American Ginseng for the Treatment of Alzheimer’s Disease: A Review
Abstract
1. Introduction
2. Pathogenic Mechanism of Alzheimer’s Disease
2.1. Amyloid Cascade Hypothesis
2.2. Tau Hyperphosphorylation Hypothesis
2.3. Cholinergic Hypothesis
2.4. Oxidative Stress Hypothesis
2.5. Neuroinflammatory Hypothesis
2.6. Other Pathogenic Hypotheses
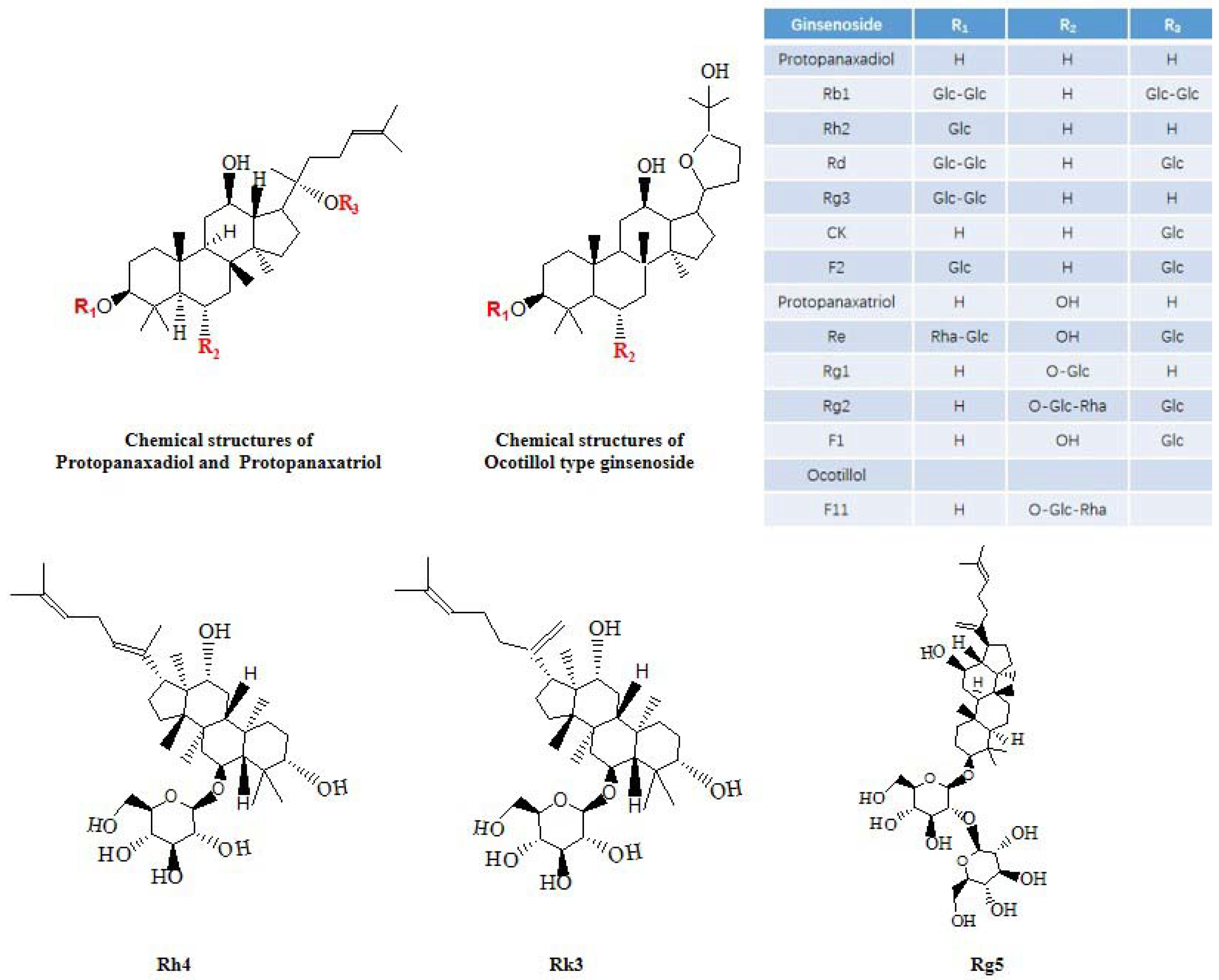
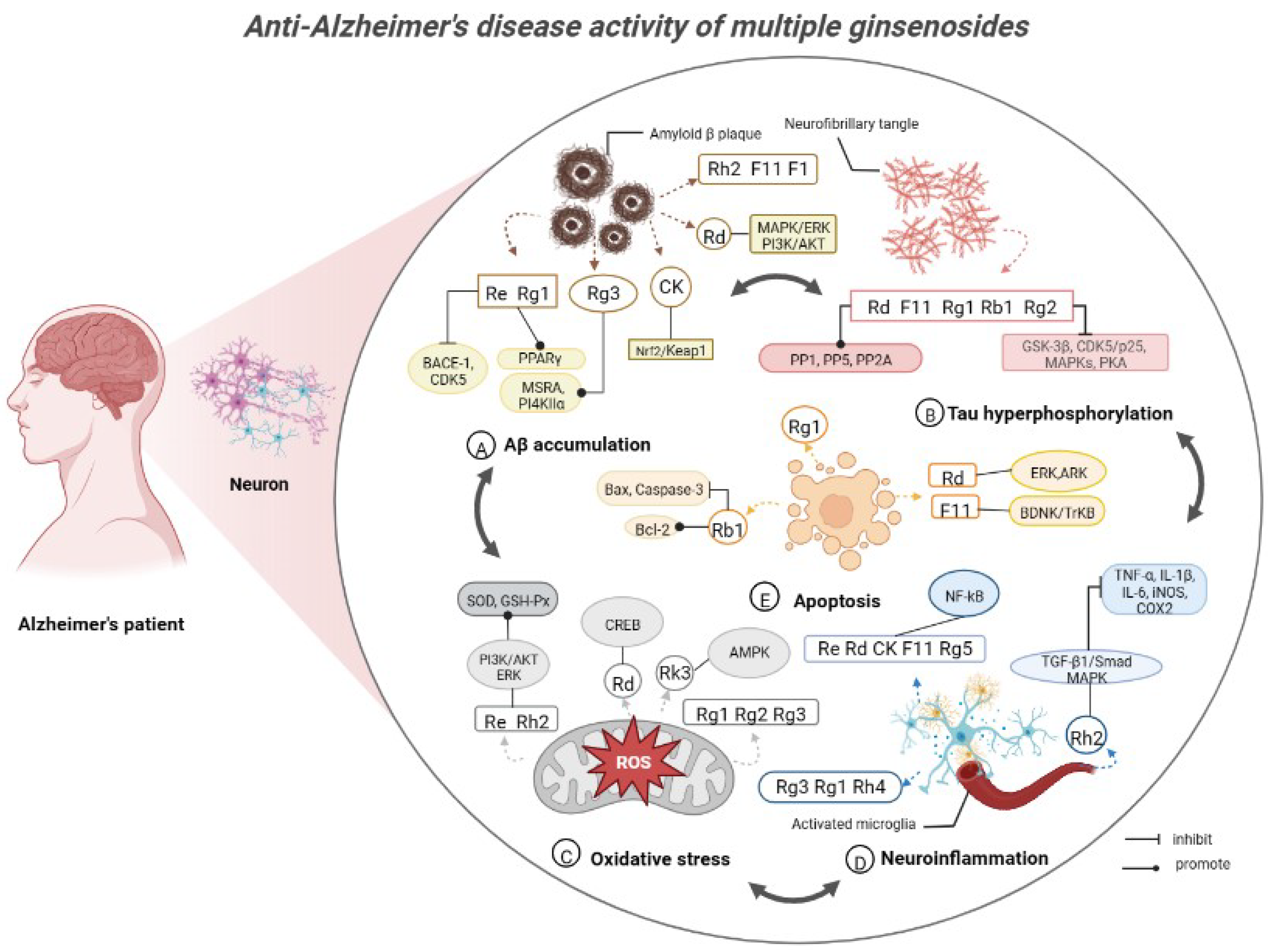
3. Anti-Alzheimer’s Disease Activity of Ginsenosides in American Ginseng
3.1. Protopanaxadiol Type
3.1.1. Ginsenoside Rb1
3.1.2. Ginsenoside Rh2
3.1.3. Ginsenoside Rd
3.1.4. Ginsenoside Rg3
3.1.5. Ginsenoside CK
3.2. Protopanaxatriol Type
3.2.1. Ginsenoside Re
3.2.2. Ginsenoside Rg1
3.2.3. Ginsenoside Rg2
3.3. Ocotillol Type
Pseudoginsenoside F11
3.4. Other Ginsenosides
Ginsenosides F1, F2, Rg5, Rh4 and Rk3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qin, Z.; Jia, C.; Liao, D.; Chen, X.; Li, X. Comparison of Serum Metabolite Changes of Radiated Mice Administered with Panax quinquefolium from Different Cultivation Regions Using UPLC-Q/TOF-MS Based Metabolomic Approach. Molecules 2018, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qu, C.Y.; Li, J.X.; Wang, Y.F.; Li, W.; Wang, C.Z.; Wang, D.S.; Song, J.; Sun, G.Z.; Yuan, C.S. Hypoglycemic and Hypolipidemic Effects of Malonyl Ginsenosides from American Ginseng (Panax quinquefolius L.) on Type 2 Diabetic Mice. ACS Omega 2021, 6, 33652–33664. [Google Scholar] [CrossRef]
- Ghosh, R.; Bryant, D.L.; Farone, A.L. Panax quinquefolius (North American Ginseng) Polysaccharides as Immunomodulators: Current Research Status and Future Directions. Molecules 2020, 25, 5854. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lim, H.J.; Jun, J.H.; Choi, J.; Lee, M.S. Ginseng for Treating Hypertension: A Systematic Review and Meta-Analysis of Double Blind, Randomized, Placebo-Controlled Trials. Curr. Vasc. Pharmacol. 2017, 15, 549–556. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.W.; Zhang, T.; Liu, R.; Wu, L.; Du, Q.; Li, Y. Anti-fatigue effects of small-molecule oligopeptides isolated from Panax quinquefolium L. in mice. Food Funct. 2018, 9, 4266–4273. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xiao, Y.K.; Hwang, E.; Haeng, J.J.; Yi, T.H. Antiphotoaging and Antimelanogenesis Properties of Ginsenoside C-Y, a Ginsenoside Rb2 Metabolite from American Ginseng PDD-ginsenoside. Photochem. Photobiol. 2019, 95, 1412–1423. [Google Scholar] [CrossRef]
- Chang, Y.D.; Smith, J.; Portman, D.; Kim, R.; Oberoi-Jassal, R.; Rajasekhara, S.; Davis, M. Single Institute Experience with Methylphenidate and American Ginseng in Cancer-Related Fatigue. Am. J. Hosp. Palliat. Care 2018, 35, 144–150. [Google Scholar] [CrossRef]
- Wang, C.M.; Liu, M.Y.; Wang, F.; Wei, M.J.; Wang, S.; Wu, C.F.; Yang, J.Y. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol. Biochem. Behav. 2013, 106, 57–67. [Google Scholar] [CrossRef]
- Sen, S.; Chen, S.; Feng, B.; Wu, Y.; Lui, E.; Chakrabarti, S. Corrigendum to: “Preventive effects of North American ginseng (Panax quinquefolium) on diabetic nephropathy” [Phytomedicine 19 (2012) 494–505]. Phytomed. Int. J. Phytother. Phytopharm. 2019, 62, 152995. [Google Scholar] [CrossRef]
- Jovanovski, E.; Lea Duvnjak, S.; Komishon, A.; Au-Yeung, F.; Zurbau, A.; Jenkins, A.L.; Sung, M.K.; Josse, R.; Vuksan, V. Vascular effects of combined enriched Korean Red ginseng (Panax Ginseng) and American ginseng (Panax Quinquefolius) administration in individuals with hypertension and type 2 diabetes: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102338. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.J.; Shui, Y.M.; Wan, J.B.; Gao, J.L. Anticancer Activities of Protopanaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evid.-Based Complement. Altern. Med. Ecam 2016, 2016, 5738694. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.Y.; Yu, X.F.; Qu, S.C.; Lu, Z.Z.; Wang, L.; Chen, M.Q. Protective effect of Panax quinquefolium 20s-proto-panaxdiolsaponins on acute myocardial infarction in dogs. China J. Chin. Mater. Medica 2001, 26, 416–419. [Google Scholar]
- Yu, C.; Wen, X.D.; Zhang, Z.; Zhang, C.F.; Wu, X.; He, X.; Liao, Y.; Wu, N.; Wang, C.Z.; Du, W.; et al. American ginseng significantly reduced the progression of high-fat-diet-enhanced colon carcinogenesis in ApcMin/+ mice. J. Ginseng Res. 2015, 39, 230–237. [Google Scholar] [CrossRef]
- Hu, J.N.; Yang, J.Y.; Jiang, S.; Zhang, J.; Liu, Z.; Hou, J.G.; Gong, X.J.; Wang, Y.P.; Wang, Z.; Li, W. Panax quinquefolium saponins protect against cisplatin evoked intestinal injury via ROS-mediated multiple mechanisms. Phytomed. Int. J. Phytother. Phytopharm. 2021, 82, 153446. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Jia, C.; Sun, P.; Qi, J.; Li, X. Quality evaluation of Panax quinquefolium from different cultivation regions based on their ginsenoside content and radioprotective effects on irradiated mice. Sci. Rep. 2019, 9, 1079. [Google Scholar] [CrossRef]
- Yu, C.; Wang, C.-Z.; Zhou, C.-J.; Wang, B.; Han, L.; Zhang, C.-F.; Wu, X.-H.; Yuan, C.-S. Adulteration and cultivation region identification of American ginseng using HPLC coupled with multivariate analysis. J. Pharm. Biomed. Anal. 2014, 99, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, L.; Wang, H.; Jiang, S.; Zhou, S. Photoperiod and Temperature as Dominant Environmental Drivers Triggering Plant Phenological Development of American Ginseng Along with Its Quality Formation. Front. Earth Sci. 2022, 10, 894251. [Google Scholar] [CrossRef]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American Ginseng (Panax quinquefolium L.) as a Source of Bioactive Phytochemicals with Pro-Health Properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.R.; Huang, J.H.; He, D.; Yi, Z.Y.; Zhao, D.; Liu, Z.; Zhang, S.H.; Huang, L.Q. Green and Efficient Extraction of Polysaccharide and Ginsenoside from American Ginseng (Panax quinquefolius L.) by Deep Eutectic Solvent Extraction and Aqueous Two-Phase System. Molecules 2022, 27, 3132. [Google Scholar] [CrossRef]
- Aminifard, T.; Razavi, B.M.; Hosseinzadeh, H. The effects of ginseng on the metabolic syndrome: An updated review. Food Sci. Nutr. 2021, 9, 5293–5311. [Google Scholar] [CrossRef]
- Guo, M.; Jin, J.; Zhao, D.; Rong, Z.; Cao, L.Q.; Li, A.H.; Sun, X.Y.; Jia, L.Y.; Wang, Y.D.; Huang, L.; et al. Research Advances on Anti-Cancer Natural Products. Front. Oncol. 2022, 12, 866154. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Zhou, J.; Wu, D.; Ye, J.; Sun, G.; Sun, X. Saponins in Chinese Herbal Medicine Exerts Protection in Myocardial Ischemia-Reperfusion Injury: Possible Mechanism and Target Analysis. Front. Pharmacol. 2020, 11, 570867. [Google Scholar] [CrossRef] [PubMed]
- Zarneshan, S.N.; Fakhri, S.; Khan, H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol. Res. 2022, 177, 106099. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X.; Wu, A.; Cao, Y.; Dai, X.; Liang, Y.; Li, X. Ginsenosides in central nervous system diseases: Pharmacological actions, mechanisms, and therapeutics. Phytother. Res. 2022, 36, 1523–1544. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Z.; Liu, J.; Chen, Z.; Huang, Q.; Li, J.; Chen, J.; Wang, M.; Zhao, D.; Wang, Z.; et al. Comparisons of Isolation Methods, Structural Features, and Bioactivities of the Polysaccharides from Three Common Panax Species: A Review of Recent Progress. Molecules 2021, 26, 4997. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Ren, R.; Qi, J.; Lin, S.; Liu, X.; Yin, P.; Wang, Z.; Tang, R.; Wang, J.; Huang, Q.; Li, J.; et al. The China Alzheimer Report 2022. Gen. Psychiatry 2022, 35, e100751. [Google Scholar] [CrossRef]
- Mumtaz, I.; Ayaz, M.O.; Khan, M.S.; Manzoor, U.; Ganayee, M.A.; Bhat, A.Q.; Dar, G.H.; Alghamdi, B.S.; Hashem, A.M.; Dar, M.J.; et al. Clinical relevance of biomarkers, new therapeutic approaches, and role of post-translational modifications in the pathogenesis of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 977411. [Google Scholar] [CrossRef]
- Fenton, L.; Weissberger, G.H.; Boyle, P.A.; Mosqueda, L.; Yassine, H.N.; Nguyen, A.L.; Lim, A.C.; Han, S.D. Cognitive and Neuroimaging Correlates of Financial Exploitation Vulnerability in Older Adults without Dementia: Implications for Early Detection of Alzheimer’s Disease. Neurosci. Biobehav. Rev. 2022, 140, 104773. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Ettcheto, M.; Cano, A.; Sanchez-López, E.; Carrasco, M.; Espinosa, T.; Beas-Zarate, C.; Gudiño-Cabrera, G.; Ureña-Guerrero, M.E.; Verdaguer, E.; et al. Impact of New Drugs for Therapeutic Intervention in Alzheimer’s Disease. Front. Biosci. Landmark Ed. 2022, 27, 146. [Google Scholar] [CrossRef]
- Hoy, S.M. Lecanemab: First Approval. Drugs 2023, 83, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Durk, M.R.; Han, K.; Chow, E.C.; Ahrens, R.; Henderson, J.T.; Fraser, P.E.; Pang, K.S. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 7091–7101. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Lee, M.; Shin, E.; Yun, M.R.; Lee, Y.H.; Moon, J.H.; Kim, E.; Lee, P.H.; Lee, B.W.; Kang, E.S.; et al. Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci. Rep. 2019, 9, 4414. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.G.; Xing, S.T.; Wang, L.W. Immunological aspects of Chinese medicinal plants as antiageing drugs. J. Ethnopharmacol. 1993, 38, 167–175. [Google Scholar] [CrossRef]
- Shin, K.; Guo, H.; Cha, Y.; Ban, Y.H.; Seo, D.W.; Choi, Y.; Kim, T.S.; Lee, S.P.; Kim, J.C.; Choi, E.K.; et al. Cereboost™, an American ginseng extract, improves cognitive function via up-regulation of choline acetyltransferase expression and neuroprotection. Regul. Toxicol. Pharmacol. 2016, 78, 53–58. [Google Scholar] [CrossRef]
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; de Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res. Bull. 2016, 125, 30–43. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liu, Q.P.; An, P.; Jia, M.; Luan, X.; Tang, J.Y.; Zhang, H. Ginsenoside Rd: A promising natural neuroprotective agent. Phytomed. Int. J. Phytother. Phytopharm. 2022, 95, 153883. [Google Scholar] [CrossRef]
- Dai, R.; Sun, Y.; Su, R.; Gao, H. Anti-Alzheimer’s disease potential of traditional chinese medicinal herbs as inhibitors of BACE1 and AChE enzymes. Biomed. Pharmacother. 2022, 154, 113576. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, Y. Nature’s Derivative(s) as Alternative Anti-Alzheimer’s Disease Treatments. J. Alzheimer’s Dis. Rep. 2019, 3, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Feng, S.; Zhu, L.; Yang, L.; Liu, T.C.; Duan, R. Therapeutic non-invasive brain treatments in Alzheimer’s disease: Recent advances and challenges. Inflamm. Regen. 2022, 42, 31. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Zlokovic, B.V. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr. Pharm. Des. 2008, 14, 1601–1605. [Google Scholar] [CrossRef]
- Scheuner, D.; Eckman, C.; Jensen, M.; Song, X.; Citron, M.; Suzuki, N.; Bird, T.D.; Hardy, J.; Hutton, M.; Kukull, W.; et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996, 2, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of beta-amyloid in Alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Klein, W.L. The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef]
- Hölttä, M.; Hansson, O.; Andreasson, U.; Hertze, J.; Minthon, L.; Nägga, K.; Andreasen, N.; Zetterberg, H.; Blennow, K. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS ONE 2013, 8, e66381. [Google Scholar]
- Imbimbo, B.P.; Watling, M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2019, 28, 967–975. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef] [PubMed]
- Madhu, P.; Mukhopadhyay, S. Distinct types of amyloid-β oligomers displaying diverse neurotoxicity mechanisms in Alzheimer’s disease. J. Cell. Biochem. 2021, 122, 1594–1608. [Google Scholar] [CrossRef]
- Stanley, M.; Macauley, S.L.; Holtzman, D.M. Changes in insulin and insulin signaling in Alzheimer’s disease: Cause or consequence? J. Exp. Med. 2016, 213, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Bloom, G.S. Tau: The Center of a Signaling Nexus in Alzheimer’s Disease. Front. Neurosci. 2016, 10, 31. [Google Scholar] [CrossRef]
- Gibbons, G.S.; Lee, V.M.Y.; Trojanowski, J.Q. Mechanisms of Cell-to-Cell Transmission of Pathological Tau: A Review. JAMA Neurol. 2019, 76, 101–108. [Google Scholar] [CrossRef]
- Stefanoska, K.; Gajwani, M.; Tan, A.R.P.; Ahel, H.I.; Asih, P.R.; Volkerling, A.; Poljak, A.; Ittner, A. Alzheimer’s disease: Ablating single master site abolishes tau hyperphosphorylation. Sci. Adv. 2022, 8, eabl8809. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Q.; Li, M.X.; Wang, C.Y.; Song, Z.S.; Ding, H.; Shi, K.J.; Li, Z.; Tao, S.Q.; Tang, W. Effects of pre-moxibustion on expression of phosphorylated Tau protein and related protein kinases in hippocampal CA3 region of AD rats. Acupunct. Res. 2022, 47, 573–579. [Google Scholar]
- Arriagada, P.V.; Marzloff, K.; Hyman, B.T. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 1992, 42, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- Regan, P.; Whitcomb, D.J.; Cho, K. Physiological and Pathophysiological Implications of Synaptic Tau. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2017, 23, 137–151. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Terro, F. Tau protein phosphatases in Alzheimer’s disease: The leading role of PP2A. Ageing Res. Rev. 2013, 12, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Hugon, J.; Paquet, C. The PKR/P38/RIPK1 Signaling Pathway as a Therapeutic Target in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 3136. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Praticò, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef]
- Llorens-Martín, M.; Jurado, J.; Hernández, F.; Avila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [PubMed]
- Chauhan, N.; Paliwal, S.; Jain, S.; Verma, K.; Paliwal, S.; Sharma, S. GSK-3β and its Inhibitors in Alzheimer’s Disease: A Recent Update. Mini Rev. Med. Chem. 2022, 22, 2881–2895. [Google Scholar]
- Ly, P.T.; Wu, Y.; Zou, H.; Wang, R.; Zhou, W.; Kinoshita, A.; Zhang, M.; Yang, Y.; Cai, F.; Woodgett, J.; et al. Inhibition of GSK3β-mediated BACE1 expression reduces Alzheimer-associated phenotypes. J. Clin. Investig. 2013, 123, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, H.L.; Xie, J.Z.; Meng, D.L.; Wang, X.C.; Ke, D.; Zeng, J.; Liu, R. Protein Phosphatase 2A as a Drug Target in the Treatment of Cancer and Alzheimer’s Disease. Curr. Med. Sci. 2020, 40, 1–8. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., III; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Candy, J.M.; Perry, R.H.; Perry, E.K.; Irving, D.; Blessed, G.; Fairbairn, A.F.; Tomlinson, B.E. Pathological changes in the nucleus of Meynert in Alzheimer’s and Parkinson’s diseases. J. Neurol. Sci. 1983, 59, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Prohovnik, I.; Arnold, S.E.; Smith, G.; Lucas, L.R. Physostigmine reversal of scopolamine-induced hypofrontality. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1997, 17, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Norman, T.A.; Haydar, T.F.; Slack, B.E.; Leeman, S.E.; Blusztajn, J.K.; Mellott, T.J. BMP9 ameliorates amyloidosis and the cholinergic defect in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2013, 110, 19567–19572. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yamagata, N.; Takasaki, K.; Sano, K.; Hayakawa, K.; Katsurabayashi, S.; Egashira, N.; Mishima, K.; Iwasaki, K.; Fujiwara, M. Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res. 2009, 1249, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, J.; Li, A.; Yao, M.; Liu, G.; Chen, S.; Luo, Y.; Wang, Z.; Gong, H.; Li, X.; et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat. Commun. 2022, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; Farlow, M.R.; Doody, R.S.; Mohs, R.; Friedhoff, L.T. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology 1998, 50, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Tholey, G.; Ledig, M. Neuronal and astrocytic plasticity: Metabolic aspects. Ann. Med. Interne 1990, 141 (Suppl. S1), 13–18. [Google Scholar]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free. Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, L.; Tu, L. GABAB Receptor-Mediated PI3K/Akt Signaling Pathway Alleviates Oxidative Stress and Neuronal Cell Injury in a Rat Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 1513–1526. [Google Scholar] [CrossRef]
- Fasano, C.; Disciglio, V.; Bertora, S.; Lepore Signorile, M.; Simone, C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells 2019, 8, 1110. [Google Scholar] [CrossRef]
- Wójtowicz, S.; Strosznajder, A.K.; Jeżyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef]
- Canseco-Rodriguez, A.; Masola, V.; Aliperti, V.; Meseguer-Beltran, M.; Donizetti, A.; Sanchez-Perez, A.M. Long Non-Coding RNAs, Extracellular Vesicles and Inflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 13171. [Google Scholar] [CrossRef]
- Garwood, C.J.; Pooler, A.M.; Atherton, J.; Hanger, D.P.; Noble, W. Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011, 2, e167. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, X.; Li, H.; Hui, S.; Zhang, Z.; Xiao, Y.; Peng, W. Non-Coding RNAs as Novel Regulators of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2022, 13, 908076. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafò, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca2+ Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Princiotta Cariddi, L.; Mauri, M.; Cosentino, M.; Versino, M.; Marino, F. Alzheimer’s Disease: From Immune Homeostasis to Neuroinflammatory Condition. Int. J. Mol. Sci. 2022, 23, 13008. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Prà, I. Danger-Sensing/Patten Recognition Receptors and Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Motolani, A.; Campos, L.; Lu, T. The Pivotal Role of NF-kB in the Pathogenesis and Therapeutics of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8972. [Google Scholar] [CrossRef]
- Seo, E.J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 262–273. [Google Scholar] [CrossRef]
- Li, T.; Lu, L.; Pember, E.; Li, X.; Zhang, B.; Zhu, Z. New Insights into Neuroinflammation Involved in Pathogenic Mechanism of Alzheimer’s Disease and Its Potential for Therapeutic Intervention. Cells 2022, 11, 1925. [Google Scholar] [CrossRef]
- Li, R.L.; Wang, L.Y.; Duan, H.X.; Zhang, Q.; Guo, X.; Wu, C.; Peng, W. Regulation of mitochondrial dysfunction induced cell apoptosis is a potential therapeutic strategy for herbal medicine to treat neurodegenerative diseases. Front. Pharmacol. 2022, 13, 937289. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Oliver, D.M. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019, 8, 488. [Google Scholar] [CrossRef]
- Pohland, M.; Pellowska, M.; Asseburg, H.; Hagl, S.; Reutzel, M.; Joppe, A.; Berressem, D.; Eckert, S.H.; Wurglics, M.; Schubert-Zsilavecz, M.; et al. MH84 improves mitochondrial dysfunction in a mouse model of early Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yang, W.; Gao, S.; Lin, J.; Wang, T.; Zhou, K.; Hu, H. Ginsenoside Rb1 inhibit apoptosis in rat model of Alzheimer’s disease induced by Aβ(1-40). Am. J. Transl. Res. 2018, 10, 796–805. [Google Scholar]
- Gasparini, L.; Netzer, W.J.; Greengard, P.; Xu, H. Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol. Sci. 2002, 23, 288–293. [Google Scholar] [CrossRef]
- Ali, S.K.; Ali, R.H. Effects of antidiabetic agents on Alzheimer’s disease biomarkers in experimentally induced hyperglycemic rat model by streptozocin. PLoS ONE 2022, 17, e0271138. [Google Scholar] [CrossRef]
- Amir Rawa, M.S.; Mazlan, M.K.N.; Ahmad, R.; Nogawa, T.; Wahab, H.A. Roles of Syzygium in Anti-Cholinesterase, Anti-Diabetic, Anti-Inflammatory, and Antioxidant: From Alzheimer’s Perspective. Plants 2022, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ramachandran, A.K.; Halder, D.; Akbar, S.; Ahmed, B.; Joseph, A. Mechanistic and Etiological Similarities in Diabetes Mellitus and Alzheimer’s Disease: Antidiabetic Drugs as Optimistic Therapeutics in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2022, 22, 973–993. [Google Scholar]
- Bagaria, J.; Bagyinszky, E.; An, S.S.A. Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene. Int. J. Mol. Sci. 2022, 23, 10970. [Google Scholar] [CrossRef]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, X.; Liu, H.; Liao, X.; Zhou, Y.; Weng, L.; Zhou, L.; Liu, X.; Bi, X.Y.; Xu, T.; et al. Clinical characteristics and genotype-phenotype correlation analysis of familial Alzheimer’s disease patients with pathogenic/likely pathogenic amyloid protein precursor mutations. Front. Aging Neurosci. 2022, 14, 1013295. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Comparison of Ginsenoside Components of Various Tissues of New Zealand Forest-Grown Asian Ginseng (Panax Ginseng) and American Ginseng (Panax Quinque folium L.). Biomolecules 2020, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Wang, C.Z.; Yuan, C.S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef]
- Liang, J.; Chen, L.; Guo, Y.H.; Zhang, M.; Gao, Y. Simultaneous Determination and Analysis of Major Ginsenosides in Wild American Ginseng Grown in Tennessee. Chem. Biodivers. 2019, 16, e1900203. [Google Scholar] [CrossRef]
- Lim, W.; Mudge, K.W.; Vermeylen, F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium). J. Agric. Food Chem. 2005, 53, 8498–8505. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Zhang, C.; Guo, Y.; Li, J.; Wu, C.; Jiao, J.; Zheng, H. Ginsenoside Rg1 improves Alzheimer’s disease by regulating oxidative stress, apoptosis, and neuroinflammation through Wnt/GSK-3β/β-catenin signaling pathway. Chem. Biol. Drug Des. 2022, 99, 884–896. [Google Scholar] [CrossRef]
- Cao, G.; Su, P.; Zhang, S.; Guo, L.; Zhang, H.; Liang, Y.; Qin, C.; Zhang, W. Ginsenoside Re reduces Aβ production by activating PPARγ to inhibit BACE1 in N2a/APP695 cells. Eur. J. Pharmacol. 2016, 793, 101–108. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Feng, J.; Wang, Z.; Cao, Y.; Liu, J.; Li, H. Fuzheng Quxie Decoction Ameliorates Learning and Memory Impairment in SAMP8 Mice by Decreasing Tau Hyperphosphorylation. Evid.-Based Complement. Altern. Med. 2017, 2017, 5934254. [Google Scholar] [CrossRef]
- Qiu, J.; Li, W.; Feng, S.H.; Wang, M.; He, Z.Y. Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid precursor protein via a cholesterol-dependent pathway. Genet. Mol. Res. 2014, 13, 3586–3598. [Google Scholar] [CrossRef]
- Yan, X.; Hu, G.; Yan, W.; Chen, T.; Yang, F.; Zhang, X.; Zhao, G.; Liu, J. Ginsenoside Rd promotes non-amyloidogenic pathway of amyloid precursor protein processing by regulating phosphorylation of estrogen receptor alpha. Life Sci. 2017, 168, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Kong, X.; Yang, Q.; Zhang, Y.; Han, J.; Li, P.; Xiong, L.; Zhao, G. Ginsenoside rd in experimental stroke: Superior neuroprotective efficacy with a wide therapeutic window. Neurother. J. Am. Soc. Exp. NeuroTher. 2011, 8, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Li, X.; Feng, J.; Hou, J.; Li, M.; Zhang, B. Ginsenoside Rg1 reduces β-amyloid levels by inhibiting CDΚ5-induced PPARγ phosphorylation in a neuron model of Alzheimer’s disease. Mol. Med. Rep. 2020, 22, 3277–3288. [Google Scholar]
- Quan, Q.; Wang, J.; Li, X.; Wang, Y. Ginsenoside Rg1 decreases Aβ(1-42) level by upregulating PPARγ and IDE expression in the hippocampus of a rat model of Alzheimer’s disease. PLoS ONE 2013, 8, e59155. [Google Scholar] [CrossRef]
- Chen, L.M.; Lin, Z.Y.; Zhu, Y.G.; Lin, N.; Zhang, J.; Pan, X.D.; Chen, X.C. Ginsenoside Rg1 attenuates β-amyloid generation via suppressing PPARγ-regulated BACE1 activity in N2a-APP695 cells. Eur. J. Pharmacol. 2012, 675, 15–21. [Google Scholar] [CrossRef]
- Yun, Y.J.; Park, B.H.; Hou, J.; Oh, J.P.; Han, J.H.; Kim, S.C. Ginsenoside F1 Protects the Brain against Amyloid Beta-Induced Toxicity by Regulating IDE and NEP. Life 2022, 12, 58. [Google Scholar] [CrossRef]
- Zhao, A.; Liu, N.; Yao, M.; Zhang, Y.; Yao, Z.; Feng, Y.; Liu, J.; Zhou, G. A Review of Neuroprotective Effects and Mechanisms of Ginsenosides from Panax Ginseng in Treating Ischemic Stroke. Front. Pharmacol. 2022, 13, 946752. [Google Scholar] [CrossRef]
- Oh, J.; Kim, J.S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yang, Q.; Lan, X.; Wang, J.; Cao, Z.; Shi, X.; Li, J.; Kan, M.; Qu, X.; et al. Ginsenoside compound K ameliorates Alzheimer’s disease in HT22 cells by adjusting energy metabolism. Mol. Biol. Rep. 2019, 46, 5323–5332. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, J.; Zhang, H.; Liu, Y.; Kan, M.; Xiu, Z.; Chen, X.; Lan, X.; Li, X.; Shi, X.; et al. Ginsenoside Compound K Regulates Amyloid β via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J. Mol. Neurosci. 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Kutscher, H.L.; Singh, A.; Cwiklinski, K.; Khechen, N.; Schwartz, S.A.; Prasad, P.N.; Mahajan, S.D. Neuroprotective effects of a biodegradable poly(lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: A potential nanotherapy for Alzheimer’s disease? J. Drug Target. 2018, 26, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.S.; Lee, D.I. Potential effects of microglial activation induced by ginsenoside Rg3 in rat primary culture: Enhancement of type A Macrophage Scavenger Receptor expression. Arch. Pharmacal Res. 2005, 28, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Baek, S.H.; Chun, Y.S.; Moore, A.Z.; Landman, N.; Berman, D.; Yang, H.O.; Morishima-Kawashima, M.; Osawa, S.; Funamoto, S.; et al. Modulation of lipid kinase PI4KIIα activity and lipid raft association of presenilin 1 underlies γ-secretase inhibition by ginsenoside (20S)-Rg3. J. Biol. Chem. 2013, 288, 20868–20882. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, J.; Zhang, J.; Xia, W.; Dong, X.; Hu, X.; Kong, F.; Cui, X. Ginsenoside Rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J. Pharm. Pharmacol. 2009, 61, 375–380. [Google Scholar] [CrossRef]
- Ahn, J.W.; Jang, S.K.; Jo, B.R.; Kim, H.S.; Park, J.Y.; Park, H.Y.; Yoo, Y.M.; Joo, S.S. A therapeutic intervention for Alzheimer’s disease using ginsenoside Rg3: Its role in M2 microglial activation and non-amyloidogenesis. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2021, 72, 185–193. [Google Scholar]
- Yao, X.C.; Xue, X.; Zhang, H.T.; Zhu, M.M.; Yang, X.W.; Wu, C.F.; Yang, J.Y. Pseudoginsenoside-F11 alleviates oligomeric β-amyloid-induced endosome-lysosome defects in microglia. Traffic 2019, 20, 61–70. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Yang, X.; Zhang, T.; Hou, Y.; Wang, P.; Liu, Y.; Yuan, L.; Zhang, H.; Wu, C.; et al. Pseudoginsenoside-F11 ameliorates thromboembolic stroke injury in rats by reducing thromboinflammation. Neurochem. Int. 2021, 149, 105108. [Google Scholar] [CrossRef]
- Han, J.; Oh, J.P.; Yoo, M.; Cui, C.H.; Jeon, B.M.; Kim, S.C.; Han, J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol. Brain 2019, 12, 77. [Google Scholar] [CrossRef]
- Chu, S.; Gu, J.; Feng, L.; Liu, J.; Zhang, M.; Jia, X.; Liu, M.; Yao, D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014, 19, 317–326. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Tian, X.; Zhao, L. Ginsenoside Rd Attenuates Tau Phosphorylation in Olfactory Bulb, Spinal Cord, and Telencephalon by Regulating Glycogen Synthase Kinase 3β and Cyclin-Dependent Kinase 5. Evid.-Based Complement. Altern. Med. 2021, 2021, 4485957. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Yan, X.; Qin, K.; Shi, M.; Lin, T.; Zhu, Y.; Kang, T.; Zhao, G. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011, 138, 135–141. [Google Scholar] [CrossRef]
- Chu, J.; Wang, J.; Cui, L.; Liu, S.; An, N.; Han, J.; Che, X.; Wu, C.; Yang, J. Pseudoginsenoside-F11 ameliorates okadiac acid-induced learning and memory impairment in rats via modulating protein phosphatase 2A. Mech. Ageing Dev. 2021, 197, 111496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, S.; Yu, H.; Duan, S.; Zhao, J. Baicalin and ginsenoside Rb1 promote the proliferation and differentiation of neural stem cells in Alzheimer’s disease model rats. Brain Res. 2018, 1678, 187–194. [Google Scholar] [CrossRef]
- Wu, S.D.; Xia, F.; Lin, X.M.; Duan, K.L.; Wang, F.; Lu, Q.L.; Cao, H.; Qian, Y.H.; Shi, M. Ginsenoside-Rd Promotes Neurite Outgrowth of PC12 Cells through MAPK/ERK- and PI3K/AKT-Dependent Pathways. Int. J. Mol. Sci. 2016, 17, 177. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, S.; Cheng, G.; Yang, X.; Zhao, N.; Chen, C. Ginsenoside Rg1 and Acori Graminei Rhizoma Attenuates Neuron Cell Apoptosis by Promoting the Expression of miR-873-5p in Alzheimer’s Disease. Neurochem. Res. 2018, 43, 1529–1538. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, S.; Pan, X.; Zheng, L.; Li, Y.; Yang, J.; Wu, C. Pseudoginsenoside-F11 improves long-term neurological function and promotes neurogenesis after transient cerebral ischemia in mice. Neurochem. Int. 2020, 133, 104586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Di, J.; Liu, W.S.; Liu, H.L.; Lai, H.; Lü, Y.L. Involvement of GSK3 and PP2A in ginsenoside Rb1′s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013, 241, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Changhong, K.; Peng, Y.; Yuan, Z.; Cai, J. Ginsenoside Rb1 protected PC12 cells from Aβ(25-35)-induced cytotoxicity via PPARγ activation and cholesterol reduction. Eur. J. Pharmacol. 2021, 893, 173835. [Google Scholar]
- Liu, M.; Bai, X.; Yu, S.; Zhao, W.; Qiao, J.; Liu, Y.; Zhao, D.; Wang, J.; Wang, S. Ginsenoside Re Inhibits ROS/ASK-1 Dependent Mitochondrial Apoptosis Pathway and Activation of Nrf2-Antioxidant Response in Beta-Amyloid-Challenged SH-SY5Y Cells. Molecules 2019, 24, 2687. [Google Scholar] [CrossRef]
- Shieh, P.C.; Tsao, C.W.; Li, J.S.; Wu, H.T.; Wen, Y.J.; Kou, D.H.; Cheng, J.T. Role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the action of ginsenoside Rh2 against beta-amyloid-induced inhibition of rat brain astrocytes. Neurosci. Lett. 2008, 434, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, J.; Zheng, M.; Gou, D.; Liu, C.; Zhou, Y. Ginsenoside Rg2 protects PC12 cells against β-amyloid(25-35)-induced apoptosis via the phosphoinositide 3-kinase/Akt pathway. Chem.-Biol. Interact. 2017, 275, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective effects of ginsenoside Rg2 against memory impairment and neuronal death induced by Aβ25-35 in rats. J. Ethnopharmacol. 2021, 266, 113466. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hu, F.; Fu, W.; Yu, X.; Zhong, W.; Liu, F.; Wang, T.; Sui, D. Ginsenoside Rg2 Ameliorates Brain Injury After Intracerebral Hemorrhage in a Rat Model of Preeclampsia. Reprod. Sci. 2021, 28, 3431–3439. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhao, Y.; Liu, Y.; Zhang, S.; Zhao, W.; Liu, S.; Liu, M. Neuroprotective effect of Ginsenoside Re against neurotoxin-induced Parkinson’s disease models via induction of Nrf2. Mol. Med. Rep. 2022, 25, 215. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, N.; Lv, J.; Huang, H.; Liu, X. Ginsenoside Rd reverses cognitive deficits by modulating BDNF-dependent CREB pathway in chronic restraint stress mice. Life Sci. 2020, 258, 118107. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Wang, Z.; Li, W. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother. Res. 2018, 32, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Fang, F.; Chen, L.; Zhu, Y.; Zhang, J.; Chen, X.; Yan, S.S. Ginsenoside Rg1 attenuates oligomeric Aβ(1-42)-induced mitochondrial dysfunction. Curr. Alzheimer Res. 2012, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, S.; Song, S. Ginsenoside Rg3 Prevents Cognitive Impairment by Improving Mitochondrial Dysfunction in the Rat Model of Alzheimer’s Disease. J. Agric. Food Chem. 2019, 67, 10048–10058. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, S.; Li, G.; Liu, Z.; Xu, B. 20(S)-ginsenoside Rg3, a neuroprotective agent, inhibits mitochondrial permeability transition pores in rat brain. Phytother. Res. 2009, 23, 486–491. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, K.C.; Zhou, Y.; Wei, H.; Qi, M.H.; Wang, Z.; Zheng, Y.N.; Chen, R.X.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomed. Int. J. Phytother. Phytopharm. 2022, 104, 154341. [Google Scholar] [CrossRef]
- She, L.; Xiong, L.; Li, L.; Zhang, J.; Sun, J.; Wu, H.; Ren, J.; Wang, W.; Zhao, X.; Liang, G. Ginsenoside Rk3 ameliorates Aβ-induced neurotoxicity in APP/PS1 model mice via AMPK signaling pathway. Biomed. Pharmacother. 2023, 158, 114192. [Google Scholar] [CrossRef]
- Lin, J.; Gao, S.; Wang, T.; Shen, Y.; Yang, W.; Li, Y.; Hu, H. Ginsenoside Rb1 improves learning and memory ability through its anti-inflammatory effect in Aβ(1-40) induced Alzheimer’s disease of rats. Am. J. Transl. Res. 2019, 11, 2955–2968. [Google Scholar] [PubMed]
- Lee, K.W.; Jung, S.Y.; Choi, S.M.; Yang, E.J. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement. Altern. Med. 2012, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Madhi, I.; Kim, J.H.; Shin, J.E.; Kim, Y. Ginsenoside Re exhibits neuroprotective effects by inhibiting neuroinflammation via CAMK/MAPK/NF-κB signaling in microglia. Mol. Med. Rep. 2021, 24, 698. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Y.; Cen, J.K.; Song, H.L.; Song, S.Y.; Zhang, Z.J.; Lu, H.J. Ginsenoside Rh2 Ameliorates Neuropathic Pain by inhibition of the miRNA21-TLR8-mitogen-activated protein kinase axis. Mol. Pain 2022, 18, 17448069221126078. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Oh, T.W.; Park, Y.K. Anti-Inflammatory Effects of Ginsenoside-Rh2 Inhibits LPS-Induced Activation of Microglia and Overproduction of Inflammatory Mediators Via Modulation of TGF-β1/Smad Pathway. Neurochem. Res. 2016, 41, 951–957. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.; Li, L.; Li, Y.; Zhou, L.; Zhang, X.; Hu, X.; Zhao, G. Ginsenoside Rd Improves Learning and Memory Ability in APP Transgenic Mice. J. Mol. Neurosci. 2015, 57, 522–528. [Google Scholar] [CrossRef]
- Jiao, H.; Jia, J. Ginsenoside compound K acts via LRP1 to alleviate Amyloid β(42)-induced neuroinflammation in microglia by suppressing NF-κB. Biochem. Biophys. Res. Commun. 2022, 590, 14–19. [Google Scholar] [CrossRef]
- Park, J.S.; Shin, J.A.; Jung, J.S.; Hyun, J.W.; Van Le, T.K.; Kim, D.H.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Ginsenoside rg3 alleviates lipopolysaccharide-induced learning and memory impairments by anti-inflammatory activity in rats. Biomol. Ther. 2013, 21, 381–390. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoo, Y.M.; Ahn, B.W.; Nam, S.Y.; Kim, Y.B.; Hwang, K.W.; Lee, D.I. Prevention of inflammation-mediated neurotoxicity by Rg3 and its role in microglial activation. Biol. Pharm. Bull. 2008, 31, 1392–1396. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, S.; Chen, Y.; Sun, Z.; Zhang, J.; Han, Y.; Dong, X.; Fang, Z.; Li, W. Ginsenoside Rg1 alleviates lipopolysaccharide-induced neuronal damage by inhibiting NLRP1 inflammasomes in HT22 cells. Exp. Ther. Med. 2021, 22, 782. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.Z.; Shen, X.Y.; Sun, L.L.; Chen, Y.L.; Zhang, B.Q.; Huang, D.K.; Li, W.Z. Ginsenoside Rg1 protects against H2O2-induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 2019, 43, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Wang, J.; Zhao, S.; Zhang, K.; Wang, J.; Zhang, W.; Wu, C.; Yang, J. Pseudoginsenoside-F11 (PF11) exerts anti-neuroinflammatory effects on LPS-activated microglial cells by inhibiting TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt signaling pathways. Neuropharmacology 2014, 79, 642–656. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, J.S.; Jung, J.S.; Kim, D.H.; Kim, H.S. Anti-inflammatory effect of ginsenoside Rg5 in lipopolysaccharide-stimulated BV2 microglial cells. Int. J. Mol. Sci. 2013, 14, 9820–9833. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, H.; Li, Q.; Ren, H.; Xu, X.; Li, X. Structural-Activity Relationship of Rare Ginsenosides from Red Ginseng in the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8625. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jiang, X.; He, X.; Liang, D.; Sun, S.; Zhou, G. Ginsenoside Rb1 Improves Cognitive Impairment Induced by Insulin Resistance through Cdk5/p35-NMDAR-IDE Pathway. BioMed Res. Int. 2020, 2020, 3905719. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Jang, S.K.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol. Pharm. Bull. 2014, 37, 826–833. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Li, K.X.; Xue, W. Pharmacokinetics and acetylcholine releasing effects of ginsenoside Rg1 in hippocampus of beta-amyloid model rats. J. Asian Nat. Prod. Res. 2019, 21, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, X.; Jin, P.; Li, N.; Zhang, Q.; Yan, W.; Zhang, H.; Sun, J. Screening and determination for potential acetylcholinesterase inhibitory constituents from ginseng stem-leaf saponins using ultrafiltration (UF)-LC-ESI-MS2. Phytochem. Anal. 2019, 30, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Xie, R.; Zhong, C.; Huang, J.; Shi, P.; Yao, H. Recent progress (2015–2020) in the investigation of the pharmacological effects and mechanisms of ginsenoside Rb(1), a main active ingredient in Panax ginseng Meyer. J. Ginseng Res. 2022, 46, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.M.; Alnasser, S.M.; Ahmed Khairy, D.; Alabiad, M.A.; Alorini, M.; Jaber, F.A.; Tawfeek, S.E. The neuroprotective effect of ginsenoside Rb1 on the cerebral cortex changes induced by aluminium chloride in a mouse model of Alzheimer’s disease: A histological, immunohistochemical, and biochemical study. J. Chem. Neuroanat. 2023, 129, 102248. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Chakrabarti, S.; Goel, K.; Bhutani, K.; Chopra, T.; Bali, S. Neuronal cell death mechanisms in Alzheimer’s disease: An insight. Front. Mol. Neurosci. 2022, 15, 937133. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.H.; Han, H.; Hu, X.D.; Shi, L.L. Protective effect of ginsenoside Rb1 on beta-amyloid protein(1-42)-induced neurotoxicity in cortical neurons. Neurol. Res. 2009, 31, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, E.; Klegeris, A. Neuroinflammation as a mechanism linking hypertension with the increased risk of Alzheimer’s disease. Neural Regen. Res. 2022, 17, 2342–2346. [Google Scholar]
- Wang, Y.; Liu, J.; Zhang, Z.; Bi, P.; Qi, Z.; Zhang, C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci. Lett. 2011, 487, 70–72. [Google Scholar] [CrossRef]
- Lü, J.M.; Weakley, S.M.; Yang, Z.; Hu, M.; Yao, Q.; Chen, C. Ginsenoside Rb1 directly scavenges hydroxyl radical and hypochlorous acid. Curr. Pharm. Des. 2012, 18, 6339–6347. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chai, X.; Meng, Q.; Jiang, S.; Zhao, F. Advances in Biocatalytic Synthesis, Pharmacological Activities, Pharmaceutical Preparation and Metabolism of Ginsenoside Rh2. Mini Rev. Med. Chem. 2022, 22, 437–448. [Google Scholar]
- Lv, J.; Lu, C.; Jiang, N.; Wang, H.; Huang, H.; Chen, Y.; Li, Y.; Liu, X. Protective effect of ginsenoside Rh2 on scopolamine-induced memory deficits through regulation of cholinergic transmission, oxidative stress and the ERK-CREB-BDNF signaling pathway. Phytother. Res. 2021, 35, 337–345. [Google Scholar] [CrossRef]
- Mai, M.; Guo, X.; Huang, Y.; Zhang, W.; Xu, Y.; Zhang, Y.; Bai, X.; Wu, J.; Zu, H. DHCR24 Knockdown Induces Tau Hyperphosphorylation at Thr181, Ser199, Ser262, and Ser396 Sites via Activation of the Lipid Raft-Dependent Ras/MEK/ERK Signaling Pathway in C8D1A Astrocytes. Mol. Neurobiol. 2022, 59, 5856–5873. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, X.; Li, L.; Zhu, Y.; Qin, K.; Zhou, L.; Sun, D.; Zhang, X.; Ye, R.; Zhao, G. Ginsennoside rd attenuates cognitive dysfunction in a rat model of Alzheimer’s disease. Neurochem. Res. 2012, 37, 2738–2747. [Google Scholar] [CrossRef]
- Liu, J.F.; Yan, X.D.; Qi, L.S.; Li, L.; Hu, G.Y.; Li, P.; Zhao, G. Ginsenoside Rd attenuates Aβ25-35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chem.-Biol. Interact. 2015, 239, 12–18. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Liu, J.; Tai, X.; Hu, X.; Liu, X.; Wu, Z.; Zhang, G.; Shi, M.; Zhao, G. Ginsenoside Rd attenuates beta-amyloid-induced tau phosphorylation by altering the functional balance of glycogen synthase kinase 3beta and protein phosphatase 2A. Neurobiol. Dis. 2013, 54, 320–328. [Google Scholar] [CrossRef]
- Liu, H.H.; Jan, Y.N. Mechanisms of neurite repair. Curr. Opin. Neurobiol. 2020, 63, 53–58. [Google Scholar] [CrossRef]
- Zhang, C.; Du, F.; Shi, M.; Ye, R.; Cheng, H.; Han, J.; Ma, L.; Cao, R.; Rao, Z.; Zhao, G. Ginsenoside Rd protects neurons against glutamate-induced excitotoxicity by inhibiting Ca2+ influx. Cell. Mol. Neurobiol. 2012, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Dumbacher, M.; Van Dooren, T.; Princen, K.; De Witte, K.; Farinelli, M.; Lievens, S.; Tavernier, J.; Dehaen, W.; Wera, S.; Winderickx, J.; et al. Modifying Rap1-signalling by targeting Pde6δ is neuroprotective in models of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Eckman, E.A.; Eckman, C.B. Reductions in levels of the Alzheimer’s amyloid beta peptide after oral administration of ginsenosides. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1269–1271. [Google Scholar]
- Wilkinson, K.; El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 489456. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.E.; Dall’Armi, C.; Voronov, S.V.; McIntire, L.B.; Zhang, H.; Moore, A.Z.; Staniszewski, A.; Arancio, O.; Kim, T.W.; Di Paolo, G. Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism. Nat. Neurosci. 2008, 11, 547–554. [Google Scholar] [CrossRef]
- Otgongerel, D.; Lee, H.J.; Jo, S.A. Induction of ICAM1 in Brain Vessels is Implicated in an Early AD Pathogenesis by Modulating Neprilysin. Neuromol. Med. 2022, 24, 35948857. [Google Scholar] [CrossRef]
- Wang, Y.; Leak, R.K.; Cao, G. Microglia-mediated neuroinflammation and neuroplasticity after stroke. Front. Cell. Neurosci. 2022, 16, 980722. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.C.; Chang, W.C.; Chang, C.L. Fraction from wax apple [Syzygium samarangense (Blume) Merrill and Perry] fruit extract ameliorates insulin resistance via modulating insulin signaling and inflammation pathway in tumor necrosis factor α-treated FL83B mouse hepatocytes. Int. J. Mol. Sci. 2012, 13, 8562–8577. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, W.; Wang, Z.; Guo, P.; Li, L.; Li, N. Metabolic profiling of the effects of ginsenoside Re in an Alzheimer’s disease mouse model. Behav. Brain Res. 2018, 337, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Ma, C.; Yan, Y.; Yang, Y.; Wang, X.; Rausch, W.D. Ginsenoside Rd and ginsenoside Re offer neuroprotection in a novel model of Parkinson’s disease. Am. J. Neurodegener. Dis. 2016, 5, 52–61. [Google Scholar]
- Yoo, M.H.; Gu, X.; Xu, X.M.; Kim, J.Y.; Carlson, B.A.; Patterson, A.D.; Cai, H.; Gladyshev, V.N.; Hatfield, D.L. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid. Redox Signal. 2010, 12, 819–827. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, W.J.; Hur, J.; Kim, E.; Lee, H.G.; Seo, H.G. Ginsenoside Re Mitigates 6-Hydroxydopamine-Induced Oxidative Stress through Upregulation of GPX4. Molecules 2020, 25, 188. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Zhang, P.P.; Zhang, X.L.; Zheng, Y.Y.; Huang, Y.R.; Zheng, G.Q.; Lin, Y. Preclinical systematic review of ginsenoside Rg1 for cognitive impairment in Alzheimer’s disease. Aging 2021, 13, 7549–7569. [Google Scholar] [CrossRef]
- Wu, J.J.; Yang, Y.; Wan, Y.; Xia, J.; Xu, J.F.; Zhang, L.; Liu, D.; Chen, L.; Tang, F.; Ao, H.; et al. New insights into the role and mechanisms of ginsenoside Rg1 in the management of Alzheimer’s disease. Biomed. Pharmacother. 2022, 152, 113207. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Lu, J.; Jiao, J.; Yang, Y.; Zhao, H.; Liang, Z.; Zheng, H. Ginsenoside Rg1 improves cognitive capability and affects the microbiota of large intestine of tree shrew model for Alzheimer’s disease. Mol. Med. Rep. 2021, 23, 291. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Huang, H.; Lv, J.; Chen, S.; Pires Dias, A.C.; Li, Y.; Liu, X.; Wang, Q. Comparison of the Protective Effects of Ginsenosides Rb1 and Rg1 on Improving Cognitive Deficits in SAMP8 Mice Based on Anti-Neuroinflammation Mechanism. Front. Pharmacol. 2020, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, J.; Xia, Z.; Chen, H. miR-363-3p attenuates the oxygen-glucose deprivation/reoxygenation-induced neuronal injury in vitro by targeting PDCD6IP. Mol. Med. Rep. 2022, 26, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, Y.; Ji, J.; Wang, L.; Xie, G.; Tang, Z.; Qu, X.; Liu, Z.; Ren, G. Microglial exosomal miR-466i-5p induces brain injury via promoting hippocampal neuron apoptosis in heatstroke. Front. Immunol. 2022, 13, 968520. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, J.; Zeng, Y.; Guo, Y.; Wu, C.; Zhao, H.; Zheng, H.; Jiao, J. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol. Lett. 2020, 367, fnaa011. [Google Scholar] [CrossRef]
- Du, J.; Cui, C.H.; Park, S.C.; Kim, J.K.; Yu, H.S.; Jin, F.X.; Sun, C.; Kim, S.C.; Im, W.T. Identification and characterization of a ginsenoside-transforming β-glucosidase from Pseudonocardia sp. Gsoil 1536 and its application for enhanced production of minor ginsenoside Rg2(S). PLoS ONE 2014, 9, e96914. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Li, W.; Zhou, L.; Li, Q.; Wang, X.; He, P. A UPLC/MS-based metabolomics investigation of the protective effect of ginsenosides Rg1 and Rg2 in mice with Alzheimer’s disease. J. Ginseng Res. 2016, 40, 9–17. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef]
- Shuangyan, W.; Ruowu, S.; Hongli, N.; Bei, Z.; Yong, S. Protective effects of Rg2 on hypoxia-induced neuronal damage in hippocampal neurons. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 142–145. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, G.; Mana, L.; Dong, Y.; Huang, S.; Wang, Y.; Wu, Y.; Shi, J.; Tian, J.; Wang, P. GAPT regulates cholinergic dysfunction and oxidative stress in the brains of learning and memory impairment mice induced by scopolamine. Brain Behav. 2020, 10, e01602. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Yang, X.; Liu, Y.; Yang, H.; Yuan, L.; Liu, Y.; Sun, S.; Yang, J. Pseudoginsenoside-F11 Protects against Transient Cerebral Ischemia Injury in Rats Involving Repressing Calcium Overload. Neuroscience 2019, 411, 86–104. [Google Scholar] [CrossRef]
- Zhu, L.; Hou, X.J.; Che, X.H.; Zhou, T.S.; Liu, X.Q.; Wu, C.F.; Yang, J.Y. Pseudoginsenoside-F11 attenuates cognitive dysfunction and tau phosphorylation in sporadic Alzheimer’s disease rat model. Acta Pharmacol. Sin. 2021, 42, 1401–1408. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Liu, C.; Xie, J.; Qiu, S.; Yang, X.; Wu, C. Pseudoginsenoside-F11 alleviates cognitive deficits and Alzheimer’s disease-type pathologies in SAMP8 mice. Pharmacol. Res. 2019, 139, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Verstak, B.; Nagpal, K.; Bottomley, S.P.; Golenbock, D.T.; Hertzog, P.J.; Mansell, A. MyD88 adapter-like (Mal)/TIRAP interaction with TRAF6 is critical for TLR2- and TLR4-mediated NF-kappaB proinflammatory responses. J. Biol. Chem. 2009, 284, 24192–24203. [Google Scholar] [CrossRef]
- Wen, Q.X.; Biao, L.; Xie, X.Y.; Zhou, G.F.; Chen, J.; Song, L.; Xie, S.Q.; Chen, L.; Li, K.Y.; Xiang, X.J.; et al. AP2S1 regulates APP degradation through late endosome-lysosome fusion in cells and APP/PS1 mice. Traffic 2022, 24, 20–33. [Google Scholar] [CrossRef]
- Wu, L.; Jin, Y.; Yin, C.; Bai, L. Co-transformation of Panax major ginsenosides Rb₁ and Rg₁ to minor ginsenosides C-K and F₁ by Cladosporium cladosporioides. J. Ind. Microbiol. Biotechnol. 2012, 39, 521–527. [Google Scholar] [CrossRef]
- Liu, M.Y.; Liu, F.; Gao, Y.L.; Yin, J.N.; Yan, W.Q.; Liu, J.G.; Li, H.J. Pharmacological activities of ginsenoside Rg5 (Review). Exp. Ther. Med. 2021, 22, 840. [Google Scholar] [CrossRef]
- Li, Z.M.; Shao, Z.J.; Qu, D.; Huo, X.H.; Hua, M.; Chen, J.B.; Lu, Y.S.; Sha, J.Y.; Li, S.S.; Sun, Y.S. Transformation Mechanism of Rare Ginsenosides in American Ginseng by Different Processing Methods and Antitumour Effects. Front. Nutr. 2022, 9, 833859. [Google Scholar] [CrossRef]
- Cheng, X.; Song, C.; Du, Y.; Gaur, U.; Yang, M. Pharmacological Treatment of Alzheimer’s Disease: Insights from Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 4621. [Google Scholar] [CrossRef]
- Bouleau, S.; Tricoire, H. Drosophila models of Alzheimer’s disease: Advances, limits, and perspectives. J. Alzheimer’s Dis. 2015, 45, 1015–1038. [Google Scholar] [CrossRef]
- Tsuda, L.; Lim, Y.M. Alzheimer’s Disease Model System Using Drosophila. Adv. Exp. Med. Biol. 2018, 1076, 25–40. [Google Scholar]
- Bell, L.; Whyte, A.; Duysburgh, C.; Marzorati, M.; Van den Abbeele, P.; Le Cozannet, R.; Fança-Berthon, P.; Fromentin, E.; Williams, C. A randomized, placebo-controlled trial investigating the acute and chronic benefits of American Ginseng (Cereboost®) on mood and cognition in healthy young adults, including in vitro investigation of gut microbiota changes as a possible mechanism of action. Eur. J. Nutr. 2022, 61, 413–428. [Google Scholar] [CrossRef]
- Ossoukhova, A.; Owen, L.; Savage, K.; Meyer, M.; Ibarra, A.; Roller, M.; Pipingas, A.; Wesnes, K.; Scholey, A. Improved working memory performance following administration of a single dose of American ginseng (Panax quinquefolius L.) to healthy middle-age adults. Hum. Psychopharmacol. 2015, 30, 108–122. [Google Scholar] [CrossRef]
- Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 2010, 212, 345–356. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, S.T.; Chu, K.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Kim, M. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer’s disease. Nutr. Neurosci. 2012, 15, 278–282. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, S.T.; Chu, K.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Kim, M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008, 15, 865–868. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mukerjee, N.; Maitra, S.; Zehravi, M.; Mukherjee, D.; Ghosh, A.; Massoud, E.E.S.; Rahman, M.H. Therapeutic Potential of Different Natural Products for the Treatment of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 6873874. [Google Scholar] [CrossRef]
- Gao, J.; Inagaki, Y.; Li, X.; Kokudo, N.; Tang, W. Research progress on natural products from traditional Chinese medicine in treatment of Alzheimer’s disease. Drug Discov. Ther. 2013, 7, 46–57. [Google Scholar]
- Li, J.; Sun, M.; Cui, X.; Li, C. Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure-Activity Relationship. Int. J. Mol. Sci. 2022, 23, 10020. [Google Scholar] [CrossRef]
- Adalier, N.; Parker, H. Vitamin E, Turmeric and Saffron in Treatment of Alzheimer’s Disease. Antioxidants 2016, 5, 40. [Google Scholar] [CrossRef]
- Popescu, A.; German, M. Vitamin K2 Holds Promise for Alzheimer’s Prevention and Treatment. Nutrients 2021, 13, 2206. [Google Scholar] [CrossRef]
- Ravi, S.K.; Narasingappa, R.B.; Vincent, B. Neuro-nutrients as anti-alzheimer’s disease agents: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2999–3018. [Google Scholar] [CrossRef]

| Physiological Effects | Type of Ginsenoside | Type of Structure | In Vivo/In Vitro | Cell Lines/Animal Models | Concentration of Ginsenosides Used | Association of Ginsenosides with BBB | Mechanism | References |
|---|---|---|---|---|---|---|---|---|
| Inhibit Aβ accumulation | Ginsenoside Re | Protopanaxatriol type | In vivo and in vitro | N2a/APP695 cell line | Effective dose: 100 μM | Ginsenoside Re can cross the BBB | Regulate amyloid formation pathway; Mediate PPARγ activation and BACE1 inhibition | [118,119] |
| Ginsenoside Rh2 | Protopanaxadiol type | In vivo | Tg2576 mice | 10 mg/kg body weight | - | Decrease cholesterol and lipid raft concentrations | [120] | |
| Ginsenoside Rd | Protopanaxadiol type | In vivo and in vitro | Sprague Dawley (SD) rats (280–300 g)/HT22 hippocampal neuronal cell | 10 mg/kg/10 μM | Ginsenoside Rd is lipophilic and readily passes through biofilms and the BBB | Regulation of α-secretase and β-secretase activities through estrogen receptor α-mediated MAPK/ERK and PI3K/AKT pathways | [121,122] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vivo and in vitro | Hippocampal neurons in 2-day-old SD rates; Establishing an AD model using healthy male SD rats (6–7 weeks, 220 ± 10 g); N2a cell | 1 μM; 10 mg/kg; Effective dose: 2.5 μM | There are different opinions about Ginsenoside passing through BBB, and Ginsenoside Rg1 can also improve nerve damage by reducing BBB permeability | Inhibit PPARγ phosphorylation by downregulating CDK5 expression, thereby affecting the expression of PPARγ target genes (IDE and BACE1) | [119,123,124,125,126,127] | |
| Ginsenoside CK | Protopanaxadiol type | In vivo and in vitro | HT22 mouse hippocampal neuron cell; Scopolamine Hydrobromic acid induced memory impairment ICR mouse model | Low dose: 2.5 μM, medium dose: 5 μM, high dose: 10 μM; Low dose: 20 mg/kg, high dose: 40 mg/kg | Uncertainty that ginsenoside CK crosses the BBB | Regulated energy metabolism signaling pathway and Nrf2/Keap1 signaling pathway | [128,129,130] | |
| Ginsenoside Rg3 | Protopanaxadiol type | In vitro | Use of Microglia isolated from the brain of newborn SD rats; SK-N-SH cell; N2a murine neuroblastoma and HMO6 human microglial cell | 25 μg/kg; 50 μM; 5 μg/mL | Ginsenoside Rg3 does not cross the BBB, but more bioavailable ginsenoside Rg3 nanopreparations can be prepared that can significantly treat AD. | Stimulates MSRA expression as well as increases PI4KIIα activity; Enhance NEP gene expression; Promote acute activation of microglia | [131,132,133,134,135] | |
| Ginsenoside F11 | Ocotillol type | In vitro | Primary rat microglial cell | Effective dose: 100 μM | Ginsenoside F11 reduces BBB damage | Regulate the aberrant expression and distribution of APP | [136,137] | |
| Ginsenoside F1 | Protopanaxatriol type | In vivo and in vitro | APP/PS1 AD model mice; N2a, SH-SY5Y/APP/PS1 AD model mice | 20 mg/kg/d; 2.5 μM/8 mg/kg/d | Present in the brain and blood, can cross the BBB | Increased pCREB and BDNF expression levels; Upregulation of IDE and NEP expression | [126,138] | |
| Ginsenoside Rg5 | Rare ginsenosid of the protopanaxadiol type | In vivo | STZ-induced memory impaired rats | 5, 10 and 20 mg/kg | - | Increased BDNF and insulin-like growth factors 1 (IGF-1) expression | [139] | |
| Inhibit tau hyperphosphorylation | Ginsenoside Rd | Protopanaxadiol type | In vivo and in vitro | APP transgenic mice; Establishing an in vivo tau hyperphosphorylation AD model in rats using okadaic acid (OA)/Cortical neurons were cultured from SD rats | 10 mg/kg; 10 mg/kg/Effective dose: 2.5 and 5 μM | - | Regulate the balance of GSK-3β and PP2A activity, as well as the balance of GSK-3β and CDK5/P25 function in the OB, spinal cord, and telencephalon | [140,141] |
| Ginsenoside F11 | Ocotillol type | In vivo | OA induced AD rat (Six-week-old male SD rats) model | 2, 4, 8 mg/kg | - | Increase PP2A activity, thereby increase methylPP2A protein expression, or directly bind to and activate PP2A | [142] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vivo | Senescence-Accelerated Mice Prone-8 (SAMP8) mice | Fuzheng Quxie Decoction (FQD) low dose (0.7 g/kg/d, extract)/FQD high dose/Rg1 accounts for 9.86% of FQD (3.5 g/kg/d, extract) | - | Regulate the levels of NMDAR/PP2A-related proteins | [119] | |
| Inhibit neuronal apoptosis and protect neurons | Ginsenoside Rb1 | Protopanaxadiol type | In vivo | AD rat (SD) modeling using Aβ1-40; AD rat (SD) modeling using Aβ1-40 | Low dose: 12.5 mg/kg/d, medium dose: 25 mg/kg/d, high dose: 50 mg/kg/d; 10 mg/kg/d | Ginsenoside Rb1 can protect BBB integrity | Increase the expression of Nestin, NSE and GFAP; Downregulate the expression of Bax and Caspase-3, increase the level of Bcl-2 | [105,127,143] |
| Ginsenoside Rd | Protopanaxadiol type | In vitro | PC12 cells | 0.1,1,10,50 and 100 μM | - | Upregulate GAP-43 expression through ERK and ARK-dependent signaling pathway | [144] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vivo | Sixteen-week-old male SAMP1 and SAMP8 mice | 15 mg/kg/d/7.5 mg/kg/d | - | Promote the expression of miR-873-5p in AD | [145] | |
| Ginsenoside F11 | Ocotillol type | In vivo | Ischemic stroke induced by transient middle cerebral artery occlusion (tMCAO) in C57BL/6 mice. | 8, 16, 32 mg/kg | - | Activate the BDNF/TrkB pathway | [146] | |
| Inhibit neurotoxicity | Ginsenoside Rb1 | Protopanaxadiol type | In vivo and in vitro | AD mice (ICR) model using aluminum-induced tau hyperphosphorylation; PC12 cell | 20 mg/kg/d; Effective dose: 50 μM | - | Reduce tau phosphorylation by reducing the level of activated p-GSK3 and increase the level of PP2A; Reduce the accumulation of ROS and lipid peroxidation induced by the enhanced cholesterol efflux | [147,148] |
| Ginsenoside Re | Protopanaxatriol type | In vitro | SH-SY5Y human neuroblastoma cells | Effective dose: 25 μM | - | Inhibite ROS-dependent ASK-1/JNK/BAX apoptosis pathway and activate Nrf2/HO-1 anti-oxidant pathway | [149] | |
| Ginsenoside Rh2 | Protopanaxadiol type | In vitro | Type I rat brain astrocytes (RBA1) cell | Effective dose: 1 μM | - | Induce the expression of PACAP further activate PAC1 | [150] | |
| Ginsenoside Rg2 | Protopanaxatriol type | In vivo and in vitro | PC12 cell; AD rat modeling using Aβ25-35 | 5, 10, and 20 mg/mL; low dose: 25 mg/kg/d, medium dose: 50 mg/kg/d, high dose: 100 mg/kg/d; 10 mg/kg/d | Ginsenoside Rg2 can improve BBB dysfunction | Activate PI3K/Akt signaling pathway | [151,152,153] | |
| Anti-oxidant | Ginsenoside Re | Protopanaxatriol type | In vivo and in vitro | SH-SY5Y cells/Drosophila | 5 μM/0.4 mM | - | Activate PI3K/AKT and ERK pathways | [154] |
| Ginsenoside Rd | Protopanaxatriol type | In vivo | Chronic constraint stress (CRS) induced Cognitive impairment in adult male C57BL/6J mice | 10, 20, 40 mg/kg | - | Upregulate BDNF-mediated CREB signaling pathway in the hippocampus | [155] | |
| Ginsenoside Rh2 | Protopanaxadiol type | In vivo | Mice (ICR) model of trimethyltin intoxication | 20 mg/kg/d | - | Regulate ERK and PI3K/Akt signaling pathways | [156] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vitro | Cortical neurons from C57BL/6 mouse fetuses at embryonic days 15–16 | Effective dose: 2.5, 5, 10 μM | - | Regulate the Wnt/GSK-3β/β-catenin signaling pathway; Inhibite intracellular mitochondrial OS | [117,157] | |
| Ginsenoside Rg3 | Protopanaxadiol type | In vivo and in vitro | D-galactose (D-gal)-induced AD rat Model; Ca2+- and H2O2-induced swelling of mitochondria isolated from rat brains | 20 mg/kg/d; 2–16 μM) | - | Improve mitochondrial dysfunction; Inhibit mitochondrial permeability transition pore opening | [158,159] | |
| Ginsenoside Rg2 | Protopanaxatriol type | In vivo | D-gal induced brain aging model (800 mg/kg for 8 weeks) | 10, 20 mg/kg for 4 weeks | - | Maintain mitochondrial function by increasing mitophagy flux | [160] | |
| Ginsenoside Rk3 | Rare ginsenosid of the protopanaxatriol type | In vivo and in vitro | PC12 cells/APP/PS1 double transgenic mouse model | 10 μM/10 mg/kg | - | Regulating the AMPK-Nrf2 signaling pathway | [161] | |
| Anti-inflammatory | Ginsenoside Rb1 | Protopanaxadiol type | In vivo | AD rat model induced by Aβ1-40 | 12.5 mg/kg/d, 25.0 mg/kg/d and 50.0 mg/kg/d | - | Change the amyloidogenic process of APP to the non-amyloidogenic process | [162] |
| Ginsenoside Re | Protopanaxatriol type | In vitro | Immortalized BV2 murine microglial cell line; ICR mouse primary microglia | 0.5, 1 and 2 μg/mL; 2.5, 5.5 and 7.5 μg/mL | - | Inhibite p-p38, iNOS and COX-2 signaling pathways; Block CAMK/ERK/JNK/NF-κB signaling | [163,164] | |
| Ginsenoside Rh2 | Protopanaxadiol type | In vivo and in vitro | spared nerve injury -induced neuropathic pain mice (ICR) model; Microglia cell | 100 μM; Effective dose: 20 and 50 μM | - | Regulate TGF-β1/Smad pathway and MAPK signaling pathway | [165,166] | |
| Ginsenoside Rd | Protopanaxadiol type | In vivo | CRS induced Cognitive impairment in adult male C57BL/6J mice; APP Tg mice | 10, 20, 40 mg/kg; low dose: 10 mg/kg/d, medium dose: 30 mg/kg/d, high dose: 50 mg/kg/d | - | Upregulate BDNF-mediated CREB signaling pathway in the hippocampus; Inhibite activation of the NF-κB pathway | [155,167] | |
| Ginsenoside CK | Protopanaxadiol type | In vitro | Microglial Cell (BV2) | 25, 50, 75 μM | Regulate the expression of LRP1 to activate the NF-κB pathway; Inhibite the activities of ROS, MAPKs, and NF-κB/AP-1, enhance the CREB and Nrf2/HO-1 signaling axis | [168,169] | ||
| Ginsenoside Rg3 | Protopanaxadiol type | In vivo and in vitro | SK-N-SH cell; N2a murine neuroblastoma and HMO6 human micro-glial cell; LPS induced learning and memory impairment and inflammation in rats; Microglial cell line (BV2) | 50 mm; 5 μg/mL; 20,50 and 21 mg/kg; 10 µg/kg | - | Inhibite microglial activation | [134,135,170,171] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vivo and in vitro | Wild-type (WT) and APP/PS1 AD mice; HT22 cell line; Primary hippocampal neurons | 10 mg/kg; 1, 5 and 10 µM; 5, 10 µM | - | Inhibite the activation of NOX2-NLRP1 inflammasome and NOX2-mediated ROS production | [172,173,174] | |
| Ginsenoside F11 | Ocotillol type | In vivo and in vitro | The murine microglia cell line N9/Thirty-six male C57BL/6 mice | 100 μM/8 mg/kg | Inhibite TLR4-mediated TAK1/IKK/NF-κB, MAPKs and Akt signaling pathways | [175] | ||
| Ginsenoside Rg5 | Rare ginsenosid of the protopanaxadiol type | In vivo and in vitro | The immortalized murine BV2 microglial cell line; STZ-induced memory impaired rats | 10–50 μM/5, 10 and 20 mg/kg | - | Regulation of MAPK and PI3K/Akt signaling pathways, inhibition of downstream transcription factors NF-κB and AP-1 exert anti-inflammatory effects to control microglia activation and exert anti-AD effects | [139,176] | |
| Ginsenoside Rh4 | Rare ginsenosid of the protopanaxatriol type | In vivo and in vitro | Microglia cell line BV-2/APP/PS1 double transgenic mice | 50 μM/20 mg/kg | Suppressing the release of inflammatory factors and the expression of apoptosis-associated speck-like protein and caspase-1 to inhibit the formation and aggregation of NLRP3 and exert anti-inflammatory effects | [177] | ||
| Reduce insulin resistance | Ginsenoside Rb1 | Protopanaxadiol type | In vivo | STZ induced high glucose model in C57BL/6N mice (150 mg/kg) | 30 mg/kg | - | Stimulate the expression of NMDAR1 and IDE by inhibiting the activity of CDK5/p35 | [178] |
| Increase production of ACh | Ginsenoside Re | Protopanaxatriol type | In vitro | N2a mouse neuroblastoma cell | Effective dose: 5 μg/mL | - | Enhance the expression of ChAT and VAChT | [179] |
| Ginsenoside Rd | Protopanaxadiol type | In vitro | N2a mouse neuroblastoma cell | Effective dose: 5 μg/mL | - | ChAT/VAChT gene-mediated ACh production | [179] | |
| Ginsenoside Rg1 | Protopanaxatriol type | In vivo | β Amyloid protein model rats (adult male SD rats) | 40 mg/kg | - | Penetrate the BBB to reach the target | [180] | |
| Ginsenoside F2 | Protopanaxadiol type | In vitro | In vitro AChE inhibition assay | 25 μg/mL | Inhibition of AChE activity | [181] | ||
| Ginsenoside Rg5 | Rare ginsenosid of the protopanaxadiol type | In vivo | STZ-induced memory impaired rats | 5, 10 and 20 mg/kg | - | Significantly reduce AChE activity | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, M.; Bai, Y.; Fang, X.; Lan, X.; Zhang, Y.; Cao, Y.; Zhu, D.; Luo, H. American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules 2023, 28, 5716. https://doi.org/10.3390/molecules28155716
Shan M, Bai Y, Fang X, Lan X, Zhang Y, Cao Y, Zhu D, Luo H. American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules. 2023; 28(15):5716. https://doi.org/10.3390/molecules28155716
Chicago/Turabian StyleShan, Mengyao, Yunfan Bai, Xiaoxue Fang, Xintian Lan, Yegang Zhang, Yiming Cao, Difu Zhu, and Haoming Luo. 2023. "American Ginseng for the Treatment of Alzheimer’s Disease: A Review" Molecules 28, no. 15: 5716. https://doi.org/10.3390/molecules28155716
APA StyleShan, M., Bai, Y., Fang, X., Lan, X., Zhang, Y., Cao, Y., Zhu, D., & Luo, H. (2023). American Ginseng for the Treatment of Alzheimer’s Disease: A Review. Molecules, 28(15), 5716. https://doi.org/10.3390/molecules28155716





