The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone
Abstract
1. Introduction
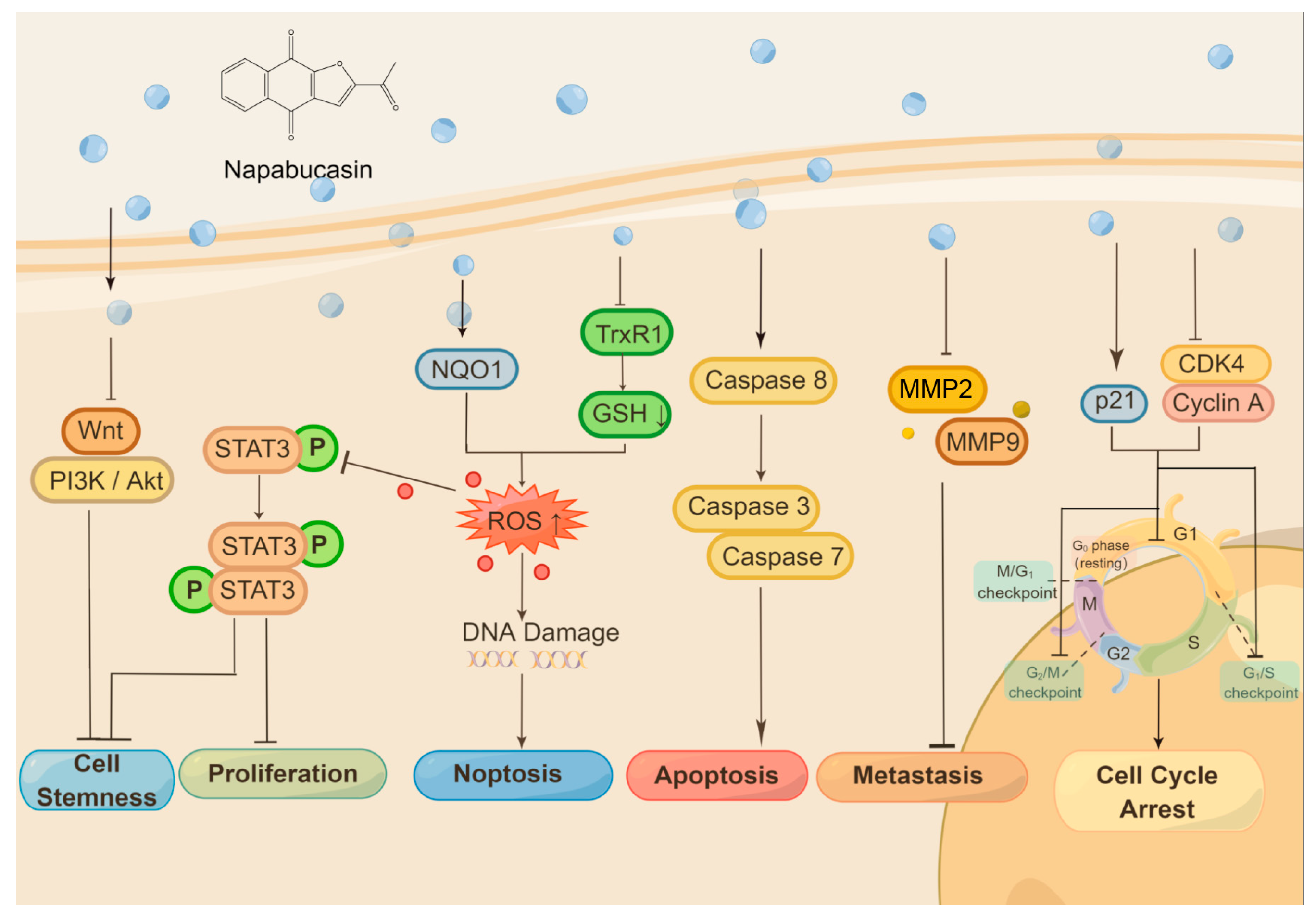
2. Anticancer Activity of Napabucasin
2.1. Cell Proliferation Inhibition and Cell Death Induction
| Cell | Pathology | Treatment | Result | Ref. |
|---|---|---|---|---|
| HuCCt-1, NOZ | Intrahepatic cholangiocarcinoma | 2 μM for 24 h | Suppresses cancer stemness | [14] |
| BxPC-3 | Pancreatic adenocarcinoma | 2.5 μM for 1 h | Resensitizaion of cells to radiation therapy (RT) and chemoradiotherapy (CRT) | [15] |
| U87MG, LN229 | Glioblastoma | 5 μM for 48 h | Induces cell cycle arrest, cell apoptosis; inhibits STAT3, cell migration, and invasion | [16] |
| 143B, MG63, U2OS, KHOS | Osteosarcoma | 5 μM for 72 h | Induces cell apoptosis; inhibits STAT3 | [11] |
| HCT116, HT29 | Colorectal carcinoma | 1 μM for 36 h | Induces ROS generation, DNA damage, suppresses angiogenesis | [17] |
| Huh7, HepG2, murine Hepa1-6 cells, HepG2.2.15 | Hepatoma | 2 μM for 48 h | Induces cell apoptosis, cell cycle arrest; suppresses cancer stemness | [9] |
| H460, H1299, SKMES-1 | Lung carcinoma | 1 μM for 72 h | Induces cell apoptosis; suppresses cancer stemness; resensates cisplatin resistance cells | [12] |
| PC-3, 22RV1 | Prostatic carcinoma | 1 μM for 2 to 5 days | Induces cell apoptosis, cell cycle arrest; suppresses cancer stemness | [18] |
| AML | Acute myeloid leukemia | Designed dose for 24 h | Induces cell cycle arrest, DNA damage, cell apoptosis; inhibits STAT3 | [19] |
| A2780, SKOV3, ID8 | Ovarian carcinoma | 2 μM for 12 h | Induces apoptosis; resensitization of cell to tamoxifen or paclitaxel; inhibits STAT3, cell migration, and invasion | [20] |
| FaDu | Pharyngeal squamous cell carcinoma | 2 μM for 24 h | Suppresses cancer stemness | [21] |
| DLD1, HCT116 | Colorectal carcinoma | 2 μM for 24 h | Blocked cell survival and self-renewal | [21] |
| Melanoma (ret melanoma cell) | Melanoma | 1 μM for 72 h | Inhibits cell proliferation | [22] |
2.2. Disruption of Cell Cycle
2.3. Suppress Metastasis and Improve Drug Resistance
2.4. Inhibition on Cancer Stemness
2.5. In Vivo Evidence
2.6. Molecular Targets
3. Clinical Trials of Napabucasin
4. Side Effects and Toxicity of Napabucasin
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Maomao, C.; He, L.; Dianqin, S.; Siyi, H.; Xinxin, Y.; Fan, Y.; Shaoli, Z.; Changfa, X.; Lin, L.; Ji, P.; et al. Current cancer burden in China: Epidemiology, etiology, and prevention. Cancer Biol. Med. 2022, 19, 1121–1138. [Google Scholar]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Recent advancements in immunotherapy of melanoma using nanotechnology-based strategies. Biomed. Pharmacother. 2023, 159, 114243. [Google Scholar] [CrossRef]
- Sun, W.; Bao, J.; Lin, W.; Gao, H.; Zhao, W.; Zhang, Q.; Leung, C.H.; Ma, D.L.; Lu, J.; Chen, X. 2-Methoxy-6-acetyl-7-methyljuglone (MAM), a natural naphthoquinone, induces NO-dependent apoptosis and necroptosis by H2O2-dependent JNK activation in cancer cells. Free Radic. Biol. Med. 2016, 92, 61–77. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.-G.; Chen, T. Antibiotics for cancer treatment: A double-edged sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Petsri, K.; Thongsom, S.; Racha, S.; Chamni, S.; Jindapol, S.; Kaekratoke, N.; Zou, H.; Chanvorachote, P. Novel mechanism of napabucasin, a naturally derived furanonaphthoquinone: Apoptosis and autophagy induction in lung cancer cells through direct targeting on Akt/mTOR proteins. BMC Complement. Med. Ther. 2022, 22, 1–20. [Google Scholar]
- Shih, P.C. The role of the STAT3 signaling transduction pathways in radioresistance. Pharmacol. Ther. 2022, 234, 108118. [Google Scholar] [CrossRef]
- Froeling, F.E.; Swamynathan, M.M.; Deschênes, A.; Chio, I.I.C.; Brosnan, E.; Yao, M.A.; Alagesan, P.; Lucito, M.; Li, J.; Chang, A.-Y.; et al. Bioactivation of Napabucasin Triggers Reactive Oxygen Species–Mediated Cancer Cell Death. Clin. Cancer Res. 2019, 25, 7162–7174. [Google Scholar] [CrossRef]
- Li, Y.; Han, Q.; Zhao, H.; Guo, Q.; Zhang, J. Napabucasin Reduces Cancer Stem Cell Characteristics in Hepatocellular Carcinoma. Front. Pharmacol. 2020, 11, 597520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Y.; Wei, X. Napabucasin, a novel inhibitor of STAT3, inhibits growth and synergises with doxorubicin in diffuse large B-cell lymphoma. Cancer Lett. 2020, 491, 146–161. [Google Scholar] [CrossRef]
- Zuo, D.; Shogren, K.L.; Zang, J.; Jewison, D.E.; Waletzki, B.E.; MillerI, A.L., 2nd; Okuno, S.H.; Cai, Z.; Yaszemski, M.J.; Maran, A. Inhibition of STAT3 blocks protein synthesis and tumor metastasis in osteosarcoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 244. [Google Scholar] [CrossRef]
- MacDonagh, L.; Gray, S.; Breen, E.; Cuffe, S.; Finn, S.P.; O’Byrne, K.J.; Barr, M.P. BBI608 inhibits cancer stemness and reverses cisplatin resistance in NSCLC. Cancer Lett. 2018, 428, 117–126. [Google Scholar] [CrossRef]
- Li, J.M.; Hsu, P.C.; Kuan, F.C.; Shi, C.S.; Yang, C.T. The cancer stemness inhibitor napabucasin suppresses small cell lung cancer growth through SOX2 expression. Am. J. Cancer Res. 2022, 12, 4637–4651. [Google Scholar] [PubMed]
- Beyreis, M.; Gaisberger, M.; Jakab, M.; Neureiter, D.; Helm, K.; Ritter, M.; Kiesslich, T.; Mayr, C. The Cancer Stem Cell Inhibitor Napabucasin (BBI608) Shows General Cytotoxicity in Biliary Tract Cancer Cells and Reduces Cancer Stem Cell Characteristics. Cancers 2019, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Flebbe, H.; Spitzner, M.; Marquet, P.E.; Gaedcke, J.; Ghadimi, B.M.; Rieken, S.; Schneider, G.; Koenig, A.O.; Grade, M. Targeting STAT3 Signaling Facilitates Responsiveness of Pancreatic Cancer Cells to Chemoradiotherapy. Cancers 2022, 14, 1301. [Google Scholar] [CrossRef]
- Han, D.; Yu, T.; Dong, N.; Wang, B.; Sun, F.; Jiang, D. Napabucasin, a novel STAT3 inhibitor suppresses proliferation, invasion and stemness of glioblastoma cells. J. Exp. Clin. Cancer Res. 2019, 38, 289. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Farran, B.; Farren, M.; Chalikonda, G.; Wu, C.; Lesinski, G.B.; El-Rayes, B.F. Napabucasin (BBI 608), a potent chemoradiosensitizer in rectal cancer. Cancer 2020, 126, 3360–3371. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Z.; Zhou, H.; Ou, X.; Xu, Y.; Li, H.; Liu, C.; Li, B. Suppression of prostate cancer progression by cancer cell stemness inhibitor napabucasin. Cancer Med. 2016, 5, 1251–1258. [Google Scholar] [CrossRef]
- Bi, S.; Chen, K.; Feng, L.; Fu, G.; Yang, Q.; Deng, M.; Zhao, H.; Li, Z.; Yu, L.; Fang, Z.; et al. Napabucasin (BBI608) eliminate AML cells in vitro and in vivo via inhibition of Stat3 pathway and induction of DNA damage. Eur. J. Pharmacol. 2019, 855, 252–261. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; Lleonart, M. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Matsui, W.H. Cancer stem cell signaling pathways. Medicine 2016, 95 (Suppl. S1), S8–S19. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Soleimanjahi, H.; Soleimani, M.; Arefian, E. The synergistic anticancer effects of ReoT3D, CPT-11, and BBI608 on murine colorectal cancer cells. Daru 2020, 28, 555–565. [Google Scholar] [CrossRef]
- Guo, G.; Gao, Z.; Tong, M.; Zhan, D.; Wang, G.; Wang, Y.; Qin, J. NQO1 is a determinant for cellular sensitivity to anti-tumor agent Napabucasin. Am. J. Cancer Res. 2020, 10, 1442–1454. [Google Scholar] [PubMed]
- Li, H.; Qian, Y.; Wang, X.; Pi, R.; Zhao, X.; Wei, X. Targeted activation of Stat3 in combination with paclitaxel results in increased apoptosis in epithelial ovarian cancer cells and a reduced tumour burden. Cell Prolif. 2019, 53, e12719. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Xu, D.; Tiek, D.; Goenka, A.; Wu, B.; Sastry, N.; Hu, B.; Cheng, S.Y. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 2020, 10, 8721–8743. [Google Scholar] [CrossRef]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Grothey, A. Napabucasin: An Update on the First-in-Class Cancer Stemness Inhibitor. Drugs 2017, 77, 1091–1103. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.; Xie, Y.; Zhou, Y.; Wang, R.; Lou, J. Napabucasin Attenuates Resistance of Breast Cancer Cells to Tamoxifen by Reducing Stem Cell-Like Properties. Experiment 2019, 25, 8905–8912. [Google Scholar] [CrossRef]
- Bitsch, R.; Kurzay, A.; Kurt, F.; De La Torre, C.; Lasser, S.; Lepper, A.; Siebenmorgen, A.; Müller, V.; Altevogt, P.; Utikal, J.; et al. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J. Immunother. Cancer 2022, 10, e004384. [Google Scholar] [CrossRef]
- Hitron, M.; Stephenson, J.; Chi, K.N.; Edenfield, W.J.; Leggett, D.; Li, Y.; Li, W.; Gada, K.; Li, C. A phase 1b study of the cancer stem cell inhibitor BBI608 administered with paclitaxel in patients with advanced malignancies. J. Clin. Oncol. 2014, 32, 2530. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Shahda, S.; Starodub, A.; O’Neil, B.H.; Hanna, W.T.; Shaib, W.L.; Oh, C.; Li, W.; Li, Y.; Borodyansky, L.; et al. A phase Ib extension study of cancer stemness inhibitor BB608 (napabucasin) in combination with gemcitabine and nab-paclitaxel (nab-PTX) in patients (pts) with metastatic pancreatic cancer. J. Clin. Oncol. 2016, 34, 4128. [Google Scholar] [CrossRef]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, Y.; Lv, H.; Li, J.; Wang, Z.; Wang, K.; Wang, L.; Li, Y.; Jiang, H.; Zhang, Y. Rapamycin targets STAT3 and impacts c-Myc to suppress tumor growth. Cell Chem. Biol. 2021, 29, 373–385.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Q.; Ye, Y.; Li, W.; Qiu, J.; Liu, J.; Zhan, R.; Chen, W.; Yu, Q. Angoline: A selective IL-6/STAT3 signaling pathway inhibitor isolated from Zanthoxylum nitidum. Phytomedicine 2014, 21, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, D.; Ma, K.; Wu, X.; Hao, H.; Jiang, S. NAD(P)H:Quinone Oxidoreductase 1 (NQO1) as a Therapeutic and Diagnostic Target in Cancer. J. Med. Chem. 2018, 61, 6983–7003. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, B.; Jin, L.; Hou, Y.; Ai, N.; Ge, W.; Li, L.; Liu, S.; Lu, J.; Chen, X. 2-Methoxy-6-acetyl-7-methyljuglone (MAM) induced programmed necrosis in glioblastoma by targeting NAD(P)H: Quinone oxidoreductase 1 (NQO1). Free. Radic. Biol. Med. 2020, 152, 336–347. [Google Scholar] [CrossRef]
- Silvers, M.A.; Deja, S.; Singh, N.; Egnatchik, R.A.; Sudderth, J.; Luo, X.; Beg, M.S.; Burgess, S.C.; DeBerardinis, R.J.; Boothman, D.A.; et al. The NQO1 bioactivatable drug, β-lapachone, alters the redox state of NQO1+ pancreatic cancer cells, causing perturbation in central carbon metabolism. J. Biol. Chem. 2017, 292, 18203–18216. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, B.; Xiao, Q.; Du, L.; Hou, Y.; Sun, H.-S.; Lu, J.-J.; Chen, X. Induction of programmed necrosis: A novel anti-cancer strategy for natural compounds. Pharmacol. Ther. 2020, 214, 107593. [Google Scholar] [CrossRef]
- Busker, S.; Page, B.; Arnér, E.S. To inhibit TrxR1 is to inactivate STAT3–Inhibition of TrxR1 enzymatic function by STAT3 small molecule inhibitors. Redox Biol. 2020, 36, 101646. [Google Scholar] [CrossRef]
- Leijten, N.M.; Bakker, P.; Spaink, H.P.; den Hertog, J.; Lemeer, S. Thermal Proteome Profiling in Zebrafish Reveals Effects of Napabucasin on Retinoic Acid Metabolism. Mol. Cell Proteomics 2021, 20, 100033. [Google Scholar] [CrossRef]
- Langleben, A.; Supko, J.G.; Hotte, S.J.; Batist, G.; Hirte, H.W.; Li, H.R.; Li, W.; Kerstein, D.; Leggett, D.; Hitron, M.J.; et al. A dose-escalation phase I study of a first-in-class cancer stemness inhibitor in patients with advanced malignancies. J. Clin. Oncol. 2013, 31, 2542. [Google Scholar] [CrossRef]
- Shah, M.A.; Yoshino, T.; Tebbutt, N.C.; Grothey, A.; Tabernero, J.; Xu, R.H.; Cervantes, A.; Oh, S.C.; Yamaguchi, K.; Fakih, M.; et al. Napabucasin Plus FOLFIRI in Patients with Previously Treated Metastatic Colorectal Cancer: Results from the Open-Label, Randomized Phase III CanStem303C Study. Clin. Colorectal Cancer 2022, 22, 100–110. [Google Scholar] [CrossRef]
- Mason, W.P.; de Robles, P.; Borodyansky, L.; Hitron, M.; Ortuzar, W.F.; Khan, W.; Xu, B.; Li, W.; Li, Y.; Li, C.J. BBI608-201GBM: A phase Ib/II clinical study of napabucasin (BBI608) in combination with temozolomide (TMZ) for adult patients with recurrent glioblastoma (GBM). J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Grothey, A.; Shah, M.A.; Yoshino, T.; Van Cutsem, E.; Taieb, J.; Xu, R.; Tebbutt, N.C.; Falcone, A.; Cervantes, A.; Borodyansky, L.; et al. CanStem303C trial: A phase III study of napabucasin (BBI-608) in combination with 5-fluorouracil (5-FU), leucovorin, irinotecan (FOLFIRI) in adult patients with previously treated metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Becerra, C.; Stephenson, J.; Jonker, D.J.; Cohn, A.L.; Asmis, T.R.; Bekaii-Saab, T.S.; Conkling, P.R.; Garbo, L.E.; Lenz, H.-J.; Richards, D.A.; et al. Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J. Clin. Oncol. 2015, 33, 4069. [Google Scholar] [CrossRef]
- Kawazoe, A.; Kuboki, Y.; Bando, H.; Fukuoka, S.; Kojima, T.; Naito, Y.; Iino, S.; Yodo, Y.; Doi, T.; Shitara, K.; et al. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2020, 85, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., 3rd; Ajani, J.A.; Catalano, R.B.; Engelking, C.; Kornblau, S.M.; Martenson JAJr McCallum, R.; Mitchell, E.P.; O’Dorisio, T.M.; Vokes, E.E.; Wadler, S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004, 22, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, S.; Qu, R.; Yang, Y.; Gong, X.; Hong, Y.; Jin, A.; Huang, X.; Dai, Q.; Jiang, L. Icariin prevents oestrogen deficiency–induced alveolar bone loss through promoting osteogenesis via STAT3. Cell Prolif. 2020, 53, e12743. [Google Scholar] [CrossRef]
- Huang, X.; Jin, A.; Wang, X.; Gao, X.; Xu, H.; Chung, M.; Dai, Q.; Yang, Y.; Jiang, L. Napabucasin Induces Mouse Bone Loss by Impairing Bone Formation via STAT3. Front. Cell Dev. Biol. 2021, 9, 648866. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Newnum, A.B.; Martin, J.R.; Li, P.; Nelson, M.T.; Moh, A.; Fu, X.-Y.; Yokota, H.; Li, J. Osteoblast/osteocyte-specific inactivation of Stat3 decreases load-driven bone formation and accumulates reactive oxygen species. Bone 2011, 49, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Thulin, M.H.; Määttä, J.; Linder, A.; Sterbova, S.; Ohlsson, C.; Damber, J.; Widmark, A.; Persson, E. Inhibition of STAT3 prevents bone metastatic progression of prostate cancer in vivo. Prostate 2021, 81, 452–462. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S.-W.; Choi, J. Drug discovery inspired by bioactive small molecules from nature. Anim. Cells Syst. 2022, 26, 254–265. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar]
- Lewis, A.M.; Ough, M.; Hinkhouse, M.M.; Tsao, M.-S.; Oberley, L.W.; Cullen, J.J. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol. Carcinog. 2005, 43, 215–224. [Google Scholar] [CrossRef]
- Quatannens, D.; Verhoeven, Y.; Van Dam, P.; Lardon, F.; Prenen, H.; Roeyen, G.; Peeters, M.; Smits, E.L.; Van Audenaerde, J. Targeting hedgehog signaling in pancreatic ductal adenocarcinoma. Pharmacol. Ther. 2022, 236, 108107. [Google Scholar] [CrossRef]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’malley, B.W. Drug Combination in Cancer Treatment—From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef] [PubMed]
| Model | Treatment | Result | Ref. |
|---|---|---|---|
| U87MG intracranially xenotransplanted in nude mice | 40 mg/kg, i.p. every other day for 2 months | Suppresses tumor growth with prolonged survival rate than vehicle | [16] |
| 143B injected into the right tibial medullary cavity of BABL/c nude mice | 10 or 20 mg/kg, i.p. every 3 days within eight injections | Decreases tumor volume; inhibits osteosarcoma lung metastasis | [11] |
| SUDHL-6 subcutaneously injected into the flank of NOD/SCID mice | 50 mg/kg, p.o. daily; doxorubicin 3 mg/kg, i.v, once weekly; combination treatment for 15 days | Suppresses tumor growth without obvious organ injury | [10] |
| Hepa1-6 subcutaneously injected into the left axilla of C57BL/6J male mice | 20 mg/kg, intraperitoneal injections every 2 days within eight injections | Decreases tumor volume; increases proportion of apoptotic tumor cells | [9] |
| PC-3 or 22RV1 subcutaneously injected into dorsal flank in nude mouse | 40 mg/kg, i.p. every 3 days | Suppresses tumor growth; inhibits spherogenesis | [18] |
| Molm-13 injected into NOD-Prkdc−/−IL2rg−/− (NPI) mice after one Gy irradiation | 60 mg/kg, p.o. for 2 weeks | Decreases tumor volume | [19] |
| Model A: SKOV3 subcutaneously injected into BALB/c mice; Model B: ID8 intraperitoneally inoculated into C57BL/6 mice | Model A: 40 mg/kg, i.g. daily for 21 days; Model B: 40 mg/kg, p.o. for 6 days; paclitaxel 12 mg/kg, i.p. once; combination treatment daily for 6 days | Decreases tumor volume and weight without noticeable ascites and toxicity Inhibits pSTAT3-positive cells, STAT3 activity, and Ki67 expression | [20] |
| Model A: PaCa-2 inoculated s.c. in athymic nude mice Model B: HT29 injected under spleen capsule of nude mice | Model A: 20 mg/kg, i.p. every 3 days for 4 weeks; Model B: 20 mg/kg, i.p. 5 days per week for 4 weeks | Inhibits cancer relapse, metastasis, and stemness Suppresses tumor growth | [21] |
| CT26 subcutaneously injected into the right flanks of BALB/c mice | 50 mg/kg of derivatives 8q, p.o. daily for 4 weeks | Suppresses tumor growth; decreases tumor volume | [34] |
| MDA-MB-231 subcutaneously injected into BALB/c nude mice | 5 or 10 mg/kg once daily of derivatives A11, i.p., for 21 days | Decreases tumor volume and weight; inhibits p-STAT3 | [35] |
| Inhibitor | Origin | Treatment of Cancer |
|---|---|---|
| Curcumin | Curcuma longa L. (turmeric) | Human prostate cancer (LNCa, C4-2B), human breast cancer (MDA-MB-231), colon carcinoma (C-26), human ovarian adenocarcinoma (SKV3), etc. |
| Cucurbitacin II | Cucumis sativus (cucumber) and Cucumis melo L. (melon), etc. | Human breast cancer (MDA-MB-231), liver cancer (HepG2), human lung cancer (A549), etc. |
| Honokiol | Officinalis, obovata and grandiflora | Human lung cancer (A549), liver cancer (HepG2), human skin cancer, etc. |
| Guggulsterone | Commiphora mukul | Pancreatic cancer, hepatocellular carcinoma, head and neck squamous cell carcinoma, cholangiocarcinoma, etc. |
| Resveratrol | Grapeskin, blueberries, raspberries, mulberries, and peanuts | Human breast cancer (MDA-MB-231, MDA-MB-453, MDA-MB-468), pancreatic cancer (Panc-1, Colo-357), prostate cancer (LNCaP, DU145), etc. |
| Berbamine | Berberis amurensis | Hepatocellular carcinoma, pancreatic cancer |
| Flavopiridol | Aphanamixis polystachya | Human chronic lymphocytic leukemia cells, human squamous cell, prostate carcinoma, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Z.; Wang, H.; Ren, H.; Sun, Y.; Chen, X. The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone. Molecules 2023, 28, 5678. https://doi.org/10.3390/molecules28155678
Shao Z, Wang H, Ren H, Sun Y, Chen X. The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone. Molecules. 2023; 28(15):5678. https://doi.org/10.3390/molecules28155678
Chicago/Turabian StyleShao, Zeyang, Heng Wang, Haiyan Ren, Yinxiang Sun, and Xiuping Chen. 2023. "The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone" Molecules 28, no. 15: 5678. https://doi.org/10.3390/molecules28155678
APA StyleShao, Z., Wang, H., Ren, H., Sun, Y., & Chen, X. (2023). The Anticancer Effect of Napabucasin (BBI608), a Natural Naphthoquinone. Molecules, 28(15), 5678. https://doi.org/10.3390/molecules28155678







