Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome
Abstract
1. Introduction
2. Bioavailability Challenges Associated with Bismuth-Based Compounds
3. Pharmacological Properties of Bismuth-Based Compounds
3.1. Antibacterial Activity
3.1.1. Modes of Action against H. pylori and Bacterial Resistance Mechanisms
3.1.2. Antibacterial Activity beyond H. pylori and Reversion of Bacterial Drug Resistance
3.2. Antifungal Activity
3.3. Antiparasitic Activity
3.4. Antitumoral Activity
3.4.1. Bismuth(III) Complexes Bearing S-Donor Ligands
3.4.2. Bismuth(III) Complexes Bearing N,S-Donor Ligands
3.4.3. Bismuth(III) Complexes Bearing N,O-Donor Ligands
3.4.4. Organobismuth(III) Compounds
3.5. Antiviral Activity
4. Strategies to Enhance the Bioavailability of Bismuth beyond the Complex Formation
4.1. Incorporation of Bismuth in Biopolymeric Systems
4.2. Bismuth-Based MOFs and Coordination Polymers
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, R.; Li, H.; Ip, T.K.Y.; Sun, H. Bismuth Drugs as Antimicrobial Agents, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 75, ISBN 9780128191965. [Google Scholar]
- Li, H.; Sun, H. Recent Advances in Bioinorganic Chemistry of Bismuth. Curr. Opin. Chem. Biol. 2012, 16, 74–83. [Google Scholar] [CrossRef]
- Yang, N.; Sun, H. Bismuth: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 414–420. [Google Scholar]
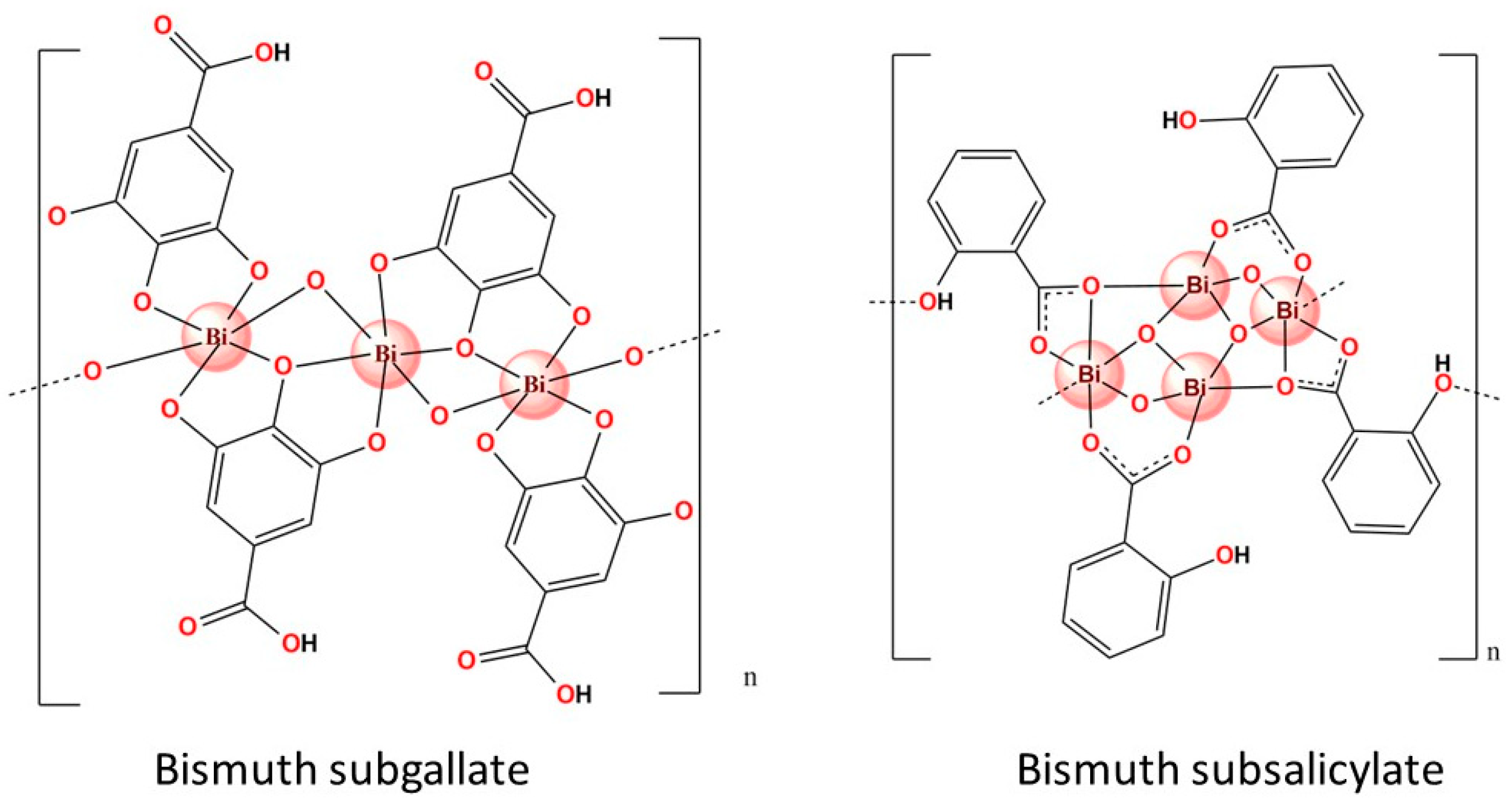
- Wang, Y.; Takki, S.; Cheung, O.; Xu, H.; Wan, W.; Öhrström, L.; Inge, A.K. Elucidation of the Elusive Structure and Formula of the Active Pharmaceutical Ingredient Bismuth Subgallate by Continuous Rotation Electron Diffraction. Chem. Commun. 2017, 53, 7018–7021. [Google Scholar] [CrossRef] [PubMed]
- Svensson Grape, E.; Rooth, V.; Nero, M.; Willhammar, T.; Inge, A.K. Structure of the Active Pharmaceutical Ingredient Bismuth Subsalicylate. Nat. Commun. 2022, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Midolo, P. The Actions of Bismuth in the Treatment of Helicobacter pylori Infection. Aliment. Pharmacol. Ther. 1997, 11, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Yang, N.; Sun, H. Biocoordination Chemistry of Bismuth: Recent Advances. Coord. Chem. Rev. 2007, 251, 2354–2366. [Google Scholar] [CrossRef]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal Chemistry and Biomedical Applications of Bismuth-Based Compounds and Nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef]
- Keogan, D.M.; Griffith, D.M. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef]
- Shetu, S.A.; Sanchez-Palestino, L.M.; Rivera, G.; Bandyopadhyay, D. Medicinal Bismuth: Bismuth-Organic Frameworks as Pharmaceutically Privileged Compounds. Tetrahedron 2022, 129, 133117. [Google Scholar] [CrossRef]
- Ong, Y.C.; Kedzierski, L.; Andrews, P.C. Do Bismuth Complexes Hold Promise as Antileishmanial Drugs? Future Med. Chem. 2018, 10, 1721–1733. [Google Scholar] [CrossRef]
- Murafuji, T.; Fujiwara, Y.; Yoshimatsu, D.; Miyakawa, I.; Migita, K.; Mikata, Y. Bismuth Heterocycles Based on a Diphenyl Sulfone Scaffold: Synthesis and Substituent Effect on the Antifungal Activity against Saccharomyces cerevisiae. Eur. J. Med. Chem. 2011, 46, 519–525. [Google Scholar] [CrossRef]
- Deng, T.; Jia, Y.; Tong, Z.; Shi, J.; Wang, Z.; Liu, Y. Bismuth Drugs Reverse Tet(X)-Conferred Tigecycline Resistance in Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e01578-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lai, T.P.; Gao, P.; Zhang, H.; Ho, P.L.; Woo, P.C.Y.; Ma, G.; Kao, R.Y.T.; Li, H.; Sun, H. Bismuth Antimicrobial Drugs Serve as Broad-Spectrum Metallo-β-Lactamase Inhibitors. Nat. Commun. 2018, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhang, L.; Du, L.; Liao, R.; Cai, H.; Lu, K.; Zhao, Z.; Xie, Y.; Wang, P.H.; Pan, J.A.; et al. Allosteric Inhibition of SARS-CoV-2 3CL Protease by Colloidal Bismuth Subcitrate. Chem. Sci. 2021, 12, 14098–14102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, R.; Chan, J.F.W.; Zhang, A.J.; Cheng, T.; Chik, K.K.H.; Ye, Z.W.; Wang, S.; Lee, A.C.Y.; Jin, L.; et al. Metallodrug Ranitidine Bismuth Citrate Suppresses SARS-CoV-2 Replication and Relieves Virus-Associated Pneumonia in Syrian Hamsters. Nat. Microbiol. 2020, 5, 1439–1448. [Google Scholar] [CrossRef]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qiu, Y.; Zhang, D.Y.; et al. SARS-Coronavirus-2 Nsp13 Possesses NTPase and RNA Helicase Activities That Can Be Inhibited by Bismuth Salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef]
- Toutain, P.L.; Bousquet-Melou, A. Bioavailability and Its Assessment. J. Vet. Pharmacol. Ther. 2004, 27, 455–466. [Google Scholar] [CrossRef]
- Himeno, S.; Fujishiro, H.; Sumi, D. Bismuth. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 121–139. [Google Scholar]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkim, C. Role of Bismuth in the Eradication of Helicobacter pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori Eradication with a Capsule Containing Bismuth Subcitrate Potassium, Metronidazole, and Tetracycline given with Omeprazole versus Clarithromycin-Based Triple Therapy: A Randomised, Open-Label, Non-Inferiority, Phase 3 Trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Guiard, E.; Lelievre, B.; Rouyer, M.; Zerbib, F.; Diquet, B.; Mégraud, F.; Tison, F.; Bignon, E.; Lassalle, R.; Perroteau, C.D.; et al. Bismuth Concentrations in Patients Treated in Real-Life Practice with a Bismuth Subcitrate-Metronidazole-Tetracycline Preparation: The SAPHARY Study. Drug Saf. 2019, 42, 993–1003. [Google Scholar] [CrossRef]
- Hong, Y.; Lai, Y.T.; Chan, G.C.F.; Sun, H. Glutathione and Multidrug Resistance Protein Transporter Mediate a Self-Propelled Disposal of Bismuth in Human Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 3211–3216. [Google Scholar] [CrossRef]
- Larsen, A.; Stoltenberg, M.; Sondergaard, C.; Bruhn, M.; Danscher, G. In Vivo Distribution of Bismuth in the Mouse Brain: Influence of Long-Term Survival and Intracranial Placement on the Uptake and Transport of Bismuth in Neuronal Tissue. Basic Clin. Pharmacol. Toxicol. 2005, 97, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, M.; Hutson, J.C. Bismuth Uptake in Rat Testicular Macrophages: A Follow-up Observation Suggesting That Bismuth Alters Interactions Between Testicular Macrophages and Leydig Cells. J. Histochem. Cytochem. 2004, 52, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.; Zhu, N.; Hou, X.; Deng, F.; Sun, H. Structure of Colloidal Bismuth Subcitrate (CBS) in Dilute HCl: Unique Assembly of Bismuth Citrate Dinuclear Units ([Bi(Cit)2Bi]2−). J. Am. Chem. Soc. 2003, 125, 12408–12409. [Google Scholar] [CrossRef]
- Spénard, J.; Aumais, C.; Massicotte, J.; Tremblay, C.; Lefebvre, M. Influence of Omeprazole on Bioavailability of Bismuth Following Administration of a Triple Capsule of Bismuth Biskalcitrate, Metronidazole, and Tetracycline. J. Clin. Pharmacol. 2004, 44, 640–645. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Fu, W.; Song, Z.; Zhou, L.; Xue, Y.; Ding, Y.; Suo, B.; Wang, L. Randomized Clinical Trial: Esomeprazole, Bismuth, Levofloxacin, and Amoxicillin or Cefuroxime as First-Line Eradication Regimens for Helicobacter pylori Infection. Dig. Dis. Sci. 2017, 62, 1580–1589. [Google Scholar] [CrossRef]
- Robinson, K.; Atherton, J.C. The Spectrum of Helicobacter-Mediated Diseases. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.; Warren, J.R. Unidentified Curved Bacilli in the Stomach of Patients with Gastritis and Peptic Ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Ge, R.; Chen, Z.; Zhou, Q. The Actions of Bismuth in the Treatment of Helicobacter pylori Infections: An Update. Metallomics 2012, 4, 239–243. [Google Scholar] [CrossRef]
- Beil, W.; Birkholz, C.; Wagner, S.; Sewing, K.-F. Bismuth Subcitrate and Omeprazole Inhibit Helicobacter pylori F1-ATPase. Pharmacology 1995, 50, 333–337. [Google Scholar] [CrossRef]
- Zhang, L.; Mulrooney, S.B.; Leung, A.F.K.; Zeng, Y.; Ko, B.B.C.; Hausinger, R.P.; Sun, H. Inhibition of Urease by Bismuth(III): Implications for the Mechanism of Action of Bismuth Drugs. BioMetals 2006, 19, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Roine, R.P.; Salmela, K.S.; Höök-Nikanne, J.; Kosunen, T.U.; Salaspuro, M. Colloidal Bismuth Subcitrate and Omeprazole Inhibit Alcohol Dehydrogenase Mediated Acetaldehyde Production by Helicobacter pylori. Life Sci. 1992, 51, PL195–PL200. [Google Scholar] [CrossRef] [PubMed]
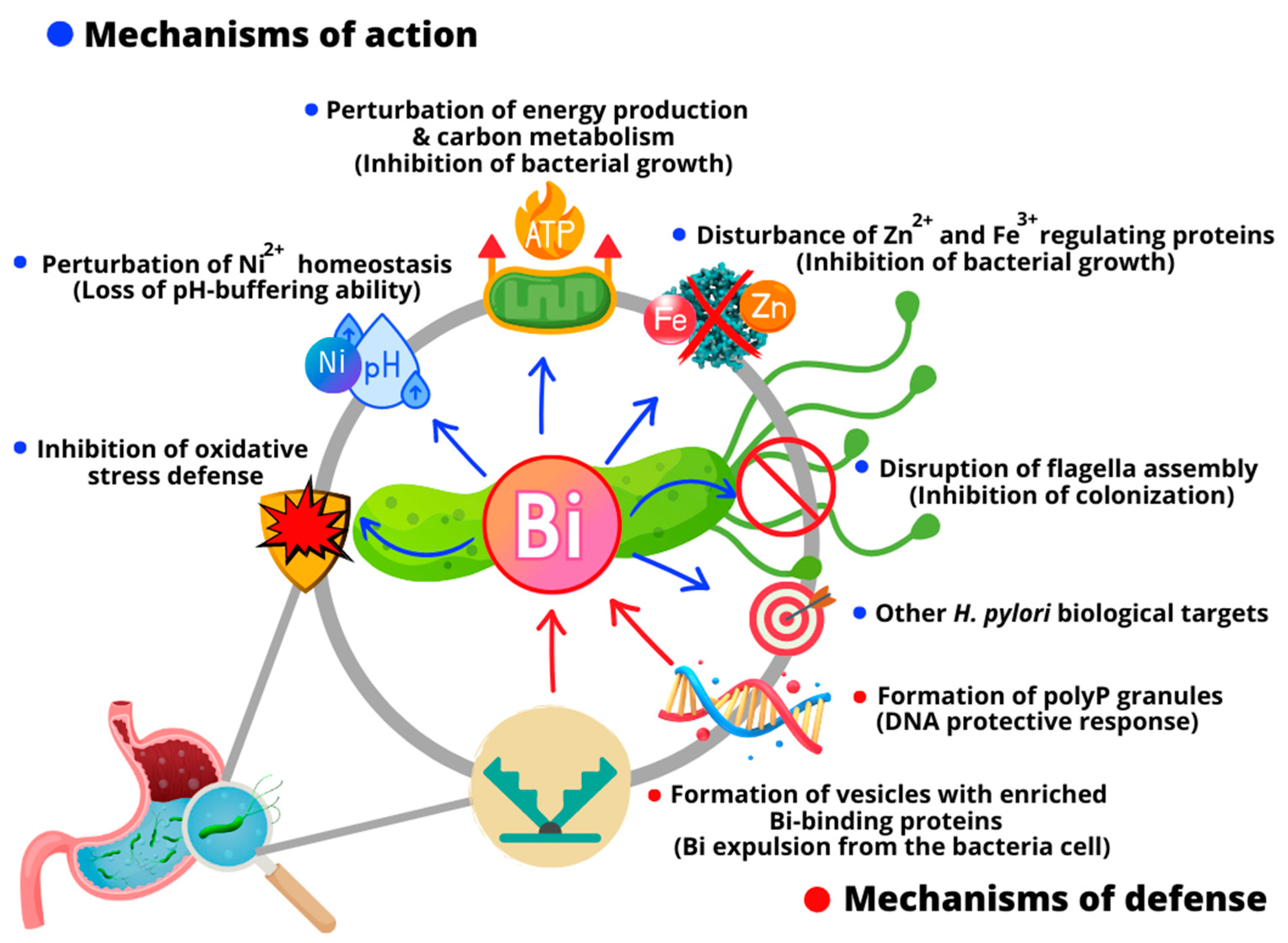
- Ge, R.; Sun, H. Bioinorganic Chemistry of Bismuth and Antimony: Target Sites of Metallodrugs. Acc. Chem. Res. 2007, 40, 267–274. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhu, Y.; Ge, R. Molecular Mechanisms of Bismuth-Containing Drugs Against Helicobacter pylori: A Further Update. Curr. Pharmacol. Rep. 2023, 9, 59–65. [Google Scholar] [CrossRef]
- Han, B.; Zhang, Z.; Xie, Y.; Hu, X.; Wang, H.; Xia, W.; Wang, Y.; Li, H.; Wang, Y.; Sun, H. Multi-Omics and Temporal Dynamics Profiling Reveal Disruption of Central Metabolism in Helicobacter pylori on Bismuth Treatment. Chem. Sci. 2018, 9, 7488–7497. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, L.; Xu, F.; Quan, Q.; Lai, Y.T.; Xia, W.; Yang, Y.; Chang, Y.Y.; Yang, X.; Chai, Z.; et al. Integrative Approach for the Analysis of the Proteome-Wide Response to Bismuth Drugs in Helicobacter pylori. Chem. Sci. 2017, 8, 4626–4633. [Google Scholar] [CrossRef]
- Yao, X.; Xiao, S.; Zhou, L. Integrative Proteomic and Metabolomic Analyses Reveal the Mechanism by Which Bismuth Enables Helicobacter pylori Eradication. Helicobacter 2021, 26, e12846. [Google Scholar] [CrossRef]
- Zhu, X.; Gerstein, M.; Snyder, M. Getting Connected: Analysis and Principles of Biological Networks. Genes Dev. 2007, 21, 1010–1024. [Google Scholar] [CrossRef]
- Salcedo, J.A.; Al-Kawas, F. Treatment of Helicobacter pylori Infection. Arch. Intern. Med. 1998, 158, 842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Schmitt, C.; Gorgette, O.; Marbouty, M.; Duchateau, M.; Gianetto, Q.G.; Matondo, M.; Guigner, J.M.; De Reuse, H. Bacterial Membrane Vesicles as a Novel Strategy for Extrusion of Antimicrobial Bismuth Drug in Helicobacter pylori. mBio 2022, 13, e01633-22. [Google Scholar] [CrossRef] [PubMed]
- Razmara, Z.; Delarami, H.S.; Eigner, V.; Dusek, M. Single Crystal Structure Feature and Quantum Mechanical Studies of a New Binuclear Bi (III) Complex and Its Activity against Helicobacter pylori. Inorg. Chem. Commun. 2022, 146, 110207. [Google Scholar] [CrossRef]
- Kowalik, M.; Masternak, J.; Łakomska, I.; Kazimierczuk, K.; Zawilak-Pawlik, A.; Szczepanowski, P.; Khavryuchenko, O.V.; Barszcz, B. Structural Insights into New Bi(III) Coordination Polymers with Pyridine-2,3-Dicarboxylic Acid: Photoluminescence Properties and Anti-Helicobacter pylori Activity. Int. J. Mol. Sci. 2020, 21, 8696. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Ferrero, R.L.; Junk, P.C.; MacLellan, J.G.; Peiris, R.M. Bismuth(III) Thiobenzoates and Their Activity against Helicobacter pylori. Aust. J. Chem. 2012, 65, 883–891. [Google Scholar] [CrossRef]
- Andrews, P.C.; Ferrero, R.L.; Junk, P.C.; Peiris, R.M. A Sweeter Way to Combat Helicobacter pylori? Bismuth(III) Complexes and Oxido-Clusters Derived from Non-Nutritive Sweeteners and Their Activity against H. Pylori. J. Organomet. Chem. 2013, 724, 88–94. [Google Scholar] [CrossRef]
- Busse, M.; Trinh, I.; Junk, P.C.; Ferrero, R.L.; Andrews, P.C. Synthesis and Characterisation of Bismuth(III) Aminoarenesulfonate Complexes and Their Powerful Bactericidal Activity against Helicobacter pylori. Chem.-Eur. J. 2013, 19, 5264–5275. [Google Scholar] [CrossRef]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Tabora, R.F.; Andrews, P.C. Synthesis and Structural Characterisation of Bismuth(III) Hydroxamates and Their Activity against Helicobacter pylori. Dalton Trans. 2015, 44, 16903–16913. [Google Scholar] [CrossRef]
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Bismuth(III) β-Thioxoketonates as Antibiotics against Helicobacter pylori and as Anti-Leishmanial Agents. Dalton Trans. 2014, 43, 1279–1291. [Google Scholar] [CrossRef]
- Busse, M.; Border, E.; Junk, P.C.; Ferrero, R.L.; Andrews, P.C. Bismuth(III) Complexes Derived from α-Amino Acids: The Impact of Hydrolysis and Oxido-Cluster Formation on Their Activity against Helicobacter pylori. Dalton Trans. 2014, 43, 17980–17990. [Google Scholar] [CrossRef]
- Duffin, R.N.; Werrett, M.V.; Andrews, P.C. Antimony and Bismuth as Antimicrobial Agents, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 75, ISBN 9780128191965. [Google Scholar]
- Burke, K.J.; Stephens, L.J.; Werrett, M.V.; Andrews, P.C. Bismuth(III) Flavonolates: The Impact of Structural Diversity on Antibacterial Activity, Mammalian Cell Viability and Cellular Uptake. Chem.-Eur. J. 2020, 26, 7657–7671. [Google Scholar] [CrossRef]
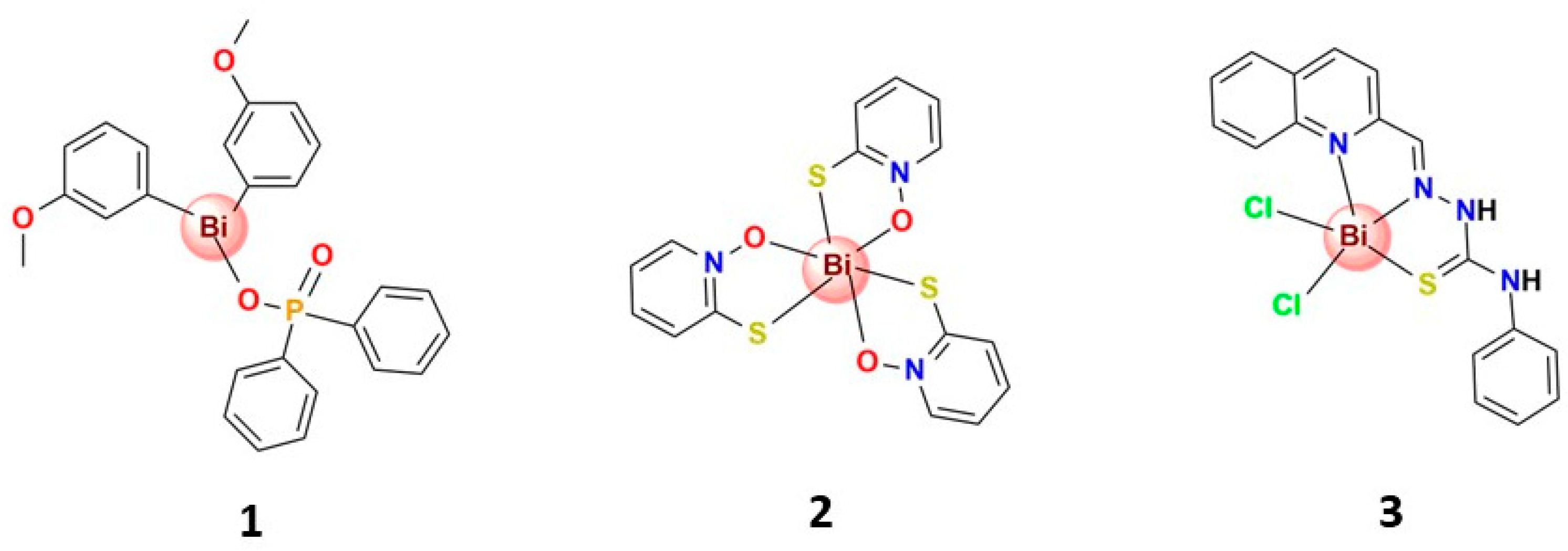
- Herdman, M.E.; Werrett, M.V.; Andrews, P.C. Aryl Bismuth Phosphinates [BiAr2(O(O)PRR′)]: Structure–Activity Relationships for Antibacterial Activity and Cytotoxicity. Dalton Trans. 2022, 51, 9323–9335. [Google Scholar] [CrossRef]
- Werrett, M.V.; Herdman, M.E.; Brammananth, R.; Garusinghe, U.; Batchelor, W.; Crellin, P.K.; Coppel, R.L.; Andrews, P.C. Bismuth Phosphinates in Bi-Nanocellulose Composites and Their Efficacy towards Multi-Drug Resistant Bacteria. Chem.-Eur. J. 2018, 24, 12938–12949. [Google Scholar] [CrossRef]
- Herdman, M.E.; Werrett, M.V.; Duffin, R.N.; Stephens, L.J.; Brammananth, R.; Coppel, R.L.; Batchelor, W.; Andrews, P.C. Impact of Structural Changes in Heteroleptic Bismuth Phosphinates on Their Antibacterial Activity in Bi-Nanocellulose Composites. Dalton Trans. 2020, 49, 7341–7354. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.P.; Carneiro, Z.A.; Ribeiro, C.M.; Lima, M.F.; Paixão, D.A.; Pivatto, M.; de Souza, M.V.N.; Teixeira, L.R.; Lopes, C.D.; de Albuquerque, S.; et al. Three New Platinum Complexes Containing Fluoroquinolones and DMSO: Cytotoxicity and Evaluation against Drug-Resistant Tuberculosis. J. Inorg. Biochem. 2018, 183, 77–83. [Google Scholar] [CrossRef] [PubMed]
- MacHado, I.; Marino, L.B.; Demoro, B.; Echeverría, G.A.; Piro, O.E.; Leite, C.Q.F.; Pavan, F.R.; Gambino, D. Bioactivity of Pyridine-2-Thiolato-1-Oxide Metal Complexes: Bi(III), Fe(III) and Ga(III) Complexes as Potent Anti-Mycobacterium tuberculosis Prospective Agents. Eur. J. Med. Chem. 2014, 87, 267–273. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Sun, H. Systems Approaches for Unveiling the Mechanism of Action of Bismuth Drugs: New Medicinal Applications beyond Helicobacter pylori Infection. Acc. Chem. Res. 2019, 52, 216–227. [Google Scholar] [CrossRef]
- Scaccaglia, M.; Rega, M.; Bacci, C.; Giovanardi, D.; Pinelli, S.; Pelosi, G.; Bisceglie, F. Bismuth Complex of Quinoline Thiosemicarbazone Restores Carbapenem Sensitivity in NDM-1-Positive Klebsiella pneumoniae. J. Inorg. Biochem. 2022, 234, 111887. [Google Scholar] [CrossRef]
- Falcao, A.; Bullón, P. A Review of the Influence of Periodontal Treatment in Systemic Diseases. Periodontology 2000 2019, 79, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, G.A.; Negi, M.; Uchida, K.; Sekine, M.; Furukawa, A.; Ito, T.; Kobayashi, D.; Suzuki, Y.; Akashi, T.; Umeda, M.; et al. Localization and Density of Porphyromonas gingivalis and Tannerella forsythia in Gingival and Subgingival Granulation Tissues Affected by Chronic or Aggressive Periodontitis. Sci. Rep. 2018, 8, 9507. [Google Scholar] [CrossRef]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug Resistance of Bacterial Dental Biofilm and the Potential Use of Natural Compounds as Alternative for Prevention and Treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Lai, Y.T.; Wang, C.; Wang, Y.; Jiang, N.; Li, H.; Sun, H.; Jin, L. Bismuth Drugs Tackle: Porphyromonas gingivalis and Attune Cytokine Response in Human Cells. Metallomics 2019, 11, 1207–1218. [Google Scholar] [CrossRef]
- Marzano, I.M.; Franco, M.S.; Silva, P.P.; Augusti, R.; Santos, G.C.; Fernandes, N.G.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Pereira-Maia, E.C. Crystal Structure, Antibacterial and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine. Molecules 2013, 18, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.N.; Nogueira, P.M.; Demicheli, C.; de Oliveira, L.G.; da Silva, M.M.; Frézard, F.; Melo, M.N.; Soares, R.P. Cytotoxicity and In Vitro Antileishmanial Activity of Antimony (V), Bismuth (V), and Tin (IV) Complexes of Lapachol. Bioinorg. Chem. Appl. 2013, 2013, 961783. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kumar, I. Making Bispirin: Synthesis, Structure and Activity against Helicobacter pylori of Bismuth(III) Acetylsalicylate. Chem. Commun. 2013, 49, 2870. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Reis, D.C.; Da Silva, J.G.; Souza-Fagundes, E.M.; Baran, E.J.; Beraldo, H. Investigation on the Bioactivities of Clioquinol and Its Bismuth(III) and Platinum(II,IV) Complexes. Polyhedron 2013, 63, 28–35. [Google Scholar] [CrossRef]
- Oliver, T.E.; Piantavigna, S.; Andrews, P.C.; Holt, S.A.; Dillon, C.T. Interactions of Non-Steroidal Anti-Inflammatory Drugs and Their Bismuth Analogues (BiNSAIDs) with Biological Membrane Mimics at Physiological PH. Langmuir 2021, 37, 1337–1352. [Google Scholar] [CrossRef]
- Oliveira, A.; Ferreira, J.; Farias, L.; Magalhães, P.; Teixeira, L.; Beraldo, H. Antimicrobial Effects of Silver(I) and Bismuth(III) Complexes with Secnidazole-Derived Schiff Base Ligands: The Role of the Nitro Group Reduction. J. Braz. Chem. Soc. 2019, 30, 2299–2307. [Google Scholar] [CrossRef]
- Frei, A.; Elliott, A.G.; Kan, A.; Dinh, H.; Bräse, S.; Bruce, A.E.; Bruce, M.R.; Chen, F.; Humaidy, D.; Jung, N.; et al. Metal Complexes as Antifungals? From a Crowd-Sourced Compound Library to the First In Vivo Experiments. JACS Au 2022, 2, 2277–2294. [Google Scholar] [CrossRef]
- Bouz, G.; Doležal, M. Advances in Antifungal Drug Development: An Up-To-Date Mini Review. Pharmaceuticals 2021, 14, 1312. [Google Scholar] [CrossRef]
- Burrell, R.E.; Corke, C.T.; Goel, R.G. Fungitoxicity of Organoantimony and Organobismuth Compounds. J. Agric. Food Chem. 1983, 31, 85–88. [Google Scholar] [CrossRef]
- Murafuji, T.; Miyoshi, Y.; Ishibashi, M.; Mustafizur Rahman, A.F.M.; Sugihara, Y.; Miyakawa, I.; Uno, H. Antifungal Activity of Organobismuth Compounds against the Yeast Saccharomyces cerevisiae: Structure–Activity Relationship. J. Inorg. Biochem. 2004, 98, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, H.P.S.; Shaik, N.M.; Singh, U.P. Synthetic, Spectroscopic and Antimicrobial Studies of Bis(Dialkyldithiocarbamato)Diorganodithiophosphatobismuth(III) Complexes. Appl. Organomet. Chem. 2005, 19, 1132–1139. [Google Scholar] [CrossRef]
- Solanki, J.S.; Tripathi, U.N.; Bhardwaj, A.; Thapak, T.R. Synthesis, Spectral Study and Antimicrobial Activity of Bismuth(III) 3(2′-Hydroxyphenyl)-5-(4-Substituted Phenyl) Pyrazolinates. J. Coord. Chem. 2008, 61, 4025–4032. [Google Scholar] [CrossRef]
- Mahajan, K.; Swami, M.; Singh, R.V. Microwave Synthesis, Spectral Studies, Antimicrobial Approach, and Coordination Behavior of Antimony(III) and Bismuth(III) Compounds with Benzothiazoline. Russ. J. Coord. Chem. 2009, 35, 179–185. [Google Scholar] [CrossRef]
- Solanki, J.S.; Thapak, T.R.; Bhardwaj, A.; Tripathi, U.N. Synthesis, Structural Characterization, and in Vitro Antimicrobial Properties of Salicylate and Pyrazoline Complexes of Bismuth(III). J. Coord. Chem. 2011, 64, 369–376. [Google Scholar] [CrossRef]
- El-Metwaly, N.M.; Refat, M.S. Elaborated 1H NMR Study for the Ligitional Behavior of Two Thiosemicarbazide Derivatives towards Some Heavy Metals (Sn(II), Sb(III), Pb(II) and Bi(III)), Thermal, Antibacterial and Antifungal Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Murafuji, T.; Kitagawa, K.; Yoshimatsu, D.; Kondo, K.; Ishiguro, K.; Tsunashima, R.; Miyakawa, I.; Mikata, Y. Heterocyclic Bismuth Carboxylates Based on a Diphenyl Sulfone Scaffold: Synthesis and Antifungal Activity against Saccharomyces cerevisiae. Eur. J. Med. Chem. 2013, 63, 531–535. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Silva, N.F.; Da Silva, J.G.; De Miranda, L.F.; Romeiro, C.F.D.; Souza-Fagundes, E.M.; Mendes, I.C.; Beraldo, H. Investigation on the Pharmacological Profile of 2,6-Diacetylpyridine Bis(Benzoylhydrazone) Derivatives and Their Antimony(III) and Bismuth(III) Complexes. Eur. J. Med. Chem. 2012, 53, 98–106. [Google Scholar] [CrossRef]
- Mehmood, M.; Imtiaz-ud-Din; Abbas, S.; Azam, S.S.; Ihsan-ul-Haq; Tahir, M.N.; Parvaiz, N.; Tameez Ud Din, A. Bioactive Heteroleptic Bismuth(V) Carboxylates: Synthetic Stratagem, Characterization and Binding Pattern Validation. J. Organomet. Chem. 2020, 921, 121357. [Google Scholar] [CrossRef]
- Cabral-Romero, C.; Hernandez-Delgadillo, R.; Velasco-Arias, D.; Martinez-Sanmiguel, J.J.; Diaz, D.; Zumeta-Dubé, I.; Arevalo-Niño, K. Bismuth Oxide Aqueous Colloidal Nanoparticles Inhibit Candida albicans Growth and Biofilm Formation. Int. J. Nanomed. 2013, 8, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Murafuji, T.; Tomura, M.; Ishiguro, K.; Miyakawa, I. Activity of Antifungal Organobismuth(III) Compounds Derived from Alkyl Aryl Ketones against S. Cerevisiae: Comparison with a Heterocyclic Bismuth Scaffold Consisting of a Diphenyl Sulfone. Molecules 2014, 19, 11077–11095. [Google Scholar] [CrossRef] [PubMed]
- Raheel, A.; Imtiaz-ud-Din; Andleeb, S.; Ramadan, S.; Tahir, M.N. Synthesis and Structural Characterization of New Bioactive Ligands and Their Bi(III) Derivatives. Appl. Organomet. Chem. 2017, 31, e3632. [Google Scholar] [CrossRef]
- Murafuji, T.; Hafizur Rahman, A.F.M.; Yamashita, K.; Narita, M.; Ishiguro, K.; Kamijo, S.; Miyakawa, I.; Mikata, Y. Synthesis and Antifungal Activities of Pyridine Bioisosteres of a Bismuth Heterocycle Derived from Diphenyl Sulfone. Heterocycles 2018, 96, 1037. [Google Scholar] [CrossRef]
- Das, P.E.; Majdalawieh, A.F.; Abu-Yousef, I.A.; Narasimhan, S.; Poltronieri, P. Use of A Hydroalcoholic Extract of Moringa oleifera Leaves for the Green Synthesis of Bismuth Nanoparticles and Evaluation of Their Anti-Microbial and Antioxidant Activities. Materials 2020, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Boadi, N.O.; Degbevi, M.; Saah, S.A.; Badu, M.; Borquaye, L.S.; Kortei, N.K. Antimicrobial Properties of Metal Piperidine Dithiocarbamate Complexes against Staphylococcus aureus and Candida albicans. Sci. Afr. 2021, 12, e00846. [Google Scholar] [CrossRef]
- Stevanović, N.; Mazzeo, P.P.; Bacchi, A.; Matić, I.Z.; Đorđić Crnogorac, M.; Stanojković, T.; Vujčić, M.; Novaković, I.; Radanović, D.; Šumar-Ristović, M.; et al. Synthesis, Characterization, Antimicrobial and Cytotoxic Activity and DNA-Binding Properties of d-Metal Complexes with Hydrazones of Girard’s T and P Reagents. JBIC J. Biol. Inorg. Chem. 2021, 26, 863–880. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zohra, T.; Alam, M.M.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Phytosynthesis of BiVO4 Nanorods Using Hyphaene thebaica for Diverse Biomedical Applications. AMB Express 2019, 9, 200. [Google Scholar] [CrossRef]
- Vazquez-Munoz, R.; Lopez, F.D.; Lopez-Ribot, J.L. Bismuth Nanoantibiotics Display Anticandidal Activity and Disrupt the Biofilm and Cell Morphology of the Emergent Pathogenic Yeast Candida auris. Antibiotics 2020, 9, 461. [Google Scholar] [CrossRef]
- Abdel-Moniem, S.M.; El-Liethy, M.A.; Ibrahim, H.S.; Ali, M.E.M. Innovative Green/Non-Toxic Bi2S3@g-C3N4 Nanosheets for Dark Antimicrobial Activity and Photocatalytic Depollution: Turnover Assessment. Ecotoxicol. Environ. Saf. 2021, 226, 112808. [Google Scholar] [CrossRef]
- World Health Organization. Neglected Tropical Diseases. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 1 May 2023).
- Carvalho, S.H.; Frézard, F.; Pereira, N.P.; Moura, A.S.; Ramos, L.M.Q.C.; Carvalho, G.B.; Rocha, M.O.C. American Tegumentary Leishmaniasis in Brazil: A Critical Review of the Current Therapeutic Approach with Systemic Meglumine Antimoniate and Short-Term Possibilities for an Alternative Treatment. Trop. Med. Int. Health 2019, 24, 380–391. [Google Scholar] [CrossRef]
- Andrews, P.C.; Frank, R.; Junk, P.C.; Kedzierski, L.; Kumar, I.; Maclellan, J.G. Anti-Leishmanial Activity of Homo- and Heteroleptic Bismuth(III) Carboxylates. J. Inorg. Biochem. 2011, 105, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.C.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Anti-Leishmanial Activity of Novel Homo- and Heteroleptic Bismuth(III) Thiocarboxylates. Aust. J. Chem. 2013, 66, 1297. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.; Monte-Neto, R.; Reis, P.; Fernandes, N.; Speziali, N.; Melo, M.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef]
- Duffin, R.N.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Comparative Stability, Toxicity and Anti-Leishmanial Activity of Triphenyl Antimony(v) and Bismuth(v) α-Hydroxy Carboxylato Complexes. Dalton Trans. 2018, 47, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Imtiaz-ud-Din; Rauf, M.K.; Azam, S.S.; Haq, I.; Tahir, M.N.; Ahmad, S. Bioactive Heteroleptic Bismuth(V) Complexes: Synthesis, Structural Analysis and Binding Pattern Validation. Appl. Organomet. Chem. 2019, 33, e5061. [Google Scholar] [CrossRef]
- Anamika, A.; Singh, R.; Manar, K.K.; Yadav, C.L.; Kumar, A.; Singh, R.K.; Drew, M.G.B.; Singh, N. Impact of Substituents on the Crystal Structures and Anti-Leishmanial Activity of New Homoleptic Bi(III) Dithiocarbamates. New J. Chem. 2019, 43, 16921–16931. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Islam, A.; Da Silva, J.; Berbet, F.; da Silva, S.; Rodrigues, B.; Beraldo, H.; Melo, M.; Frézard, F.; Demicheli, C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules 2014, 19, 6009–6030. [Google Scholar] [CrossRef]
- Ong, Y.C.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Stability and Toxicity of Heteroleptic Organometallic Bi(V) Complexes towards Leishmania major. Dalton Trans. 2014, 43, 12904–12916. [Google Scholar] [CrossRef]
- Loh, A.; Ong, Y.C.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Bismuth(III) α-Hydroxy Carboxylates: Highly Selective Toxicity of Glycolates towards Leishmania major. JBIC J. Biol. Inorg. Chem. 2015, 20, 1193–1203. [Google Scholar] [CrossRef]
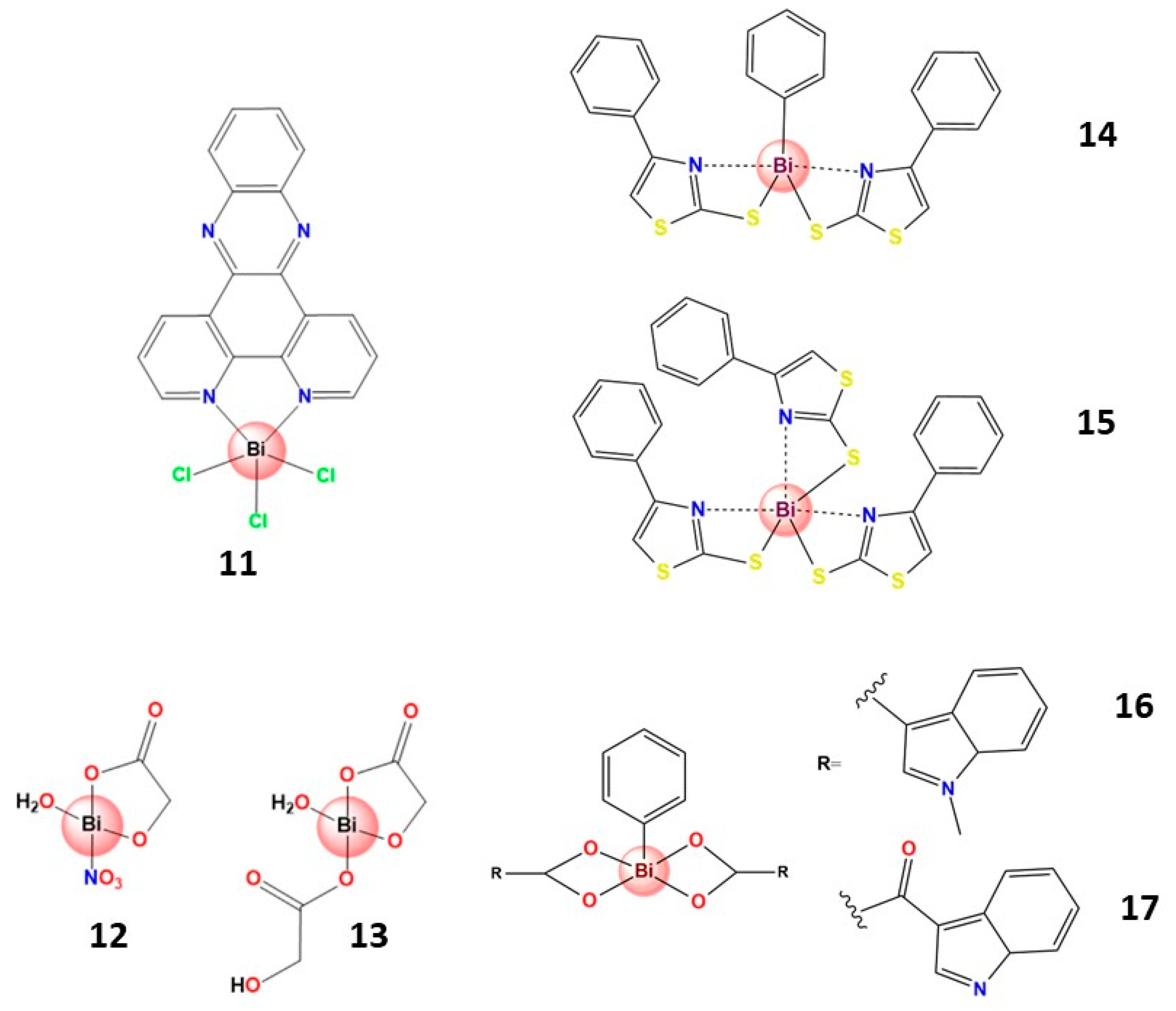
- Luqman, A.; Blair, V.L.; Brammananth, R.; Crellin, P.K.; Coppel, R.L.; Kedzierski, L.; Andrews, P.C. Homoleptic and Heteroleptic Bismuth(III) Thiazole–Thiolates and the Influence of Ring Substitution on Their Antibacterial and Antileishmanial Activity. Eur. J. Inorg. Chem. 2015, 2015, 725–733. [Google Scholar] [CrossRef]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Kedzierski, L.; Andrews, P.C. Structural Influences on the Activity of Bismuth(III) Indole-Carboxylato Complexes towards Helicobacter pylori and Leishmania. J. Inorg. Biochem. 2017, 177, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Blair, V.L.; Kedzierski, L.; Tuck, K.L.; Andrews, P.C. Stability and Toxicity of Tris-Tolyl Bismuth(v) Dicarboxylates and Their Biological Activity towards Leishmania major. Dalton Trans. 2015, 44, 18215–18226. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Imtiaz-ud-Din; Rauf, M.K.; Azam, S.S.; Haq, I.; Tahir, M.N.; Zaman, N. Structural Characterization and Antileishmanial Activity of Newly Synthesized Organo-Bismuth(V) Carboxylates: Experimental and Molecular Docking Studies. JBIC J. Biol. Inorg. Chem. 2022, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Paixão, D.A.; Lopes, C.D.; Carneiro, Z.A.; Sousa, L.M.; de Oliveira, L.P.; Lopes, N.P.; Pivatto, M.; Chaves, J.D.S.; de Almeida, M.V.; Ellena, J.; et al. In Vitro Anti-Trypanosoma cruzi Activity of Ternary Copper(II) Complexes and in Vivo Evaluation of the Most Promising Complex. Biomed. Pharmacother. 2019, 109, 157–166. [Google Scholar] [CrossRef]
- Paixão, D.A.; de Oliveira, L.P.; Maia, P.I.d.S.; Deflon, V.M.; Carneiro, Z.A.; de Almeida, K.J.; Lopes, N.P.; Pivatto, M.; Chaves, J.D.S.; de Albuquerque, S.; et al. Crystal Structure of Two New Polymeric Copper(II) Complexes Active against Trypanosoma cruzi. J. Saudi Chem. Soc. 2018, 22, 809–815. [Google Scholar] [CrossRef]
- Urbina, J.A. Specific Chemotherapy of Chagas Disease: Relevance, Current Limitations and New Approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.A.; Recio-Despaigne, A.A.; Ferreira, I.P.; Diniz, R.; Sousa, K.A.F.; Bastos, T.M.; Pereira Soares, M.B.; Moreira, D.R.M.; Beraldo, H. Investigation of the Antitrypanosomal Effects of 2-Formyl-8-Hydroxyquinoline-Derived Hydrazones and Their Antimony(III) and Bismuth(III) Complexes. New J. Chem. 2019, 43, 18996–19002. [Google Scholar] [CrossRef]
- Neamati, F.; Kodori, M.; Feizabadi, M.M.; Abavisani, M.; Barani, M.; Khaledi, M.; Moghadaszadeh, M.; Azadbakht, M.K.; Zeinali, M.; Fathizadeh, H. Bismuth Nanoparticles against Microbial Infections. Nanomedicine 2023, 17, 2109–2122. [Google Scholar] [CrossRef]
- Rodríguez-Luis, O.E.; Hernández-Delgadillo, R.; Pineda-Aguilar, N.; Vargas-Villarreal, J.; González-Salazar, F.; Garza-González, J.N.; Hernández-García, M.E.; Chellam, S.; Cabral-Romero, C. Effect of Bismuth Lipophilic Nanoparticles (BisBAL NPs) on Trichomonas Vaginalis Growth. J. Nanosci. Nanotechnol. 2017, 17, 4618–4622. [Google Scholar] [CrossRef]
- Souza, W.A.; Ramos, L.M.S.; de Almeida, A.M.; Tezuka, D.Y.; Lopes, C.D.; Moreira, M.B.; Zanetti, R.D.; Netto, A.V.G.; Ferreira, F.B.; de Oliveira, R.J.; et al. Preparation, Cytotoxic Activity and DNA Interaction Studies of New Platinum(II) Complexes with 1,10-Phenanthroline and 5-Alkyl-1,3,4-Oxadiazol-2(3H)-Thione Derivatives. J. Inorg. Biochem. 2022, 237, 111993. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, R.K. Metal Complexes in Medicine: An Overview and Update from Drug Design Perspective. Cancer Ther. Oncol. Int. J. 2019, 14, 555883. [Google Scholar] [CrossRef]
- Yun, U.-J.; Lee, J.-H.; Shim, J.; Yoon, K.; Goh, S.-H.; Yi, E.H.; Ye, S.-K.; Lee, J.-S.; Lee, H.; Park, J.; et al. Anti-Cancer Effect of Doxorubicin Is Mediated by Downregulation of HMG-Co A Reductase via Inhibition of EGFR/Src Pathway. Lab. Investig. 2019, 99, 1157–1172. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Chan, S.; Wang, Y.; Zhang, Y.; Zuo, Z.; Chan, G.C.-F.; Li, H.; Sun, H. Bismuth Porphyrin Antagonizes Cisplatin-Induced Nephrotoxicity via Unexpected Metallothionein-Independent Mechanisms. iScience 2020, 23, 101054. [Google Scholar] [CrossRef] [PubMed]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common Themes in Antimicrobial and Anticancer Drug Resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef]
- Iuchi, K.; Yagura, T. Heterocyclic Organobismuth (III) Compounds Containing an Eight-Membered Ring: Inhibitory Effects on Cell Cycle Progression. Toxicol. Vitr. 2018, 50, 172–178. [Google Scholar] [CrossRef]
- Chan, P.F.; Ang, K.P.; Hamid, R.A. A Bismuth Diethyldithiocarbamate Compound Induced Apoptosis via Mitochondria-Dependent Pathway and Suppressed Invasion in MCF-7 Breast Cancer Cells. BioMetals 2021, 34, 365–391. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Synthesis, Characterization, Cytotoxicity and Antimicrobial Studies on Bi(III) Dithiocarbamate Complexes Containing Furfuryl Group and Their Use for the Preparation of Bi2O3 nanoparticles. Polyhedron 2017, 121, 70–79. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Manos, M.J.; Tasiopoulos, A.J.; Owczarzak, A.M.; Kubicki, M.; Hadjikakou, S.K. Synthesis, Characterization and Biological Activity of Antimony(III) or Bismuth(III) Chloride Complexes with Dithiocarbamate Ligands Derived from Thiuram Degradation. Polyhedron 2014, 67, 89–103. [Google Scholar] [CrossRef]
- Arda, M.; Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Manoli, M.; Tasiopoulosd, A.J.; Hadjikakou, S.K. Novel Bismuth Compounds: Synthesis, Characterization and Biological Activity against Human Adenocarcinoma Cells. RSC Adv. 2016, 6, 29026–29044. [Google Scholar] [CrossRef]
- Das, A.; Das, A.; Banik, B.K. Influence of Dipole Moments on the Medicinal Activities of Diverse Organic Compounds. J. Indian Chem. Soc. 2021, 98, 100005. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lei, J.; Tang, L.W.; Peng, Y.; Au, C.T.; Chen, Y.; Yin, S.F. Studies on the Cytotoxicity and Anticancer Performance of Heterocyclic Hypervalent Organobismuth(III) Compounds. Eur. J. Med. Chem. 2017, 139, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Douke, M.; Nakamura, T.; Ochiai, Y.; Kakusawa, N.; Yasuike, S.; Kurita, J.; Yamamoto, C.; Kawahata, M.; Yamaguchi, K.; et al. A Novel Organobismuth Compound, 1-[(2-Di-p-Tolylbismuthanophenyl)Diazenyl] Pyrrolidine, Induces Apoptosis in the Human Acute Promyelocytic Leukemia Cell Line NB4 via Reactive Oxygen Species. J. Inorg. Biochem. 2012, 117, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Rodrigues, B.L.; Marzano, I.M.; Perreira-Maia, E.C.; Dittz, D.; Lopes, M.T.P.; Ishfaq, M.; Frezard, F.; Demicheli, C. Cytotoxicity and Apoptotic Activity of Novel Organobismuth(V) and Organoantimony(V) Complexes in Different Cancer Cell Lines. Eur. J. Med. Chem. 2016, 109, 254–267. [Google Scholar] [CrossRef]
- Yi, N.; Jiang, Z.J.; Wang, L.; Luo, P.; Peng, F. Crystal Structure and Anti-Breast Cancer Activity Evaluation of a Nanosized Bismuth(V)-Containing Coordination Complex Based on the F-Decorated Ligand. Inorg. Nano-Metal Chem. 2020, 50, 562–568. [Google Scholar] [CrossRef]
- Cui, L.; Bi, C.; Fan, Y.; Li, X.; Meng, X.; Zhang, N.; Zhang, Z. Synthesis, Crystal Structures, DNA Interaction and Anticancer Activity of Organobismuth(V) Complexes. Inorganica Chim. Acta 2015, 437, 41–46. [Google Scholar] [CrossRef]
- Zhang, N.; Tai, Y.; Li, M.; Ma, P.; Zhao, J.; Niu, J. Main Group Bismuth(III), Gallium(III) and Diorganotin(IV) Complexes Derived from Bis(2-Acetylpyrazine)Thiocarbonohydrazone: Synthesis, Crystal Structures and Biological Evaluation. Dalton Trans. 2014, 43, 5182–5189. [Google Scholar] [CrossRef]
- Li, Y.K.; Yang, M.; Li, M.X.; Yu, H.; Wu, H.C.; Xie, S.Q. Synthesis, Crystal Structure and Biological Evaluation of a Main Group Seven-Coordinated Bismuth(III) Complex with 2-Acetylpyridine N4-Phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2013, 23, 2288–2292. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.T.; Zhao, M.; Lu, Y.L.; Li, M.X.; Zhang, Y.H. Bismuth(III) and Diorganotin(IV) Complexes of Bis(2-Acetylpyridine) Thiocarbonohydrazone: Synthesis, Characterization, and Apoptosis Mechanism of Action In Vitro. Polyhedron 2018, 155, 254–260. [Google Scholar] [CrossRef]
- Iuchi, K.; Hatano, Y.; Yagura, T. Heterocyclic Organobismuth(III) Induces Apoptosis of Human Promyelocytic Leukemic Cells through Activation of Caspases and Mitochondrial Perturbation. Biochem. Pharmacol. 2008, 76, 974–986. [Google Scholar] [CrossRef]
- Ishak, D.H.A.; Ooi, K.K.; Ang, K.P.; Akim, A.M.; Cheah, Y.K.; Nordin, N.; Halim, S.N.B.A.; Seng, H.L.; Tiekink, E.R.T. A Bismuth Diethyldithiocarbamate Compound Promotes Apoptosis in HepG2 Carcinoma, Cell Cycle Arrest and Inhibits Cell Invasion through Modulation of the NF-ΚB Activation Pathway. J. Inorg. Biochem. 2014, 130, 38–51. [Google Scholar] [CrossRef]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-Apoptotic Cell Death-Based Cancer Therapy: Molecular Mechanism, Pharmacological Modulators, and Nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef]
- Iuchi, K.; Shirai, S.; Tasaki, Y.; Hisatomi, H. Heterocyclic Organobismuth(III) Compound Induces Nonapoptotic Cell Death via Lipid Peroxidation. Anti-Cancer Drugs 2020, 31, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Marzano, I.M.; Tomco, D.; Staples, R.J.; Lizarazo-Jaimes, E.H.; Gomes, D.A.; Bucciarelli-Rodriguez, M.; Guerra, W.; de Souza, Í.P.; Verani, C.N.; Pereira Maia, E.C. Dual Anticancer and Antibacterial Activities of Bismuth Compounds Based on Asymmetric [NN′O] Ligands. J. Inorg. Biochem. 2021, 222, 111522. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.P.; Piló, E.D.L.; Recio-Despaigne, A.A.; Da Silva, J.G.; Ramos, J.P.; Marques, L.B.; Prazeres, P.H.D.M.; Takahashi, J.A.; Souza-Fagundes, E.M.; Rocha, W.; et al. Bismuth(III) Complexes with 2-Acetylpyridine- and 2-Benzoylpyridine-Derived Hydrazones: Antimicrobial and Cytotoxic Activities and Effects on the Clonogenic Survival of Human Solid Tumor Cells. Bioorg. Med. Chem. 2016, 24, 2988–2998. [Google Scholar] [CrossRef]
- Li, C.H.; Jiang, J.H.; Lei, Y.H.; Li, X.; Yao, F.H.; Ji, M.H.; Zhang, K.W.; Tao, L.M.; Ye, L.J.; Li, Q.G. Design, Synthesis, and Biological Evaluation of Dinuclear Bismuth(III) Complexes with Isoniazid-Derived Schiff Bases. J. Inorg. Biochem. 2022, 235, 111931. [Google Scholar] [CrossRef]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mukherjee, S. Cancer Therapy Using Antibiotics. J. Cancer Ther. 2015, 6, 849–858. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, Y.; Feng, K.; Zong, T.; Cao, P.; Xiong, F.; Guo, N.; Li, Y.; Miao, Y.; Tong, X.; et al. Potent Anticancer Activity of a New Bismuth (III) Complex against Human Lung Cancer Cells. J. Inorg. Biochem. 2017, 168, 18–26. [Google Scholar] [CrossRef]
- Li, M.X.; Yang, M.; Niu, J.Y.; Zhang, L.Z.; Xie, S.Q. A Nine-Coordinated Bismuth(III) Complex Derived from Pentadentate 2,6-Diacetylpyridine Bis(4N-Methylthiosemicarbazone): Crystal Structure and Both in Vitro and in Vivo Biological Evaluation. Inorg. Chem. 2012, 51, 12521–12526. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, Y.; Tong, X.; Yang, Y.; Tao, H.; Zong, T.; Feng, K.; Jia, P.; Cao, P.; Guo, N.; et al. Potential Anti-Cancer Activity of a Novel Bi(III) Containing Thiosemicarbazone Derivative. Inorg. Chem. Commun. 2016, 73, 138–141. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Yang, M.; Li, Y.; Zhang, L.; Xie, S. One Dodecahedral Bismuth(Iii) Complex Derived from 2-Acetylpyridine N(4)-Pyridylthiosemicarbazone: Synthesis, Crystal Structure and Biological Evaluation. Dalton Trans. 2012, 41, 12882–12887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; An, G.Y.; Yang, M.; Li, M.X.; Zhu, X.F. Synthesis, Characterization, Crystal Structure and Biological Activities of the Unusual Main Group 8-Coordinate Bismuth (III) Complex Derived from 2-Acetylpyrazine N4- Pyridylthiosemicarbazone. Inorg. Chem. Commun. 2012, 20, 37–40. [Google Scholar] [CrossRef]
- Li, C.H.; Ji, M.H.; Zhang, K.W.; Sun, S.Y.; Jiang, J.H. Dinuclear Bismuth(III) Complex Constructed by Isoniazid-Derived Schiff Base: Synthesis, Crystal Structure, and Biological Activity. Appl. Organomet. Chem. 2022, 36, e6876. [Google Scholar] [CrossRef]
- De Carvalho, A.B.; de Souza, Í.P.; de Andrade, L.M.; Binatti, I.; Pedroso, E.F.; Krambrock, K.; Oliveira, W.X.C.; Pereira-Maia, E.C.; Silva-Caldeira, P.P. Novel Copper(II) Coordination Polymer Containing the Drugs Nalidixic Acid and 8-Hydroxyquinoline: Evaluation of the Structural, Magnetic, Electronic, and Antitumor Properties. Polyhedron 2018, 156, 312–319. [Google Scholar] [CrossRef]
- Li, H.; Lai, C.S.; Wu, J.; Ho, P.C.; de Vos, D.; Tiekink, E.R.T. Cytotoxicity, Qualitative Structure-Activity Relationship (QSAR), and Anti-Tumor Activity of Bismuth Dithiocarbamate Complexes. J. Inorg. Biochem. 2007, 101, 809–816. [Google Scholar] [CrossRef] [PubMed]
- López-Cardoso, M.; Tlahuext, H.; Pérez-Salgado, M.; Vargas-Pineda, D.G.; Román-Bravo, P.P.; Cotero-Villegas, A.M.; Acevedo-Quiroz, M.; Razo-Hernández, R.S.; Alvarez-Fitz, P.; Mendoza-Catalán, M.A.; et al. Synthesis, Crystal Structure, Antibacterial, Antiproliferative and QSAR Studies of New Bismuth(III) Complexes of Pyrrolidineditiocarbamate of Dithia-Bismolane and Bismane, Oxodithia- and Trithia-Bismocane. J. Mol. Struct. 2020, 1217, 128456. [Google Scholar] [CrossRef]
- Ucar, O.; Grześkiewicz, A.M.; Banti, C.; Hadjikakou, S.K.; Ozturk, I.I. Structural Characterization and Biological Evaluation of Antimony(III) and Bismuth(III) Complexes with Imidazolidine-2-Thione. J. Mol. Struct. 2021, 1235, 130270. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Hadjikakou, S.K.; Panagiotou, N.; Tasiopoulos, A.J. Bismuth(III) Halide Complexes of Aromatic Thiosemicarbazones: Synthesis, Structural Characterization and Biological Evaluation. Polyhedron 2021, 208, 115388. [Google Scholar] [CrossRef]
- Aygun, O.; Grześkiewicz, A.M.; Banti, C.N.; Hadjikakou, S.K.; Kubicki, M.; Ozturk, I.I. Monomeric Octahedral Bismuth(III) Benzaldehyde-N1-Alkyl Thiosemicarbazones: Synthesis, Characterization and Biological Properties. Polyhedron 2022, 215, 115683. [Google Scholar] [CrossRef]
- Turk, K.; Grześkiewicz, A.M.; Banti, C.N.; Hadjikakou, S.K.; Kubicki, M.; Ozturk, I.I. Synthesis, Characterization, and Biological Properties of Mono-, Di- and Poly-Nuclear Bismuth(III) Halide Complexes Containing Thiophene-2-Carbaldehyde Thiosemicarbazones. J. Inorg. Biochem. 2022, 237, 111987. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Turk, K.; Grześkiewicz, A.M.; Kubicki, M.; Banti, C.N.; Hadjikakou, S.K. Heteroleptic Six-Coordinate Bismuth(III) Complexes with 2-Acetylthiophene Thiosemicarbazones: Synthesis, Characterization, and Biological Properties. New J. Chem. 2023, 47, 12779–12789. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Hadjikakou, S.K.; Panagiotou, N.; Tasiopoulos, A.J. Structural Architectures and Biological Properties of Main Group Bismuth(III) Iodide Complexes with Heterocyclic Thioamides. Inorganica Chim. Acta 2019, 497, 119094. [Google Scholar] [CrossRef]
- Cakmak, M.; Ozturk, I.I.; Banti, C.N.; Manoli, M.; Moushi, E.; Tasiopoulos, A.J.; Grzeskiewicz, A.M.; Kubicki, M.; Hadjikakou, S.K. Bismuth(III) Bromide-Thioamide Complexes: Synthesis, Characterization and Cytotoxic Properties. Main Group Met. Chem. 2018, 41, 143–154. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, S.; Ouyang, R.; Yang, Y.; Tao, H.; Feng, K.; Zhang, X.; Xiong, F.; Guo, N.; Zong, T.; et al. Improvement in the Anticancer Activity of 6-Mercaptopurine via Combination with Bismuth(III). Chem. Pharm. Bull. 2016, 64, 1539–1545. [Google Scholar] [CrossRef]
- Almeida, J.C.L.; Amim, R.S.; Pessoa, C.; Lourenço, M.C.S.; Mendes, I.C.; Lessa, J.A. Bismuth(III) Complexes with Pyrazineformamide Thiosemicarbazones: Investigation on the Antimicrobial and Cytotoxic Effects. Polyhedron 2020, 189, 114709. [Google Scholar] [CrossRef]
- Li, M.X.; Zhang, L.Z.; Yang, M.; Niu, J.Y.; Zhou, J. Synthesis, Crystal Structures, in Vitro Biological Evaluation of Zinc(II) and Bismuth(III) Complexes of 2-Acetylpyrazine N(4)-Phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2012, 22, 2418–2423. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Akagi, K.; Yagura, T. Heterocyclic Organobismuth(III) Compound Targets Tubulin to Induce G2/M Arrest in HeLa Cells. J. Pharmacol. Sci. 2009, 109, 573–582. [Google Scholar] [CrossRef]
- Shang, X.; Zhao, B.; Xiang, G.; Guedes Da Silva, M.F.C.; Pombeiro, A.J.L. Dimeric Diorganotin(IV) Complexes with Arylhydrazones of β-Diketones: Synthesis, Structures, Cytotoxicity and Apoptosis Properties. RSC Adv. 2015, 5, 45053–45060. [Google Scholar] [CrossRef]
- Gorbach, S.L.; Cornick, N.A.; Silva, M. Effect of Bismuth Subsalicylate on Fecal Microflora. Clin. Infect. Dis. 1990, 12, S21–S23. [Google Scholar] [CrossRef]
- Brum, J.M.; Gibb, R.D.; Ramsey, D.L.; Balan, G.; Yacyshyn, B.R. Systematic Review and Meta-Analyses Assessment of the Clinical Efficacy of Bismuth Subsalicylate for Prevention and Treatment of Infectious Diarrhea. Dig. Dis. Sci. 2021, 66, 2323–2335. [Google Scholar] [CrossRef]
- Ward, R.L.; Sander, D.S.; Knowlton, D.R. In Vitro Activities of Bismuth Salts against Rotaviruses and Other Enteric Viruses. Antimicrob. Agents Chemother. 1985, 27, 306–308. [Google Scholar] [CrossRef]
- Yang, N.; Tanner, J.A.; Zheng, B.J.; Watt, R.M.; He, M.L.; Lu, L.Y.; Jiang, J.Q.; Shum, K.T.; Lin, Y.P.; Wong, K.L.; et al. Bismuth Complexes Inhibit the SARS Coronavirus. Angew. Chem. Int. Ed. 2007, 46, 6464–6468. [Google Scholar] [CrossRef]
- Yang, N.; Tanner, J.A.; Wang, Z.; Huang, J.D.; Zheng, B.J.; Zhu, N.; Sun, H. Inhibition of SARS Coronavirus Helicase by Bismuth Complexes. Chem. Commun. 2007, 42, 4413–4415. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.A.; Douangamath, A.; Yadzani, S.; Yosaatmadja, Y.; Aimon, A.; Brandão-neto, J.; Dunnett, L.; Gorrie-stone, T.; Skyner, R.; Fearon, D.; et al. Structure, Mechanism and Crystallographic Fragment Screening of the SARS-CoV-2 NSP13 Helicase. Nat. Commun. 2021, 12, 4848. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.A.; Watt, R.M.; Chai, Y.-B.; Lu, L.-Y.; Lin, M.C.; Peiris, J.S.M.; Poon, L.L.M.; Kung, H.-F.; Huang, J.-D. The Severe Acute Respiratory Syndrome (SARS) Coronavirus NTPase/Helicase Belongs to a Distinct Class of 5′ to 3′ Viral Helicases. J. Biol. Chem. 2003, 278, 39578–39582. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Harvey, I.; Sadler, P.J. Interactions of Bismuth Complexes with Metallothionein(II). J. Biol. Chem. 1999, 274, 29094–29101. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chan, J.F.W.; Wang, S.; Li, H.; Zhao, J.; Ip, T.K.Y.; Zuo, Z.; Yuen, K.Y.; Yuan, S.; Sun, H. Orally Administered Bismuth Drug Together with N -Acetyl Cysteine as a Broad-Spectrum Anti-Coronavirus Cocktail Therapy. Chem. Sci. 2022, 13, 2238–2248. [Google Scholar] [CrossRef]
- Tolbatov, I.; Storchi, L.; Marrone, A. Structural Reshaping of the Zinc-Finger Domain of the SARS-CoV-2 Nsp13 Protein Using Bismuth(III) Ions: A Multilevel Computational Study. Inorg. Chem. 2022, 61, 15664–15677. [Google Scholar] [CrossRef]
- De Paiva, R.E.F.; Marçal Neto, A.; Santos, I.A.; Jardim, A.C.G.; Corbi, P.P.; Bergamini, F.R.G. What Is Holding Back the Development of Antiviral Metallodrugs? A Literature Overview and Implications for SARS-CoV-2 Therapeutics and Future Viral Outbreaks. Dalton Trans. 2020, 49, 16004–16033. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Shukla, S.N.; Gaur, P. Metallo-Antiviral Aspirants: Answer to the Upcoming Virus Outbreak. Eur. J. Med. Chem. Rep. 2023, 8, 100104. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.M.M.N.; Costa, B.L.; Nunes Dourado, L.F.; Silva, R.O.; Silva-Cunha, A.; Santos, A.K.; Resende, R.R.; Faria, P.E.; Campos Rubio, J.C.; Goulart, G.A.C.; et al. Four Modified Sodium Alginate/Carboxymethylcellulose Blends for Prednisone Delivery. J. Appl. Polym. Sci. 2021, 138, 50383. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Li, Q.; Zhao, T.; Zhang, M.; Feng, W.; Takase, M.; Wu, X.; Zhou, Z.; Yang, L.; et al. Preparation, Characterization, and Anti-Helicobacter pylori Activity of Bi3+-Hericium Erinaceus Polysaccharide Complex. Carbohydr. Polym. 2014, 110, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.M.M.N.; de Carvalho, D.É.L.; Valente, V.M.M.; Rubio, J.C.C.; Faria, P.E.; Silva-Caldeira, P.P. Concomitant and Controlled Release of Furazolidone and Bismuth(III) Incorporated in a Cross-Linked Sodium Alginate-Carboxymethyl Cellulose Hydrogel. Int. J. Biol. Macromol. 2019, 126, 359–366. [Google Scholar] [CrossRef]
- Jiang, W.-X.; Qi, J.-R.; Liao, J.-S.; Wan, Z.-L.; Liang, W.-L.; Huang, J.-Y.; Cao, Y.; Xiao, J.; Yang, X.-Q. Structural Characterization of Pectin-Bismuth Complexes and Their Aggregation in Acidic Conditions. Int. J. Biol. Macromol. 2020, 154, 788–794. [Google Scholar] [CrossRef]
- Maliha, M.; Brammananth, R.; Dyson, J.; Coppel, R.L.; Werrett, M.; Andrews, P.C.; Batchelor, W. Biocompatibility and Selective Antibacterial Activity of a Bismuth Phosphinato-Nanocellulose Hydrogel. Cellulose 2021, 28, 4701–4718. [Google Scholar] [CrossRef]
- Dantas, K.C.F.; Rosário, J.d.S.; Silva-Caldeira, P.P. Polymeric Nanosystems Applied for Metal-Based Drugs and Photosensitizers Delivery: The State of the Art and Recent Advancements. Pharmaceutics 2022, 14, 1506. [Google Scholar] [CrossRef]
- Gomez, C.; Hallot, G.; Laurent, S.; Port, M. Medical Applications of Metallic Bismuth Nanoparticles. Pharmaceutics 2021, 13, 1793. [Google Scholar] [CrossRef]
- Gonçalves, I.K.V.; Oliveira, W.X.C.; de Almeida, F.B.; Marinho, M.V.; do Pim, W.D.; Silva-Caldeira, P.P. The Versatile Coordination Chemistry of 1,3-Benzenedicarboxylate in the Last 20 Years: An Investigation from the Coordination Modes to Spectroscopic Insights. Polyhedron 2021, 198, 115068. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E.; Mustansar, C. Application of MOF Materials as Drug Delivery Systems for Cancer Therapy and Dermal Treatment. Coord. Chem. Rev. 2022, 451, 214262. [Google Scholar] [CrossRef]
- Orellana-Tavra, C.; Köppen, M.; Li, A.; Stock, N.; Fairen-Jimenez, D. Biocompatible, Crystalline, and Amorphous Bismuth-Based Metal-Organic Frameworks for Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 5633–5641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. A Biocompatible Bismuth Based Metal-Organic Framework as Efficient Light-Sensitive Drug Carrier. J. Colloid Interface Sci. 2022, 617, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, Z.; Lan, X.; Tang, F.K.; Cheng, T.; Sun, H.; Leung, K.C.-F.; Li, X.; Jin, L. Rapid Synthesis of Bismuth-Organic Frameworks as Selective Antimicrobial Materials against Microbial Biofilms. Mater. Today Bio 2023, 18, 100507. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.D.; Jurcic, M.; Mahon, M.F.; Pierrat, S.; Roffe, G.W.; Windle, H.J.; Spencer, J. Bismuth Coordination Networks Containing Deferiprone: Synthesis, Characterisation, Stability and Antibacterial Activity. Dalton Trans. 2015, 44, 13814–13817. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The Versatile Biomedical Applications of Bismuth-Based Nanoparticles and Composites: Therapeutic, Diagnostic, Biosensing, and Regenerative Properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]




| Bismuth Compound | Brand Name | Clinical Use |
|---|---|---|
| Bismuth subgallate | – | Improving stool consistency and odor in colostomy and ileostomy patients |
| Bismuth oxide | – | Wound infection |
| Bismuth subnitrate | – | Irritable colon, gastric disorders, constipation |
| Bismuth phosphate, aluminate, and subcarbonate | – | Several gastrointestinal disorders |
| Sodium bismuth tartrate | – | Syphilis and canker sores |
| Colloidal bismuth subcitrate (CBS) | De-Nol® | Gastric and duodenal ulcers, non-ulcer dyspepsia, H. pylori |
| Bismuth subsalicylate (BSS) | Pepto-Bismol® | Dyspepsia, diarrhea, H. pylori |
| Ranitidine bismuth citrate (RBC) | Tritec®, Pylorid® | Gastric and duodenal ulcers, H. pylori |
| Tribromophenatobismuth(III) | Xeroform® | Antibiotic in wound dressings |
| Compounds | HepG2 | MCF-7 | HeLa | K562 | A549 | H460 | B16F10 | BT474 | MDA-MB-231 | SNU-16 | HCT116 | HL60 | THP-1 | NB4 | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 0.5 * | 1.26 * | – | – | – | – | – | – | – | – | – | – | – | – | [137] |
| 31–33 | – | – | 0.40 *–0.95 * | – | – | – | – | – | – | – | – | – | – | – | [124] |
| 34, 35 | – | 0.023 §; 0.043 § | 0.33 §; 0.19 § | – | – | – | – | – | – | – | – | – | – | – | [125] |
| 36–40 | – | 0.070 §–0.100 § | 0.05 §–0.30 § | – | – | – | – | – | – | – | – | – | – | – | [126] |
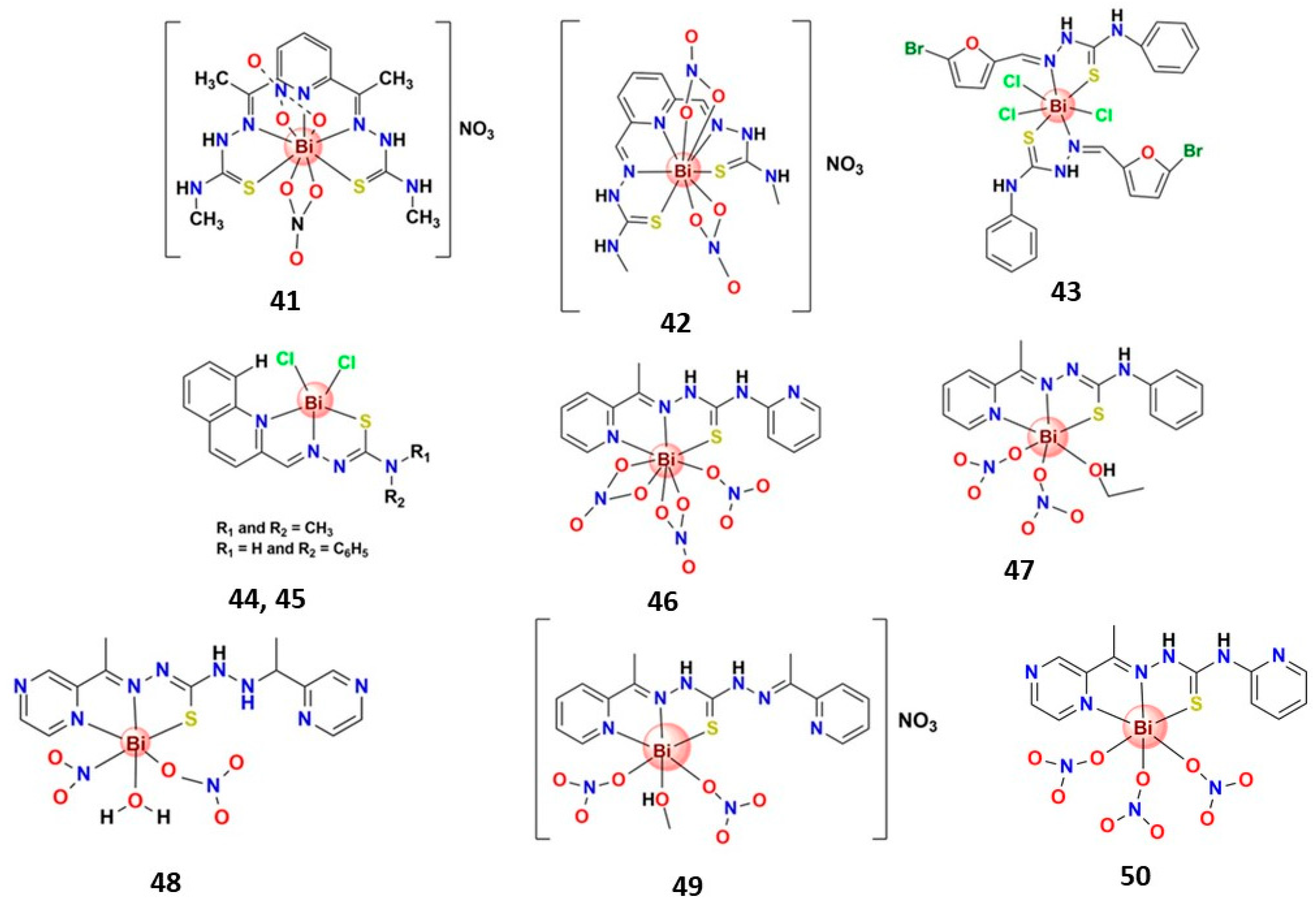
| 41 | – | – | – | – | 3.2 * | 3.6 * | – | – | – | – | – | – | – | – | [145] |
| 42 | – | – | – | 26.8 * | – | – | – | – | – | – | – | – | – | – | [146] |
| 43 | – | – | – | – | 16.4 * | 20.0 * | – | – | – | – | – | – | – | – | [147] |
| 44, 45 | – | – | – | – | 5.0 * | – | – | – | – | – | – | – | – | – | [62] |
| 46, 47 | 1.6 *, 3.4 * | – | 2.7 *, 9.0 * | 1.8 *, 5.2 * | – | – | – | – | – | – | 1.6 *, 5.57 * | – | – | – | [134,148] |
| 48, 49 | 2.96 *, 3.42 * | – | – | – | – | – | – | – | – | – | – | – | – | – | [133,135] |
| 50 | – | – | – | 1.6 * | – | – | – | – | – | – | – | – | – | – | [149] |
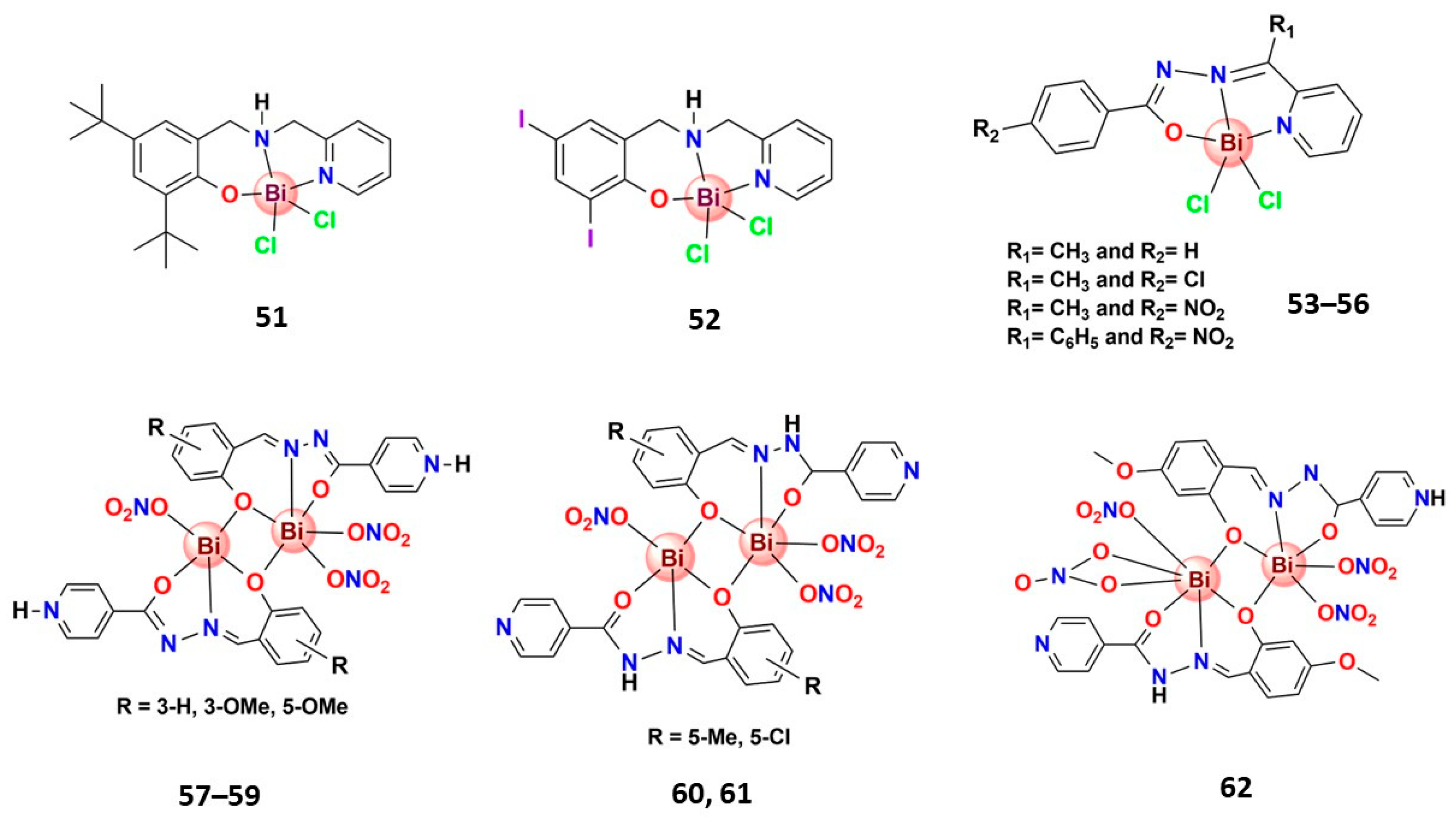
| 51, 52 | – | – | – | 0.30 †, 0.38 † | – | – | – | – | – | – | – | – | – | – | [140] |
| 53–56 | – | 0.23 §–10 § | – | – | – | – | – | – | – | – | 1.42 §–4.47 § | 0.20 §–1.18 § | 0.43 §–2.78 § | – | [141] |
| 3–6 | – | 0.27 §–1.07 § | – | – | – | – | – | – | – | – | 2.83 §–10.22 § | 0.09 §–0.23 § | – | – | [83] |
| 57–62 | – | – | – | – | – | – | – | – | – | 0.3–1.6 † | – | – | – | – | [142,150] |
| 63–65 | – | – | 4.27–4.85 ᴥ | 0.69–0.95 ᴥ | 1.15–4.29 ᴥ | – | – | – | – | – | – | 0.15–1.13 ᴥ | – | 0.05–0.25 ᴥ | [136] |
| 66–71 | – | – | – | – | 2.2 *–26.7 *; 1.8 §–18.6 §; 0.8 †–12.6 † | – | – | – | – | – | – | – | – | – | [128] |
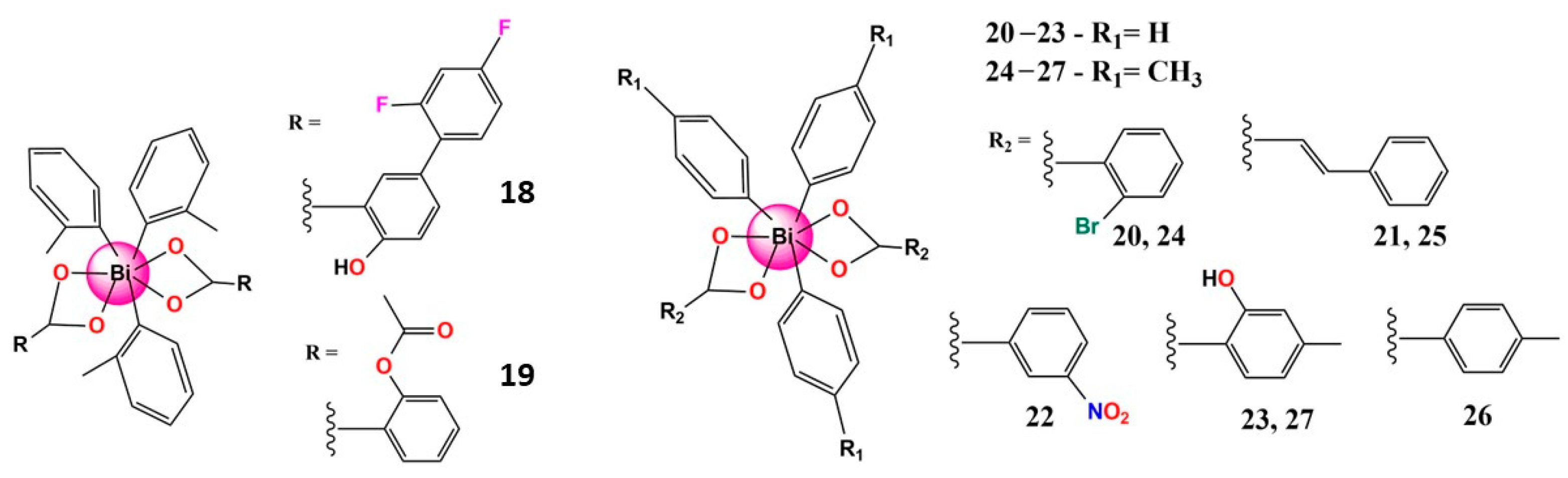
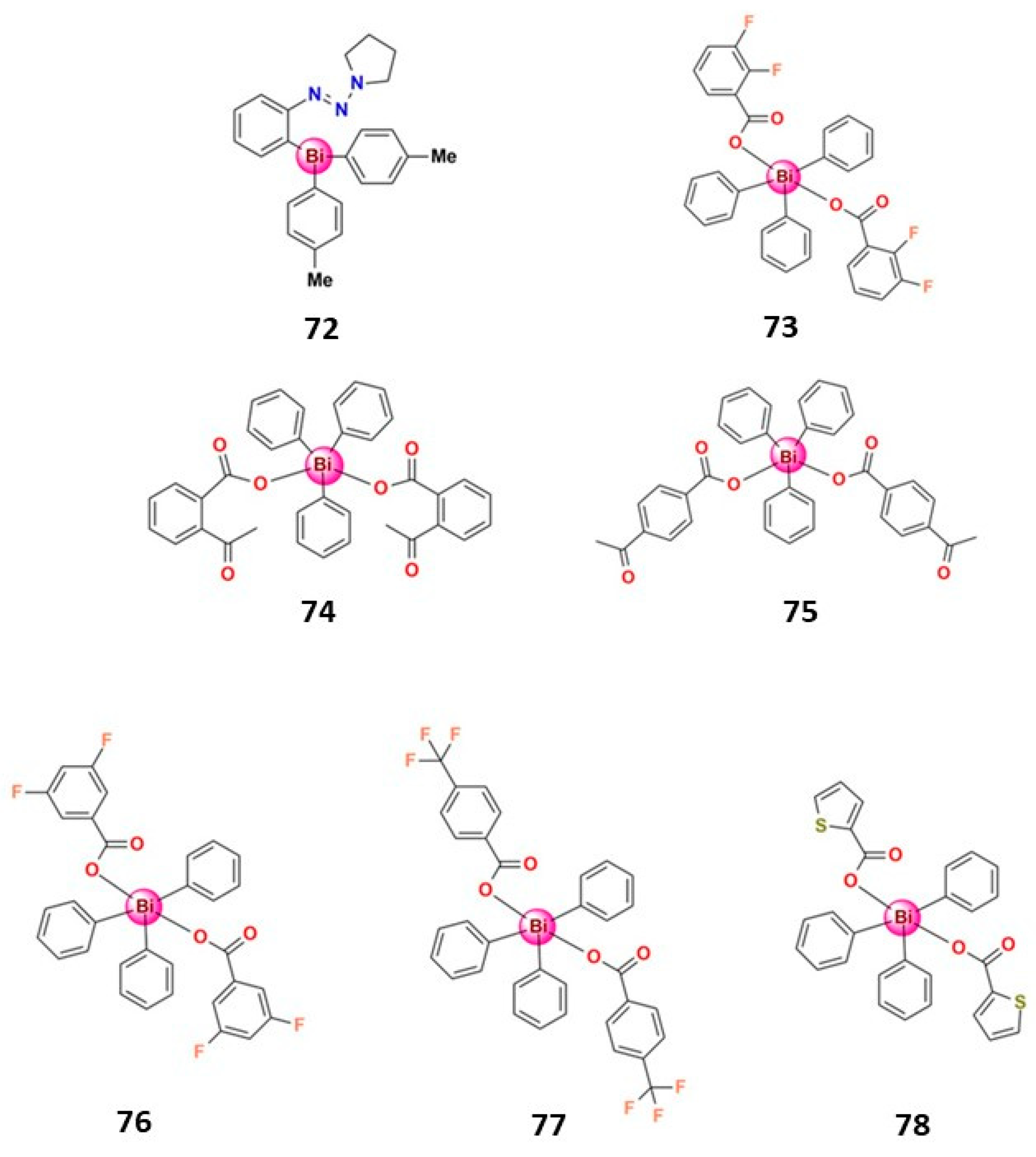
| 72 | – | – | 5.36 ᴥ | 3.33 ᴥ | 3.34 ᴥ | – | – | – | – | – | – | 1.44 ᴥ | – | 0.88 ᴥ | [129] |
| 73, 74 | – | – | – | 3.0 †; 19.6 † | – | – | 11.9 †; 11.4 † | – | – | – | – | – | – | – | [130] |
| 75 | – | – | – | – | – | – | – | 3.9 * | – | – | – | – | – | – | [131] |
| 76–78 | – | – | – | – | – | – | – | – | <20 * | – | – | – | – | – | [132] |
| CDDP | 152.0 * | 25.9 *; 5.5 §–6.8 § | 10.0 § | 1.10 † | (23.8–39.8) *; 10.7 §; 9.8 † | 46.2 * | – | – | – | – | >100 § | 0.05 § | >100 § | – | [125,128,137,141,151] |
| DOX | 4.6 * | 0.018 § | 1.12 § | – | – | – | – | – | – | 0.23 † | – | – | – | – | [125,137,142] |
| TMX | – | 0.046 § | – | – | – | – | – | – | – | – | – | – | – | – | [126] |
| MIT | 5.3 * | 5.98 * | – | – | – | – | – | – | – | 8.34 * | – | – | – | [148] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosário, J.d.S.; Moreira, F.H.; Rosa, L.H.F.; Guerra, W.; Silva-Caldeira, P.P. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules 2023, 28, 5921. https://doi.org/10.3390/molecules28155921
Rosário JdS, Moreira FH, Rosa LHF, Guerra W, Silva-Caldeira PP. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules. 2023; 28(15):5921. https://doi.org/10.3390/molecules28155921
Chicago/Turabian StyleRosário, Jânia dos Santos, Fábio Henrique Moreira, Lara Hewilin Fernandes Rosa, Wendell Guerra, and Priscila Pereira Silva-Caldeira. 2023. "Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome" Molecules 28, no. 15: 5921. https://doi.org/10.3390/molecules28155921
APA StyleRosário, J. d. S., Moreira, F. H., Rosa, L. H. F., Guerra, W., & Silva-Caldeira, P. P. (2023). Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules, 28(15), 5921. https://doi.org/10.3390/molecules28155921