Utilizing Proteomic Approaches to Uncover the Neuroprotective Effects of ACE Inhibitors: Implications for Alzheimer’s Disease Treatment
Abstract
1. Introduction
2. Results
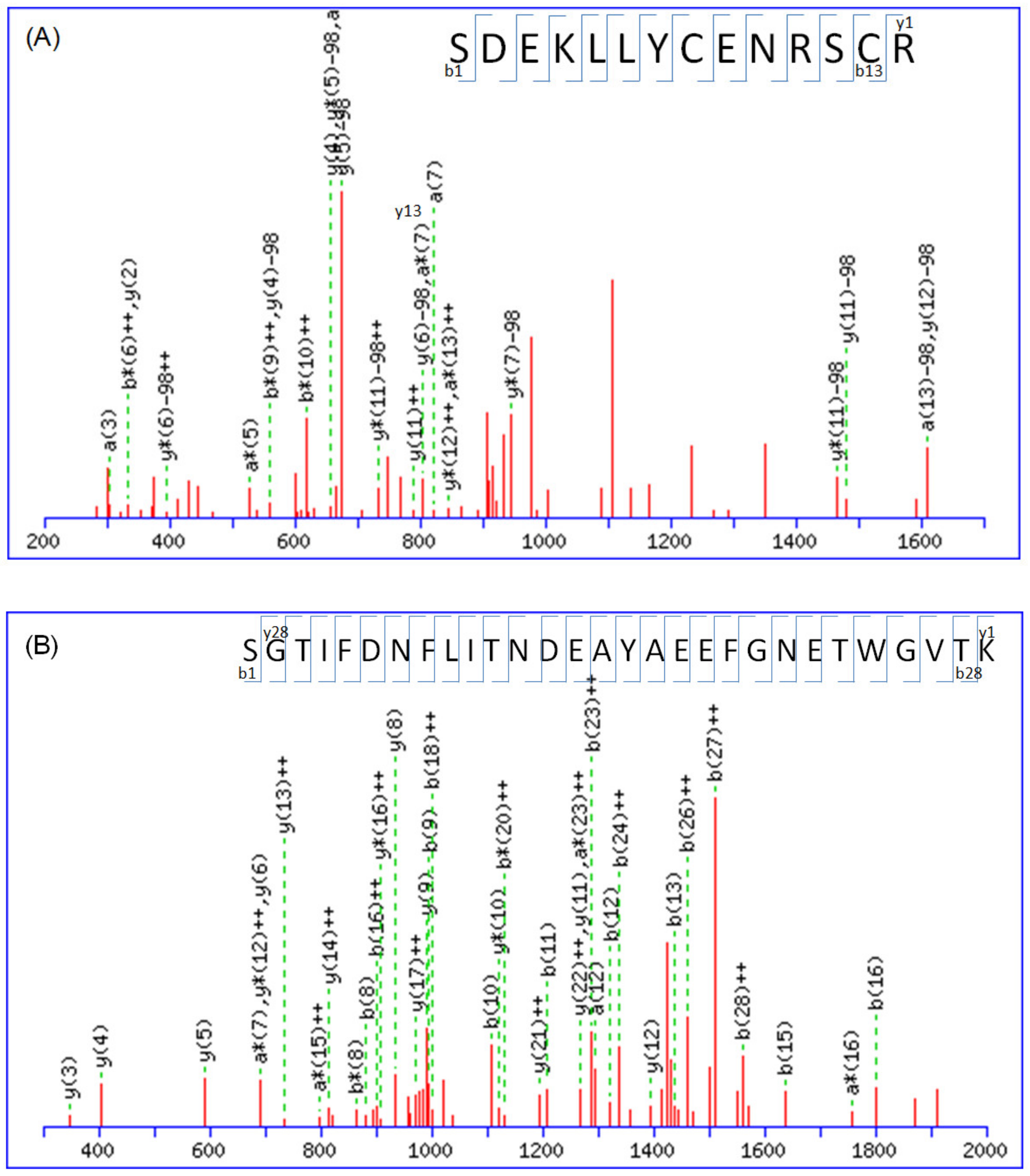
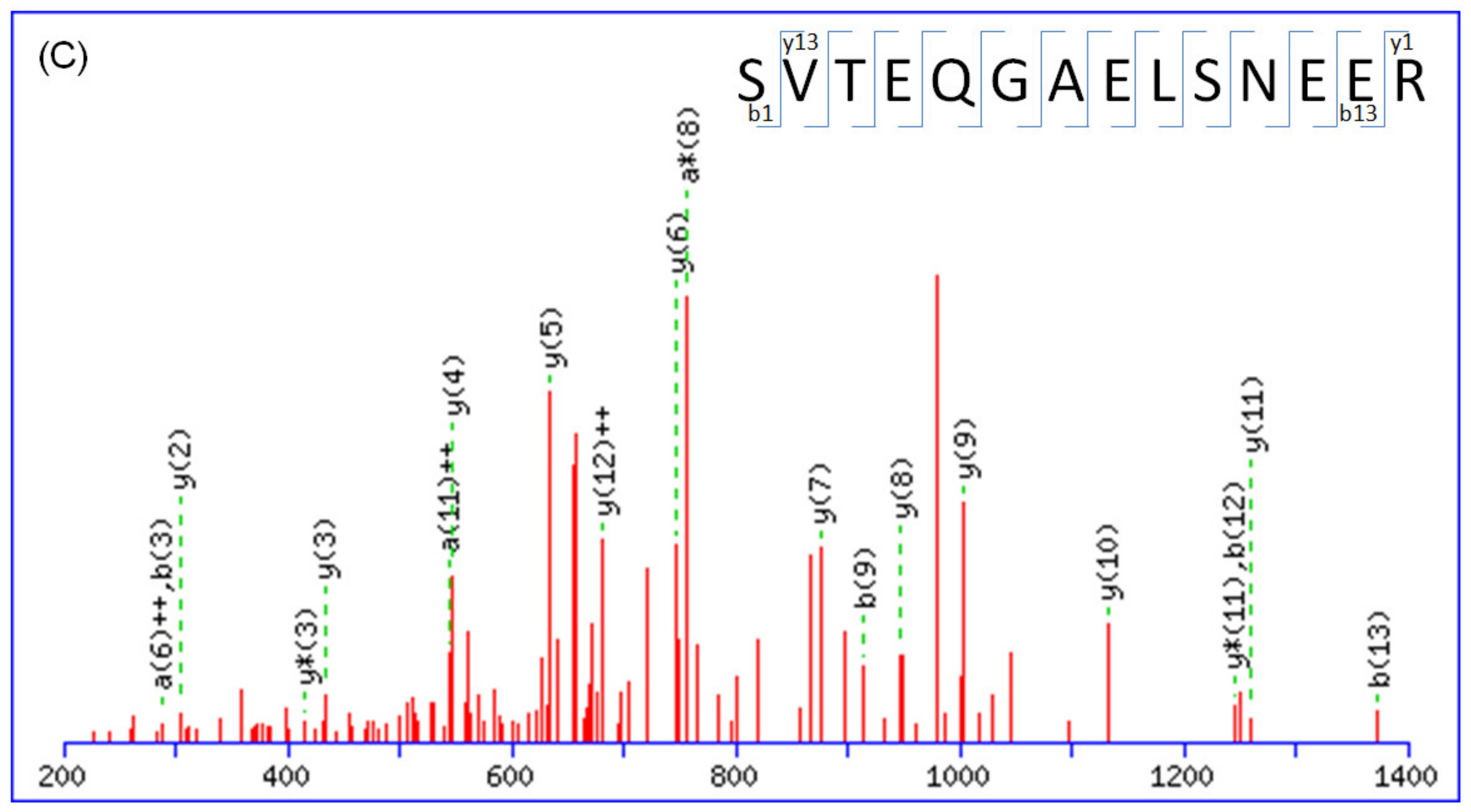
2.1. Protein Identification by Mass Spectrometry
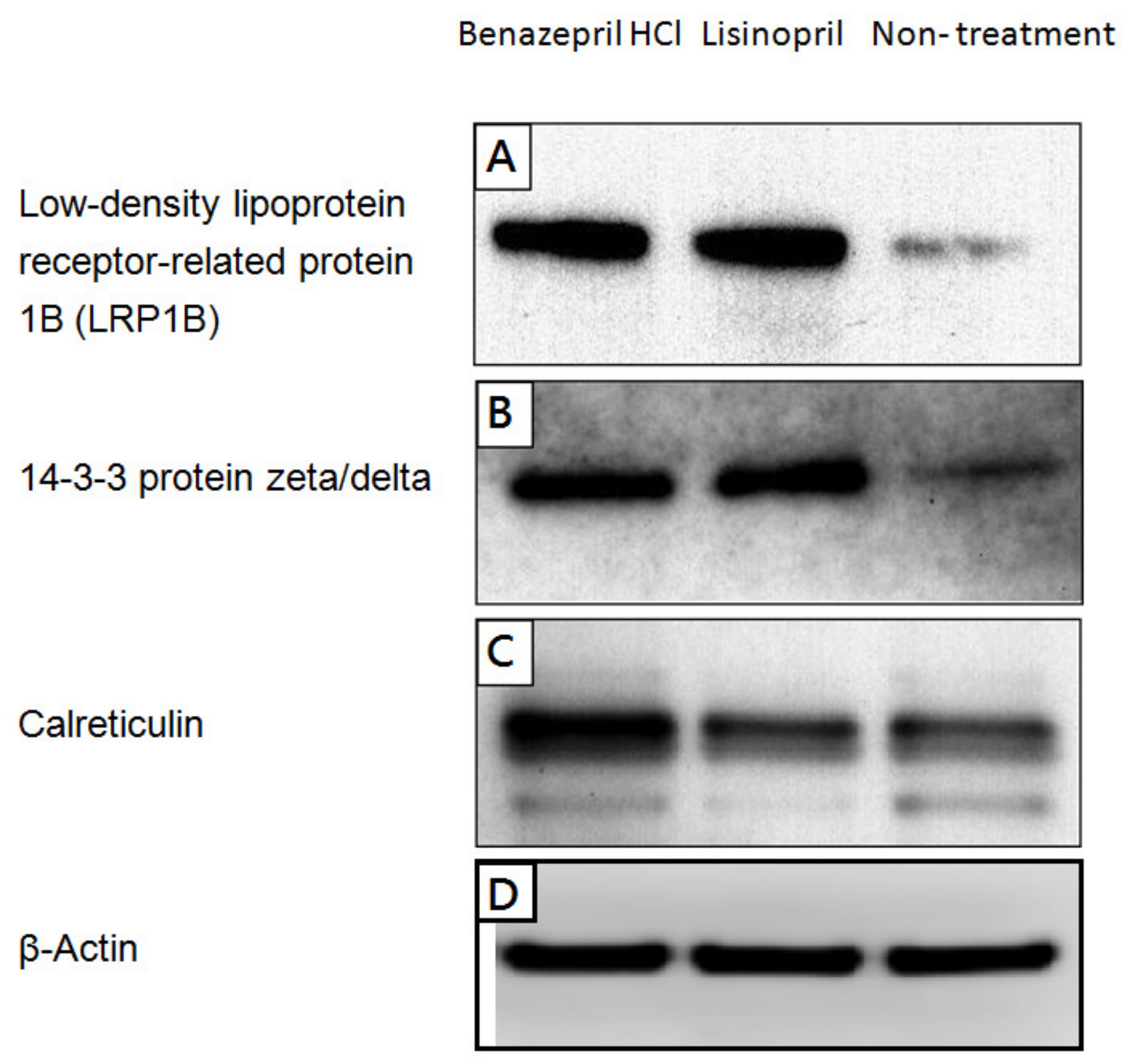
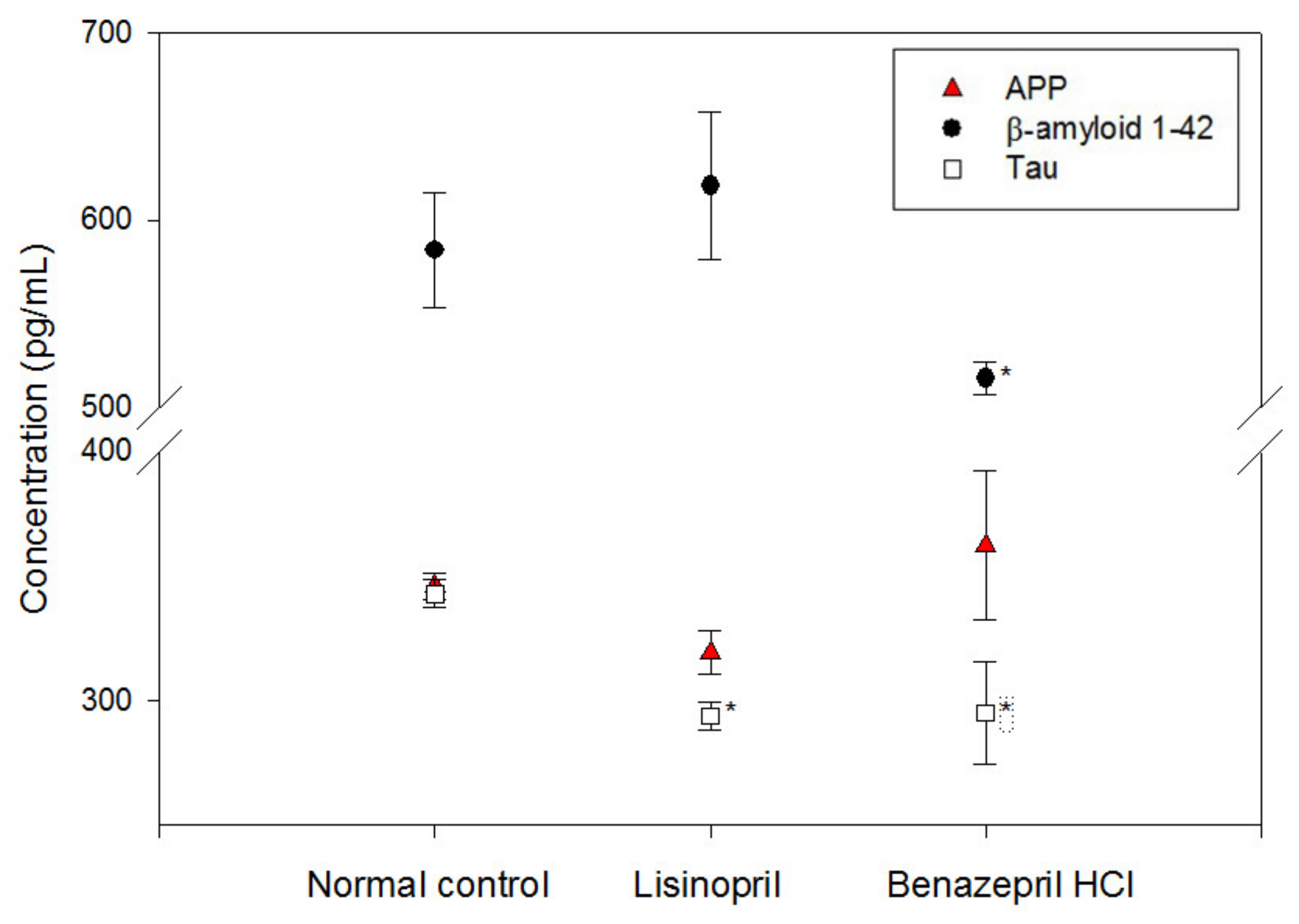
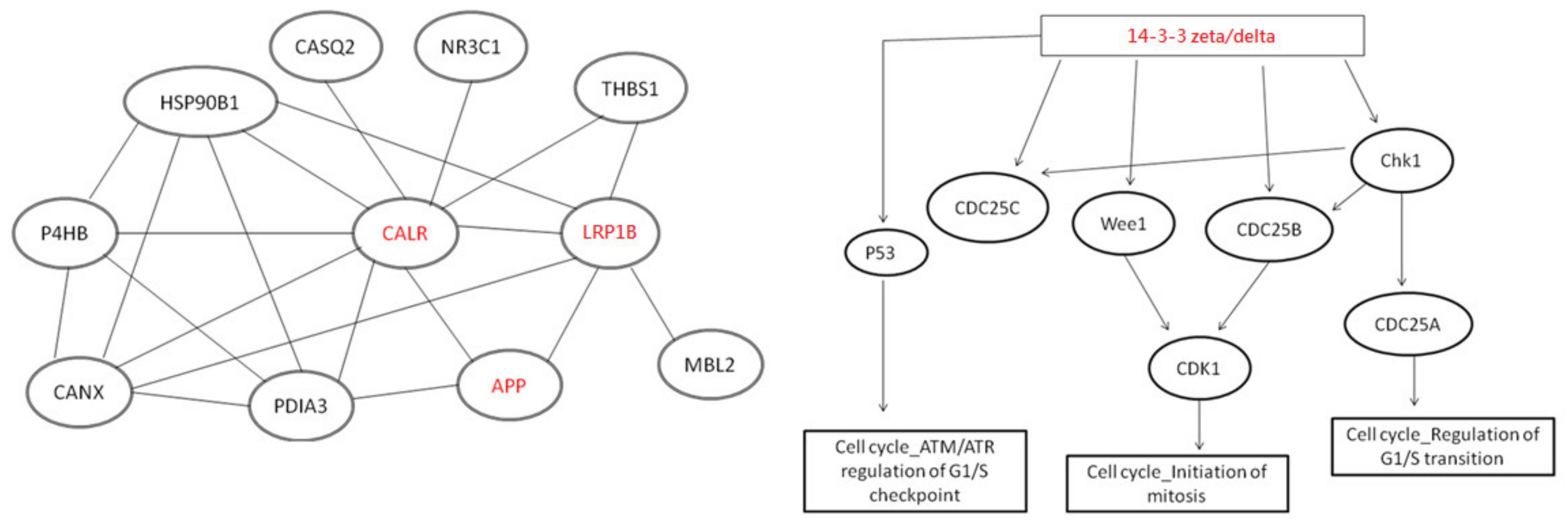
2.2. Alteration of Cellular Neuropathogenic Proteins by ACE Inhibitors
3. Discussion
3.1. Potential Neuroprotective Effect Mediated by ACE Inhibitors
3.2. The role of ACE in ACE-Inhibitor-Mediated Amelioration of Cognitive Deficits
4. Materials and Methods
4.1. Cell Culture of SH-SY5Y Neuroblastoma Cell Line
4.2. Treatment of ACE Inhibitors
4.3. Protein Identification
4.4. Western Blotting
4.5. ELISA Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patterson, C. World Alzheimer Report 2018; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Dong, Y.F.; Kataoka, K.; Tokutomi, Y.; Nako, H.; Nakamura, T.; Toyama, K.; Sueta, D.; Koibuchi, N.; Yamamoto, E.; Ogawa, H.; et al. Perindopril, a centrally active angiotensin-converting enzyme inhibitor, prevents cognitive impairment in mouse models of Alzheimer’s disease. FASEB J. 2011, 25, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Uchida, S.; Takahashi, S.; Takayama, M.; Nagata, Y.; Suzuki, N.; Shirakura, S.; Kanda, T. Effect of a centrally active angiotensin-converting enzyme inhibitor, perindopril, on cognitive performance in a mouse model of Alzheimer’s disease. Brain Res. 2010, 1352, 176–186. [Google Scholar] [CrossRef]
- AbdAlla, S.; Langer, A.; Fu, X.; Quitterer, U. ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 16917–16942. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Passmore, P.A. The renin-angiotensin system and antihypertensive drugs in Alzheimer’s disease: Current standing of the angiotensin hypothesis? J. Alzheimers Dis. 2012, 30, S251–S268. [Google Scholar] [CrossRef]
- Gao, Y.; O’Caoimh, R.; Healy, L.; Kerins, D.M.; Eustace, J.; Guyatt, G.; Sammon, D.; Molloy, D.W. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open 2013, 3, e002881. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Healy, L.; Gao, Y.; Svendrovski, A.; Kerins, D.M.; Eustace, J.; Kehoe, P.G.; Guyatt, G.; Molloy, D.W. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J. Alzheimers Dis. 2014, 40, 595–603. [Google Scholar] [CrossRef]
- de Oliveira, F.F.; Bertolucci, P.H.; Chen, E.S.; Smith, M.C. Brain-penetrating angiotensin-converting enzyme inhibitors and cognitive change in patients with dementia due to Alzheimer’s disease. J. Alzheimers Dis. 2014, 42, S321–S324. [Google Scholar] [CrossRef] [PubMed]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kuller, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef]
- Soto, M.E.; van Kan, G.A.; Nourhashemi, F.; Gillette-Guyonnet, S.; Cesari, M.; Cantet, C.; Rolland, Y.; Vellas, B. Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: Results from the Réseau sur la Maladie d’Alzheimer Français cohort. J. Am. Geriatr. Soc. 2013, 61, 1482–1488. [Google Scholar] [CrossRef]
- Hemming, H.L.; Selkoe, D.J. Amyloid veta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J. Biol. Chem. 2005, 280, 37644–37650. [Google Scholar] [CrossRef]
- Hu, J.; Igarashi, A.; Kamata, M.; Nakagawa, H. Angiotensin-converting enzyme degrades Alzheimer amyloid β-peptide (Aβ); Retards A beta 48 aggregation, deposition, fibril formation; and inhibits cytotoxicity. J. Biol. Chem. 2001, 276, 47863–47868. [Google Scholar] [CrossRef] [PubMed]
- Oba, R.; Igarashi, A.; Kamata, M.; Nagata, K.; Takano, S.; Nakagawa, H. The N-terminal active centre of human angiotensin-convertingenzyme degrades Alzheimer amyloid beta-peptide. Eur. J. Neurosci. 2005, 21, 733–740. [Google Scholar] [CrossRef]
- Baranello, R.J.; Bharani, K.L.; Padmaraju, V.; Chopra, N.; Lahiri, D.K.; Greig, N.H.; Pappolla, M.A.; Sambamurti, K. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 32–46. [Google Scholar] [CrossRef]
- Bernstein, K.E.; Koronyo, Y.; Salumbides, B.C.; Sheyn, J.; Pelissier, L.; Lopes, D.H.; Shah, K.H.; Bernstein, E.A.; Fuchs, D.T.; Yu, J.J.; et al. Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline. J. Clin. Investig. 2014, 124, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, H.; Ogawa, N.; Kanesaka, T.; Hori, A.; Yamamoto, T.; Matsukawa, N.; Michikawa, M. Higher activity of peripheral blood angiotensin-converting enzyme is associated with later-onset of Alzheimer’s disease. J. Neurol. Sci. 2011, 300, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jochemsen, H.M.; van der Flier, W.M.; Ashby, E.L.; Teunissen, C.E.; Jones, R.E.; Wattjes, M.P.; Scheltens, P.; Geerlings, M.I.; Kehoe, P.G.; Muller, M. Angiotensin-converting enzyme in cerebrospinal fluid and risk of brain atrophy. J. Alzheimers Dis. 2015, 44, 153–162. [Google Scholar] [CrossRef]
- Jochemsen, H.M.; Teunissen, C.E.; Ashby, E.L.; van der Flier, W.M.; Jones, R.E.; Geerlings, M.I.; Scheltens, P.; Kehoe, P.G.; Muller, M. The association of angiotensin-converting enzyme with biomarkers for Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 27. [Google Scholar] [CrossRef]
- Yamada, K.; Horita, T.; Takayama, M.; Takahashi, S.; Takaba, K.; Nagata, Y.; Suzuki, N.; Kanda, T. Effect of a centrally active angiotensin converting enzyme inhibitor, perindopril, on cognitive performance in chronic cerebral hypo-perfusion rats. Brain Res. 2011, 1421, 110–120. [Google Scholar] [CrossRef]
- Ríos, J.C.; Repetto, G.; Jos, A.; del Peso, A.; Salguero, M.; Cameán, A.; Repetto, M. Tribromophenol induces the differentiation of SH-SY5Y human neuroblastoma cells in vitro. Toxicol. Vitr. 2003, 17, 635–641. [Google Scholar] [CrossRef]
- Skandrani, D.; Gaubin, Y.; Beau, B.; Murat, J.C.; Vincent, C.; Croute, F. Effect of selected insecticides on growth rate and stress protein expression in cultured human A549 and SH-SY5Y cells. Toxicol. Vitr. 2006, 20, 1378–1386. [Google Scholar] [CrossRef]
- Marzolo, M.P.; Bu, G. Lipoprotein receptors and cholesterol in APP trafficking and proteolytic processing, implications for Alzheimer’s disease. Semin. Cell Dev. Biol. 2009, 20, 191–200. [Google Scholar] [CrossRef]
- Cam, J.A.; Zerbinatti, C.V.; Knisely, J.M.; Hecimovic, S.; Li, Y.; Bu, G. The low density lipoprotein receptor-related protein 1B retains beta-amyloid precursor protein at the cell surface and reduces amyloid-beta peptide production. J. Biol. Chem. 2004, 279, 29639–29646. [Google Scholar] [CrossRef]
- Benoit, M.E.; Hernandez, M.X.; Dinh, M.L.; Benavente, F.; Vasquez, O.; Tenner, A.J. C1q-induced LRP1B and GPR6 proteins expressed early in Alzheimer disease mouse models, are essential for the C1q-mediated protection against amyloid-β neurotoxicity. J. Biol. Chem. 2013, 288, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Investig. 2000, 106, 1489–1499. [Google Scholar] [CrossRef]
- Hollenbach, E.; Ackermann, S.; Hyman, B.T.; Rebeck, G.W. Confirmation of an association between a polymorphism in exon 3 of the low-density lipoprotein receptor-related protein gene and Alzheimer’s disease. Neurology 1998, 50, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Fournier, A.E.; Yamagata, K. Neuroprotective Function of 14-3-3 Proteins in Neurodegeneration. BioMed Res. Int. 2013, 79, 564534. [Google Scholar] [CrossRef] [PubMed]
- Agarwal-Mawal, A.; Qureshi, H.Y.; Cafferty, P.W.; Yuan, Z.; Han, D.; Lin, R.; Paudel, H.K. 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. J. Biol. Chem. 2003, 278, 12722–12728. [Google Scholar] [CrossRef]
- Sadik, G.; Tanaka, T.; Kato, K.; Yanagi, K.; Kudo, T.; Takeda, M. Differential interaction and aggregation of 3-repeat and 4-repeat tau isoforms with 14-3-3zeta protein. Biochem. Biophys. Res. Commun. 2009, 383, 37–41. [Google Scholar] [CrossRef]
- Yuan, Z.; Agarwal-Mawal, A.; Paudel, H.K. 14-3-3 binds to and mediates phosphorylation of microtubule-associated tau protein by Ser9-phosphorylated glycogen synthase kinase 3 beta in the brain. J. Biol. Chem. 2004, 279, 26105–26114. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jeong, W.S.; Jun, M. Protective effects of the key compounds isolated from Corni fructus against β-amyloid-induced neurotoxicity in PC12 cells. Molecules 2012, 17, 10831–10845. [Google Scholar] [CrossRef]
- Jang, H.; Arce, F.T.; Capone, R.; Ramachandran, S.; Lal, R.; Nussinov, R. Misfolded amyloid ion channels present mobile beta-sheet subunits in contrast to conventional ion channels. Biophys. J. 2009, 97, 3029–3037. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Xiao, G.; Shanmugaratnam, J.; Fine, R.E. Calreticulin functions as a molecular chaperone for the beta-amyloid precursor protein. Neurobiol. Aging 2001, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Pedraza, C.E.; Pallero, M.A.; Elzie, C.A.; Goicoechea, S.; Strickland, D.K.; Murphy-Ullrich, J.E. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J. Cell Biol. 2003, 161, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Cao, Y.; Gao, J. Serum calreticulin is a negative biomarker in patients with Alzheimer’s disease. Int. J. Mol. Sci. 2014, 15, 21740–21753. [Google Scholar] [CrossRef]
- Ferrington, L.; Miners, J.S.; Palmer, L.E.; Bond, S.M.; Povey, J.E.; Kelly, P.A.; Love, S.; Horsburgh, K.J.; Kehoe, P.G. Angiotensin II-inhibiting drugs have no effect on intraneuronal Aβ or oligomeric Aβ levels in a triple transgenic mouse model of Alzheimer’s disease. Am. J. Transl. Res. 2011, 3, 197–208. [Google Scholar]
- Hemming, M.L.; Selkoe, D.J.; Farris, W. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol. Dis. 2007, 26, 273–281. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Z.; Sun, Q.; Orsi, A.; Rees, D. A new approach to the pharmacological regulation of memory: Sarsasapogenin improves memory by elevating the low muscarinic acetylcholine receptor density in brains of memory-deficit rat models. Brain Res. 2005, 1060, 26–39. [Google Scholar] [CrossRef]
- Wang, X.B.; Cui, N.H.; Gao, J.J.; Qiu, X.P.; Yang, N.; Zheng, F. Angiotensin-converting enzyme gene polymorphisms and risk for sporadic Alzheimer’s disease: A meta-analysis. J. Neural Transm. 2015, 122, 211–224. [Google Scholar] [CrossRef]
- Wang, X.B.; Cui, N.H.; Yang, J.; Qiu, X.P.; Gao, J.J.; Yang, N.; Zheng, F. Angiotensin-converting enzyme insertion/deletion polymorphism is not a major determining factor in the development of sporadic Alzheimer disease: Evidence from an updated meta-analysis. PLoS ONE 2014, 9, e111406. [Google Scholar] [CrossRef]
- Yuan, Y.; Piao, J.H.; Ma, K.; Lu, N. Angiotensin-converting enzyme gene insertion-deletion polymorphism is a risk marker for Alzheimer’s disease in a Chinese population: A meta-analysis of case-control studies. J. Neural Transm. 2015, 122, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
| Accession No. a | Protein Name | MW (KDa) | Mascot Score | Match Queries | PI | Sequence Coverage | Peptide b |
|---|---|---|---|---|---|---|---|
| P63104 | 14-3-3 protein zeta/delta | 27,728 | 66 | 4 | 4.76 | 15% | R.NLLSVAYKNVVGARR.S K.SVTEQGAELSNEER.N R.SSWRVVSSIEQK.T + deamidated (NQ); 2 phospho (ST) K.SVTEQGAELSNEER.N + 2 phospho (ST) |
| P27797 | Calreticulin | 123,665 | 32 | 6 | 5.5 | 6% | K.NVLINKDIR.C + deamidated (NQ) K.DKGLQTSQDAR.F + 2 deamidated (NQ); phospho (ST) K.GQTLVVQFTVK.H + deamidated (NQ); Phospho (ST) K.IDNSQVESGSLEDDWDFLPPKK.I K.IDNSQVESGSLEDDWDFLPPKK.I + deamidated (NQ) K.SGTIFDNFLITNDEAYAEEFGNETWGVTK.A |
| Q9NZR2 | Low-density lipoprotein-receptor-related protein 1B | 515,159 | 26 | 23 | 5.09 | 6% | K.CIPVNLR.C + carbamidomethyl (C) K.SCEPASPTCSSR.E + carbamidomethyl (C); 3 Phospho (ST) R.TCLSNCTASQFR.C + carbamidomethyl (C); deamidated (NQ); 2 Phospho (ST) K.CSQVCEQHKHTVK.C + carboxymethyl (C); 2 deamidated (NQ); phospho (ST) R.EYICASDGCISASLK.C + carbamidomethyl (C); 2 phospho (ST); phospho (Y) K.SDEKLLYCENRSCR.R + 2 carbamidomethyl (C); deamidated (NQ); phospho (ST) K.DQDECAVYGTCSQTCR.N + carbamidomethyl (C); carboxymethyl (C); phospho (ST) K.NCNNTDCTHFYKLGVK.T + carbamidomethyl (C); 3 deamidated (NQ); phospho (ST) K.DQDECAVYGTCSQTCR.N + carboxymethyl (C); 2 deamidated (NQ); 2 phospho (ST); phospho (Y) R.IIEVSKLNGLYPTILVSK.R + phospho (ST); phospho (Y) R.TNTLSKANKWTGQNVSVIQK.T + 2 deamidated (NQ); phospho (ST) K.CKSAEQSCNSSFFMCKNGR.C + carboxymethyl (C); 2 deamidated (NQ); 2 phospho (ST) K.CKSAEQSCNSSFFMCKNGR.C + carboxymethyl (C); 3 deamidated (NQ); Oxidation (M); 3 phospho (ST) K.LYWTDGNTINMANMDGSNSK.I + 2 deamidated (NQ); Oxidation (M); 3 phospho (ST) K.CKSAEQSCNSSFFMCKNGR.C + 2 carboxymethyl (C); 2 deamidated (NQ); Oxidation (M); 3 phospho (ST) R.GKLYWTDGNTINMANMDGSNSK.I + deamidated (NQ); 2 Oxidation (M); 3 phospho (ST) R.NTHGSYTCSCVEGYLMQPDNR.S + 2 carbamidomethyl (C); 2 deamidated (NQ); 3 phospho (ST) R.CIPKRWLCDGANDCGSNEDESNQTCTAR.T + carbamidomethyl (C); 2 deamidated (NQ); phospho (ST) R.NCHINECLSKKVSGCSQDCQDLPVSYK.C + 3 deamidated (NQ); 3 phospho (ST) K.SCEPASPTCSSREYICASDGCISASLK.C + carbamidomethyl (C); 2 carboxymethyl (C); 5 phospho (ST); phospho (Y) R.CNSTSLCVLPTWICDGSNDCGDYSDELK.C + carbamidomethyl (C); 5 phospho (ST) K.DDGKTCVDIDECSSGFPCSQQCINTYGTYK.C + carbamidomethyl (C); carboxymethyl (C); 3 deamidated (NQ); 5 phospho (ST); phospho (Y) |
| Protein Name | Subcellular Location | Biological Process | Molecular Function | Protein Function |
|---|---|---|---|---|
| 14-3-3 protein zeta/delta | Cytoplasm | Cytoplasmic sequestering of protein | Histone deacetylase binding | Adapter protein implicated in the regulation of a large spectrum of both general and specialized signaling pathways. Binds to a large number of partners, usually by recognition of a phosphoserine or phosphothreonine motif. Binding generally results in the modulation of the activity of the binding partner. Negative regulator of osteogenesis. Blocks the nuclear translocation of the phosphorylated form (by AKT1) of SRPK2 and antagonizes its stimulatory effect on cyclin D1 expression resulting in blockage of neuronal apoptosis elicited by SRPK2 |
| Calreticulin | Endoplasmic reticulum lumen | Cellular senescence | DNA binding | Calcium-binding chaperone that promotes folding, oligomeric assembly and quality control in the endoplasmic reticulum (ER) via the calreticulin/calnexin cycle. This lectin interacts transiently with almost all of the monoglucosylated glycoproteins that are synthesized in the ER. Interacts with the DNA-binding domain of NR3C1 and mediates its nuclear export. Involved in maternal gene expression regulation. May participate in oocyte maturation via the regulation of calcium homeostasis |
| Low-density lipoprotein-receptor-related protein 1B | Membrane | Protein transport | Calcium ion binding | Potential cell surface proteins that bind and internalize ligands in the process of receptor-mediated endocytosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.-H.; Ho, T.-C.; Chang, C.-C.; Su, Y.-S.; Yuan, C.-H.; Chuang, K.-P.; Tyan, Y.-C. Utilizing Proteomic Approaches to Uncover the Neuroprotective Effects of ACE Inhibitors: Implications for Alzheimer’s Disease Treatment. Molecules 2023, 28, 5938. https://doi.org/10.3390/molecules28165938
Yang M-H, Ho T-C, Chang C-C, Su Y-S, Yuan C-H, Chuang K-P, Tyan Y-C. Utilizing Proteomic Approaches to Uncover the Neuroprotective Effects of ACE Inhibitors: Implications for Alzheimer’s Disease Treatment. Molecules. 2023; 28(16):5938. https://doi.org/10.3390/molecules28165938
Chicago/Turabian StyleYang, Ming-Hui, Tzu-Chuan Ho, Chin-Chuan Chang, Yuh-Shan Su, Cheng-Hui Yuan, Kuo-Pin Chuang, and Yu-Chang Tyan. 2023. "Utilizing Proteomic Approaches to Uncover the Neuroprotective Effects of ACE Inhibitors: Implications for Alzheimer’s Disease Treatment" Molecules 28, no. 16: 5938. https://doi.org/10.3390/molecules28165938
APA StyleYang, M.-H., Ho, T.-C., Chang, C.-C., Su, Y.-S., Yuan, C.-H., Chuang, K.-P., & Tyan, Y.-C. (2023). Utilizing Proteomic Approaches to Uncover the Neuroprotective Effects of ACE Inhibitors: Implications for Alzheimer’s Disease Treatment. Molecules, 28(16), 5938. https://doi.org/10.3390/molecules28165938









