In Vivo Zymosan Treatment Induces IL15-Secreting Macrophages and KLRG1-Expressing NK Cells in Mice
Abstract
1. Introduction
2. Results
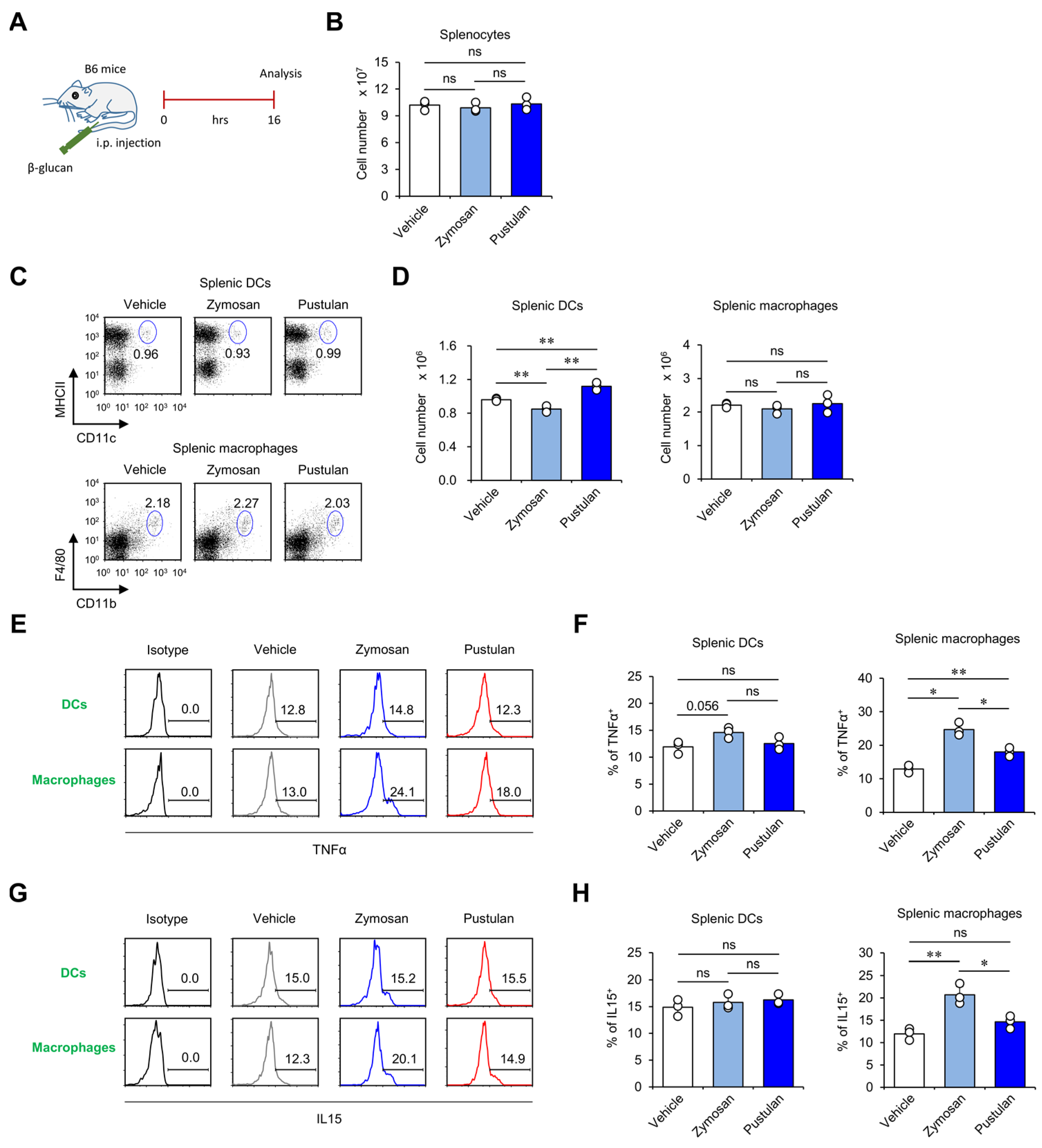
2.1. In Vivo Zymosan Treatment Activates Splenic Macrophages to Produce High Levels of IL15
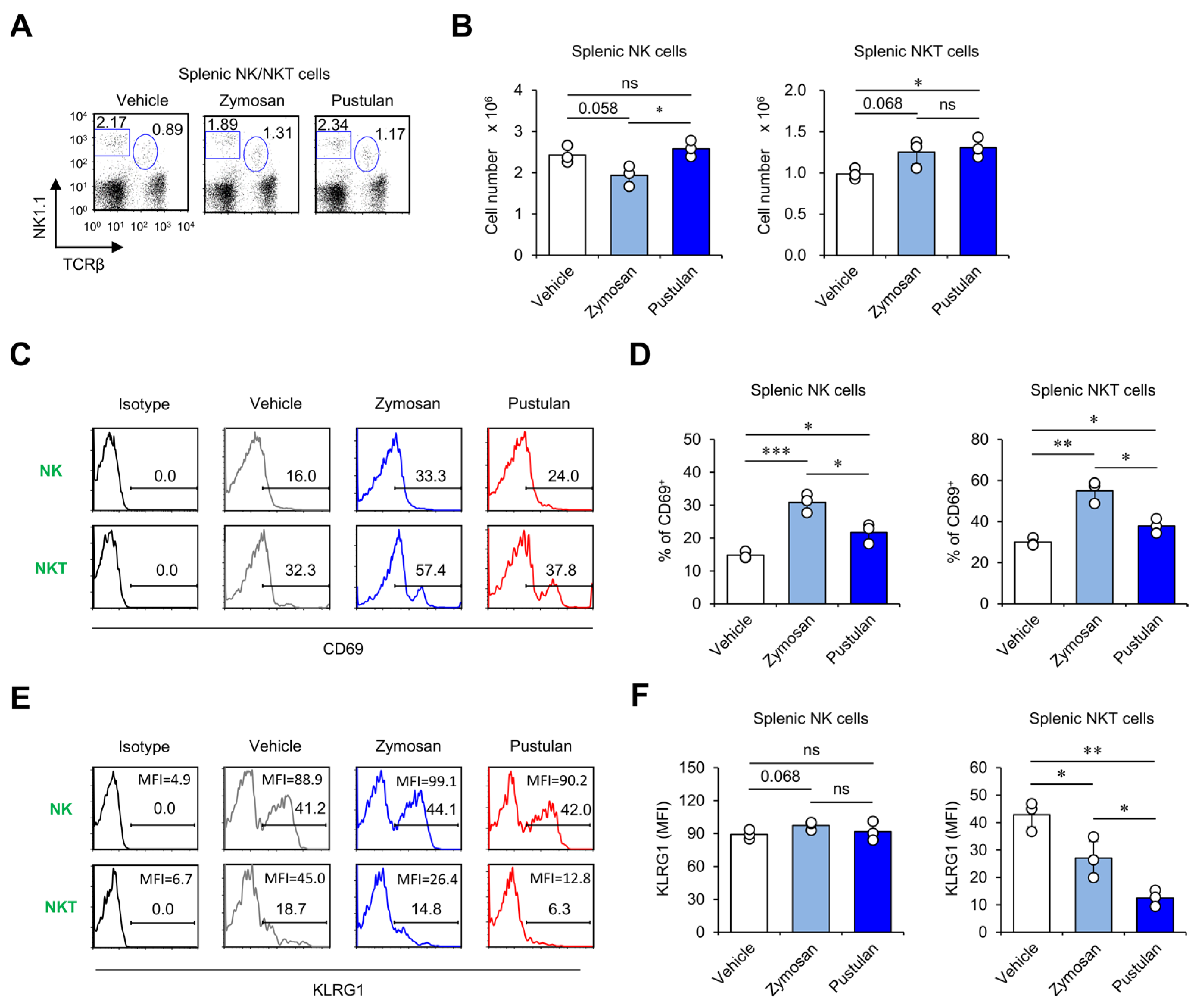
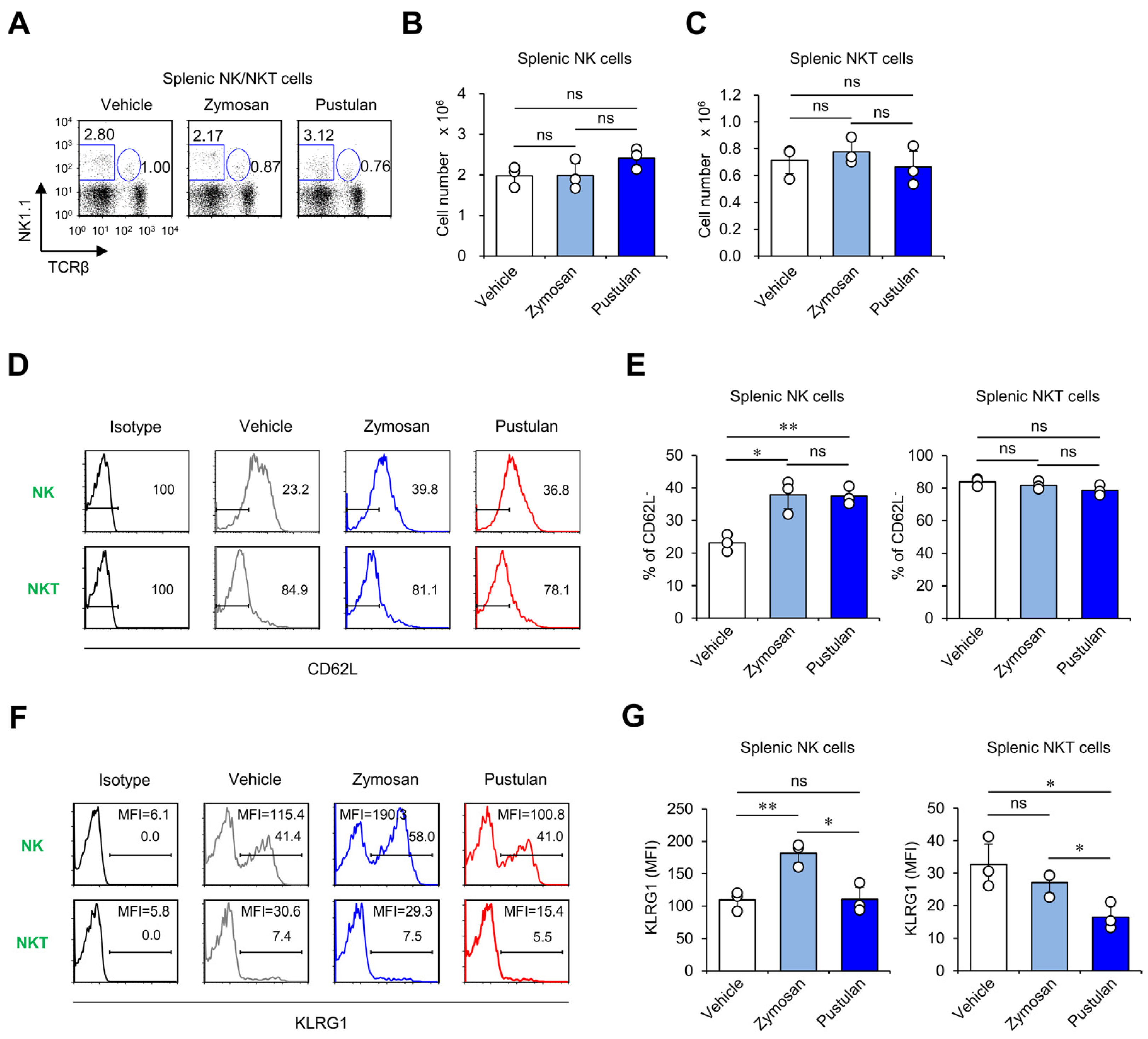
2.2. In Vivo Zymosan Treatment Induces the Activation of NK and NKT Cells
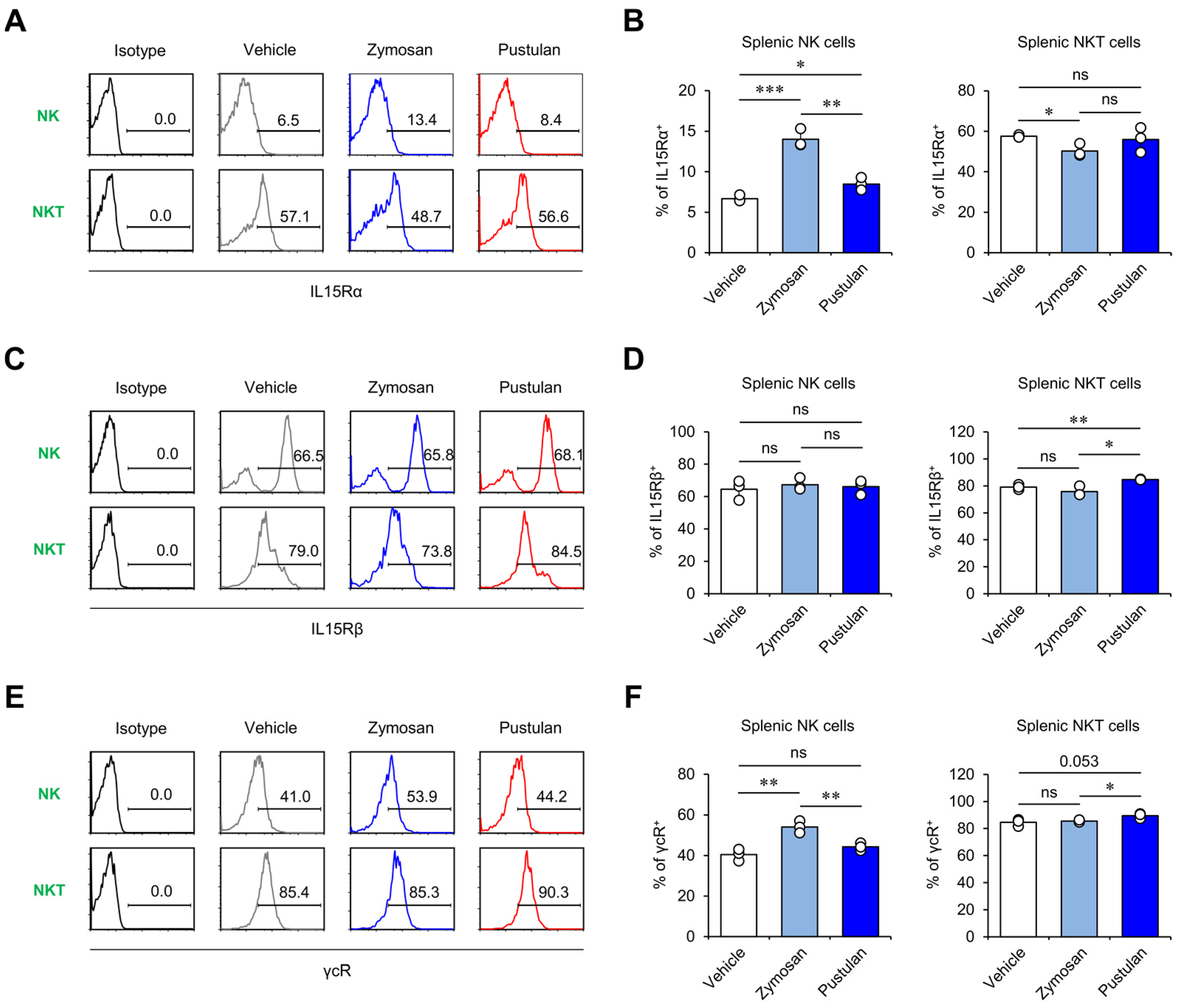
2.3. In Vivo Zymosan Treatment Increases IL15 Receptor Expression on NK Cells
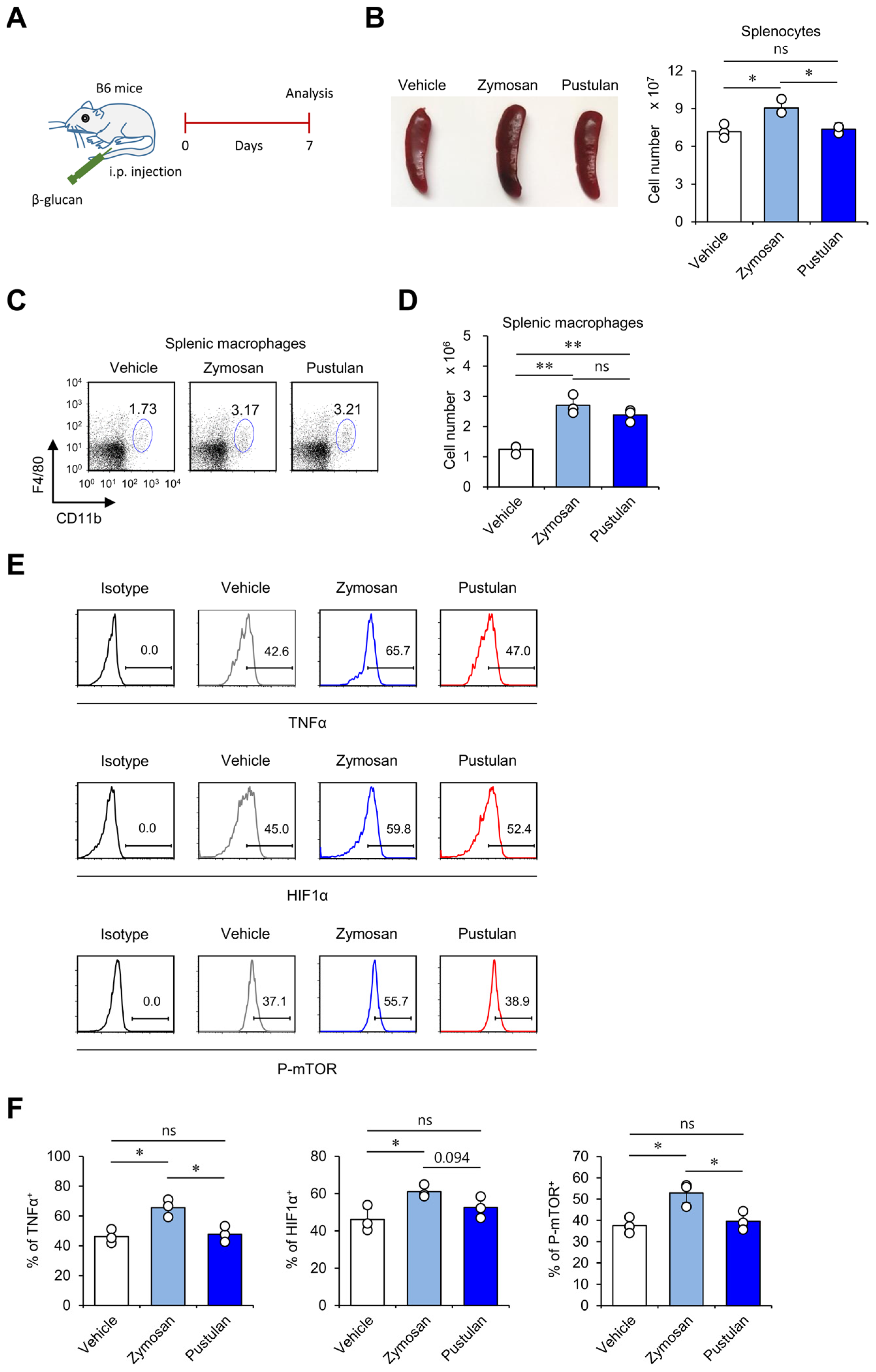
2.4. In Vivo Zymosan Treatment Upregulates Innate Memory-Related Markers on Macrophages
2.5. In Vivo Zymosan Treatment Upregulates Expression of Memory Markers on NK Cells
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mice and Reagents
4.3. Flow Cytometry
4.4. Intracellular Cytokine Staining
4.5. Phosphoflow Analysis of Protein Phosphorylation Levels
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Hong, J.T.; Kim, Y.; Han, S.B. Stimulatory Effect of beta-glucans on Immune Cells. Immune Netw. 2011, 11, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained immunity, tolerance, priming and differentiation: Distinct immunological processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed]
- Fahlquist-Hagert, C.; Sareila, O.; Rosendahl, S.; Holmdahl, R. Variants of beta-glucan polysaccharides downregulate autoimmune inflammation. Commun. Biol. 2022, 5, 449. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kim, Y.J.; Han, K.U.; Yoon, B.D.; Kim, J.W. Zymosan and PMA activate the immune responses of Mutz3-derived dendritic cells synergistically. Immunol. Lett. 2015, 167, 41–46. [Google Scholar] [CrossRef]
- Sas, A.R.; Carbajal, K.S.; Jerome, A.D.; Menon, R.; Yoon, C.; Kalinski, A.L.; Giger, R.J.; Segal, B.M. A new neutrophil subset promotes CNS neuron survival and axon regeneration. Nat. Immunol. 2020, 21, 1496–1505. [Google Scholar] [CrossRef]
- Geary, C.D.; Sun, J.C. Memory responses of natural killer cells. Semin. Immunol. 2017, 31, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Malaise, M.; Rovira, J.; Renner, P.; Eggenhofer, E.; Sabet-Baktach, M.; Lantow, M.; Lang, S.A.; Koehl, G.E.; Farkas, S.A.; Loss, M.; et al. KLRG1+ NK cells protect T-bet-deficient mice from pulmonary metastatic colorectal carcinoma. J. Immunol. 2014, 192, 1954–1961. [Google Scholar] [CrossRef]
- Elpek, K.G.; Rubinstein, M.P.; Bellemare-Pelletier, A.; Goldrath, A.W.; Turley, S.J. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc. Natl. Acad. Sci. USA 2010, 107, 21647–21652. [Google Scholar] [CrossRef] [PubMed]
- Victorino, F.; Bigley, T.M.; Park, E.; Yao, C.H.; Benoit, J.; Yang, L.P.; Piersma, S.J.; Lauron, E.J.; Davidson, R.M.; Patti, G.J.; et al. HIF1alpha is required for NK cell metabolic adaptation during virus infection. eLife 2021, 10, e68484. [Google Scholar] [CrossRef] [PubMed]
- Javmen, A.; Nemeikaite-Ceniene, A.; Bratchikov, M.; Grigiskis, S.; Grigas, F.; Jonauskiene, I.; Zabulyte, D.; Mauricas, M. beta-Glucan from Saccharomyces cerevisiae Induces IFN-gamma Production In Vivo in BALB/c Mice. In Vivo 2015, 29, 359–363. [Google Scholar] [PubMed]
- Park, H.J.; Lee, S.W.; Van Kaer, L.; Hong, S. CD1d-Dependent iNKT Cells Control DSS-Induced Colitis in a Mouse Model of IFNgamma-Mediated Hyperinflammation by Increasing IL22-Secreting ILC3 Cells. Int. J. Mol. Sci. 2021, 22, 1250. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Cheon, J.H.; Wu, L.; Van Kaer, L.; Hong, S. iNKT Cells Suppress Pathogenic NK1.1(+)CD8(+) T Cells in DSS-Induced Colitis. Front. Immunol. 2018, 9, 2168. [Google Scholar] [CrossRef]
- Huntington, N.D.; Puthalakath, H.; Gunn, P.; Naik, E.; Michalak, E.M.; Smyth, M.J.; Tabarias, H.; Degli-Esposti, M.A.; Dewson, G.; Willis, S.N.; et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 2007, 8, 856–863. [Google Scholar] [CrossRef]
- Ma, S.; Caligiuri, M.A.; Yu, J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. 2022, 43, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, C.; Zhang, J.; Tian, Z. Macrophages help NK cells to attack tumor cells by stimulatory NKG2D ligand but protect themselves from NK killing by inhibitory ligand Qa-1. PLoS ONE 2012, 7, e36928. [Google Scholar] [CrossRef]
- Nedvetzki, S.; Sowinski, S.; Eagle, R.A.; Harris, J.; Vely, F.; Pende, D.; Trowsdale, J.; Vivier, E.; Gordon, S.; Davis, D.M. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 2007, 109, 3776–3785. [Google Scholar] [CrossRef]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2012, 3, 403. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, M.; Mortaz, E.; Khosravi, A.; Vahedi, G.; Folkerts, G.; Varahram, M.; Kazempour-Dizaji, M.; Garssen, J.; Adcock, I.M. Zymosan attenuates melanoma growth progression, increases splenocyte proliferation and induces TLR-2/4 and TNF-alpha expression in mice. J. Inflamm. 2018, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, P.; Berrevoets, C.; Rӧsch, C.; Schols, H.A.; Verhoef, K.; Wichers, H.J.; Debets, R.; Govers, C. Curdlan, zymosan and a yeast-derived beta-glucan reshape tumor-associated macrophages into producers of inflammatory chemo-attractants. Cancer Immunol. Immunother. 2021, 70, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.W.; Hong, S. Regulation of Allergic Immune Responses by Microbial Metabolites. Immune Netw. 2018, 18, e15. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Van Kaer, L.; Hong, S. Roles and therapeutic potential of CD1d-Restricted NKT cells in inflammatory skin diseases. Front. Immunol. 2022, 13, 979370. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Park, S.H.; Van Kaer, L.; Hong, S. Selective Expansion of Double-Negative iNKT Cells Inhibits the Development of Atopic Dermatitis in Valpha14 TCR Transgenic NC/Nga Mice by Increasing Memory-Type CD8(+) T and Regulatory CD4(+) T Cells. J. Invest. Dermatol. 2021, 141, 1512–1521. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, T.C.; Park, Y.H.; Lee, S.W.; Jeon, J.; Park, S.H.; Van Kaer, L.; Hong, S. Repeated alpha-GalCer Administration Induces a Type 2 Cytokine-Biased iNKT Cell Response and Exacerbates Atopic Skin Inflammation in Valpha14(Tg) NC/Nga Mice. Biomedicines 2021, 9, 1619. [Google Scholar] [CrossRef]
- Katsuta, M.; Takigawa, Y.; Kimishima, M.; Inaoka, M.; Takahashi, R.; Shiohara, T. NK cells and gamma delta+ T cells are phenotypically and functionally defective due to preferential apoptosis in patients with atopic dermatitis. J. Immunol. 2006, 176, 7736–7744. [Google Scholar] [CrossRef]
- Mack, M.R.; Brestoff, J.R.; Berrien-Elliott, M.M.; Trier, A.M.; Yang, T.B.; McCullen, M.; Collins, P.L.; Niu, H.; Bodet, N.D.; Wagner, J.A.; et al. Blood natural killer cell deficiency reveals an immunotherapy strategy for atopic dermatitis. Sci. Transl. Med. 2020, 12, eaay1005. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Qin, H.; Dahut, M.; Iida, M.; Yacubovich, D.; Hamilton, D.H.; Thomas, A.; Schlom, J.; Palena, C. An Interleukin-15 Superagonist Enables Antitumor Efficacy of Natural Killer Cells Against All Molecular Variants of SCLC. J. Thorac. Oncol. 2023, 18, 350–368. [Google Scholar] [CrossRef]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of beta-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, F.X.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.M.; Deng, X.; et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jeung, I.C.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Wang, F. beta-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. beta-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Song, J.G.; Van Kaer, L.; Cheon, J.H.; Lim, S.J.; Han, H.K.; Hong, S. Aminoclay Nanoparticles Induce Anti-Inflammatory Dendritic Cells to Attenuate LPS-Elicited Pro-Inflammatory Immune Responses. Molecules 2022, 27, 8743. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; Van Kaer, L.; Hong, S. Opposing Roles of DCs and iNKT Cells in the Induction of Foxp3 Expression by MLN CD25(+)CD4(+) T Cells during IFNgamma-Driven Colitis. Int. J. Mol. Sci. 2022, 23, 15316. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Park, Y.H.; Kim, T.C.; Van Kaer, L.; Hong, S. CD1d-independent NK1.1(+) Treg cells are IL2-inducible Foxp3(+) T cells co-expressing immunosuppressive and cytotoxic molecules. Front. Immunol. 2022, 13, 951592. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, H.J.; Pei, Y.; Yeo, Y.; Hong, S. Topical application of zwitterionic chitosan suppresses neutrophil-mediated acute skin inflammation. Int. J. Biol. Macromol. 2020, 158, 1184–1193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Lee, S.W.; Park, Y.H.; Kim, T.-C.; Lee, S.; Lee, S.; Van Kaer, L.; Hong, S. In Vivo Zymosan Treatment Induces IL15-Secreting Macrophages and KLRG1-Expressing NK Cells in Mice. Molecules 2023, 28, 5779. https://doi.org/10.3390/molecules28155779
Park HJ, Lee SW, Park YH, Kim T-C, Lee S, Lee S, Van Kaer L, Hong S. In Vivo Zymosan Treatment Induces IL15-Secreting Macrophages and KLRG1-Expressing NK Cells in Mice. Molecules. 2023; 28(15):5779. https://doi.org/10.3390/molecules28155779
Chicago/Turabian StylePark, Hyun Jung, Sung Won Lee, Yun Hoo Park, Tae-Cheol Kim, Sujin Lee, Seyeong Lee, Luc Van Kaer, and Seokmann Hong. 2023. "In Vivo Zymosan Treatment Induces IL15-Secreting Macrophages and KLRG1-Expressing NK Cells in Mice" Molecules 28, no. 15: 5779. https://doi.org/10.3390/molecules28155779
APA StylePark, H. J., Lee, S. W., Park, Y. H., Kim, T.-C., Lee, S., Lee, S., Van Kaer, L., & Hong, S. (2023). In Vivo Zymosan Treatment Induces IL15-Secreting Macrophages and KLRG1-Expressing NK Cells in Mice. Molecules, 28(15), 5779. https://doi.org/10.3390/molecules28155779








