Purification of Coffee Polyphenols Extracted from Coffee Pulps (Coffee arabica L.) Using Aqueous Two-Phase System
Abstract
1. Introduction
2. Results and Discussion
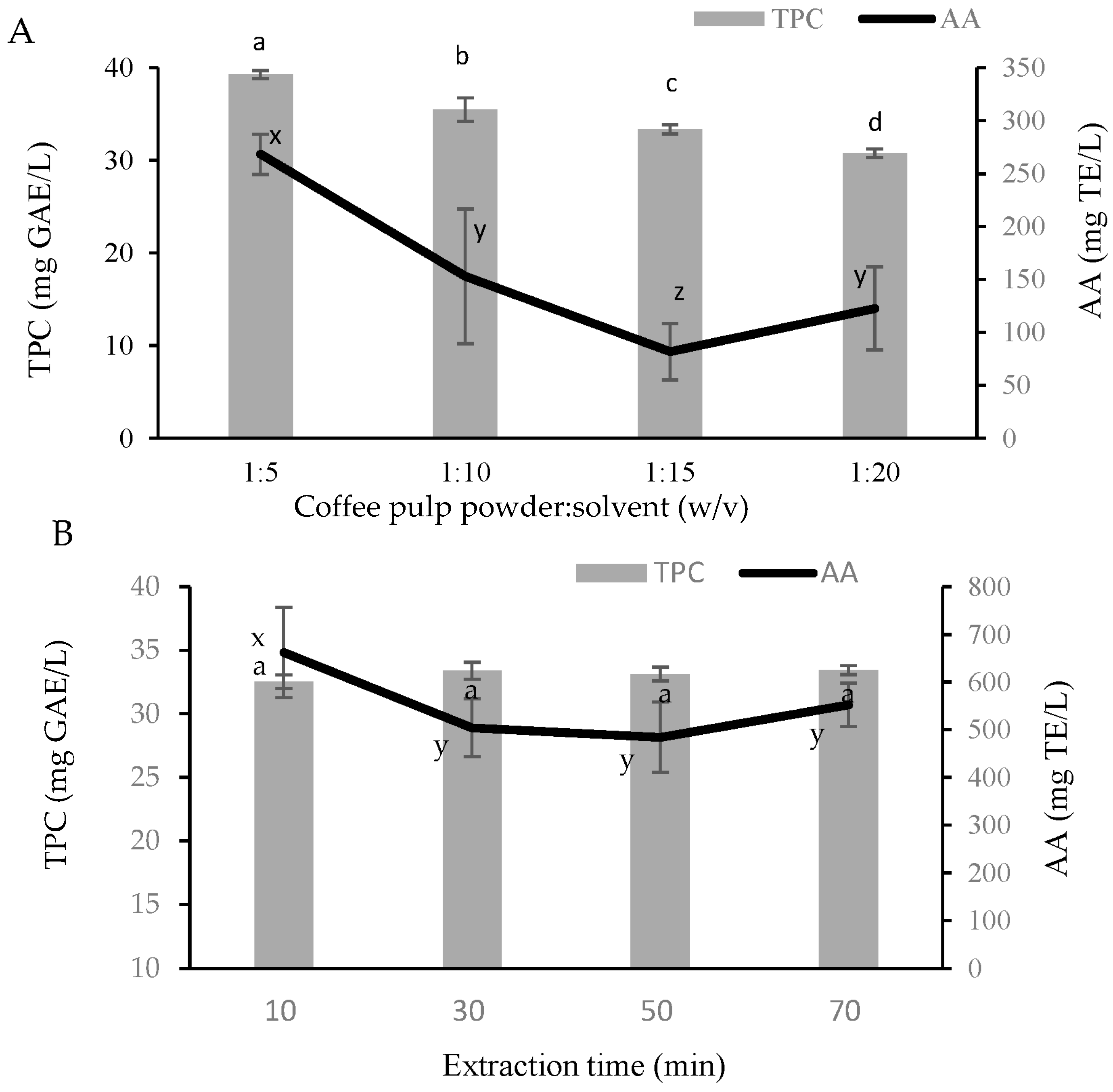
2.1. Crude Extract of Coffee Pulp
2.2. Phase Ratio (R), Partition Coefficient (K) and Purification Efficiency (E)
2.3. Total Polyphenol Content, Antioxidant Activity and Characterized Polyphenols in the Purified Coffee Pulp Extract
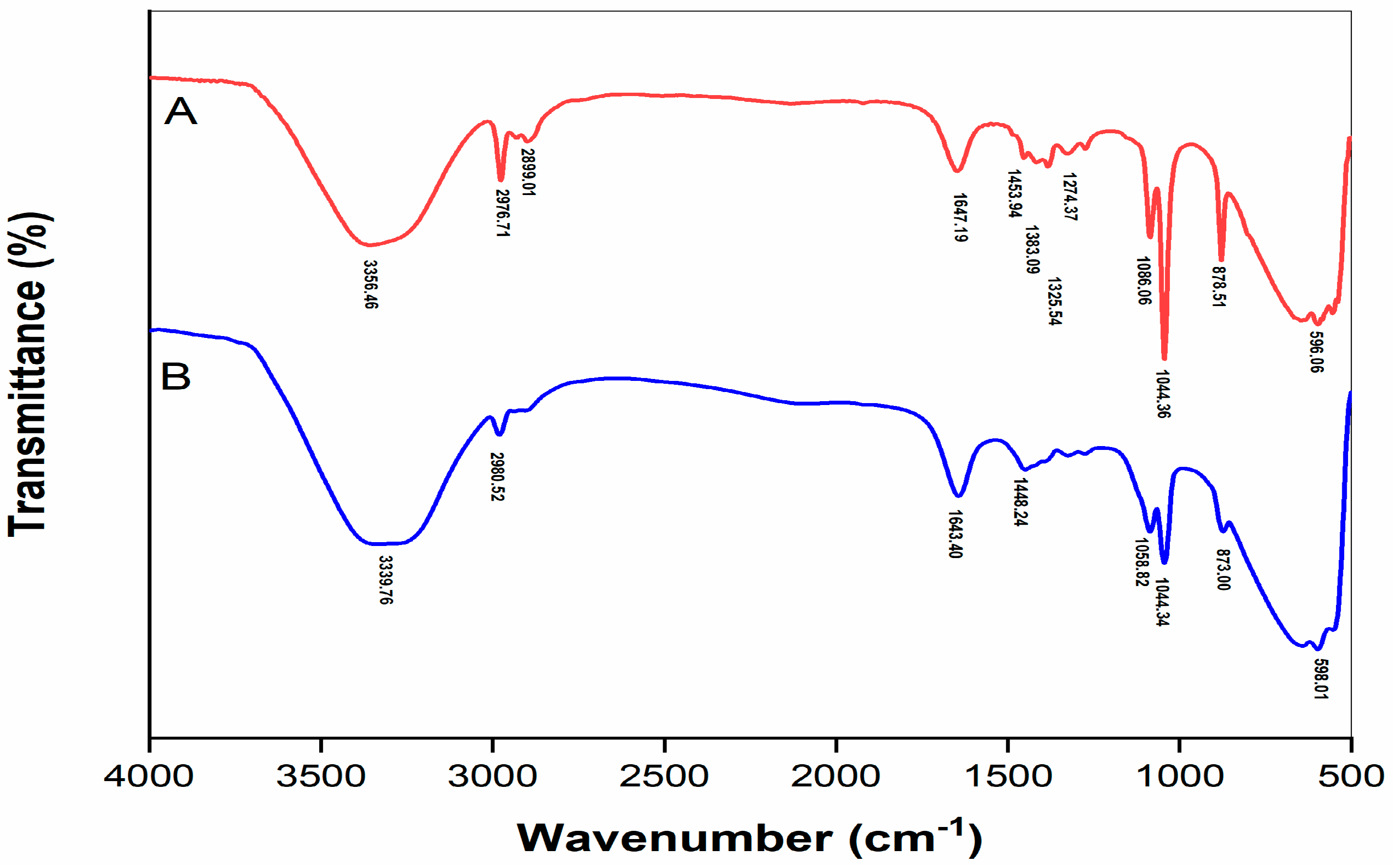
2.4. FTIR Spectrum of Polyphenol Extracts before and after Purification
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of the Crude Extract
3.3. Phase Diagram Building and ATPS Preparation
3.4. Analysis Methods
3.4.1. Determination of Volume ratio (R), Partition Coefficient (K) and Purification Efficiency (E)
3.4.2. Total Phenolic Content
3.4.3. Antioxidant Activity Determination
3.4.4. Analysis of CFA and CGA
3.4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Loukri, A.; Tsitlakidou, P.; Goula, A.; Assimopoulou, A.N.; Kontogiannopoulos, K.N.; Mourtzinos, I. Green Extracts from Coffee Pulp and Their Application in the Development of Innovative Brews. Appl. Sci. 2020, 10, 6982. [Google Scholar] [CrossRef]
- Dao, D.N.; Le, P.H.; Do, D.X.; Dang, T.M.Q.; Nguyen, S.K.; Nguyen, V. Pectin and Cellulose Extracted from Coffee Pulps and Their Potential in Formulating Biopolymer Films. Biomass Convers. Biorefin 2022. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, F.; He, C.; Yu, Y.; Wang, M. Optimization of Ultrasonic-Assisted Aqueous Two-Phase Extraction of Phloridzin from Malus Micromalus Makino with Ethanol/Ammonia Sulfate System. J. Food Sci. 2017, 82, 2944–2953. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous Two-Phase System (ATPS): An Overview and Advances in Its Applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef]
- Le, P.H.; Dao, D.N.; Huynh, T.Q.; Tran, T.T.T.; Nguyen, V. Extraction and Purification of Anthocyanins from Peristrophe bivalvis (L.) Merr. Leaf (Acanthaceae) Using Aqueous Two-Phase Systems. Nat. Prod. Res. 2023, 37, 154–158. [Google Scholar] [CrossRef]
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.L. Aqueous Two-Phase Extraction of Bioactive Compounds from Haskap Leaves (Lonicera caerulea): Comparison of Salt/Ethanol and Sugar/Propanol Systems. Sep. Purif. Technol. 2020, 252, 117399. [Google Scholar] [CrossRef]
- Babu, B.R.; Rastogi, N.K.; Raghavarao, K.S.M.S. Liquid-Liquid Extraction of Bromelain and Polyphenol Oxidase Using Aqueous Two-Phase System. Chem. Eng. Process. Process. Intensif. 2008, 47, 83–89. [Google Scholar] [CrossRef]
- Chethana, S.; Nayak, C.A.; Raghavarao, K.S.M.S. Aqueous Two Phase Extraction for Purification and Concentration of Betalains. J. Food Eng. 2007, 81, 679–687. [Google Scholar] [CrossRef]
- Rito-Palomares, M. Practical Application of Aqueous Two-Phase Partition to Process Development for the Recovery of Biological Products. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 807, 3–11. [Google Scholar] [CrossRef]
- Cheng, Z.; Cheng, L.; Song, H.; Yu, L.; Zhong, F.; Shen, Q.; Hu, H. Aqueous Two-Phase System for Preliminary Purification of Lignans from Fruits of Schisandra Chinensis Baill. Sep. Purif. Technol. 2016, 166, 16–25. [Google Scholar] [CrossRef]
- Ma, L.; Tong, W.; Du, L.; Huang, S.; Wei, J.; Xiao, D. Optimization of an Aqueous Two-Phase System for the Determination of Trace Ethyl Carbamate in Red Wine. J. Food Prot. 2019, 82, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Qiu, Y.; Ren, H.; Ju, D.H.; Jia, H.L. Optimization of Ultrasound-Assisted Aqueous Two-Phase System Extraction of Polyphenolic Compounds from Aronia Melanocarpa Pomace by Response Surface Methodology. Prep. Biochem. Biotechnol. 2017, 47, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Salazar, N.; Valle-Guadarrama, S. Separation of Phenolic Compounds from Roselle (Hibiscus Sabdariffa) Calyces with Aqueous Two-Phase Extraction Based on Sodium Citrate and Polyethylene Glycol or Acetone. Sep. Sci. Technol. 2020, 55, 2313–2324. [Google Scholar] [CrossRef]
- Montalvo-Hernández, B.; Rito-Palomares, M.; Benavides, J. Recovery of Crocins from Saffron Stigmas (Crocus Sativus) in Aqueous Two-Phase Systems. J. Chromatogr. A 2012, 1236, 7–15. [Google Scholar] [CrossRef]
- Santos, S.B.; Reis, I.A.O.; Silva, C.P.C.; Campos, A.F.; Ventura, S.P.M.; Soares, C.M.F.; Lima, Á.S. Selective Partition of Caffeine from Coffee Bean and Guaraná Seed Extracts Using Alcohol–Salt Aqueous Two-Phase Systems. Sep. Sci. Technol. 2016, 51, 2008–2019. [Google Scholar] [CrossRef]
- Cienfuegos, N.E.C.; Santos, P.L.; García, A.R.; Soares, C.M.F.; Lima, A.S.; Souza, R.L. Integrated Process for Purification of Capsaicin Using Aqueous Two-Phase Systems Based on Ethanol. Food Bioprod. Process. 2017, 106, 1–10. [Google Scholar] [CrossRef]
- Sang, J.; Dang, K.-K.; Ma, Q.; Li, B.; Huang, Y.-Y.; Li, C. qin Partition Behaviors of Different Polar Anthocyanins in Aqueous Two-Phase Systems and Extraction of Anthocyanins from Nitraria Tangutorun Bobr. and Lycium Ruthenicum Murr. Food Anal. Methods 2018, 11, 980–991. [Google Scholar] [CrossRef]
- Huang, A.; Deng, W.; Wu, D.; Wu, S.; Xiao, Y. Hexafluoroisopropanol-Salt Aqueous Two-Phase System for Extraction and Purification of Chlorogenic Acid from Ramie Leaves. J. Chromatogr. A 2019, 1597, 196–201. [Google Scholar] [CrossRef]
- Chong, K.Y.; Brooks, M.S.L. Effects of Recycling on the Aqueous Two-Phase Extraction of Bioactives from Haskap Leaves. Sep. Purif. Technol. 2021, 255, 117755. [Google Scholar] [CrossRef]
- Xie, L.; Chong, K.Y.; Stefanova, R.; Hui, J.P.M.; Zhang, J.; Brooks, M.S.L. Recovery of Chlorogenic Acid from Haskap Leaves (Lonicera caerulea) Using Aqueous Two-Phase Extraction. Biomass Convers. Biorefinery 2021, 13, 3741–3750. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, C.; Yi, Y.; Wang, H.; Li, M.; Zhou, W.; Tan, S.; Li, F. Extraction and Purification of Chlorogenic Acid from Ramie (Boehmeria nivea L. Gaud) Leaf Using an Ethanol/Salt Aqueous Two-Phase System. Sep. Purif. Technol. 2014, 132, 396–400. [Google Scholar] [CrossRef]
- Phuong, N.N.M.; Le, T.T.; Dang, M.Q.; Van Camp, J.; Raes, K. Selection of Extraction Conditions of Phenolic Compounds from Rambutan (Nephelium lappaceum L.) Peel. Food Bioprod. Process. 2020, 122, 222–229. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, D.; Fan, H.; Liu, X.; Wan, Q.; Wu, X.; Liu, P.; Tang, J.Z. Simultaneous Extraction and Purification of Alkaloids from Sophora Flavescens Ait. by Microwave-Assisted Aqueous Two-Phase Extraction with Ethanol/Ammonia Sulfate System. Sep. Purif. Technol. 2015, 141, 113–123. [Google Scholar] [CrossRef]
- Xavier, L.; Freire, M.S.; Vidal-Tato, I.; González-Álvarez, J. Recovery of Phenolic Compounds from Eucalyptus Globulus Wood Wastes Using PEG/Phosphate Aqueous Two-Phase Systems. Waste Biomass Valorization 2017, 8, 443–452. [Google Scholar] [CrossRef]
- Simental-Martínez, J.; Montalvo-Hernández, B.; Rito-Palomares, M.; Benavides, J. Application of Aqueous Two-Phase Systems for the Recovery of Bioactive Low-Molecular Weight Compounds. Sep. Sci. Technol. 2014, 49, 1872–1882. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T.; et al. Recovery of Polyphenolic Fraction from Arabica Coffee Pulp and Its Antifungal Applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yan, C.; Zhang, K.; Xie, J.; Zhang, Y.; Wen, Z. Comprehensive Determination of Nine Polyphenols in Polygoni Avicularis Herba with a New HPLC–DAD Method and Their Correlation with the Antioxidant Activities. J. Food Meas. Charact. 2018, 12, 1593–1600. [Google Scholar] [CrossRef]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR Characteristics, Phenolic Profiles and Inhibitory Potential against Digestive Enzymes of 25 Herbal Infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; Van Dam, J.E.G. Characterisation of Structure-Dependent Functional Properties of Lignin with Infrared Spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- John Cordero, C.G.; Marquez, K.P. Extraction of Milled Wood Lignin from Coffee Husk (Coffea Arabica L.) and the Analysis of Its Potential as a UV-Protective Component of Lotion and Sunscreen. KIMIKA 2021, 32, 1–10. [Google Scholar] [CrossRef]
- Reis, R.S.; Tienne, L.G.P.; de Souza, D.H.S.; Marques, M.d.F.V.; Monteiro, S.N. Characterization of Coffee Parchment and Innovative Steam Explosion Treatment to Obtain Microfibrillated Cellulose as Potential Composite Reinforcement. J. Mater. Res. Technol. 2020, 9, 9412–9421. [Google Scholar] [CrossRef]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of Hemicellulose, Cellulose, Holocellulose and Lignin Content Using FTIR in Calycophyllum spruceanum (Benth.) K. schum. And Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Atlabachew, M.; Abebe, A.; Alemneh Wubieneh, T.; Tefera Habtemariam, Y. Rapid and Simultaneous Determination of Trigonelline, Caffeine, and Chlorogenic Acid in Green Coffee Bean Extract. Food Sci. Nutr. 2021, 9, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
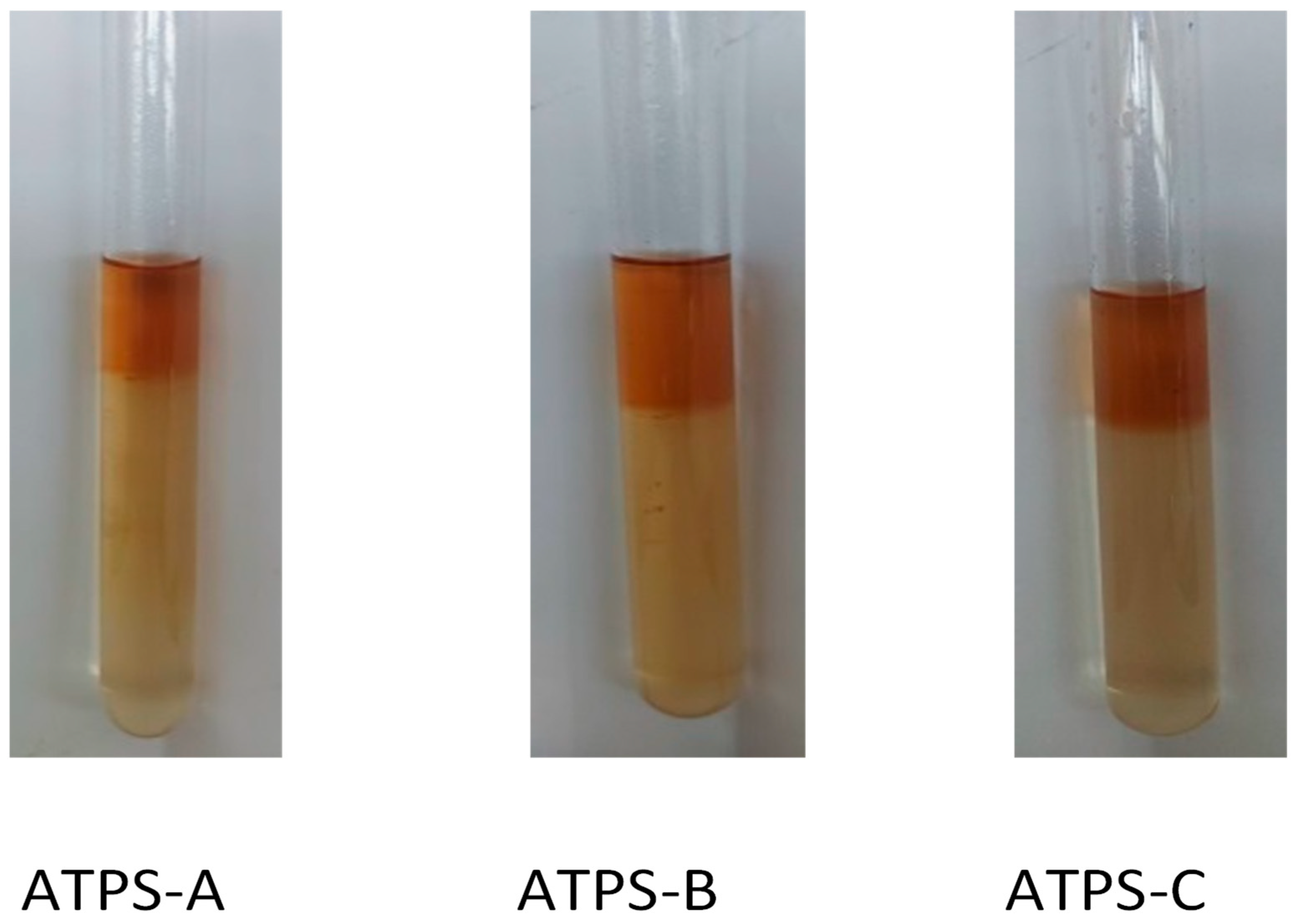
| Phase System | Plant Materials | Purified Compounds | References |
|---|---|---|---|
| EtOH/K3PO4 | Saffron stigmas (Crocus sativus) | Crocins | [15] |
| EtOH/(NH4)SO4 | Fruits of Schisandra chinensis Baill | Lignan | [11] |
| 2-propanol/K3PO4 MeOH/K2HPO4 (KH2PO4) | Coffee bean Guarana seed | Caffeine | [16] |
| EtOH/NaH2PO4 EtOH/Na2S2O3 EtOH/Na2SO4 EtOH/Na2CO3 | Chili | Capsaicin | [17] |
| EtOH/(NH4)2SO4 EtOH/NaH2PO4 | Nitraria tangutorun Bobr Lycium ruthenicum Murr. | Anthocyanins | [18] |
| Hexafluoroisopropanol/NaCl | Ramie leaves | Chlorogenic acid | [19] |
| Polyethylen glycol/Na Citrate Acetone/Na citrate | Roselle (Hibiscus sabdariffa) calyces | Phenolic compounds | [14] |
| EtOH/(NH4)SO4 EtOH/NaH2PO4 1-propanol/glucose 1-propanol/mantose | Haskap leaves | Bioactive compounds | [7,20] |
| Type of ATPS | Volume Ratio (R) | Partition Coefficient (K) | Purification Efficiency (E, %) |
|---|---|---|---|
| ATPS-A | 0.33 ± 0.02 a | 22.10 ± 1.54 a | 88.01 ± 1.42 a |
| ATPS-B | 0.47 ± 0.08 b | 17.82 ± 1.99 b | 89.19 ± 0.63 a |
| ATPS-C | 0.44 ± 0.02 b | 21.08 ± 0.34 a | 90.35 ± 0.47 a |
| APTS EtOH/(NH4)2SO4 | TPC (mg GAE/L) | AA (mg TE/L) | Chlorogenic Acid (mmol/L) | Caffeic Acid (mmol/L) |
|---|---|---|---|---|
| Before purification | 37.54 ± 0.96 a | 584.50 ± 22.24 a | 138.59 ± 7.77 a | 218.66 ± 19.94 a |
| A | 38.70 ± 0.84 a | 778.05 ± 21.11 b | 149.63 ± 12.78 a | 233.13 ± 24.80 a |
| B | 38.65 ± 0.96 a | 775.39 ± 32.49 b | 140.68 ± 19.43 a | 209.74 ± 43.16 a |
| C | 38.60 ± 0.83 a | 774.06 ± 21.11 b | 149.15 ± 19.66 a | 218.16 ± 31.97 a |
| ATPS | (NH4)2SO4 (%) | EtOH (%) | (NH4)2SO4 (g) | EtOH (mL) | H2O (mL) | Coffee Pulp Crude Extract (mL) | Top Phase pH | Bottom Phase pH |
|---|---|---|---|---|---|---|---|---|
| ATPS-A | 18 | 26 | 3.6 | 1.6 | 9.8 | 4.8 | 6.09 | 5.26 |
| ATPS-B | 16 | 32 | 3.2 | 1.9 | 9.0 | 5.7 | 5.92 | 5.21 |
| ATPS-C | 20 | 28 | 4.0 | 1.7 | 8.8 | 5.2 | 6.02 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.H.; Ho, L.T.T.; Le, D.H.T.; Nguyen, V. Purification of Coffee Polyphenols Extracted from Coffee Pulps (Coffee arabica L.) Using Aqueous Two-Phase System. Molecules 2023, 28, 5922. https://doi.org/10.3390/molecules28155922
Le PH, Ho LTT, Le DHT, Nguyen V. Purification of Coffee Polyphenols Extracted from Coffee Pulps (Coffee arabica L.) Using Aqueous Two-Phase System. Molecules. 2023; 28(15):5922. https://doi.org/10.3390/molecules28155922
Chicago/Turabian StyleLe, Phuong Hong, Linh Thuy Thi Ho, Dao Hong Thi Le, and Viet Nguyen. 2023. "Purification of Coffee Polyphenols Extracted from Coffee Pulps (Coffee arabica L.) Using Aqueous Two-Phase System" Molecules 28, no. 15: 5922. https://doi.org/10.3390/molecules28155922
APA StyleLe, P. H., Ho, L. T. T., Le, D. H. T., & Nguyen, V. (2023). Purification of Coffee Polyphenols Extracted from Coffee Pulps (Coffee arabica L.) Using Aqueous Two-Phase System. Molecules, 28(15), 5922. https://doi.org/10.3390/molecules28155922






