Caspase-1 Regulates the Apoptosis and Pyroptosis Induced by Phthalocyanine Zinc-Mediated Photodynamic Therapy in Breast Cancer MCF-7 Cells
Abstract
1. Introduction
2. Results
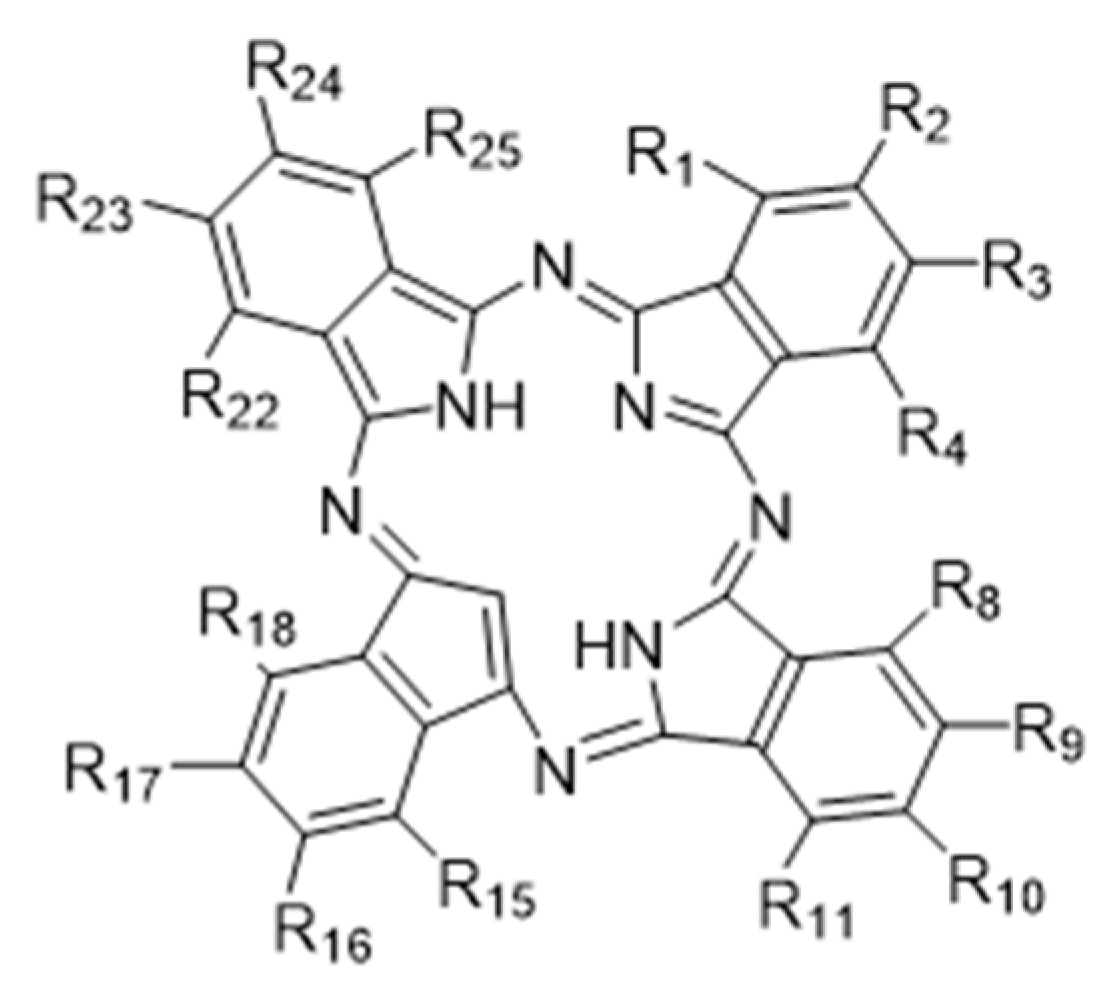
2.1. TαPcZn-PDT Produces Cytotoxicity in Breast Cancer MCF-7 Cells
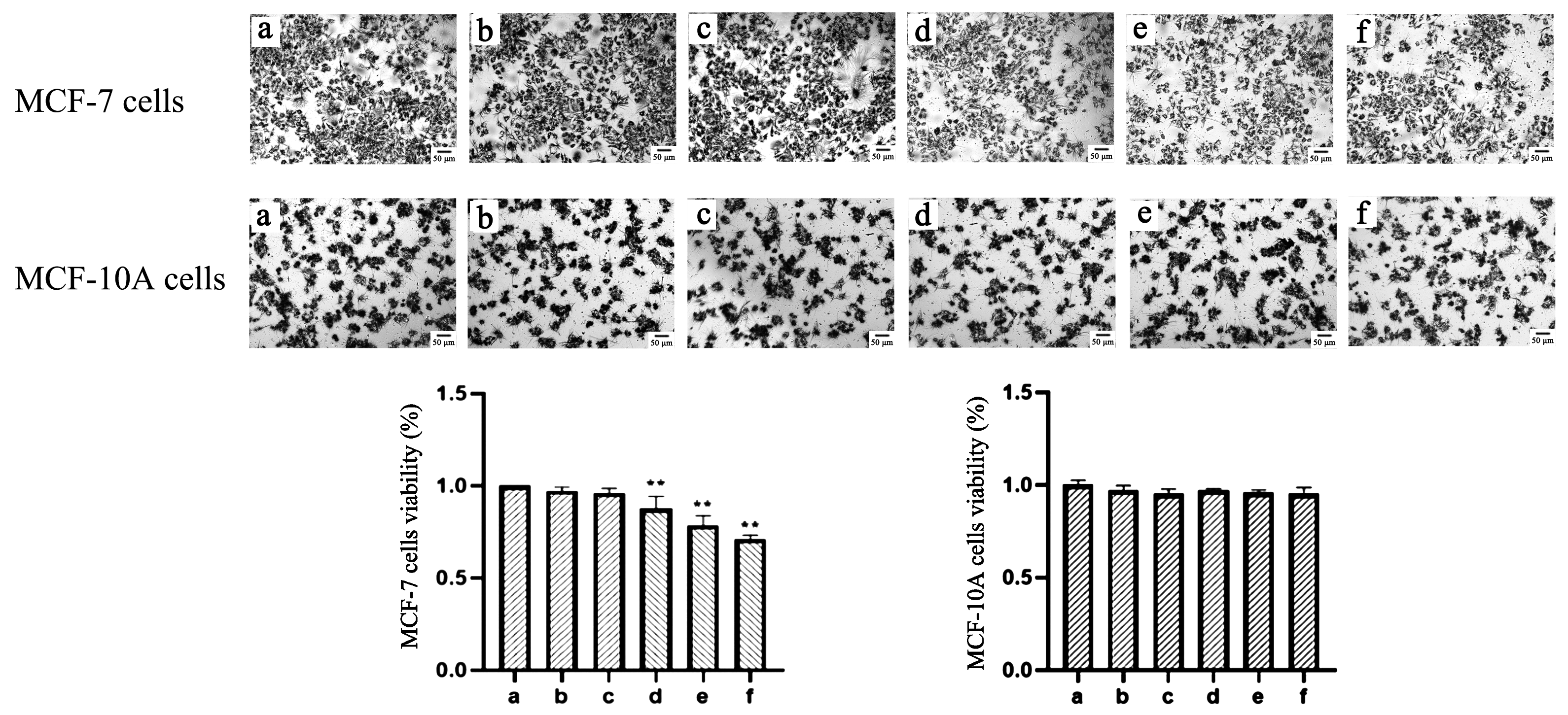
2.2. TαPcZn-PDT Reduces Cell Viability of MCF-7 Cells and Has Little Effect on Mammary Epithelial MCF-10A Cells
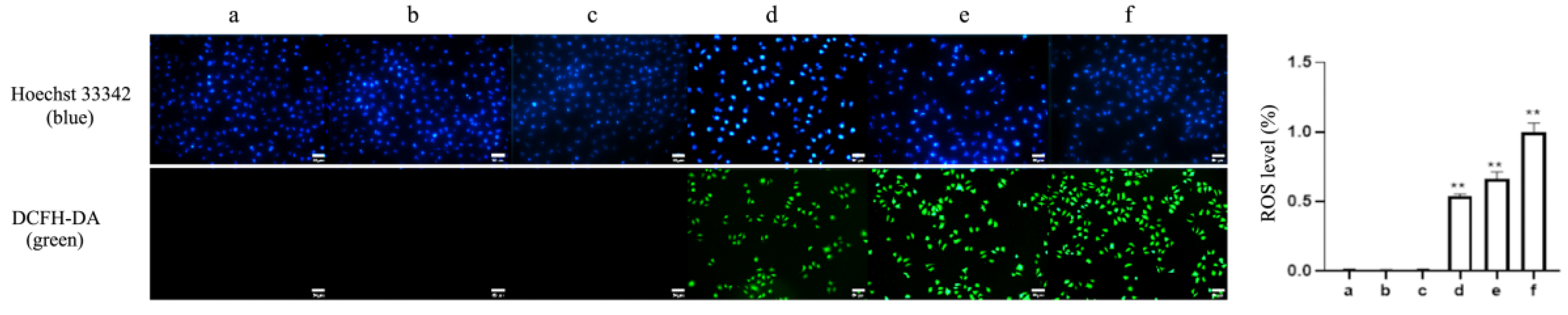
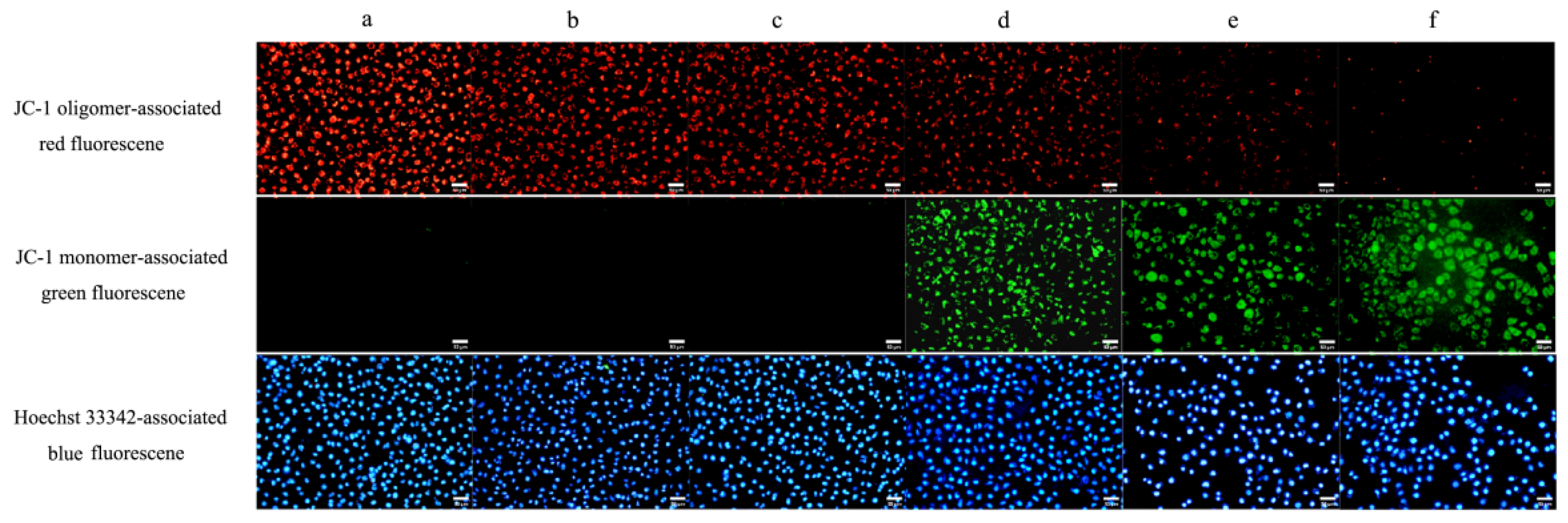
2.3. ROS and ∆Ψm Are Involved in Apoptosis and Pyroptosis Induced by TαPcZn-PDT in MCF-7 Cells
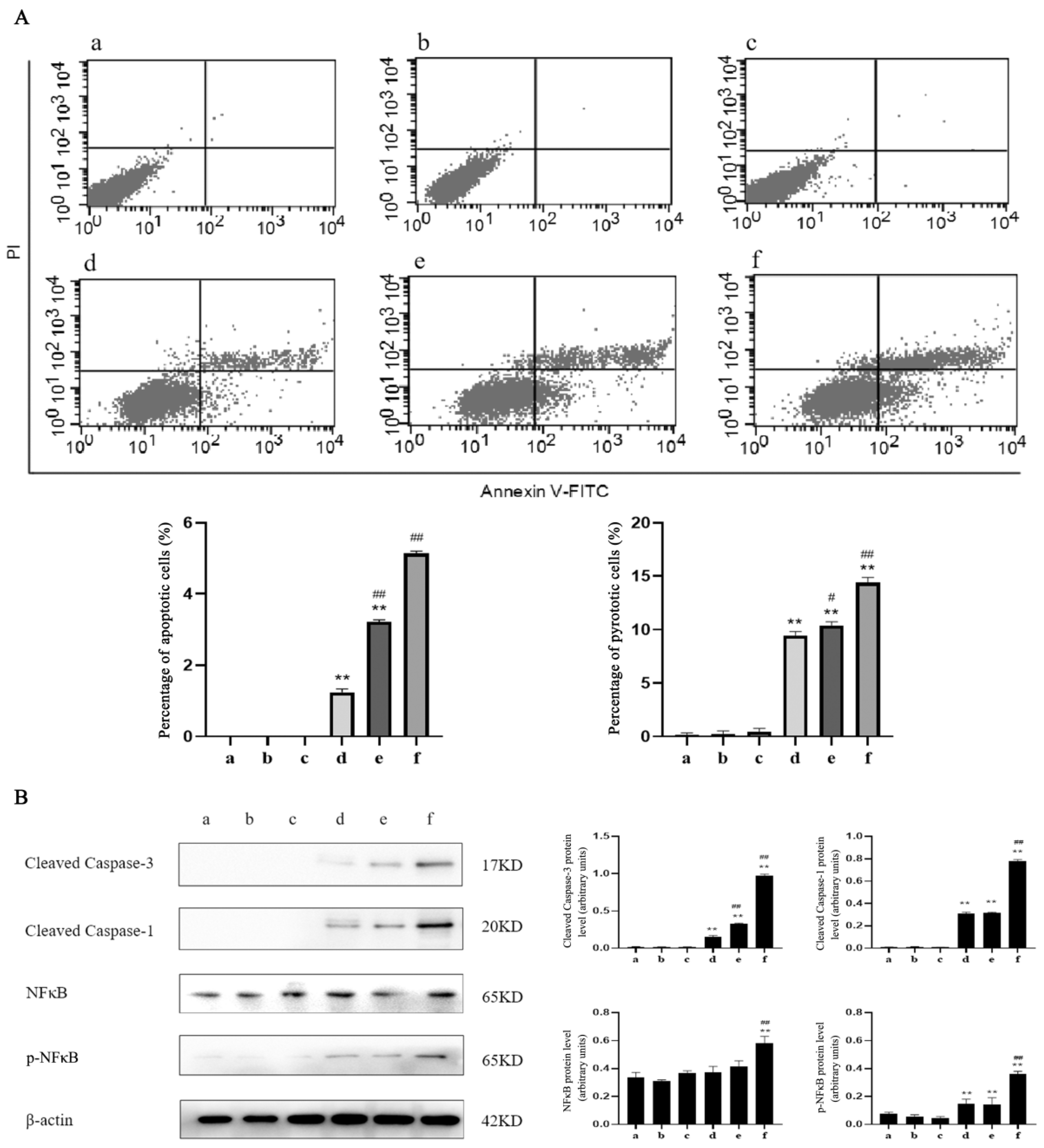
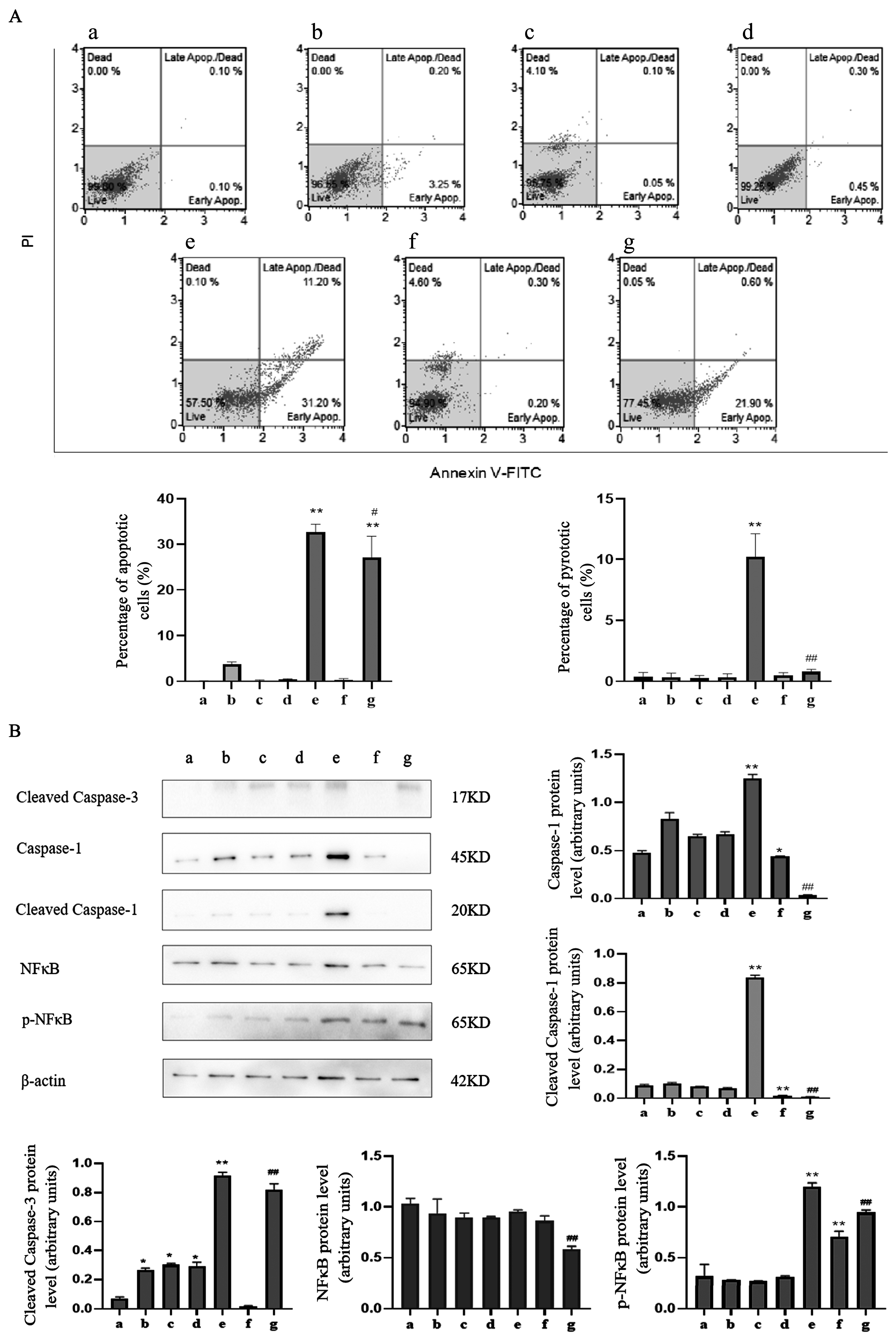
2.4. Caspase-1 Modulates Apoptosis and Pyroptosis Induced by TαPcZn-PDT in MCF-7 Cells
2.4.1. siRNA Silencing of Caspase-1
2.4.2. siRNA-caspase-1 Inhibits Apoptosis and Pyroptosis Induced by TαPcZn-PDT in MCF-7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Processing
4.3. Calcein AM/PI Assay for Cytotoxicity
4.4. MTT Assay for Cell Viability
4.5. DCFH-DA Assay for Cellular ROS Production Levels
4.6. JC-1/Hoechst 33342 Assay for Intracellular Mitochondrial Membrane Potential (∆Ψm)
4.7. Annexin V/Propidium Iodide (PI) Double-Staining Analysis of Apoptosis and Pyroptosis
4.8. Western Blot Assay for Protein Expression
4.9. siRNA Transfection
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O.; et al. A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Narayan, V.; Ky, B. Common Cardiovascular Complications of Cancer Therapy: Epidemiology, Risk Prediction, and Prevention. Annu. Rev. Med. 2018, 69, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Feng, L.Z. Perspectives on photofunctional nanomaterials and tumor optical therapy. J. Photoluminescence 2020, 41, 1339–1342. [Google Scholar]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Cao, H.; Wang, L.; Yang, Y.; Li, J.; Qi, Y.; Li, Y.; Li, Y.; Wang, H.; Li, J. An Assembled Nanocomplex for Improving both Therapeutic Efficiency and Treatment Depth in Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 7759–7763. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, Q.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.X. Synthesis and characterization of alkoxy-substituted phthalocyanine compounds. Chem. Bond. 2021, 43, 89–94. [Google Scholar]
- Lv, F.; Cao, B.; Cui, Y.; Liu, T. Zinc phthalocyanine labelled polyethylene glycol: Preparation, characterization, interaction with bovine serum albumin and near infrared fluorescence imaging in vivo. Molecules 2012, 17, 6348–6361. [Google Scholar]
- Rajabi, N.; Mohammadnejad, F.; Doustvandi, M.A.; Shadbad, M.A.; Amini, M.; Tajalli, H.; Mokhtarzadeh, A.; Baghbani, E.; Silvestris, N.; Baradaran, B. Photodynamic therapy with zinc phthalocyanine enhances the anti-cancer effect of tamoxifen in breast cancer cell line: Promising combination treatment against triple-negative breast cancer. Photodiagnosis Photodyn. Ther. 2023, 41, 103212. [Google Scholar]
- Gholizadeh, M.; Doustvandi, M.A.; Mohammadnejad, F.; Shadbad, M.A.; Tajalli, H.; Brunetti, O.; Argentiero, A.; Silvestris, N.; Baradaran, B. Photodynamic Therapy with Zinc Phthalocyanine Inhibits the Stemness and Development of Colorectal Cancer: Time to Overcome the Challenging Barriers. Molecules 2021, 26, 6877. [Google Scholar] [CrossRef]
- Doustvandi, M.A.; Mohammadnejad, F.; Mansoori, B.; Tajalli, H.; Mohammadi, A.; Mokhtarzadeh, A.; Baghbani, E.; Khaze, V.; Hajiasgharzadeh, K.; Moghaddam, M.M.; et al. Photodynamic therapy using zinc phthalocyanine with low dose of diode laser combined with doxorubicin is a synergistic combination therapy for human SK-MEL-3 melanoma cells. Photodiagnosis Photodyn. Ther. 2019, 28, 88–97. [Google Scholar] [CrossRef]
- Nowara, E. Rilotumumab extends PFS in gastric cancer. Cancer Discov. 2014, 4, 14–20. [Google Scholar]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xie, W.; He, M.; Li, H.; Xiao, W.; Li, Y. Pyroptosis and Sarcopenia: Frontier Perspective of Disease Mechanism. Cells 2022, 11, 1078–1095. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Song, A.; Zhang, C. Modes of podocyte death in diabetic kidney disease: An update. J. Nephrol. 2022, 35, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128–140. [Google Scholar]
- Mamun, A.A.; Mimi, A.A.; Aziz, M.A.; Zaeem, M.; Ahmed, T.; Munir, F.; Xiao, J. Role of pyroptosis in cancer and its therapeutic regulation. Eur. J. Pharmacol. 2021, 910, 174444. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, M.; Zhou, C.; Yin, F.; Wang, Z.; Hua, Y.; Cai, Z. Hematoporphyrin monomethyl ether-mediated photodynamic therapy selectively kills sarcomas by inducing apoptosis. PLoS ONE 2013, 8, e77727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K.; Xue, Z.; Yin, H.; Dong, H.; Jin, W.; Shi, X.; Wang, H.; Wang, H. High-voltage pulsed electric field plus photodynamic therapy kills breast cancer cells by triggering apoptosis. Am. J. Transl. Res. 2018, 10, 334–351. [Google Scholar] [PubMed]
- Korbelik, M.; Banáth, J.; Zhang, W.; Gallagher, P.; Hode, T.; Lam, S.S.K.; Chen, W.R. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int. Immunopharmacol. 2019, 75, 105764. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, Z.; Huang, H.; Xu, Z.; Chen, Z.; Shen, G.; Li, Z.; Ren, Y.; Li, G.; Hu, Y. VB12-Sericin-PBLG-IR780 Nanomicelles for Programming Cell Pyroptosis via Photothermal (PTT)/Photodynamic (PDT) Effect-Induced Mitochondrial DNA (mitoDNA) Oxidative Damage. ACS Appl. Mater. Interfaces 2022, 14, 17008–17021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, C.; Lun, Z.; Lv, Y.; Chen, W.; Li, T. Crosstalk between p38 MAPK and caspase-9 regulates mitochondria-mediated apoptosis induced by tetra-α-(4-carboxyphenoxy) phthalocyanine zinc photodynamic therapy in LoVo cells. Oncol. Rep. 2018, 39, 61–70. [Google Scholar] [PubMed]
- Xia, C.; Wang, Y.; Chen, W.; Yu, W.; Wang, B.; Li, T. New hydrophilic/lipophilic tetra-α-(4-carboxyphenoxy) phthalocyanine zinc-mediated photodynamic therapy inhibits the proliferation of human hepatocellular carcinoma Bel-7402 cells by triggering apoptosis and arresting cell cycle. Molecules 2011, 16, 1389–1401. [Google Scholar] [CrossRef]
- Wang, H.; Jing, G.; Niu, J.; Yang, L.; Li, Y.; Gao, Y.; Wang, H.; Xu, X.; Qian, Y.; Wang, S. A mitochondria-anchored supramolecular photosensitizer as a pyroptosis inducer for potent photodynamic therapy and enhanced antitumor immunity. J. Nanobiotechnol. 2022, 20, 513. [Google Scholar]
- Yao, W.Y.; Xu, M.; Li, J.; Hu, W.B.; Huang, W. Revealing excited-state dynamics of type I zinc phthalocyanine photosensitizer for photodynamic therapy. SCIENTIA SINICA Chim. 2022, 52, 1384–1392. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Chen, W.; Xia, C.H.; Lun, Z.Q. Phthalocyanine zinc photodynamic therapy-induced ROS of Lovo cells. Tianjin Med. J. 2020, 48, 253–257. [Google Scholar]
- Chen, K.; Hou, J.; Huang, B.; Xiao, S.; Li, X.; Sun, H.; Peng, Y. Bromopropylate Imidazoliumyl Substituted Silicon Phthalocyanine for Mitochondria-Targeting, Two-Photon Imaging Guided in Vitro Photodynamic Therapy. Front. Pharmacol. 2022, 13, 921718. [Google Scholar] [CrossRef]
- Cheng, Y.-Q.; Yue, Y.-X.; Cao, H.-M.; Geng, W.-C.; Wang, L.-X.; Hu, X.-Y.; Li, H.-B.; Bian, Q.; Kong, X.-L.; Liu, J.-F.; et al. Coassembly of hypoxia-sensitive macrocyclic amphiphiles and extracellular vesicles for targeted kidney injury imaging and therapy. J. Nanobiotechnol. 2021, 9, 451. [Google Scholar] [CrossRef]
- Tudor, D.; Nenu, I.; Filip, G.A.; Olteanu, D.; Cenariu, M.; Tabaran, F.; Ion, R.M.; Gligor, L.; Baldea, I. Combined regimen of photodynamic therapy mediated by Gallium phthalocyanine chloride and Metformin enhances anti-melanoma efficacy. PLoS 2017, 2, e0173241. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Wang, X.; Sun, N.; Gong, Y.-H. Pathway network of pyroptosis and its potential inhibitors in acute kidney injury. Pharmacol. Res. 2022, 175, 106033. [Google Scholar] [CrossRef]
- de Malmanche, H.; Marcellin, E.; Reid, S. Knockout of Sf-Caspase-1 generates apoptosis-resistant Sf9 cell lines: Implications for baculovirus expression. Biotechnol. J. 2022, 17, e2100532. [Google Scholar] [CrossRef]
- Liao, J.; Yang, F.; Tang, Z.; Yu, W.; Han, Q.; Hu, L.; Li, Y.; Guo, J.; Pan, J.; Ma, F.; et al. Inhibition of Caspase-1-dependent pyroptosis attenuates copper-induced apoptosis in chicken hepatocytes. Ecotoxicol. Environ. Saf. 2019, 174, 110–119. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Nakajima, S.; Hosojima, S.; Nguyen, D.T.; Hattori, T.; Le, T.M.; Hori, O.; Mahib, M.R.; Yamaguchi, Y.; Miura, M.; et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 2019, 10, 2091. [Google Scholar] [CrossRef]
- Wu, H.Y.; Chen, W.; Li, T.; Wang, Y.; Xia, C.H.; Li, X.L. Study on synthesis and antineoplastic activity of α-tetra-(4-carboxyphenoxy)phthalocyanine zinc. Liaoning Norm. Univ. Nat. Sci. 2009, 32, 94–97. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Wang, Y.; Chen, W.; Hou, T.; Zhang, H.; Zhang, H.; Yao, X.; Xia, C. Caspase-1 Regulates the Apoptosis and Pyroptosis Induced by Phthalocyanine Zinc-Mediated Photodynamic Therapy in Breast Cancer MCF-7 Cells. Molecules 2023, 28, 5934. https://doi.org/10.3390/molecules28165934
Ma C, Wang Y, Chen W, Hou T, Zhang H, Zhang H, Yao X, Xia C. Caspase-1 Regulates the Apoptosis and Pyroptosis Induced by Phthalocyanine Zinc-Mediated Photodynamic Therapy in Breast Cancer MCF-7 Cells. Molecules. 2023; 28(16):5934. https://doi.org/10.3390/molecules28165934
Chicago/Turabian StyleMa, Chunjie, Yu Wang, Wei Chen, Ting Hou, Honglian Zhang, Hongguang Zhang, Xu Yao, and Chunhui Xia. 2023. "Caspase-1 Regulates the Apoptosis and Pyroptosis Induced by Phthalocyanine Zinc-Mediated Photodynamic Therapy in Breast Cancer MCF-7 Cells" Molecules 28, no. 16: 5934. https://doi.org/10.3390/molecules28165934
APA StyleMa, C., Wang, Y., Chen, W., Hou, T., Zhang, H., Zhang, H., Yao, X., & Xia, C. (2023). Caspase-1 Regulates the Apoptosis and Pyroptosis Induced by Phthalocyanine Zinc-Mediated Photodynamic Therapy in Breast Cancer MCF-7 Cells. Molecules, 28(16), 5934. https://doi.org/10.3390/molecules28165934






