The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods
Abstract
1. Introduction
2. Results
2.1. Comparison between the Study and the Control Group
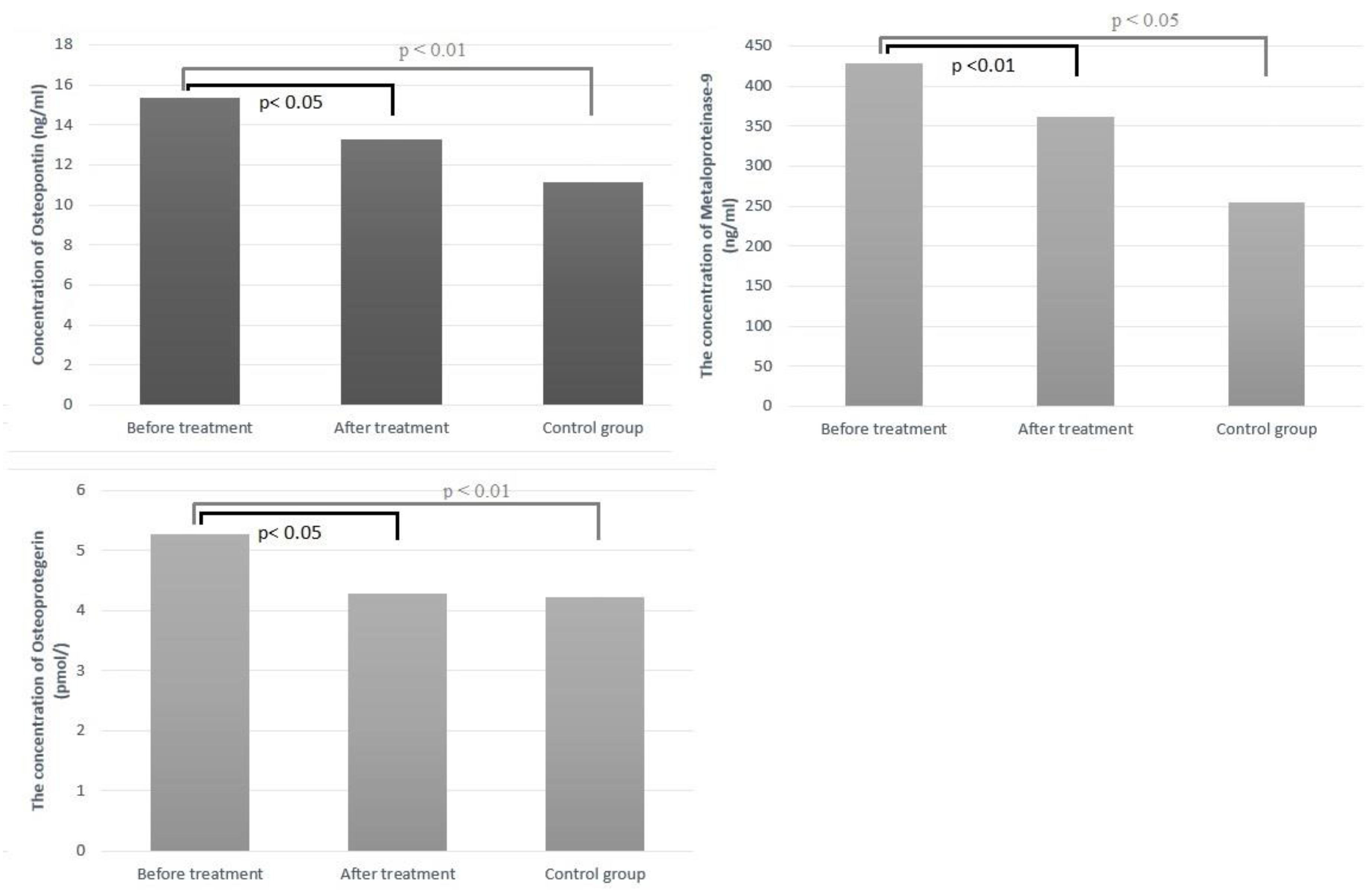
2.2. Changes in Serum Biomarkers
2.3. Correlation of Concentrations of Arteriosclerotic Markers with Results of Carotid Artery MRI Examination
3. Discussion
4. Materials and Methods
4.1. Inclusion and Exclusion Criteria
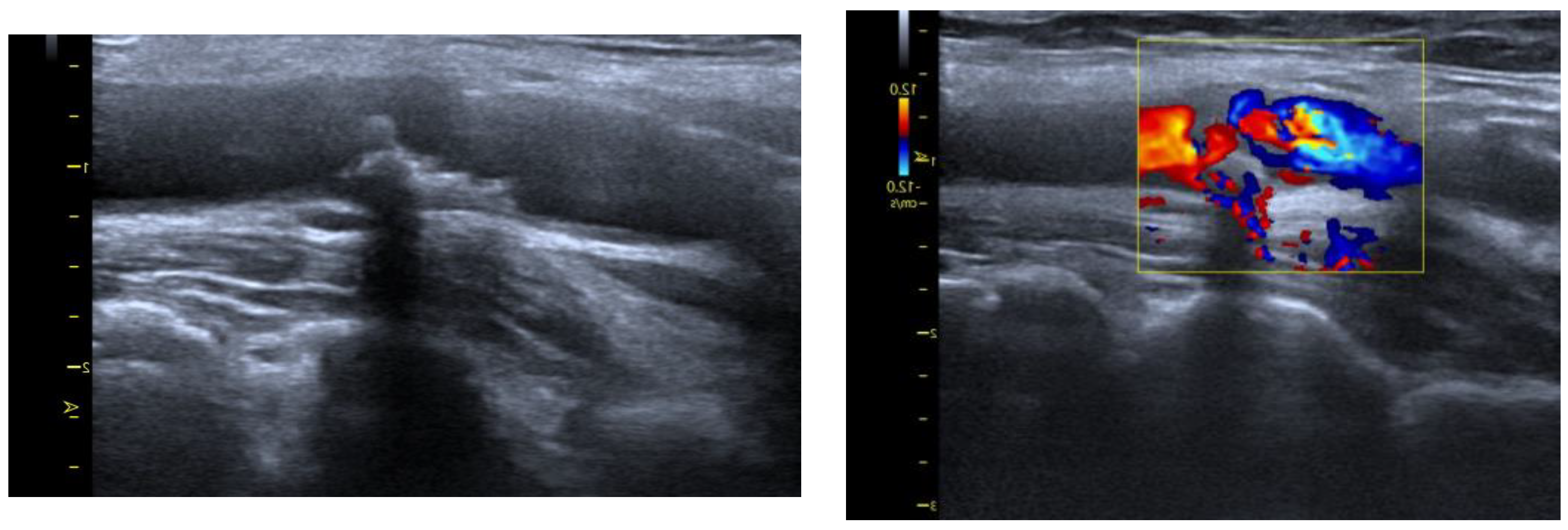
4.2. Arteriosclerotic Plaque Examination
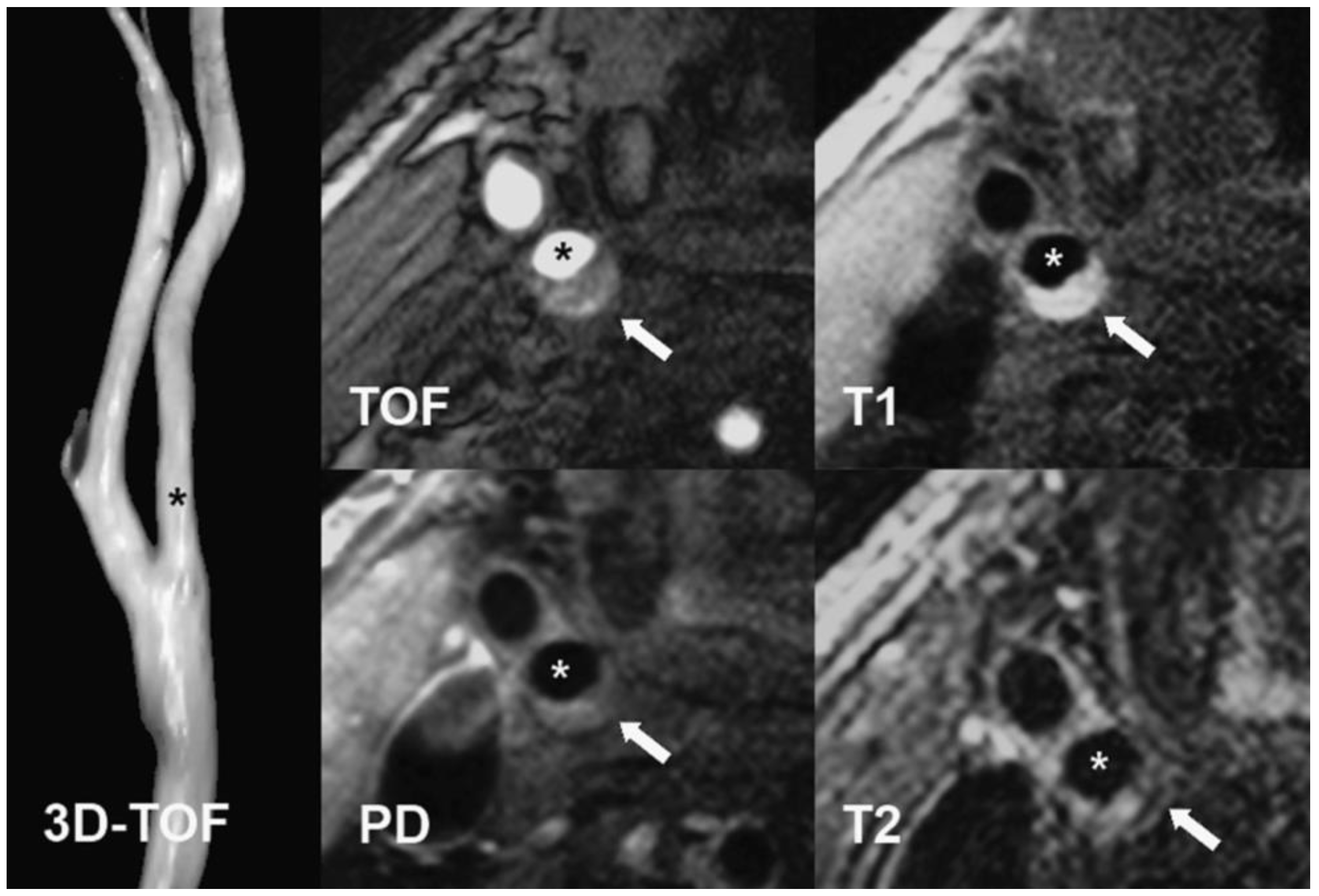
4.3. MRI Protocol
4.4. Serum Arteriosclerotic Markers Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lloyd-Jones, D.; Adams, R.J.; Brown, T.M.; Carnethon, M.; Dai, S.; De Simone, G.; Ferguson, T.B.; Ford, E.; Furie, K.; Gillespie, C.; et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010, 121, 948–954. [Google Scholar] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunität und Entzündung bei Arteriosklerose [Immunity and inflammation in atherosclerosis]. Herz 2019, 44, 107–120. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Ley, K.; Miller, Y.I.; Hedrick, C.C. Monocyte and macrophage dynamics during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1506–1516. [Google Scholar] [CrossRef]
- Flores-Gomez, D.; Bekkering, S.; Netea, M.G.; Riksen, N.P. Trained Immunity in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 62–69. [Google Scholar] [CrossRef]
- Thim, T.; Hagensen, M.K.; Bentzon, J.F.; Falk, E. From vulnerable plaque to atherothrombosis. J. Intern. Med. 2008, 263, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Libby, P.; Falk, E.; Casscells, S.W.; Litovsky, S.; Rumberger, J.; Badimon, J.J.; Stefanadis, C.; Moreno, P.; Pasterkamp, G.; et al. From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation 2003, 108, 1664–1672. [Google Scholar] [PubMed]
- Nezu, T.; Hosomi, N.; Aoki, S.; Matsumoto, M. Carotid Intima-Media Thickness for Atherosclerosis. J. Atheroscler. Thromb. 2016, 23, 18–31. [Google Scholar] [CrossRef]
- Wüst, R.C.I.; Calcagno, C.; Daal, M.R.R.; Nederveen, A.J.; Coolen, B.F.; Strijkers, G.J. Emerging Magnetic Resonance Imaging Techniques for Atherosclerosis Imaging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K.; et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Tuzcu, E.M.; Sipahi, I.; Grasso, A.W.; Schoenhagen, P.; Hu, T.; Wolski, K.; Crowe, T.; Desai, M.Y.; Hazen, S.L.; et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA 2007, 297, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Nicholls, S.J.; Sipahi, I.; Libby, P.; Raichlen, J.S.; Ballantyne, C.M.; Davignon, J.; Erbel, R.; Fruchart, J.C.; Tardif, J.C.; et al. ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA 2006, 295, 1556–1565. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tuzcu, E.M.; Schoenhagen, P.; Brown, B.G.; Ganz, P.; Vogel, R.A.; Crowe, T.; Howard, G.; Cooper, C.J.; Brodie, B.; et al. REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: A randomized controlled trial. JAMA 2004, 291, 1071–1080. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights from Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar]
- Degnan, A.J.; Young, V.E.; Gillard, J.H. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev. Cardiovasc. Ther. 2012, 10, 37–53. [Google Scholar] [CrossRef]
- Chappell, F.M.; Wardlaw, J.M.; Young, G.R.; Gillard, J.H.; Roditi, G.H.; Yip, B.; Pell, J.P.; Rothwell, P.M.; Brown, M.M.; Gough, M.J.; et al. Carotid artery stenosis: Accuracy of noninvasive tests--individual patient data meta-analysis. Radiology 2009, 251, 493–502. [Google Scholar] [CrossRef]
- Makowski, M.R.; Henningsson, M.; Spuentrup, E.; Kim, W.Y.; Maintz, D.; Manning, W.J.; Botnar, R.M. Characterization of coronary atherosclerosis by magnetic resonance imaging. Circulation 2013, 128, 1244–1255. [Google Scholar] [CrossRef]
- Henningsson, M.; Malik, S.; Botnar, R.; Castellanos, D.; Hussain, T.; Leiner, T. Black-Blood Contrast in Cardiovascular MRI. J. Magn. Reson. Imaging 2022, 55, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, L.M.; Hatsukami, T.S.; Ferguson, M.S.; Kerwin, W.S.; Cai, J.; Yuan, C. In vivo accuracy of multisequence MR imaging for identifying unstable fibrous caps in advanced human carotid plaques. J. Magn. Reson. Imaging 2003, 17, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hatsukami, T.S.; Ferguson, M.S.; Kerwin, W.S.; Saam, T.; Chu, B.; Takaya, N.; Polissar, N.L.; Yuan, C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: Comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005, 112, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Cappendijk, V.C.; Cleutjens, K.B.; Kessels, A.G.; Heeneman, S.; Schurink, G.W.; Welten, R.J.; Mess, W.H.; Daemen, M.J.; van Engelshoven, J.M.; Kooi, M.E. Assessment of human atherosclerotic carotid plaque components with multisequence MR imaging: Initial experience. Radiology 2005, 234, 487–492. [Google Scholar] [CrossRef]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Underhill, H.; Cai, J.; Tran, N.; Polissar, N.L.; Isaac, C.; Ferguson, M.S.; et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with MRI--initial results. Stroke 2006, 37, 818–823. [Google Scholar] [CrossRef]
- Parmar, J.P.; Rogers, W.J.; Mugler, J.P.; Baskurt, E.; Altes, T.A.; Nandalur, K.R.; Stukenborg, G.J.; Phillips, C.D.; Hagspiel, K.D.; Matsumoto, A.H.; et al. Magnetic resonance imaging of carotid atherosclerotic plaque in clinically suspected acute transient ischemic attack and acute ischemic stroke. Circulation 2010, 122, 2031–2038. [Google Scholar] [CrossRef]
- Gergei, I.; Kälsch, T.; Scharnagl, H.; Kleber, M.E.; Zirlik, A.; März, W.; Krämer, B.K.; Kälsch, A.I. Association of soluble CD40L with short-term and long-term cardiovascular and all-cause mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis 2019, 291, 127–131. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef]
- Sugiyama, S.; Okada, Y.; Sukhova, G.K.; Virmani, R.; Heinecke, J.W.; Libby, P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am. J. Pathol. 2001, 158, 879–891. [Google Scholar] [CrossRef]
- Chen, J.; Mohler, E.R.; Xie, D.; Shlipak, M.; Townsend, R.R.; Appel, L.J.; Ojo, A.; Schreiber, M.; Nessel, L.; Zhang, X.; et al. CRIC Study Investigators. Traditional and non-traditional risk factors for incident peripheral arterial disease among patients with chronic kidney disease. Nephrol. Dial. Transplant. 2016, 31, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, S.; Lundberg, A.K.; Jonasson, L. Overexpression of MMP-9 and its inhibitors in blood mononuclear cells after myocardial infarction--is it associated with depressive symptomatology? PLoS ONE 2014, 25, e105572. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.J.; Gomez, I.G.; Wille, P.T.; Raines, E.W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Investig. 2006, 116, 59–69. [Google Scholar] [CrossRef]
- Zouridakis, E.; Avanzas, P.; Arroyo-Espliguero, R.; Fredericks, S.; Kaski, J.C. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation 2004, 110, 1747–1753. [Google Scholar] [CrossRef]
- Schönbeck, U.; Libby, P. The CD40/CD154 receptor/ligand dyad. Cell Mol. Life Sci. 2001, 58, 4–43. [Google Scholar] [PubMed]
- Tousoulis, D.; Androulakis, E.; Papageorgiou, N.; Briasoulis, A.; Siasos, G.; Antoniades, C.; Stefanadis, C. From atherosclerosis to acute coronary syndromes: The role of soluble CD40 ligand. Trends Cardiovasc. Med. 2010, 20, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Shui, C.; Riggs, B.L.; Dunstan, C.R.; Spelsberg, T.C.; O’Brien, T.; Khosla, S. Effects of immunosuppressants on receptor activator of NF-kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem. Biophys. Res. Commun. 2001, 280, 334–339. [Google Scholar] [CrossRef]
- Tousoulis, D.; Siasos, G.; Maniatis, K.; Oikonomou, E.; Kioufis, S.; Zaromitidou, M.; Paraskevopoulos, T.; Michalea, S.; Kollia, C.; Miliou, A.; et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int. J. Cardiol. 2013, 167, 1924–1928. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll Cardiol. 2019, 73, 3168–3209. [Google Scholar]
- Stein, E.A.; Gipe, D.; Bergeron, J.; Gaudet, D.; Weiss, R.; Dufour, R.; Wu, R.; Pordy, R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: A phase 2 randomised controlled trial. Lancet 2012, 380, 29–36. [Google Scholar]
- Tang, Z.; Jiang, L.; Peng, J.; Ren, Z.; Wei, D.; Wu, C.; Pan, L.; Jiang, Z.; Liu, L. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-κB activation in THP-1-derived macrophages. Int. J. Mol. Med. 2012, 30, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Peng, J.; Ren, Z.; Yang, J.; Li, T.T.; Li, T.H.; Wang, Z.; Wei, D.H.; Liu, L.S.; Zheng, X.L.; et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-κB pathway. Atherosclerosis 2017, 262, 113–122. [Google Scholar] [CrossRef]
- Gencer, B.; Montecucco, F.; Nanchen, D.; Carbone, F.; Klingenberg, R.; Vuilleumier, N.; Aghlmandi, S.; Heg, D.; Räber, L.; Auer, R.; et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur. Heart J. 2016, 37, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Y.-L.; Xu, R.-X.; Zhang, Y.; Zhu, C.-G.; Sun, J.; Qing, P.; Wu, N.-Q.; Jiang, L.-X.; Li, J.-J. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis 2014, 234, 441–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.-G.; Xu, R.-X.; Li, S.; Guo, Y.-L.; Sun, J.; Li, J.-J. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J. Clin. Lipidol. 2014, 8, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Xu, R.-X.; Guo, Y.-L.; Zhu, C.-G.; Wu, N.-Q.; Qing, P.; Liu, G.; Dong, Q.; Li, J.-J. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann. Med. 2015, 47, 386–393. [Google Scholar] [CrossRef]
- Jones, P.H.; Bays, H.E.; Chaudhari, U.; Pordy, R.; Lorenzato, C.; Miller, K.; Robinson, J.G. Safety of Alirocumab (A PCSK9 Monoclonal Antibody) from 14 Randomized Trials. Am. J. Cardiol. 2016, 118, 1805–1811. [Google Scholar] [CrossRef]
- Toth, P.P.; Descamps, O.; Genest, J.; Sattar, N.; Preiss, D.; Dent, R.; Djedjos, C.; Wu, Y.; Geller, M.; Uhart, M.; et al. PROFICIO Investigators. Pooled Safety Analysis of Evolocumab in Over 6000 Patients from Double-Blind and Open-Label Extension Studies. Circulation 2017, 135, 1819–1831. [Google Scholar] [CrossRef]
- Gürgöze, M.T.; Muller-Hansma, A.H.G.; Schreuder, M.M.; Galema-Boers, A.M.H.; Boersma, E.; Roeters van Lennep, J.E. Adverse Events Associated With PCSK9 Inhibitors: A Real-World Experience. Clin. Pharmacol. Ther. 2019, 105, 496–504. [Google Scholar] [CrossRef]
- Hovingh, G.K.; Raal, F.J.; Dent, R.; Stefanutti, C.; Descamps, O.; Masana, L.; Lira, A.; Bridges, I.; Coll, B.; Sullivan, D. Long-term safety, tolerability, and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia. J. Clin. Lipidol. 2017, 11, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Zhu, Q.Q.; Zhu, L.; Chen, J.Z.; Chen, Q.H.; Li, G.N.; Xie, J.; Kang, L.N.; Xu, B. Safety and efficacy of anti-PCSK9 antibodies: A meta-analysis of 25 randomized, controlled trials. BMC Med. 2015, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Guedeney, P.; Sorrentino, S.; Giustino, G.; Chapelle, C.; Laporte, S.; Claessen, B.E.; Ollier, E.; Camaj, A.; Kalkman, D.N.; Vogel, B.; et al. Indirect comparison of the efficacy and safety of alirocumab and evolocumab: A systematic review and network meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 225–235. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Park, H.J. New Therapeutic Approaches to the Treatment of Dyslipidemia 2: LDL-C and Lp(a). J. Lipid Atheroscler. 2023, 12, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.J.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Lawrence, D.; Friedman, A.; et al. ORION Phase III investigators. Inclisiran and cardiovascular events: A patient-level analysis of phase III trials. Eur. Heart J. 2023, 44, 129–138. [Google Scholar] [CrossRef]
- Merćep, I.; Friščić, N.; Strikić, D.; Reiner, Ž. Advantages and Disadvantages of Inclisiran: A Small Interfering Ribonucleic Acid Molecule Targeting PCSK9—A Narrative Review. Cardiovasc. Ther. 2022, 2022, 8129513. [Google Scholar] [CrossRef]
- Banerjee, Y.; Pantea Stoian, A.; Cicero, A.F.G.; Fogacci, F.; Nikolic, D.; Sachinidis, A.; Rizvi, A.A.; Janez, A.; Rizzo, M. Inclisiran: A small interfering RNA strategy targeting PCSK9 to treat hypercholesterolemia. Expert. Opin. Drug Saf. 2022, 21, 9–20. [Google Scholar] [CrossRef]
- Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 10 May 2022).
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef]
- Chiba, S.; Okamoto, H.; Kon, S.; Kimura, C.; Murakami, M.; Inobe, M.; Matsui, Y.; Sugawara, T.; Shimizu, T.; Uede, T.; et al. Development of atherosclerosis in osteopontin transgenic mice. Heart Vessel. 2002, 16, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kurata, M.; Okura, T.; Watanabe, S.; Fukuoka, T.; Higaki, J. Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin. Sci. 2006, 111, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; McCann, M.; Mangan, S.; Lam, A.; Karan, M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004, 35, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.M.; Hatsukami, T.S.; Ferguson, M.S.; Small, R.; Polissar, N.L.; Yuan, C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002, 106, 1368–1373. [Google Scholar] [CrossRef]
- Polonskaya, Y.V.; Kashtanova, E.V.; Murashov, I.S.; Kurguzov, A.V.; Sadovski, E.V.; Maslatsov, N.A.; Stakhneva, E.M.; Chernyavskii, A.M.; Ragino, Y.I. The Influence of Calcification Factors and Endothelial-Dysfunction Factors on the Development of Unstable Atherosclerotic Plaques. Diagnostics 2020, 10, 1074. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Gerasimidis, T.; Golemati, S.; Kapelouzou, A.; Karayannacos, P.E.; Liapis, C.D. The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. J. Vasc. Surg. 2008, 47, 55–62. [Google Scholar] [CrossRef]
- Mazzone, A.; Parri, M.S.; Giannessi, D.; Ravani, M.; Vaghetti, M.; Altieri, P.; Casalino, L.; Maltinti, M.; Balbi, M.; Barsotti, A.; et al. Osteopontin plasma levels and accelerated atherosclerosis in patients with CAD undergoing PCI: A prospective clinical study. Coron. Artery Dis. 2011, 22, 179–187. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Kim, J.; Song, T.J.; Yang, S.H.; Lee, O.H.; Nam, H.S.; Kim, Y.D.; Kim, E.H.; Lee, H.S.; Nam, C.M.; Heo, J.H. Plasma osteoprotegerin levels increase with the severity of cerebral artery atherosclerosis. Clin. Biochem. 2013, 46, 1036–1040. [Google Scholar] [CrossRef]
- Halak, S.; Östling, G.; Edsfeldt, A.; Kennbäck, C.; Dencker, M.; Gonçalves, I.; Asciutto, G. Spotty Carotid Plaques Are Associated with Inflammation and the Occurrence of Cerebrovascular Symptoms. Cerebrovasc. Dis. Extra 2018, 8, 16–25. [Google Scholar] [CrossRef]
- Kiechl, S.; Schett, G.; Wenning, G.; Redlich, K.; Oberhollenzer, M.; Mayr, A.; Santer, P.; Smolen, J.; Poewe, W.; Willeit, J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004, 109, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Mathiesen, E.B.; Notø, A.T.; Sveinbjørnsson, B.; Brox, J.; Hansen, J.B. Serum osteoprotegerin is inversely associated with carotid plaque echogenicity in humans. Atherosclerosis 2007, 191, 128–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Browner, W.S.; Lui, L.Y.; Cummings, S.R. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes. Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef]
- Abedin, M.; Omland, T.; Ueland, T.; Khera, A.; Aukrust, P.; Murphy, S.A.; Jain, T.; Gruntmanis, U.; McGuire, D.K.; de Lemos, J.A. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am. J. Cardiol. 2007, 99, 513–518. [Google Scholar] [CrossRef]
- Dekker, M.; Waissi, F.; Silvis, M.J.M.; Bennekom, J.V.; Schoneveld, A.H.; de Winter, R.J.; Isgum, I.; Lessmann, N.; Velthuis, B.K.; Pasterkamp, G.; et al. High levels of osteoprotegerin are associated with coronary artery calcification in patients suspected of a chronic coronary syndrome. Sci. Rep. 2021, 11, 18946. [Google Scholar] [CrossRef]
- Cottin, Y.; Issa, R.; Benalia, M.; Mouhat, B.; Meloux, A.; Tribouillard, L.; Bichat, F.; Rochette, L.; Vergely, C.; Zeller, M. Association between Serum Osteoprotegerin Levels and Severity of Coronary Artery Disease in Patients with Acute Myocardial Infarction. J. Clin. Med. 2021, 10, 4326. [Google Scholar] [CrossRef]
- Fuernau, G.; Zaehringer, S.; Eitel, I.; de Waha, S.; Droppa, M.; Desch, S.; Schuler, G.; Adams, V.; Thiele, H. Osteoprotegerin in ST-elevation myocardial infarction: Prognostic impact and association with markers of myocardial damage by magnetic resonance imaging. Int. J. Cardiol. 2013, 167, 2134–2139. [Google Scholar] [CrossRef]
- Huang, Q.X.; Li, J.B.; Huang, N.; Huang, X.W.; Li, Y.L.; Huang, F.X. Elevated Osteoprotegerin Concentration Predicts Increased Risk of Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press Res. 2020, 45, 565–575. [Google Scholar]
- Marques, G.L.; Hayashi, S.; Bjällmark, A.; Larsson, M.; Riella, M.; Olandoski, M.; Lindholm, B.; Nascimento, M.M. Osteoprotegerin is a marker of cardiovascular mortality in patients with chronic kidney disease stages 3–5. Sci. Rep. 2021, 11, 2473. [Google Scholar] [CrossRef]
- Strobescu-Ciobanu, C.; Giuşcă, S.E.; Căruntu, I.D.; Amălinei, C.; Rusu, A.; Cojocaru, E.; Popa, R.F.; Lupaşcu, C.D. Osteopontin and osteoprotegerin in atherosclerotic plaque—Are they significant markers of plaque vulnerability? Rom. J. Morphol. Embryol. 2020, 61, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Langley, S.R.; Willeit, K.; Didangelos, A.; Matic, L.P.; Skroblin, P.; Barallobre-Barreiro, J.; Lengquist, M.; Rungger, G.; Kapustin, A.; Kedenko, L.; et al. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J. Clin. Investig. 2017, 127, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, D.; Shimada, K.; Tanaka, A.; Kusuyama, T.; Yamashita, H.; Ehara, S.; Nakamura, Y.; Kawarabayashi, T.; Iida, H.; Yoshiyama, M.; et al. Comparison of levels of serum matrix metalloproteinase-9 in patients with acute myocardial infarction versus unstable angina pectoris versus stable angina pectoris. Am. J. Cardiol. 2006, 97, 175–180. [Google Scholar] [CrossRef]
- Eldrup, N.; Grønholdt, M.L.; Sillesen, H.; Nordestgaard, B.G. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation 2006, 114, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Olson, F.J.; Schmidt, C.; Gummesson, A.; Sigurdardottir, V.; Hulthe, J.; Wiklund, O.; Fagerberg, B. Circulating matrix metalloproteinase 9 levels in relation to sampling methods, femoral and carotid atherosclerosis. J. Intern. Med. 2008, 263, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Liu, Y.; Li, W.; Deng, F.; Liu, X.; Wang, X.; Gui, Y.; Qin, L.; Hu, C.; Chen, L. Associations of matrix metalloproteinase-9 and monocyte chemoattractant protein-1 concentrations with carotid atherosclerosis, based on measurements of plaque and intima-media thickness. Atherosclerosis 2014, 232, 199–203. [Google Scholar] [CrossRef]
- Guardiola, M.; Plana, N.; Ibarretxe, D.; Cabré, A.; González, M.; Ribalta, J.; Masana, L. Circulating PCSK9 levels are positively correlated with NMR-assessed atherogenic dyslipidaemia in patients with high cardiovascular risk. Clin. Sci. 2015, 128, 877–882. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef]
- Sun, J.; Lepor, N.E.; Cantón, G.; Contreras, L.; Hippe, D.S.; Isquith, D.A.; Balu, N.; Kedan, I.; Simonini, A.A.; Yuan, C.; et al. Serial magnetic resonance imaging detects a rapid reduction in plaque lipid content under PCSK9 inhibition with alirocumab. Int. J. Cardiovasc. Imaging 2021, 37, 1415–1422. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Anderson, T.; Ballantyne, C.M.; Cho, L.; Kastelein, J.J.; Koenig, W.; Somaratne, R.; Kassahun, H.; Yang, J.; et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA 2016, 316, 2373–2384. [Google Scholar] [CrossRef]
- Leucker, T.M.; Gerstenblith, G.; Schär, M.; Brown, T.T.; Jones, S.R.; Afework, Y.; Weiss, R.G.; Hays, A.G. Evolocumab, a PCSK9-Monoclonal Antibody, Rapidly Reverses Coronary Artery Endothelial Dysfunction in People Living With HIV and People With Dyslipidemia. J. Am. Heart Assoc. 2020, 9, e016263. [Google Scholar] [CrossRef] [PubMed]
- Otake, H.; Sugizaki, Y.; Toba, T.; Nagano, Y.; Tsukiyama, Y.; Yanaka, K.I.; Yamamoto, H.; Nagasawa, A.; Onishi, H.; Takeshige, R.; et al. Efficacy of alirocumab for reducing plaque vulnerability: Study protocol for ALTAIR, a randomized controlled trial in Japanese patients with coronary artery disease receiving rosuvastatin. J. Cardiol. 2019, 73, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Elseweidy, M.M.; Mohamed, H.E.; Elrashidy, R.A.; Atteia, H.H.; Elnagar, G.M. Inhibition of Aortic Calcification by Policosanol in Dyslipidemic Rabbits Is Enhanced by Pentoxifylline: Potential Role of PCSK9. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 551–560. [Google Scholar] [CrossRef]
- Bond, M.G.; Barnes, R.W.; Riley, W.A.; Wilmoth, S.K.; Chambless, L.E.; Howard, G.; Owens, B.; The ARIC Study Group. High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities study (ARIC). J. NeuroImage 1991, 1, 68–73. [Google Scholar] [CrossRef]
| Author | Study Design | Inflammatory Marker | Coefficient (r) | p-Value |
|---|---|---|---|---|
| Gencer et al. [44]/SPUM-ACS study | Multi-centre prospective cohort study (2168 ACS patients) | hs-CRP | 0.077 | 0.006 |
| Li et al. [45] | Single-centre cross-sectional study (251 stable CAD patients) | WBC | 0.167 | 0.008 |
| Zhang et al. [46] | Cross-sectional study (219 stable CAD patients) | Fibrinogen hs-CRP | 0.211 0.153 | 0.002 0.023 |
| Li et al. [47] | Prospective study (552 CAD patients) | WBC Fibrinogen hs-CRP | 0.077 0.181 0.101 | 0.014 <0.001 0.003 |
| Control Group | Study Group | |
|---|---|---|
| Number of patients | 12 | 16 |
| Age, years | 57 ± 5 | 58 ± 6 |
| Body mass index | 27.2 ± 2.6 | 27.8 ± 2.0 |
| Women, % | 37 | 38 |
| BMI | 27.4 ± 2.7 | 28.1 ± 2.2 |
| WHO guidelines on physical activity, % | 84 | 81 |
| Smokers, % | 26 | 25 |
| Alcohol abuse | No | No |
| Systolic blood pressure, mmHg | 132 ± 6 | 134 ± 5 |
| Diastolic blood pressure, mmHg | 84 ± 4 | 83 ± 4 |
| White blood cell count, ×109/L | 5.2 ± 1.1 | 8.0 ± 1.4 |
| High-sensitivity C-reactive protein, mg/L | 1.86 ± 0.96 | 2.84 ± 1.14 |
| Control Group | Study Group | p Value | |
|---|---|---|---|
| Total cholesterol, mg/dL | 158.2 ± 10.6 | 242.7 ± 11.8 | p < 0.001 |
| Low-density lipoprotein cholesterol, mg/dL | 94.4 ± 8.7 | 181.2 ± 10.2 | p < 0.001 |
| High-density lipoprotein cholesterol, mg/dL | 47.1 ± 4.4 | 46.1 ± 4.3 | p < 0.001 |
| Triglicerydes, mg/dL | 112.2 ± 9.6 | 198.6 ± 13.2 | p < 0.001 |
| Osteopontin, ng/mL | 11.12 ± 4.30 | 15.32 ± 3.20 | p < 0.01 |
| Osteoprotegerin, pmol/L | 4.23 ± 1.20 | 5.28 ± 1.11 | p < 0.01 |
| Metaloproteinase-9, ng/mL | 255 ± 86 | 428 ± 82 | p < 0.05 |
| Soluble CD40 ligand, ng/mL | 2.14 ± 0.80 | 3.69 ± 0.69 | p > 0.05 |
| Myeloperoxidase, ng/mL | 426 ± 112 | 560 ± 96 | p > 0.05 |
| Marker | Before Treatment | After Treatment | p Value |
|---|---|---|---|
| sCD40L (ng/mL) | 3.69 ± 0.9 | 3.11 ± 0.55 | p = 0.094 |
| OPN (ng/mL) | 15.32 ± 3.20 | 13.24 ± 3.18 | p < 0.05 |
| OPG (pmol/L) | 5.28 ± 1.11 | 4.28 ± 1.02 | p < 0.05 |
| MMP 9 (ng/mL) | 428 ± 82 | 362 ± 64 | p < 0.01 |
| MPO (ng/mL) | 560 ± 96 | 460 ± 82 | p = 0.082 |
| Type of Carotid Atherosclerotic Lesions: | ||||
|---|---|---|---|---|
| Marker | Type IV-V (n = 10) | Type VI (n = 6) | Control Group (n = 12) | p Value |
| OPN (ng/mL) | 15.86 ± 3.42 | 14.94 ± 3.02 | 11.12 ± 4.30 | p < 0.01 |
| OPG (pmol/L) | 5.64 ± 1.18 | 5.02 ± 1.0 | 4.23 ± 1.20 | p < 0.01 |
| MMP-9 (ng/mL) | 398 ± 60 | 468 ± 94 | 255 ± 86 | p < 0.05 |
| CD40L (ng/mL) | 3.56 ± 0.62 | 3.81 ± 0.72 | 2.14 ± 0.80 | p > 0.05 |
| MPO (ng/mL) | 522 ± 63 | 592 ± 104 | 426 ± 112 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiak, M.; Hachula, M.; Kosowski, M.; Machnik, G.; Maliglowka, M.; Dziubinska-Basiak, M.; Krysiak, R.; Okopien, B. The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods. Molecules 2023, 28, 5928. https://doi.org/10.3390/molecules28155928
Basiak M, Hachula M, Kosowski M, Machnik G, Maliglowka M, Dziubinska-Basiak M, Krysiak R, Okopien B. The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods. Molecules. 2023; 28(15):5928. https://doi.org/10.3390/molecules28155928
Chicago/Turabian StyleBasiak, Marcin, Marcin Hachula, Michal Kosowski, Grzegorz Machnik, Mateusz Maliglowka, Maria Dziubinska-Basiak, Robert Krysiak, and Boguslaw Okopien. 2023. "The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods" Molecules 28, no. 15: 5928. https://doi.org/10.3390/molecules28155928
APA StyleBasiak, M., Hachula, M., Kosowski, M., Machnik, G., Maliglowka, M., Dziubinska-Basiak, M., Krysiak, R., & Okopien, B. (2023). The Effect of PCSK9 Inhibition on the Stabilization of Atherosclerotic Plaque Determined by Biochemical and Diagnostic Imaging Methods. Molecules, 28(15), 5928. https://doi.org/10.3390/molecules28155928






