Abstract
Wild Asparagus shoots are consumed worldwide, although most species remain understudied. In this work, a total of four wild Asparagus species were collected from different locations and analyzed compared with farmed A. officinalis. Shoots were screened for (i) phenolic compounds by HPLC-DAD and LC-MS; (ii) total phenolic acids and total flavonoid content by the Folin–Ciocalteu and aluminum chloride methods; (iii) vitamin C by HPLC-DAD; (iv) antioxidant activity by the DPPH and ABTS•+ methods; and (v) the in vitro antiproliferative activities against HT-29 colorectal cancer cells by the MTT assay. Phenolics ranged from 107.5 (A. aphyllus) to 605.4 mg/100 g dry weight (dw) (A. horridus). Vitamin C ranged from 15.8 (A. acutifolius) to 22.7 mg/100 g fresh weight (fw) (A. officinalis). The antioxidant activity was similar in all species, standing out in A. officinalis with 5.94 (DPPH) and 4.64 (ABTS) mmol TE/100 g dw. Among phenolics, rutin reached the highest values (574 mg/100 g dw in A. officinalis), followed by quercetin, nicotiflorin, asterin, and narcissin. The MTT assay revealed the inhibitory effects of ethanol extracts against HT-29 cancer cells, highlighting the cell growth inhibition exercised by A. albus (300 µg/mL after 72 h exposure to cells). This work improves knowledge on the phytochemicals and bioactivities of the shoots of wild Asparagus species and confirms their suitability for use as functional foods.
Keywords:
Asparagus; phenolic compounds; flavonoids; vitamin C; antioxidant activity; MTT; HT-29 cells 1. Introduction
Traditional knowledge about wild edible plants has been seriously threatened for several generations, due to the progressive abandonment of conventional ways of life and the massive exodus of the population from the countryside to the city. Considering that the population is currently aware of the relationship between diet and health and that plant-based diets are increasing among consumers worldwide, it is relevant to boost the potential of wild plants as a source of bioactive compounds to promote their consumption in modern societies, which would contribute to the preservation of traditional knowledge, improve biodiversity, sustainability, and food security, and generate employment opportunities in traditional collecting areas [1,2].
Asparagus L. 1753 is a genus belonging to the family Asparagaceae Juss. 1789 (Class Liliopsida Batsch 1802), which includes over 250 species, from which A. officinalis L. is the only cultivated species. However, several wild species are traditionally collected for consumption and medicinal purposes in the Mediterranean Basin, such as A. aphyllus L., A. acutifolius L., and A. horridus L. (syn. A. stipularis Forssk) [3].
Assessed through color, A. officinalis can be eaten as green, white, purple-green, purple-blue, and pink asparagus. White asparagus turns into green asparagus when exposed to sunlight after harvesting. Besides as canned food, these shoots can be marketed as fresh green to be eaten as vegetables [4]. The protein of Asparagus contains all essential amino acids in a suitable proportion, and green Asparagus contains relatively higher amounts of nutritional components than white ones, except for available carbohydrates. Moreover, this vegetable stimulates intestinal transit due to its high fiber concentration [5].
Asparagus shoots, besides being a highly appreciated vegetable due to their appropriate organoleptic and nutritional characteristics, have noticeable functional value since they are reported to contain bioactive compounds, and among these, steroidal saponins are highlighted. Such spears contain vitamins A, B1, B2, C, and E and folic acid [5], as well as several other healthy bioactive constituents, such as Mg, P, Ca, Fe, flavonoids (kaempferol, quercetin, and rutin), arginine, asparagine, tyrosine, resin, essential oils, and tannin [6].
Among bioactive compounds, phenolics have redox properties, which allow them to act as antioxidants [7]. Flavonoids, including flavones and flavanols, are plant secondary metabolites that have antioxidant activity, which depends on the presence of free OH groups, especially 3-OH. These suppress and scavenge reactive oxygen species, and up-regulate and protect antioxidant defenses. Similarly, phenolics confer oxidative stress tolerance to plant tissues [8].
Although the most widely studied species is the cultivated A. officinalis, there is an increasing interest in wild edible Asparagus taxa. An exhaustive review on important bioactive compounds of Asparagus shoots collected from the wild or cultured in Southern Europe is detailed in Supplementary Table S1. It highlights that A. acutifolius has been previously researched in Italy, and it has been reported to be a good source of valuable nutrients including phenolic compounds, carotenoids, and saponins, while its antioxidative and antiproliferative properties were favorable [9,10]. This taxon has been widely scrutinized to establish its biological activities, especially antitumor ones (e.g., [11]). On the other hand, A. horridus has been researched looking to establish its antioxidant activity; α-glucosidase inhibitory potential and in vivo protective effect [3]; and anti-inflammatory and anti-cancer activities [12,13]. Among its various phytochemicals, phenolics are highlighted [3]. Most of the research regarding the antioxidant activity of A. acutifolius extracts available in the literature was carried out on shoots, which are the edible organs [10,14].
Although the phytochemical composition and bioactivity of cultivated A. officinalis and wild A. acutifolius are relatively well known, there is a lack of knowledge on the biochemical composition and biological properties of the shoots of A. horridus, A. albus, and A. aphyllus. Moreover, the variability of the phytochemical composition of the various Asparagus taxa depending on their ecogeographic locations constitutes an unexplored and interesting task. Therefore, this work was designed to determine in wild and cultivated Asparagus species collected in several locations of South Spain valuable phytochemicals, such as vitamin C (Vit C) and phenolic compounds, as well as their in vitro biological activities, such as antioxidant and antitumor ones, seeking to unravel the health benefits of the more relevant shoots from commonly consumed species of this genus.
2. Results
2.1. Phytochemical Characterization
Moisture, Vit C, total phenolics and flavonoids, and the antioxidant activity measured by the DPPH and ABTS methodologies are detailed in Table 1, the phenolic compound profiles quantified by HPLC-DAD are summarized in Table 2, and the occurrence of phenolic compounds detected in selected Asparagus samples by LC-MS is detailed in Supplementary Table S2.

Table 1.
Moisture, antioxidant activity, vitamin C, and total phenolic and flavonoid content of Asparagus samples 1,2,3.

Table 2.
Phenolic compounds of the ethanol extracts of Asparagus shoots 1,2,3.
2.1.1. Moisture Content
Moisture ranged from 80.7 in A. horridus H1 (from Cabo de Gata) to 91.1 g/100 g in A. officinalis O1 (from Láchar). Mean values for the moisture content of Asparagus shoots ranged from 84.5 (A. acutifolius) to 91.0% (A. officinalis).
2.1.2. Vitamin C Content
Table 1 shows the Vit C content of the analyzed Asparagus samples. Amounts ranged from 9.5 (AC3, A. acutifolius from Navas de San Juan) to 27.1 mg/100 g fw (AC1, A. acutifolius from Arroyo Blanco). Mean values for Vit C among Asparagus species ranged from 15.8 in A. acutifolius to 22.7 mg/100 g fw in A. officinalis.
2.1.3. Total Phenolic Compound and Flavonoid Contents
TPC ranged between 238.0 and 468.2 mg GAE/100 g fw in A. albus AL2 from Sierra de Cabrera and A. horridus H1 from Cabo de Gata. Mean values for Asparagus were between 296.6 (A. albus) and 402.6 mg GAE/100 g fw (A. officinalis). TFC was between 56.9 (A. aphyllus AP1, from Alcalá de los Gazules) and 430.8 mg QE/100 g fw (A. officinalis O2, from Loja). Mean values for TFC ranged from 96.1 in A. aphyllus to 348.3 mg QE/100 g fw in A. officinalis.
2.1.4. Antioxidant Activity
The antioxidant activities of the phenolic-containing extracts of Asparagus spp. are detailed in Table 1. Values for all samples ranged between 0.72 in A. albus AL3 (from Sierra Morena) and 6.08 mmol TE/100 g dw in A. officinalis O2 (from Loja) according to the DPPH assay. Considering species, the antioxidant activity was between 2.34 (A. albus) and 5.94 mmol TE/100 g dw (A. officinalis). In the case of ABTS•+, there were no significant differences in the mean values among the different Asparagus taxa. Considering samples, the lowest and highest antioxidant activities were recorded for A. horridus H3 (1.86 mmol TE/100 g dw) and A. officinalis O1 (4.23 mmol TE/100 g dw). Overall, the highest antioxidant activities determined by the DPPH and ABTS•+ methods were recorded in cultivated A. officinalis. The antioxidant activity of pure compounds assessed with comparative purposes was between 17.54 and 23.34 mmol of TE/100 g for α-tocopherol and ascorbic acid (DPPH methodology), while for the ABTS•+ assay, the values ranged between 8.78 (α-tocopherol) and 10.43 mmol TE/100 g (ascorbic acid).
2.1.5. Phenolic Compound Profiles
The phenolic compound profiles and total phenolic contents quantified by HPLC-DAD are detailed in Table 2, and those detected for selected Asparagus species by LC-MS are listed in Supplementary Table S2. Chromatograms were screened at 254, 280, and 320 nm (Supplementary Table S3), where they showed good molar extinction coefficients. The phenolic compounds were identified by comparing the recorded absorption spectra of all peaks from the various chromatograms with pure standards. Quantified phenolic compounds were rutin, asterin, nicotiflorin, narcissin, and quercetin, and values for these were characteristic of each species. Rutin ranged from 59.5 (A. aphyllus) to 574.3 mg/100 g dw (A. officinalis); nicotiflorin was between 8.4 (A. albus) and 72.5 mg/100 g dw (A. horridus); asterin ranged from 1.3 (A. albus) to 33.2 mg/100 g dw (A. acutifolius); and narcissin and quercetin were within ~5–15 mg/100 g dw for all studied species, but narcissin was below the detection limit in A. officinalis.
The structures of all identified compounds that occurred in Asparagus shoots were confirmed by LC-MS. The precision/injection repeatability test showed good precision in peak area (standard deviation < 1%) and peak retention time (±2%). The results of the linearity range, the regression equation, LOD, LOQ, and recoveries for all quantified compounds are presented in Supplementary Table S3. Besides the compounds quantified with the HPLC-DAD system, several phenolic compounds were identified using the LC-MS system from the m/z value of the molecular ion, but some of these compounds could not be clearly attributed to any of the chromatographic peaks obtained with the HPLC-DAD system.
2.2. Antitumor Activity
The MTT assay was accomplished to evaluate the inhibitory effects of selected Asparagus extracts on HT-29 human colorectal cancer cell viability. Extracts having the highest antioxidant activity (one of each species) were selected for this assay. Figure 1A,B show the activity of such extracts against cancer cell viability after 48 and 72 h of treatment, respectively. Cell growth inhibition was exercised much better by A. acutifolius, A. albus, and A. aphyllus, which at 600 µg/mL and 72 h of cell exposure induced 22.1, 6.2, and 37.6% of cancer cell viability in comparison with controls without extract addition. GI50 values, i.e., the doses of extracts that inhibited cell growth by 50%, of selected samples and those of two pure phenolic compounds occurring in samples are shown in Figure 1C. After a 72 h incubation period, GI50 for A. acutifolius (AC4), A. albus (AL4), A. aphyllus (AP3), rutin, and quercetin were 369, 303, 488, 680, and 76 µg/mL, respectively.
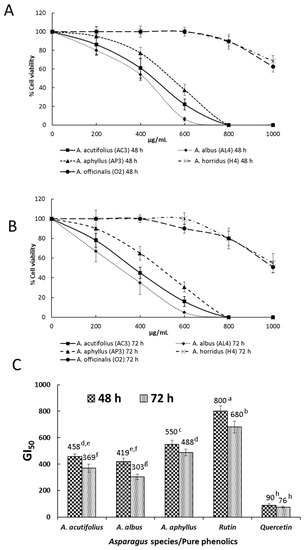
Figure 1.
MTT assay. (A) Dose–response curves of HT-29 cell viability after treatment with different concentrations of ethanol extracts of Asparagus shoots for 48 h. (B) Dose–response curves of HT-29 cell viability after treatment with different concentrations of ethanol extracts of Asparagus shoots for 72 h. (C) GI50 of HT-29 cells after treatment with ethanol extracts of Asparagus shoots, rutin, and quercetin for 48 and 72 h. The GI50 value is detailed over each column. Data represent the mean of three complete independent experiments ± SD (error bars). In a bar, means followed by different letters are significantly different at p < 0.05.
3. Discussion
3.1. Vitamin C Content
Vit C, i.e., ascorbic acid, is a water-soluble vitamin found in certain foods. It has a role as an antioxidant in the human organism, helping to protect cells against damage caused by free radicals and allowing the synthesis of collagen [15,16]. The mean values of Vit C were quite similar for the different analyzed species, and the highest value was obtained in cultivated A. officinalis (22.7 mg/100 g fw). The remaining species showed Vit C content in the ~16–17 mg/100 g fw range. It is not surprising that the Vit C content in cultivated Asparagus was higher than that in wild ones, considering that cultivated plants receive a greater supply of nutrients and are not subjected to environmental stress. On the other hand, it has been suggested that Vit C in cultivated Asparagus depends mainly on the harvesting season [17]; however, in the case of wild Asparagus, it is difficult to determine it, since the various samples were collected from locations having different ecogeographical features.
Some studies suggest that the features of the cultivation area influence the chemical composition of Asparagus shoots. Hence, the Vit C content of eight white A. officinalis varieties cultivated in Greece was 14.1–20.2 mg/100 g fw [18], in line with the results of this work. Moreover, an investigation on the chemical composition of wild A. acutifolius shoots from Portugal and its comparison with similar samples from different origins concluded that there were great differences in Vit C contents [19].
3.2. Phenolic Compound Content
TPC values showed no significant differences among species when assessed through the F-C method (Table 1), although a wide range of variation within species depending on the collecting area was noted. For instance, TPC ranged between 238.0 and 515.2 mg GAE/100 g fw in A. albus. TFC was typically half of TPC in most cases, and a wide variation in TFC was also noted within species; for instance, A. officinalis showed significantly higher values than those of A. aphyllus.
In this study, most Asparagus shoots, all of them collected in Southern Spain, showed significant amounts of phenolic compounds. This fact can be due to the stressful conditions of a warm-summer Mediterranean climate, which can activate a multi-gene response that leads to changes in secondary metabolite accumulation, including the synthesis of phenolic compounds [20,21]. Phenolics are chemical mediators between plants and their environment, and consistently, both phenolics and antioxidant compounds in Asparagus vary widely depending on the phenological stage, environmental conditions (seasonal changes, radiation, soil type, and water availability), and chemotypes [22,23].
TPC and TFC of the shoots of three Asparagus species analyzed in the current work have been previously reported (Supplementary Table S1). Data from A. acutifolius and A. officinalis showed a wider range of variation than that obtained here. Such variation can be due to climatic features, analytical procedures, different varieties under study, and the analysis of shoots having different stages of development. The mean values obtained here had intermediate values when compared with those of Supplementary Table S1 for A. acutifolius (TPC 352.9 mg GAE/100 g fw and TFC 257.6 mg QE/100 g fw) and for A. officinalis (TPC 402.6 mg GAE/100 g fw and TFC 348.3 mg QE/100 g fw). Regarding A. horridus, Adouni et al. [3] reported slightly lower values for TPC and TFC than those found in this work (398.4 mg GAE and 143.6 mg QE by 100 g fw).
The phenolic profiles of all samples analyzed and quantified by HPLC-DAD are given in Table 2, while the compounds identified by LC-MS are detailed in Supplementary Table S2. Notice that there were several phenolic compounds identified by their m/z ions (Supplementary Table S3) but lacking quantification considering an absence of specific peaks attributed in the HPLC-DAD chromatograms. Overall, the TPC obtained by the sum of quantified phenolics by HPLC-DAD was significantly lower than that obtained by the F-C methodology (which provides GAE). This fact is explained by the following: (i) the F-C method informs on total phenolic compounds, while chromatographic methods report only the concentration of identified compounds; and (ii) there is an overestimation of the actual phenolic content of the F-C method due to its unspecificity toward targeted phenolics [24].
The identification of peaks detected with the LC-MS system was based on the m/z ion. For instance, the compound with m/z = 595 and MS2 ion at 271 due to a loss of 324 amu corresponding to two glucose moieties was confirmed as pelargonidin 3-O-diglucoside. As the ion at 271 is the most abundant one in MS2, it indicates that the two glucose moieties are linked to each other and correspond to a diglucoside derivative of pelargonidin. Among others, it highlights the occurrence of chelidonic acid, which is a heterocyclic organic acid with a pyran skeleton, which was detected in all samples; chlorogenic acid (an ester of caffeic acid and (−)-quinic acid) was absent in A. horridus; p-coumaric acid hexoside (a hydroxycinnamic acid) and the flavonoids nicotiflorin, rutin, and quercetin were present in all samples; syringic acid (a gallic acid derivative); and 5-O-p-coumaroyl quinic acid (a cinnamate ester), (−)-epicatechin gallate (a flavan-3-ol, a type of flavonoid), and isoquercetin (a flavonoid) were restricted to A. albus samples. The remaining phenolics had a more random distribution among the different species. In all taxa, rutin reached the highest values, especially in A. officinalis and A. acutifolius, followed by nicotiflorin, which had the highest values in A. horridus, while narcissin was undetected in A. officinalis. Asterin and quercetin were minor contributors to this profile. This situation agrees with previously reported values (Supplementary Table S1), although it can be noted that a great variety of phenolics was previously reported in minor amounts. The concentration obtained for rutin in A. officinalis agrees with most values given by various authors, such as Motoki et al. [25], and such an amount suggests that wild edible shoots can be used as functional foods, considering the bioactivity of this flavonoid. It develops potent antioxidant and anti-inflammatory actions and prevents neurodegenerative and cardiovascular disorders, as well as skin cancer, among other diseases [26].
It was reported that medicinal plants having high amounts of phenolic compounds develop potent antioxidant actions [27], and ethanol is a suitable solvent for their extraction given that polyphenols are linked to the cell-wall matrix through a glycosidic/ester linkage; thus, alcohol-based solvents are appropriate for the extraction of all types of phenolic compounds [28]. Then, this solvent was selected for extraction in this study, and it might be used in the food industry for rutin-rich extract obtainment.
3.3. Antioxidant Activity
Two different methodologies were accomplished in this work for assessing this property, and differences in values obtained from both methods were noted. DPPH· is a relatively stable nitrogen radical, whereas ABTS•+ is more unstable and is produced upon reaction with potassium persulfate. It has been reported that some compounds that react rapidly with peroxyl radicals do not react well with the DPPH radical, due to steric hindrance between the compounds [29]. The results obtained by the two methods are shown in Table 1. Overall, the results of this study agree with the highest values reported for both A. acutifolius and A. officinalis (Supplementary Table S1, e.g., [30,31]). The lowest antioxidant activity determined by the DPPH· and ABTS•+ methods was recorded in some samples of A. albus, and the highest one was obtained in A. officinalis samples. The results obtained by the ABTS•+ and DPPH methods for A. officinalis samples were higher than those reported by Sun et al. [31] for juice samples of the same species (DPPH 1.74 and ABTS•+ 2.64 mmol TE/100 g) and those reported by Fan et al. [29] for A. officinalis residues (ABTS•+ 1.42 and DPPH 0.62 mmol TE/100 g). Such differences in bioactivity could be because plants grown under different environmental conditions were analyzed.
The relationship between phenolic compound content and antioxidant activity has been extensively studied [32,33], and the antioxidant activity found in Asparagus shoots has been attributed to flavonoids [34]. Kulczyński et al. [35] identified a positive correlation between ABTS•+ and phenolic compounds in A. officinalis samples; however, this relationship changes according to the type of phenolic compound considered, and quercetin and rutin showed the highest values. Fan et al. [29] evidenced a positive correlation between antioxidant activity determined by the ABTS•+ and DPPH methods and both TPC and TFC, being higher for ABTS•+, due to the above-exposed reasons. Moreover, the ABTS•+ radical is more soluble in organic compounds and water than DPPH; thus, the ABTS•+ method may have higher sensitivity in samples with high water content, such as Asparagus samples [36].
Recently, it has been demonstrated that different cultivation systems have a significant effect on the bioactive compounds, antioxidant enzymes, and anti-cancer activity of A. officinalis [37]. In this research, spears grown in an open field (OF) and a rain-shelter house (RSH) system were analyzed. Results showed that rutin and trans-p-coumaric acid contents, as well as the ABTS•+ and DPPH radical scavenging activity of Asparagus spears grown in the OF, were higher than those grown in the RSH system. Given that the samples analyzed in the present work were collected from the wild, i.e., OF, this factor would presumably not affect the results obtained here.
The antioxidant activity of pure compounds (α-tocopherol, ascorbic acid, and caffeic acid) was also checked with comparative purposes, and the results obtained by the DPPH method were approximately double those of the ABTS•+ procedure, although values obtained by each method for all these compounds were quite similar (Table 1). Interestingly, the highest antioxidant activity, which was found in A. officinalis, was approximately between half and one-third of that showed by such pure molecules by the DPPH method and approximately half when checked by the ABTS•+ methodology. Other Asparagus species showed much lower values, but A. aphyllus had also a high antioxidant activity, approximately half that of A. officinalis.
Figure 2 shows the correlations between the antioxidant activity and bioactive compounds (TPC, TFC, Vit C, rutin, quercetin, rosmarinic acid, and total identified phenolics) in Asparagus samples. Notice that TFC, TPC, and Vit C have a high and positive correlation with the antioxidant activity determined by the ABTS•+ method. This correlation indicated that the compounds that have significant reducing ability quantified by the F-C methodology and Vit C contributed largely to the antioxidant activity of the various Asparagus samples.
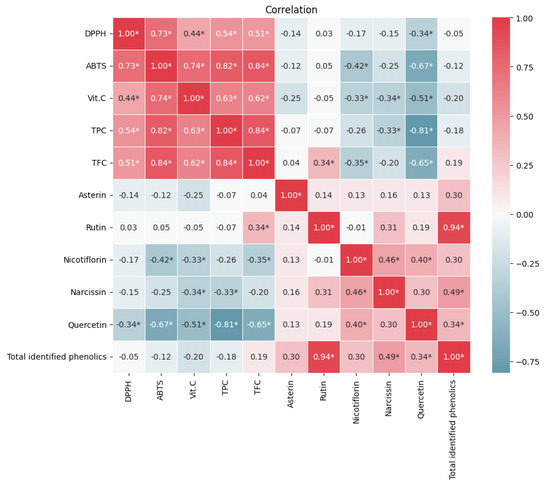
Figure 2.
Heat map of the correlation between the different variables. The redder colors indicate a stronger and positive correlation, while the bluer colors indicate a stronger and negative correlation. Correlations were evaluated with a significance of p < 0.05 (*).
3.4. Antiproliferative Activity of the Ethanol Extracts of Asparagus Shoots on HT-29 Cancer Cells
The antitumor actions of Asparagus extracts on cancer cells constitute a hot topic. Previous studies indicated that saponins from Asparagus are responsible for a reduced risk of colorectal cancer by inducing cytotoxicity and apoptosis [38,39]. Recently, the synergistic action of an A. officinalis extract combined with paclitaxel at low concentrations has been evidenced, which inhibited cell proliferation and induced apoptosis in paclitaxel-sensitive and -resistant ovarian cancer cell lines. Such treatment exercised DNA damage and suppressed microtubule dynamics [40].
The antitumor activities of different A. acutifolius organs were previously tested in human cancer cell lines (HCT-116, colon) and (HepG2, liver) using the MTT assay [11], and antitumor activities were characteristic for tested organs. The ethanol extract of the rhizome showed activity against both cell lines tested, while the leaf extract was more cytotoxic than the rhizome. However, the stem and pericarp extracts lack antitumor activity. Likewise, the rhizome from A. acutifolius showed an important cytotoxic activity against the HepG2 cell line, and similar values were reported by other authors for A. adscendent [41] and A. filicinius [42], who related this activity to the presence of saponins and their genins. As for A. albus, low IC50 values (40 µg/mL) were found after HCT-116 colon cancer cell exposure to the rhizome extracts, while the leaf extracts had similar IC50 values against such cells [43].
Adouni et al. [3] reported for the hydroalcoholic extract of A. horridus shoots in cell cultures GI50 of 298.63 in MCF-7 (breast carcinoma); 244.26 in NCI-H460 (non-small-cell lung cancer); 208.24 in HeLa (cervical carcinoma); 200.77 in HepG2 (hepatocellular carcinoma); and >400 µg/mL in PLP2 (liver primary culture).
Among the various antitumor phytochemicals contained in different organs of Asparagus, polysaccharides are highlighted. The antitumor activity of Asparagus tissues was partially related to the presence of polysaccharides, which also exhibit antioxidant activity. Then, it is likely that the polysaccharides present in the extracts tested in this study against HT-29 cells contributed, at least partially, to the noted activity. These molecules demonstrated significant inhibition of human cervical (HeLa) and liver (BEL-7404) cancer cell lines after protein elimination [44]. Moreover, among the various pectic polysaccharide sub-fractions from Asparagus, the fractions with the higher degree of esterification showed a stronger immunomodulatory activity on RAW 264.7 macrophages [45].
Interestingly, it has been reported that the cultivation system of A. officinalis had a significant effect on the survival rate of MCF-7 breast cancer cells treated with Asparagus shoot extracts. Cells treated with extracts of plants grown in an OF had a lower survival rate than cells treated with extracts from plants grown in the RSH system. However, cultivation systems lack effects on quercetin, sinapic acid, ferulic acid, and caffeic acid contents, as well as catalase activity and Calu-6 cell viability [37].
Given the nature of the extracts tested in this work against HT-29 cells (ethanol 96%), it is likely that besides phenolic compounds, some saponins and polysaccharides were present in such extracts. After 48 and 72 h of treatment (Figure 1A and Figure 1B, respectively), the MTT assay revealed concentration- and time-dependent inhibitory effects on HT-29 cells for all assayed extracts. Cell viability after 72 h of treatment at the maximum concentration tested (1000 μg/mL) and for the different species was 15–20% higher than that obtained at 48 h.
After 72 h cell culture, cell growth inhibition was exercised much better by A. acutifolius (AC3), A. albus (AL4), and A. aphyllus (AP3), which reached GI50 of between 300 and 500 µg/mL of extract, with null cell viability at 800 µg/mL, and GI50 were 369, 303, and 488 µg/mL, respectively. The antiproliferative activity against HT-29 cells of some pure phenolic compounds occurring at high concentrations in Asparagus extracts was also checked. Rutin and quercetin showed GI50 values of 680 and 76 µg/mL. Considering that rutin was a major phenolic present in the ethanol extract and its low activity, the recorded antitumor activity was likely due to a synergy between polysaccharides, saponins, and some phenolic compounds, i.e., quercetin, as previously described in Buglossoides seed extracts when tested against HT-29 cancer cells, since these cells are not very sensitive to phenolics [46]. In contrast to rutin, quercetin has been previously demonstrated to inhibit colon cancer cell growth through the induction of apoptosis. It decreases the expression of Bcl-2, a protein that acts as an inhibitor of programmed cell death [47]. Even though the concentration of rutin is much higher than that of quercetin in all checked species, given the low GI50 of the latter (76 µg/mL), it could have influenced the noted antitumor activity. However, quercetin concentrations vary slightly between the different samples, so the antitumor effects would probably be due to a synergy among several compounds present in the ethanol extract, as previously mentioned.
4. Materials and Methods
4.1. Reagents and Chemicals
Unless otherwise indicated, all chemicals and solvents were purchased from Merck (Madrid, Spain). L-Ascorbic acid was obtained from Labkem (Barcelona, Spain). Aluminum chloride and sodium carbonate were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Sodium nitrite, sodium hydroxide, and oxalic acid were purchased from Panreac (Barcelona, Spain). Water was purified using a Milli-Q system (Millipore, Burlington, MA, USA). All the chemicals used, including the solvents, were of analytical grade.
4.2. Samples
Data on shoots collected for this work are shown in Table 3. Upon arrival at the laboratory, the shoots were labeled, weighed, measured, and frozen at −20 °C until analysis. Approximately 2 g of each fresh sample was used for moisture analysis, which was carried out in a forced air oven at 100 °C until constant weight. Just before analysis, shoots were ground with a mortar.

Table 3.
Data on sample collection of Asparagus species.
4.3. Extraction of Phenolic Compounds from Asparagus Species
This methodology is fully detailed in Supplementary File S1. Extraction and analysis of phenolic compounds from Asparagus species were carried out according to Lyashenko et al. [48].
4.4. Characterization of Phenolic Compounds by HPLC-DAD
This methodology is fully detailed in Supplementary File S1. HPLC analyses of phenolics were carried out using a Finnigan Surveyor chromatograph equipped with a diode-array detector (DAD) and a reverse-phase C18 column (Hypersil Gold, 250 × 4.6 mm i.d., 5 µm particle size) (Thermo Electron, Cambridge, UK). The compounds were separated with a gradient elution using acidified water (1% acetic acid) (A) and acetonitrile (B) as mobile phase at 25 °C. The running time was 100 min. A 254 nm-HPLC-DAD chromatogram of A. horridus (H2) is depicted in Figure 3. Quantification of the compounds was made using external calibration curves obtained from pure standards (Sigma-Aldrich, St. Louis, MO, USA) in the HPLC-DAD system.
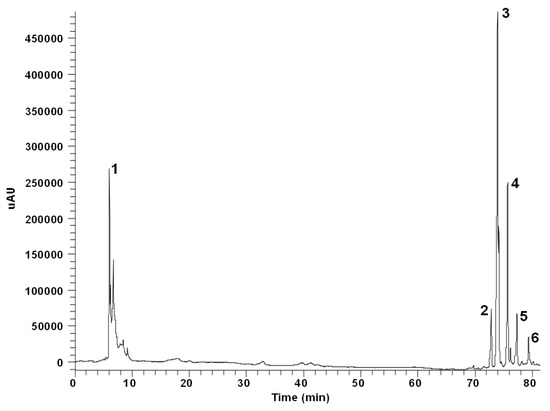
Figure 3.
A 254 nm-HPLC chromatogram of the phenolic-containing ethanol extract of Asparagus horridus (H2). 1. Ascorbic acid. 2. Asterin. 3. Rutin. 4. Nicotiflorin. 5. Narcissin. 6. Quercetin.
4.5. Characterization of Phenolic Compounds by LC-MS
This methodology is fully detailed in Supplementary File S1, and the HPLC-DAD and LC-MS parameters for the analysis of phenolic-rich extracts of Asparagus shoots are detailed in Supplementary Table S3. The chromatographic separations were performed on a Vanquish Flex Quaternary LC equipped with a reverse-phase C18 column (Hypersil Gold, 100 mm × 2.1 mm, 1.9 μm) at a flow rate of 0.2 mL/min. The total running time was 39 min. The LC system was coupled to a hybrid mass spectrometer Q-Orbitrap Thermo Fisher Scientific using electrospray ionization (ESI) in positive and negative ion mode.
4.6. Determination of Total Phenolic Content
Total phenolic content (TPC) was measured using the F-C assay, as reported by Singleton et al. [49] with minor modifications. This methodology is fully detailed in Supplementary File S1. The results were expressed as mg of gallic acid equivalents (GAE) per 100 g of fw using a standard curve of GA. Determinations were performed in triplicate.
4.7. Determination of Total Flavonoid Content
Total flavonoid content (TFC) of Asparagus samples was determined according to Zou et al. [50] with some modifications, and this methodology is fully detailed in Supplementary File S1. The results were expressed as mg of quercetin equivalents (QE) per 100 g fw using a standard curve of quercetin (10–500 μg/mL). Determinations were performed in triplicate.
4.8. Extraction and Quantification of Vitamin C
Vit C (L-ascorbic acid) content was determined according to Volden et al. [51] with minor modifications. This methodology is fully explained in Supplementary File S1. The analysis of Vit C was carried out using the previously described HPLC-DAD system. Ascorbic acid was quantified by external calibration, and results were recorded as mg/100 g fw. All data are presented as mean ± standard deviation of samples analyzed in triplicate.
4.9. Antioxidant Activity
Extraction was carried out with ethanol (96%) according to the methodology described by Forbes-Hernández et al. [52] with some modifications. This methodology is fully explained in Supplementary File S1. The antioxidant activity using the ABTS method was determined using a solution of ABTS•+ radical 2,2′-azinobis (3-Ethylbenzothiazoline-6-sulfonic acid) in ethanol (2.45 mM). The DPPH method was carried out according to Skenderidis et al. [53], and is fully described in Supplementary File S1. The absorbance of the solution was measured at 517 nm. The values of ABTS•+ and DPPH were expressed as mmol of Trolox Equivalent/100 g dw (mmol TE/100 g dw).
4.10. Antitumor Assay
This methodology is fully detailed in Supplementary File S1. The antiproliferative activity of the 96%-ethanol extract from Asparagus shoots was assayed on the HT-29 human colon cancer cell line as described by Lyashenko et al. [48].
4.11. Statistical Analysis
All samples were analyzed in triplicate. Data were assessed for normality using a Shapiro–Wilk test and submitted to one-way ANOVA, and the comparison of means was made using Duncan’s multiple-range test. Statistical analyses were performed using Statgraphics© Centurion XVI (StatPoint Technologies, Warrenton-Virginia, VA, USA).
5. Conclusions
The levels of phytochemicals and biological activities found in the shoots of four wild Asparagus species lead us to consider them as functional foods. Cultured A. officinalis is highlighted considering its values for Vit C, TPC, TFC, antioxidant activity, rutin, and total phenolics quantified through the HPLC-DAD system. This fact was probably due to that farmed plants receive a controlled supply of water and nutrients, which prevents stressful situations. Therefore, it would be expected that agronomic research on the four wild taxa analyzed here could lead to equalizing the phytochemical content and the biological activities of cultured A. officinalis. TFC, TPC, and Vit C have a clear and positive correlation with the antioxidant activity determined by the ABTS•+ method, which indicated that the compounds quantified by the F-C methodology and Vit C contributed largely to the high antioxidant activity detected in the various Asparagus samples. Regarding phenolics, rutin reached the highest values in all analyzed species, especially in A. officinalis and A. acutifolius, followed by nicotiflorin, which had the highest values in A. horridus, while narcissin was undetected in A. officinalis. The LC-MS system allowed the detection of several phenolic glycosides, and some of them had a characteristic distribution among species. Extracts from wild Asparagus species (A. acutifolius, A. albus, and A. aphyllus) showed higher antitumor activity against HT-29 cells than that of cultured A. officinalis. Future research should be focused on the study of other phytochemicals, such as sterols and tocols, and checking the phytochemicals occurring in wild Asparagus species after they have been cultivated in optimized farming systems. Furthermore, extensive research on the bioactivity of the various Asparagus extracts against other cell lines, including normal colorectal cells, and the mechanisms through which the extracts exercise antitumor actions will be welcomed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28155786/s1, Supplementary File S1: Methods, Supplementary Table S1: Selected data on ascorbic acid, phenolics, and antioxidant activity of green Asparagus spp. shoots, Supplementary Table S2: Compounds detected in Asparagus samples by LC-MS, Supplementary Table S3: HPLC-DAD and LC-MS parameters used for the analysis of phenolic-enriched extracts of Asparagus shoots. References [3,9,10,11,14,19,25,31,37,48,49,50,51,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, J.L.G.-G.; Data curation, J.L.G.-G., T.C.C., M.A.R.-C., F.G.-M., R.L.-R., M.G.-B. and M.E.; Formal analysis, J.L.G.-G., T.C.C., M.A.R.-C., R.L.-R., M.G.-B. and M.E.; Funding acquisition, J.L.G.-G. and R.L.-R.; Investigation, J.L.G.-G. and F.G.-M.; Methodology, J.L.G.-G., T.C.C., M.A.R.-C., F.G.-M., R.L.-R., M.G.-B. and M.E.; Project administration, J.L.G.-G.; Resources, J.L.G.-G. and R.L.-R.; Software, T.C.C., M.A.R.-C., R.L.-R. and M.E.; Validation, J.L.G.-G.; Visualization, T.C.C. and F.G.-M.; Writing—original draft, T.C.C. and M.G.-B.; Writing—review and editing, J.L.G.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vicerrectorado de Investigación e Innovación of the University of Almería (Project P_LANZ_2023/003), Junta de Andalucía (Project P20_00806), and the Andalusia Ministry of Economic Transformation, Industry, Knowledge and Universities for financial support from “Ayudas para Captación, Incorporación y Movilidad de Capital Humano de I+D+i (PAIDI 2020)”.
Informed Consent Statement
The HT-29 colorectal cancer cell line was supplied by the Technical Instrumentation Service of the University of Granada (Granada, Spain).
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Compound samples are not available from the authors.
References
- Mokria, M.; Gebretsadik, Y.; Birhane, E.; McMullin, S.; Ngethe, E.; Hadgu, K.M.; Hagazi, N.; Tewolde-Berhan, S. Nutritional and ecoclimatic importance of indigenous and naturalized wild edible plant species in Ethiopia. Food Chem. Mol. Sci. 2022, 4, 100084. [Google Scholar] [CrossRef] [PubMed]
- Schunko, C.; Li, X.; Klappoth, B.; Lesi, F.; Porcher, V.; Porcuna-Ferrer, A.; Reyes-García, V. Local communities’ perceptions of wild edible plant and mushroom change: A systematic review. Glob. Food Secur. 2022, 32, 100601. [Google Scholar] [CrossRef]
- Adouni, K.; Chahdoura, H.; Mosbah, H.; Santos-Buelga, C.; González-Paramás, A.M.; Cuidad-Mulero, M.; Fernandes, Â.; Calhelha, R.C.; Morales, P.; Flamini, G.; et al. Revalorization of wild Asparagus stipularis Forssk. as a traditional vegetable with nutritional and functional properties. Food Funct. 2018, 9, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Zafiriou, P.; Mamolos, A.P.; Menexes, G.C.; Siomos, A.S.; Tsatsarelis, C.A.; Kalburtji, K.L. Analysis of energy flow and greenhouse gas emissions in organic, integrated and conventional cultivation of white asparagus by PCA and HCA: Cases in Greece. J. Clean. Prod. 2012, 29, 20–27. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, N.; Liu, H.; Li, Z.; Lu, L.; Wang, C. The bioactive compounds and biological functions of Asparagus officinalis L.—A review. J. Funct. Foods 2020, 65, 103727. [Google Scholar] [CrossRef]
- Negi, J.S.; Singh, P.; Joshi, G.P.; Rawat, M.S.; Bisht, V.K. Chemical constituents of Asparagus. Pharmacogn. Rev. 2010, 4, 215. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, O.T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Di Maro, A.; Pacifico, S.; Fiorentino, A.; Galasso, S.; Gallicchio, M.; Guida, V.; Severino, V.; Monaco, P.; Parente, A. Raviscanina wild asparagus (Asparagus acutifolius L.): A nutritionally valuable crop with antioxidant and antiproliferative properties. Food Res. Int. 2013, 53, 180–188. [Google Scholar] [CrossRef]
- Ferrara, L.; Dosi, R.; Di Maro, A.; Guida, V.; Cefarelli, G.; Pacifico, S.; Mastellone, C.; Fiorentino, A.; Rosati, A.; Parente, A. Nutritional values, metabolic profile and radical scavenging capacities of wild asparagus (A. acutifolius L.). J. Food Compos. Anal. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Hamdi, A.; Jaramillo-Carmona, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Lachaal, M.; Karray-Bouraoui, N.; Guillén-Bejarano, R. Phytochemical characterization and bioactivity of Asparagus acutifolius: A focus on antioxidant, cytotoxic, lipase inhibitory and antimicrobial activities. Molecules 2021, 26, 3328. [Google Scholar] [CrossRef] [PubMed]
- Altundag, E.M.; Gençalp, D.; Özbilenler, C.; Toprak, K.; Kerküklü, N. In vitro antioxidant, anti-inflammatory and anti-cancer activities of methanolic extract of Asparagus horridus grows in North Cyprus Kuzey Kıbrıs da yetişen Asparagus horridus metanolik ekstraktının in-vitro antioksidan, anti-enflamatuar ve anti-kanser aktivitesi. Turk. J. Biochem. 2020, 45, 365–372. [Google Scholar] [CrossRef]
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Muñoz, E.; Heinrich, M. Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol. 2009, 124, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Palfi, M.; Jurković, Z.; Ćosić, J.; Tomić-Obrdalj, H.; Jurković, V.; Knežević, N.; Vrandečić, K. Total polyphenol content and antioxidant activity of wild and cultivated asparagus in Croatia. Poljoprivreda 2017, 23, 56–62. [Google Scholar] [CrossRef]
- Englard, S.; Seifter, S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 1986, 6, 365–406. [Google Scholar] [CrossRef]
- Padh, H. Cellular functions of ascorbic acid. Biochem. Cell Biol. 1990, 6, 1166–1173. [Google Scholar] [CrossRef]
- Esteve, M.J.; Farre, R.; Frigola, A.; Clemente, G. Changes in ascorbic acid content of green asparagus during the harvesting period and storage. J. Agric. Food Chem. 1995, 43, 2058–2061. [Google Scholar] [CrossRef]
- Moreno, R.; Castro, P.; Rubio, J.; Rodriguez-Arcos, R.; Gil, J. Desarrollo de una nueva variedad de espárrago octo-ploide ’HT801’. Mejor. Genética Veg. 2012, 60, 105–108. [Google Scholar]
- Martins, D.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional and in vitro antioxidant properties of edible wild greens in Iberian Peninsula traditional diet”. Food Chem. 2011, 125, 488–494. [Google Scholar] [CrossRef]
- Beck, H.; Zimmermann, N.; McVicar, T.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E. Present and future Kö-ppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Stavroula, M.; Rahul, J. Mediterranean climate affects the biosynthesis of secondary metabolites in common medicinal plants. Int. J. Bus. Syst. Res. 2016, 6, 17–28. [Google Scholar]
- Maeda, T.; Honda, K.; Sonoda, T.; Motoki, S.; Inoue, K.; Suzuki, T.; Oosawa, K.; Suzuki, M. Light condition influences rutin and polyphenol contents in asparagus spears in the mother-fern culture system during the summer-autumn harvest. J. Jpn. Soc. Hortic. Sci. 2010, 79, 161–167. [Google Scholar] [CrossRef]
- Medina-Medrano, J.R.; Almaraz-Abarca, N.; González-Elizondo, M.S.; Uribe-Soto, J.N.; González-Valdez, L.S.; Herrera-Arrieta, Y. Phenolic constituents and antioxidant properties of five wild species of Physalis (Solanaceae). Bot. Stud. 2015, 56, 24. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef] [PubMed]
- Motoki, S.; Kitazawa, H.; Maeda, T.; Suzuki, T.; Chiji, H.; Nishihara, E.; Shinohara, Y. Effects of various asparagus production methods on rutin and protodioscin contents in spears and cladophylls. Biosci. Biotechnol. Biochem. 2012, 76, 1047–1050. [Google Scholar] [CrossRef]
- Frutos, M.J.; Rincón-frutos, L.; Valero-cases, E. Chapter 2.14: Rutin. In Nonvitamin and Nonmineral Nutritional Suplements; Nabavi, S.M., Silva, A.S., Eds.; Charlotte Cockle: London, UK, 2019; pp. 111–117. [Google Scholar] [CrossRef]
- Dehshiri, M.M.; Aghamollaei, H.; Zarini, M.; Nabavi, S.M.; Mirzaei, M.; Loizzo, M.R.; Nabavi, S.F. Antioxidant activity of different parts of Tetrataenium lasiopetalum. Pharm. Biol. 2013, 51, 1081–1085. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosein-Farzaei, M.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, S1700–S1741. [Google Scholar] [CrossRef]
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Huang, Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 2015, 52, 2690–2700. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Rodríguez, G.; Espejo, J.A.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A.; Jiménez, A. Antioxidant Activity of Ethanolic Extracts from Several Asparagus Cultivars. J. Agric. Food Chem. 2005, 53, 5212–5217. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007, 105, 101–106. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Flaczyk, E.; Rudzińska, M.; Kmiecik, D. Antioxidant properties of extracts from Ginkgo biloba leaves in meatballs. Meat Sci. 2014, 97, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Valencia, D.; Alday, E.; Robles-Zepeda, R.; Garibay-Escobar, A.; Galvez-Ruiz, J.C.; Salas-Reyes, M.; Jiménez-Estrada, M.; Velazquez-Contreras, E.; Hernandez, J.; Velazquez, C. Seasonal effect on chemical composition and biological activi-ties of Sonoran propolis. Food Chem. 2012, 131, 645–651. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Domestic Processing of Onion Bulbs (Allium cepa) and Asparagus Spears (Asparagus officinalis): Effect on Flavonol Content and Antioxidant Status. J. Agric. Food Chem. 2001, 49, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Kobus-Cisowska, J.; Kmiecik, D.; Gramza-Michałowska, A.; Golczak, D.; Korczak, J. Antiradical capacity and polyphenol composition of asparagus spears varieties cultivated under diff erent sunlight conditions. Acta Sci. Pol. Technol. Aliment. 2016, 15, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Pathiraja, D.; Wanasundara, J.P.D.; Elessawy, F.M.; Purves, R.W.; Vandenberg, A.; Shand, P.J. Water-soluble phenolic compounds and their putative antioxidant activities in the seed coats from different lentil (Lens culinaris) genotypes. Food Chem. 2023, 407, 135145. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.L.; Cho, N.; Lee, T.H.; Ahn, S.J.; Lee, D.J.; Ku, Y.G. Bioactive substances, antioxidant enzymes, and anti-cancer activity of asparagus ‘atlas’ grown in an open field and rain-shelter house system. Hortic. Environ. Biotechnol. 2022, 63, 809–821. [Google Scholar] [CrossRef]
- Bousserouel, S.; Le Grandois, J.; Gossé, F.; Werner, D.; Barth, S.W.; Marchioni, E.; Marescaux, J.; Raul, F. Methanolic extract of white asparagus shoots activates TRAIL apoptotic death pathway in human cancer cells and inhibits colon carcin-ogenesis in preclinical model. Int. J. Oncol. 2013, 43, 394–404. [Google Scholar] [CrossRef]
- Jaramillo, S.; Muriana, F.J.G.; Guillen, R.; Jimenez-Araujo, A.; Rodríguez-Arcos, R.; López, S. Saponins from edible spears of wild asparagus inhibit AKT, p70S6K, and ERK signalling, and induce apoptosis through G0/G1 cell cycle arrest in human colon cancer HCT-116 cells. J. Funct. Foods 2016, 26, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Fan, Y.; Zhao, Z.; Paraghamian, S.E.; Hawkins, G.M.; Buckingham, L.; O’Donnell, J.; Hao, T.; Suo, H.; et al. Asparagus officinalis combined with paclitaxel exhibited synergistic anti-tumor activity in paclitaxel-sensitive and-resistant ovarian cancer cells. J. Cancer Res. Clin. Oncol. 2022, 149, 3871–3883. [Google Scholar] [CrossRef]
- Khan, K.M.; Nahar, L.; Mannan, A.; Arfan, M.; Khan, G.A.; Al-Groshi, A.; Evans, A.; Dempster, N.M.; Ismail, F.M.D.; Sarker, S.D. Liquid chromatography mass spectrometry analysis and cytotoxicity of Asparagus adscendens roots against human cancer cell lines. Pharmacogn. Mag. 2017, 13, S890–S894. [Google Scholar] [CrossRef]
- Liu, W.; Ning, R.; Chen, R.N.; Huang, X.F.; Dai, Q.S.; Hu, J.H.; Wang, Y.W.; Wu, L.L.; Xiong, J.; Hu, G.; et al. Aspafilioside B induces G2/M cell cycle arrest and apoptosis by up-regulating H-Ras and N-Ras via ERK and p38 MAPK signaling pathways in human hepatoma HepG2 cells. Mol. Carcinog. 2015, 55, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Jaramillo-Carmona, S.; Srairi Beji, R.; Tej, R.; Zaouia, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Kasri, M.; Lachaal, M.; Karray Bouraoui, N.; et al. The phytochemical and bioactivity profiles of wild Asparagus albus L. Food Res. Int. 2017, 99, 720–729. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, B.; Yan, J.; Zhao, F.; Xiao, J.; Yao, L.; Zhao, B.; Huang, Y. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr. Polym. 2012, 87, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jia, G.; Wang, X.; Liu, Y.; Li, Z.; Bao, H.; Guo, Q.; Wang, C.; Xiao, D. Fractionation, structural characteris-tics and immunomodulatory activity of polysaccharide fractions from asparagus (Asparagus officinalis L.) skin. Carbohydr. Polym. 2021, 256, 117514. [Google Scholar] [CrossRef] [PubMed]
- Chileh-Chelh, T.; Lyashenko, S.; Lahlou, A.; Belarbi, E.H.; Rincón-Cervera, M.Á.; Rodríguez-García, I.; Urrestarazu-Gavilán, M.; López-Ruiz, R.; Guil-Guerrero, J.L. Buglossoides spp. seeds, a land source of health-promoting n-3 PUFA and phenolic compounds. Food Res. Int. 2022, 157, 111421. [Google Scholar] [CrossRef]
- Kim, R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem. Biophys. Res. Commun. 2005, 333, 336–343. [Google Scholar] [CrossRef]
- Lyashenko, S.; Fabrikov, D.; González-Fernández, M.J.; Gómez-Mercado, F.; López-Ruiz, R.; Fedorov, A.; Guil-Guerrero, J.L. Phenolic composition and in vitro antiproliferative activity of Borago spp. seed extracts on HT-29 cancer cells. Food Biosci. 2021, 42, 101043. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of flavonoid-rich extracts of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, L-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients 2017, 9, 621. [Google Scholar] [CrossRef]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; Gonnella, M.; Renna, M.; Linsalata, V.; Gatto, M.A.; Boari, F.; Di Venere, D. Biochemical traits of aspara-gus cultivars and quality changes in two differently coloured genotypes during cold storage. LWT 2019, 101, 427–434. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; González-Paramás, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F.; et al. Strawberry (cv. Romina) methanolic extract and anthocyanin-enriched fraction improve lipid profile and antioxidant status in HepG2 cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, A.; Chileh-Chelh, T.; Lyashenko, S.; Rincón-Cervera, M.Á.; Rodríguez-García, I.; López-Ruiz, R.; Urrestarazu, M.; Guil-Guerrero, J.L. Arecaceae fruits: Fatty acids, phenolic compounds and in vitro antitumor activity. Food Biosci. 2022, 50, 102181. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Guil-Guerrero, J.L. Various acylglycerols from common oils exert different antitumor activities on colorectal cancer cells. Nutr. Cancer 2016, 68, 518–529. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Romero-González, R.; González-Fernández, M.J.; Guil-Guerrero, J.L. Phytochemical composition and in vitro anti-tumour activities of selected tomato varieties. J. Sci. Food Agric. 2017, 97, 488–496. [Google Scholar] [CrossRef]
- Ayhan, N.K.; Rosenberg, E. Development of comprehensive liquid chromatography with diode array and mass spectrometric detection for the characterization of (poly-) phenolic and flavonoid compounds and application to asparagus. Food Chem. 2021, 354, 129518. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.; Carvalho, A.M.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Chen, X.H.; Ma, L.H.; Dong, Y.W.; Song, H.; Pu, Y.; Zhou, Q.Y. Evaluation of the differences in phenolic compounds and antioxidant activities of five green asparagus (Asparagus officinalis L.) cultivars. Qual. Assur. Saf. Crops Foods 2017, 9, 479–487. [Google Scholar] [CrossRef]
- Eum, H.L.; Yi, T.G.; Hong, S.J.; Park, N.I. Variations of bioactive compound contents and antioxidant capacity of asparagus seedlings in 23 varieties. Hortic. Sci. Technol. 2020, 38, 291–302. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Szczepaniak, O.M.; Gramza-Michałowska, A.; Kmiecik, D.; Kulczyński, B.; Szulc, P.; Górnaś, P. Composition of polyphenols of asparagus spears (Asparagus officinalis) and their antioxidant potential. Ciência Rural 2019, 49. [Google Scholar] [CrossRef]
- Ku, Y.G.; Kang, D.H.; Lee, C.K.; Lee, S.Y.; Ryu, C.; Kim, D.E.; Polovka, M.; Namieśnik, J.; Gorinstein, S. Influence of different cultivation systems on bioactivity of asparagus. Food Chem. 2018, 244, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Yoo, K.S.; Patil, B.S. Development of a rapid HPLC-UV method for simultaneous quantification of protodioscin and rutin in white and green asparagus spears. J. Food Sci. 2010, 75, C703–C709. [Google Scholar] [CrossRef] [PubMed]
- Nindo, C.; Sun, T.; Wang, S.W.; Tang, J.; Powers, J.R. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis L.). LWT-Food Sci. Technol. 2003, 36, 507–516. [Google Scholar] [CrossRef]
- Noperi-Mosqueda, L.C.; López-Moreno, F.J.; Navarro-León, E.; Sánchez, E.; Blasco, B.; Moreno, D.A.; Soriano, T.; Ruiz, J.M. Effects of asparagus decline on nutrients and phenolic compounds, spear quality, and allelopathy. Sci. Hortic. 2020, 261, 109029. [Google Scholar] [CrossRef]
- Poljuha, D.; Šola, I.; Bilić, J.; Dudaš, S.; Bilušić, T.; Markić, J.; Rusak, G. Phenolic composition, antioxidant capacity, energy content and gastrointestinal stability of Croatian wild edible plants. Eur. Food Res. Technol. 2015, 241, 573–585. [Google Scholar] [CrossRef]
- Redondo-Cuenca, A.; García-Alonso, A.; Rodríguez-Arcos, R.; Castro, I.; Alba, C.; Rodríguez, J.M.; Goñi, I. Nutritional composition of green asparagus (Asparagus officinalis L.), edible part and by-products, and assessment of their effect on the growth of human gut-associated bacteria. Food Res. Int. 2023, 163, 112284. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jaramillo, S.; Guillén, R.; Jiménez, A.; Fernández-Bolaños, J.; Heredia, A. Cell wall phenolics of white and green asparagus. J. Sci. Food Agric. 2005, 85, 971–978. [Google Scholar] [CrossRef]
- Salvatore, S.; Pellegrini, N.; Brenna, O.V.; Del Rio, D.; Frasca, G.; Brighenti, F.; Tumino, R. Antioxidant characterization of some Sicilian edible wild greens. J. Agric. Food Chem. 2005, 53, 9465–9471. [Google Scholar] [CrossRef]
- Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive phenolics and antioxidant capacity of some wild edible greens as affected by different cooking treatments. Foods 2020, 9, 1320. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; Boari, F.; Di Venere, D.; Gonnella, M.; Cantore, V.; Renna, M. Quality evaluation of wild and cultivated asparagus: A comparison between raw and steamed spears. Agriculture 2021, 11, 1213. [Google Scholar] [CrossRef]
- Slatnar, A.; Petkovsek, M.M.; Stampar, F.; Veberic, R.; Horvat, J.; Jakse, M.; Sircelj, H. Game of tones: Sugars, organic acids, and phenolics in green and purple asparagus (Asparagus officinalis L.) cultivars. Turk. J. Agric. For. 2018, 42, 55–66. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Wang, M.; Tadmor, Y.; Wu, Q.L.; Chin, C.K.; Garrison, S.A.; Simon, J.E. Quantification of protodioscin and rutin in asparagus shoots by LC/MS and HPLC methods. J. Agric. Food Chem. 2003, 51, 6132–6136. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Su, Y.; Liu, D.; Ye, X. Effect of salicylic acid treatment on postharvest quality, antioxidant activities, and free polyamines of asparagus. J. Food Sci. 2011, 76, S126–S132. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Fan, L. Effect of drying methods on the microstructure, bioactivity substances, and antityrosinase activity of asparagus stems. J. Agric. Food Chem. 2019, 67, 1537–1545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).