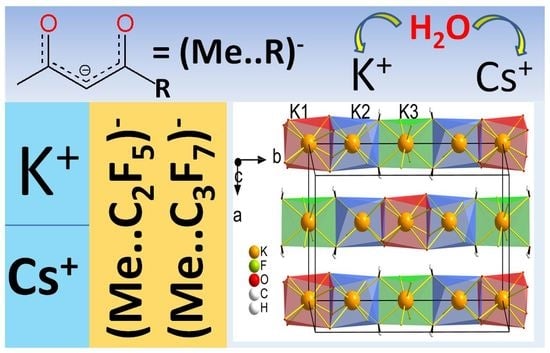
Potassium and Cesium Fluorinated β-Diketonates: Effect of a Cation and Terminal Substituent on Structural and Thermal Properties
Abstract
1. Introduction
2. Results and Discussion
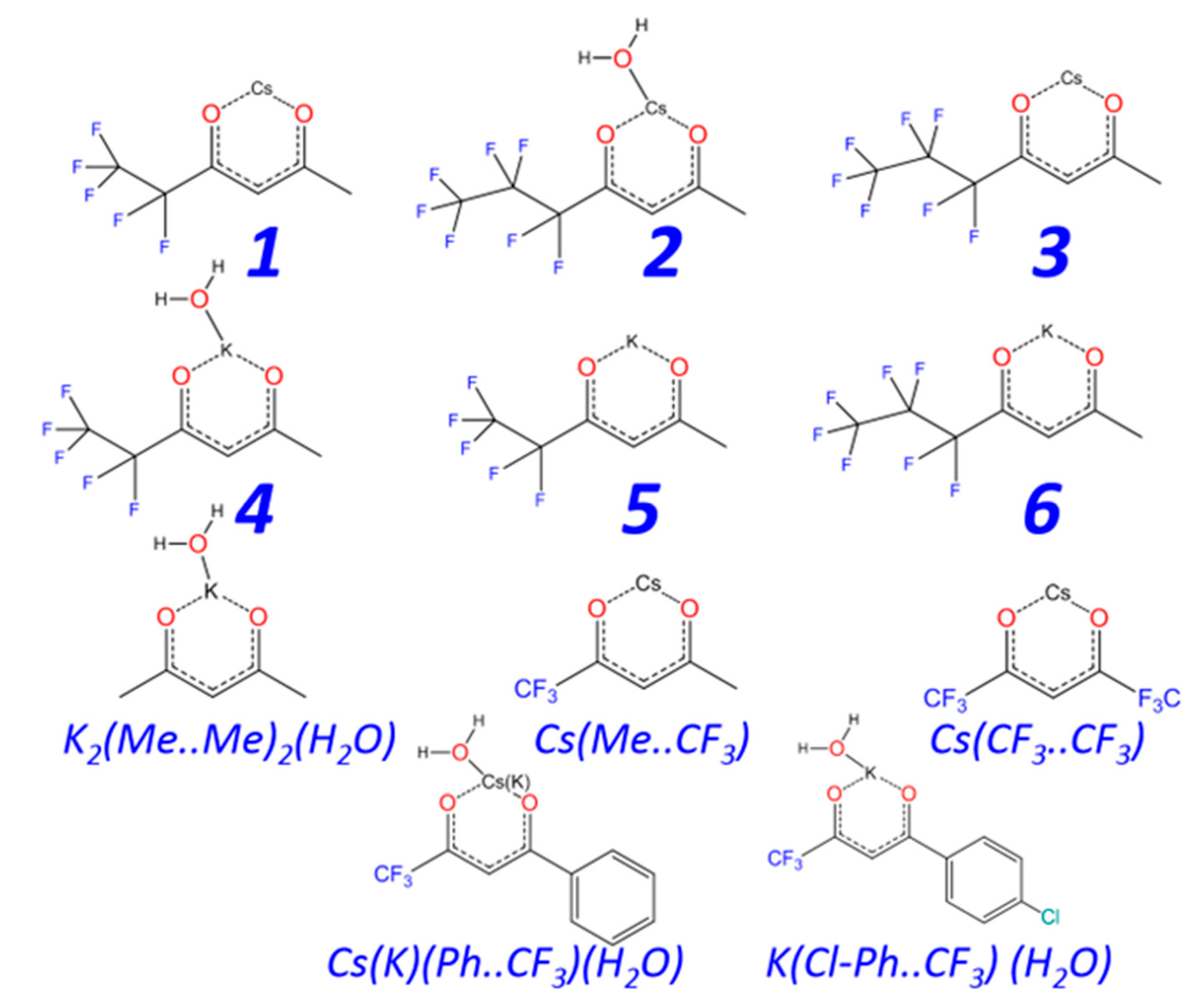
2.1. Synthesis and Characterization
2.2. Crystal Structure Investigation
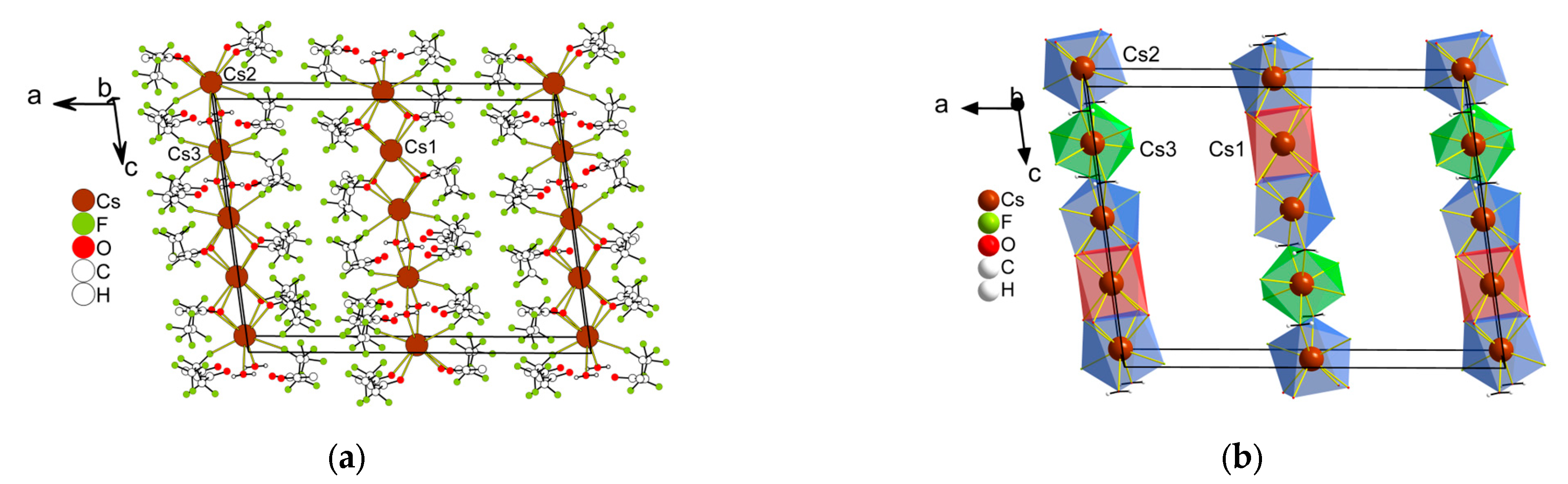
2.2.1. Structure Description
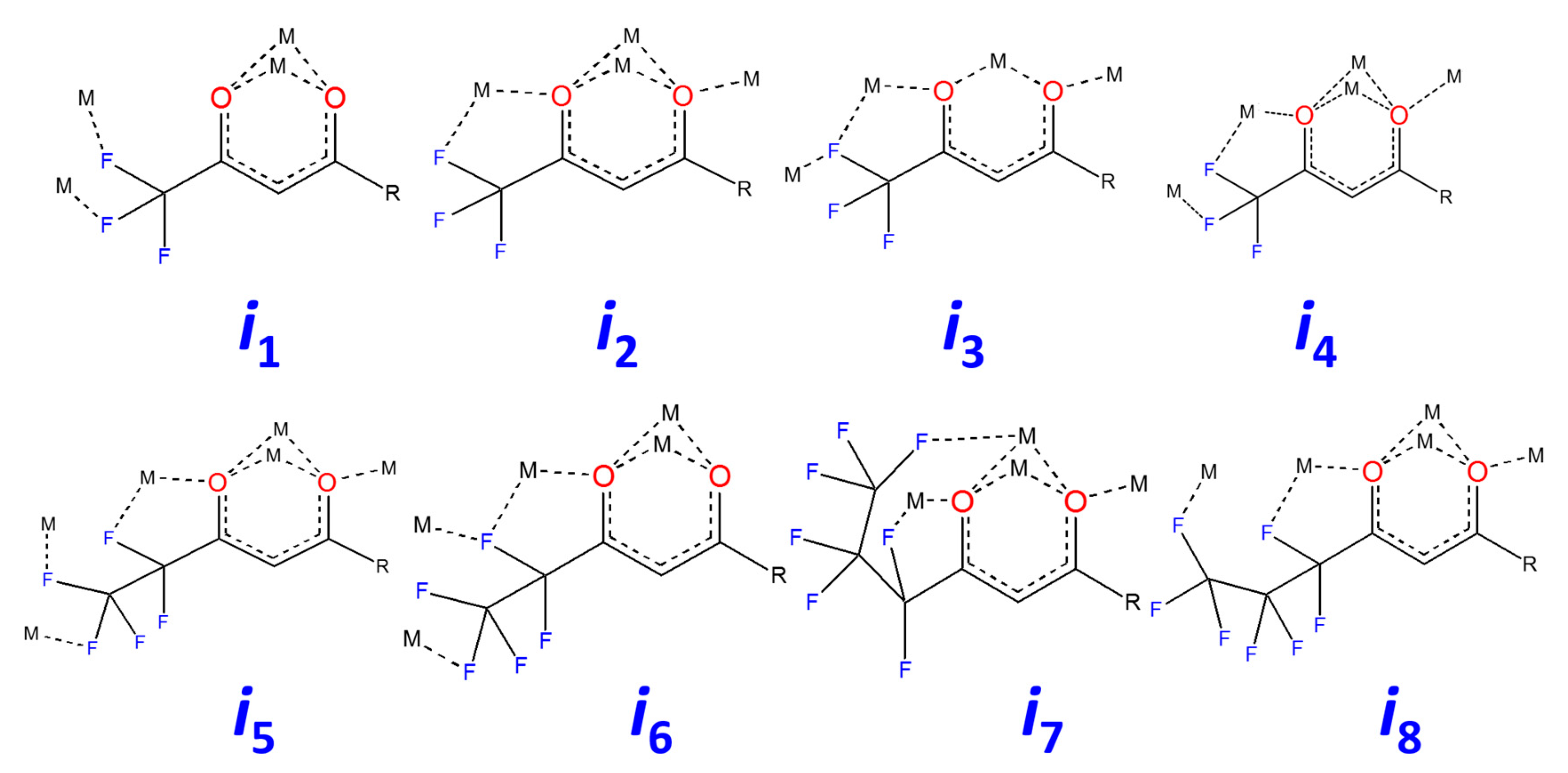
2.2.2. Structure Discussion
Features of Aqua-Complexes
Features of Anhydrous Complexes
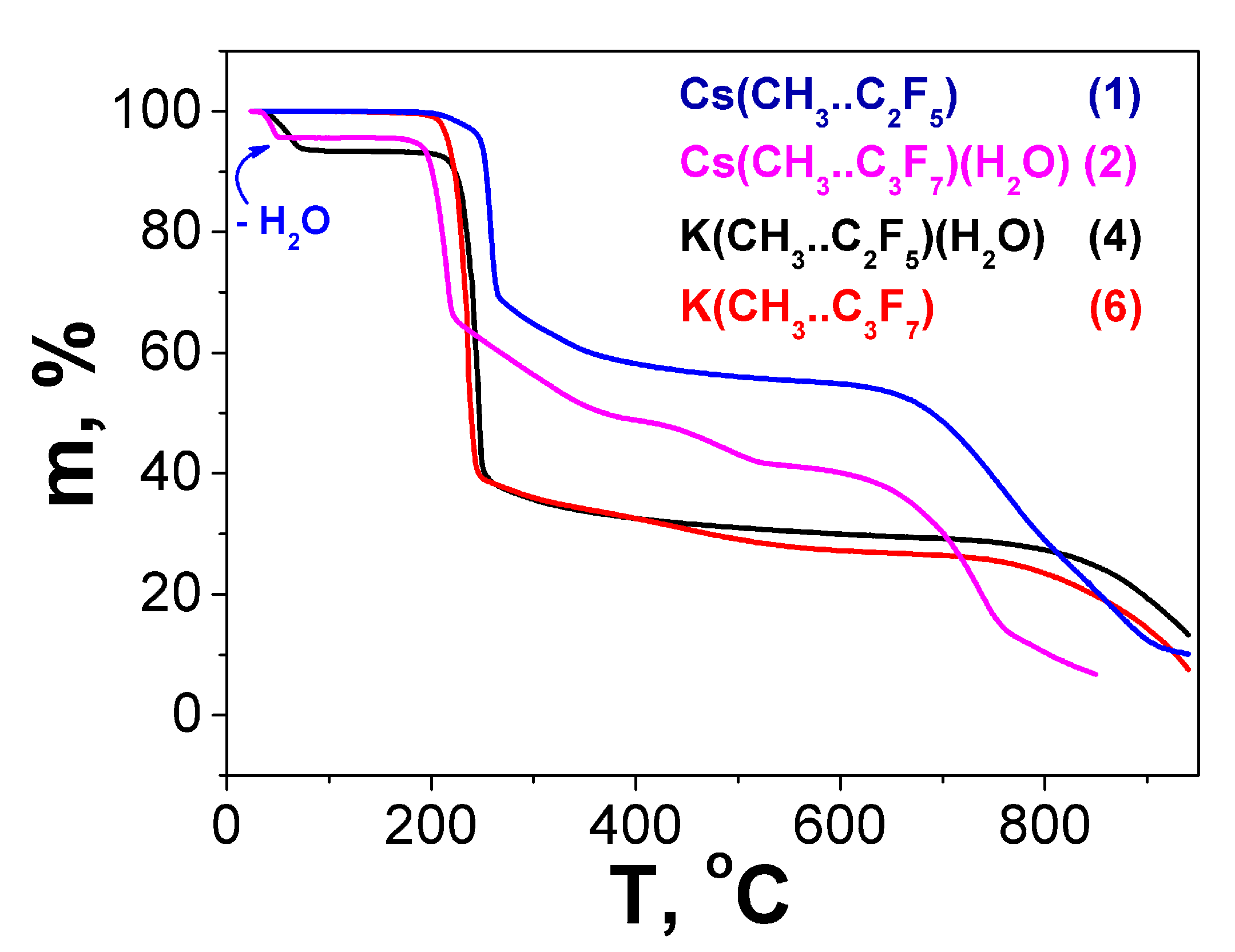
2.3. Thermal Properties
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis
3.3. Structural Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Isayama, S.; Mukaiyama, T. Hydration of Olefins with Molecular Oxygen and Triethylsilane Catalyzed by Bis(trifluoroacetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 569–572. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Nehra, K.; Dalal, A.; Hooda, A.; Bhagwan, S.; Saini, R.K.; Mari, B.; Kumar, S.; Singh, D. Lanthanides β-diketonate complexes as energy-efficient emissive materials: A review. J. Mol. Struct. 2022, 1249, 131531. [Google Scholar] [CrossRef]
- Mishra, S.; Daniele, S. Metal-organic derivatives with fluorinated ligands as precursors for inorganic nanomaterials. Chem. Rev. 2015, 115, 8379–8448. [Google Scholar] [CrossRef] [PubMed]
- Dar, W.A.; Ganaie, A.B.; Iftikhar, K. Synthesis and photoluminescence study of two new complexes [Sm(hfaa)3(impy)2] and [Eu(hfaa)3(impy)2] and their PMMA based hybrid films. J. Lumin. 2018, 202, 438–449. [Google Scholar] [CrossRef]
- DuChane, C.M.; Merola, J.S. Hexafluoroacetylacetonate (hfac) as ligand for pentamethylcyclopentadienyl (Cp*) rhodium and iridium complexes: Some surprising results, including an Ir3Na1O4 cubane structure. J. Organomet. Chem. 2020, 929, 121552. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. The impact of alkali metal ion on the crystal structure and (mechano)luminescence of terbium(III) tetrakis(β-diketonates). Eur. J. Inorg. Chem. 2019, 2020, 523–531. [Google Scholar] [CrossRef]
- Devi, A. ‘Old Chemistries’ for new applications: Perspectives for development of precursors for MOCVD and ALD applications. Coord. Chem. Rev. 2013, 257, 3332–3384. [Google Scholar] [CrossRef]
- Saloutin, V.I.; Edilova, Y.O.; Kudyakova, Y.S.; Burgart, Y.V.; Bazhin, D.N. Heterometallic Molecular Architectures Based on Fluorinated β-Diketone Ligands. Molecules 2022, 27, 7894. [Google Scholar] [CrossRef]
- Salmankurt, B.; Duman, S. Investigation of the structural, mechanical, dynamical and thermal properties of CsCaF3 and CsCdF3. Mater. Res. Express. 2016, 3, 045903. [Google Scholar] [CrossRef]
- Raja, A.; Nagaraj, R.; Ramachandran, K.; Sivasubramani, V.; Annadurai, G.; Joseph, D.D.; Ramasamy, P. A novel bifunctional Dy3+ activated RbCaF3 single phase phosphor: Facile synthesis and dual-luminescence properties for WLEDs and dosimetry applications. Adv Powder Technol. 2020, 31, 2597. [Google Scholar] [CrossRef]
- Quan, Z.; Yang, P.; Li, C.; Yang, J.; Yang, D.; Jin, Y.; Lian, H.; Li, H.; Lin, J. Shape and Phase-Controlled Synthesis of KMgF3 Colloidal Nanocrystals via Microwave Irradiation. J. Phys. Chem. C. 2009, 113, 4018–4025. [Google Scholar] [CrossRef]
- Hua, R.N.; Yu, J.C.; Jiang, H.; Shi, C. Solvothermal synthesis and luminescent properties of the complex fluorides KMgF3: Eu and KZnF3:RE (RE = Eu, Ce). J. Alloys Compd. 2007, 432, 253–257. [Google Scholar] [CrossRef]
- Sommerdijk, J.L.; Bril, A. On the position of the 5D0 level of Eu3+ in AMgF3 (A = K, Rb, Cs). J. Lumin. 1976, 12–13, 669–673. [Google Scholar] [CrossRef]
- Alcala, R.; Sardar, D.K.; Sibley, W.A. Optical transitions of Eu2+ IONS in RbMgF3 crystals. J. Lumin. 1982, 27, 273–284. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Wang, C.; Liu, Z.; Liu, X.G. Single-band Upconversion Emission in Lanthanide-Doped KMnF3 Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 10369–10372. [Google Scholar] [CrossRef] [PubMed]
- Lopez Ortiz, J.C.; Guerra, G.A.F.; Machado, F.L.A.; Rezende, S.M. Magnetic Anisotropy of Antiferromagnetic RbMnF3. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 054402. [Google Scholar] [CrossRef]
- Seavey, M.H. Nuclear and Electronic Spin-Wave Relaxation Rates in the Hexagonal Antiferromagnet CsMnF3. J. Appl. Phys. 1969, 40, 1597–1598. [Google Scholar] [CrossRef]
- Pellegrino, A.L.; Lo Presti, F.; Malandrino, G. A molecular route to fluoro-perovskite materials: Synthesis of CsCaF3 films through a sol-gel/spin-coating process. Discov. Mater. 2022, 2, 4. [Google Scholar] [CrossRef]
- Park, H.H.; Senegas, J.; Reau, J.M.; Pezat, M.; Darriet, B.; Hagenmuller, P. The CsCaF3-xHx solid solution (0 ≤ x ≤ 1.70): Structural characteristics and hydrogen diffusion investigation. Mat Res Bull. 1988, 23, 1127. [Google Scholar] [CrossRef]
- Usman, M.; Ayer, G.B.; Smith, M.D.; zur Loye, H.-C. Synthesis and Crystal Structure of a 6H Hexagonal Fluoro-Perovskite: RbMgF3. J. Chem. Crystallogr. 2021, 51, 9–13. [Google Scholar] [CrossRef]
- Okazaki, A.; Suemune, Y. The Crystal Structures of KMnF3, KFeF3, KCoF3, KNiF3 and KCuF3 above and below their Néel Temperatures. J. Phys. Soc. Jpn. 1961, 16, 671–675. [Google Scholar] [CrossRef]
- Pellegrino, A.L.; Malandrino, G. Surfactant-free synthesis of the full inorganic perovskite CsPbBr3: Evolution and phase stability of CsPbBr3 vs. CsPb2Br5 and their photocatalytic properties. ACS Appl. Energy Mater. 2021, 4, 9431–9439. [Google Scholar] [CrossRef]
- Peddagopu, N.; Sanzaro, S.; Rossi, P.; Paoli, P.; Malandrino, G. A one-pot synthesis of “K(hfa) glyme” adducts: Effect of the polyether length on the ion coordination sphere. Eur. J. Inorg. Chem. 2021, 2021, 3776–3780. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Zherikova, K.V.; Kuratieva, N.V.; Morozova, N.B.; Igumenov, I.K. Synthesis, structure, and thermal properties of fluorinated cesium beta-diketonates. J. Coord. Chem. 2013, 66, 2235–2249. [Google Scholar] [CrossRef]
- Kochelakov, D.V.; Vikulova, E.S.; Kuratieva, N.V. Potassium and Rubidium Benzoyltrifluoroacetonates: A Crystal Chemical Study and Thermal Properties. J. Struct. Chem. 2020, 61, 439–448. [Google Scholar] [CrossRef]
- Kochelakov, D.V.; Vikulova, E.S.; Kuratieva, N.V.; Sukhikh, A.S.; Gromilov, S.A. Study of potassium, rubidium hexafluoroacetylacetonates and by-products of their synthesis and crystallization. J. Struct. Chem. 2023, 64, 82–96. [Google Scholar] [CrossRef]
- Tsymbarenko, D.M.; Korsakov, I.E.; Lyssenko, K.A.; Troyanov, S.I. One-dimensional coordination polymers in the crystal structures of sodium and potassium acetylacetonates. Polyhedron 2015, 92, 68–76. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Ali, A.; Iqbal, M.S. Synergistic extraction of Ce(III), Tb(III) and Lu(III) with a mixture of hexafluoroacetylacetone and triphenylphosphineoxide in benzene. Radiochim. Acta 2007, 95, 733–737. [Google Scholar] [CrossRef]
- Martins, J.P.; Arranja, C.C.; Sobral, A.J.F.N.; Ramos Silva, M. Poly[μ2-aqua-μ4-[1-(4-chlorophenyl)-4,4, 4-trifluorobutane-1,3-dionato]-potassium]. Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69, 422–423. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Alkali and alkaline-earth metals. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 602–625. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Grebenyuk, D.; Zobel, M.; Polentarutti, M.; Ungur, L.; Kendin, M.; Zakharov, K.; Degtyarenko, P.; Vasiliev, A.; Tsymbarenko, D. A Family of Lanthanide Hydroxo Carboxylates with 1D Polymeric Topology and Ln4 Butterfly Core Exhibits Switchable Supramolecular Arrangement. Inorg. Chem. 2021, 60, 8049–8061. [Google Scholar] [CrossRef]
- Kendin, M.; Tsymbarenko, D. 2D-Coordination Polymers Based on Rare-Earth Propionates of Layered Topology Demonstrate Polytypism and Controllable Single-Crystal-to-Single-Crystal Phase Transitions. Cryst. Growth Des. 2020, 20, 3316–3324. [Google Scholar] [CrossRef]
- Peddagopu, N.; Rossi, P.; Bonaccorso, C.; Bartasyte, A.; Paoli, P.; Malandrino, G. Facile synthesis of novel lithium β-diketonate glyme adducts: The effect of molecular engineering on the thermal properties. Dalton Trans. 2020, 49, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Peddagopu, N.; Pellegrino, A.L.; Bonaccorso, C.; Rossi, P.; Paoli, P.; Malandrino, G. Sodium β-Diketonate Glyme Adducts as Precursors for Fluoride Phases: Synthesis, Characterization and Functional Validation. Molecules 2022, 27, 6282. [Google Scholar] [CrossRef]
- Mikhailovskaya, T.F.; Makarov, A.G.; Selikhova, N.Y.; Makarov, A.Y.; Pritchina, E.A.; Bagryanskaya, I.Y.; Vorontsova, E.V.; Ivanov, I.G.; Tikhova, V.D.; Gritsan, N.P.; et al. Carbocyclic functionalization of quinoxalines, their chalcogen congeners 2,1,3-benzothia/selenadiazoles, and related 1,2-diaminobenzenes based on nucleophilic substitution of fluorine. J. Fluor. Chem. 2016, 183, 44–58. [Google Scholar] [CrossRef]
- Tikhova, V.D.; Fadeeva, V.P.; Nikulicheva, O.N.; Dobinskaya, T.A.; Deryabina, Y.M. Determination of fluorine in organic functional materials. Chem. Sust. Develop. 2022, 30, 640–653. [Google Scholar] [CrossRef]
- Li, T.; Gamer, M.T.; Scheer, M.; Konchenko, S.N.; Roesky, P.W. Intramolecular Phosphorus–Phosphorus Bond Formation within a Co2P4 Core. Chem. Comm. 2013, 49, 2183–2185. [Google Scholar] [CrossRef]
- Bruker Advanced X-ray Solutions. APEX2 (Version 1.08), SAINT (Version 7.03), and SADABS (Version 2.11); Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. IUCr Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Ferraris, G.; Franchini-Angela, M. Survey of the geometry and environment of water molecules in crystalline hydrates studied by neutron diffraction. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 3572–3583. [Google Scholar] [CrossRef]

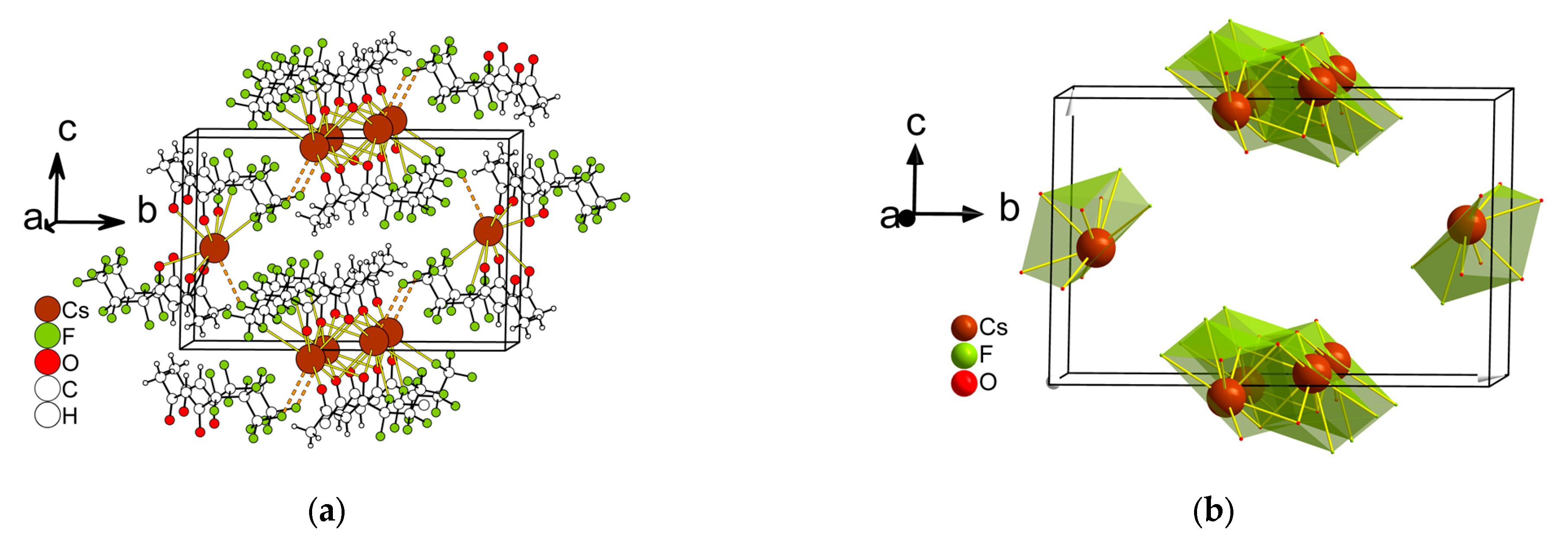

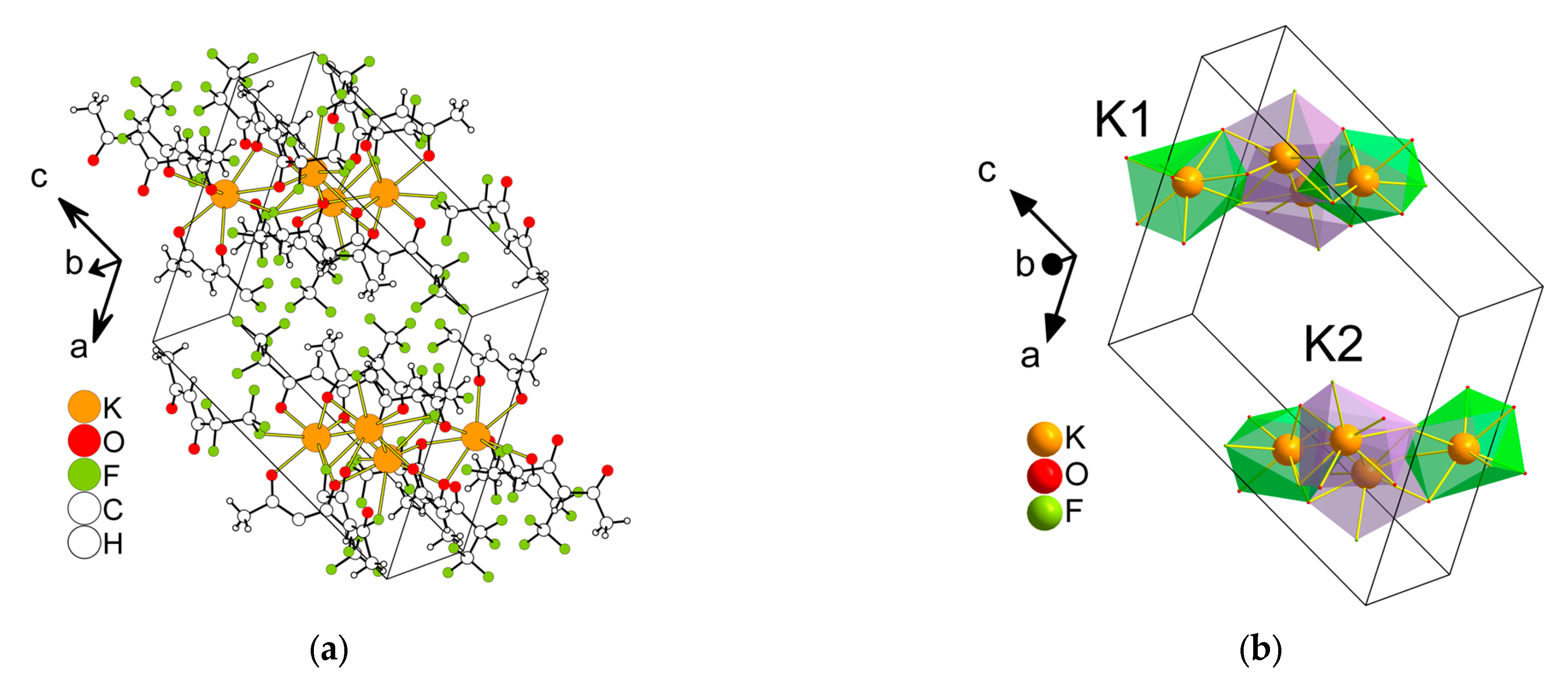
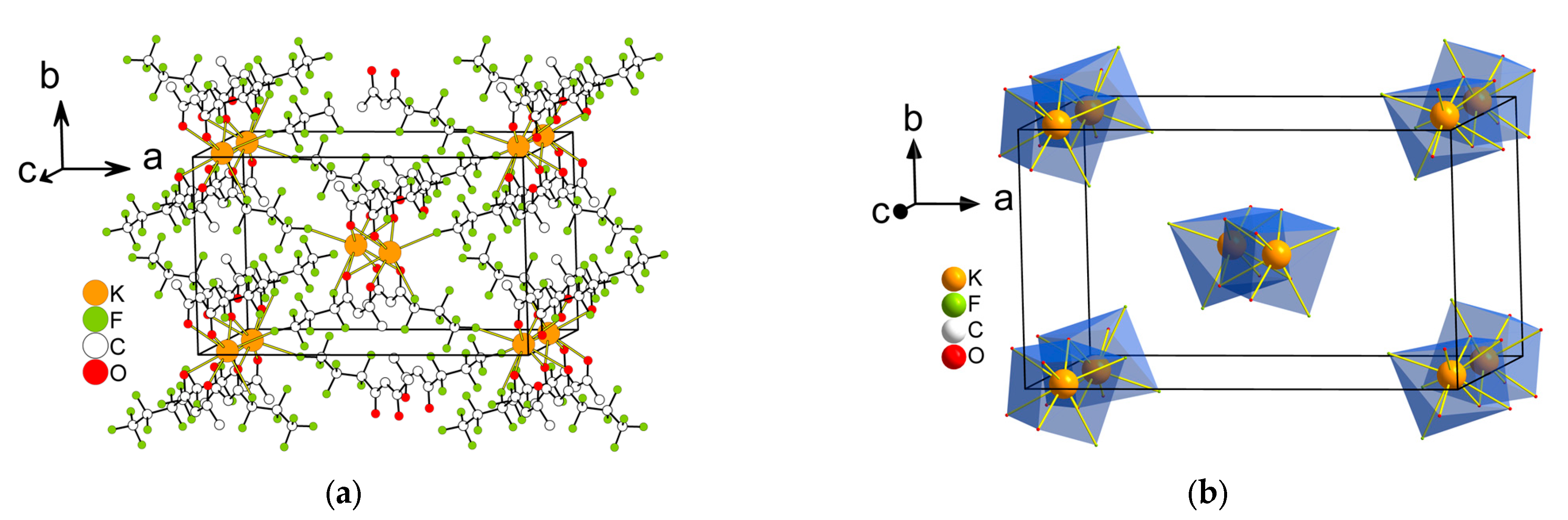
| Compound | Coordination Number | Environment | Polyhedra | Reference |
|---|---|---|---|---|
| Cs(Me..C2F5) 1 | 9 | 6O3F | CCU-9 | This work |
| Cs(Me..C3F7)(H2O) 2 | 8 | 6O2F | CU-8 | |
| 8 | 8O | CU-8 | ||
| 8 | 4O4F | SAPR-8 or TDD-8 | ||
| Cs(Me..C3F7) 3 | 8 | 6O2F | HPY-8 | |
| K(Me..C2F5)(H2O) 4 | 8 | 8O | TDD-8 | |
| 8 | 6O2F | TDD-8 | ||
| 8 | 4O4F | SAPR-8 or TDD-8 | ||
| K(Me..C2F5) 5 | 8 | 6O2F | TDD-8 | |
| 7 | 5O2F | CTPR-7 | ||
| K(Me..C3F7) 6 | 8 | 6O2F | CU-8 | |
| K(Ph..CF3)(H2O) | 8 | 8O | TDD-8 | [26] |
| 8 | 4O4F | TDD-8 | ||
| K(Cl-Ph..CF3)(H2O) | 8 | 6O2F | CU-8 | [30] |
| Cs(Ph..CF3)(H2O) | 8 | 8O | TDD-8 | [25] |
| 8 | 4O4F | TDD-8 | ||
| Cs(Me..CF3) | 8 | 6O2F | TT-8 or CU-8 or HBPY-8 | [25] |
| Compound | Twater loss | Water Loss Exp.(Calc.), Mass.% | Tdecomp. | Decomposition Products (Residues) Exp.(Calc.), Mass.% |
|---|---|---|---|---|
| Cs(Me..C2F5) 1 | – | – | 195 °C | 57.0 (42.7, CsF/48.5, ½ Cs2CO3) |
| Cs(Me..C3F7)(H2O) 2 | 25–61 °C | 5.0 (4.5, -H2O) | 170 °C | 41.4 (37.6, CsF/40.3, ½ Cs2CO3) |
| K(Me..C2F5)(H2O) 4 | 25–82 °C | 6.4 (6.9, -H2O) | 200 °C | 28.9 (22.3, KF/26.6 ½ K2CO3) |
| K(Me..C3F7) 6 | – | – | 195 °C | 26.7 (19.9, KF/23.7 ½ K2CO3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochelakov, D.V.; Vikulova, E.S.; Kuratieva, N.V.; Korolkov, I.V. Potassium and Cesium Fluorinated β-Diketonates: Effect of a Cation and Terminal Substituent on Structural and Thermal Properties. Molecules 2023, 28, 5886. https://doi.org/10.3390/molecules28155886
Kochelakov DV, Vikulova ES, Kuratieva NV, Korolkov IV. Potassium and Cesium Fluorinated β-Diketonates: Effect of a Cation and Terminal Substituent on Structural and Thermal Properties. Molecules. 2023; 28(15):5886. https://doi.org/10.3390/molecules28155886
Chicago/Turabian StyleKochelakov, Danil V., Evgeniia S. Vikulova, Natalia V. Kuratieva, and Ilya V. Korolkov. 2023. "Potassium and Cesium Fluorinated β-Diketonates: Effect of a Cation and Terminal Substituent on Structural and Thermal Properties" Molecules 28, no. 15: 5886. https://doi.org/10.3390/molecules28155886
APA StyleKochelakov, D. V., Vikulova, E. S., Kuratieva, N. V., & Korolkov, I. V. (2023). Potassium and Cesium Fluorinated β-Diketonates: Effect of a Cation and Terminal Substituent on Structural and Thermal Properties. Molecules, 28(15), 5886. https://doi.org/10.3390/molecules28155886