Abstract
An oxidant-free and highly efficient synthesis of phenolic quinazolin-4(3H)-ones was achieved by simply stirring a mixture of 2-aminobenzamides, sulfonyl azides, and terminal alkynes. The intermediate N-sulfonylketenimine underwent two nucleophilic additions and the sulfonyl group eliminated through the power of aromatization. The natural product 2-(4-hydroxybenzyl)quinazolin-4(3H)-one can be synthesized on a large scale under mild conditions with this method.
1. Introduction
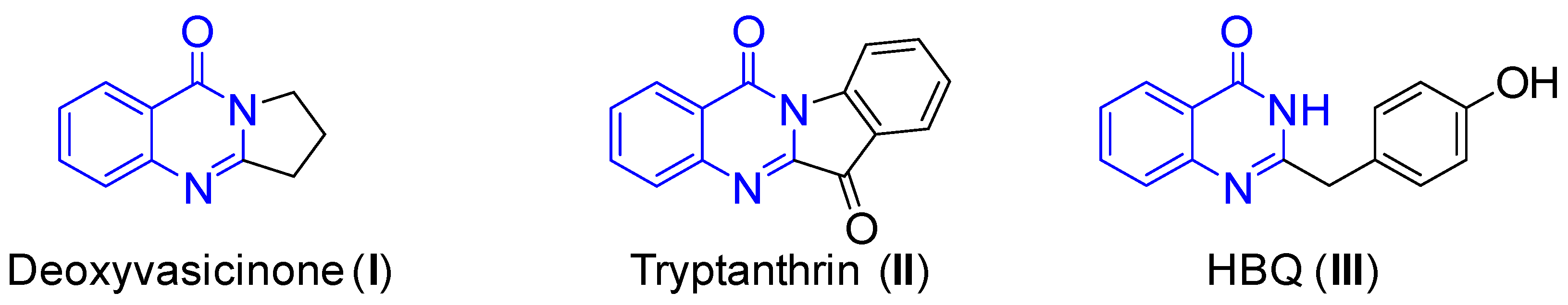
Due to their great physiological importance and pharmaceutical usefulness for fighting tumors, quinazolin-4(3H)-ones are promising compounds for biological and medicinal applications [1,2,3,4]. Some natural and synthetic quinazolin-4(3H)-ones with therapeutic properties are already being tested in clinical trials as potential drugs. For instance, natural products like deoxyvasicinone (I) [5] and tryptanthrin (II) [6,7,8] (Figure 1) have demonstrated antibacterial, antidepressant, and anti-inflammatory properties. The compound 2-(4-hydroxybenzyl) quinazolin-4(3H)-one (HBQ, III) [9,10], which is obtained from a fungus found in marine sediment, has been shown to have significant cytotoxic activity against certain cancer cell lines as well as strong inhibitory effects on the replication of tobacco mosaic virus (TMV). Given their versatile pharmacological and biological characteristics, there is always an urgent need for the synthesis of quinazolin-4(3H)-one products.
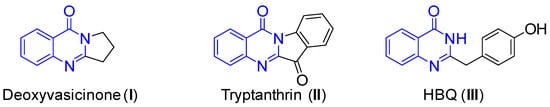
Figure 1.
Natural products containing the quinazolin-4(3H)-one skeleton.
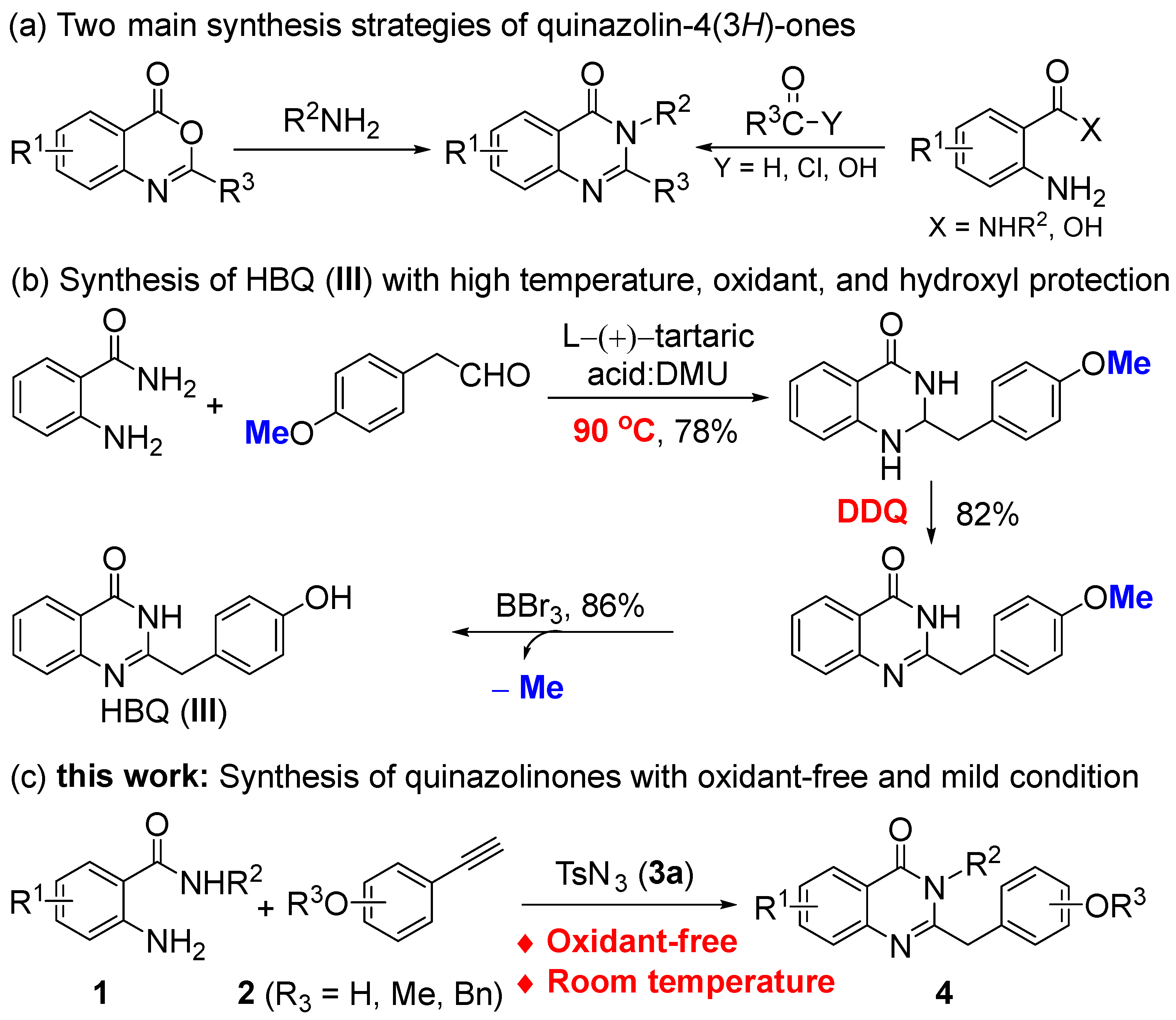
Traditional methods for synthesizing quinazolin-4(3H)-ones involve two main approaches. One method is the amidation of benzoxazinones with arylamines [11,12]. The other method is the condensation of 2-aminobenzoyl derivatives with carbonyl derivatives (Scheme 1a). The latter method is the primary approach; the 2-aminobenzoyl derivatives included are 2-aminobenzamides [13,14,15,16,17,18,19,20,21,22,23,24], 2-aminobenzoic acid [11,25,26,27], 2-nitrobenzamides [28,29], and methyl anthranilate [30,31,32], while the carbonyl derivatives included are aldehydes [14,15], 1,3-diketones [16,17], orthoesters [18,19], benzyl alcohols [20,21,22], benzyl halides [23,24], acetophenones [33,34,35,36], methylarenes [37], and others [38,39]. Although most of these synthetic methods have their own merits, they often require extreme reaction conditions such as heating, using dehydration reagents, and adding an oxidant, which limits the synthesis of phenolic quinazolin-4(3H)-ones, or the phenol hydroxyl group needs to be protected in advance. For example, HBQ needs to be synthesized by oxidation from 4 (1H) quinazolinone using DDQ, which also requires high temperatures and protection of the phenol hydroxyl group from oxidation (Scheme 1b) [15].
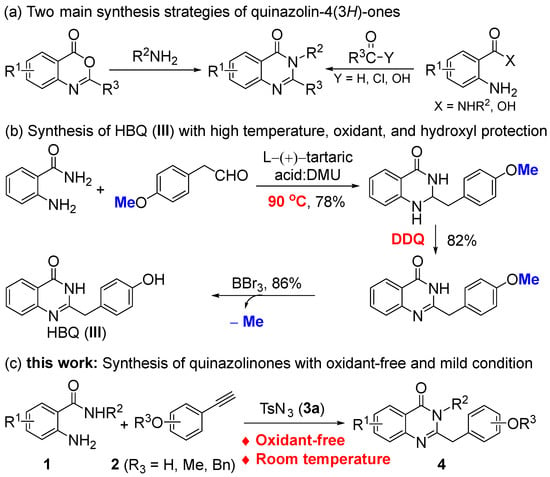
Scheme 1.
Strategies for the synthesis of quinazolin-4(3H)-ones.
Since it was reported by Chang’s group [40,41], the copper-catalyzed sulfonyl azide−alkyne cycloaddition/ring cleavage reaction (CuAAC/ring cleavage reaction) has been acknowledged as a gentle and effective method for synthesizing various nitrogenated compounds. It has also been used for modifying the structure of natural products, drugs, and biological macromolecules [42,43,44]. Our group has delved into this area and utilized the CuAAC/ring cleavage reaction to synthesize pyridine derivates, fused heterocycles, coumarins, indoles, and other nitrogenated compounds [45,46,47,48,49].
Therefore, in this study, we present a highly efficient and oxidant-free approach to synthesize phenolic quinazolin-4(3H)-ones using the CuAAC/ring cleavage reaction (Scheme 1c). This method involves stirring a mixture of 2-aminobenzamides, sulfonyl azides, and terminal alkynes in the presence of a copper(I) catalyst under mild conditions.
2. Results
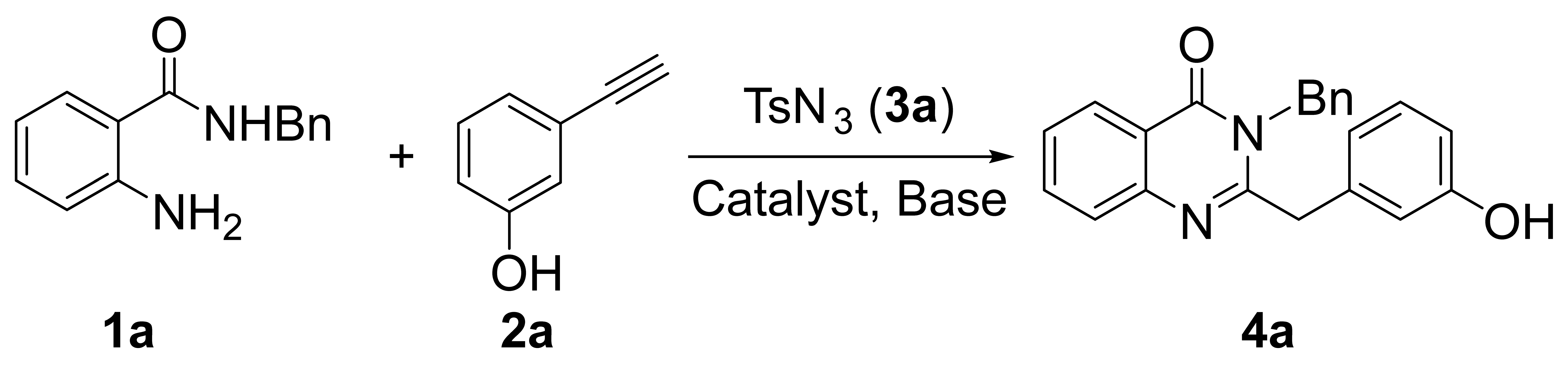
Our investigations began with an examination of the synthesis of the parent and previously unreported system 3-benzyl-2-(3-hydroxybenzyl)quinazolin-4(3H)-one 4a via 2-amino-N-benzylbenzamide 1a, 3-ethynylphenol 2a, and tosyl azide 3a (Table 1). After an initial screening using CuI as a catalyst with the additive Et3N in a variety of solvents, we found that the desired conversion was affected by different solvents (Table 1, entries 1−10). The results revealed that MeCN generated product 4a in the highest yield of 85%, the other solvents gave comparable yields, and EtOH generated product 4a with the lowest yield of 34%. Encouraged by these promising results, a variety of catalysts were then evaluated, as shown in Table 1 (entries 11–17). Among the copper catalysts used, CuI catalysts (Table 1, entries 11–12) exhibited higher catalytic reactivity than CuII catalysts (Table 1, entries 13–16), and Cu(OTf)2 (Table 1, entry 17) was the least efficient for this reaction. Additional screening revealed that the other additives used were less efficient than Et3N (Table 1, entries 18–20). It is worth noting that the other sulfonyl azides such as MsN3 or PhSO2N3 were also suitable for this reaction (Table 1, entry 21).

Table 1.
Optimization of catalytic conditions a.
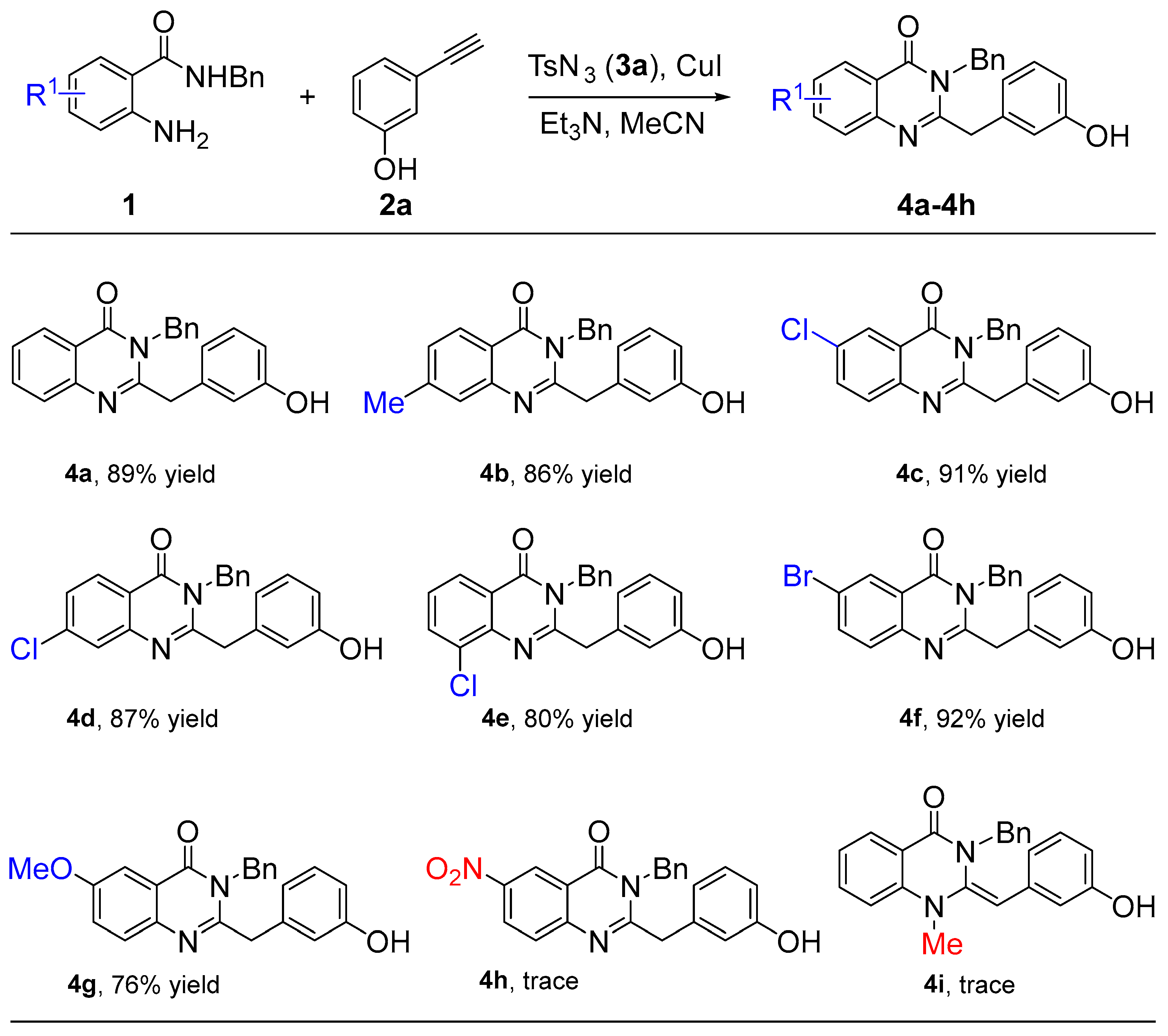
After the optimized reaction condition was established (Table 1, entry 5), the capacity of these reactions to affect the coupling of a range of different 2-aminobenzamides 1 was investigated. As shown in Scheme 2, the electronic effects of the substituents 2-aminobenzamides 1 had an obvious influence. For example, the substrate bearing a –Me group was examined, and an 82% yield of 4b was isolated, which is the same efficiency as 4a. When 2-aminobenzamides 1 carried halogen substituents including Cl or Br, the anticipated products (4c–4f) were also obtained in good yields ranging from 80% to 92%. However, the strong electron-donating substituent gave the corresponding product 4g with a moderate yield of 76%, while the strongly electron-withdrawing substituent did not obtain the target product 4h due to the weak nucleophilic activity of the amino group. Finally, when the –NH2 group was replaced by –NHMe, the target product 4i was not obtained.
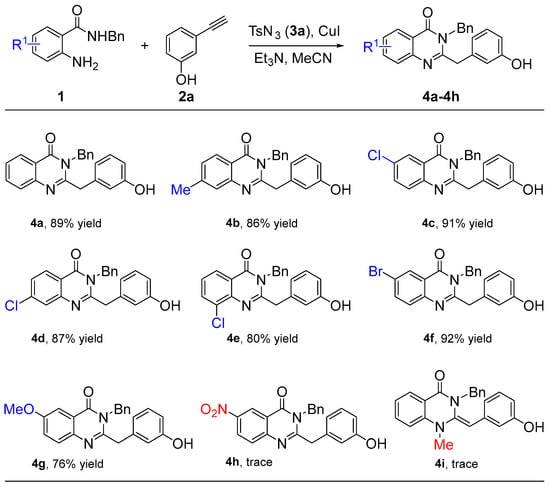
Scheme 2.
Substrate scope of 2-aminobenzamides 1.
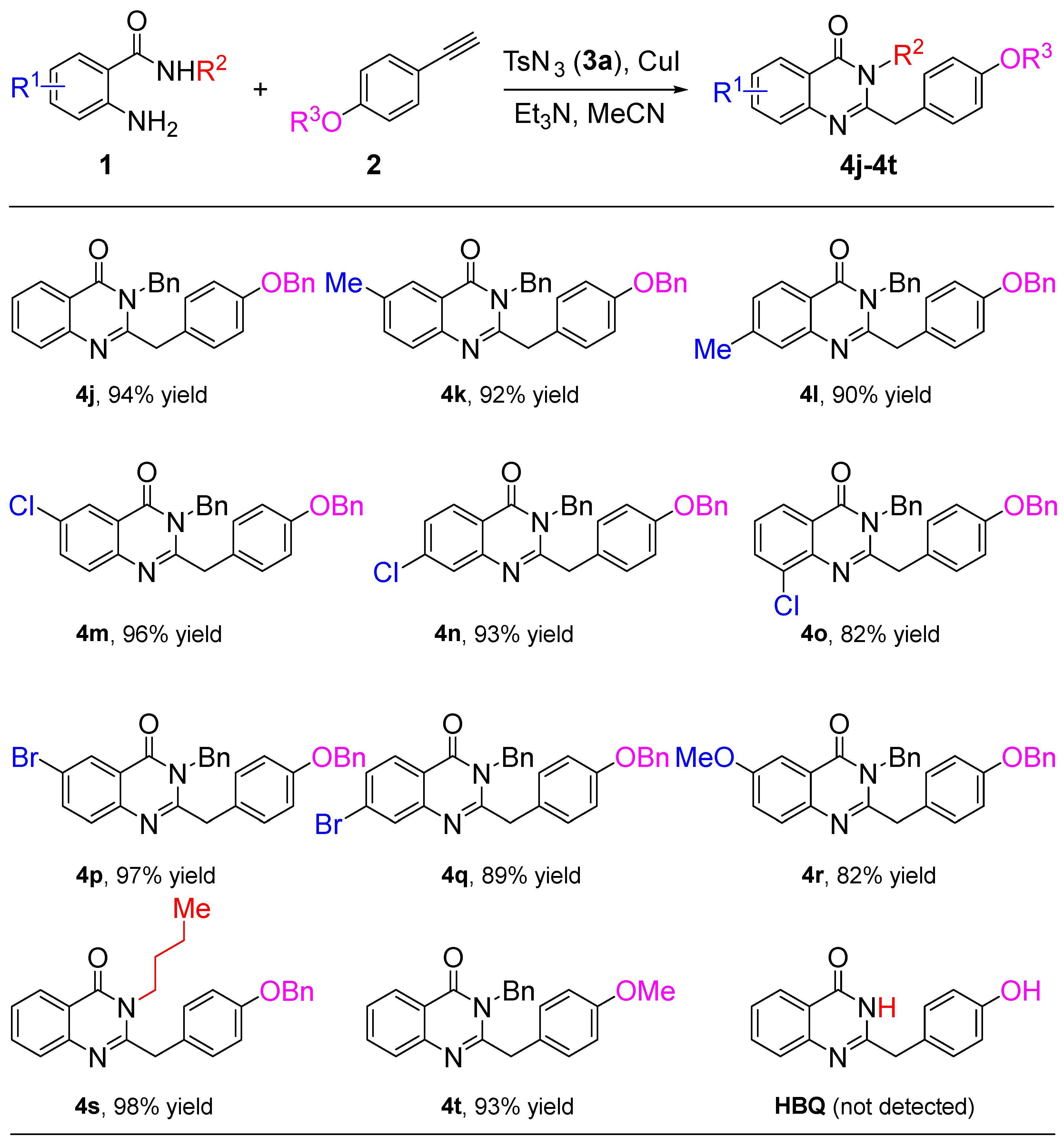
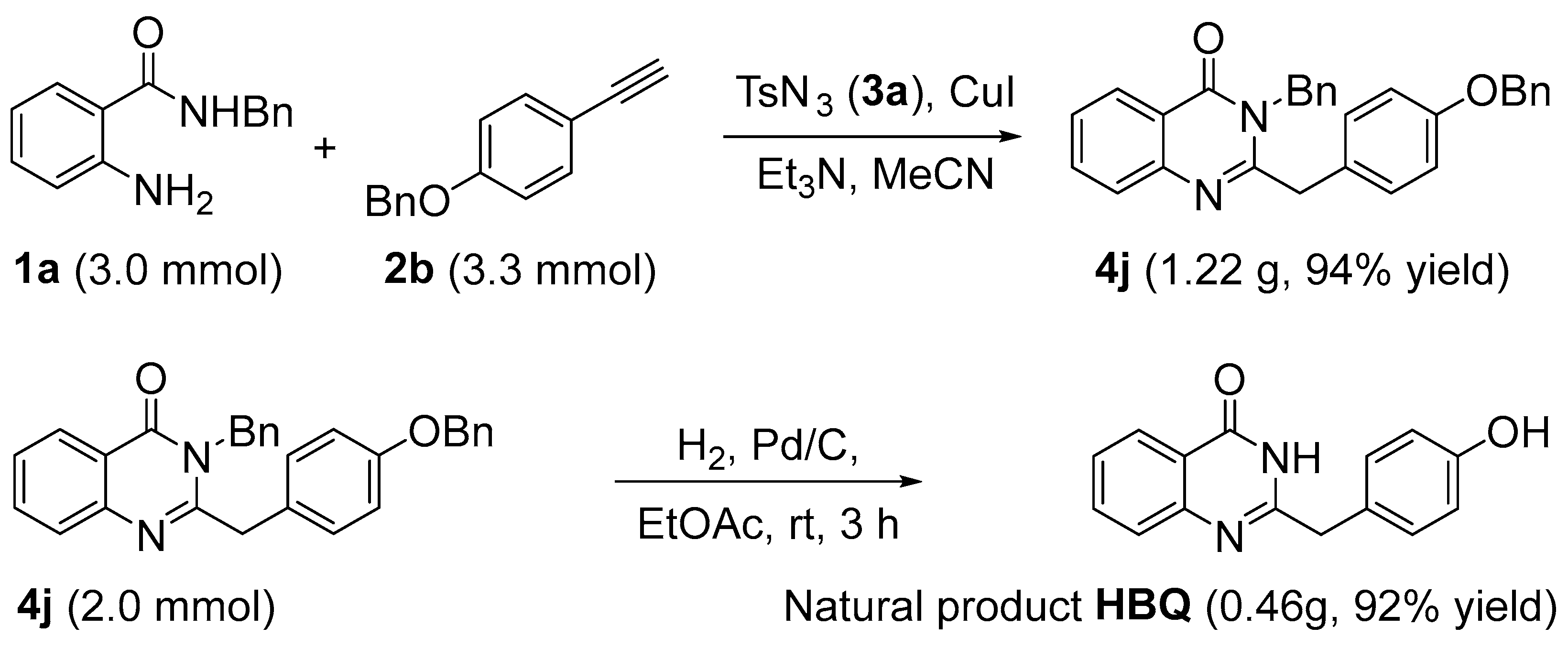
The scope and limitations of different substrates with 2-aminobenzamides 1 and terminal alkynes 2 were also tested. As shown in Scheme 3, 2-aminobenzamides 1 exhibit the same electronic effect when 1-(benzyloxy)-4-ethynylbenzene is involved as a terminal alkyne in this reaction. The effect of the –Me group on the reaction is relatively small (4j–4l), the halogen groups are the most effective (4m–4q), and the strong electron-donating group is poor (4r). Expectedly, with R2 bearing an n-butyl or R3 bearing a –Me group, the corresponding quinazolin-4(3H)-one derivatives 4s or 4t are formed in an excellent yield of 98% and 93%, respectively. Disappointingly, the natural product 2-(4-hydroxybenzyl)quinazolin-4(3H)-one (HBQ, Figure 1, III) was not obtained when the R2 group changed to H of 1a, which shows that the proton in this situation interferes with the reaction.
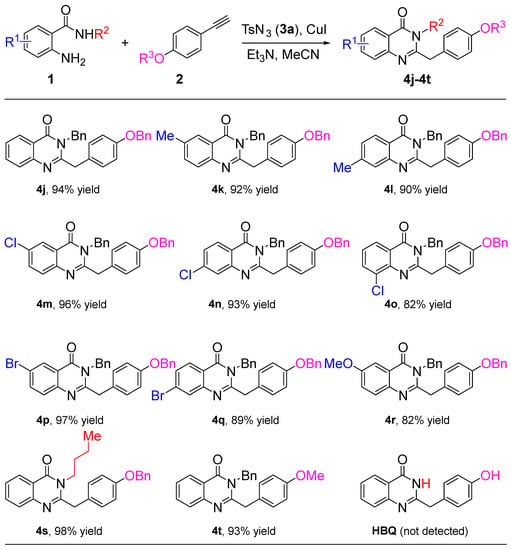
Scheme 3.
Substrate scope of 2-aminobenzamides 1 and terminal alkynes 2.
Although the natural product HBQ cannot be directly obtained by the above method, it can be obtained by a simple reduction of product 4j and can also be prepared in large quantities under mild conditions (Scheme 4).
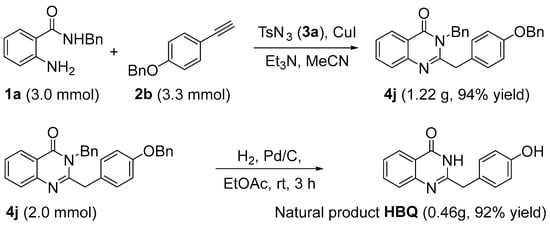
Scheme 4.
Synthesis of natural product HBQ.
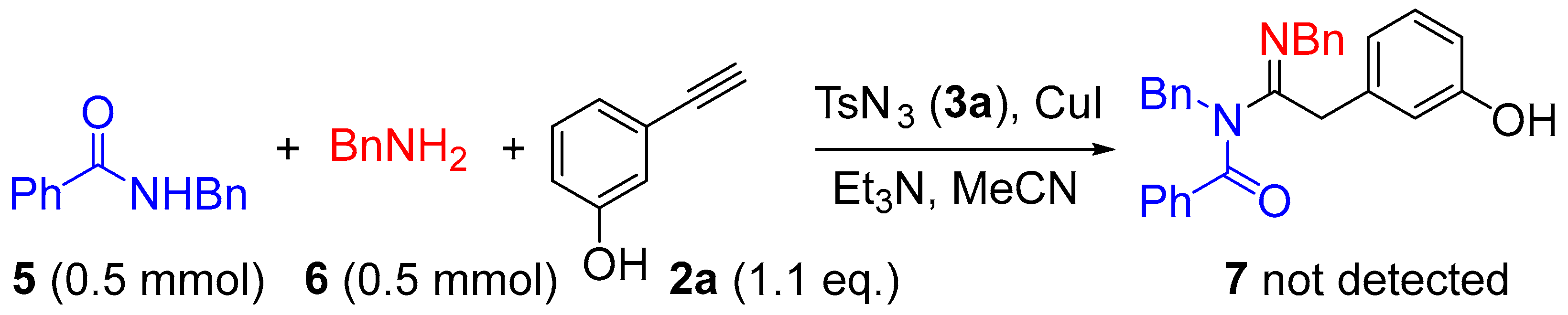
What is interesting to us is that there was no sulfonyl group in the target products, and we could detect the other undesired product TsNH2, which we compared with standard samples by thin-layer chromatography (TLC) and confirmed by NMR. Moreover, unlike the other products, compound 4i, which was difficult to synthesize (Scheme 2), was unaromatized. Therefore, we concluded that the product had aromatic properties. To confirm this fact and elucidate the mechanism, an intermolecular control experiment was performed under the optimized reaction condition (Table 1, entry 5). As shown in Scheme 5, N-Phenylbenzamide 5 and benzylamine 6 were tested for the intermolecular reaction. After being detected by TLC and confirmed by NMR, the N-sulfonylamidine product, which has been reported previously [41], was formed instead of the desired compound 7. The above experiments show that aromaticity is indispensable.
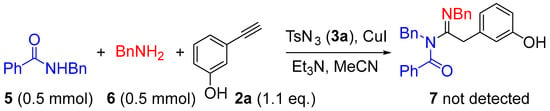
Scheme 5.
Control experiment.
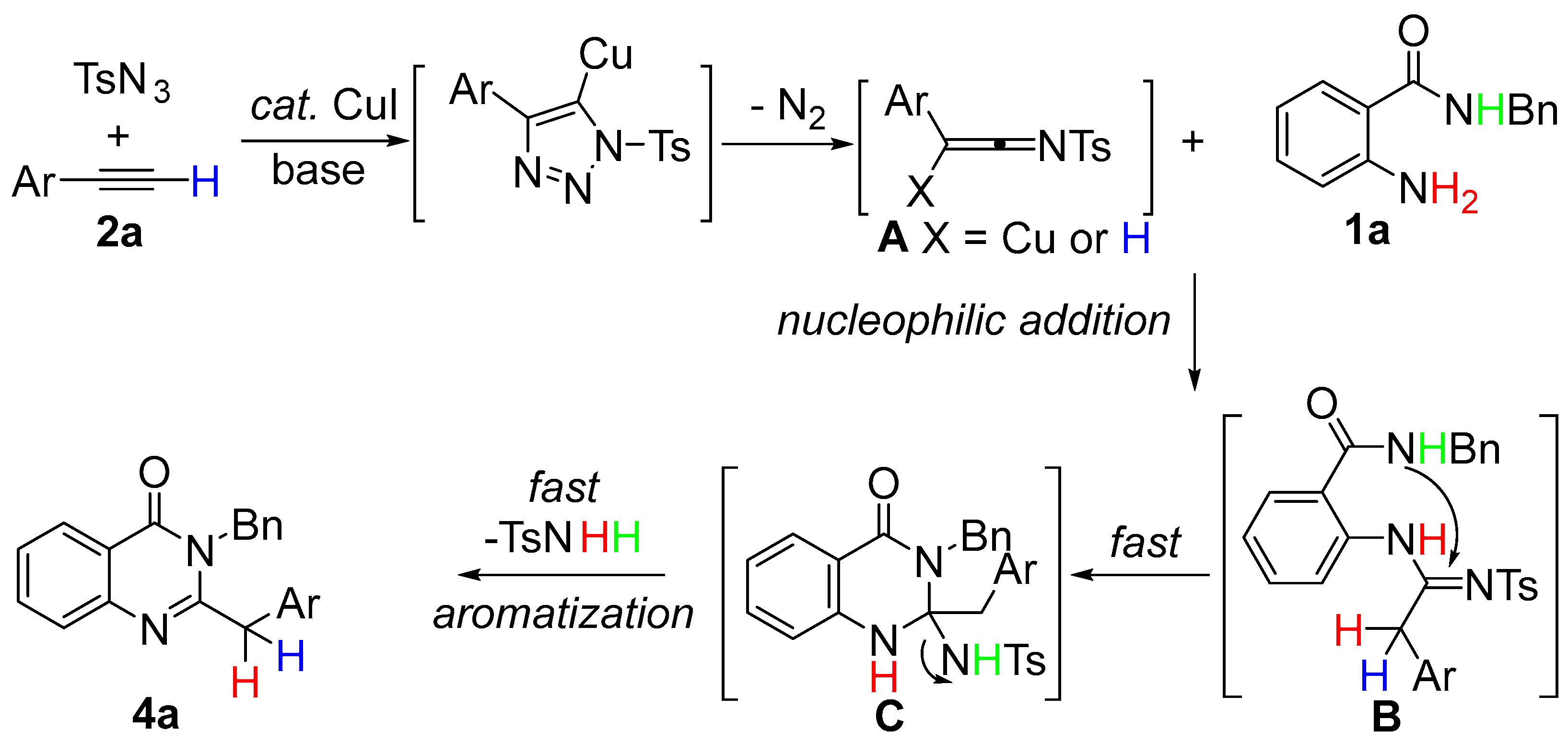
Based on the above experiments, a possible reaction pathway for the synthesis of quinazolin-4(3H)-one 4a was proposed (Scheme 6). According to the previous proposal [41,42,43,44,45,46,47,48,49,50], N-sulfonylketenimine A was generated first by the reaction of TsN3 and 2a. Then, A underwent a nucleophilic addition reaction with 1a to generate the intermediate B. Subsequently, intermediate B underwent an intramolecular cascade addition to generate the intermediate C. Lastly, the desired product 4a and product TsNH2 were obtained by aromatization of intermediate C. We could not detect intermediates B and C during the experiment, which indicated that the procedure from B to 4a was fast and almost simultaneous. The sulfonyl group was eliminated through the power of aromatization and activated the decomposition of the terminal alkynes into TsNH2 and N2.
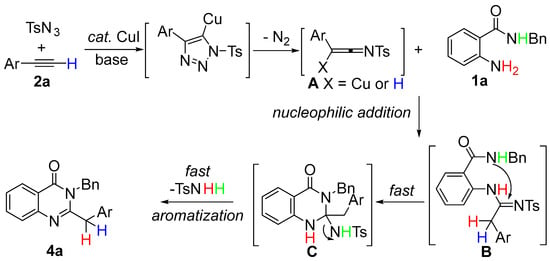
Scheme 6.
Plausible reaction mechanism.
3. Experimental Procedure
3.1. General Information
The 1H NMR spectra were recorded on a Bruker DPX 400 MHz spectrometer in CDCl3. Chemical shifts are reported in ppm with the internal TMS signal at 0.0 ppm as a standard. The spectra were interpreted as s, singlet; bs, broad singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, double doublet; ddd, double double doublet; dt, double triplet; ddt, double double triplet; tt, triple triplet; td, triple doublet. Coupling constant(s) J are reported in Hz and relative integrations are reported. The 13C NMR (100 MHz) spectra were recorded on a Bruker DPX 400 MHz spectrometer in CDCl3. Chemical shifts are reported in ppm with the internal chloroform signal at 77.16 ppm as a standard and HBQ using CD3OD residual nondeuterated solvent as internal standard (CD3OD: δ 3.31 for 1H and 49.00 ppm for 13C). Melting points were obtained in open capillary tubes using the SGW X-4 micro melting point apparatus and were uncorrected. IR spectra were obtained with the Bruker Tensor-27 FT-IR spectrometer. Mass spectra were recorded on a TOF mass spectrometer. The starting materials, 2-amino-N-benzylbenzamide derivatives 1, were all known and prepared according to the literature procedures [50,51]. Terminal alkynes 2, TsN3 3a, and other reagents were purchased from Adamas-beta and other suppliers and used without further purification.
3.2. Compound Characterization and Preparations
At room temperature, to a solution of 2-amino-N-benzylbenzamides 1 (0.1 mmol, 1.0 equiv.), phenyl acetylenes 2 (0.11 mmol, 1.1 equiv.), CuI (1.9 mg, 10 mol%), TsN3 3a (21.7 mg, 0.11 mmol, 1.1 equiv.), and Et3N (11.1 mg, 0.11 mmol, 1.1 equiv.) in MeCN (2 mL) was added. The reaction mixture was stirred for 12 h. After completion of the reaction as indicated by TLC, the solvent was removed by evaporation in a vacuum. The residue was directly purified by flash column chromatography on silica gel (eluting with hexanes/EtOAc = 2:1) to form the corresponding product 4. Some products contained the impurity sulfonamide which is difficult to separate when generated in this reaction.
- 3-Benzyl-2-(3-hydroxybenzyl)quinazolin-4(3H)-one (4a). White solid, 30.5 mg, yield: 89%, m.p: 180–182 °C. 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H), 8.08–7.93 (m, 1H), 7.59–7.52 (m, 1H), 7.42 (dt, J = 9.6, 4.7 Hz, 2H), 7.36–7.20 (m, 4H), 7.10 (d, J = 7.2 Hz, 2H), 6.82 (d, J = 5.4 Hz, 2H), 6.74 (d, J = 7.7 Hz, 1H), 5.13 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.8, 158.3, 156.8, 145.2, 135.8 (2C), 134.9, 131.0, 129.2 (2C), 128.0, 127.9, 127.5, 126.4 (2C), 124.9, 119.8, 119.7, 115.4, 113.6, 46.3, 41.5; IR νmax (KBr): 3308, 2928, 1682, 1591, 1456, 1265, 1165, 976, 775, 731 cm−1; HRMS (ESITOF) m/z calcd for C22H18N2O2, [M + H]+ 343.1441, found 343.1443.
- 3-Benzyl-2-(3-hydroxybenzyl)-7-methylquinazolin-4(3H)-one (4b). White solid, 30.6 mg, yield: 86%, m.p: 184–186 °C. 1H NMR (400 MHz, CDCl3) δ 9.86 (s, 1H), 7.90 (dd, J = 8.5, 2.9 Hz, 1H), 7.38–7.28 (m, 3H), 7.27–7.20 (m, 2H), 7.15 (d, J = 2.5 Hz, 1H), 7.13–7.08 (m, 2H), 6.80 (d, J = 4.9 Hz, 2H), 6.73 (d, J = 7.6 Hz, 1H), 5.12 (s, 2H), 3.97 (s, 2H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.7, 158.4, 156.8, 146.4, 145.4, 136.0, 135.9, 130.9, 129.9, 129.2, 129.0, 128.0, 127.8, 126.6, 126.4, 124.6, 119.7, 117.3, 115.3, 113.6, 46.0, 41.4, 22.1; IR νmax (KBr): 3055, 1682, 1592, 1456, 1342, 1265, 1163, 974, 879, 737 cm−1; HRMS (ESITOF) m/z calcd for C23H20N2O2, [M + H]+ 357.1598, found 357.1599.
- 3-Benzyl-6-chloro-2-(3-hydroxybenzyl)quinazolin-4(3H)-one (4c). white solid, 34.2 mg, yield: 91%, m.p: 197–199 °C. 1H NMR (400 MHz, CDCl3) δ 9.23 (s, 1H), 8.11 (d, J = 2.6 Hz, 1H), 7.50 (dt, J = 8.3, 2.5 Hz, 1H), 7.37–7.21 (m, 5H), 7.10 (dd, J = 7.7, 3.2 Hz, 2H), 6.81 (d, J = 8.5 Hz, 1H), 6.78–6.72 (m, 2H), 5.15 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 160.9, 158.1, 157.2, 143.9, 135.7, 135.5, 135.4, 133.5, 131.1, 129.3 (2C), 128.2, 127.0, 126.7, 126.5 (2C), 120.9, 120.0, 115.5, 113.5, 46.5, 41.5; IR νmax (KBr): 3034, 2947, 1688, 1587, 1473, 1277, 1155, 980, 764, 717 cm−1; HRMS (ESITOF) m/z calcd for C22H17ClN2O2, [M + H]+ 377.1051, found 377.1056.
- 3-Benzyl-7-chloro-2-(3-hydroxybenzyl)quinazolin-4(3H)-one (4d). white solid, 32.7 mg, yield: 87%, m.p: 190–192 °C. 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H), 8.14–8.03 (m, 1H), 7.47 (d, J = 2.7 Hz, 1H), 7.44–7.38 (m, 1H), 7.37–7.29 (m, 3H), 7.28–7.22 (m, 1H), 7.13 (d, J = 7.1 Hz, 2H), 6.81 (d, J = 8.3 Hz, 1H), 6.75 (d, J = 15.3 Hz, 2H), 5.17 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.3, 156.0, 157.7, 146.8, 141.3, 135.8, 135.7, 131.0, 129.3 (2C), 129.2, 128.1 (2C), 126.4 (2C), 125.3, 120.2, 118.5, 115.4, 113.9, 46.5, 41.7; IR νmax (KBr): 2924, 1684, 1601, 1456, 1331, 1232, 1265, 1159, 974, 731 cm−1; HRMS (ESITOF) m/z calcd for C22H17ClN2O2, [M + H]+ 377.1051, found 377.1056.
- 3-Benzyl-8-chloro-2-(3-hydroxybenzyl)quinazolin-4(3H)-one (4e). white solid, 30.1 mg, yield: 80%, m.p: 197–199 °C. 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.1, 3.0 Hz, 1H), 7.75 (dd, J = 7.9, 3.0 Hz, 1H), 7.42–7.27 (m, 4H), 7.18 (td, J = 7.7, 7.2, 4.2 Hz, 1H), 7.13 (d, J = 7.5 Hz, 2H), 6.83 (s, 1H), 6.75 (t, J = 9.9 Hz, 2H), 5.98 (s, 1H), 5.25 (s, 2H), 4.09 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 162.2, 156.9, 156.7, 143.9, 136.5, 135.8, 134.9, 131.4, 130.5, 129.2 (2C), 128.0, 127.1, 126.3 (2C), 126.2, 122.2, 120.5, 115.1, 114.8, 46.6, 41.9; IR νmax (KBr): 3007, 1676, 1580, 1445, 1389, 1275, 1159, 980, 849, 764 cm−1; HRMS (ESITOF) m/z calcd for C22H17ClN2O2, [M + H]+ 377.1051, found 377.1056.
- 3-Benzyl-6-bromo-2-(3-hydroxybenzyl)quinazolin-4(3H)-one (4f). white solid, 38.6 mg, yield: 92%, m.p: 176–178 °C. 1H NMR (400 MHz, CDCl3) δ 9.33 (s, 1H), 8.30 (q, J = 2.2 Hz, 1H), 7.68–7.57 (m, 1H), 7.38–7.21 (m, 5H), 7.10 (d, J = 7.2 Hz, 2H), 6.81 (d, J = 8.4 Hz, 1H), 6.75 (d, J = 10.2 Hz, 2H), 5.15 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 160.7, 158.2, 157.4, 144.2, 138.2, 135.6, 135.5, 131.1, 130.3, 129.3 (2C), 128.2, 126.7, 126.5 (2C), 121.3, 121.2, 120.0, 115.5, 113.4, 46.5, 41.5; IR νmax (KBr): 3026, 1684, 1587, 1456, 1389, 1277, 1153, 966, 831, 750 cm−1; HRMS (ESITOF) m/z calcd for C22H17BrN2O2, [M + H]+ 421.0546, found 421.0551.
- 3-Benzyl-2-(3-hydroxybenzyl)-6-methoxyquinazolin-4(3H)-one (4g). White solid, 28.3 mg, yield: 76%, m.p: 183–185 °C. 1H NMR (400 MHz, CDCl3) δ 10.06 (s, 1H), 7.42 (q, J = 2.7 Hz, 1H), 7.37–7.19 (m, 5H), 7.11 (td, J = 5.6, 2.7 Hz, 3H), 6.81 (d, J = 2.8 Hz, 2H), 6.73 (d, J = 7.6 Hz, 1H), 5.13 (s, 2H), 3.99 (s, 2H), 3.97 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.7, 158.5, 158.4, 154.4, 139.6, 136.1, 136.0, 130.9, 129.8, 129.2, 128.0, 126.6, 126.4 (2C), 124.1, 120.8, 119.7, 115.4, 113.6, 108.1, 55.8, 46.4, 41.2; IR νmax (KBr): 3005, 1670, 1593, 1495, 1456, 1362, 1275, 1155, 1028, 750 cm−1; HRMS (ESITOF) m/z calcd for C23H20N2O3, [M + H]+ 373.1547, found 373.1549.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)quinazolin-4(3H)-one (4j). Oil, 40.6 mg, yield: 94%. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.1, 2.8 Hz, 1H), 7.82–7.70 (m, 2H), 7.50 (d, J = 8.1 Hz, 1H), 7.44–7.21 (m, 8H), 7.17–7.11 (m, 4H), 6.93 (dd, J = 7.8, 3.1 Hz, 2H), 5.26 (s, 2H), 5.04 (s, 2H), 4.02 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 162.8, 158.2, 155.9, 147.4, 136.9, 136.3, 134.6, 129.3 (2C), 129.1 (2C), 128.7 (2C), 128.1, 127.7, 127.6 (2C), 127.5, 127.4, 127.3, 127.0, 126.3 (2C), 120.6, 115.6 (2C), 70.2, 46.3, 41.6; IR νmax (KBr): 3032, 1672, 1591, 1508, 1454, 1240, 1172, 1013, 750, 694 cm−1; HRMS (ESITOF) m/z calcd for C29H24N2O2, [M + H]+ 433.1911, found 433.1910.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-6-methylquinazolin-4(3H)-one (4k). White solid, 41.1 mg, yield: 92%, m.p: 137–139 °C. 1H NMR (400 MHz, CDCl3) δ 8.12 (s, 1H), 7.64 (dd, J = 8.3, 2.8 Hz, 1H), 7.61–7.57 (m, 1H), 7.45–7.22 (m, 8H), 7.13 (d, J = 7.3 Hz, 4H), 6.92 (dd, J = 8.3, 3.0 Hz, 2H), 5.26 (s, 2H), 5.04 (s, 2H), 4.01 (s, 2H), 2.50 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 158.2, 155.0, 145.5, 137.2, 137.0, 136.4, 136.0, 129.2 (2C), 129.1 (2C), 128.7 (2C), 128.1, 127.7 (2C), 127.6 (2C), 127.2, 126.7, 126.3 (2C), 120.4, 115.6 (2C), 70.2, 46.2, 41.5, 21.5; IR νmax (KBr): 3032, 1670, 1591, 1508, 1454, 1340, 1275, 1013, 831, 750 cm−1; HRMS (ESITOF) m/z calcd for C30H26N2O2, [M + H]+ 447.2067, found 447.2069.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-7-methylquinazolin-4(3H)-one (4l). Oil, 40.2 mg, yield: 90%. 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.5, 3.0 Hz, 1H), 7.54 (s, 1H), 7.44–7.23 (m, 9H), 7.13 (d, J = 7.2 Hz, 4H), 6.95–6.89 (m, 2H), 5.25 (s, 2H), 5.03 (s, 2H), 4.00 (s, 2H), 2.51 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.8, 158.2, 155.9, 147.5, 145.6, 137.0, 136.5, 129.3 (2C), 129.1 (2C), 128.7 (2C), 128.5, 128.1, 127.7, 127.6, 127.5 (2C), 127.1, 127.0, 126.3 (2C), 118.2, 115.6 (2C), 70.2, 46.1, 41.6, 22.0; IR νmax (KBr): 3032, 1672, 1593, 1508, 1454, 1259, 1173, 1011, 750, 696 cm−1; HRMS (ESITOF) m/z calcd for C30H26N2O2, [M + H]+ 447.2067, found 447.2069.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-6-chloroquinazolin-4(3H)-one (4m). White solid, 44.7 mg, yield: 96%, m.p: 174–176 °C. 1H NMR (400 MHz, CDCl3) δ 8.20 (s, 1H), 7.66–7.55 (m, 2H), 7.38–7.15 (m, 8H), 7.04 (dd, J = 8.1, 2.9 Hz, 4H), 6.85 (dd, J = 8.7, 3.0 Hz, 2H), 5.17 (s, 2H), 4.96 (s, 2H), 3.93 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.9, 158.3, 156.2, 146.0, 136.9, 136.0, 135.0, 132.7, 129.3 (2C), 129.2 (2C), 129.1, 128.7 (2C), 128.2, 127.9, 127.6 (2C), 127.2, 126.6, 126.3 (2C), 121.7, 115.6 (2C), 70.2, 46.4, 41.5; IR νmax (KBr): 3034, 1676, 1591, 1508, 1472, 1335, 1275, 1013, 835, 750 cm−1; HRMS (ESITOF) m/z calcd for C29H23ClN2O2, [M + H]+ 467.1521, found 467.1528.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-7-chloroquinazolin-4(3H)-one (4n). Oil, 43.4 mg, yield: 93%. 1H NMR (400 MHz, CDCl3) δ 8.24 (dd, J = 9.0, 3.1 Hz, 1H), 7.73 (d, J = 3.0 Hz, 1H), 7.47–7.23 (m, 9H), 7.13 (d, J = 6.8 Hz, 4H), 6.93 (dd, J = 8.1, 3.2 Hz, 2H), 5.25 (s, 2H), 5.04 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 162.3, 158.3, 157.3, 148.4, 140.8, 136.9, 136.1, 129.3 (2C), 129.2 (2C), 128.8, 128.7 (2C), 128.2, 127.9, 127.6 (2C), 127.5, 127.2, 127.0, 126.3 (2C), 119.1, 115.6 (2C), 70.2, 46.3, 41.5; IR νmax (KBr): 3034, 1676, 1591, 1508, 1454, 1383, 1240, 1013, 748, 694 cm−1; HRMS (ESITOF) m/z calcd for C29H23ClN2O2, [M + H]+ 467.1521, found 467.1528.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-8-chloroquinazolin-4(3H)-one (4o). White solid, 38.2 mg, yield: 82%, m.p: 111–113 °C. 1H NMR (400 MHz, CDCl3) δ 8.22 (dd, J = 8.0, 2.8 Hz, 1H), 7.84 (dd, J = 7.9, 2.8 Hz, 1H), 7.44–7.35 (m, 5H), 7.34–7.26 (m, 4H), 7.16 (d, J = 7.8 Hz, 2H), 7.12 (d, J = 6.7 Hz, 2H), 6.93 (dd, J = 8.0, 2.6 Hz, 2H), 5.26 (s, 2H), 5.04 (s, 2H), 4.07 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 162.3, 158.3, 156.8, 144.2, 137.0, 135.9, 134.8, 129.5 (2C), 129.2 (2C), 128.91, 128.7 (2C), 128.1, 127.9, 127.6 (2C), 127.3, 126.9, 126.3 (2C), 126.1, 122.2, 115.6 (2C), 70.2, 46.5, 41.7; IR νmax (KBr): 3030, 1676, 1591, 1508, 1445, 1261, 1163, 987, 748, 696 cm−1; HRMS (ESITOF) m/z calcd for C29H23ClN2O2, [M + H]+ 467.1521, found 467.1528.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-6-bromoquinazolin-4(3H)-one (4p). White solid, 49.5 mg, yield: 97%, m.p: 167–169 °C. 1H NMR (400 MHz, CDCl3) δ 8.45 (t, J = 2.4 Hz, 1H), 7.83 (dd, J = 8.7, 2.6 Hz, 1H), 7.59 (dd, J = 9.4, 3.0 Hz, 1H), 7.44–7.29 (m, 8H), 7.12 (d, J = 6.8 Hz, 4H), 6.93 (dd, J = 7.8, 2.8 Hz, 2H), 5.25 (s, 2H), 5.04 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 161.7, 158.3, 156.3, 146.3, 137.7, 136.9, 136.0, 129.8, 129.3 (2C), 129.2, 129.1 (2C), 128.7 (2C), 128.2, 127.9, 127.6 (2C), 127.2, 126.3 (2C), 122.0, 120.4, 115.6 (2C), 70.2, 46.4, 41.5; IR νmax (KBr): 2905, 1676, 1589, 1510, 1467, 1333, 1275, 985, 750, 692 cm−1; HRMS (ESITOF) m/z calcd for C29H23BrN2O2, [M + H]+ 511.1016, found 511.1021.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-7-bromoquinazolin-4(3H)-one (4q). Oil, 45.4 mg, yield: 89%. 1H NMR (400 MHz, CDCl3) δ 8.16 (dd, J = 8.8, 3.0 Hz, 1H), 7.91 (s, 1H), 7.58 (dd, J = 8.6, 2.7 Hz, 1H), 7.45–7.23 (m, 8H), 7.13 (d, J = 6.9 Hz, 4H), 6.97–6.90 (m, 2H), 5.25 (s, 2H), 5.04 (s, 2H), 4.00 (s, 2H); 13C NMR (100 MHz, CDCl3) δ 162.4, 158.3, 157.3, 148.4, 136.9, 136.0, 130.3, 130.2, 129.4 (2C), 129.3, 129.2 (2C), 128.8, 128.7 (2C), 128.2, 127.9, 127.6 (2C), 127.2, 126.3 (2C), 119.5, 115.6 (2C), 70.2, 46.4, 41.5; IR νmax (KBr): 3032, 1676, 1591, 1508, 1454, 1259, 1013, 883, 750, 694 cm−1; HRMS (ESITOF) m/z calcd for C29H23BrN2O2, [M + H]+ 511.1016, found 511.1021.
- 3-Benzyl-2-(4-(benzyloxy)benzyl)-6-methoxyquinazolin-4(3H)-one (4r). Oil, 37.9 mg, yield: 82%. 1H NMR (400 MHz, CDCl3) δ 7.72–7.64 (m, 2H), 7.44–7.25 (m, 9H), 7.13 (dd, J = 8.3, 3.0 Hz, 4H), 6.96–6.90 (m, 2H), 5.27 (s, 2H), 5.03 (s, 2H), 4.01 (s, 2H), 3.91 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.7, 158.5, 158.2, 153.5, 142.1, 137.0, 136.4, 129.2 (2C), 129.1 (2C), 129.0, 128.7 (2C), 128.1, 127.8, 127.7, 127.6 (2C), 126.3 (2C), 124.9, 121.4, 115.5 (2C), 106.5, 70.2, 55.9, 46.4, 41.4; IR νmax (KBr): 3032, 1667, 1591, 1489, 1360, 1240, 1026, 837, 750, 694 cm−1; HRMS (ESITOF) m/z calcd for C30H26N2O3, [M + H]+ 463.2016, found 463.2022.
- 2-(4-(Benzyloxy)benzyl)-3-butylquinazolin-4(3H)-one (4s). Oil, 39.0 mg, yield: 98%. 1H NMR (400 MHz, CDCl3) δ 8.26 (dd, J = 8.1, 2.9 Hz, 1H), 7.72 (tt, J = 8.3, 5.3 Hz, 2H), 7.49–7.43 (m, 1H), 7.43–7.28 (m, 5H), 7.18 (d, J = 7.7 Hz, 2H), 6.93 (dd, J = 7.9, 2.8 Hz, 2H), 5.04 (s, 2H), 4.18 (s, 2H), 4.01–3.88 (m, 2H), 1.60–1.49 (m, 2H), 1.36 (q, J = 7.5 Hz, 2H), 0.92 (td, J = 7.8, 2.5 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 162.5, 158.2, 155.6, 147.4, 137.0, 134.3, 129.4 (2C), 128.7 (2C), 128.1, 127.8, 127.6 (2C), 127.2, 126.9, 126.7, 120.9, 115.5 (2C), 70.2, 44.4, 41.8, 30.9, 20.4, 13.8; IR νmax (KBr): 3034, 1672, 1589, 1510, 1474, 1259, 1175, 1022, 750, 696 cm−1; HRMS (ESITOF) m/z calcd for C26H26N2O2, [M + H]+ 399.2067, found 399.2065.
- 3-Benzyl-2-(4-methoxybenzyl)quinazolin-4(3H)-one (4t). Oil, 33.1 mg, yield: 93%. 1H NMR (400 MHz, CDCl3) δ 8.33 (dd, J = 8.1, 3.0 Hz, 1H), 7.75 (td, J = 9.6, 8.2, 3.9 Hz, 2H), 7.50 (t, J = 7.1 Hz, 1H), 7.37–7.23 (m, 3H), 7.16–7.12 (m, 4H), 6.86 (dt, J = 8.8, 2.1 Hz, 2H), 5.26 (s, 2H), 4.03 (s, 2H), 3.79 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.9, 159.0, 155.9, 147.5, 136.3, 134.6, 129.2 (2C), 129.1 (2C), 127.7, 127.4, 127.3, 127.2, 127.0, 126.3 (2C), 120.7, 114.7 (2C), 55.4, 46.3, 41.6; IR νmax (KBr): 3032, 1672, 1593, 1510, 1454, 1246, 1175, 1030, 750, 694 cm−1; HRMS (ESITOF) m/z calcd for C23H20N2O2, [M + H]+ 357.1598, found 357.1599.
Gram-Scale Synthesis and Synthesis of HBQ
- 3-Benzyl-2-(4-(benzyloxy)benzyl)quinazolin-4(3H)-one (4j). CuI (48 mg, 10 mol%) was added to an oven-dried 50 mL round-bottomed flask containing a mixture of 2-amino-N-benzylbenzamide 1a (678 mg, 3.0 mmol, 1.0 equiv.), 1-(benzyloxy)-4-ethynylbenzene 2b (686 mg, 3.3 mmol, 1.1 equiv.), TsN3 3a (650 mg, 3.3 mmol, 1.1 equiv.), and Et3N (333 mg, 3.3 mmol, 1.1 equiv.) in MeCN (20 mL). The reaction mixture was stirred for 12 h. After completion of the reaction as indicated by TLC, the solvent was removed by evaporation in a vacuum. The residue was directly purified by flash column chromatography on silica gel (eluting with hexanes/EtOAc = 2:1) to obtain 4j (1.22 g, 94% yield) as oil.
- 2-(4-Hydroxybenzyl)quinazolin-4(3H)-one (HBQ). To a stirred solution of 4j (0.86 g, 2.0 mmol, 1.0 equiv.) in dry EtOAc (15 mL) was added palladium (10%) on carbon (15.0 mg). Then, the reaction mixture was stirred under an atmosphere of H2 at room temperature for 3 h. The reaction mixture was then filtered on a silica pad and rinsed with EtOAc. After evaporation of the solvent, the residue was purified by flash column chromatography on silica gel (eluting with petroleum ether/EtOAc = 1:1) to obtain HBQ as a white solid, 460 mg, yield: 92%, m.p: 210–212 °C (literature [15], m.p: no report). 1H NMR (400 MHz, CD3OD) δ 8.17 (dd, J = 8.2, 2.9 Hz, 1H), 7.84–7.76 (m, 1H), 7.71–7.65 (m, 1H), 7.50 (td, J = 7.8, 2.9 Hz, 1H), 7.22–7.15 (m, 2H), 6.75 (dt, J = 8.7, 2.1 Hz, 2H), 4.57 (s, 1H), 3.90 (s, 2H); 13C NMR (100 MHz, CD3OD) δ 164.4, 158.4, 157.8, 150.1, 136.0, 130.9 (2C), 127.8, 127.7, 127.6, 127.1, 121.8, 116.6 (2C), 41.5; IR νmax (KBr): 3383, 2492, 1682, 1609, 1452, 1269, 1119, 972, 827, 756 cm−1; HRMS (ESITOF) m/z calcd for C15H12N2O2, [M + H]+ 253.0972, found 253.0969.
4. Conclusions
We have developed an oxidant-free and highly effective approach to synthesize phenolic quinazolin-4(3H)-ones via the CuAAC/ring cleavage reaction. N-sulfonylketenimine, generated by TsN3 and terminal alkynes, undergoes two nucleophilic additions by benzamides and anilines, and the sulfonyl group is eliminated through aromatization. More importantly, the protocol can be used to synthesize the natural product 2-(4-hydroxybenzyl)quinazolin-4(3H)-one and scaled up under mild conditions. Moreover, we expect that this methodology can be applied to building phenolic quinazolin-4(3H)-one block facility.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28155734/s1. References [50,51] are cited in the supplementary materials.
Author Contributions
Conceptualization, methodology and supervision, X.L., X.C. and W.Y.; experiment, Y.H. and Z.Y.; spectroscopic characterization, Z.Y. and D.L.; writing—review and editing, X.L., X.C. and W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Weiguang Yang’s project of Technology Planning Program of Zhanjiang (2021A05247) and Medical Scientific Research Foundation of Guangdong Province (A2021037); and Xiai Luo’s project of Natural Science Foundation of Hunan Province, China (2023JJ40465) for support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We are grateful to Zunnan Huang’s Key Discipline Construction Project of Guangdong Medical University (4SG23004G) and Yun Liu’s project of Innovation and Entrepreneurship Team Leads the Pilot Program of Zhanjiang (2020LHJH005) for support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 4a–4t are available from the authors.
References
- Li, H.; Fu, G.; Zhong, W. Natural quinazolinones: From a treasure house to promising anticancer leads. Eur. J. Med. Chem. 2023, 245, 114915. [Google Scholar] [CrossRef] [PubMed]
- El-Subbagh, H.; Sabry, M. 2-Substituted-mercapto-quinazolin-4(3H)-ones as DHFR inhibitors. Mini Rev. Med. Chem. 2021, 21, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Gatadi, S.; Lakshmi, T.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Auti, P.S.; George, G.; Paul, A.T. Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Al-Shamma, A.; Drake, S.; Flynn, D.L.; Mitscher, L.A.; Park, Y.H.; Rao, G.S.R.; Simpson, A.; Swayze, J.K.; Veysoglu, T.; Wu, S.T.S. Antimicrobial agents from higher plants. Antimicrobial agents from Peganum harmala seeds. J. Nat. Prod. 1981, 44, 745–747. [Google Scholar] [CrossRef]
- Iwaki, K.; Ohashi, E.; Arai, N.; Kohno, K.; Ushio, S.; Taniguchi, M.; Fukuda, S.J. Tryptanthrin inhibits Th2 development, and IgE-mediated degranulation and IL-4 production by rat basophilic leukemia RBL-2H3 cells. J. Ethnopharmacol. 2011, 134, 450–459. [Google Scholar] [CrossRef]
- Moon, S.Y.; Lee, J.H.; Choi, H.Y.; Cho, I.J.; Kim, S.C.; Kim, Y.W. Tryptanthrin protects hepatocytes against oxidative stress via activation of the extracellular signal-regulated kinase/NF-E2-related factor 2 pathway. Biol. Pharm. Bull. 2014, 37, 1633–1640. [Google Scholar] [CrossRef]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of natural products as potential inhibitors of COVID-19 main protease (Mpro): In-silico evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Li, C.S.; An, C.Y.; Li, X.M.; Gao, S.S.; Cui, C.M.; Sun, H.F.; Wang, B.G. Triazole and dihydroimidazole alkaloids from the marine sediment-derived fungus penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Shen, S.; Li, W.; Wang, J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312F1. Nat. Prod. Res. 2013, 27, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, R.A.K.; Albadrani, R.F.N.; Abbas, S.Y. Synthesis and characterization of 2-trifluoromethyl-4(3H)-quinazolinone derivatives with various 3-substituents. J. Heterocycl. Chem. 2023, 60, 614–622. [Google Scholar] [CrossRef]
- Snodgrass, H.M.; Mondal, D.; Lewis, J.C. Directed evolution of flavin-dependent halogenases for site- and atroposelective halogenation of 3-aryl-4(3H)-quinazolinones via kinetic or dynamic kinetic resolution. J. Am. Chem. Soc. 2022, 144, 16676–16682. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, K.; Reddy, M.B.; Anandhan, R. Visible-light-induced photocatalyst-free oxidative cyclization of primary alcohols by selectfluor via HAT process: Synthesis of quinazolinones and benzothiadiazines. Asian J. Org. Chem. 2022, 11, e202100590. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Win, K.M.; Ninomiya, M.; Koketsu, M. In situ air oxidation and photophysical studies of isoquinoline-fused N-heteroacenes. Org. Biomol. Chem. 2020, 18, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Nagarajan, R. Total synthesis of penipanoid C, 2-(4-hydroxybenzyl) quinazolin-4-(3H)-one and NU1025. Tetrahedron Lett. 2016, 57, 4277–4279. [Google Scholar] [CrossRef]
- Shen, G.; Zhou, H.; Sui, Y.; Liu, Q.; Zou, K. FeCl3-catalyzed tandem condensation/intramolecular nucleophilic addition/C–C bond cleavage: A concise synthesis of 2-substitued quinazolinones from 2-aminobenzamides and 1,3-diketones in aqueous media. Tetrahedron Lett. 2016, 57, 587–590. [Google Scholar]
- Li, Z.; Dong, J.; Chen, X.; Li, Q.; Zhou, Z.; Yin, S. Metal- and oxidant-free synthesis of quinazolinones from β-ketoesters with o-aminobenzamides via phosphorous acid-catalyzed cyclocondensation and selective C–C bond cleavage. J. Org. Chem. 2015, 80, 9392–9400. [Google Scholar] [CrossRef] [PubMed]
- Abuelhassan, S.; Bakhite, E.A.; Abdel-Rahman, A.E.; El-Mahdy, A.F.M.; Saddik, A.A.; Marae, I.S.; Abdel-Hafez, S.H.; Tolba, M. Synthesis, photophysical properties, and biological activities of some new thienylpyridines, thienylthieno[2.3-b]pyridines and related fused heterocyclic compounds. J. Heterocycl. Chem. 2023, 60, 458–470. [Google Scholar] [CrossRef]
- Yang, W.; Qiao, R.; Chen, J.; Huang, X.; Liu, M.; Gao, W.; Ding, J.; Wu, H. Palladium-catalyzed cascade reaction of 2-amino-N′-arylbenzohydrazides with triethyl orthobenzoates to construct indazolo[3,2-b]quinazolinones. J. Org. Chem. 2015, 80, 482–489. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Xiao, F.; Liu, S.; Deng, G.J. Iron-catalyzed one-pot 2,3-diarylquinazolinone formation from 2-Nitrobenzamides and alcohols. Org. Lett. 2013, 15, 4900–4903. [Google Scholar] [CrossRef]
- Hikawa, H.; Ino, Y.; Suzuki, H.; Yokoyama, Y. Pd-catalyzed benzylic C–H amidation with benzyl alcohols in water: A strategy to construct quinazolinones. J. Org. Chem. 2012, 77, 7046–7051. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, J. One-Pot Synthesis of Quinazolinones via Iridium-Catalyzed Hydrogen Transfers. J. Org. Chem. 2011, 76, 7730–7736. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.; Sheikhi, E.; Bijanzadeh, H.R. One-pot three-component synthesis of 4-(3H)-quinazolinones from benzyl halides, isatoic anhydride, and primary amines. Synlett 2012, 1, 85–88. [Google Scholar] [CrossRef]
- Jayaram, A.; Govindan, K.; Kannan, V.R.; Seenivasan, V.T.; Chen, N.Q.; Lin, W.Y. Iodine-promoted oxidative cyclization of acylated and alkylated derivatives from epoxides toward the synthesis of aza heterocycles. J. Org. Chem. 2023, 88, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Seifu, G.W.; Birhan, Y.S.; Beshay, B.Y.; Hymete, A.; Bekhit, A.A. Synthesis, antimalarial, antileishmanial evaluation, and molecular docking study of some 3-aryl-2-styryl substituted-4-(3H)-quinazolinone derivatives. BMC Chem. 2022, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Chiba, Y.; Kawaguchi, S.; Koitaya, Y.; Yoneta, Y.; Yamada, K.; Abe, T. Total synthesis of pyrano[3,2-e] indole alkaloid fontanesine B by a double cyclization strategy. RSC Adv. 2019, 9, 10420–10424. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.S.; Degani, M.S. Magnetically recoverable nano sulfated titania catalysed one pot synthesis of 4-(3H)-quinazolinone derivatives. Curr. Catal. 2018, 7, 167–175. [Google Scholar] [CrossRef]
- Schroeder, C.E.; Neuenswander, S.A.; Yao, T.; Aube, J.; Golden, J.E. One-pot, regiospecific assembly of (E)-benzamidines from δ- and γ-amino acids via an intramolecular aminoquinazolinone rearrangement. Org. Biomol. Chem. 2016, 14, 3950–3955. [Google Scholar] [CrossRef]
- Zhichkin, P.; Kesicki, E.; Treiberg, J.; Bourdon, L.; Ronsheim, M.; Ooi, H.C.; White, S.; Judkins, A.; Fairfax, D. A novel highly stereoselective synthesis of 2,3-disubstituted 3H-quinazoline-4-one derivatives. Org. Lett. 2007, 9, 1415–1418. [Google Scholar] [CrossRef]
- Ravindran, N.E.A.; Yadav, M.; Tamizh, M.M.; Bhuvanesh, N.; Sarkar, S.; Karvembu, R. Solvent-free synthesis of substituted benzimidazoles and quinazolinones via acceptorless dehydrogenative coupling using ferrocene-hydrazone-based Ru(II)-p-cymene catalysts. Asian J. Org. Chem. 2023, 12, e202200675. [Google Scholar] [CrossRef]
- Pinkerton, A.B.; Peddibhotla, S.; Yamamoto, F.; Slosky, L.M.; Bai, Y.; Maloney, P.; Hershberger, P.; Hedrick, M.P.; Hedrick, M.P.; Falter, B.; et al. Discovery of β-arrestin biased, orally bioavailable, and CNS penetrant neurotensin receptor 1 (NTR1) allosteric modulators. J. Med. Chem. 2019, 62, 8357–8363. [Google Scholar] [CrossRef] [PubMed]
- Phakhodee, W.; Wangngae, S.; Pattarawarapan, M. Approach to the synthesis of 2,3-disubstituted-3H-quinazolin-4-ones mediated by Ph3P-I2. J. Org. Chem. 2017, 82, 8058–8066. [Google Scholar] [CrossRef]
- Lv, X.Y.; Abrams, R.; Martin, R. Copper-catalyzed C(sp3)-amination of ketone-derived dihydroquinazolinones by aromatization-driven C−C bond scission. Angew. Chem., Int. Ed. 2023, 62, e202217386. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Li, L.Y.; Tsai, Z.N.; Lee, Y.H.; Tsao, Y.T.; Huang, P.G.; Cheng, C.K.; Lin, H.B.; Chen, T.W.; Yang, C.H.; et al. Aromatization as an impetus to harness ketones for metallaphotoredox-catalyzed benzoylation/benzylation of (hetero) arenes. Org. Lett. 2022, 24, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Hou, J.Y.; Retailleau, P. Sulfur-promoted synthesis of 2-aroylquinazolin-4-(3H)-ones by oxidative condensation of anthranilamide and acetophenones. Adv. Synth. Catal. 2019, 361, 3337–3341. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Fei, Z.; Liu, M.C.; Jia, F.C.; Wu, A.X. Direct one-pot synthesis of luotonin F and analogues via rational logical design. Org. Lett. 2013, 15, 378–381. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, T.; Li, J.X. Metal-free oxidative synthesis of quinazolinones via dual amination of sp3 C–H bonds. Chem. Commun. 2014, 50, 6471–6474. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, X.; Zhang, J.; Yu, J.T.; Pan, C. Metal-free photoinduced hydrocyclization of unactivated alkenes toward ring-fused quinazolin-4-(3H)-ones via intermolecular hydrogen atom transfer. Org. Lett. 2023, 25, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, K.; Ye, C.; Yu, W.; Chang, J. Iodine-mediated C=C double bond cleavage toward pyrido[2,1-b]quinazolinones. Org. Lett. 2022, 24, 3286–3290. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Han, H.; Chang, S. Highly efficient one-pot synthesis of N-sulfonylamidines by Cu-catalyzed three-component coupling of sulfonyl azide, alkyne, and amine. J. Am. Chem. Soc. 2005, 127, 2038–2039. [Google Scholar] [CrossRef]
- Cho, S.H.; Yoo, E.J.; Bae, I.; Chang, S. Copper-catalyzed hydrative amide synthesis with terminal alkyne, sulfonyl azide, and water. J. Am. Chem. Soc. 2005, 127, 16046–16047. [Google Scholar] [CrossRef] [PubMed]
- Bahadorikhalili, S.; Divar, M.; Damghani, T.; Moeini, F.; Ghassamipour, S.; Iraji, A.; Miller, M.A.; Larijani, B.; Mahdavi, M. N-sulfonyl ketenimine as a versatile intermediate for the synthesis of heteroatom containing compounds. J. Organomet. Chem. 2021, 939, 121773. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y. The thriving chemistry of ketenimines. Chem. Soc. Rev. 2012, 41, 5687–5705. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, S.H.; Choi, J.H.; Chang, S. Sulfonyl and phosphoryl azides: Going further beyond the click realm of alkyl and aryl azides. Chem. Asian J. 2011, 6, 2618–2634. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, Y.; Bu, Q.; Li, L.; Zhou, B.; Huang, Z. Tandem CuAAC/ring cleavage/[4+2] annulation reaction to synthesize dihydrooxazines and conversion to 2-aminopyrimidines. Org. Lett. 2022, 24, 457–461. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Z.; Zheng, J.; Liang, G.; Luo, H.; Yang, W. CuX dual catalysis: Construction of oxazolo[2,3-b][1,3]oxazines via a tandem CuAAC/ring cleavage/[4+2+3] annulation reaction. Org. Lett. 2022, 24, 7300–7304. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, X.; Zhang, Z.; Luo, X.; Zheng, J.; Luo, H.; Yang, W. Silver-catalyzed [3+2] cycloaddition for the diastereoselective synthesis of anti-3-substituted hydroindolin-2-imines. Adv. Synth. Catal. 2022, 364, 4433–4439. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, H.; Yi, W.; Li, G.; Chen, L.; Yang, W. Synthesis of isoxazolidine by tandem CuAAC/ring cleavage/5-endo-trig cyclization. Eur. J. Org. Chem. 2022, 2022, e202201214. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Z.; Liu, L.; Chen, M.; Yang, W.; Chen, Q.; Gardiner, M.G.; Banwell, M.G. The copper-catalyzed reaction of 2-(1-hydroxyprop-2-yn-1-yl)phenols with sulfonyl azides leading to C3-unsubstituted N-sulfonyl-2-iminocoumarins. J. Org. Chem. 2021, 86, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Yan, Y.; Xu, K.; Su, J.; Zha, Z.; Wang, Z. Copper-catalyzed radical methylation/C–H amination/oxidation cascade for the synthesis of quinazolinones. J. Org. Chem. 2015, 80, 4736–4742. [Google Scholar] [CrossRef]
- Patel, S.M.; Chada, H.; Biswal, S.; Sharma, S.; Sharada, D.S. Copper-catalyzed intramolecular α-C–H amination via ring opening cyclization strategy to quinazolin-4-ones: Development and application in rutaecarpine synthesis. Synthesis 2019, 51, 3160–3170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).