Phosphodiesterase 5 and Arginase Inhibitory Activities of the Extracts from Some Members of Nelumbonaceae and Nymphaeaceae Families
Abstract
1. Introduction
2. Results
2.1. Phosphodiesterase-5 Inhibition of the Extracts
2.2. Arginase Inhibition of the Extracts
2.3. Isolation and Identification of PDE5 Inhibitors from N. pubescens Petal Ethanolic Extract
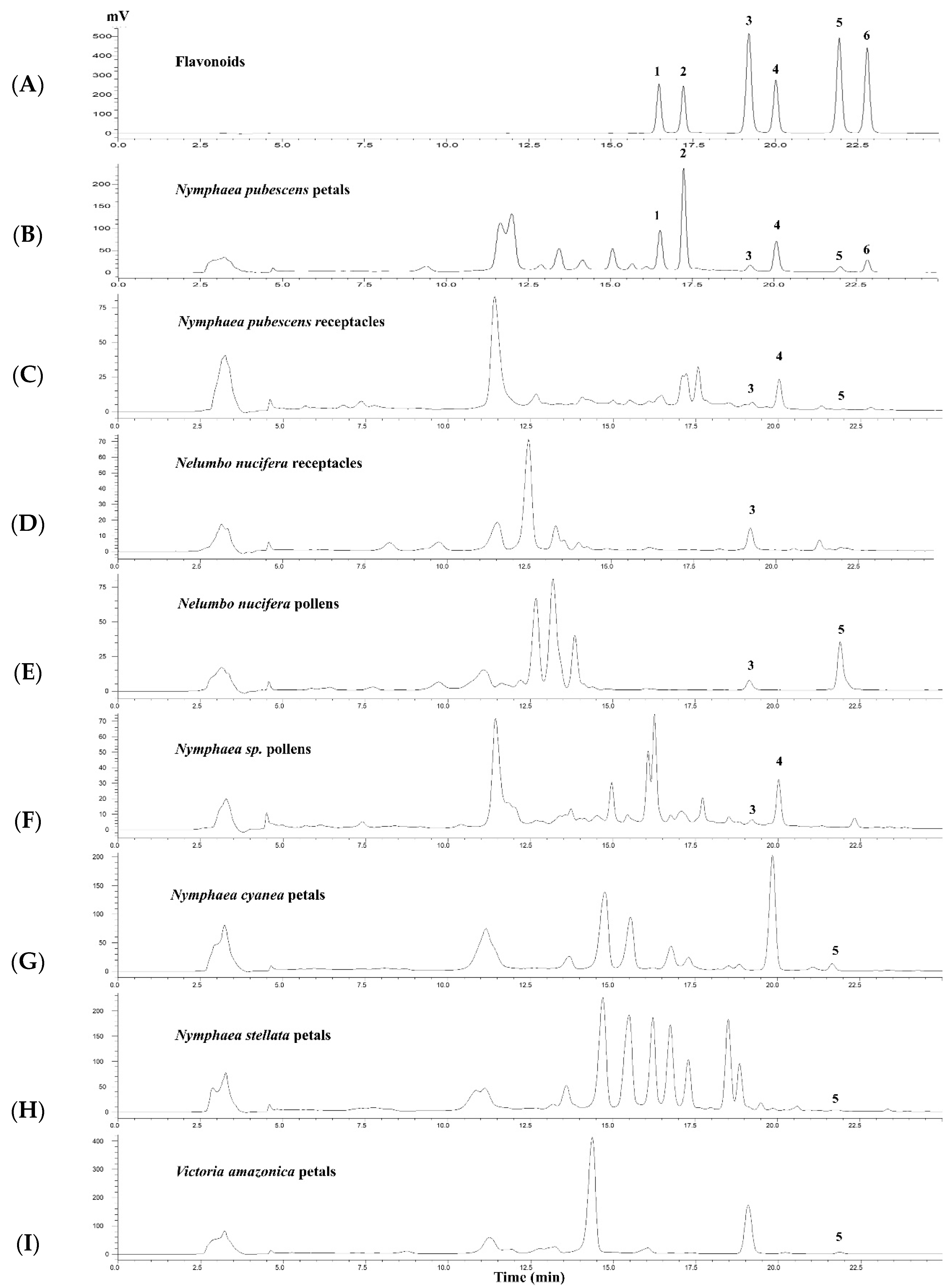
2.4. The HPLC Method for Quantitative Analysis of Flavonoids in Plant Extracts
2.5. Contents of 6 Flavonoids in Some Plant Members of Nelumbonaceae and Nymphaeaceae
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction and Isolation
4.3. Chemicals
4.4. Phosphodiesterase 5 Inhibition Assay
4.4.1. Sample Preparation
4.4.2. Enzyme Preparation
4.4.3. Experimental Protocols
4.5. Arginase Inhibition Assay
4.5.1. Sample Preparation
4.5.2. Experimental Protocols
4.6. Sample Preparation for HPLC Analysis
4.7. Instrumentation and Chromatographic Conditions
4.8. Method Validation
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mazzilli, F. Erectile dysfunction: Causes, diagnosis and treatment: An update. J. Clin. Med. 2022, 11, 6429. [Google Scholar] [CrossRef]
- Goldstein, I.; Goren, A.; Li, V.W.; Tang, W.Y.; Hassan, T.A. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex. Med. Rev. 2020, 8, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A. Prevalence of erectile dysfunction in Thailand. Int. J. Androl. 2000, 23, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kulshreshtha, M.; Saha, S.; Shankar, S.; Singh, K.; Kumar, S.; Singh, M. Contribution of phosphodiesterase-5 (PDE5) inhibitors in the various diseases. Int. J. Behav. Sci. Healthc. Res. 2022, 7, 164–172. [Google Scholar] [CrossRef]
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile dysfunction: AUA guideline. J. Urol. 2018, 200, 633–641. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Corbin, J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int. J. Impot. Res. 2004, 16, S4–S7. [Google Scholar] [CrossRef]
- Bouchera, J.L.; Moalia, C.; Tenub, J.P. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol. Life Sci. 1999, 55, 1015–1028. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Taskforce, T.I.N.P.S.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Chaichamnong, N.; Temkitthawon, P.; Khorana, N.; Pitpakdeeanan, P.; Taepavarapruk, P.; Nuengchamnong, N.; Siriwattanasathien, Y.; Suksamrarn, A.; Ingkaninan, K. Phosphodiesterase 5 Inhibitors from Derris scandens. Planta Med. 2018, 84, 1134–1140. [Google Scholar] [CrossRef]
- Choonong, R.; Chaingam, J.; Chantakul, R.; Mukda, S.; Temkitthawon, P.; Ingkaninan, K.; Juengwatanatrakul, T.; Yusakul, G.; Kanchanapoom, T.; Putalun, W. Phosphodiesterase-5 Inhibitory Activity of Canthin-6-One Alkaloids and the Roots of Eurycoma longifolia and Eurycoma harmandiana. Chem. Biodivers. 2022, 19, e202200121. [Google Scholar] [CrossRef] [PubMed]
- Kruangtip, O.; Chootip, K.; Temkitthawon, P.; Changwichit, K.; Chuprajob, T.; Changtam, C.; Suksamrarn, A.; Khorana, N.; Scholfield, C.N.; Ingkaninan, K. Curcumin analogues inhibit phosphodiesterase-5 and dilate rat pulmonary arteries. J. Pharm. Pharmacol. 2015, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Molee, W.; Phanumartwiwath, A.; Kesornpun, C.; Sureram, S.; Ngamrojanavanich, N.; Ingkaninan, K.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Naphthalene derivatives and quinones from Ventilago denticulata and their nitric oxide radical scavenging, antioxidant, cytotoxic, antibacterial, and phosphodiesterase inhibitory activities. Chem. Biodivers. 2018, 15, 1700537. [Google Scholar] [CrossRef] [PubMed]
- Sabphon, C.; Temkitthawon, P.; Ingkaninan, K.; Sawasdee, P. Phosphodiesterase inhibitory activity of the flavonoids and xanthones from Anaxagorea Luzonensis Nat. Prod. Commun. 2015, 10, 301–303. [Google Scholar]
- Temkitthawon, P.; Changwichit, K.; Khorana, N.; Viyoch, J.; Suwanborirux, K.; Ingkaninan, K. Phenanthrenes from Eulophia macrobulbon as novel phosphodiesterase-5 inhibitors. Nat. Prod. Commun. 2017, 12, 79–82. [Google Scholar] [CrossRef]
- Temkitthawon, P.; Viyoch, J.; Limpeanchob, N.; Pongamornkul, W.; Sirikul, C.; Kumpila, A.; Suwanborirux, K.; Ingkaninan, K. Screening for phosphodiesterase inhibitory activity of Thai medicinal plants. J. Ethnopharmacol. 2008, 119, 214–217. [Google Scholar] [CrossRef]
- La-ongsri, W.; Trisonthi, C.; Balslev, H. A synopsis of Thai Nymphaeaceae. Nord. J. Bot. 2009, 27, 97–114. [Google Scholar] [CrossRef]
- Debnath, S.; Ghosh, S.; Hazra, B. Inhibitory effect of Nymphaea pubescens Willd. flower extract on carrageenan-induced inflammation and CCl4-induced hepatotoxicity in rats. Food Chem. Toxicol. 2013, 59, 485–491. [Google Scholar] [CrossRef]
- Singh, M.; Jain, A.P. A review on genus Nymphaea: Multi-potential medicinal plant. Asian J. Pharm. Educ. Res. 2017, 6, 1–9. [Google Scholar]
- Dkhar, J.; Kumariaa, S.; Rao, S.R.; Tandon, P. Molecular phylogenetics and taxonomic reassessment of four Indian representatives of the genus Nymphaea. Aquat. Bot. 2010, 93, 135–139. [Google Scholar] [CrossRef][Green Version]
- Selvakumari, E.; Shantha, A.; Kumar, C.S.; Prabhu, T.P. Phytochemistry and pharmacology of the genus Nymphaea. J. Acad. Ind. Res. 2016, 5, 98–108. [Google Scholar]
- Kite, G.; Reynolds, T.; Prance, G.T. Potential pollinator-attracting chemicals from Victoria (Nymphaeaceae). Biochem. Syst. Ecol. 1991, 19, 535–539. [Google Scholar] [CrossRef]
- Smith, L.T.; Magdalena, C.; Przelomska, N.A.S.; Pérez-Escobar, O.A.; Melgar-Gómez, D.G.; Beck, S.; Negrão, R.; Mian, S.; Leitch, I.J.; Dodsworth, S.; et al. Revised species delimitation in the Giant Water Lily Genus Victoria (Nymphaeaceae) confirms a new species and has implications for its conservation. Front. Plant Sci. 2022, 13, 883151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Yeh, H.C.; Li, H.T.; Wu, M.D.; Cheng, M.J.; Li, W.J. A new ketone derivative from Victoria amazonica. Chem. Nat. Compd. 2022, 58, 385–386. [Google Scholar] [CrossRef]
- Hassan, M.A.; Alfasane, M.A.; Uddin, M.Z. Taxonomic notes on Nelumbo Adans. with a new cultivar ‘Gomoti’ from Bangladesh. Bangladesh J. Plant Taxon. 2020, 27, 225–231. [Google Scholar] [CrossRef]
- Li, Y.; Svetlana, P.; Yao, J.; Li, C. A review on the taxonomic, evolutionary and phytogeographic studies of the lotus plant (Nelumbonaceae: Nelumbo). Acta Geol. Sin. 2014, 88, 1252–1261. [Google Scholar]
- Chen, G.; Zhu, M.; Guo, M. Research advances in traditional and modern use of Nelumbo nucifera: Phytochemicals, health promoting activities and beyond. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S189–S209. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid. Based Complement. Altern. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef]
- Ganapathy, A.A.; Priya, V.M.H.; Kumaran, A. Medicinal plants as a potential source of Phosphodiesterase-5 inhibitors: A review. J. Ethnopharmacol. 2021, 267, 113536. [Google Scholar] [CrossRef]
- Moretto, J.; Girard, C.; Demougeot, C. The role of arginase in aging: A systematic review. Exp. Gerontol. 2019, 116, 54–73. [Google Scholar] [CrossRef]
- Fossen, T.; Frøystein, N.Å.; Andersen, Ø.M. Myricetin 3-rhamnosyl(1→6)galactoside from Nymphaéa × marliacea. Phytochem. 1998, 49, 1997–2000. [Google Scholar] [CrossRef]
- Ganbolda, M.; Shimamotob, Y.; Ferdousic, F.; Tominagab, K.; Isoda, H. Antifibrotic effect of methylated quercetin derivatives on TGFβ-induced hepatic stellate cells. Biochem. Biophys. Rep. 2019, 20, 100678. [Google Scholar] [CrossRef] [PubMed]
- Csapi, B.; Hajdú, Z.; Zupkó, I.; Berényi, Á.; Forgo, P.; Szabó, P.; Hohmann, J. Bioactivity-guided isolation of antiproliferative compounds from Centaurea arenaria. Phytother. Res. 2010, 24, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Nuengchamnong, N.; Chaichamnong, N.; Seasong, T.; Ingkaninan, K.; Temkitthawon, P. At-line LC-QTOF-MS micro-fractionation of Derris scandens (Roxb.) Benth, coupled to radioassay for the early identification of PDE5A1 inhibitors. Phytochem. Anal. 2019, 31, 297–305. [Google Scholar] [CrossRef]
- Raja, M.K.M.M.; Devarajan, A.; Madhu, B.H.; Mallikarjuna, M.; Sowjanya, P.J.S. Aphrodisiac activity of ethanolic extract of Nymphaea stellata leaves in male rats. Contemp. Investig. Obs. Pharm. 2012, 1, 24–30. [Google Scholar]
- Chen, J.; Qi, J.; Chen, F.; Liu, J.-H.; Wang, T.; Yang, J.; Yin, C.-P. Relaxation mechanisms of neferine on the rabbit corpus cavernosum tissue in vitro. Asian J. Androl. 2007, 9, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.K.M.M.; Sethiya, N.K.; Mishra, S.H. A comprehensive review on Nymphaea stellata: A traditionally used bitter. J. Adv. Pharm. Technol. Res. 2010, 1, 311–319. [Google Scholar] [CrossRef]
- Acharya, J.; Dutta, M.; Chaudhury, K.; De, B. Metabolomics and chemometric study for identification of acetylcholinesterase inhibitor(s) from the flower extracts of Nymphaea pubescens. J. Food Biochem. 2018, 42, e12575. [Google Scholar] [CrossRef]
- Pokhrel, T.; Shrestha, D.; Dhakal, K.; Yadav, P.M.; Adhikari, A. Comparative analysis of the antioxidant and antidiabetic potential of Nelumbo nucifera Gaertn. and Nymphaea lotus L. var. pubescens (Willd.). J. Chem. 2022, 2022, 4258124. [Google Scholar] [CrossRef]
- Verma, A.; Ahmed, B.; Upadhyay, R.; Soni, N. Nymphasterol, a new steroid from Nymphaea stellata. Med. Chem. Res. 2012, 21, 783–787. [Google Scholar] [CrossRef]
- Shakya, A.K. Drug-induced hepatotoxicity and hepatoprotective medicinal plants: A review. Indian. J. Pharm. Educ. Res. 2020, 54, 234–250. [Google Scholar] [CrossRef]
- Bakr, R.O.; El-Naa, M.M.; Zaghloul, S.S.; Omar, M.M. Profile of bioactive compounds in Nymphaea alba L. leaves growing in Egypt: Hepatoprotective, antioxidant and anti-inflammatory activity. BMC Complement. Med. Ther. 2017, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Chen, M.-C.; Wang, S.-H.; Lai, Y.-H.; Chen, J.-H.; Lin, C.-N. 3-O-methylquercetin more selectively inhibits phosphodiesterase subtype 3. Planta Med. 2003, 69, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Shih, C.-M.; Lai, Y.-H.; Chen, J.-H.; Huang, H.-L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem. Pharmacol. 2004, 68, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Mykoniatis, I.; Pyrgidis, N.; Sokolakis, I.; Ouranidis, A.; Sountoulides, P.; Haidich, A.-B.; Renterghem, K.; Hatzichristodoulou, G.; Hatzichristou, D. Assessment of combination therapies vs. monotherapy for erectile dysfunction a systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e2036337. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Zhao, J.; Wang, Q.; Zhou, L.; Han, J.; Yu, Z.; Yang, F. Preparative separation of phenolic compounds from Halimodendron halodendron by high-speed counter-current chromatography. Molecules 2010, 15, 5998–6007. [Google Scholar] [CrossRef]
- Kim, B.-G.; Joe, E.J.; Ahn, J.-H. Molecular characterization of flavonol synthase from poplar and its application to the synthesis of 3-O-methylkaempferol. Biotechnol. Lett. 2010, 32, 579–584. [Google Scholar] [CrossRef]
- Temkitthawon, P.; Hinds, T.R.; Beavo, J.A.; Viyoch, J.; Suwanborirux, K.; Pongamornkul, W.; Sawasdee, P.; Ingkaninan, K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J. Ethnopharmacol. 2011, 137, 1437–1441. [Google Scholar] [CrossRef]
- Bordage, S.; Pham, T.-N.; Zedet, A.; Gugglielmetti, A.-S.; Nappey, M.; Demougeot, C.; Girard-Thernier, C. Investigation of mammal arginase inhibitory properties of natural ubiquitous polyphenols by using an optimized colorimetric microplate assay. Planta Med. 2017, 83, 647–653. [Google Scholar] [CrossRef]

| No. | Samples | Part Used | % PDE5 Inhibitory Activity at 50 µg/mL | % Arginase Inhibitory Activity at 100 µg/mL |
|---|---|---|---|---|
| 1 | Nelumbo nucifera | petals | 75.03 ± 1.77 | 17.70 ± 0.84 |
| pollens | 85.07 ± 6.08 | 21.39 ± 3.82 | ||
| seeds | 74.66 ± 4.39 | 22.25 ± 10.84 | ||
| receptacles | 94.26 ± 0.74 | 45.30 ± 5.89 | ||
| peduncles | 68.37 ± 0.50 | 19.98 ± 4.70 | ||
| petioles | 56.71 ± 4.90 | 2.83 ± 5.08 | ||
| leaves | 71.46 ± 0.54 | 21.84 ± 4.92 | ||
| 2 | Nymphaea sp. | petals | 78.28 ± 4.43 | 31.47 ± 7.71 |
| pollens | 82.04 ± 2.06 | 36.91 ± 2.51 | ||
| receptacles | 54.20 ± 3.26 | 22.07 ± 3.85 | ||
| peduncles | 34.55 ± 4.37 | 15.62 ± 4.62 | ||
| petioles | 43.54 ± 3.48 | 15.03 ± 2.77 | ||
| leaves | 62.31 ± 4.35 | 30.19 ± 4.85 | ||
| 3 | Nymphaea cyanea | petals | 86.54 ± 1.63 | 31.28 ± 4.75 |
| pollen | 73.16 ± 2.38 | 38.73 ± 2.17 | ||
| receptacle | 62.61 ± 1.26 | 27.76 ± 3.66 | ||
| peduncles | 34.40 ± 3.06 | 10.44 ± 9.32 | ||
| petioles | 38.26 ± 9.82 | 12.09 ± 3.85 | ||
| leaves | 55.53 ± 5.43 | 23.81 ± 3.87 | ||
| 4 | Nymphaea stellata | petals | 84.92 ± 1.86 | 28.45 ± 5.24 |
| pollens | 38.05 ± 5.83 | 27.23 ± 14.29 | ||
| receptacles | 46.07 ± 4.03 | 23.50 ± 6.07 | ||
| peduncles | 13.36 ± 6.59 | 0 | ||
| petioles | 29.17 ± 7.58 | 9.86 ± 4.59 | ||
| leaves | 44.89 ± 7.26 | 21.13 ± 19.60 | ||
| 5 | Nymphaea pubescens | petals | 98.97 ± 0.26 | 39.49 ± 4.53 |
| pollens | 74.86 ± 2.45 | 15.20 ± 1.87 | ||
| receptacles | 82.69 ± 3.36 | 23.53 ± 3.57 | ||
| peduncles | 58.90 ± 4.60 | 23.36 ± 2.52 | ||
| petioles | 50.23 ± 7.61 | 11.96 ± 2.65 | ||
| leaves | 73.26 ± 3.29 | 37.71 ± 3.23 | ||
| 6 | Victoria amazonica | petals | 86.67 ± 2.65 | 30.49 ± 3.22 |
| pollens | 63.35 ± 5.45 | 27.61 ± 1.47 | ||
| receptacles | 34.78 ± 6.41 | 13.30 ± 5.48 | ||
| peduncles | 8.23 ± 3.92 | 16.87 ± 4.80 | ||
| petioles | 9.16 ± 0.73 | 14.39 ± 5.57 | ||
| leaves | 43.90 ± 3.78 | 21.40 ± 4.61 |
| No. | Samples | Part Used | IC50 Values (μg/mL) |
|---|---|---|---|
| 1 | Nelumbo nucifera | Receptacles | 10.50 ± 3.65 |
| Pollens | 14.15 ± 3.97 | ||
| 2 | Nymphaea sp. | Pollens | 25.61 ± 2.74 |
| 3 | Nymphaea cyanea | Petals | 8.07 ± 1.33 |
| 4 | Nymphaea stellata | Petals | 13.28 ± 0.25 |
| 5 | Nymphaea pubescens | Petals | 6.37 ± 0.65 |
| Receptacles | 18.61 ± 4.03 | ||
| 6 | Victoria amazonica | Petals | 21.54 ± 4.23 |
| Sample | Contents of the Flavonoid Constituents (Mean ± SD) (mg/g Ethanolic Extract) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Nelumbo nucifera | ||||||
| Receptacles | - | - | 4.55 ± 0.25 | - | - | - |
| Pollens | - | - | 2.55 ± 0.18 | - | 7.34 ± 0.35 | - |
| Nymphaea sp. | ||||||
| Pollens | - | - | 3.09 ± 0.13 | 16.67 ± 056 | - | - |
| Nymphaea cyanea | ||||||
| Petals | - | - | - | - | 2.71 ± 0.18 | - |
| Nymphaea stellata | ||||||
| Petals | - | - | - | - | 0.82 ± 0.01 | - |
| Nymphaea pubescens | ||||||
| Petals | 7.10 ± 0.10 | 17.31 ± 0.05 | 0.61 ± 0.00 | 4.72 ± 0.08 | 0.40 ± 0.00 | 1.04 ± 0.01 |
| Receptacles | - | - | 6.38 ± 035 | 8.32 ± 0.67 | 1.40 ± 0.19 | - |
| Victoria amazonica | ||||||
| Petals | - | - | - | - | 1.77 ± 0.16 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panklai, T.; Suphrom, N.; Temkitthawon, P.; Totoson, P.; Chootip, K.; Yang, X.-L.; Ge, H.-M.; Yao, Z.-J.; Chaichamnong, N.; Ingkaninan, K.; et al. Phosphodiesterase 5 and Arginase Inhibitory Activities of the Extracts from Some Members of Nelumbonaceae and Nymphaeaceae Families. Molecules 2023, 28, 5821. https://doi.org/10.3390/molecules28155821
Panklai T, Suphrom N, Temkitthawon P, Totoson P, Chootip K, Yang X-L, Ge H-M, Yao Z-J, Chaichamnong N, Ingkaninan K, et al. Phosphodiesterase 5 and Arginase Inhibitory Activities of the Extracts from Some Members of Nelumbonaceae and Nymphaeaceae Families. Molecules. 2023; 28(15):5821. https://doi.org/10.3390/molecules28155821
Chicago/Turabian StylePanklai, Teerapap, Nungruthai Suphrom, Prapapan Temkitthawon, Perle Totoson, Krongkarn Chootip, Xiao-Liang Yang, Hui-Ming Ge, Zhu-Jun Yao, Nattiya Chaichamnong, Kornkanok Ingkaninan, and et al. 2023. "Phosphodiesterase 5 and Arginase Inhibitory Activities of the Extracts from Some Members of Nelumbonaceae and Nymphaeaceae Families" Molecules 28, no. 15: 5821. https://doi.org/10.3390/molecules28155821
APA StylePanklai, T., Suphrom, N., Temkitthawon, P., Totoson, P., Chootip, K., Yang, X.-L., Ge, H.-M., Yao, Z.-J., Chaichamnong, N., Ingkaninan, K., & Girard, C. (2023). Phosphodiesterase 5 and Arginase Inhibitory Activities of the Extracts from Some Members of Nelumbonaceae and Nymphaeaceae Families. Molecules, 28(15), 5821. https://doi.org/10.3390/molecules28155821







