Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells
Abstract
1. Introduction
2. Results
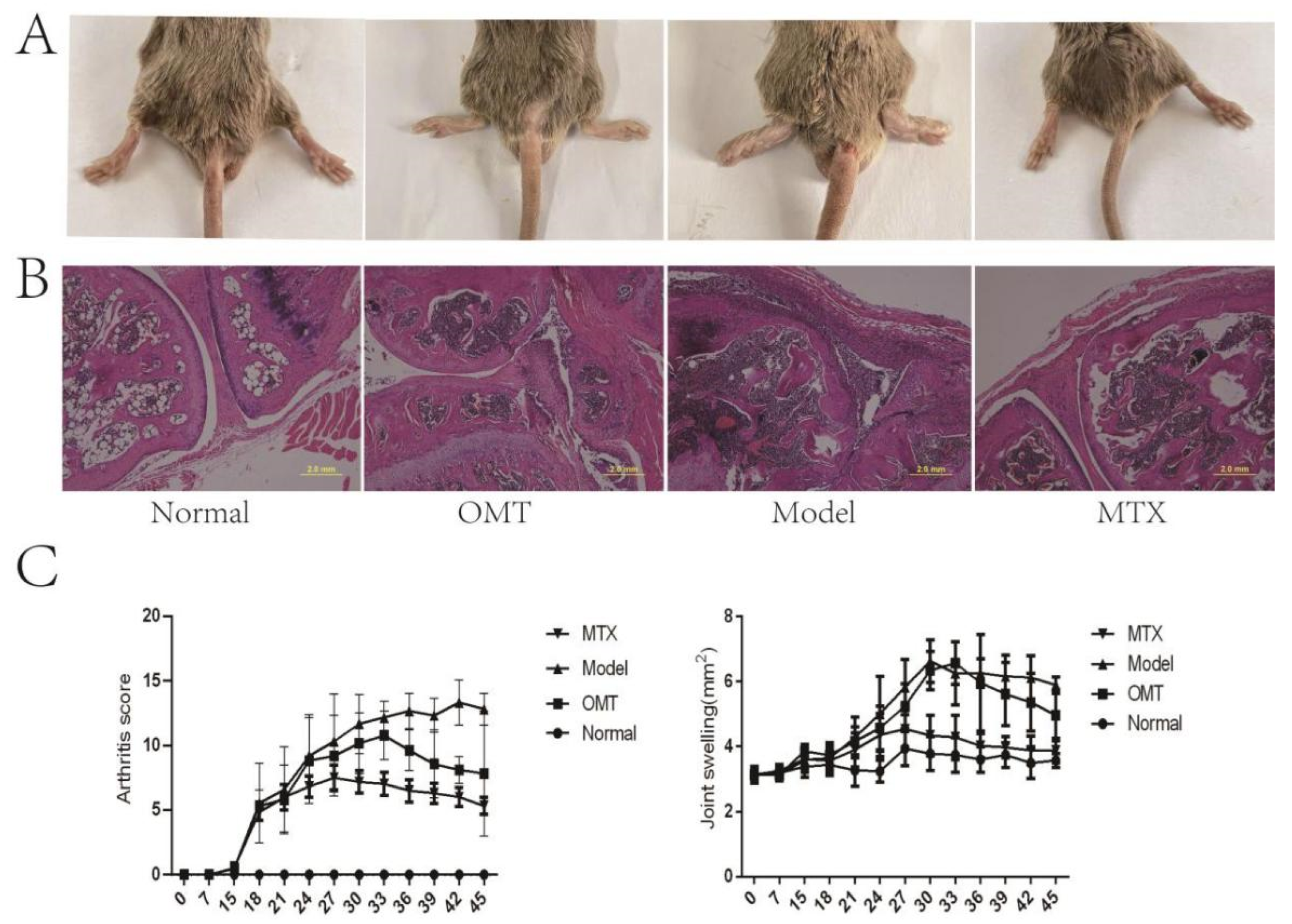
2.1. OMT Alleviates Ankle Swelling, Arthritis Score, and Joint Histopathological Changes of CIA Mice
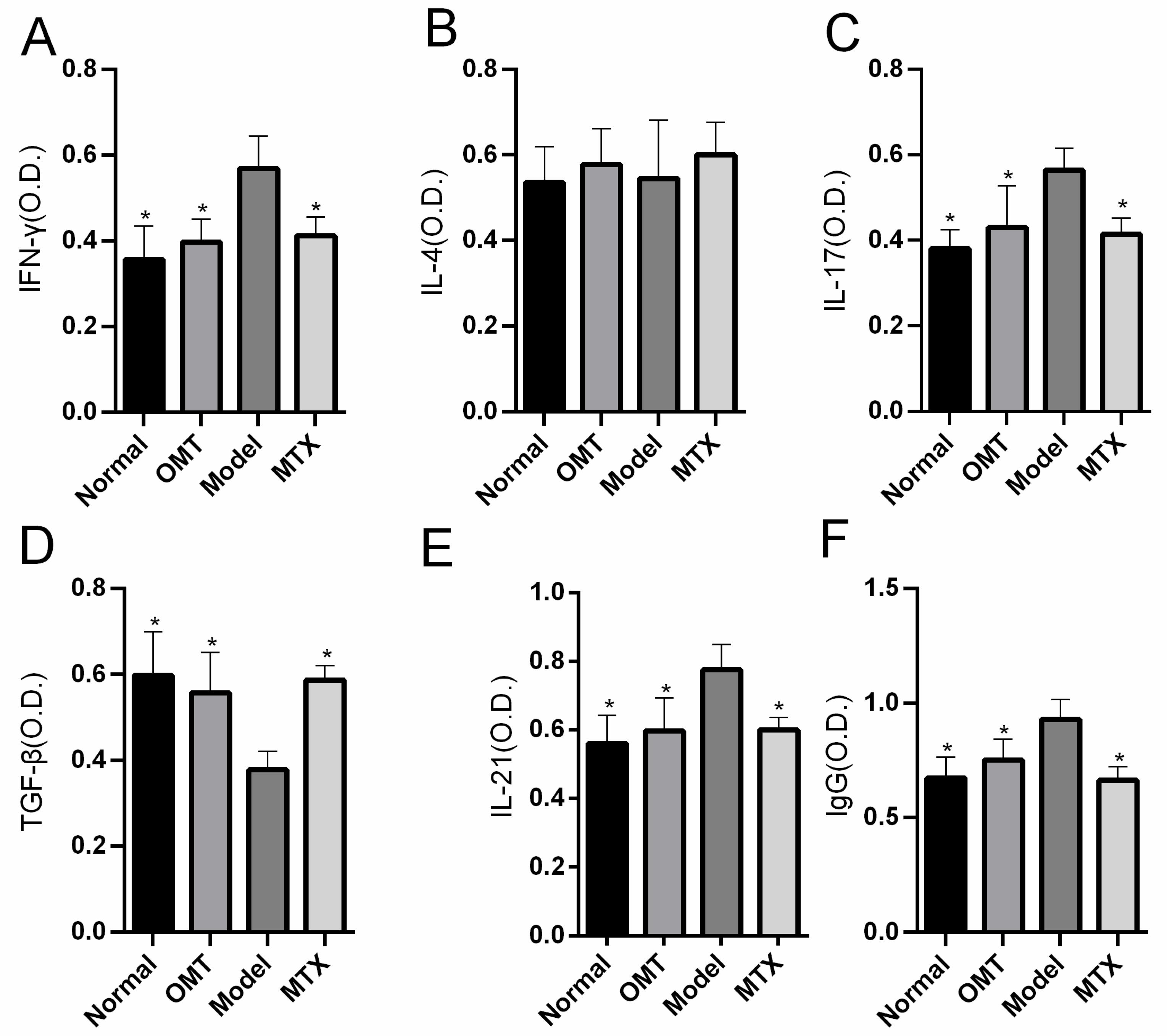
2.2. OMT Treatment Reduces the Level of Inflammatory Factors
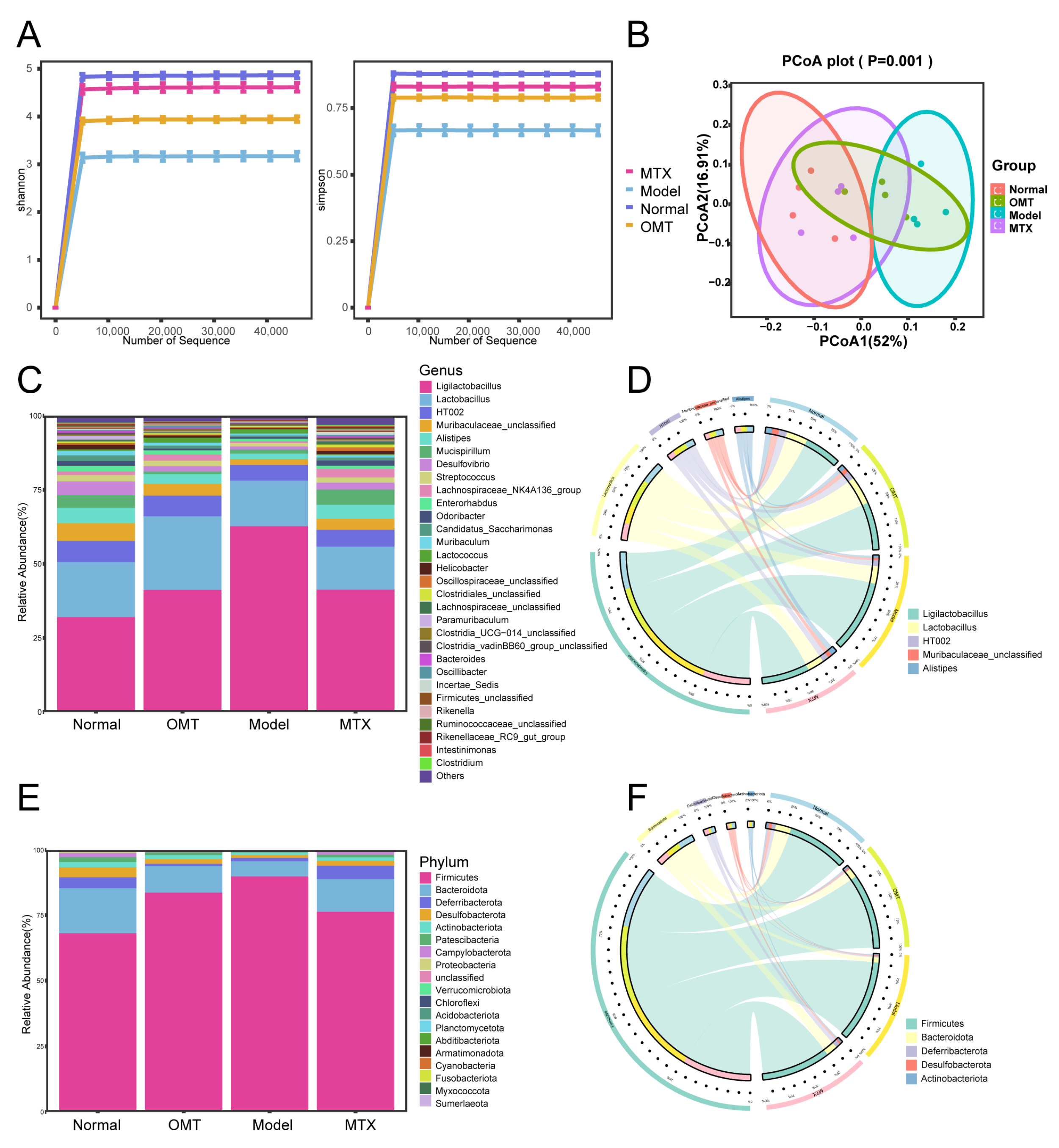
2.3. OMT Treatment Changes the Gut Microbiota Composition in CIA Mice
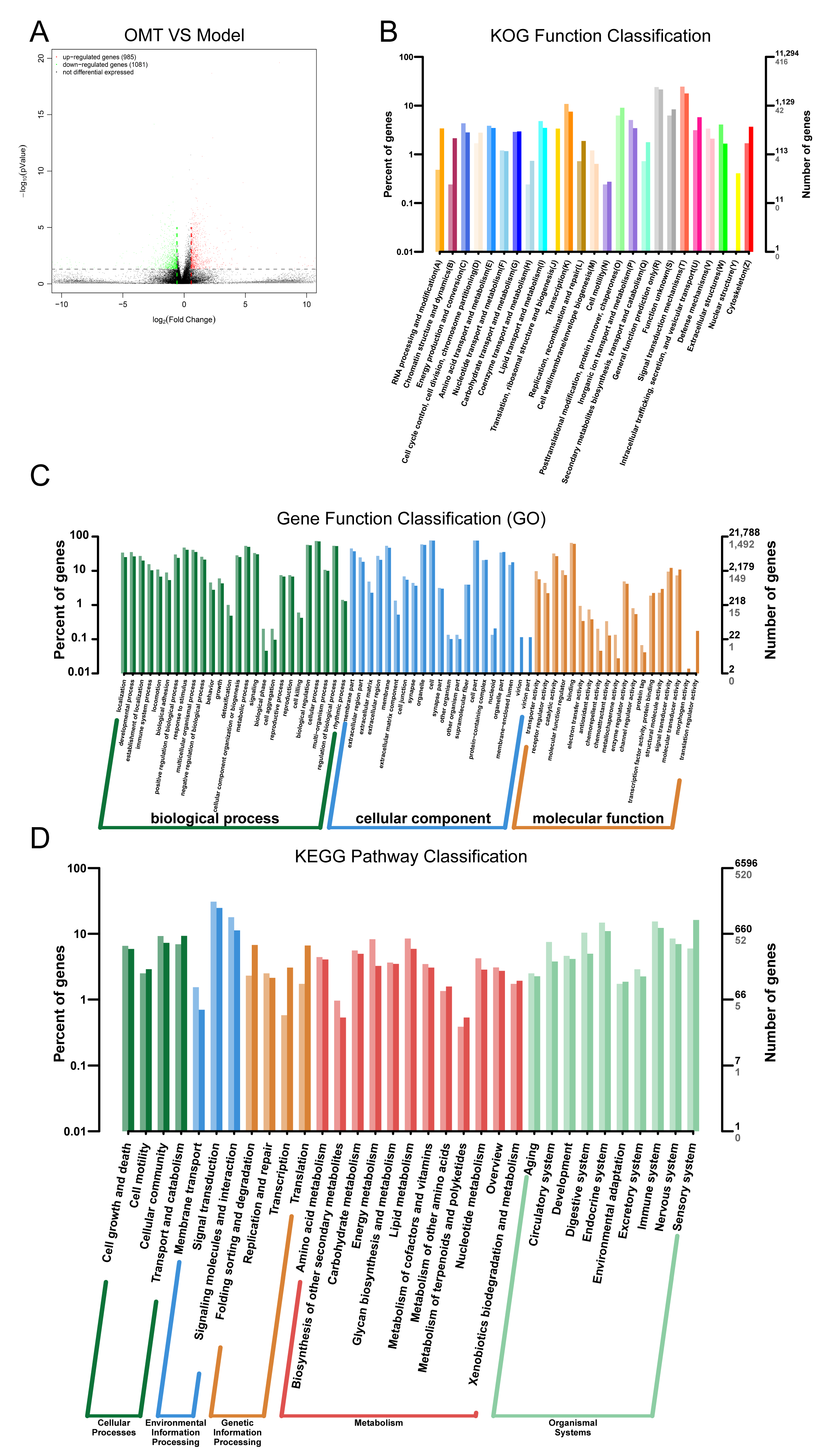
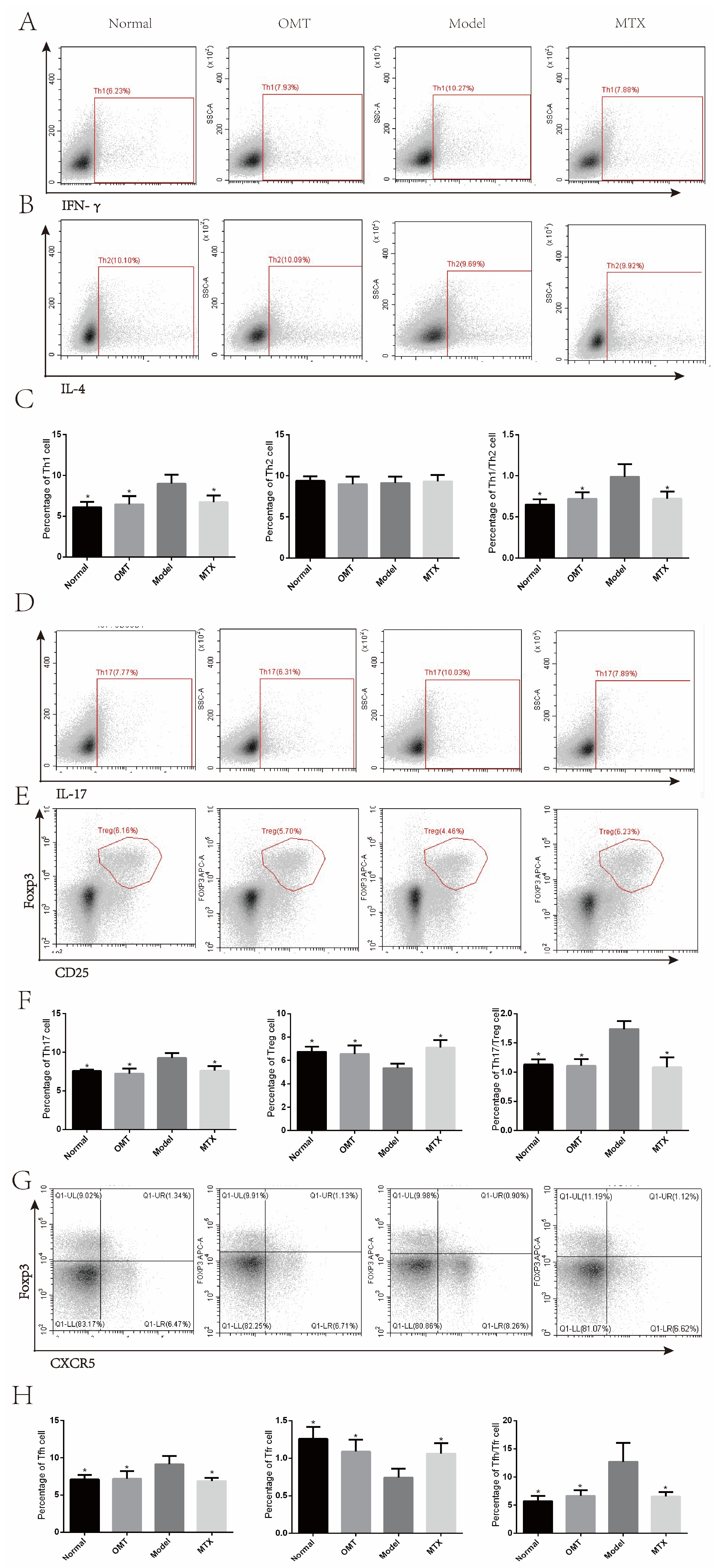
2.4. OMT May Regulate Th Cells Imbalance through Immune System Processes
2.5. OMT Could Balance Th Cells in CIA Mice
3. Methods
3.1. Animal and Reagents
3.2. Establishment of Collagen-Induced Arthritis (CIA) Mouse Model
3.3. Grouping and Administration
3.4. Evaluation of Rheumatoid Arthritis
3.5. HE Staining of Joint Tissues
3.6. Analysis of CD4+T Cell Subsets in Mouse Lymph Nodes by Flow Cytometry
3.7. Detection of Inflammatory Factors by Enzyme Linked Immunosorbent Assay (ELISA)
3.8. 16S rRNA Sequencing
3.9. RNA-Sequencing
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zuo, Y.; Xu, H.; Li, Y.; Zhang, Z.; Tao, R.; Wang, M. Hsa_circ_0007707 participates in PDE3B-mediated apoptosis inhibition and inflammation promotion in fibroblast-like synoviocytes. Int. Immunopharmacol. 2023, 119, 110157. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, J.; Wang, Q.; Cai, J.; Yu, B.; Dai, Q.; Bao, Y.; Chen, R.; Zhang, Z.; Zhang, D.; et al. Glucocorticoid-loaded pH/ROS dual-responsive nanoparticles alleviate joint destruction by downregulating the NF-κB signaling pathway. Acta Biomater. 2023, 164, 458–473. [Google Scholar] [CrossRef] [PubMed]
- Langbour, C.; Rene, J.; Goupille, P.; Alegria, G.C. Efficacy of Janus kinase inhibitors in rheumatoid arthritis. Inflamm. Res. 2023, 72, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Wei, H.; Wang, X.; Xie, M.; Jiang, Y.; Zhou, J. Matrine-induced nephrotoxicity via GSK-3β/nrf2-mediated mitochondria-dependent apoptosis. Chem. Interact. 2023, 378, 110492. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Y.; Zhang, Z.; Chen, Y.; Chen, N.; Chen, F.; Qi, Y.; Han, C.; Xu, Y.; Chen, M.; et al. Oxymatrine boosts hematopoietic regeneration by modulating MAPK/ERK phosphorylation after irradiation-induced hematopoietic injury. Exp. Cell Res. 2023, 427, 113603. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.C.-L.; Barua, N.; Lau, C.B.-S.; Leung, P.-C.; Fung, K.-P.; Ip, M. Enhancing Antibiotics Efficacy by Combination of Kuraridin and Epicatechin Gallate with Antimicrobials against Methicillin-Resistant Staphylococcus aureus. Antibiotics 2023, 12, 117. [Google Scholar] [CrossRef]
- Hu, H.-F.; Wang, Z.; Tang, W.-L.; Fu, X.-M.; Kong, X.-J.; Qiu, Y.-K.; Xi, S.-Y. Effects of Sophora flavescens aiton and the absorbed bioactive metabolite matrine individually and in combination with 5-fluorouracil on proliferation and apoptosis of gastric cancer cells in nude mice. Front. Pharmacol. 2022, 13, 1047507. [Google Scholar] [CrossRef]
- Huang, P.; Hu, F.; Yang, Z.; Pan, Y.; Zhou, R.; Yan, Y.; Wang, H.; Wang, C. Matrine regulates Th1/Th2 inflammatory responses by inhibiting the Hsp90/NF-κB signaling axis to alleviate atopic dermatitis. Kaohsiung J. Med Sci. 2023, 39, 501–510. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Horta-Baas, G.; Romero-Figueroa, M.D.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J. Immunol. Res. 2017, 2017, 4835189. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and Role of Regulatory T Cells in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Zhu, J.; Su, W.; Jian, C.; Zhang, J.; Wu, J.; Wang, T.; Zhang, W.; Zeng, F.; et al. Multi-omics profiling reveals potential alterations in rheumatoid arthritis with different disease activity levels. Thromb. Haemost. 2023, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Fu, Y.; Zhou, K.; Song, H.; Fang, G.; Chen, Q.; Pang, Y. Toddalia asiatica extract attenuates adjuvant-induced arthritis by modulating colon Th17/Treg balance and colony homeostasis. J. Ethnopharmacol. 2023, 313, 116542. [Google Scholar] [CrossRef]
- Lai, W.; Wang, C.; Lai, R.; Peng, X.; Luo, J. Lycium barbarum polysaccharide modulates gut microbiota to alleviate rheumatoid arthritis in a rat model. Npj Sci. Food 2022, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- D’amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2017, 102, 415–425. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Li, D.; Yan, J.; Yang, J.; Zhong, X.; Xu, Q.; Xu, Y.; Xia, Y.; Wang, Q.; et al. Combined treatment with glucosamine and chondroitin sulfate improves rheumatoid arthritis in rats by regulating the gut microbiota. Nutr. Metab. 2023, 20, 1–12. [Google Scholar] [CrossRef]
- Niu, S.; Zhu, X.; Zhang, J.; Ma, Y.; Lang, X.; Luo, L.; Li, W.; Zhao, Y.; Zhang, Z. Arsenic trioxide modulates the composition and metabolic function of the gut microbiota in a mouse model of rheumatoid arthritis. Int. Immunopharmacol. 2022, 111, 109159. [Google Scholar] [CrossRef]
- Yang, P.; Qian, F.-Y.; Zhang, M.-F.; Xu, A.-L.; Wang, X.; Jiang, B.-P.; Zhou, L.-L. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J. Leukoc. Biol. 2019, 106, 1233–1240. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wang, W.; Wang, H.; Zhang, Y.; Zhang, Z. Data on arsenic trioxide modulates Treg/Th17/Th1/Th2 cells in treatment-naïve rheumatoid arthritis patients and collagen-induced arthritis model mice. Data Brief 2019, 27, 104615. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, B.; Zhang, M.; Wang, X.; Zhu, H.; Gu, Z.; Zhou, X.; Lu, Y.; Zhou, L. MicroRNA-143-3p ameliorates collagen-induced arthritis by polarizing naive CD4+T cells into Treg cells. J. Clin. Lab. Anal. 2023, 37, e24845. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, X.; Bai, Z.; Lu, Z.; Zhu, X.; Liu, J.; Wang, J.; Jin, M.; Liu, C.; Li, X. FcγRIIIA activation-mediated up-regulation of glycolysis alters MDSCs modulation in CD4+ T cell subsets of Sjögren syndrome. Cell Death Dis. 2023, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Da Silva, G.A.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2020, 147, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Wajda, A.; Romanowska-Próchnicka, K.; Walczuk, E.; Kuca-Warnawin, E.; Kmiolek, T.; Stypinska, B.; Rzeszotarska, E.; Majewski, D.; Jagodzinski, P.P.; et al. Th17/Treg-Related Transcriptional Factor Expression and Cytokine Profile in Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 572858. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Xu, L.; Zhang, R.; Jin, Y.; Jiang, P.; Wei, K.; Xu, L.; Shi, Y.; Zhao, J.; Xiong, M.; et al. MicroRNA-Mediated Epigenetic Regulation of Rheumatoid Arthritis Susceptibility and Pathogenesis. Front. Immunol. 2022, 13, 838884. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Su, J.; Ma, Q.; Zhang, F.; Wang, J.; Feng, L.; Jia, X.; Tan, X. Erteng Tongbi Decoction ameliorates collagen-induced arthritis in mice via modulating T cell differentiation and cytokines balance. J. Ethnopharmacol. 2021, 286, 114928. [Google Scholar] [CrossRef]
- Walker, L.S.K. The link between circulating follicular helper T cells and autoimmunity. Nat. Rev. Immunol. 2022, 22, 567–575. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.; Zhang, H.; Chen, C.; Zeng, M.; Yunis, J.; Wei, Y.; Wan, Y.; Wang, N.; Zhou, M.; et al. Selenium–GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021, 22, 1127–1139. [Google Scholar] [CrossRef]
- Gonzalez-Figueroa, P.; Roco, J.A.; Papa, I.; Villacís, L.N.; Stanley, M.; Linterman, M.A.; Dent, A.; Canete, P.F.; Vinuesa, C.G. Follicular regulatory T cells produce neuritin to regulate B cells. Cell 2021, 184, 1775–1789.e19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, G.; Li, J.; Mao, Z.; Zhang, Y. Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells. Molecules 2023, 28, 5879. https://doi.org/10.3390/molecules28155879
Cao G, Li J, Mao Z, Zhang Y. Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells. Molecules. 2023; 28(15):5879. https://doi.org/10.3390/molecules28155879
Chicago/Turabian StyleCao, Gan, Jing Li, Zhuhan Mao, and Yanli Zhang. 2023. "Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells" Molecules 28, no. 15: 5879. https://doi.org/10.3390/molecules28155879
APA StyleCao, G., Li, J., Mao, Z., & Zhang, Y. (2023). Oxymatrine Alleviates Collagen-Induced Arthritis in Mice by Regulating the Immune Balance of T Cells. Molecules, 28(15), 5879. https://doi.org/10.3390/molecules28155879





