Collection of Partition Coefficients in Hexadecyltrimethylammonium Bromide, Sodium Cholate, and Lithium Perfluorooctanesulfonate Micellar Solutions: Experimental Determination and Computational Predictions
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental logP Values of SC, HTAB, and LPFOS Micelles
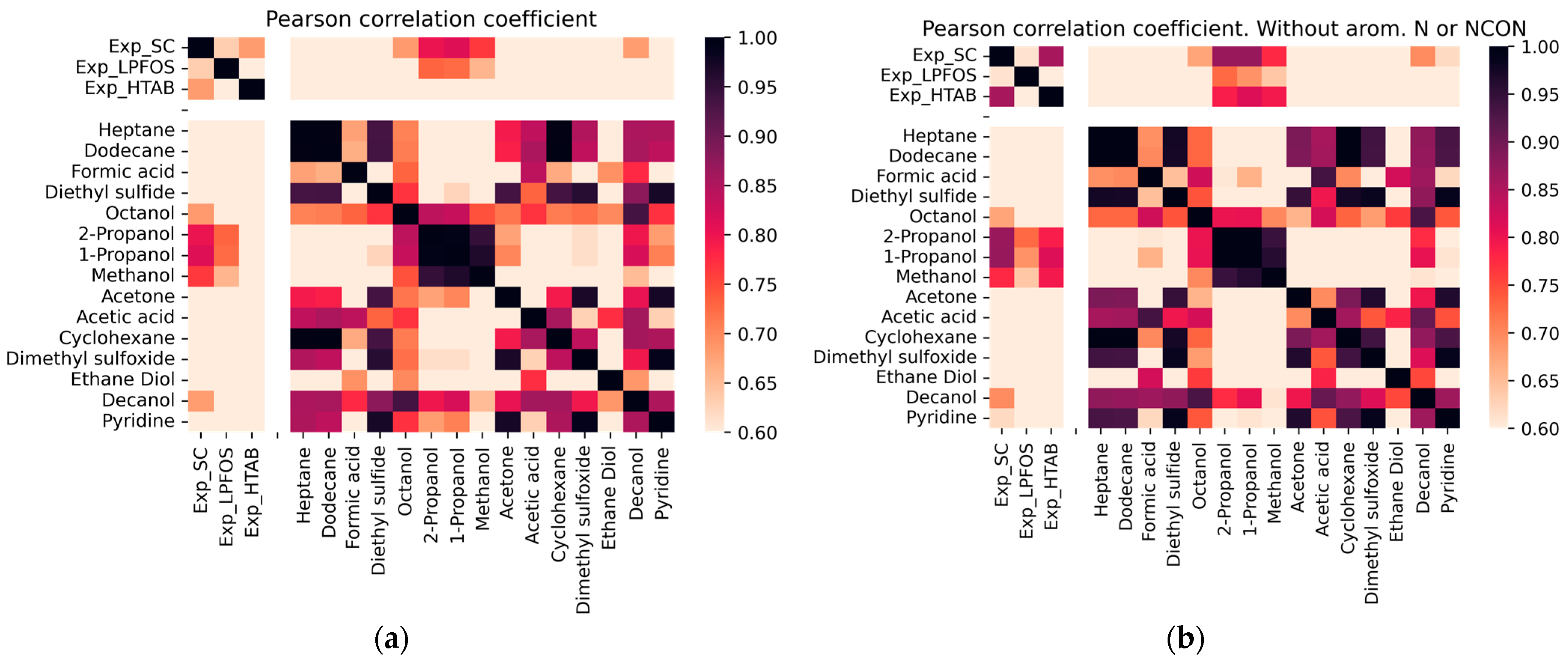
2.2. Correlation of logP Values in Micelles Using DFT Calculations
2.3. Estimation of logP Values in Micelles Using SVM Calculations
3. Materials and Methods
3.1. Regents and Materials
3.2. Determination of Partition Coefficients in Systems of SC, LPFOS, and HTAB Micelles
3.3. QM Computational Determination of Partition Coefficients
3.4. Correlation Analysis
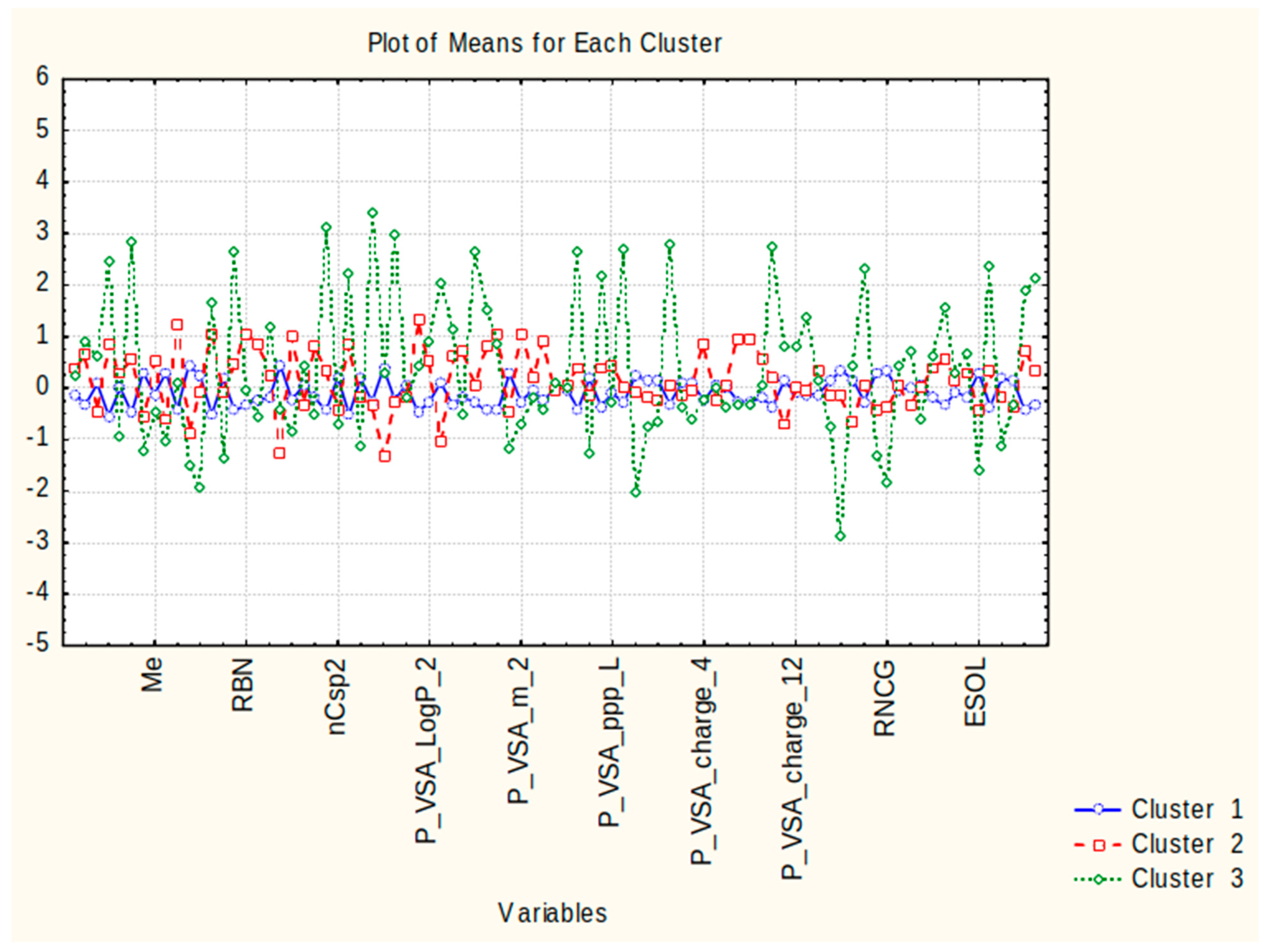
3.5. Supervised and Unsupervised Methods
3.6. K-Means Clustering
3.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Paprikar, A.; Soni, A.; Kaushal, N.; Lin, S. Polymeric Micelles for Drug Delivery. In Smart Nanomaterials in Biomedical Applications. Nanotechnology in the Life Sciences; Kim, J.C., Alle, M., Husen, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 345–372. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Yagui, C.O.; Pessoa, A.; Tavares, L.C.; Becke, A.D. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Ingram, T.; Storm, S.; Kloss, L.; Mehling, T.; Jakobtorweihen, S.; Smirnova, I. Prediction of micelle/water and liposome/water partition coefficients based on molecular dynamics simulations, COSMO-RS, and COSMOmic. Langmuir 2013, 29, 3527–3537. [Google Scholar] [CrossRef] [PubMed]
- Mehling, T.; Ingram, T.; Smirnova, I. Experimental methods and prediction with COSMO-RS to determine partition coefficients in complex surfactant systems. Langmuir 2012, 28, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Singh, O.; Sharma, S.; Betlem, K.; Aswal, V.K.; Peeters, M.; Mahajan, R.K. Temperature-Dependent Solubilization of the Hydrophobic Antiepileptic Drug Lamotrigine in Different Pluronic Micelles—A Spectroscopic, Heat Transfer Method, Small Angle Neutron Scattering, Dynamic Light Scattering, and in Vitro Release Study. ACS Omega 2019, 4, 11251–11262. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Chabba, S.; Aswal, V.K.; Mahajan, R.K. Sodium deoxycholate mediated enhanced solubilization and stability of hydrophobic drug Clozapine in pluronic micelles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 143–154. [Google Scholar] [CrossRef]
- Mehling, T.; Kloss, L.; Ingram, T.; Smirnova, I. Partition coefficients of ionizable solutes in mixed nonionic/ionic micellar systems. Langmuir 2013, 29, 1035–1044. [Google Scholar] [CrossRef]
- Khan, A.M.; Bashir, S.; Shah, A.; Nazar, M.F.; Rahman, H.M.A.; Shah, S.S.; Khan, A.Y.; Khan, A.R.; Shah, F. Spectroscopically probing the effects of Holmium(III) based complex counterion on the dye-cationic surfactant interactions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 407–415. [Google Scholar] [CrossRef]
- Materna, K.; Goralska, E.; Sobczynska, A.; Szymanowski, J. Recovery of various phenols and phenylamines by micellar enhanced ultrafiltration and cloud point separation. Green Chem. 2004, 6, 176–182. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Torres-Lapasió, J.R.; García-Álvarez-Coque, M.C.; Bosch, E.; Rosés, M. Solute-solvent interactions in micellar electrokinetic chromatography. 6. Optimization of the selectivity of lithium dodecyl sulfate-lithium perfluorooctanesulfonate mixed micellar buffers. Anal. Chem. 2002, 74, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Fuguet, E.; Rafols, C.; Bosch, E.; Abraham, M.H.; Roses, M. Solute-solvent interactions in micellar electrokinetic chromatography III. Characterization of the selectivity of micellar electrokinetic chromatography systems. J. Chromatogr. A 2002, 942, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Terabe, S.; Otsuka, K.; Ichikawa, K.; Tsuchiya, A.; Teiichi, A. Electrokinetic separations with micellar solutions and open-tubular capillaries. Anal. Chem. 1984, 56, 111–113. [Google Scholar] [CrossRef]
- Baker, D.R. Capillary Electrophoresis; Wiley–Interscience: New York, NY, USA, 1995. [Google Scholar]
- Azeem, W.; John, P.; Nazar, M.F.; Khan, I.U.; Riaz, A.; Sharif, S. Spectral and chromatographic characterization of fixed dose combination norfloxacin and metronidazole interacting with cetyltrimethylammonium bromide. J. Mol. Liquids 2017, 244, 135–140. [Google Scholar] [CrossRef]
- Maeder, C.; Beaudoin III, G.M.J.; Hsu, E.; Escobar, V.A.; Chambers, S.M.; Kurtin, W.E.; Bushey, M.M. Measurement of bilirubin partition coefficients in bile salt micelle/aqueous buffer solutions by micellar electrokinetic chromatography. Electrophoresis 2000, 21, 706–714. [Google Scholar] [CrossRef]
- Herbert, B.J.; Dorsey, J.G. n-Octanol-water partition coefficient estimation by micellar electrokinetic capillary chromatography. Anal. Chem. 1995, 67, 744–749. [Google Scholar] [CrossRef]
- Mrestani, Y.; Marestani, Z.; Neubert, R.H.H. Characterization of micellar solubilization of antibiotics using micellar electrokinetic chromatography. J. Pharm. Biomed. Anal. 2001, 26, 883–889. [Google Scholar] [CrossRef]
- Godyn, J.; Hebda, M.; Wieckowska, A.; Wieckowski, K.; Malawska, B.; Bajda, M. Lipophilic properties of anti-Alzheimer’s agents determined by micellar electrokinetic chromatography and reversed-phase thin-layer chromatography. Electrophoresis 2017, 38, 1268–1275. [Google Scholar] [CrossRef]
- Storm, S.; Jakobtorweihen, S.; Smirnova, I. Solubilization in mixed micelles studied by molecular dynamics simulations and COSMOmic. J. Phys. Chem. B 2014, 118, 3593–3604. [Google Scholar] [CrossRef]
- Storm, S.; Jakobtorweihen, S.; Smirnova, I.; Panagiotopoulos, A.Z. Molecular dynamics simulation of SDS and CTAB micellization and prediction of partition equilibria with COSMOmic. Langmuir 2013, 29, 11582–11592. [Google Scholar] [CrossRef]
- Yordanova, D.; Smirnova, I.; Jakobtorweihen, S. Molecular modeling of Triton X micelles: Force field parameters, self-assembly, and partition equilibria. J. Chem. Theory Comput. 2015, 11, 2329–2340. [Google Scholar] [CrossRef] [PubMed]
- Turchi, M.; Kognole, A.A.; Kumar, A.; Cai, Q.; Lian, G.; Mackerell, A.D. Predicting Partition Coefficients of Neutral and Charged Solutes in the Mixed SLES-Fatty Acid Micellar System. J. Phys. Chem. B 2020, 124, 1653–1664. [Google Scholar] [CrossRef]
- Yordanova, D.; Ritter, E.; Gerlach, T.; Jensen, J.H.; Smirnova, I.; Jakobtorweihen, S. Solute Partitioning in Micelles: Combining Molecular Dynamics Simulations, COSMOmic, and Experiments. J. Phys. Chem. B 2017, 121, 5794–5809. [Google Scholar] [CrossRef]
- Burns, S.T.; Khaledi, M.G. Predictions of Micelle-Water Partition Coefficients and Retention in Micellar Electrokinetic Chromatography from Solute Structure. 2. Fragmental Constant Approach. Anal. Chem. 2004, 76, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F. In Silico Log P Prediction for a Large Data Set with Support Vector Machines, Radial Basis Neural Networks and Multiple Linear Regression. Chem. Biol. Drug Des. 2009, 74, 142–147. [Google Scholar] [CrossRef]
- Liao, Q.; Yao, J.; Yuan, S. SVM approach for predicting LogP. Mol. Divers. 2006, 10, 301–309. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, Z.; Wang, R.; Wei, G.-W. TopP–S: Persistent Homology-Based Multi-Task DeepNeural Networks for Simultaneous Predictions of PartitionCoefficient and Aqueous Solubility. J. Comput. Chem. 2018, 39, 1444–1454. [Google Scholar] [CrossRef]
- Popova, M.; Isayev, O.; Tropsha, A. Deep reinforcement learning for de novo drug design. Sci. Adv. 2018, 4, eaap7885. [Google Scholar] [CrossRef]
- Donyapour, N.; Hirn, M.; Dickson, A. ClassicalGSG: Prediction of log P using classical molecular force fields and geometric scattering for graphs. J. Comput. Chem. 2021, 42, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asympotic behaviour. Phys. Rev. A 1998, 38, 3098–3100. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Perspective on foundations of solvation modeling: The electrostatic contribution to the free energy. J. Chem. Theory Comput. 2008, 4, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of hydrogen bonding: Their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Labute, P. A widely applicable set of descriptors. J. Mol. Graph. Model. 2000, 18, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Fuguet, E.; Ràfols, C.; Rosés, M.; Bosch, E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered systems. Anal. Chim. Acta. 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Zana, R. The role of hydrogen bonding in the formation of bile salt micelles. J. Phys. Chem. 1978, 82, 2440–2443. [Google Scholar] [CrossRef]
- Fuguet, E.; Ràfols, C.; Rosés, M. Characterization of the solvation properties of surfactants by solvatochromic indicators. Langmuir 2003, 19, 6685–6692. [Google Scholar] [CrossRef]
- Velegol, S.B.; Fleming, B.D.; Biggs, S.; Wanless, E.J.; Tilton, R.D. Counterion effects on hexadecyltrimethylammonium surfactant adsorption and self-assembly on silica. Langmuir 2000, 16, 2548–2556. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Saranjam, L.; Fuguet, E.; Nedyalkova, M.; Simeonov, V.; Mas, F.; Madurga, S. Prediction of Partition Coefficients in SDS Micelles by DFT Calculations. Symmetry 2021, 13, 1750. [Google Scholar] [CrossRef]
- Ikotun, A.M.; Ezugwu, A.E.; Abualigah, L.; Abuhaija, B.; Heming, J. K-means clustering algorithms: A comprehensive review, variants analysis, and advances in the era of big data. Inf. Sci. 2023, 622, 178–210. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Madurga, S.; Simeonov, V. Combinatorial K-Means Clustering as a Machine Learning Tool Applied to Diabetes Mellitus Type 2. Int. J. Environ. Res. Public Health 2021, 18, 1919. [Google Scholar] [CrossRef] [PubMed]
- Mauri, A. alvaDesc: A Tool to Calculate and Analyze Molecular Descriptors and Fingerprints. In Ecotoxicological QSARs; Springer: Berlin/Heidelberg, Germany, 2020; pp. 801–820. [Google Scholar] [CrossRef]

| Micelle Name | Symbol | Structure | Schematic Representation of Formed Micelles |
|---|---|---|---|
| Hexadecyltrimethyl- ammonium bromide | HTAB |  |  |
| Lithium perfluorooctanesulfonate | LPFOS |  |  |
| Sodium cholate | SC | 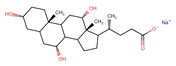 |  |
| Compound | logPSC | logPHTAB | logPLPFOS |
|---|---|---|---|
| Ethylbenzene | 2.50 | 3.00 | 2.06 |
| Propylbenzene | 2.94 | 3.42 | 2.39 |
| Butylbenzene | 3.26 | 3.71 | 2.71 |
| 1-Phenylethanone | 1.33 | 2.03 | 2.19 |
| 1-Phenylpropan-1-one | 1.65 | 2.42 | 2.44 |
| 1-Phenylbutan-1-one | 2.01 | 2.80 | 2.72 |
| 1-Phenylpentan-1-one | 2.41 | 3.24 | 3.01 |
| 1-Phenylheptan-1-one | 3.15 | - | 3.68 |
| Furan | 0.77 | 1.48 | 1.19 |
| 2-Nitroaniline | 1.59 | 2.67 | 1.80 |
| 2,3-Benzofuran | 2.12 | 2.82 | 1.82 |
| Diphenylmethanone | 2.48 | 3.28 | 3.01 |
| Benzamide | 1.06 | 1.72 | 1.50 |
| 4-Chloroaniline | 1.69 | 2.69 | 1.44 |
| 2,3-Dimethylphenol | 1.90 | 3.15 | 1.66 |
| Naphtalen-2-ol | 2.31 | - | 1.73 |
| 4-Aminobenzamide | 0.98 | 1.11 | 1.76 |
| 3-Methylphenol | 1.53 | 2.78 | 1.43 |
| 2,4-Dimethylphenol | 1.93 | 3.17 | 1.02 |
| Naphthalene | 2.67 | 3.47 | 2.09 |
| Pyrimidine | 0.56 | - | 1.27 |
| Benzaldehyde | 1.20 | 1.91 | 1.91 |
| 3-Chloroaniline | 1.63 | 2.72 | 1.41 |
| Pyrrole | 0.68 | 1.65 | 0.72 |
| 3-Nitroaniline | 1.38 | 2.42 | 1.53 |
| 4-Chlorophenol | 2.00 | 3.24 | 1.30 |
| Phenol | 1.21 | 2.35 | 1.08 |
| Methylbenzoate | 1.71 | 2.39 | 2.36 |
| Bromobenzene | 2.37 | 2.95 | 1.80 |
| 1,4-Xylene | 2.51 | 3.04 | 2.10 |
| Benzene-1,3-diol | 1.21 | 2.48 | 0.75 |
| 2-Methylaniline | 1.17 | 2.15 | 1.59 |
| Aniline | 0.92 | 1.83 | 1.34 |
| Nitrobenzene | 1.47 | 2.21 | 1.94 |
| Chlorobenzene | 2.21 | 2.77 | 1.77 |
| N-4-chlorophenylacetamide | 2.03 | 2.80 | 1.84 |
| N-Phenylacetamide | 1.25 | 1.98 | 1.58 |
| 4-Nitroaniline | 1.52 | 2.50 | 1.45 |
| Anisole | 1.66 | 2.31 | 1.83 |
| Benzonitrile | 1.21 | 1.96 | 1.95 |
| 1-Ethyl-4-nitrobenzene | 2.19 | 3.02 | 2.68 |
| Benzyl benzoate | 2.99 | - | 3.18 |
| Caffeine | 1.11 | 1.32 | 1.85 |
| Corticosterone | 1.94 | 3.69 | 3.64 |
| Cortisone | 1.72 | 3.16 | 3.37 |
| β-Estradiol | 2.77 | - | 2.84 |
| Estriol | 2.32 | 3.52 | 2.01 |
| Cortisol | 1.83 | 3.39 | 2.89 |
| Hydroquinone | 1.09 | 1.94 | 0.19 |
| Quinoline | 1.65 | 2.36 | 2.68 |
| Atrazine | 1.86 | 1.90 | 2.71 |
| Diuron | 2.46 | 2.19 | 2.34 |
| Isoproturon | 2.19 | 1.95 | 2.61 |
| Linuron | 2.59 | 2.24 | 2.50 |
| Metobromuron | 2.16 | 2.03 | 2.22 |
| Monuron | 1.81 | 1.73 | 2.03 |
| Metoxuron | 1.69 | 1.46 | 2.34 |
| Phenylurea | 1.20 | 1.20 | 1.38 |
| Propazine | 2.02 | 2.08 | 3.03 |
| Fluometuron | 2.01 | 1.92 | 2.57 |
| N,N-Diethyl-4-nitroaniline | 2.44 | 3.56 | 3.36 |
| 1-Methoxy-4-nitrobenzene | 1.69 | 2.58 | 2.20 |
| 1-Methoxy-2-nitrobenzene | 1.55 | 2.37 | 2.26 |
| Micelle | Solvent | B3LYP |
|---|---|---|
| LPFOS | Propan-1-ol | y = 0.46x + 0.77 R2 = 0.52 MAE = 0.87 |
| Propan-2-ol | y = 0.49x + 0.61 R2 = 0.53 MAE = 0.92 | |
| Methanol | y = 0.41x + 0.90 R2 = 0.43 MAE = 0.86 | |
| SC | Propan-1-ol | y = 0.47x + 0.55 R2 = 0.67 MAE = 0.92 |
| Propan-2-ol | y = 0.46x + 0.51 R2 = 0.64 MAE = 1.08 | |
| Methanol | y = 0.41x + 0.68 R2 = 0.58 MAE = 0.89 | |
| HTAB | Propan-1-ol | y = 0.23x + 1.80 R2 = 0.13 MAE = 0.74 |
| Propan-2-ol | y = 0.22x + 1.83 R2 = 0.1 MAE = 0.72 | |
| Methanol | y = 0.24x + 1.78 R2 = 0.13 MAE = 0.72 | |
| HTAB without N set * | Propan-1-ol | y = 0.56x + 1.24 R2 = 0.66 MAE = 0.45 |
| Propan-2-ol | y = 0.54x + 1.24 R2 = 0.62 MAE = 0.43 | |
| Methanol | y = 0.56x + 1.26 R2 = 0.63 MAE = 0.46 |
| SC | HTAB | LPFOS | |
|---|---|---|---|
| Variables (descriptors) | Mv, RBN, RBF, H%, N%, O%, NRS, nR09, nR10, X4Av, P_VSA_LogP_2, P_VSA_s_4, P_VSA_ppp_P, P_VSA_charge_1, P_VSA_charge_3, P_VSA_charge_4, P_VSA_charge_5, P_VSA_charge_13, P_VSA_charge_14 | nSK, nH, N%, Xu, S1K, DELS, BAC, X0, X0sol, P_VSA_LogP_1, P_VSA_LogP_4, P_VSA_LogP_6, P_VSA_LogP_8, P_VSA_MR_5, P_VSA_m_5, P_VSA_s_3, P_VSA_ppp_D, P_VSA_charge_2, P_VSA_charge_4, P_VSA_charge_5, P_VSA_charge_6, P_VSA_charge_9, P_VSA_charge_12, P_VSA_charge_14, qpmax, qnmax, Qpos, Qneg, Qtot, Qmean, Q2, RPCG, RNCG, TPSA(NO), TPSA(Tot) | RBF, nTB, MaxTD, P_VSA_LogP_2, P_VSA_LogP_3, P_VSA_LogP_4, P_VSA_MR_2, P_VSA_s_3, P_VSA_charge_2, P_VSA_charge_6, P_VSA_charge_7, P_VSA_charge_9, P_VSA_charge_13, P_VSA_charge_14, Qmean |
| R2 | 0.693 | 0.565 | 0.783 |
| RMSE | 0.369 | 0.248 | 0.202 |
| MAE | 0.318 | 0.241 | 0.304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saranjam, L.; Nedyalkova, M.; Fuguet, E.; Simeonov, V.; Mas, F.; Madurga, S. Collection of Partition Coefficients in Hexadecyltrimethylammonium Bromide, Sodium Cholate, and Lithium Perfluorooctanesulfonate Micellar Solutions: Experimental Determination and Computational Predictions. Molecules 2023, 28, 5729. https://doi.org/10.3390/molecules28155729
Saranjam L, Nedyalkova M, Fuguet E, Simeonov V, Mas F, Madurga S. Collection of Partition Coefficients in Hexadecyltrimethylammonium Bromide, Sodium Cholate, and Lithium Perfluorooctanesulfonate Micellar Solutions: Experimental Determination and Computational Predictions. Molecules. 2023; 28(15):5729. https://doi.org/10.3390/molecules28155729
Chicago/Turabian StyleSaranjam, Leila, Miroslava Nedyalkova, Elisabet Fuguet, Vasil Simeonov, Francesc Mas, and Sergio Madurga. 2023. "Collection of Partition Coefficients in Hexadecyltrimethylammonium Bromide, Sodium Cholate, and Lithium Perfluorooctanesulfonate Micellar Solutions: Experimental Determination and Computational Predictions" Molecules 28, no. 15: 5729. https://doi.org/10.3390/molecules28155729
APA StyleSaranjam, L., Nedyalkova, M., Fuguet, E., Simeonov, V., Mas, F., & Madurga, S. (2023). Collection of Partition Coefficients in Hexadecyltrimethylammonium Bromide, Sodium Cholate, and Lithium Perfluorooctanesulfonate Micellar Solutions: Experimental Determination and Computational Predictions. Molecules, 28(15), 5729. https://doi.org/10.3390/molecules28155729