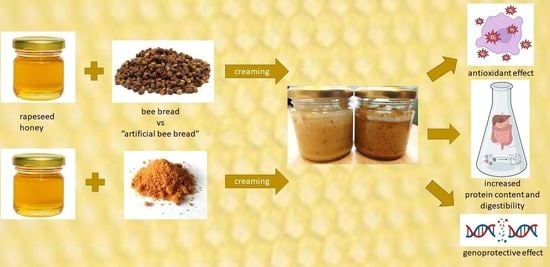
The Comparison of Honey Enriched with Laboratory Fermented Pollen vs. Natural Bee Bread in Terms of Nutritional and Antioxidant Properties, Protein In Vitro Bioaccessibility, and Its Genoprotective Effect in Yeast Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Evaluation
2.2. Total Phenolic Content and Antioxidant Properties
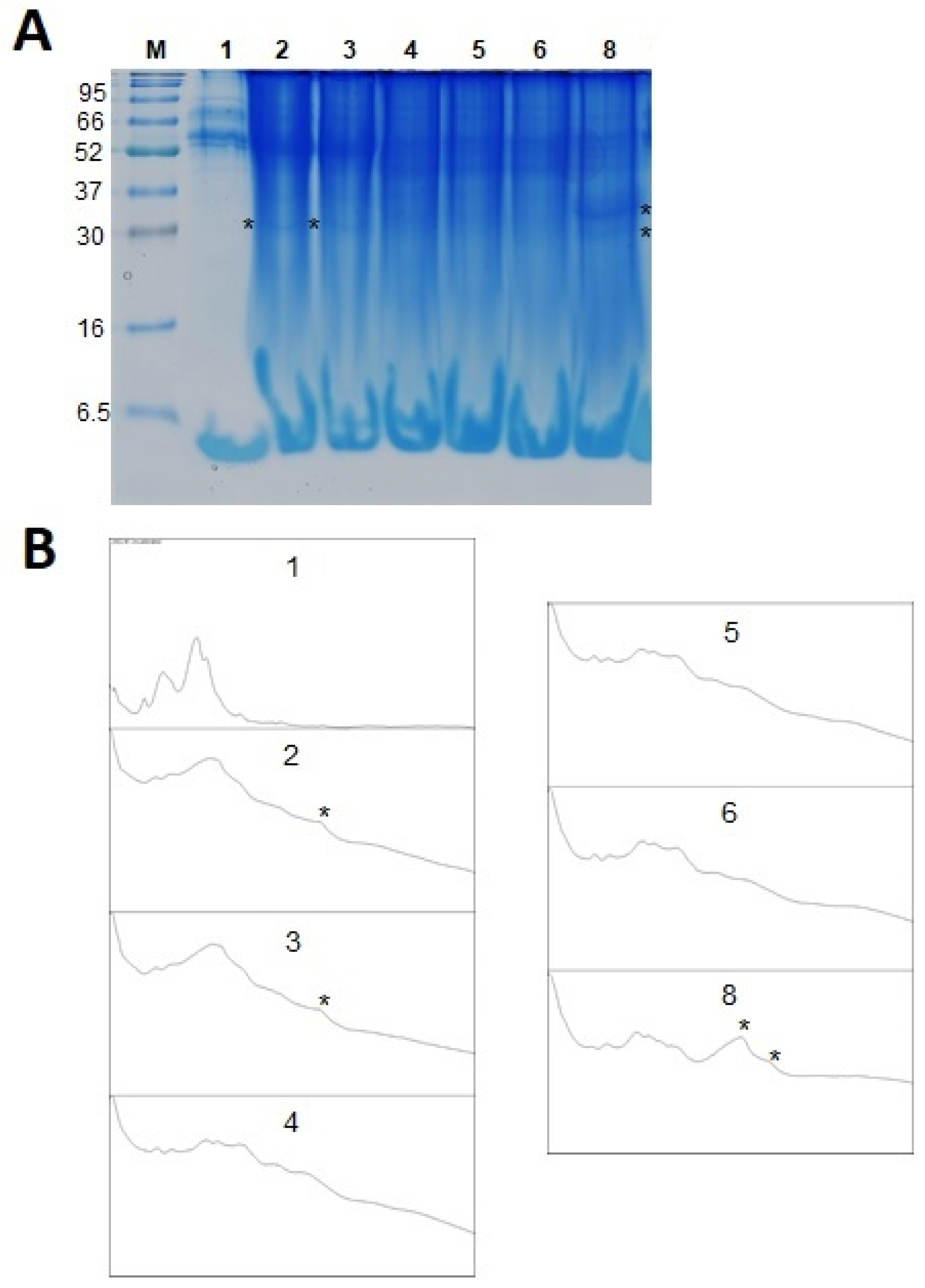
2.3. Protein Content and SDS PAGE Analysis
2.4. Protein In Vitro Digestibility
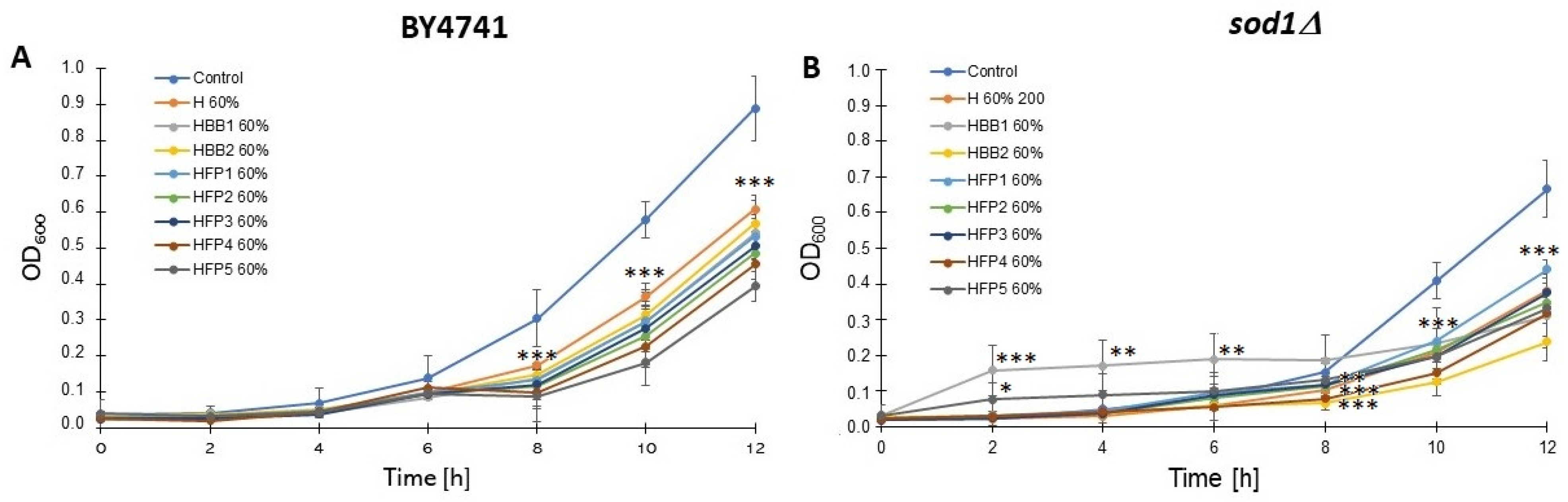
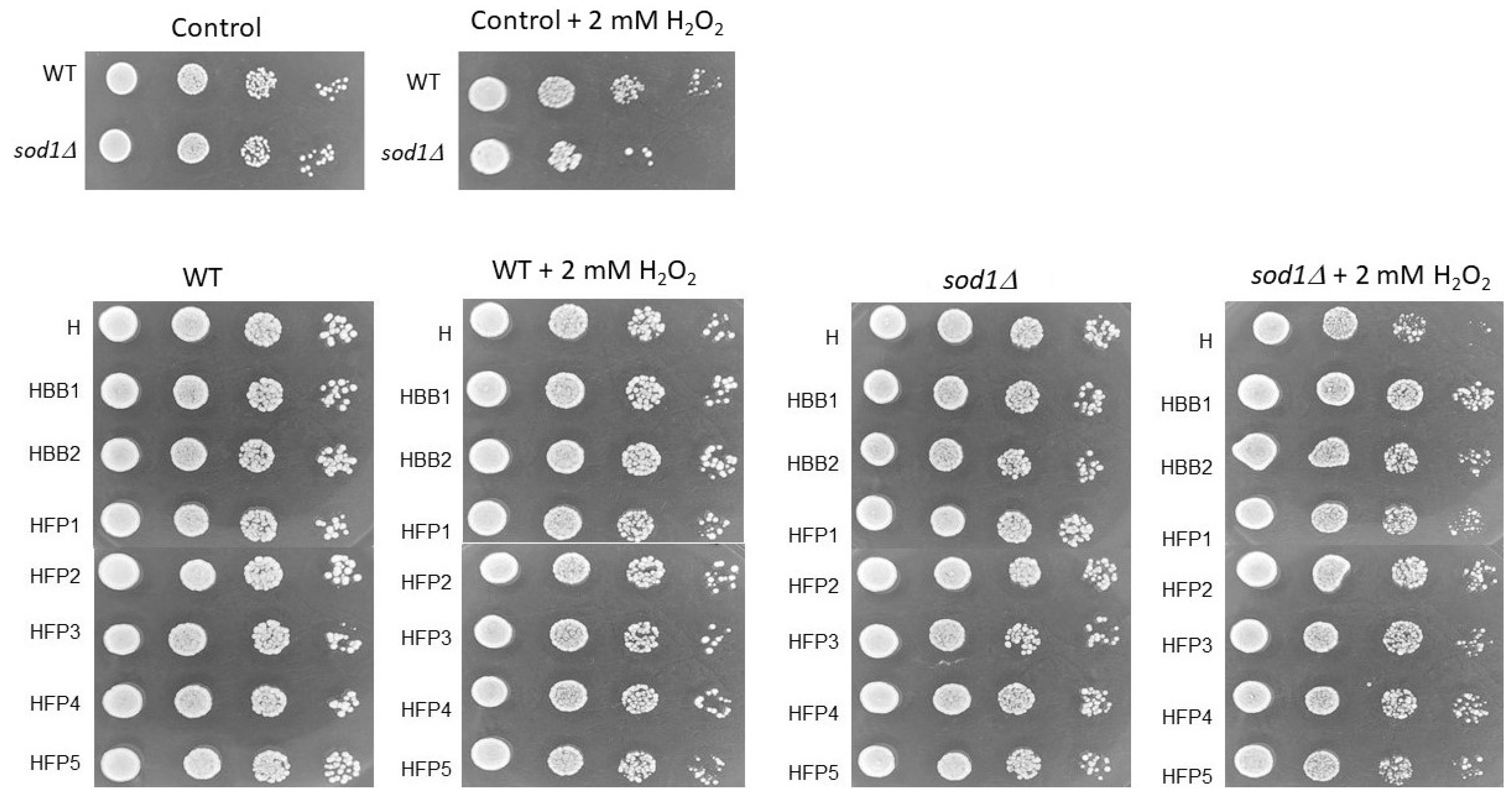
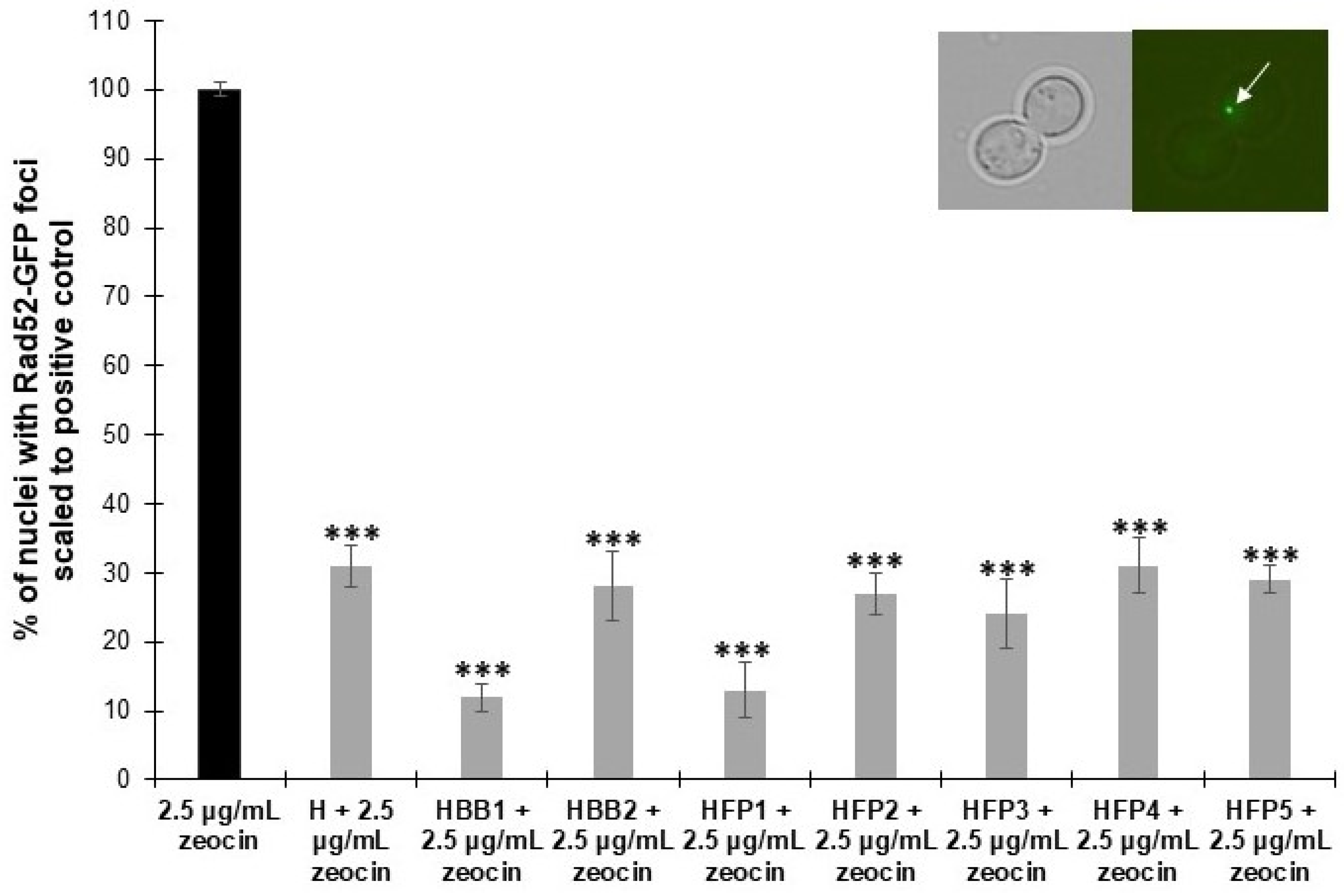
2.5. Biological Activity Using the Yeast Model
3. Materials and Methods
3.1. Honey, Bee Pollen, and Bee Bread
3.2. Bee Pollen Fermentation in Laboratory Conditions
3.3. Preparation of Honey Enriched with Bee Bread and Fermented Pollen
3.4. Enriched Honey Analysis
3.4.1. Physicochemical Parameters
3.4.2. Total Phenolic Content and Antioxidant Properties
3.4.3. Total and Soluble Protein Determination
3.4.4. SDS PAGE Protein Profile Analysis
3.4.5. In Vitro Bioaccessibility of the Enriched Honeys
3.5. Yeast Strain and Growth Conditions
3.6. Kinetics of the Growth Assay
3.7. Spot Tests
3.8. Cellular Localization of the Rad52-GFP Proteins
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Juszczak, L.; Gałkowska, D.; Ostrowska, M.; Socha, R. Antioxidant activity of honey supplemented with bee products. Nat. Prod. Res. 2016, 30, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Štajner, D.; Popović, B.M.; Čanadanović-Brunet, J.; Dilas, S.; Ćetković, G. Nutritive composition and free radical scavenger activity of honey enriched with of Rosa spp. LWT—Food Sci. Technol. 2014, 55, 408–413. [Google Scholar] [CrossRef]
- Wilczyńska, A.; Newerli-Guz, J.; Szweda, P. Influence of the addition of selected spices on sensory quality and biological activity of honey. J. Food Qual. 2017, 2017, 6963904. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. The Influence of Bee Bread on Antioxidant Properties, Sensory and Quality Characteristics of Multifloral Honey. Appl. Sci. 2023, 13, 7913. [Google Scholar] [CrossRef]
- Kowalski, S.; Makarewicz, M. Functional properties of honey supplemented with bee bread and propolis. Nat. Prod. Res. 2017, 31, 2680–2683. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Xiao, J.; Zou, X.; Khatib, A.; Göransson, U.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Ćirić, J.; Haneklaus, N.; Rajić, S.; Baltić, T.; Lazić, I.B.; Đorđević, V. Chemical composition of bee bread (perga), a functional food: A review. J. Trace Elem. Miner. 2022, 2, 100038. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-Safi, I.; Mechchate, H.; Lyoussi, B. Bee Bread as a Promising Source of Bioactive Molecules and Functional Properties: An Up-to-Date Review. Antibiotics 2022, 11, 203. [Google Scholar] [CrossRef]
- Socha, R.; Habryka, C.; Juszczak, L. Effect of bee bread additive on content of phenolic compounds and antioxidant activity of honey. Zywn. Nauk. Technol. Jakosc/Food Sci. Technol. Qual. 2018, 25, 108–119. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. The Influence of Honey Enrichment with Bee Pollen or Bee Bread on the Content of Selected Mineral Components in Multifloral Honey. Potravin. Slovak J. Food Sci. 2020, 14, 874–880. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P.; Medagli, P. Aegilops (Poaceae) in Italy: Taxonomy, geographical distribution, ecology, vulnerability and conservation. Syst. Biodivers. 2014, 12, 331–349. [Google Scholar] [CrossRef]
- Casella, F.; Vurro, M.; Valerio, F.; Perrino, E.V.; Mezzapesa, G.N.; Boari, A. Phytotoxic Effects of Essential Oils from Six Lamiaceae Species. Agronomy 2023, 13, 257. [Google Scholar] [CrossRef]
- Knazovická, V.; Mašková, Z.; Vlková, E.; Švejstil, R.; Salmonová, H.; Ivanišová, E.; Gažarová, M.; Gamráthová, I.; Repková, M.; Tokár, M.; et al. Pollen can—Testing of bee pollen fermentation in model conditions. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 805–811. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Katilevičiūtė, A.; Kaškonas, P.; Maruška, A. The impact of solid-state fermentation on bee pollen phenolic compounds and radical scavenging capacity. Chem. Pap. 2018, 72, 2115–2120. [Google Scholar] [CrossRef]
- Shirsat, D.V.; Kad, S.K.; Wakhle, D.M. Solid State Fermentation of Bee-Collected Pollen. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1557–1563. [Google Scholar] [CrossRef]
- Mora-Adames, W.I.; Fuenmayor, C.A.; Benavides-Martín, M.A.; Algecira-Enciso, N.A.; Quicazán, M.C. Bee pollen as a novel substrate in pilot-scale probiotic-mediated lactic fermentation processes. LWT 2021, 141, 110868. [Google Scholar] [CrossRef]
- Miłek, M.; Mołoń, M.; Kula-Maximenko, M.; Sidor, E.; Zaguła, G.; Dżugan, M. Chemical Composition and Bioactivity of Laboratory-Fermented Bee Pollen in Comparison with Natural Bee Bread. Biomolecules 2023, 13, 1025. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Plutino, M.; Lucini, L.; Aromolo, R.; Martinelli, E.; Souto, E.B.; Santini, A.; Pignatti, G. Bee products: A representation of biodiversity, sustainability, and health. Life 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Loukas, P.; Maria, T. The Application of Honeybee Products in the Health Sector. Adv. Biol. Chem. 2023, 13, 1–16. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Islam, M.T.; Quispe, C.; Mubarak, M.S.; Salehi, B.; Reiner, Ž.; Martorell, M.; Sharifi-Rad, J.; Setzer, W.N. Protective effects of natural products and their derivatives on genetic material: A critical review. Rec. Nat. Prod. 2021, 15, 433–462. [Google Scholar] [CrossRef]
- Bagatir, G.; Kaya, M.; Suer, I.; Cefle, K.; Palanduz, A.; Palanduz, S.; Becerir, H.B.; Koçyiğit, M.; Ozturk, S. The effect of Anzer honey on X-ray induced genotoxicity in human lymphocytes: An in vitro study. Microsc. Res. Tech. 2022, 85, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Ratanavalachai, T.; Thitiorul, S.; Jenkhetkan, W.; Jansom, C.; Itharat, A. Genoprotective Effects of Crude Thai Bee Pollen and Its Extracts Against Doxorubicin, A Potent Genotoxic Compound in Human Lymphocytes. In Proceedings of the 65th International Congress and Annual Meeting of the Society for Medicinal Plant and Natural Product Research (GA 2017), Basel, Switzerland, 3–7 September 2017; Volume 4. [Google Scholar] [CrossRef]
- Jenkhetkhan, W.; Thitiorul, S.; Jansom, C.; Ratanavalachai, T. Genoprotective Effects of Thai Royal Jelly against Doxorubicin in Human Lymphocytes in Vitro. Nat. Prod. Commun. 2018, 13, 79–84. [Google Scholar] [CrossRef]
- Ratanavalachai, T.; Jenkhetkan, W.; Jansom, C.; Itharat, A.; Thitiorul, S. Genotoxic, Antigenotoxic, and Antioxidative Potentials of Thai Bee Products. Asian Med. J. Altern. Med. 2022, 22, 219–229. [Google Scholar]
- Sánchez-Martín, V.; Haza, A.I.; Iriondo-DeHond, A.; del Castillo, M.D.; Hospital, X.F.; Fernández, M.; Hierro, E.; Morales, P. Protective Effect of Thyme and Chestnut Honeys Enriched with Bee Products against Benzo(a)pyrene-Induced DNA Damage. Int. J. Environ. Res. Public Health 2022, 19, 16969. [Google Scholar] [CrossRef] [PubMed]
- Directive 2001/110/EC of the European Council relating to honey. Off. J. Eur. Communities 2002, L010, 0047–0052. Available online: https://eur-lex.europa.eu/eli/dir/2001/110/oj (accessed on 1 July 2023).
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef]
- Szczesna, T.; Rybak-Chmielewska, H.; Waś, E.; Kachaniuk, K.; Teper, D. Characteristics of Polish unifloral honeys. I. Rape honey (Brassica napus L. var. oleifera Metzger). J. Apic. Sci. 2011, 55, 111–119. [Google Scholar]
- Majewska, E.; Kowalska, J. Badanie korelacji pomiędzy przewodnością elektryczną i zawartością popiołu w wybranych miodach pszczelich. Acta Agrophys. 2011, 17, 369–376. (In Polish) [Google Scholar]
- Dżugan, M.; Zaguła, G.; Wesołowska, M.; Sowa, P.; Puchalski, C. Levels of toxic and essential metals in varietal honey form Podkarpacie. J. Elem. 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Habryka, C.; Socha, R.; Juszczak, L. Effect of bee pollen addition on the polyphenol content, antioxidant activity, and quality parameters of honey. Antioxidants 2021, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Physical properties of honey: Honey composition. In The Honey Book; University of Belgrade: Belgrade, Serbia, 2011. [Google Scholar]
- Poyraz, F.; Yalmanci, D.; Ispirli, H.; Dertli, E. Characterization of Bee Bread Produced with Defined Starter Cultures Mimickingthe Natural Fermentation Process. Fermentation 2023, 9, 174. [Google Scholar] [CrossRef]
- Bocian, A.; Buczkowicz, J.; Jaromin, M.; Hus, K.K.; Legáth, J. An effective method of isolating honey proteins. Molecules 2019, 24, 2399. [Google Scholar] [CrossRef]
- Nagai, T.; Nagashima, T.; Myoda, T.; Inoue, R. Preparation and functional properties of extracts from bee bread. Nahrung/Food 2004, 48, 226–229. [Google Scholar] [CrossRef]
- Tawfik, A.I.; Ahmed, Z.H.; Abdel-Rahman, M.F.; Moustafa, A.M. Effect of some bee bread quality on protein content and antioxidant system of honeybee workers. Int. J. Trop. Insect Sci. 2022, 43, 93–105. [Google Scholar] [CrossRef]
- Darwish, A.M.G.; Abd El-Wahed, A.A.; Shehata, M.G.; El-Seedi, H.R.; Masry, S.H.D.; Khalifa, S.A.M.; Mahfouz, H.M.; El-Sohaimy, S.A. Chemical Profiling and Nutritional Evaluation of Bee Pollen, Bee Bread, and Royal Jelly and Their Role in Functional Fermented Dairy Products. Molecules 2023, 28, 227. [Google Scholar] [CrossRef]
- Wu, W.; Qiao, J.; Xiao, X.; Kong, L.; Dong, J.; Zhang, H. In vitro and In vivo digestion comparison of bee pollen with or without wall-disruption. J. Sci. Food Agric. 2021, 101, 2744–2755. [Google Scholar] [CrossRef]
- Aylanc, V.; Falcão, S.I.; Vilas-Boas, M. Bee pollen and bee bread nutritional potential: Chemical composition and macronutrient digestibility under in vitro gastrointestinal system. Food Chem. 2023, 413, 135597. [Google Scholar] [CrossRef]
- Aylanc, V.; Tomás, A.; Russo-Almeida, P.; Falcão, S.I.; Vilas-Boas, M. Assessment of bioactive compounds under simulated gastrointestinal digestion of bee pollen and bee bread: Bioaccessibility and antioxidant activity. Antioxidants 2021, 10, 651. [Google Scholar] [CrossRef] [PubMed]
- Baky, M.H.; Abouelela, M.B.; Wang, K.; Farag, M.A. Bee Pollen and Bread as a Super-Food: A Comparative Review of Their Metabolome Composition and Quality Assessment in the Context of Best Recovery Conditions. Molecules 2023, 28, 715. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Miłek, M.; Grabek-Lejko, D.; Stȩpień, K.; Sidor, E.; Mołoń, M.; Dżugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef]
- Fernandes, L.; Ribeiro, H.; Oliveira, A.; Sanches Silva, A.; Freitas, A.; Henriques, M.; Rodrigues, M.E. Portuguese honeys as antimicrobial agents against Candida species. J. Tradit. Complement. Med. 2021, 11, 130–136. [Google Scholar] [CrossRef]
- Condon, R.E. Curious interaction of bugs and bees. Surgery 1993, 113, 234–235. [Google Scholar]
- Czachor, J.; Miłek, M.; Galiniak, S.; Stępień, K.; Dżugan, M.; Mołoń, M. Coffee extends yeast chronological lifespan through antioxidant properties. Int. J. Mol. Sci. 2020, 21, 9510. [Google Scholar] [CrossRef]
- ISO 18125:2017; Solid Biofuels—Determination of Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2017.
- PN-75/A-04018; Agricultural and Food Products. Determination of Nitrogen by the Kjeldahl Method and Conversion to Protein. Polish Committee for Standardization: Warszawa, Poland, 2002.
- Rabie, A.L.; Wells, J.D.; Dent, L.K. The Nitrogen Content of Pollen Protein. J. Apic. Res. 1983, 22, 119–123. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Sidor, E.; Miłek, M.; Tomczyk, M. The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy. Appl. Sci. 2023, 13, 4687. [Google Scholar] [CrossRef]
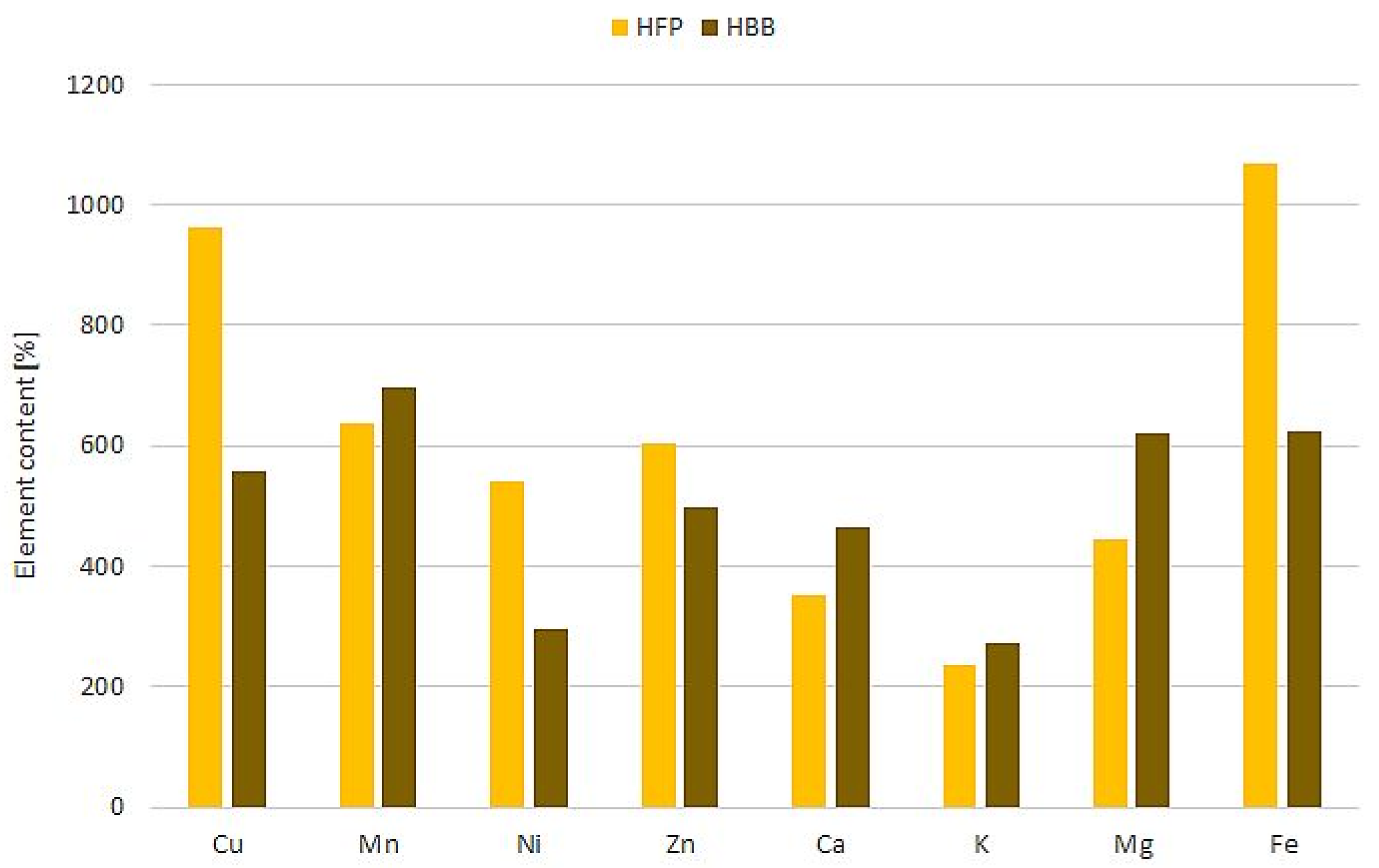

| Sample | Water Content [%] | Water Activity | Conductivity [mS/cm] | pH | Free Acidity [mval/kg] | Energy Value [kcal/100 g] |
|---|---|---|---|---|---|---|
| H | 17.80 ± 0.00 a | 0.5676 ± 0.0006 a | 0.149 ± 0.000 a | 4.25 ± 0.01 a | 8.95 ± 0.35 a | 330.6 ± 2.3 a |
| HBB1 | 17.40 ± 0.00 b | 0.5712 ± 0.0025 b | 0.528 ± 0.001 b | 3.97 ± 0.00 b | 99.26 ± 1.07 b | 342.7 ± 3.1 bc |
| HBB2 | 16.90 ± 0.28 c | 0.5673 ± 0.0000 a | 0.537 ± 0.000 c | 3.96 ± 0.01 c | 97.25 ± 2.47 b | 339.0 ± 3.5 bc |
| HFP1 | 17.15 ± 0.21 bc | 0.5566 ± 0.0005 c | 0.424 ± 0.000 d | 4.35 ± 0.01 d | 65.85 ± 0.21 c | 340.5 ± 4.0 bc |
| HFP2 | 17.85 ± 0.07 a | 0.5708 ± 0.0020 b | 0.426 ± 0.002 d | 4.38 ± 0.01 e | 62.95 ± 0.35 d | 338.2 ± 1.8 ab |
| HFP3 | 16.95 ± 0.07 c | 0.5729 ± 0.0020 b | 0.459 ± 0.001 e | 4.48 ± 0.00 f | 62.20 ± 0.28 d | 341.1 ± 1.3 bc |
| HFP4 | 15.75 ± 0.35 d | 0.5531 ± 0.0005 d | 0.601 ± 0.001 f | 4.15 ± 0.00 g | 90.00 ± 0.71 e | 347.0 ± 4.0 c |
| HFP5 | 16.45 ± 0.07 e | 0.5569 ± 0.0000 c | 0.665 ± 0.001 g | 4.41 ± 0.01 h | 70.45 ± 0.49 f | 345.6 ± 2.4 bc |
| Sample | TPC [mg GAE/100 g] | FRAP [μmol TE/100 g] | DPPH [μmol TE/100 g] |
|---|---|---|---|
| H | 17.51 ± 0.90 a | 18.87 ± 0.94 a | 3.84 ± 5.28 a |
| HBB1 | 80.43 ± 9.56 b | 105.15 ± 8.92 bc | 41.76 ± 1.95 b |
| HBB2 | 88.91 ± 8.43 b | 133.00 ± 13.10 c | 46.07 ± 7.08 bc |
| HFP1 | 81.67 ± 7.06 b | 117.71 ± 6.59 bc | 44.71 ± 2.85 bc |
| HFP2 | 82.76 ± 10.39 b | 97.92 ± 7.46 b | 53.40 ± 11.66 bc |
| HFP3 | 97.89 ± 19.05 bc | 111.57 ± 13.71 bc | 55.64 ± 10.77 bc |
| HFP4 | 119.77 ± 9.60 c | 124.40 ± 13.73 bc | 65.21 ± 0.53 bc |
| HFP5 | 101.64 ± 10.59 bc | 122.37 ± 9.18 bc | 68.40 ± 16.66 c |
| Sample | Total Protein (Kjeldahl Method) [g/100 g] | Soluble Protein (Bradford Method) [g/100 g] |
|---|---|---|
| H | 0.220 ± 0.006 a | 0.174 ± 0.004 a |
| HBB1 | 2.207 ± 0.026 b | 0.858 ± 0.009 b |
| HBB2 | 2.107 ± 0.041 b | 0.818 ± 0.021 bc |
| HFP1 | 1.633 ± 0.067 cd | 0.863 ± 0.027 b |
| HFP2 | 1.707 ± 0.056 c | 0.782 ± 0.038 c |
| HFP3 | 1.692 ± 0.035 cd | 0.714 ± 0.010 d |
| HFP4 | 1.729 ± 0.050 d | 0.494 ± 0.036 e |
| HFP5 | 1.698 ± 0.008 cd | 0.616 ± 0.022 f |
| Sample | Bee Pollen | Multifloral Honey | Water | Starter Culture | Fermentation Time and Temperature |
|---|---|---|---|---|---|
| FP1 | 10 g | 7.5 g | 12.5 mL | L. rhamnosus GG 1 g | 48 h in 32 °C and 4 weeks in 25 °C |
| FP2 | 10 gultrasound treated (2 × 15 min., 700 W) | 7.5 g | 12.5 mL | L. rhamnosus GG 1 g | 48 h in 32 °C and 4 weeks in 25 °C |
| FP3 | 10 g | 7.5 g | 12.5 mL | - | 48 h in 32 °C and 4 weeks in 25 °C |
| FP4 | 10 g | 3.33 mL (60% solution) | - | L. acidophilus 1 g | 120 h in 37 °C |
| FP5 | 10 g | 3.33 mL (60% solution) | 10 mL | L. acidophilus 1 g | 120 h in 37 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, M.; Mołoń, M.; Kielar, P.; Sidor, E.; Bocian, A.; Marciniak-Lukasiak, K.; Pasternakiewicz, A.; Dżugan, M. The Comparison of Honey Enriched with Laboratory Fermented Pollen vs. Natural Bee Bread in Terms of Nutritional and Antioxidant Properties, Protein In Vitro Bioaccessibility, and Its Genoprotective Effect in Yeast Cells. Molecules 2023, 28, 5851. https://doi.org/10.3390/molecules28155851
Miłek M, Mołoń M, Kielar P, Sidor E, Bocian A, Marciniak-Lukasiak K, Pasternakiewicz A, Dżugan M. The Comparison of Honey Enriched with Laboratory Fermented Pollen vs. Natural Bee Bread in Terms of Nutritional and Antioxidant Properties, Protein In Vitro Bioaccessibility, and Its Genoprotective Effect in Yeast Cells. Molecules. 2023; 28(15):5851. https://doi.org/10.3390/molecules28155851
Chicago/Turabian StyleMiłek, Michał, Mateusz Mołoń, Patrycja Kielar, Ewelina Sidor, Aleksandra Bocian, Katarzyna Marciniak-Lukasiak, Anna Pasternakiewicz, and Małgorzata Dżugan. 2023. "The Comparison of Honey Enriched with Laboratory Fermented Pollen vs. Natural Bee Bread in Terms of Nutritional and Antioxidant Properties, Protein In Vitro Bioaccessibility, and Its Genoprotective Effect in Yeast Cells" Molecules 28, no. 15: 5851. https://doi.org/10.3390/molecules28155851
APA StyleMiłek, M., Mołoń, M., Kielar, P., Sidor, E., Bocian, A., Marciniak-Lukasiak, K., Pasternakiewicz, A., & Dżugan, M. (2023). The Comparison of Honey Enriched with Laboratory Fermented Pollen vs. Natural Bee Bread in Terms of Nutritional and Antioxidant Properties, Protein In Vitro Bioaccessibility, and Its Genoprotective Effect in Yeast Cells. Molecules, 28(15), 5851. https://doi.org/10.3390/molecules28155851