One Material-Opposite Triboelectrification: Molecular Engineering Regulated Triboelectrification on Silica Surface to Enhance TENG Efficiency
Abstract
1. Introduction
2. Results and Discussions
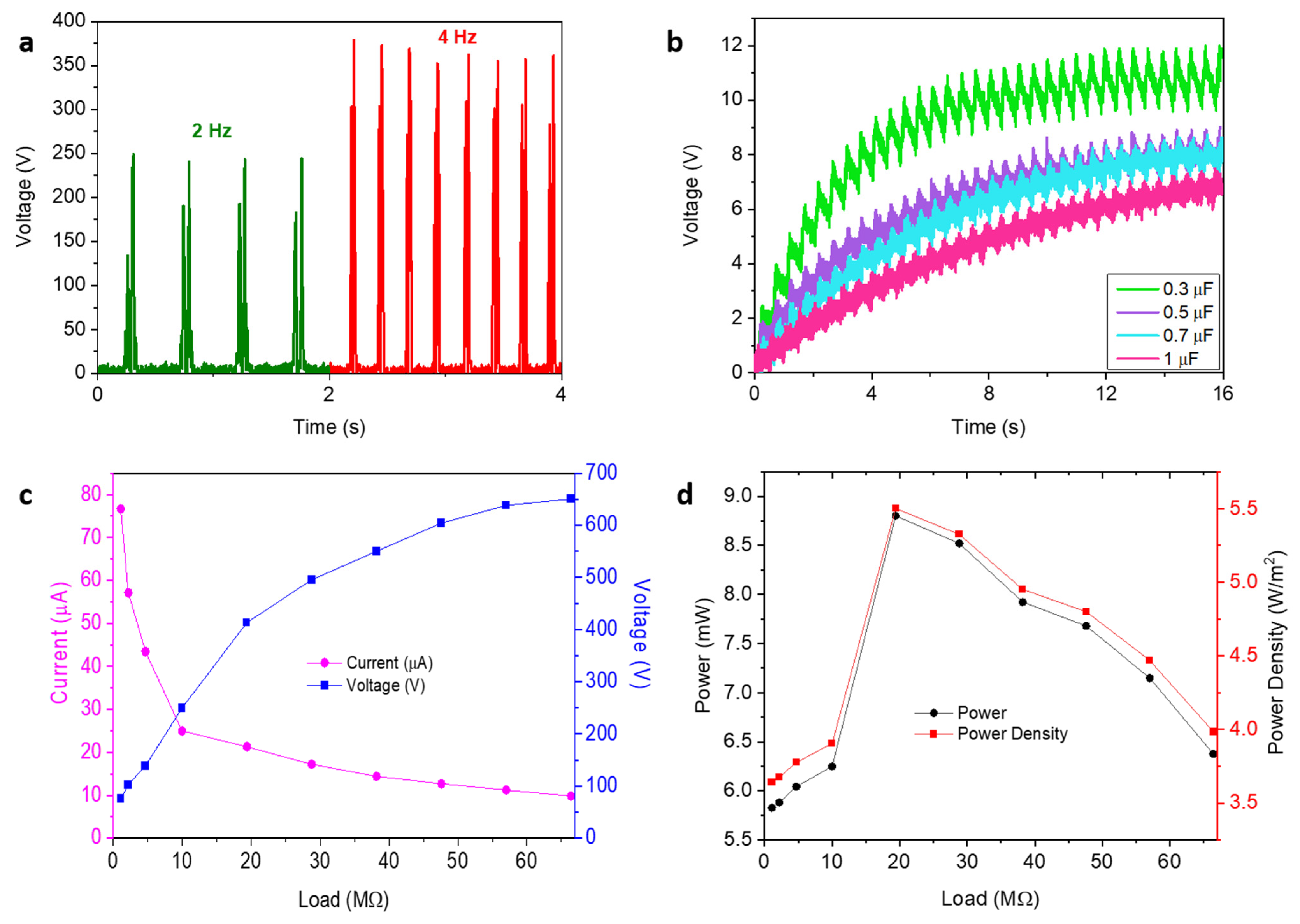
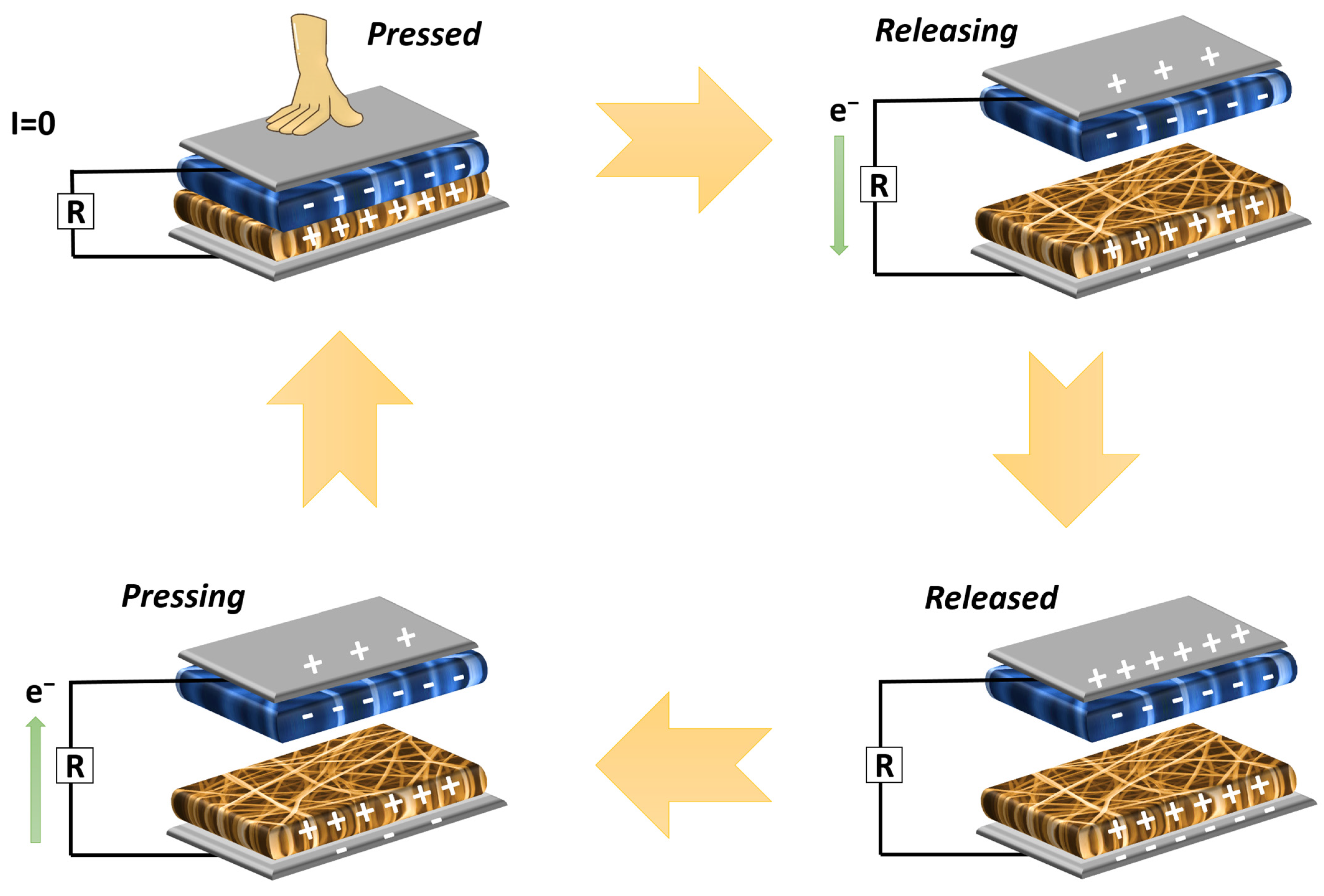
TENG Performance
3. Materials and Methods
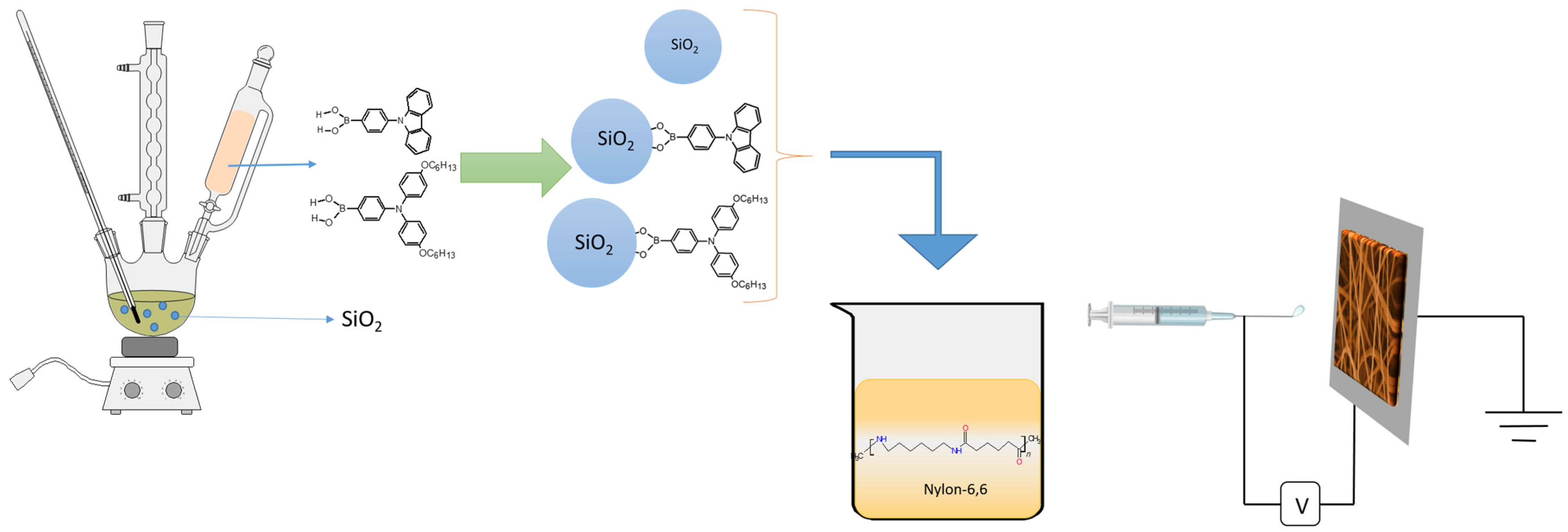
3.1. Materials
3.2. Synthesis of Hybrid Nanoparticles
3.3. Electrospinning Process
3.4. Device Fabrication
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Schoenefeld, J.J. The European Green Deal: What prospects for governing climate change with policy monitoring? Politics Gov. 2021, 9, 370–379. [Google Scholar]
- Zhu, G.; Peng, B.; Chen, J.; Jing, Q.; Wang, Z.L. Triboelectric nanogenerators as a new energy technology: From fundamentals, devices, to applications. Nano Energy 2015, 14, 126–138. [Google Scholar] [CrossRef]
- Walden, R.; Kumar, C.; Mulvihill, D.M.; Pillai, S.C. Opportunities and challenges in triboelectric nanogenerator (TENG) based sustainable energy generation technologies: A mini-review. Chem. Eng. J. Adv. 2022, 9, 100237. [Google Scholar]
- Morone, J.; Ivins, R. Problems and opportunities in technology transfer from the national laboratories to industry. Res. Manag. 1982, 25, 35–44. [Google Scholar] [CrossRef]
- Yiğit Arkan, M.Z.; Kınas, Z.; Arkan, E.; Gökçe, C.O.; Kara, K.; Karabiber, A.; Kuş, M.; Özel, F. Molecular engineering-device efficiency relation: Performance boosting of triboelectric nanogenerator through doping of small molecules. Int. J. Energy Res. 2022, 46, 23517–23529. [Google Scholar] [CrossRef]
- Wu, H.S.; Wei, S.M.; Chen, S.W.; Pan, H.C.; Pan, W.P.; Huang, S.M.; Tsai, M.L.; Yang, P.K. Metal-Free Perovskite Piezoelectric Nanogenerators for Human–Machine Interfaces and Self-Powered Electrical Stimulation Applications. Adv. Sci. 2022, 9, 2105974. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Lang, X.; Singh, R.; Marques, C.; Zhang, B.; Kumar, S. Novel Optical Fiber-Based Structures for Plasmonics Sensors. Biosensors 2022, 12, 1016. [Google Scholar]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Arkan, E.; Karabiber, A.; Topçu, M.A.; Kinas, Z.; Sarilmaz, A.; Ozel, S.S.; Ozel, F. Nano-ceria based TENGs: Effect of dopant structure on energy harvesting performance. Surf. Interfaces 2023, 37, 102683. [Google Scholar] [CrossRef]
- Zhou, Y.; Deng, W.; Xu, J.; Chen, J. Engineering materials at the nanoscale for triboelectric nanogenerators. Cell Rep. Phys. Sci. 2020, 1, 100142. [Google Scholar]
- Liu, Y.; Liu, W.; Wang, Z.; He, W.; Tang, Q.; Xi, Y.; Wang, X.; Guo, H.; Hu, C. Quantifying contact status and the air-breakdown model of charge-excitation triboelectric nanogenerators to maximize charge density. Nat. Commun. 2020, 11, 1599. [Google Scholar] [PubMed]
- Lone, S.A.; Lim, K.C.; Kaswan, K.; Chatterjee, S.; Fan, K.-P.; Choi, D.; Lee, S.; Zhang, H.; Cheng, J.; Lin, Z.-H. Recent advancements for improving the performance of triboelectric nanogenerator devices. Nano Energy 2022, 99, 107318. [Google Scholar]
- Chen, J.; Guo, H.; He, X.; Liu, G.; Xi, Y.; Shi, H.; Hu, C. Enhancing performance of triboelectric nanogenerator by filling high dielectric nanoparticles into sponge PDMS film. ACS Appl. Mater. Interfaces 2016, 8, 736–744. [Google Scholar] [PubMed]
- Nurmakanov, Y.; Kalimuldina, G.; Nauryzbayev, G.; Adair, D.; Bakenov, Z. Structural and chemical modifications towards high-performance of triboelectric nanogenerators. Nanoscale Res. Lett. 2021, 16, 122. [Google Scholar]
- Saqib, Q.; Chougale, M.; Khan, M.; Shaukat, R.; Kim, J.; Bhat, K.; Bae, J. Triboelectric nanogenerator based on lignocellulosic waste fruit shell tribopositive material: Comparative analysis. Mater. Today Sustain. 2022, 18, 100146. [Google Scholar]
- Kim, W.; Park, J.H.; Hwang, H.J.; Rim, Y.S.; Choi, D. Interfacial molecular engineering for enhanced polarization of negative tribo-materials. Nano Energy 2022, 96, 107110. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, Y.D. Tuning electrical properties of 2d materials by self-assembled monolayers. Adv. Mater. Interfaces 2018, 5, 1700316. [Google Scholar] [CrossRef]
- Arkan, E.; Unal, M.; Yalcin, E.; Arkan, M.Z.Y.; Yurtdas, S.; Can, M.; Tozlu, C.; Demic, S. Influence of end groups variation of self assembled monolayers on performance of planar perovskite solar cells by interface regulation. Mater. Sci. Semicond. Process. 2021, 123, 105514. [Google Scholar]
- Yalcin, E.; Can, M.; Rodriguez-Seco, C.; Aktas, E.; Pudi, R.; Cambarau, W.; Demic, S.; Palomares, E. Semiconductor self-assembled monolayers as selective contacts for efficient PiN perovskite solar cells. Energy Environ. Sci. 2019, 12, 230–237. [Google Scholar] [CrossRef]
- Mruthunjaya, A.K.; Torriero, A.A. Mechanistic Aspects of the Electrochemical Oxidation of Aliphatic Amines and Aniline Derivatives. Molecules 2023, 28, 471. [Google Scholar]
- Amthor, S.; Noller, B.; Lambert, C. UV/Vis/NIR spectral properties of triarylamines and their corresponding radical cations. Chem. Phys. 2005, 316, 141–152. [Google Scholar] [CrossRef]
- Rissner, F.; Rangger, G.M.; Hofmann, O.T.; Track, A.M.; Heimel, G.; Zojer, E. Understanding the electronic structure of metal/SAM/Organic−semiconductor heterojunctions. ACS Nano 2009, 3, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Zuo, L. Self-assembly monolayers boosting organic–inorganic halide perovskite solar cell performance. J. Mater. Res. 2018, 33, 387–400. [Google Scholar] [CrossRef]
- Arkan, E.; Yalcin, E.; Unal, M.; Arkan, M.Z.Y.; Can, M.; Tozlu, C.; Demic, S. Effect of functional groups of self assembled monolayer molecules on the performance of inverted perovskite solar cell. Mater. Chem. Phys. 2020, 254, 123435. [Google Scholar] [CrossRef]
- Greenwood, P. Surface Modifications and Applications of Aqueous Silica Sols; Chalmers Tekniska Hogskola: Gothenburg, Sweden, 2010. [Google Scholar]
- Nayl, A.; Abd-Elhamid, A.; Aly, A.A.; Bräse, S. Recent progress in the applications of silica-based nanoparticles. RSC Adv. 2022, 12, 13706–13726. [Google Scholar]
- Tilli, M.; Paulasto-Krockel, M.; Petzold, M.; Theuss, H.; Motooka, T.; Lindroos, V. Handbook of Silicon Based MEMS Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Campos, R.; Guenthner, A.J.; Haddad, T.S.; Mabry, J.M. Fluoroalkyl-functionalized silica particles: Synthesis, characterization, and wetting characteristics. Langmuir 2011, 27, 10206–10215. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.; Kınas, Z.; Karabiber, A.; Ozen, A.; Okbaz, A.; Ozel, F. Enhanced performance of triboelectric nanogenerator based on polyamide-silver antimony sulfide nanofibers for energy harvesting. Renew. Energy 2021, 179, 1781–1792. [Google Scholar]
- Ozen, A.; Ozel, F.; Kınas, Z.; Karabiber, A.; Polat, S. Spring assisted triboelectric nanogenerator based on sepiolite doped polyacrylonitrile nanofibers. Sustain. Energy Technol. Assess. 2021, 47, 101492. [Google Scholar] [CrossRef]
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings. Nanomaterials 2018, 8, 530. [Google Scholar]
- Cañón, J.; Teplyakov, A.V. XPS characterization of cobalt impregnated SiO2 and γ-Al2O3. Surf. Interface Anal. 2021, 53, 475–481. [Google Scholar]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Lin, K.; Chua, K.-N.; Christopherson, G.T.; Lim, S.; Mao, H.-Q. Reducing electrospun nanofiber diameter and variability using cationic amphiphiles. Polymer 2007, 48, 6384–6394. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.W.; Jeong, L.; Bae, S.-H.; Min, B.C.; Youk, J.H.; Park, W.H. Effect of organosoluble salts on the nanofibrous structure of electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Int. J. Biol. Macromol. 2004, 34, 249–256. [Google Scholar] [PubMed]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine electrospun polyamide-6 fibers: Effect of solution conditions on morphology and average fiber diameter. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Semnani, D. Geometrical characterization of electrospun nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 151–180. [Google Scholar]
- Tantraviwat, D.; Ngamyingyoud, M.; Sripumkhai, W.; Pattamang, P.; Rujijanagul, G.; Inceesungvorn, B. Tuning the dielectric constant and surface engineering of a BaTiO3/Porous PDMS composite film for enhanced triboelectric nanogenerator output performance. ACS Omega 2021, 6, 29765–29773. [Google Scholar] [CrossRef]
- Wang, Z.L.; Lin, L.; Chen, J.; Niu, S.; Zi, Y.; Wang, Z.L.; Lin, L.; Chen, J.; Niu, S.; Zi, Y. Triboelectric nanogenerator: Lateral sliding mode. In Triboelectric Nanogenerators; Springer: Berlin/Heidelberg, Germany, 2016; pp. 49–90. [Google Scholar]
- Khandelwal, G.; Chandrasekhar, A.; Maria Joseph Raj, N.P.; Kim, S.J. Metal–organic framework: A novel material for triboelectric nanogenerator–based self-powered sensors and systems. Adv. Energy Mater. 2019, 9, 1803581. [Google Scholar]
- Lee, S.; Lee, Y.; Kim, D.; Yang, Y.; Lin, L.; Lin, Z.-H.; Hwang, W.; Wang, Z.L. Triboelectric nanogenerator for harvesting pendulum oscillation energy. Nano Energy 2013, 2, 1113–1120. [Google Scholar] [CrossRef]
- Wen, R.; Guo, J.; Yu, A.; Zhang, K.; Kou, J.; Zhu, Y.; Zhang, Y.; Li, B.-W.; Zhai, J. Remarkably enhanced triboelectric nanogenerator based on flexible and transparent monolayer titania nanocomposite. Nano Energy 2018, 50, 140–147. [Google Scholar] [CrossRef]
- De Gennes, P.-G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827. [Google Scholar]
- Jańczuk, B.; Białlopiotrowicz, T. Surface free-energy components of liquids and low energy solids and contact angles. J. Colloid Interface Sci. 1989, 127, 189–204. [Google Scholar] [CrossRef]
- Quéré, D. Rough ideas on wetting. Phys. A Stat. Mech. Its Appl. 2002, 313, 32–46. [Google Scholar] [CrossRef]
- Bico, J.; Marzolin, C.; Quéré, D. Pearl drops. Europhys. Lett. 1999, 47, 220. [Google Scholar] [CrossRef]
- Akın Kara, D.; Kara, K.; Oylumluoglu, G.; Yigit, M.Z.; Can, M.; Kim, J.J.; Burnett, E.K.; Gonzalez Arellano, D.L.; Buyukcelebi, S.m.; Ozel, F. Enhanced device efficiency and long-term stability via boronic acid-based self-assembled monolayer modification of indium tin oxide in a planar perovskite solar cell. ACS Appl. Mater. Interfaces 2018, 10, 30000–30007. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Chen, Q.; Yang, Y.; Liu, Y.; Hong, Z.; Liu, Z.; Hsieh, Y.-T.; Meng, L.; Li, Y. Multifunctional fullerene derivative for interface engineering in perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 15540–15547. [Google Scholar] [CrossRef]
- Arkan, E.; Arkan, M.Z.Y.; Unal, M.; Yalcin, E.; Aydin, H.; Çelebi, C.; Can, M.; Tozlu, C.; Demic, S. Performance enhancement of inverted perovskite solar cells through interface engineering by TPD based bidentate self-assembled monolayers. Opt. Mater. 2020, 105, 109910. [Google Scholar] [CrossRef]
- Firdous, I.; Fahim, M.; Daoud, W.A. Performance enhancement of triboelectric nanogenerator through hole and electron blocking layers-based interfacial design. Nano Energy 2021, 82, 105694. [Google Scholar] [CrossRef]
- Shen, W.-W.; Mu, H.-B.; Zhang, G.-J.; Deng, J.-B.; Tu, D.-M. Identification of electron and hole trap based on isothermal surface potential decay model. J. Appl. Phys. 2013, 113, 083706. [Google Scholar] [CrossRef]
- Li, J.; Zhou, F.; Min, D.; Li, S.; Xia, R. The energy distribution of trapped charges in polymers based on isothermal surface potential decay model. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1723–1732. [Google Scholar] [CrossRef]
- Takada, T.; Kikuchi, H.; Miyake, H.; Tanaka, Y.; Yoshida, M.; Hayase, Y. Determination of charge-trapping sites in saturated and aromatic polymers by quantum chemical calculation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1240–1249. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Liang, Z.; Gao, D.; Huang, W. Interfacial engineering by using self-assembled monolayer in mesoporous perovskite solar cell. RSC Adv. 2015, 5, 94290–94295. [Google Scholar] [CrossRef]
- Zuo, L.; Gu, Z.; Ye, T.; Fu, W.; Wu, G.; Li, H.; Chen, H. Enhanced photovoltaic performance of CH3NH3PbI3 perovskite solar cells through interfacial engineering using self-assembling monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mei, A.; Liu, T.; Jiang, P.; Sheng, Y.; Zhang, L.; Han, H. Fully printable mesoscopic perovskite solar cells with organic silane self-assembled monolayer. J. Am. Chem. Soc. 2015, 137, 1790–1793. [Google Scholar] [CrossRef]
- Karon, K.; Lapkowski, M. Carbazole electrochemistry: A short review. J. Solid State Electrochem. 2015, 19, 2601–2610. [Google Scholar] [CrossRef]
- Mark, J. Polymer Data Handbook; Oxford University Press: New York, NY, USA, 1999; p. 363. [Google Scholar]
- Polyacrylonitrile (PAN) Carbon Fibers Industrial Capability Assessment OUSD(AT&L) Industrial Policy; U.S. Department of Defense: Washington, DC, USA, 2005.
- Paulauskas, F.L.; Warren, C.D.; Eberle, C.C.; Naskar, A.K.; Ozcan, S.; da Costa Mendes Fagundes, A.P.V.V.; Barata Dias, R.M.; de Magalhães, P.F.M. Novel Precursor Materials and Approaches for Producing Lower Cost Carbon Fiber for High Volume Industries. In Proceedings of the ICCM-17-17th International Conference on Composite Materials, Edinburgh, UK, 27–31 July 2009. [Google Scholar]
- Liu, D.; Chen, H.; Yin, P.; Ji, N.; Zong, G.; Qu, R. Synthesis of polyacrylonitrile by single-electron transfer-living radical polymerization using Fe(0) as catalyst and its adsorption properties after modification. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2916–2923. [Google Scholar] [CrossRef]
- Serkov, A.; Radishevskii, M. Status and prospects for production of carbon fibres based on polyacrylonitrile. Fibre Chem. 2008, 40, 24–31. [Google Scholar] [CrossRef]
- Bergna, H.E. Colloid Chemistry of Silica: An Overview; ACS Publications: Washington, DC, USA, 1994; p. 15. [Google Scholar]
- Iler, K.R. The Chemistry of Silica. In Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; Wiley: Hoboken, NJ, USA, 1979; p. 424. [Google Scholar]
- Balcı, M. Organik Kimya: Reaksiyon Mekanizmaları; Türkiye Bilimler Akademisi: Ankara, Türkiye, 2012; pp. 21, 25. [Google Scholar]
- Hathaway, B.A. Organic Chemistry, (Solomons, TW Graham); ACS Publications: Washington, DC, USA, 1992; p. 53. [Google Scholar]
- Takimiya, K.; Osaka, I.; Nakano, M. π-Building blocks for organic electronics: Revaluation of “inductive” and “resonance” effects of π-electron deficient units. Chem. Mater. 2014, 26, 587–593. [Google Scholar] [CrossRef]
- Domenicano, A.; Murray-Rust, P. Geometrical substituent parameters for benzene derivatives: Inductive and resonance effects. Tetrahedron Lett. 1979, 20, 2283–2286. [Google Scholar] [CrossRef]
- Went, C. Structure and Physical Properties: Inductive and Mesomeric Effects. In Work Out Organic Chemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 1–17. [Google Scholar]
- Daley, R. Organic Chemistry, Part 1 of 3; Lulu Press: Morrisvill, NC, USA, 2005; p. 58. [Google Scholar]
- Pauling, L. The nature of the chemical bond—1992. J. Chem. Educ. 1992, 69, 519. [Google Scholar] [CrossRef]
- Salvatella, L. The alkyl group is a-I+ R substituent. Educ. Química 2017, 28, 232–237. [Google Scholar] [CrossRef]
- Smith, M.B. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 19–20. [Google Scholar]
- Li, S.; Nie, J.; Shi, Y.; Tao, X.; Wang, F.; Tian, J.; Lin, S.; Chen, X.; Wang, Z.L. Contributions of different functional groups to contact electrification of polymers. Adv. Mater. 2020, 32, 2001307. [Google Scholar] [CrossRef]
- Tripp, C.; Veregin, R.; McDougall, M.; Osmond, D. Reaction of alkylchlorosilanes with silica: Importance of a surface attachment in defining the triboelectrification of the modified silica. Langmuir 1995, 11, 1858–1859. [Google Scholar] [CrossRef]
- Yigit, M.Z.; Bilgili, H.; Sefer, E.; Demic, S.; Zafer, C.; Can, M.; Koyuncu, S. Effect of a pi-bridging unit in triphenylamine-benzothiadiazole based donor acceptor chromophores for dye-sensitized solar cells. Electrochim. Acta 2014, 147, 617–625. [Google Scholar] [CrossRef]
- Alptekin, H.; Arkan, E.; Özbek, C.; Can, M.; Farzaneh, A.; Sütçü, M.; Okur, S.; Cobley, A.J. Water affinity guided tunable superhydrophobicity and optimized wettability of selected natural minerals. J. Coat. Technol. Res. 2019, 16, 199–211. [Google Scholar] [CrossRef]
- Bhadra, P.; Siu, S.W. Comparison of biomolecular force fields for alkanethiol self-assembled monolayer simulations. J. Phys. Chem. C 2017, 121, 26340–26349. [Google Scholar] [CrossRef]






| Samples | C1s (eV) | O1s (eV) | N1s | |||
|---|---|---|---|---|---|---|
| C–C/C–H | C–O–C | O–C=O | O–Si | C=O | ||
| ME-86 | 284.6 | 285.9 | 287.4 | 533.6 | — | — |
| ME-103 | 284.4 | 285.9 | 287.9 | 532.9 | 531.2 | 399.8 |
| ME-111 | 284.5 | 286.2 | 288.1 | 533.2 | 531.6 | 400.0 |
| Blank | 285.1 | 286.5 | 288.4 | 533.5 | — | — |
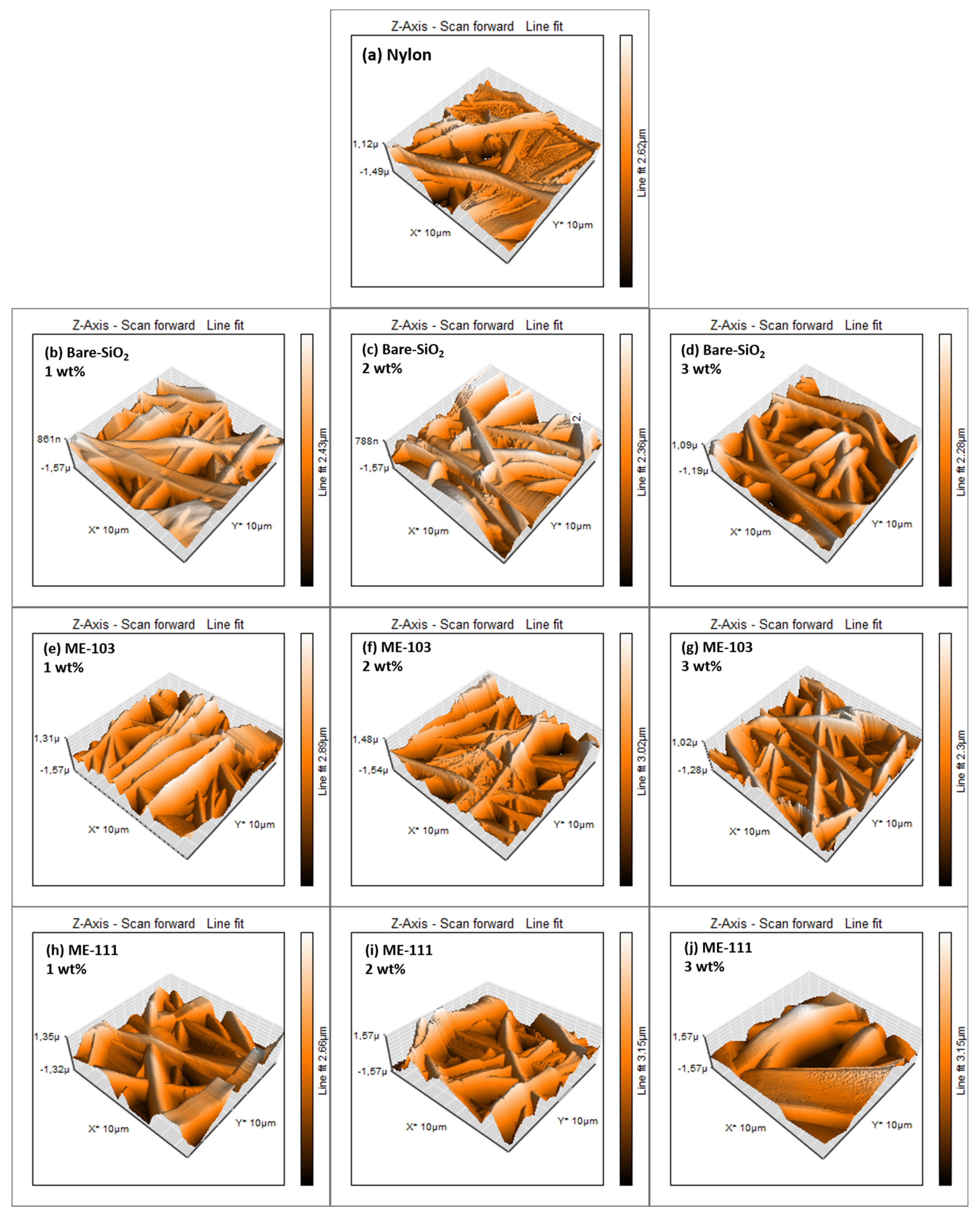
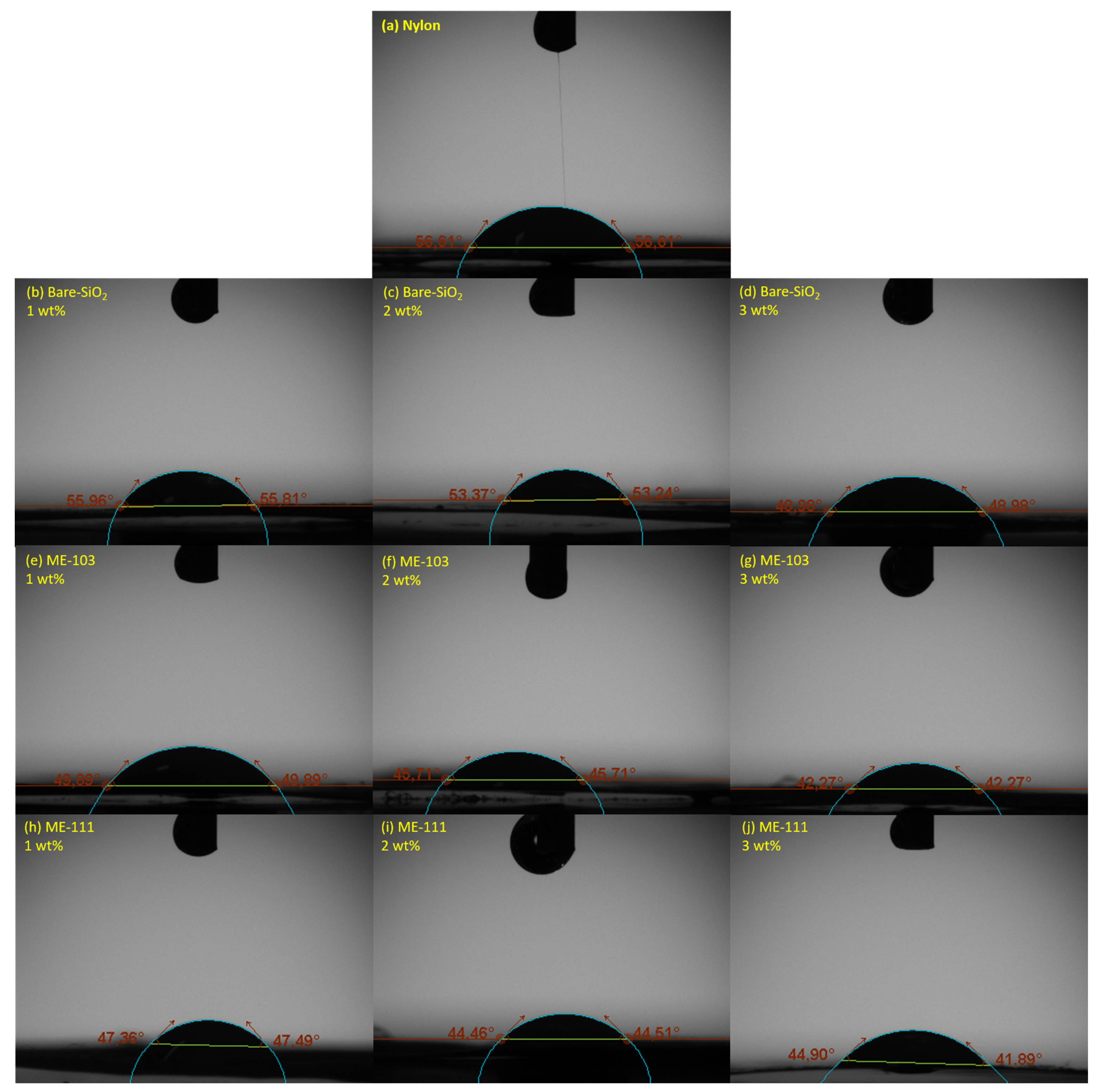
| Structure | Surface Energy (mN/m) | Contact Angle (°C) | Roughness-Sq (nm) |
|---|---|---|---|
| Nylon 6.6 | 43.53 | 56.51 | 326.52 ± 34.55 |
| SiO2 (1 wt%) | 44.03 | 55.89 | 342.97 ± 92.9 |
| SiO2 (2 wt%) | 46.13 | 53.31 | 485.93 ± 30.62 |
| SiO2 (3 wt%) | 49.59 | 48.98 | 360.76 ± 33.4 |
| ME-103 (1 wt%) | 48.87 | 49.89 | 483.32 ± 13.68 |
| ME-103 (2 wt%) | 52.13 | 45.71 | 420.02 ± 66.02 |
| ME-103 (3 wt%) | 54.72 | 42.27 | 414.66 ± 23.91 |
| ME-111 (1 wt%) | 50.80 | 47.43 | 387.29 ± 71 |
| ME-111 (2 wt%) | 53.06 | 44.49 | 528.04 ± 64.47 |
| ME-111 (3 wt%) | 53.88 | 43.40 | 550.55 ± 72.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkan, M.Z.; Kinas, Z.; Yalcin, E.; Arkan, E.; Özel, F.; Karabiber, A.; Chorążewski, M. One Material-Opposite Triboelectrification: Molecular Engineering Regulated Triboelectrification on Silica Surface to Enhance TENG Efficiency. Molecules 2023, 28, 5662. https://doi.org/10.3390/molecules28155662
Arkan MZ, Kinas Z, Yalcin E, Arkan E, Özel F, Karabiber A, Chorążewski M. One Material-Opposite Triboelectrification: Molecular Engineering Regulated Triboelectrification on Silica Surface to Enhance TENG Efficiency. Molecules. 2023; 28(15):5662. https://doi.org/10.3390/molecules28155662
Chicago/Turabian StyleArkan, Mesude Zeliha, Zeynep Kinas, Eyup Yalcin, Emre Arkan, Faruk Özel, Abdulkerim Karabiber, and Mirosław Chorążewski. 2023. "One Material-Opposite Triboelectrification: Molecular Engineering Regulated Triboelectrification on Silica Surface to Enhance TENG Efficiency" Molecules 28, no. 15: 5662. https://doi.org/10.3390/molecules28155662
APA StyleArkan, M. Z., Kinas, Z., Yalcin, E., Arkan, E., Özel, F., Karabiber, A., & Chorążewski, M. (2023). One Material-Opposite Triboelectrification: Molecular Engineering Regulated Triboelectrification on Silica Surface to Enhance TENG Efficiency. Molecules, 28(15), 5662. https://doi.org/10.3390/molecules28155662







