustain. Chem. Pharm. 2020, 17, 100306. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Kaur, C. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wei, Y.; Wang, S.; Zuo, X.; Shen, B. Sustainable ultrasound-assisted ultralow liquor ratio dyeing of cotton fabric with natural turmeric dye. Text. Res. J. 2020, 90, 685–694. [Google Scholar] [CrossRef]
- Sadeghi-Kiakhani, M.; Hashemi, E.; Gharanjig, K. Inorganic nanoparticles and natural dyes for production of antimicrobial and antioxidant wool fiber. 3 Biotech 2019, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Qadariyah, L.; Mahfud, M.; Sulistiawati, E.; Swastika, P. Natural dye extraction from teak leves (Tectona Grandis) using ultrasound assisted extraction method for dyeing on cotton fabric. In MATEC Web of Conferences; EDP Sciences: Lisses, France, 2018; Volume 156, p. 05004. [Google Scholar]
- Wizi, J.; Wang, L.; Hou, X.; Tao, Y.; Ma, B.; Yang, Y. Ultrasound-microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind. Crops Prod. 2018, 120, 203–213. [Google Scholar] [CrossRef]
- Tylor, M.; Atri, B.S.; Minhas, S. Development in Microwave Chemistry; Evalueserve: Zürich, Switzerland, 2005; pp. 5–7. [Google Scholar]
- Chaturvedi, A.K. Extraction of Nutraceuticals from Plants by Microwave Assisted Extraction. Syst. Rev. Pharm. 2018, 9, 31–35. [Google Scholar] [CrossRef]
- Akhtar, I.; Javad, S.; Yousaf, Z.; Iqbal, S.; Jabeen, K. Microwave assisted extraction of phytochemicals: An efficient and modern approach for botanicals and pharmaceuticals. Pak. J. Pharm. Sci. 2019, 32, 223–230. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; AbertVian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Gardossi, L.; Poulsen, P.B.; Ballesteros, A.; Hult, K.; Švedas, V.K.; Vasić-Rački, Đ.; Carrea, G.; Magnusson, A.; Schmid, A.; Wohlgemuth, R.; et al. Guidelines for reporting of biocatalytic reactions. Trends Biotechnol. 2010, 28, 171–180. [Google Scholar] [CrossRef]
- Tiwari, H.C.; Singh, P.; Kumar Mishra, P.; Srivastava, P. Evaluation of various techniques for extraction of natural colorants from pomegranate rind—Ultrasound and enzyme assisted extraction. Indian J. Fibre Text. Res. 2010, 35, 272. [Google Scholar]
- Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. A 2007, 1163, 2–24. [Google Scholar] [CrossRef]
- Mitra, S. Sample Preparation Techniques in Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Brunner, G. Supercritical gases as solvents: Phase equilibria. In Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Steinkopff-Verlag: New York, NY, USA, 1994; pp. 59–146. [Google Scholar]
- Elmaaty, T.A.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Björklund, E.; Sparr-Eskilsson, C. Extraction, Supercritical Fluid Extraction. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 597–604. [Google Scholar]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2016, 38, 146–166. [Google Scholar] [CrossRef]
- Borges, M.E.; Tejera, R.L.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Iovine, A.; Leone, G.P.; Larocca, V.; Di Sanzo, G.; Casella, P.; Marino, T.; Musmarra, D.; Molino, A. Risk analysis of a supercritical fluid extraction plant using a safety software. Chem. Eng. 2020, 79, 79–84. [Google Scholar]
- Kabir SM, M.; Hasan, M.M.; Uddin, M.Z. Novel approach to dye polyethylene terephthalate (PET) fabric in supercritical carbon dioxide with natural curcuminoid dyes. Fibres Text. East. Eur. 2019, 27, 65–70. [Google Scholar] [CrossRef]
- Vankar, P.S. Natural Dyes for Textiles: Sources, Chemistry and Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Boutrop, J.; Ellis, C. The Art and Science of Natural Dyes: Principles, Experiments, and Results; Schiffer Publishing, Ltd.: Atglen, PA, USA, 2018. [Google Scholar]
- Pisitsak, P.; Hutakamol, J.; Jeenapak, S.; Wanmanee, P.; Nuammaiphum, J.; Thongcharoen, R. Natural dyeing of cotton with Xylocarpus granatum bark extract: Dyeing, fastness, and ultraviolet protection properties. Fibers Polym. 2016, 17, 560–568. [Google Scholar] [CrossRef]
- Singh, M.; Vajpayee, M.; Ledwani, L. Eco-friendly surface modification of natural fibres to improve dye uptake using natural dyes and application of natural dyes in fabric finishing: A review. Mater. Today Proc. 2021, 43, 2868–2871. [Google Scholar] [CrossRef]
- Broadbent, A.D. Basic Principles of Textile Coloration; Society of Dyers and Colorists: Bradford, UK, 2001; Volume 132, pp. 332–357. [Google Scholar]
- Prabhu, K.H.; Bhute, A.S. Plant based natural dyes and mordants: A Review. J. Nat. Prod. Plant Resour. 2012, 2, 649–664. [Google Scholar]
- İşmal, Ö.E.; Yıldırım, L. Metal mordants and biomordants. In The Impact and Prospects of Green Chemistry for Textile Technology; Woodhead Publishing: Sawston, UK, 2019; pp. 57–82. [Google Scholar]
- Rahman, N.A.; Tajuddin, R.; Tumin, S. Optimization of natural dyeing using ultrasonic method and biomordant. Int. J. Chem. Eng. Appl. 2013, 4, 161. [Google Scholar] [CrossRef]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Human exposure to trace elements through the skin by direct contact with clothing: Risk assessment. Env. Res. 2015, 140, 308–316. [Google Scholar] [CrossRef]
- Guesmi, A.; Hamadi, N.B.; Ladhari, N.; Sakli, F. Dyeing properties and color fastness of wool dyed with indicaxanthin natural dye. Ind. Crop Prod. 2012, 37, 493.e499. [Google Scholar] [CrossRef]
- İşmal, Ö.E. Greener natural dyeing pathway using a by-product of olive oil; prina and biomordants. Fibers Polym. 2017, 18, 773–785. [Google Scholar] [CrossRef]
- Shahmoradi Ghaheh, F.; Moghaddam, M.K.; Tehrani, M. Comparison of the effect of metal mordants and bio-mordants on the colorimetric and antibacterial properties of natural dyes on cotton fabric. Color. Technol. 2021, 137, 689–698. [Google Scholar] [CrossRef]
- Hosen, M.D.; Rabbi, M.F.; Raihan, M.A.; Al Mamun, M.A. Effect of turmeric dye and biomordants on knitted cotton fabric coloration: A promising alternative to metallic mordanting. Clean. Eng. Technol. 2021, 3, 100124. [Google Scholar] [CrossRef]
- Pinheiro, L.; Kohan, L.; Duarte, L.O.; Garavello, M.E.D.P.E.; Baruque-Ramos, J. Biomordants and new alternatives to the sustainable natural fiber dyeings. SN Appl. Sci. 2019, 1, 1356. [Google Scholar] [CrossRef]
- Rani, N.; Jajpura, L.; Butola, B.S. Ecological dyeing of protein fabrics with Carica papaya L. leaf natural extract in the presence of bio-mordants as an alternative copartner to metal mordants. J. Inst. Eng. Ser. E 2020, 101, 19–31. [Google Scholar] [CrossRef]
- Haji, A.; Shahmoradi Ghaheh, F.; Mohammadi, L. Dyeing of polyamide 6 fabric with new bio-colorant and bio-mordants. Environ. Sci. Pollut. Res. 2023, 30, 37981–37996. [Google Scholar] [CrossRef]
- Islam, M.R.; Khan, A.N.N.; Mahmud, R.U.; Haque, S.M.N.; Khan, M.M.I. Sustainable dyeing of jute-cotton union fabrics with onion skin (allium CEPA) dye using banana peel (Musa) and guava leaves (Psidium guajava) extract as biomordants. Pigment. Resin Technol. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Phromphen, P. Optimization of Marigold Flower Dye Using Banana Peel as a Biomordant. J. Nat. Fibers 2023, 20, 2153193. [Google Scholar] [CrossRef]
- Rather, L.J.; Shabbir, M.; Bukhari, M.N.; Shahid, M.; Khan, M.A.; Mohammad, F. Ecological dyeing of woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. J. Environ. Chem. Eng. 2016, 4, 3041–3049. [Google Scholar] [CrossRef]
- Madhu, A.; Chakraborty, J.N. Developments in application of enzymes for textile processing. J. Clean. Prod. 2017, 145, 114–133. [Google Scholar] [CrossRef]
- Quandt, C.; Kuhl, B. Enzymatic processes: Operational possibilities and optimization (Enzymes Possibilite’set perspectives). L’Industrie Text. Issue 2001, 1334–1335, 116–119. [Google Scholar]
- Kumar, D.; Bhardwaj, R.; Jassal, S.; Goyal, T.; Khullar, A.; Gupta, N. Application of enzymes for an eco-friendly approach to textile processing. Environ. Sci. Pollut. Res. 2021, 30, 71838–71848. [Google Scholar] [CrossRef]
- Duran, N.; Duran, M. Enzyme applications in the textile industry. Rev. Prog. Color. Relat. Top. 2000, 30, 41–44. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shanker, R.; Srivastava, J. Ultrasonic dyeing of cotton fabric with aqueous extract of Eclipta alba. Dye. Pigment. 2007, 72, 33–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Rather, L.J.; Li, Q. Recent advances in the surface modification strategies to improve functional finishing of cotton with natural colourants—A review. J. Clean. Prod. 2022, 335, 130313. [Google Scholar] [CrossRef]
- Samant, L.; Jose, S.; Rose, N.M.; Shakyawar, D.B. Antimicrobial and UV protection properties of cotton fabric using enzymatic pretreatment and dyeing with Acacia catechu. J. Nat. Fibers 2022, 19, 2243–2253. [Google Scholar] [CrossRef]
- Benli, H.; Bahtiyari, M.İ. Use of ultrasound in biopreparation and natural dyeing of cotton fabric in a single bath. Cellulose 2015, 22, 867–877. [Google Scholar] [CrossRef]
- Raja AS, M.; Thilagavathi, G. Influence of enzyme and mordant treatments on the antimicrobial efficiency of natural dyes on wool materials. Asian J. Text 2011, 1, 138–144. [Google Scholar] [CrossRef]
- Zhang, R.P.; Cai, Z.S. Study on the natural dyeing of wool modified with enzyme. Fibers Polym. 2011, 12, 478–483. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F. High-energy radiation induced sustainable coloration and functional finishing of textile materials. Ind. Eng. Chem. Res. 2015, 54, 3727–3745. [Google Scholar] [CrossRef]
- Adeel, S.; Gulzar, T.; Azeem, M.; Saeed, M.; Hanif, I.; Iqbal, N. Appraisal of marigold flower-based lutein as natural colorant for textile dyeing under the influence of gamma radiations. Radiat. Phys. Chem. 2017, 130, 35–39. [Google Scholar] [CrossRef]
- Moholkar, V.S.; Nierstrasz, V.A.; Warmoeskerken, M.M.C.G. Intensification of mass transfer in wet textile processes by power ultrasound. Autex Res. J. 2003, 3, 129–138. [Google Scholar]
- Czaplicki, Z.; Matyjas-Zgondek, E.; Strzelecki, S. Scouring of Sheep Wool Using an Acoustic Ultrasound Wave. Fibres Text. East. Eur. 2021, 29, 44–48. [Google Scholar] [CrossRef]
- Peila, R.; Grande, G.A.; Giansetti, M.; Rehman, S.; Sicardi, S.; Rovero, G. Washing off intensification of cotton and wool fabrics by ultrasounds. Ultrason. Sonochemistry 2015, 23, 324–332. [Google Scholar] [CrossRef]
- Davulcu, A.; Eren, H.A.; Avinc, O.; Erişmiş, B. Ultrasound assisted biobleaching of cotton. Cellulose 2014, 21, 2973–2981. [Google Scholar] [CrossRef]
- Kadam, V.V.; Goud, V.; Shakyawar, D.B. Ultrasound Scouring of Wool and Its Effects on Fibre Quality; NISCAIR-CSIR: Delhi, India, 2013. [Google Scholar]
- Al Kashouty, M.; Elsayad, H.; Twaffiek, S.; Salem, T.; Elhadad, S. An Overview: Textile Surface Modification by Using Sol-gel Technology. Egypt. J. Chem. 2020, 63, 3301–3311. [Google Scholar] [CrossRef]
- Ferrero, F.; Migliavacca, G.; Periolatto, M. UV treatments on cotton fibers. In Cotton Research; IntechOpen Limited: London, UK, 2016; pp. 235–256. [Google Scholar]
- Adeel, S.; Bhatti, I.A.; EL-Nagar, K.; Alam, M.M.; Ali, N. Dyeing of cotton fabric using UV irradiated turmeric (Curcuma longa L.) as natural dye. Res. J. Text. Appar. 2011, 15, 71–76. [Google Scholar] [CrossRef]
- Gulzar, T.; Adeel, S.; Hanif, I.; Rehman, F.; Hanif, R.; Zuber, M.; Akhtar, N. Eco-friendly dyeing of gamma ray induced cotton using natural quercetin extracted from acacia bark (A. nilotica). J. Nat. Fibers 2015, 12, 494–504. [Google Scholar] [CrossRef]
- Rehman, F.; Adeel, S.; Hanif, R.; Muneer, M.; Zia, K.M.; Zuber, M.; Khosa, M.K. Modulation of marigold based lutein dye and its dyeing behaviour using UV radiation. J. Nat. Fibers 2017, 14, 63–70. [Google Scholar] [CrossRef]
- Bhatti, I.A.; Adeel, S.; Ur Fazal, R.; Irshad, M.; Abbas, M. Effect of mercerization and gamma irradiation on the dyeing behaviour of cotton using stilbene based direct dye. Radiat. Phys. Chem. 2012, 81, 823. [Google Scholar] [CrossRef]
- Ajmal, M.; Adeel, S.; Azeem, M.; Zuber, M.; Akhtar, N.; Iqbal, N. Modulation of pomegranate peel colourant characteristics for textile dyeing using high energy radiations. Ind. Crops Prod. 2014, 58, 188–193. [Google Scholar] [CrossRef]
- Batool, F.; Adeel, S.; Azeem, M.; Khan, A.A.; Bhatti, I.A.; Ghaffar, A.; Iqbal, N. Gamma radiations induced improvement in dyeing properties and colorfastness of cotton fabrics dyed with chicken gizzard leaves extracts. Radiat. Phys. Chem. 2013, 89, 33–37. [Google Scholar] [CrossRef]
- Chirila, L.; Popescu, A.; Cutrubinis, M.; Stanculescu, I.; Moise, V.I. The influence of gamma irradiation on natural dyeing properties of cotton and flax fabrics. Radiat. Phys. Chem. 2018, 145, 97–103. [Google Scholar] [CrossRef]
- Shahidi, S.; Wiener, J. Radiation effects in textile materials. In Radiation Effects in Materials; IntechOpen Limited: London, UK, 2016; pp. 309–328. [Google Scholar]
- Van der Sluijs, M.H.; Church, J.S. The effect of quarantine-level gamma irradiation on cotton fiber and its subsequent textile processing performance. Text. Res. J. 2013, 83, 197–207. [Google Scholar] [CrossRef]
- Khan, A.A.; Iqbal, N.; Adeel, S.; Azeem, M.; Batool, F.; Bhatti, I.A. Extraction of natural dye from red calico leaves: Gamma ray assisted improvements in colour strength and fastness properties. Dye. Pigment. 2014, 103, 50–54. [Google Scholar] [CrossRef]
- Henniges, U.; Okubayashi, S.; Rosenau, T.; Potthast, A. Irradiation of cellulosic pulps: Understanding its impact on cellulose oxidation. Biomacromol 2012, 13, 4171–4178. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, M.; Hoshi, Y. Electron beam processing system and its application. SEI Tech. Rev. 2012, 75, 47–54. [Google Scholar]
- Elmaaty, T.A.; Okubayashi, S.; Elsisi, H.; Abouelenin, S. Electron beam irradiation treatment of textiles materials: A review. J. Polym. Res. 2022, 29, 117. [Google Scholar] [CrossRef]
- Elmaaty, T.A.; Abouelenin, S.; Elsisi, H.; Okubayashi, S. Ecofriendly approach for dyeing synthetic fabrics with natural dyes using electron beam irradiation. Fibers Polym. 2022, 23, 759–767. [Google Scholar] [CrossRef]
- Samanta, K.K.; Basak, S.; Chattopadhyay, S.K. Environment-friendly textile processing using plasma and UV treatment. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Springer: Berlin/Heidelberg, Germany, 2014; pp. 161–201. [Google Scholar]
- Vesel, A.; Mozetic, M.; Strnad, S.; Peršin, Z.; Stana-Kleinschek, K.; Hauptman, N. Plasma modification of viscose textile. Vacuum 2009, 84, 79–82. [Google Scholar] [CrossRef]
- Molina, R.; Teixidó, J.M.; Kan, C.W.; Jovančić, P. Hydrophobic coatings on cotton obtained by in situ plasma polymerization of a fluorinated monomer in ethanol solutions. ACS Appl. Mater. Interfaces 2017, 9, 5513–5521. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.Y.H.Y.T.; Yasuda, T.; Yasuda, H. Effect of crystallinity of PET and nylon 66 fibers on plasma etching and dyeability characteristics. Text. Res. J. 1992, 62, 474–480. [Google Scholar] [CrossRef]
- Shah, J.; Shah, S. Innovative plasma technology in textile processing: A step towards a green environment. Res. J. Eng. Sci 2013, 2278, 9472. [Google Scholar]
- Haji, A. Dyeing of cotton fabric with natural dyes improved by mordants and plasma treatment. Prog. Color Color. Coat. 2019, 12, 191–201. [Google Scholar]
- Haji, A.; Qavamnia, S.S.; Bizhaem, F.K. Optimization of oxygen plasma treatment to improve the dyeing of wool with grape leaves. Ind. Textila 2016, 67, 244–249. [Google Scholar]
- Ahmed, N.S.E.; El-Shishtawy, R.M. The use of new technologies in coloration of textile fibers. J. Mater. Sci. 2010, 45, 1143–1153. [Google Scholar] [CrossRef]
- Baig, U.; Khatri, A.; Ali, S.; Sanbhal, N.; Ishaque, F.; Junejo, N. Ultrasound-assisted dyeing of cotton fabric with natural dye extracted from marigold flower. J. Text. Inst. 2021, 112, 801–808. [Google Scholar] [CrossRef]
- Babar, A.A.; Peerzada, M.H.; Naveed, T.; Dayo, A.Q. Exhaust reactive dyeing of lyocell fabric with ultrasonic energy. Ultrason. Sonochem. 2019, 58, 104611. [Google Scholar] [CrossRef]
- Udrescu, C.; Ferrero, F.; Periolatto, M. Ultrasound-assisted dyeing of cellulose acetate. Ultrason. Sonochem. 2014, 21, 1477–1481. [Google Scholar] [CrossRef]
- Xue, Z. Study of dyeing properties of wool fabrics treated with microwave. J. Text. Inst. 2016, 107, 258–263. [Google Scholar] [CrossRef]
- Arain, R.A.; Ahmad, F.; Khatri, Z.; Peerzada, M.H. Microwave assisted henna organic dyeing of polyester fabric: A green, economical and energy proficient substitute. Nat. Prod. Res. 2021, 35, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Van Der Kraan, M. Equilibrium study on the disperse dyeing of polyester textile in supercritical carbon dioxide. Text. Res. J. 2007, 77, 550–558. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Yan, J.; Zheng, L. An industrial scale multiple supercritical carbon dioxide apparatus and its eco-friendly dyeing production. J. CO2 Util. 2016, 16, 272–281. [Google Scholar] [CrossRef]
- Penthala, R.; Kumar, R.S.; Heo, G.; Kim, H.; Lee, I.Y.; Ko, E.H.; Son, Y.A. Synthesis and efficient dyeing of anthraquinone derivatives on polyester fabric with supercritical carbon dioxide. Dye. Pigment. 2019, 166, 330–339. [Google Scholar] [CrossRef]
- Eren, H.A.; Yiğit, İ.; Eren, S.; Avinc, O. Sustainable textile processing with zero water utilization using supercritical carbon dioxide technology. In Sustainability in the Textile and Apparel Industries: Production Process Sustainability; Springer: Berlin/Heidelberg, Germany, 2020; pp. 179–196. [Google Scholar]
- Banchero, M. Recent advances in supercritical fluid dyeing. Color. Technol. 2020, 136, 317–335. [Google Scholar] [CrossRef]
- Meksi, N.; Moussa, A. A review of progress in the ecological application of ionic liquids in textile processes. J. Clean. Prod. 2017, 161, 105–126. [Google Scholar] [CrossRef]
- Ragheb, A.A.; Tawfik, S.; Abd-El Thalouth, J.I.; Mosaad, M.M. Development of printing natural fabrics with curcuma natural dye via nanotechnology. Int. J. Pharm. Sci. Res. 2017, 8, 611–620. [Google Scholar]
- Ragheb, A.; Mosaad, M.; Mahmoud, S.; Abd Thaloth, J.I. The Impact of Nanotechnologies on developing the printing of natural fabrics with pomegranate peel. Egypt. J. Chem. 2019, 62, 1249–1261. [Google Scholar]
- Wasserscheid, P. Ionic Liquids in Synthesis; Welton, T., Ed.; Wiley-Vch: Weinheim, Germany, 2018; Volume 1, p. 145. [Google Scholar]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.L.; Venkatachalam, M.; Fouillaud, M.; Dufossé, L.; Caro, Y. Extraction of fungal polyketide pigments using ionic liquids. In Proceedings of the 8th International Conference of Pigments in Food, Coloured Foods for Health Benefits, Cluj-Napoca, Romania, 15 November 2016; Volume 150, pp. 2405–2426. [Google Scholar]
- Bosiljkov, T.; Dujmic, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brncic, M.; Zlatic, E.; Redovnikovic, I.R.; Jokic, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, G.; Guo, B.; Zhou, Y. Study on the ionic liquid-based extraction technology of total flavonoids from Broussonetia papyrifera leave. Chem. Ind. Eng. Process 2016, 35, 328–331. [Google Scholar]
- Mketo, N.; Nomngongo, P.N. Application of ionic liquids for extraction of phenolic compounds and dyes: A critical review. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–408. [Google Scholar]
- Bianchini, R.; Cevasco, G.; Chiappe, C.; Pomelli, C.S.; Rodríguez Douton, M.J. Ionic liquids can significantly improve textile dyeing: An innovative application assuring economic and environmental benefits. ACS Sustain. Chem. Eng. 2015, 3, 2303–2308. [Google Scholar] [CrossRef]
- Andrade, R.S.; Torres, D.; Ribeiro, F.R.; Chiari-Andréo, B.G.; Oshiro Junior, J.A.; Iglesias, M. Sustainable cotton dyeing in nonaqueous medium applying protic ionic liquids. ACS Sustain. Chem. Eng. 2017, 5, 8756–8765. [Google Scholar] [CrossRef]
- Opwis, K.; Benken, R.; Knittel, D.; Gutmann, J.S. Dyeing of PET fibers in ionic liquids. Int. J. New Technol. Res. 2017, 3, 263192. [Google Scholar]

| Advantages | Disadvantages |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules 2023, 28, 5954. https://doi.org/10.3390/molecules28165954
Pizzicato B, Pacifico S, Cayuela D, Mijas G, Riba-Moliner M. Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules. 2023; 28(16):5954. https://doi.org/10.3390/molecules28165954
Chicago/Turabian StylePizzicato, Barbara, Severina Pacifico, Diana Cayuela, Gabriela Mijas, and Marta Riba-Moliner. 2023. "Advancements in Sustainable Natural Dyes for Textile Applications: A Review" Molecules 28, no. 16: 5954. https://doi.org/10.3390/molecules28165954
APA StylePizzicato, B., Pacifico, S., Cayuela, D., Mijas, G., & Riba-Moliner, M. (2023). Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules, 28(16), 5954. https://doi.org/10.3390/molecules28165954








