Abstract
A series of new cluster compounds with {Re4Mo2S8} and {Re3Mo3S8} cores has been obtained and investigated. The clusters with different Re/Mo ratios were isolated as individual compounds, which made it possible to study their spectroscopic and electrochemical properties. The geometry of the new clusters was studied using a combination of X-ray diffraction analysis, XAS and quantum chemical DFT calculations. It was shown that the properties of the new clusters, such as the number and position of electrochemical transitions, electronic structure and change in geometry with a change in charge, are similar to the properties of clusters based on the {Re4Mo2Se8} and {Re3Mo3Se8} cores described earlier.
1. Introduction
Chemical and physical properties, as well as the application potential of octahedral cluster complexes based on {M6(µ3-Q)8} cores (M = Re, Q = S, Se, or Te; M = Mo or W, Q = Cl, Br, or I), attract great interest and have been intensively studied over the past few decades [1,2,3,4]. Within the {M6Q8} core, the octahedral M6 metal cluster is surrounded by eight inner ligands Q, which are bound to the faces of the octahedron. Each metal atom is coordinated by one apical ligand L, forming a [{M6Q8}L6]n cluster unit. The chemical composition of the {M6Q8} cluster core and, in particular, the nature of metal atoms largely determine the physical properties of the cluster-based compounds, such as their redox properties, phosphorescence, or solar light-harvesting performances [5,6,7,8,9]. The synthesis of heterometallic clusters based on M6−xM′x clusters with a controlled M/M′ ratio may lead to a smooth and predictable change in the properties of clusters. This is a promising approach for the design of cluster-based materials with predefined functionality. However, this approach may be quite difficult to realize since the octahedral cluster cores are usually formed during high-temperature synthesis and are chemically inert to the substitution of metal atoms at lower temperatures.
Solid-state Chevrel phases with the composition Mo2Re4Q8 (Q = S, Se) were the first published examples of compounds based on heterometallic octahedral clusters. They were obtained by the high-temperature reaction between stoichiometric quantities of simple substances [10]. Similar experimental protocols were used to prepare other {Mo6−xM′xQ8} heterometallic polymeric cluster-based phases (M′ = Re, Q = Te, x = 2; M′ = Ru, Q = Te, 1.5 ≤ x ≤ 2, Q = Se, x = 2; M′ = Rh, Q = Te, x = 0.5, 1.33) [11,12,13,14,15,16], as well as Cs3Re5OsS11 [17]. Note that, for Mo-M′-Q systems, well-defined compositions with integer x values (Mo4Ru2Te8; Mo4Ru2Se8; Mo5Ru1Te8) could be prepared, as well as compositions with non-integer x values (Mo4.5Ru1.5Te8; Mo1.5Re4.5Se8; Mo4.66Rh1.33Te8). This indicates the coexistence of at least two cluster cores with different Mo/M′ ratios in the form of a solid solution. The separation of the different cluster cores {Mo6−xM′xQ8} for each integer value of x from the solid-state precursor requires a combination of solid-state and solution chemistry techniques in order to form [Mo6−xM′xQ8L6] soluble cluster units.
Soluble heterometallic octahedral clusters were obtained for the following metal combinations: rhenium/osmium [18,19,20], molybdenum/niobium [21] and rhenium/molybdenum [22,23]. All the compounds were obtained by high-temperature reactions between simple substances and binary precursors. Clusters with {Re6−xOsxSe8} (x = 1–3) and {Mo5NbI8} cores can be isolated as individual species directly from the reaction mixtures. Meanwhile, clusters with {Re6−xMoxQ8} (Q = S, Se; x = 1–3) cores were formed as apparent single-phase solid solutions but contained simultaneously several cluster cores with the same charges and different values of x. The separation of the different cluster cores proved to be a non-trivial task since they have similar geometric parameters and charges and do not separate chromatographically. Very recently, the separation and detailed investigation of [Re6−xMoxSe8(CN)6]n− cluster units (x = 1–3) were reported [24,25]. It was shown that the variation in the Re/Mo ratio led to drastic changes in the redox and spectroscopic properties of the clusters, which may allow for the obtaining of sensor and electrochromic materials based on these functionalized species. The aforementioned complicated nature of solid-state phases based on {Re6−xMoxSe8} units led us to re-investigate the “sulfide” solid-state phases formed as a result of the reactions between ReS2, MoS2 and KCN and reported initially as constant composition phase K6[Re3Mo3S8(CN)5] [23]. Recently, it was found that the synthesis of that chemical composition at different temperatures led to the formation of K6[Re6−xMoxS8(CN)5] solid solutions with 2.75 < x < 3.63 [26]. Here we report on the separation procedures that allowed us to obtain salts of [Re4Mo2S8(CN)6]n− and [Re3Mo3S8(CN)6]n− clusters as individual species. Moreover, we report on the spectroscopic properties and crystal structure investigations and compare them with the properties of the corresponding {Re4Mo2Se8} and {Re3Mo3Se8} cluster cores.
2. Results and Discussion
2.1. Synthesis
The reaction of ReS2 and MoS2 with KCN at 700 °C for 7 days led to the formation of the polymeric compound K6[Re3.16Mo2.84S8(CN)5] (1) [26]. The crystal structure of this compound was described earlier [15]. It is based on cluster units polymerized by means of bridging cyanide groups in trans-position to form chains. It should be noted that the ReS2 and MoS2 precursors used in the synthesis of 1 were obtained at 1000 °C. The preparation of metal sulfides at lower temperatures may lead to the formation of polymer K6[Re6-xMoxS8(CN)5] with different Re/Mo ratio.
Compound 1 is insoluble in deaerated water. The treatment of the reaction products with boiling Ar-saturated H2O led to the dissolution of the KCN excess. The interaction of 1 with O2 in an aerated KCN solution resulted in depolymerization accompanied by two-electron oxidation of the cluster core and the formation of the discrete cluster anion [Re3.2Mo2.8S8(CN)6]5−, which was isolated and crystallized as potassium salt 2. Note that the Re/Mo ratio in compound 2 was the same as in compound 1, confirming the co-crystallization of several clusters with different cores in the reaction mixture. To determine the composition of the mixture of clusters, compound 2 was converted to (Ph4P)+ salt by mixing the aqueous solutions of 2 and Ph4PCl. The precipitate formed had the composition (Ph4P)4[Re3.2Mo2.8S8(CN)6] (3) (Scheme 1). The decoloration of the solution indicates the quantitative precipitation of the cluster products. Based on the results of the mass spectrometric analysis of a solution of this salt in CH3CN, it was demonstrated that compound 3 contains cluster units based on three different cluster cores, namely, [Re4Mo2S8(CN)6]n−, [Re3Mo3S8(CN)6]n− and [Re2Mo4S8(CN)6]n− (Figure 1).
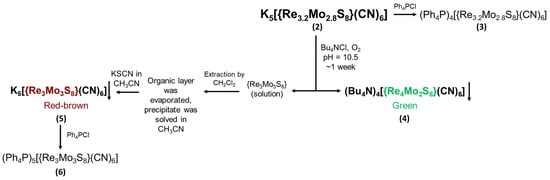
Scheme 1.
The scheme of procedure for separating individual compounds containing {Re3Mo3S8} and {Re4Mo2S8} cluster cores.
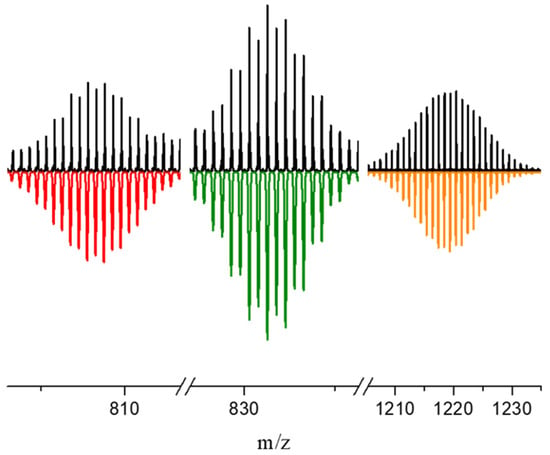
Figure 1.
Observed (top) vs. calculated (bottom) isotopic distribution peak sets corresponding to the cluster anionic adducts (Ph4P)[Re3Mo3S8(CN)6]·(H2O)2− (m/zcalcd = 808.76 Da), (Ph4P)[Re4Mo2S8(CN)5]2− (m/zcalcd = 831.28 Da) and H2[Re2Mo4S8(CN)4]·(H2O)(CH3CN)2− (m/zcalcd = 1220.40 Da) in the CH3CN solution of compound 3.
To separate clusters with different Re/Mo ratios, an excess of Bu4NCl was added to the solution of compound 2 in H2O (Scheme 1). It was found that this reaction is pH-dependent. In the case of {Re4Mo2S8}, the addition of KOH can promote oxidation. Using a KOH solution, the pH value of the reaction mixture was adjusted to 10.5. The reaction mixture was left in a glass with air access for one week. As a result, a green crystalline precipitate of compound (Bu4N)4[Re4Mo2S8(CN)6] (4) was formed.
One can see that during the reaction, one-electron oxidation of the [Re4Mo2S8(CN)6]5− cluster (23 cluster skeletal electrons—CSEs, Table 1) to [Re4Mo2S8(CN)6]4− (22 CSEs) occurred, causing the precipitation of salt 4, which is insoluble in H2O. Further holding of the filtrate solution in air did not lead to the precipitation of other products; therefore, we can assume that quantitative separation of the cluster with {Re4Mo2S8} core took place. This was confirmed by the investigation of the solution of compound 4 by mass spectrometry. In the mass spectrum (Figure 2), all intense signals correspond to adducts of the [Re4Mo2S8(CN)6]n− cluster. After the isolation of compound 4, the aqueous reaction mixture remained colored, indicating the presence of other cluster compounds. This colored species was successfully extracted with CH2Cl2, then the organic layer was separated, evaporated to dryness in air and dissolved in CH3CN. A solution of KSCN in CH3CN was added, causing the quick precipitation of brown powder of K5[Re3Mo3S8(CN)6] (5) (Scheme 1). The aqueous solution of compound 5 was mixed with the aqueous solution of Ph4PCl, causing the immediate precipitation of compound 6. As in the case of compound 3, compound 6 is soluble in CH3CN, allowing it to be examined by ESI-MS. The main isotopic distribution peak sets found match well the {Re3Mo3S8}-based cluster adducts (Figure 3). Therefore, the separation and selective isolation of clusters with the {Re4Mo2S8} and {Re3Mo3S8} cores were carried out.

Table 1.
Correlation between CSE number and charge (n) of the [Re4Mo2Se8(CN)6]n and [Re3Mo3S8(CN)6]n cluster anions.
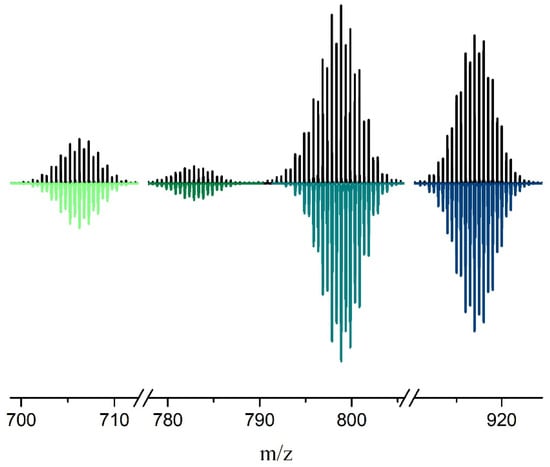
Figure 2.
Observed (top) vs. calculated (bottom) isotopic distribution peak sets corresponding to the cluster adducts H4[Re4Mo2S8(CN)6]·(H2O)(CH3CN)2− (706.24 Da), (Bu4N)[Re4Mo2S8(CN)5]2− (782.85 Da), (Bu4N)[Re4Mo2S8(CN)4(OH)]·(CH3CN)2− (798.86 Da) and (Bu4N)2[Re4Mo2S8(CN)6]2− (916.99 Da) in the ESI mass spectrum of the CH3CN solution of compound 4.
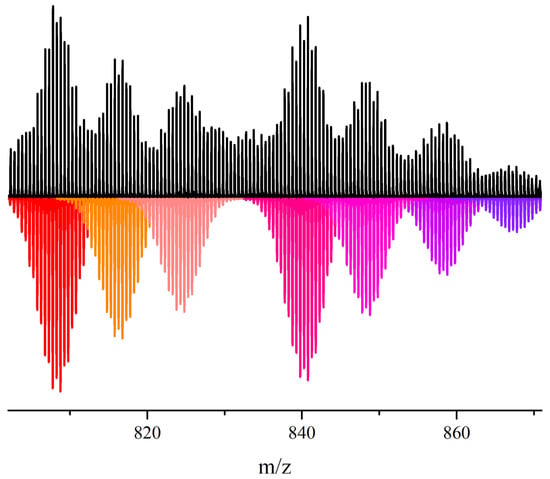
Figure 3.
Observed (top) vs. calculated (bottom) isotopic distribution peak sets corresponding to the cluster adducts (Ph4P)H3[Re3Mo3S8(CN)5]·(CH3CN)2− (808.27 Da), (Ph4P)H4[Re3Mo3S8(CN)4]·(CH3CN)22− (816.29 Da), (Ph4P)H2[Re3Mo3S8(CN)4]·(CH3CN)2(H2O)2− (824.29 Da), (Ph4P)[Re3Mo3S8(CN)6]·(CH3CN)22− (840.28 Da), (Ph4P)H[Re3Mo3S8(CN)5]·(CH3CN)32− (848.29 Da), (Ph4P)[Re3Mo3S8(CN)6]·(CH3CN)2(H2O)22− (858.29 Da) and (Ph4P)[Re3Mo3S8(CN)6]·(CH3CN)2(H2O)32− (867.29 Da) in the ESI mass spectrum of the CH3CN solution of compound 6.
2.2. Crystal Structures
Compounds (Bu4N)4[Re4Mo2S8(CN)6]·2H2O (4) and K5[Re3Mo3S8(CN)6]·8H2O (5) were investigated by single-crystal X-ray diffraction. The crystal structures of these compounds are based on cluster anions [Re4Mo2S8(CN)6]4− and [Re3Mo3S8(CN)6]5−, respectively, both having 22 CSEs. The anions exhibit the typical geometry of octahedral cluster complexes of the [M6Q8L6] type (Figure 4). The rhenium and molybdenum atoms in both structures are disordered over all positions of the metal atoms. The refinement of the site occupancies leads to chemical compositions Re3.87(3)Mo2.13(3) and Re2.94(12)Mo3.06(12), which are close to the integer values {Re4Mo2} and {Re3Mo3}. As XRD analysis provides only an average structure, it was impossible to establish the isomeric composition of the obtained clusters as well as the local Re–Re, Mo–Mo and Re–Mo interatomic distances. Nevertheless, an increase in the average interatomic M–M (2.615(1) Å and 2.623(2) Å) and M–Q (2.427(2) Å and 2.430(4) Å) distances correlated with a decrease in the Re/Mo ratio in the cluster core (compounds 4 and 5, respectively, Table 2). This correlation was found earlier for selenide heterometallic clusters [24]. M–Q and M–M are shorter for sulfide heterometallic clusters than for selenide ones, in accordance with the difference in the atomic radii of sulfur and selenium.
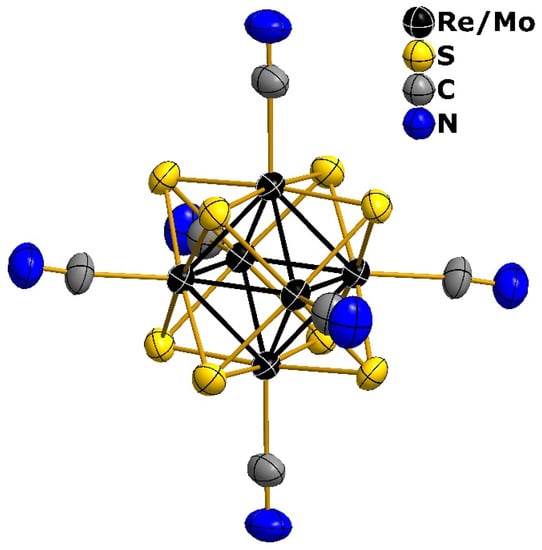
Figure 4.
Geometry of the [Re4Mo2S8(CN)6]4− cluster anion in the structure of compound 4. Thermal ellipsoids of 50% probability are given.

Table 2.
Mean bond lengths from single-crystal XRD data for Re/Mo cyanide octahedral cluster complexes.
2.3. Electrochemical Properties
The separation of the octahedral heterometallic clusters using the differences in their redox properties and solubility was first described for the clusters with {Re4Mo2Se8} and {Re3Mo3Se8} cores [24]. In that case, the process of precipitation of the (Bu4N)4[Re4Mo2Se8(CN)6] salt took only about one hour to be completed, as opposed to the one week needed to obtain compound 4. Attempts to speed up the precipitation of compound 4 by heating the solution and adding excess Bu4N+ or other alkylammonium cations have not been successful. In the CH3CN solution, the [Re4Mo2S8(CN)6]4− cluster shows two quasi-reversible transitions with E1/2 = −0.303 V and −1.186 V vs. Ag/AgCl (Figure 5a). These transitions correspond to the reduction of [Re4Mo2S8(CN)6]4− (22 CSEs) in [Re4Mo2S8(CN)6]5− (23 CSEs) and then in [Re4Mo2S8(CN)6]6− (24 CSEs, Table 1), respectively. In comparison with the [Re4Mo2S8(CN)6]n− clusters, the potentials of the corresponding transitions are shifted by 0.173 and 0.108 V to the anodic region (Table 3). This shift correlates well with the known electrochemical data for homometallic octahedral clusters of rhenium [Re6Q8(CN)6]4−/3−, where a successive cathodic shift of the oxidation potentials occurs upon changing the nature of the inner ligands from S to Se and Te [27,28]. Salt 4 can be transformed to the water-soluble salt K5[Re4Mo2S8(CN)6] by adding a solution of KSCN in CH3CN to the solution of 4 in CH3CN. The cyclic voltammetry curve for the K5[Re4Mo2S8(CN)6] salt in aqueous solution is characterized by one reversible redox transition with E1/2 at 0.073 V vs. Ag/AgCl (Figure S7). This transition corresponds to the oxidation of [Re4Mo2S8(CN)6]5− to obtain [Re4Mo2S8(CN)6]4− (22 to 23 CSEs).
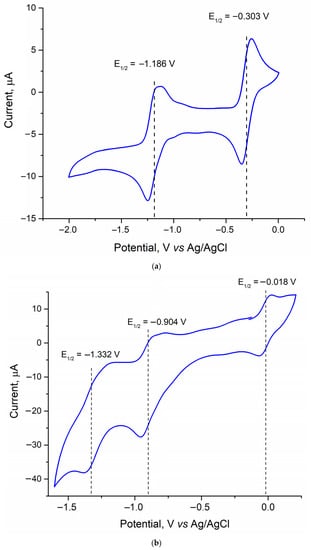
Figure 5.
Cyclic voltammograms of compound 3 in acetonitrile solution (a) and compound 5 in acetone solution (b) vs. Ag/AgCl reference electrode. Scan rate was 100 mV·s−1.

Table 3.
Redox potentials (E1/2) for {M6Q8}-type cyanide clusters (M = Re, Mo; Q = S, Se; potentials are normalized vs. Ag/AgCl/3.5M KCl electrode).
The cyclic voltammogram of the [Re3Mo3S8(CN)6]5− anion (22 CSEs, compound 5) in acetone contains three quasi-reversible redox waves (Figure 5b). One of them corresponds to the oxidation process, while the two remaining waves correspond to reduction processes. Therefore, four charge states for [Re3Mo3S8(CN)6]n− anions are electrochemically evidenced, namely, [Re3Mo3S8(CN)6]4−/5−/6−/7−, containing 21 to 24 CSEs, respectively. The half-wave potentials of these processes are −0.018, −0.904 and −1.332 V, respectively, vs. Ag/AgCl. These values are close to the previously reported ones for [Re3Mo3Se8(CN)6]4−/5−/6−/7− anions [24], with the exception of the 21/22 CSE transition for [Re3Mo3Q8(CN)6]4−/5− anions (Table 3). One can notice that the nature of the inner chalcogenide ligand has much less impact on the electrochemical behavior of clusters with a higher content of molybdenum forming the cluster.
One may see that embedding molybdenum atoms instead of rhenium ones within the {Re6} metal core increases the number of electrochemically accessible redox transitions of the resulting compounds and shifts them to the negative potential region (Table 3. The [Re6S8(CN)6]4− complex is characterized by one redox transition corresponding to a change in the number of CSEs from 24 to 23 at +0.60 V vs. Ag/AgCl [27]. In the same conditions, the [Re4Mo2S8(CN)6]6−/5− redox transition (24 to 23 CSEs) displayed a potential of −1.186 V. The substitution of one more rhenium atom for molybdenum led to the shift of the 24-to-23-CSE transition ([Re3Mo3S8(CN)6]7−/6− redox pair) to −1.332 V. Moreover, {Re3Mo3} heterometallic cluster complexes are accessible as stable compounds in highly oxidized forms up to 21 CSEs. Thus, the substitution of metals in the metal cores significantly affects the redox properties of cluster complexes, making it possible to vary the optical and electronic properties of cluster-based materials by applying voltage.
2.4. Electronic Structure
In order to analyze the geometry and electronic structure of novel heterometallic cores {Re4Mo2S8} and {Re3Mo3S8}, DFT calculations were performed for the cis- and trans-isomers of [Re4Mo2S8(CN)6]n− anions (n = 4–6, Figure 6), as well as for the fac- and mer-isomers of [Re3Mo3S8(CN)6]n− anions (n = 5–7, Figure 7). The CSE count for these anions varied from 22 to 24. The formation energies of all 24-electron anions were similar and equal to −226.518, −226.639, −228.969 and −228.855 eV for cis-[Re4Mo2S8(CN)6]6−, trans-[Re4Mo2S8(CN)6]6−, fac-[Re3Mo3S8(CN)6]7− and mer-[Re3Mo3S8(CN)6]7−, respectively. The structure of the molecular orbitals for electron-saturated heterometallic clusters shows that the two upper occupied orbitals (HOMO and HOMO-1) are separated from the lower orbitals by a relatively large energy gap. This was also mentioned for the clusters with {Re3Mo3Se8} and {Re4Mo2Se8} cores. The value of this energy gap is in the range of 0.67–0.80 eV. Frontier orbitals of clusters with 24 CSEs have bonding nature relative to metal–metal interactions below the Fermi energy level and anti-bonding characteristics above the Fermi energy level (LUMO, LUMO+1, etc.). HOMO and HOMO-1 are composed mostly of rhenium and molybdenum atomic orbitals, with some contribution of sulfur atomic orbitals (about 20%), while LUMO also has a notable contribution of C and N atomic orbitals. The HOMO–LUMO gaps are equal to about 2.2 eV for clusters with {Re3Mo3S8} cores and 2.0 eV for clusters with {Re4Mo2S8} cores.
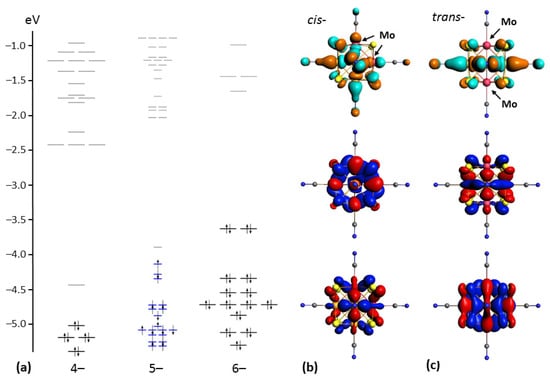
Figure 6.
Energy level diagrams of trans-isomer of [Re4Mo2S8(CN)6]n− anions (n = 4–6 from left to right, (a)). Typical view of HOMO–1, HOMO and LUMO for cis-[Re4Mo2S8(CN)6]6− anion (b). Typical view of HOMO–1, HOMO and LUMO for trans-[Re4Mo2S8(CN)6]6− anion (c). Isosurface isovalues are ±0.03 a.u.
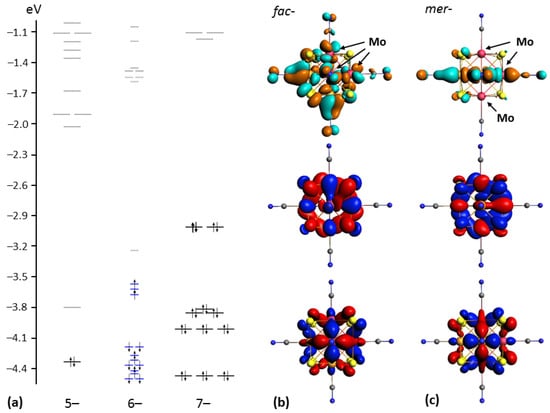
Figure 7.
Energy level diagrams of fac-isomer of [Re3Mo3S8(CN)6]n− anions (n = 5–7 from left to right, (a)). Typical view of HOMO–1, HOMO and LUMO for fac-[Re3Mo3S8(CN)6]7− anion (b). Typical view of HOMO–1, HOMO and LUMO for mer-[Re3Mo3S8(CN)6]7− anion (c). Isosurface isovalues are ±0.03 a.u.
The removal of electrons from HOMO causes a shift of HOMO–1 down in energy. The energy gap between SOMO and LUMO remains almost the same as the HOMO–LUMO gap for 24-electron anions. Further oxidation of the clusters causes a lowering of HOMO in energy. As a result, 22-electron clusters do not show a large gap between HOMO and the lower orbitals. On the contrary, the energy gap between HOMO and LUMO in 22-electron clusters (HOMO and HOMO–1 in 24-electron clusters) increases and reaches 0.5 eV. The gap between LUMO and LUMO+1 (HOMO–LUMO gap in 24-electron clusters) remains almost unchanged.
2.5. Geometry Optimization
According to the calculation data, the 24-electron metal core {Re3Mo3} is close to octahedron shape, especially for the mer-isomer and, to a lesser extent, for the fac-isomer, with little difference in M-M bond distances (Figure 8, 24 CSEs). The two-electron removal leads to a significant shortening of the Re-Re distances and an elongation of the Mo-Mo distances in the metal core in the case of both isomers (Figure 8, 22 CSEs). The distortion of the octahedron leads to C2v and Cs metal cores for mer- and fac-isomers, respectively. The difference between the longest and shortest metal–metal distances for the 22-electron mer-isomer is 0.133 Å (for the fac-isomer, 0.182 Å).
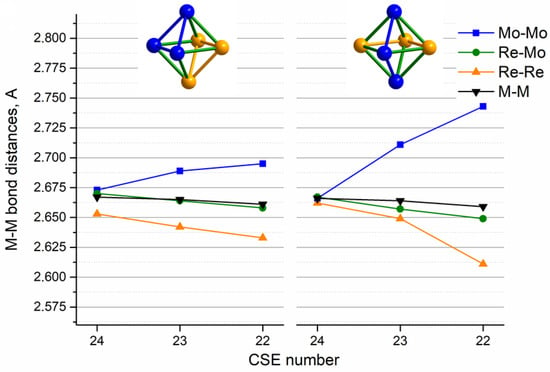
Figure 8.
M–M and M–M′ bond distances (average Mo-Mo, Re-Mo, Re-Re and overall average M-M) of [Re3Mo3S8(CN)6]n− (Å) according to optimized geometry from DFT calculations vs. CSE value of cluster anions.
A similar distortion character is observed for the {Re4Mo2} cluster, where two-electron removal also leads to the shortening of the Re–Re distances and the elongation of the Mo–Mo distances, giving C2v and D4h metal cores for cis- and trans-isomers, respectively (Figure 9).
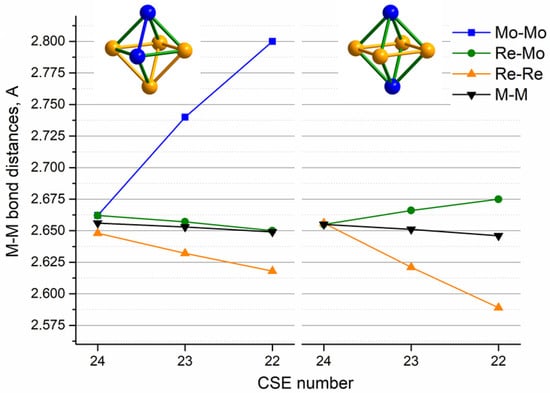
Figure 9.
Core M-M bond distances (average Mo–Mo, Re–Mo, Re–Re and overall average M–M) of [Re4Mo2S8(CN)6]n− (Å) according to optimized geometry from DFT calculations vs. CSE value of cluster anions.
The local coordination environment of rhenium and molybdenum atoms in the 22-electron {Re3Mo3} and {Re4Mo2} clusters was experimentally investigated by using the EXAFS method. It can be seen that, in both cases, the Re–Re distances are shorter than the average M–M distances, and the Mo–Mo distances are noticeably longer (Table 4 and Table 5, EXAFS column). It was shown that the interatomic M–M distances in the [Re3Mo3S8(CN)6]5− and [Re4Mo2S8(CN)6]4− cluster-based anionic units, determined theoretically by the method of quantum-chemical DFT calculations and experimentally from the EXAFS spectra, agree well with each other. Both approaches show that the {Re3Mo3} and {Re4Mo2} clusters in the cluster-based anionic units are strongly distorted in comparison with the experimental structural data obtained by X-ray diffraction. The resulting distortion cannot be determined from the structural data obtained by X-ray diffraction analysis since the symmetry of the cluster does not coincide with the symmetry of the structures, and the diffraction data contain the average positions of the metal atoms and, hence, the average interatomic distances. However, it was not possible to unambiguously determine by EXAFS the type of core isomerism from the obtained data due to the close values of the r-factor from fitting modeled data with different isomers (Tables S1–S4 in Supplementary Materials, r-factor values).

Table 4.
M-M distances (Å) in [Re3Mo3Se8(CN)6]5− by EXAFS, DFT and SC XRD.

Table 5.
M-M distances (Å) in [Re4Mo2Se8(CN)6]4− by EXAFS, DFT and SC XRD.
3. Experimental Section
3.1. Materials and Methods
All reagents and solvents were used as purchased. Elemental analysis was made on a EuroVector EA3000 analyzer (EuroVector, Pavia, Italy). Energy dispersive spectroscopy (EDS) was performed on a Hitachi TM3000 TableTop SEM (Hitachi, Ltd., Chiyoda City, Tokyo, Japan) with Bruker QUANTAX 70 EDS equipment (Bruker Corporation, Billerica, MA, USA). FT-IR spectra in KBr pellets were recorded on a Bruker Scimitar FTS 2000 spectrometer in the range 4000–375 cm−1. UV-Vis absorption measurements were performed in the wavelength range of 400–800 nm on an Agilent Cary 60 spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA). Electrospray ionization mass spectrometry was performed on a Bruker maXis 4G high-resolution ESI-q-TOF spectrometer. The mass spectra were recorded under the following conditions: registration of negative ions from m/z = 600 Da to 4000 Da, voltage +2500 V, pressure on the nebulizer of 0.8 bar, drying gas flow of 4 L/min and drying gas temperature of 180 °C. Cyclic voltammetry was carried out on an Electrochemical Instruments Elins P-20 × 8 voltammetry analyzer (Electrochemical Instruments, Chernogolovka, Russia) using a three-electrode scheme with glassy carbon working, Pt auxiliary and Ag/AgCl/3.5M KCl reference electrodes. Investigations were carried out for 2.5·10−3 M solution of cluster salts 3 and 6 in a 0.1 M solution of Bu4NClO4 in CH3CN or (CH3)2O, respectively, under an Ar atmosphere. The registered value of E1/2 for the Fc0/+ couple was 0.440 V in the same conditions. Powder X-ray diffraction (PXRD) data were collected using a Philips PW 1820/1710 diffractometer (Cu, Kα radiation, graphite monochromator, Si as external reference).
3.2. Synthesis of Compounds
ReS2 and MoS2 were synthesized by the reaction between stoichiometric amounts of corresponding metal and sulfur in evacuated silica ampoules at 1000 °C for 48 h. Phase purity of binary precursors was confirmed by PXRD.
- Preparation of K6[Re3.2Mo2.8S8(CN)5] (1). Compound 1 was prepared using a technique derived from that previously described [15]. ReS2 (1.02 g, 4 mmol), MoS2 (0.65 g, 4 mmol) and KCN (1.68 g, 25 mmol) were mixed in a silica ampoule. The ampoule was evacuated and sealed. The reaction was carried out at 700 °C for 8 days, and then the ampoule was cooled down to room temperature in 12 h. The reaction mixture was stirred in Ar-saturated H2O (250 mL) under Ar gas flow at room temperature for 1 h to remove unreacted KCN and was filtered off. The resulting solid residue was washed on a glass filter with an EtOH/H2O mixture (7/1 vol., 2 portions of 20 mL) and EtOH (30 mL). The mixture containing octahedral black crystals of 1 with an admixture of unreacted metal sulfides was separated by sonication in EtOH with subsequent decantation, and then product 1 was dried in air. Yield: 1.30 g (66% based on total amount of metals). EDS: K:Re:Mo:S = 5.7:3.2:2.8:8.3. FT-IR (KBr, cm−1): 2088 (C≡N). Phase purity of the product was confirmed by PXRD. PXRD also revealed that compound 1 is isostructural to the K6[Re3Mo3S8(CN)5] phase reported previously [15].
- Preparation of K5[Re3.2Mo2.8S8(CN)6] (2). Compound 1 (0.50 g, 0.33 mmol) and KCN (0.05 g, 0.77 mmol) were dissolved in 20 mL of H2O. The solution was evaporated on heating in a glass to a volume of about 5 mL and then 10 mL of EtOH was added, causing immediate precipitation of compound 2. The precipitate was separated by centrifugation, washed with an EtOH/H2O mixture (7/1 vol., 20 mL) and EtOH (20 mL) and dried in air. Yield: 0.34 g (70%). EDS: K:Re:Mo:S = 4.8:3.1:2.9:8.0. FT-IR (KBr, cm−1): v = 2103 (C≡N); v = 3572, 1620 (O-H). UV-vis (H2O; λmax, nm (ε, M−1cm−1)): 454 (973), 482 (1056), 804 (255). Elemental analysis: Anal. Calcd. for C6N6Re3.2Mo2.8S8 (%): C, 4.89; N, 5.71; H, 0; S, 17.42. Found: C, 4.96; N, 5.82; H, 0.14; S, 17.28. PXRD pattern shows that compound 2 is isostructural to the CaK4[Re3Mo3S8(CN)6]·8H2O phase reported previously [23] and does not present additional diffraction peaks.
- Preparation of (Ph4P)4[Re3.2Mo2.8S8(CN)6] (3). Compound 2 (0.10 g, 0.03 mmol) was dissolved in 10 mL of H2O. Ph4PCl (0.10 g, 0.26 mmol) was dissolved in H2O (10 mL) and added to the solution of compound 2, leading to the precipitation of compound 3. The precipitate was separated by centrifugation, washed with H2O and dried in air. Single crystals for X-ray diffraction studies were obtained by slow diffusion of H2O layered under the solution of compound 3 in CH3CN (15 mg mL−1) in a thin glass tube. EDS: Re:Mo:S = 3.2:2.8:7.6. FT-IR (KBr, cm−1): v = 2096 (C≡N); 1436, 1107, 995, 756, 721, 686, 522 (Ph4P+). UV-vis absorption (CH3CN; λmax, nm (ε, M−1cm−1)): 475 (1238), 483 (1266), 813 (406). Elemental analysis: Anal. Calcd. for C102N6H80Re3.2Mo2.8S8 (%): C, 46.50; N, 3.19; H, 3.06; S, 9.74. Found: C, 46.43; N, 3.32; H, 3.35; S, 9.53.
- Preparation of (Bu4N)4[Re4Mo2S8(CN)6]·2H2O (4). Compound 2 (1.00 g, 0.68 mmol) was stirred in 20 mL of H2O for 2 h, resulting in a dark-brown solution. The solution was filtered and added to the solution of n-Bu4NCl (1.00 g, 3.6 mmol) in 20 mL of H2O (the pH of the resulting solution was 9.6). KOH was added to the reaction mixture until it reached a pH of 11.0. The reaction mixture stayed at room temperature in the open glass and slowly evaporated. During the evaporation, a green precipitate of compound 4 was formed. The end of precipitation occurred in about 7 days. The precipitate was filtered, washed with H2O and dried in air. Yield: 0.52 g (33% based on initial amount of compound 2). EDS: Re:Mo:S = 4.0:2.0:7.6. FT-IR (KBr, cm−1): 2115 (C≡N). UV–vis absorption (H2O; λmax, nm (ε, M−1cm−1)): 495 (425), 452 (375), 807 (140). Elemental analysis: Anal. Calcd. for C70N10H148O2Re4Mo2S8 (%): C, 35.7; N, 5.9; H, 6.3; S, 10.9. Found: C, 34.3; N, 5.6; H, 6.1; S, 10.9. ESI-MS in CH3CN, negative ion mode, m/z (Figure 2): H4[{Re4Mo2S8}(CN)6](H2O)(CH3CN)2− (obs. 706.24, calcd. 706.24), (Bu4N)[{Re4Mo2S8}(CN)5]2− (obs. 782.85, calcd. 782.85), (Bu4N)[{Re4Mo2S8}(CN)4(OH)](CH3CN)2− (obs. 798.84, calcd. 798.86) and (Bu4N)2[{Re4Mo2S8}(CN)6]2− (obs. 916.99, calcd. 916.99). PXRD pattern of polycrystalline phase matched the calculated pattern from single-crystal X-ray diffraction data. Single crystals for X-ray diffraction studies were obtained by recrystallization of 4 from CH3CN.
- Preparation of K5[Re3Mo3S8(CN)6]·8H2O (5). The aqueous solution formed after separation of solid compound 4 was extracted with 40 mL of CH2Cl2. The organic layer was separated and evaporated in air to dryness, and the solid residue was dissolved in 15 mL of CH3CN. This solution was mixed with a solution of 0.30 g (3.1 mmol) of KSCN in 15 mL of CH3CN. A brown precipitate quickly formed after mixing the two solutions. The precipitate was dissolved in H2O, and KCN was added to the solution (0.01 g, 0.15 mmol). The solution was evaporated to 5 mL, which led to the precipitation of red-brown crystals. Yield: 0.17 g (25% based on initial amount of compound 2). Elemental analysis: Anal. Calcd. for C6N6H16O8K5Re3Mo3S8 (%): C, 4.48; N, 5.23; H, 1.00; S, 15.93. Found: C, 4.71; N, 5.53; H, 0.89; S, 16.14. FT-IR (KBr, cm−1): 2102 (C≡N). UV–vis absorption (H2O; λmax, nm (ε, M−1cm−1)): 480 (1736), 739 (565).
- Preparation of (Ph4P)5[Re3Mo3S8(CN)6] (6). A total of 300 mg (0.2 mmol) of compound 5 was dissolved in 20 mL of water. Aqueous solution of Ph4PCl (0.30 g, 0.8 mmol) was added, causing the formation of a brown-red precipitate. The precipitate was separated by centrifugation, washed with H2O and dried in air. EDS: Re:Mo:S = 2.9:3.1:8.2. FT-IR (KBr, cm−1): v = 2052 (C≡N); 1435, 1107, 995, 750, 721, 686, 522 (Ph4P+). Elemental analysis: Anal. Calcd. for C126N6H100P5Re3Mo3S8 (%): C, 51.04; N, 2.83; H, 3.40; S, 8.63. Found: C, 51.23; N, 2.78; H, 3.56; S, 8.49. ESI-MS in CH3CN, negative ion mode, m/z (Figure 3): (Ph4P)H3[{Re3Mo3S8}(CN)5]·(CH3CN)2− (obs. 808.31, calcd. 808.27), (Ph4P)H4[{Re3Mo3S8}(CN)4]·(CH3CN)22− (obs. 816.31, calcd. 816.29), (Ph4P)H2[{Re3Mo3S8}(CN)4]·(CH3CN)2(H2O)2− (obs. 824.30, calcd. 824.29), (Ph4P)[{Re3Mo3S8}(CN)6]·(CH3CN)22− (obs. 840.29, calcd. 840.28), (Ph4P)H[{Re3Mo3S8}(CN)5]·(CH3CN)32− (obs. 848.29, calcd. 848.29), (Ph4P)[{Re3Mo3S8(CN)6]·(CH3CN)2(H2O)22− (obs. 858.29, calcd. 858.29) and (Ph4P)[Re3Mo3S8(CN)6]·(CH3CN)2(H2O)32− (obs. 867.29, calcd. 867.29).
3.3. Single-Crystal Diffraction Studies
Single-crystal X-ray diffraction data were collected at room temperature on an APEX II Bruker AXS diffractometer using a Mo-Kα X-ray wavelength (λ = 0.71073 Å) and processed with the APEX 2 program suite [29]. Frame integration and data reduction were carried out with the program SAINT [30]. The program SADABS [31] was employed for multi-scan absorption corrections. The structures of compounds 4 and 5 were solved by direct methods using the SHELXT program [32] and refined with full-matrix least-squares methods based on F2 (SHELXL) [33] with the aid of the WinGX platform [34]. All non-hydrogen atoms were refined with anisotropic atomic displacement parameters. The statistical distribution of rhenium and molybdenum atoms on their respective crystallographic sites was considered by constraining equivalent atomic coordinates and anisotropic atomic displacement parameters. From preliminary refinements, a Re/Mo ratio close to 4/2 was refined for 4 and close to 3/3 for 5. Considering that these ratios are in agreement with elemental compositions determined from experimental chemical analyses, final refinements were conducted by using the SUMP command, restraining a Re/Mo ratio of 4/2 for 4 and of 3/3 for 5. This restriction has no influence on the reliability factors or the largest difference in peak and hole values. Hydrogen atoms were included in the structural model considering their calculated positions, and their equivalent isotropic displacement parameters were constrained to be equal to 1.5 times that of the linked atom for methyl hydrogens and 1.2 times for others. Hydrogen atoms in the water molecules were not localized. Publishing data were computed using CRYSCALC program [35]. Selected crystal, collection and refinement data for 4 and 5 are gathered in Table 6. Selected bond distances are listed in Table 2. Crystallographic data for the structures of the title compounds have been deposited at the Cambridge Crystallographic Data Center under reference numbers CCDC 2267663 and 2267664. Copies of this information may be obtained free of charge from the CCDC, 12 Union Road, Cambridge CB2 1 EZ, UK (Fax: +44-1223-336033; http://www.ccdc.cam.ac.uk/conts/retrieving.html; accessed on 6 June 2023).

Table 6.
Summary of single-crystal data collections and structure refinement conditions of (Bu4N)4[Re4Mo2S8(CN)6]·2H2O and K5[Re3Mo3S8(CN)6]·8H2O.
3.4. Computational Details
Density functional theory (DFT) calculations for cis- and trans-[Re4Mo2Se8(CN)6]n− cluster anions (n = 4–6) and gran- and mer-[Re3Mo3Se8(CN)6]n− cluster anions (n = 5–7) were carried out in the ADF2021 program package [36,37]. Geometric parameters were optimized with the PW92+revPBE density functional [38,39] and all-electron TZ2P basis set [40]. The zero-order regular approximation (ZORA) [41] for the scalar relativistic effects and the Conductor-like Screening Model (COSMO) [42] for water environment were used in all calculations. Spin-unrestricted calculations were used for cis- and trans-[Re4Mo2Se8(CN)6]5− and gran- and mer-[Re3Mo3Se8(CN)6]6− cluster anions containing 23 CSEs (one unpaired electron). Calculations were performed using C1 symmetry. In order to facilitate comparisons with similar works in the literature, MO units are a.u. instead of e1/2·Å−3/2, which is common practice in coordination chemistry [43,44].
3.5. EXAFS
X-ray absorption spectroscopy (XAS) measurements were carried out at room temperature in transmission mode at the K edge of Mo and L3-absorption edge of Re at the beamline SAMBA [45] in the Soleil Synchrotron, France (proposal 20210623). Radiation coming from a bending magnet source was monochromatized by a Si(220) fixed-exit sagittally focusing double-crystal monochromator. Harmonic rejection was performed using a couple of mirrors that also focused the monochromatic beam vertically (spot area around 300 × 300 μm2). The samples were examined as self-supporting pellets (matrix cellulose), for which the amount of sample was optimized in order to have a proper XAS signal. The extended X-ray absorption fine structure (EXAFS) signal treatment was performed according to standard procedures: subtraction of the pre-edge and post-edge backgrounds, edge normalization and extraction of EXAFS signal χ(k) and its Fourier transformation, which provides a map in the real space of the distribution of the distances R around the absorber atom. The Demeter software package was used to perform data treatment and fitting [46] using the phase and amplitude calculated using the FEFF-10lite code [47]. Structure models for the {Re3Mo3S8} and {Re4Mo2S8} cores were obtained from crystallographic data. The occupancies of the metal positions were changed so that the rhenium and molybdenum atoms were in different positions, corresponding to the isomerism of the core (Figures S1 and S4 in Supplementary Materials). EXAFS measurements and Fourier transform magnitudes for samples 4 and 5 are given in Figures S2, S3, S5 and S6 in the Supplementary Materials, respectively. The final refinement parameters are given in Tables S1–S4 in the Supplementary Materials.
4. Conclusions
We have demonstrated the possibility of separating anionic complexes based on {Re4Mo2S8} and {Re3Mo3S8} heterometallic clusters and their isolation as individual compounds. These clusters are obtained from the K6[{Re3.2Mo2.8S8}(CN)5] polymer synthesized at high temperature. The electrochemical behavior of the [Re3Mo3S8(CN)6]5− and [Re4Mo2S8(CN)6]4− cluster-based anionic units is different, which makes possible the selective oxidation of the {Re4Mo2S8} cluster with atmospheric oxygen in an aqueous solution and the isolation of its insoluble form (Bu4N)4[{Re4Mo2S8}(CN)6]. A comparison of the properties of anionic complexes based on the {Re4Mo2S8}, {Re3Mo3S8}, {Re4Mo2Se8} and {Re3Mo3Se8} clusters shows that the charge states and spectroscopic properties of heterometallic clusters are determined by the ratio of rhenium and molybdenum atoms in the core to a much greater extent than by the type of internal chalcogenide ligands. Such anionic complexes could be further used as building blocks for the design of photoelectrodes for solar cells. Homometallic clusters have already been shown to be promising solar cell components. For example, FTO film coated by the Cs2[{Mo6I8}I6] complex displayed extremely rare ambipolar semiconducting properties, i.e., the ability to generate both holes and electrons as main charge carriers [8]. According to the published data, molybdenum cluster complexes have an energy gap of 1.9 eV, which is close to the values for heterometallic clusters—2.2 eV for {Re3Mo3S8} and 2.0 eV for {Re4Mo2S8}. Materials based on (Bu4N)3[{Re6Q8}Cl6] (Q = S, Se) cluster complexes also exhibit ambipolar properties [9]. The energy gaps for these clusters are 2.1 and 1.9 eV for Q = S and Se, respectively. The deposition of the clusters on the FTO surface has little effect on the energy gaps. According to the published data, we assume that heterometallic cluster complexes have similar semiconductor properties but may have different optical properties in comparison with homometallic clusters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28155875/s1, Figure S1: Cluster core isomerism in [Re4Mo2S8(CN)6]; Table S1. Final fitting parameters of EXAFS spectra of (Bu4N)4[Re4Mo2S8(CN)6] (4) with trans-[Re4Mo2S8(CN)6] model; Figure S2: EXAFS measurements for (Bu4N)4[Re4Mo2S8(CN)6] (4) (weighted by k2) fitting with trans-model; Table S2: Final fitting parameters of EXAFS spectra of (Bu4N)4[Re4Mo2S8(CN)6] (4) with cis-[Re4Mo2S8(CN)6] model; Figure S3: EXAFS measurements for (Bu4N)4[Re4Mo2S8(CN)6] (4) (weighted by k2) fitting with cis-model; Figure S4: Cluster core isomerism in [Re3Mo3S8(CN)6]; Table S3: Final fitting parameters of the EXAFS spectra of K5[Re3Mo3S8(CN)6] (5) with fac-[Re3Mo3S8(CN)6] model; Figure S5: EXAFS measurements for K5[Re3Mo3S8(CN)6] (5) (weighted by k2), fiting with fac-model; Table S4: Final fitting parameters of the EXAFS spectra of K5[Re3Mo3S8(CN)6] (5) with mer-[Re3Mo3S8(CN)6] model; Figure S6: EXAFS measurements for K5[Re3Mo3S8(CN)6] (5) (weighted by k2), fitting with mer-model; Figure S7: Cyclic voltammetry curve for the K5[Re4Mo2S8(CN)6] salt in aqueous solution vs. Ag/AgCl reference electrode.
Author Contributions
Data curation, S.C. and N.G.N.; Formal analysis, C.P., P.L. and Y.M.G.; Funding acquisition, S.C. and N.G.N.; Investigation, T.I.L., V.K.M., Y.M.G., P.L., C.P. and V.V.Y.; Methodology, A.R., V.K.M. and C.P.; Resources, S.C., C.P. and N.G.N.; Supervision, Y.M.G., S.C. and N.G.N.; Visualization, T.I.L., Y.M.G., C.P. and V.K.M.; Writing—original draft, T.I.L., V.K.M. and Y.M.G.; Writing—review and editing, A.R., P.L., S.C. and N.G.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Russian Science Foundation (project 22-73-10181).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
ESI-MS was performed at the Center of Collective Use “Mass spectrometric investigations” SB RAS. We acknowledge SOLEIL for their provision of synchrotron radiation facilities and assistance in using the “SAMBA” beamline (proposal number 20210623). T. Lappi thanks the French Embassy for providing the scholarship for the co-tutelle PhD program between France and Russia. The authors are grateful to the Ministry of Science and Higher Education of the Russian Federation (projects nos. 121031700321-3, 121031700315-2 and AAAA-A21-121012290043-3).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Nguyen, N.T.K.; Lebastard, C.; Wilmet, M.; Dumait, N.; Renaud, A.; Cordier, S.; Ohashi, N.; Uchikoshi, T.; Grasset, F. A review on functional nanoarchitectonics nanocomposites based on octahedral metal atom clusters (Nb6, Mo6, Ta6, W6, Re6): Inorganic 0D and 2D powders and films. Sci. Technol. Adv. Mater. 2022, 23, 547–578. [Google Scholar] [CrossRef] [PubMed]
- Kirakci, K.; Shestopalov, M.A.; Lang, K. Recent developments on luminescent octahedral transition metal cluster complexes towards biological applications. Coord. Chem. Rev. 2023, 481, 215048. [Google Scholar] [CrossRef]
- Molard, Y. Clustomesogens: Liquid Crystalline Hybrid Nanomaterials Containing Functional Metal Nanoclusters. Acc. Chem. Res. 2016, 49, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Gray, T.G. Hexanuclear and higher nuclearity clusters of the Groups 4–7 metals with stabilizing π-donor ligands. Coord. Chem. Rev. 2003, 243, 213–235. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ishizaka, S.; Umakoshi, K.; Sasaki, Y.; Kim, H.-B.; Kitamura, N. Hexarhenium(III) Clusters [Re6(μ3-S)8X6]4− (X− = Cl−, Br−, I−) are Luminescent at Room Temperature. Chem. Lett. 1999, 28, 697–698. [Google Scholar] [CrossRef]
- Krasilnikova, A.A.; Shestopalov, M.A.; Brylev, K.A.; Kirilova, I.A.; Khripko, O.P.; Zubareva, K.E.; Khripko, Y.I.; Podorognaya, V.T.; Shestopalova, L.V.; Fedorov, V.E.; et al. Prospects of molybdenum and rhenium octahedral cluster complexes as X-ray contrast agents. J. Inorg. Biochem. 2015, 144, 13–17. [Google Scholar] [CrossRef]
- Akagi, S.; Fujii, S.; Kitamura, N. A study on the redox, spectroscopic, and photophysical characteristics of a series of octahedral hexamolybdenum(ii) clusters: [{Mo6X8}Y6]2− (X, Y = Cl, Br, or I). Dalton Trans. 2018, 47, 1131–1139. [Google Scholar] [CrossRef]
- Renaud, A.; Nguyen, T.K.N.; Grasset, F.; Raissi, M.; Guillon, V.; Delabrouille, F.; Dumait, N.; Jouan, P.Y.; Cario, L.; Jobic, S.; et al. Preparation by electrophoretic deposition of molybdenum iodide cluster-based functional nanostructured photoelectrodes for solar cells. Electrochim. Acta 2019, 317, 737–745. [Google Scholar] [CrossRef]
- Lappi, T.; Cordier, S.; Gayfulin, Y.; Ababou-Girard, S.; Grasset, F.; Uchikoshi, T.; Naumov, N.G.; Renaud, A. Nanoarchitectonics of Metal Atom Cluster-Based Building Blocks Applied to the Engineering of Photoelectrodes for Solar Cells. Solar RRL 2023, 7, 2370064. [Google Scholar] [CrossRef]
- Perrin, A.; Sergent, M.; Fischer, O. New compounds of the type Mo2Re4X8 (M = S, Se) containing octahedral Mo2Re4 clusters. Mater. Res. Bull. 1978, 13, 259–264. [Google Scholar] [CrossRef]
- Perrin, A.; Chevrel, R.; Sergent, M.; Fischer, Ø. Synthesis and electrical properties of new chalcogenide compounds containing mixed (Mo, Me)6 octahedral clusters (Me = Ru or Rh). J. Solid State Chem. 1980, 33, 43–47. [Google Scholar] [CrossRef]
- Hönle, W.; Flack, H.D.; Yvon, K. Single crystal X-ray study of Mo6Se8-type selenides containing partially substituted (Mo,Me)6 clusters (Me = Ru,Re). J. Solid State Chem. 1983, 49, 157–165. [Google Scholar] [CrossRef]
- Berry, F.J.; Gibbs, C.D.; Greaves, C. Structural properties of the molybdenum-ruthenium telluride of composition Mo4.5Ru1.5Te8. J. Solid State Chem. 1991, 92, 148–153. [Google Scholar] [CrossRef]
- Berry, F.J.; Gibbs, C.D. Synthesis of metal molybdenum tellurides of composition Mo6–MTe8(M = Ru or Rh). J. Chem. Soc. Dalton Trans. 1991, 57–59. [Google Scholar] [CrossRef]
- Caillat, T.; Fleurial, J.P. Thermoelectric properties of the semiconducting Chevrel phase Mo2Re4Se8. J. Phys. Chem. Solids 1998, 59, 1139–1144. [Google Scholar] [CrossRef]
- McGuire, M.A.; Schmidt, A.M.; Gascoin, F.; Jeffrey Snyder, G.; DiSalvo, F.J. Thermoelectric and structural properties of a new Chevrel phase: Ti0.3Mo5RuSe8. J. Solid State Chem. 2006, 179, 2158–2163. [Google Scholar] [CrossRef]
- Bronger, W.; Koppe, C.; Loevenich, M.; Schmitz, D.; Schuster, T. Cs3Re5OsS11, eine Verbindung mit gemischten Rhenium–Osmium-Clustern. Z. Anorg. Allg. Chem. 1997, 623, 695–698. [Google Scholar] [CrossRef]
- Tulsky, E.G.; Long, J.R. Heterometal Substitution in the Dimensional Reduction of Cluster Frameworks: Synthesis of Soluble [Re6-nOsnSe8Cl6](4−n)− (n = 1–3) Cluster-Containing Solids. Inorg. Chem. 2001, 40, 6990–7002. [Google Scholar] [CrossRef]
- Tulsky, E.G.; Crawford, N.R.M.; Baudron, S.A.; Batail, P.; Long, J.R. Cluster-to-Metal Magnetic Coupling: Synthesis and Characterization of 25-Electron [Re6-nOsnSe8(CN)6](5−n)− (n = 1, 2) Clusters and {Re6-nOsnSe8[CNCu(Me6tren)]6}9+ (n = 0, 1, 2) Assemblies. J. Am. Chem. Soc. 2003, 125, 15543–15553. [Google Scholar] [CrossRef]
- Brylev, K.A.; Naumov, N.G.; Kozlova, S.G.; Ryzhikov, M.R.; Kim, S.J.; Kitamura, N. Synthesis and structures of new octahedral heterometal rhenium-osmium cluster complexes. Russ. J. Coord. Chem. 2012, 38, 183–191. [Google Scholar] [CrossRef]
- Artemkina, S.B.; Naumov, N.G.; Kondrakov, K.N.; Virovets, A.V.; Kozlova, S.G.; Fedorov, V.E. Cluster Complexes with the Novel Heterometallic Cluster Core {Mo5NbI8}: Synthesis, Excision Reactions, and Crystal Structures. Z. Anorg. Allg. Chem. 2010, 636, 483–491. [Google Scholar] [CrossRef]
- Muravieva, V.K.; Gayfulin, Y.M.; Ryzhikov, M.R.; Novozhilov, I.N.; Samsonenko, D.G.; Piryazev, D.A.; Yanshole, V.V.; Naumov, N.G. Mixed-metal clusters with a {Re3Mo3Se8} core: From a polymeric solid to soluble species with multiple redox transitions. Dalton Trans. 2018, 47, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Gayfulin, Y.M.; Naumov, N.G.; Rizhikov, M.R.; Smolentsev, A.I.; Nadolinny, V.A.; Mironov, Y.V. Heterometallic clusters with a new {Re3Mo3S8} core: Direct synthesis, properties and DFT calculations. Chem. Commun. 2013, 49, 10019–10021. [Google Scholar] [CrossRef] [PubMed]
- Muravieva, V.K.; Gayfulin, Y.M.; Prestipino, C.; Lemoine, P.; Ryzhikov, M.R.; Yanshole, V.V.; Cordier, S.; Naumov, N.G. Tailoring Heterometallic Cluster Functional Building Blocks: Synthesis, Separation, Structural and DFT Studies of [Re6−xMoxSe8(CN)6]n−. Chem. Eur. J. 2019, 25, 15040–15045. [Google Scholar] [CrossRef] [PubMed]
- Muravieva, V.K.; Loginov, I.P.; Sukhikh, T.S.; Ryzhikov, M.R.; Yanshole, V.V.; Nadolinny, V.A.; Dorcet, V.; Cordier, S.; Naumov, N.G. Synthesis, Structure, and Spectroscopic Study of Redox-Active Heterometallic Cluster-Based Complexes [Re5MoSe8(CN)6]n. Inorg. Chem. 2021, 60, 8838–8850. [Google Scholar] [CrossRef] [PubMed]
- Lappi, T.I.; Gaifulin, Y.M.; Yanshole, V.V.; Cordier, S.; Naumov, N.G. Evidences of the non-stoichiometry and control of the composition of the cluster-based solid solution K6[Re6−xMoxS8(CN)5] (x = 2.75–3.63). J. Solid State Chem. 2023, 319, 123785. [Google Scholar] [CrossRef]
- Yoshimura, T.; Ishizaka, S.; Sasaki, Y.; Kim, H.-B.; Kitamura, N.; Naumov, N.G.; Sokolov, M.N.; Fedorov, V.E. Unusual Capping Chalcogenide Dependence of the Luminescence Quantum Yield of the Hexarhenium(III) Cyano Complexes [Re6(μ3-E)8(CN)6]4−, E2− = Se2− > S2− > Te2−. Chem. Lett. 1999, 28, 1121–1122. [Google Scholar] [CrossRef]
- Gabriel, J.-C.P.; Boubekeur, K.; Uriel, S.; Batail, P. Chemistry of Hexanuclear Rhenium Chalcohalide Clusters. Chem. Rev. 2001, 101, 2037–2066. [Google Scholar] [CrossRef]
- APEX2; Version 2.0; Bruker Advanced X-ray Solutions: Madison, WI, USA, 2000.
- SAINT; Version 8.18c; Bruker Advanced X-ray Solutions: Madison, WI, USA, 2012.
- SADABS; Version 2.11; Bruker Advanced X-ray Solutions: Madison, WI, USA, 2000.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Roisnel, T. CRYSCALC (Crystallographic Calculations), Rennes, France. Available online: www.cdifx.univ-rennes1.fr/cryscalc (accessed on 6 June 2023).
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comp. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF 2023.1, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: https://www.scm.com (accessed on 6 June 2023).
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Van Lenthe, E.; Baerends, E.J. Optimized Slater-type basis sets for the elements 1–118. J. Comput. Chem. 2003, 24, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- van Lenthe, E.; Ehlers, A.; Baerends, E.-J. Geometry optimizations in the zero order regular approximation for relativistic effects. J. Chem. Phys. 1999, 110, 8943–8953. [Google Scholar] [CrossRef]
- Pye, C.C.; Ziegler, T. An implementation of the conductor-like screening model of solvation within the Amsterdam density functional package. Theor. Chem. Acc. 1999, 101, 396–408. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Davydova, M.P.; Berezin, A.S.; Ryzhikov, M.R.; Samsonenko, D.G. Dicopper(I) Paddle-Wheel Complexes with Thermally Activated Delayed Fluorescence Adjusted by Ancillary Ligands. Inorg. Chem. 2020, 59, 10699–10706. [Google Scholar] [CrossRef]
- Gushchin, A.L.; Laricheva, Y.A.; Sokolov, M.N.; Llusar, R. Tri- and tetranuclear molybdenum and tungsten chalcogenide clusters: On the way to new materials and catalysts. Russ. Chem. Rev. 2018, 87, 670–706. [Google Scholar] [CrossRef]
- Briois, V.; Fonda, E.; Belin, S.; Barthe, L.; La Fontaine, C.; Langlois, F.; Ribbens, B.; Villain, F. SAMBA: The 4–40 keV X-ray absorption spectroscopy beamline at SOLEIL. In UVX 2010; EDP Sciences: Les Ulis, France, 2011; pp. 41–47. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Ankudinov, A.L.; Ravel, B.; Rehr, J.J.; Conradson, S.D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 1998, 58, 7565–7576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).