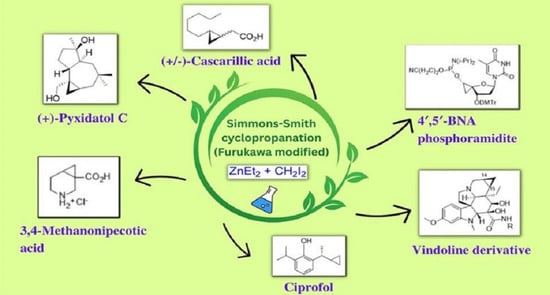
Simmons–Smith Cyclopropanation: A Multifaceted Synthetic Protocol toward the Synthesis of Natural Products and Drugs: A Review
Abstract
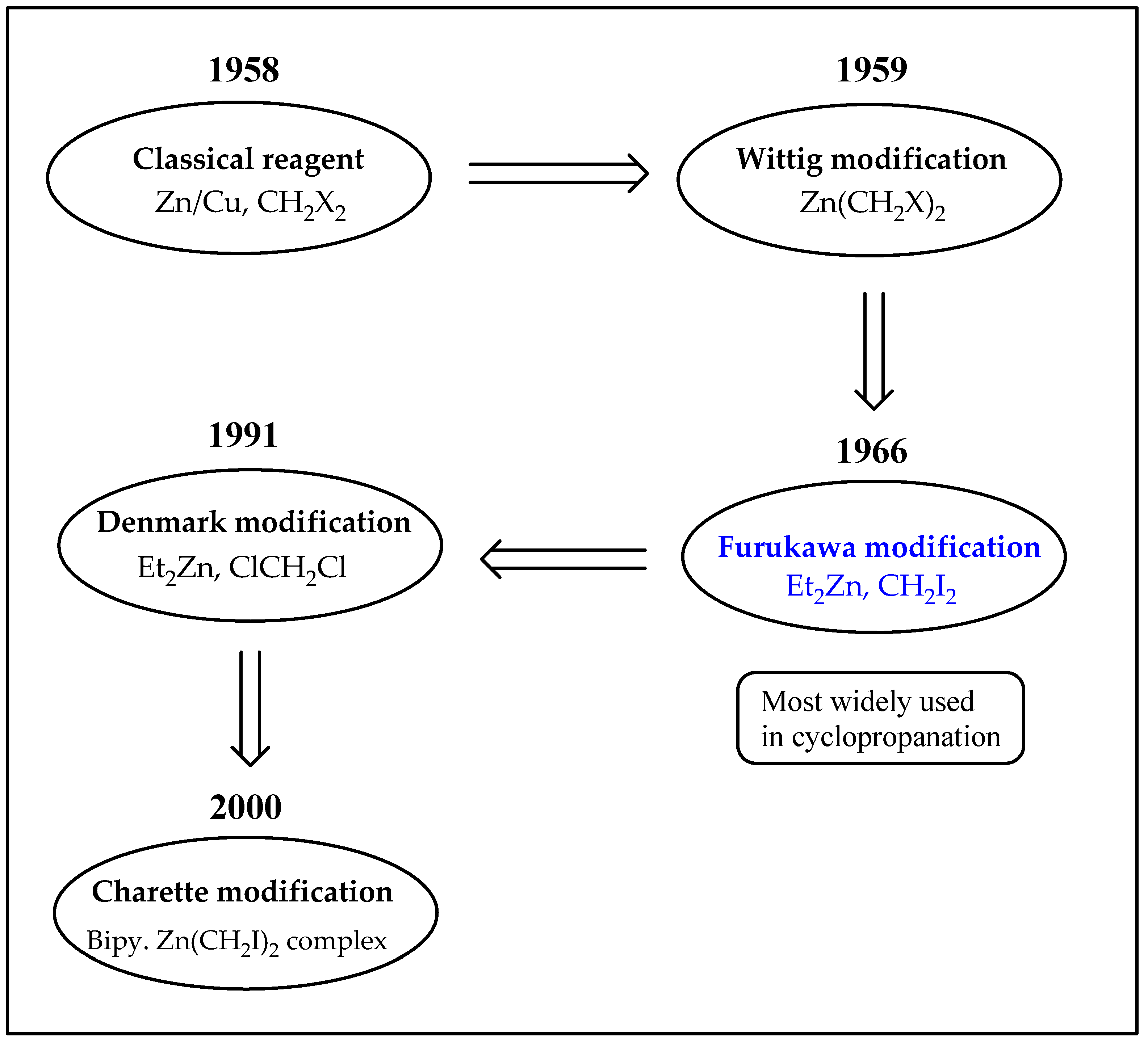
1. Introduction
2. Review of Literature
2.1. Synthesis of Alkaloids Based Natural Products
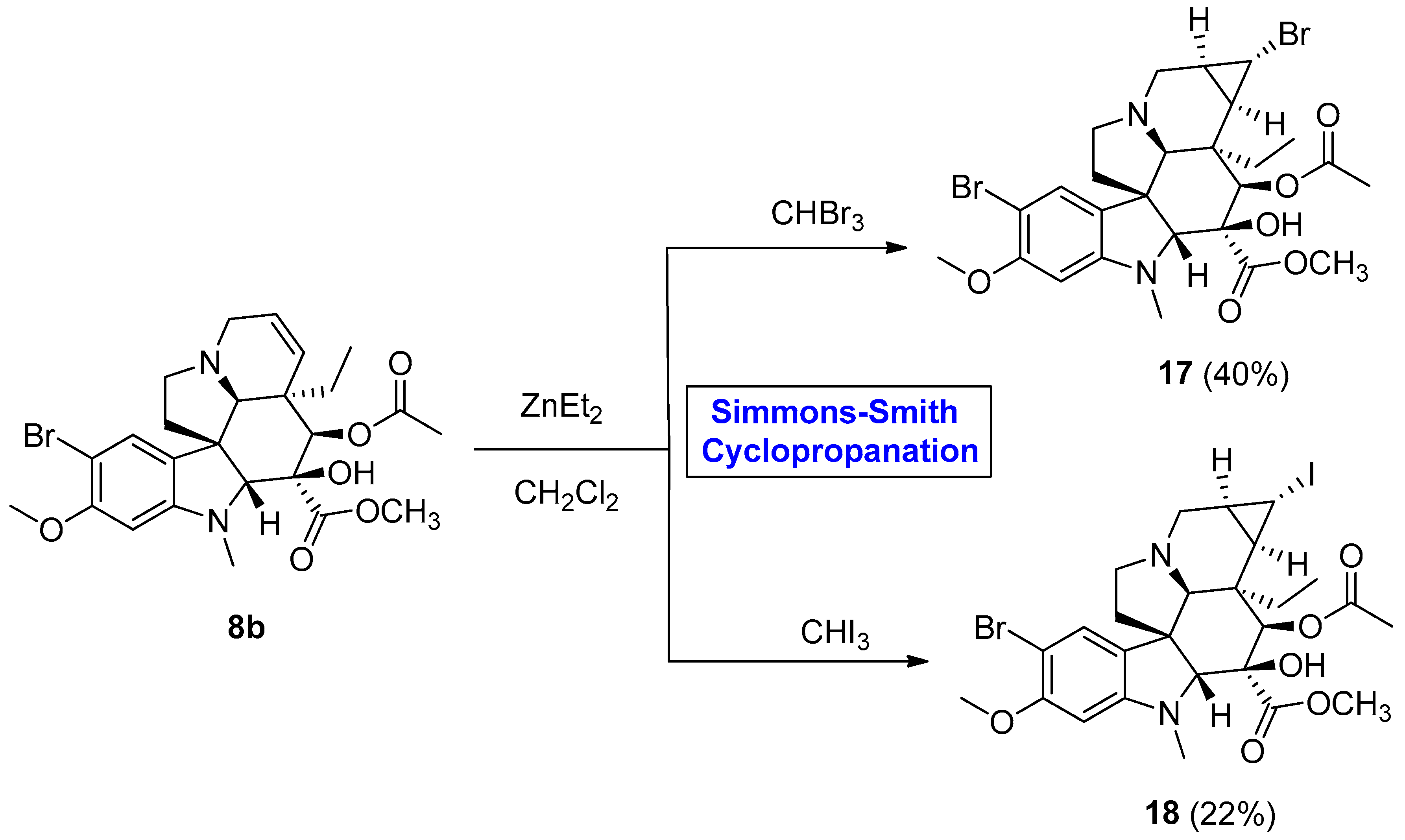
2.1.1. Bisindole Alkaloids
2.1.2. Kopsia Alkaloids
2.1.3. Limonoid Alkaloids
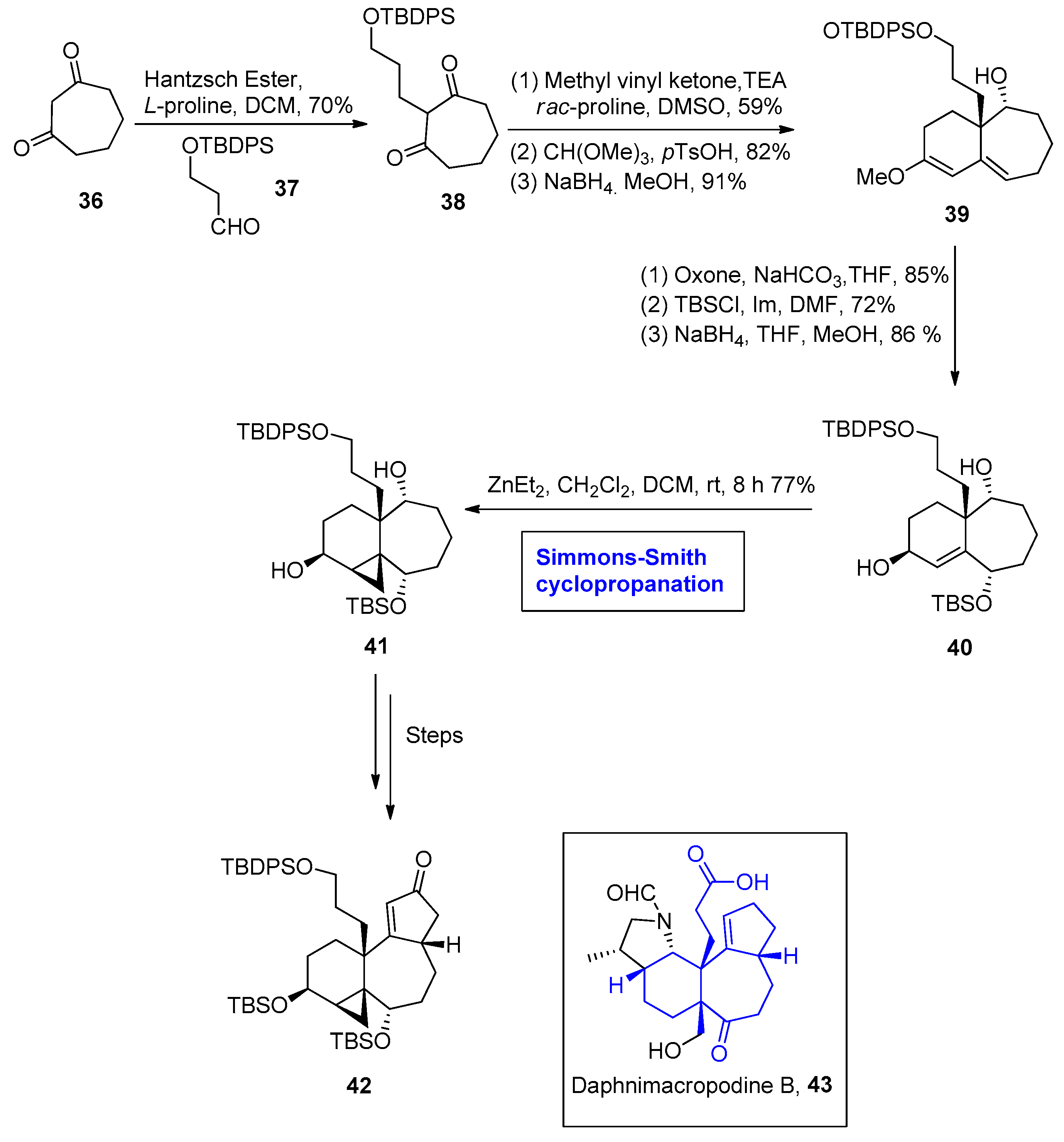
2.1.4. Daphniphyllum Alkaloids
2.2. Synthesis of Terpenoid-Based Natural Products
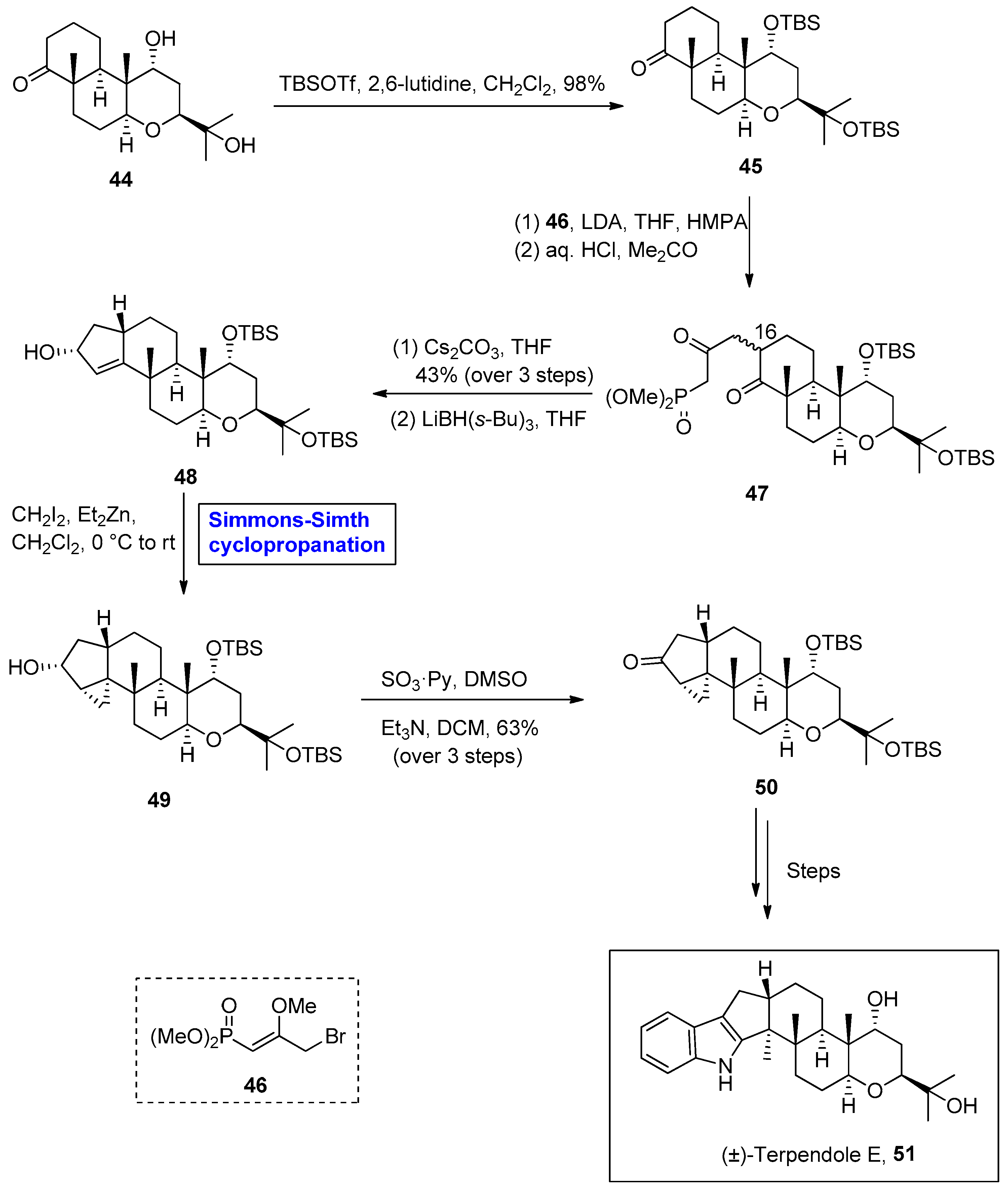
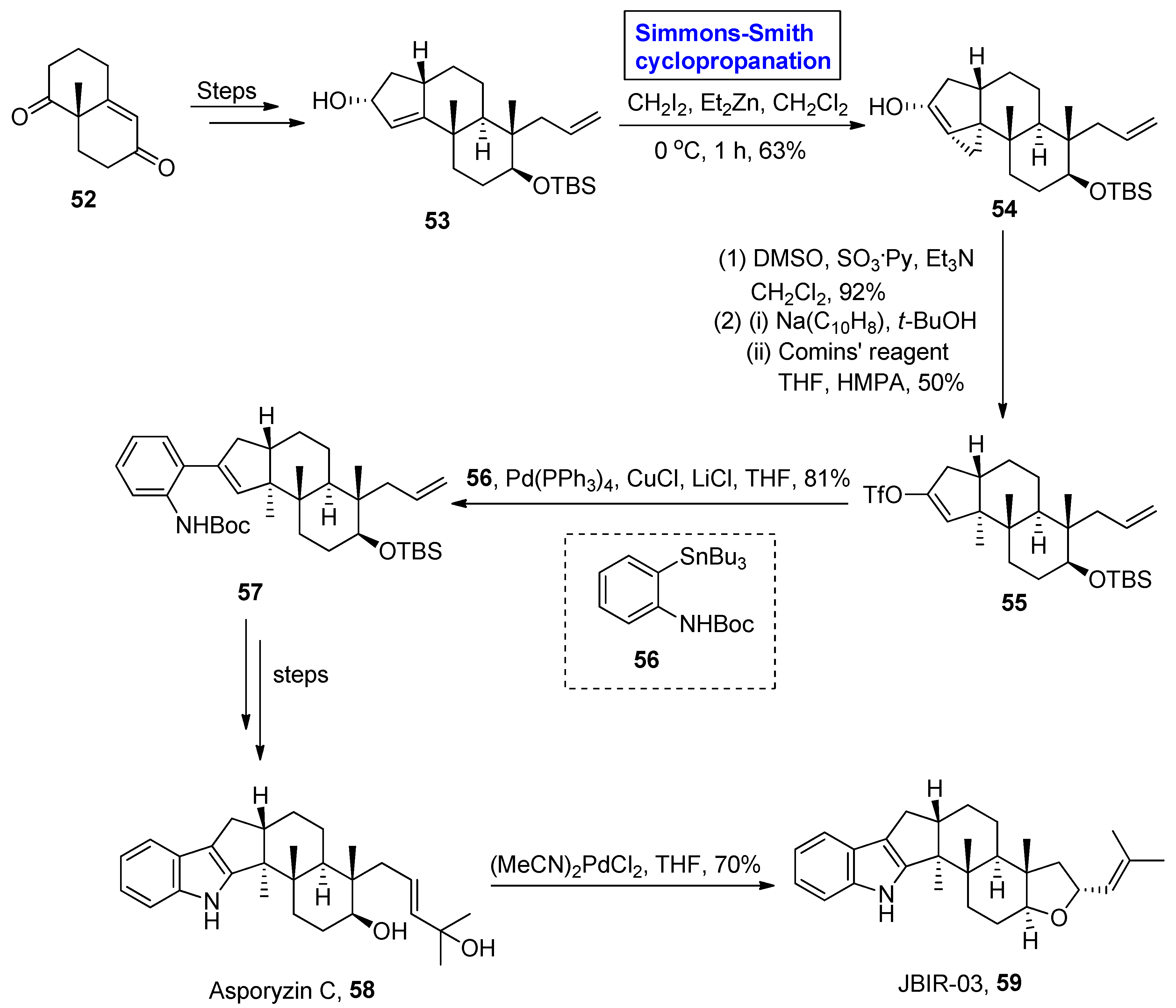
2.2.1. Indole Terpenoids
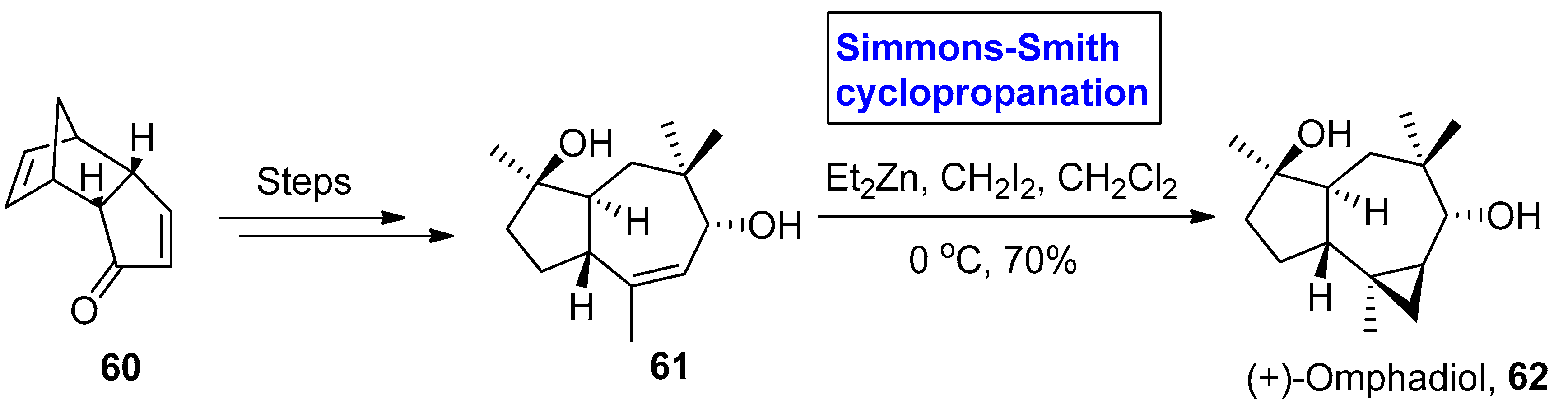
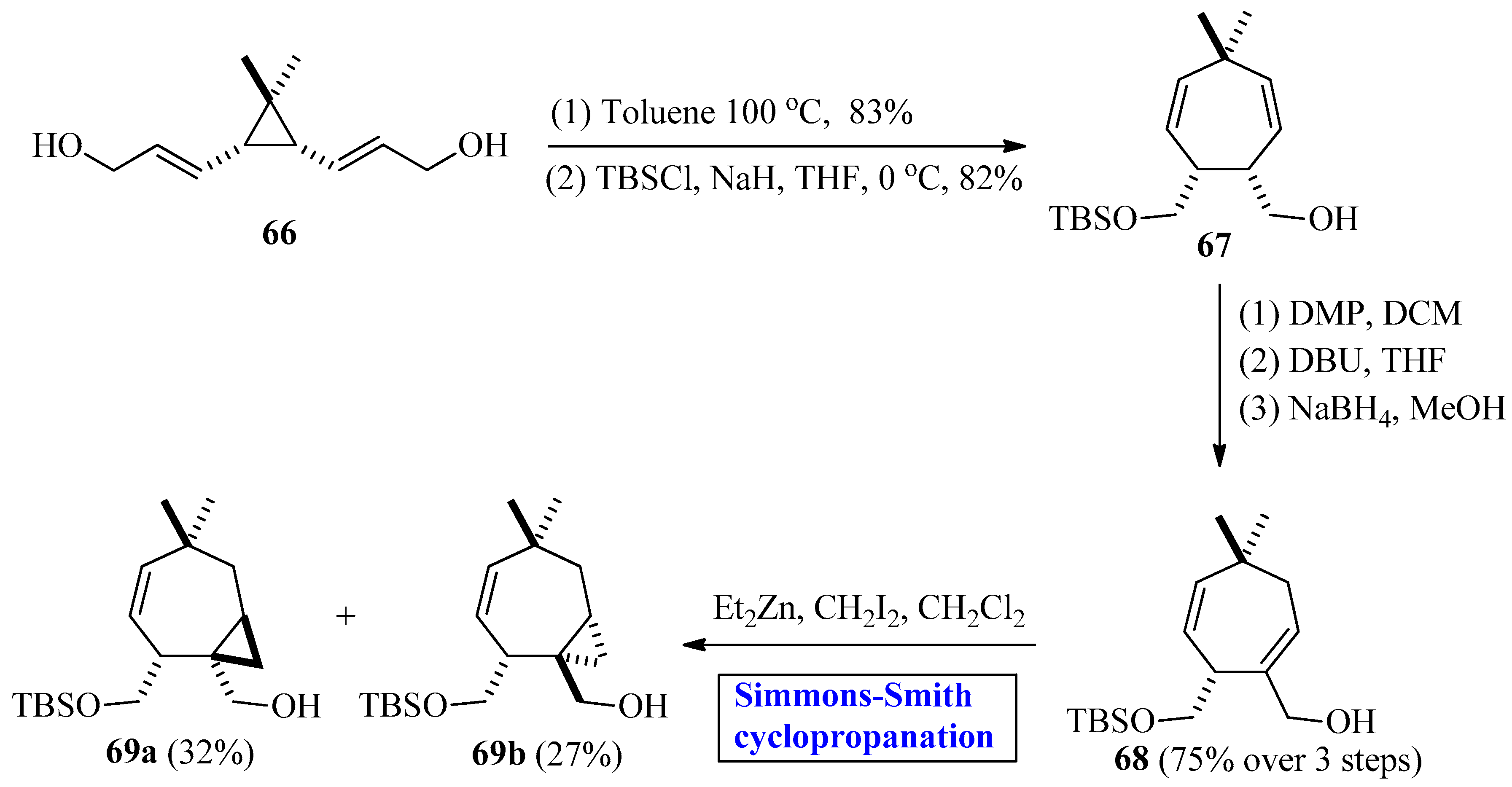
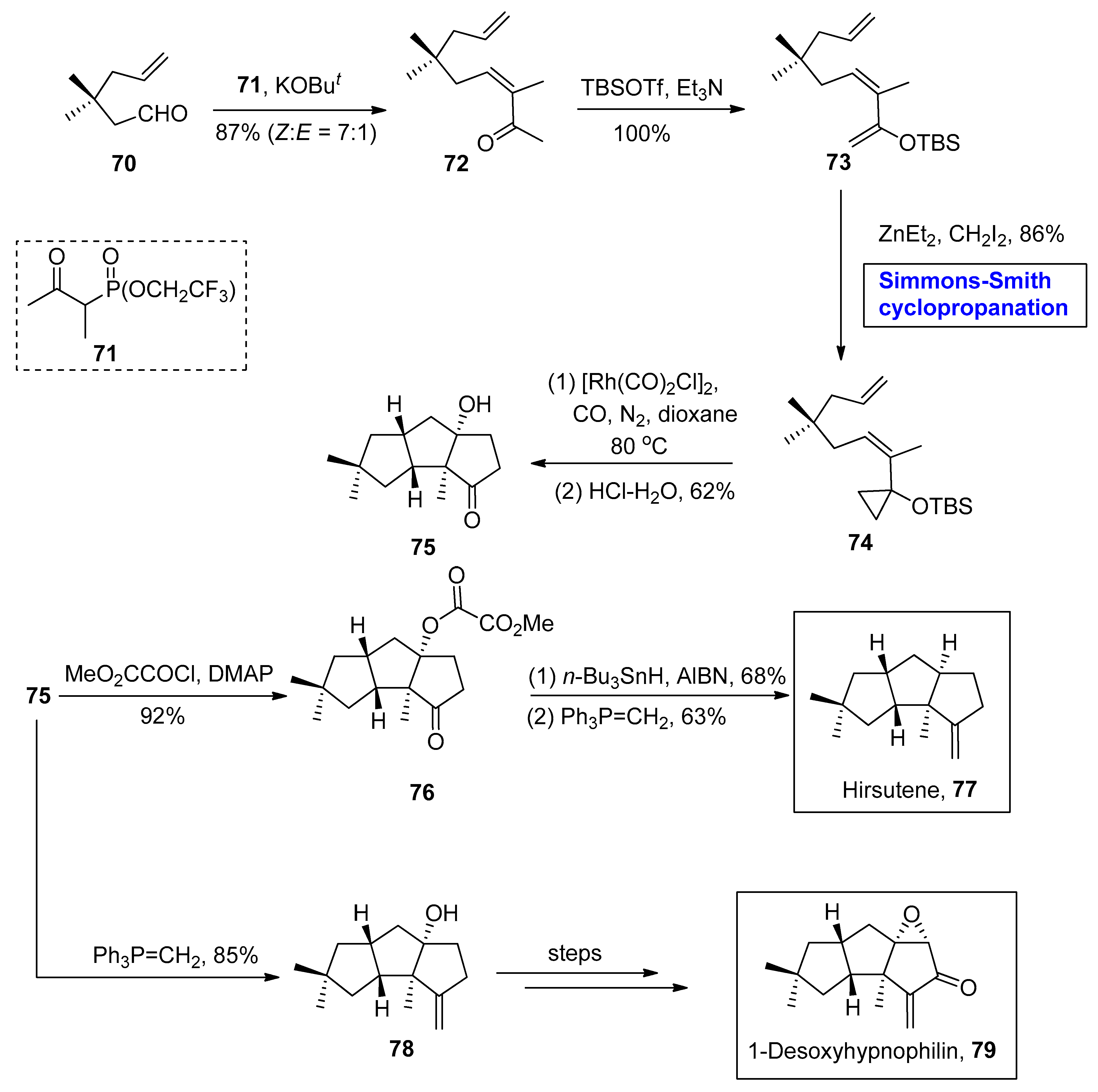
2.2.2. Sesquiterpenes
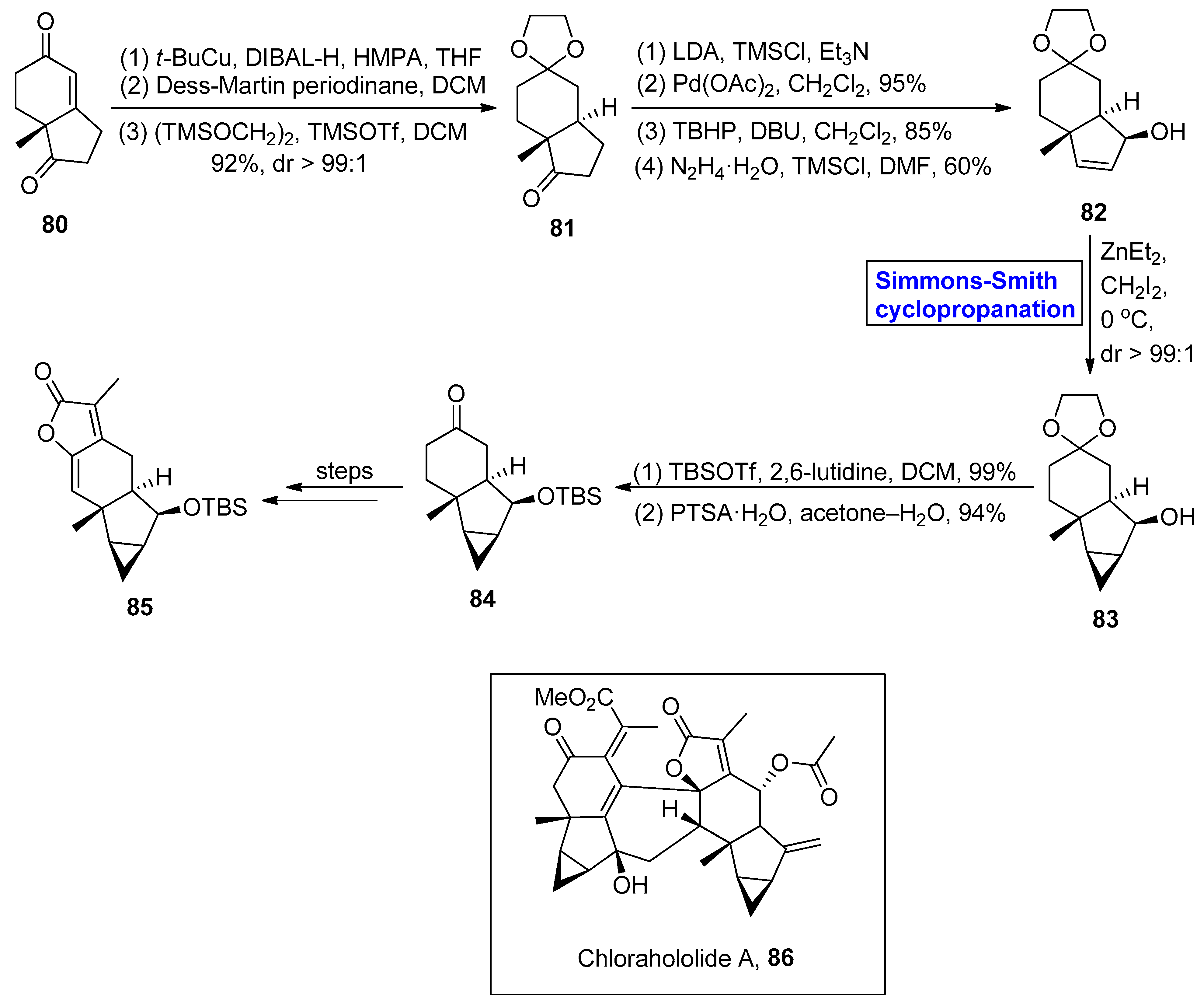
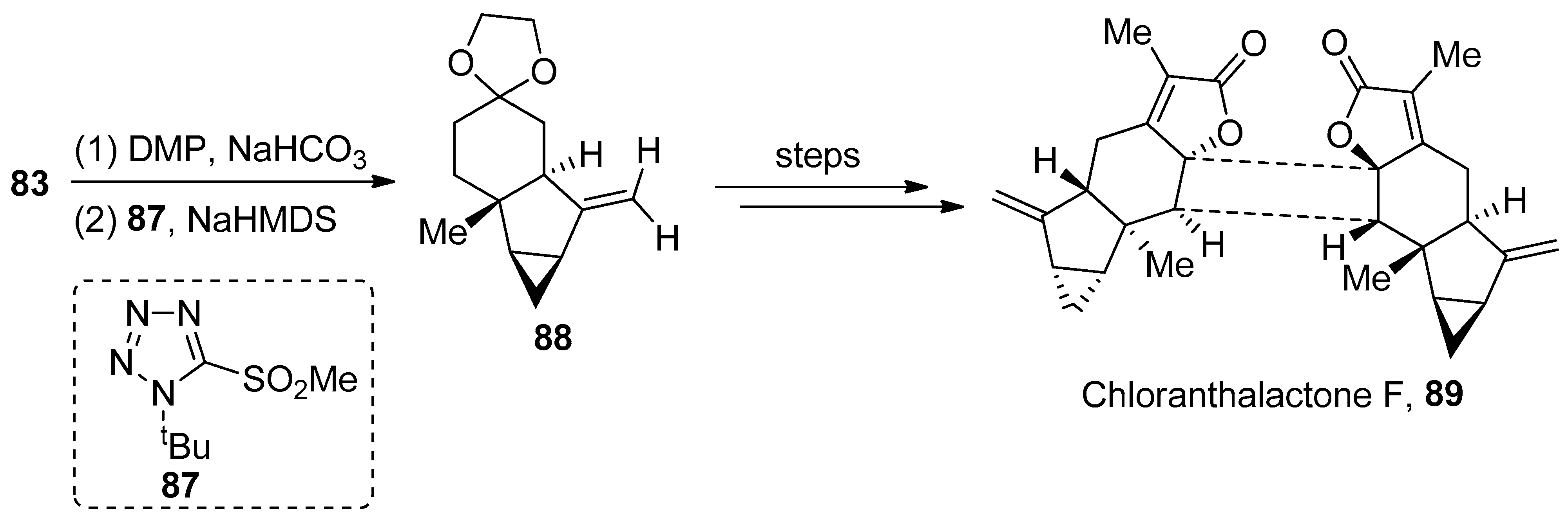
2.2.3. Diterpenoids
2.2.4. Triterpenoids
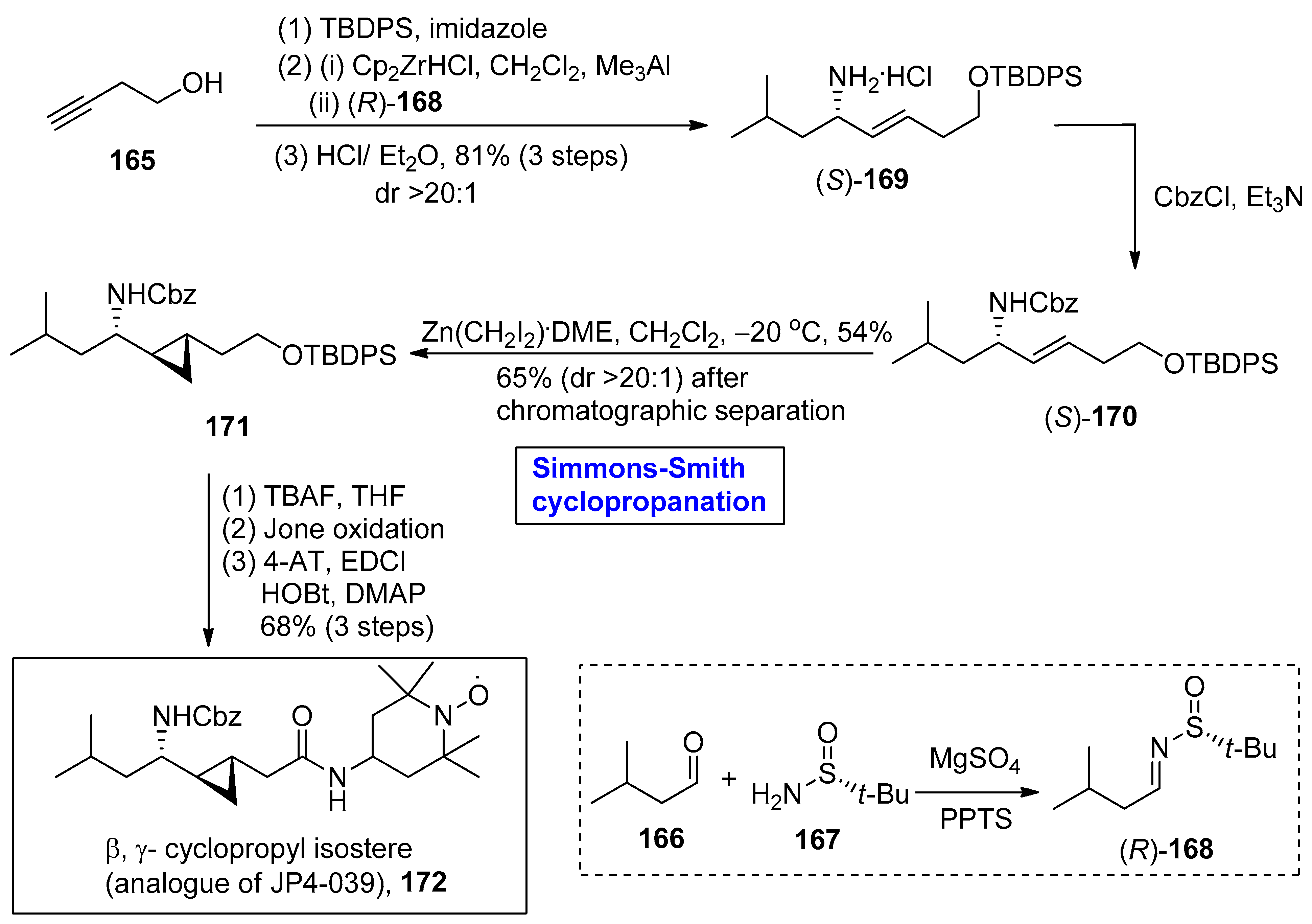
2.3. Synthesis of Amino-Acid-Based Natural Products
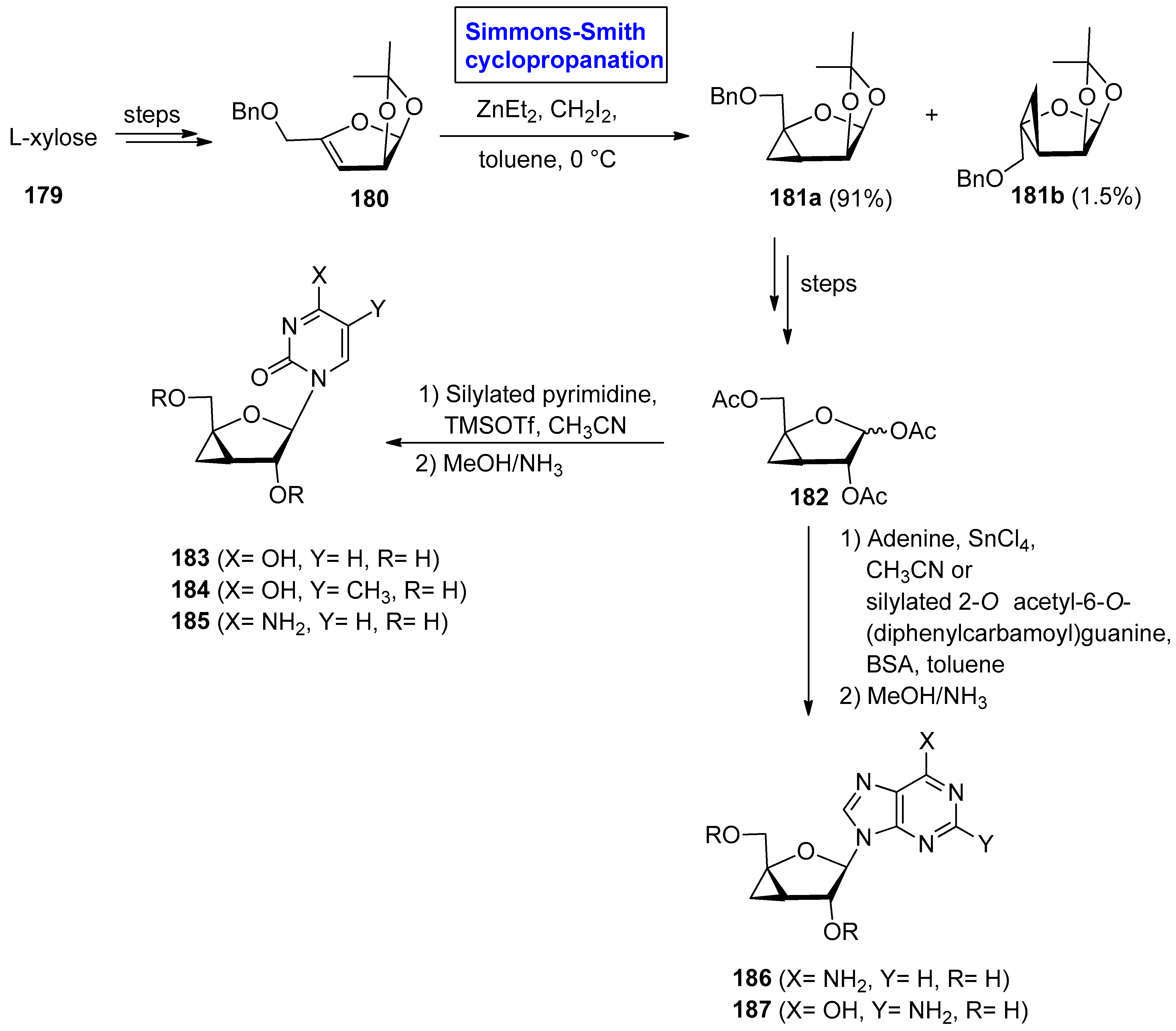
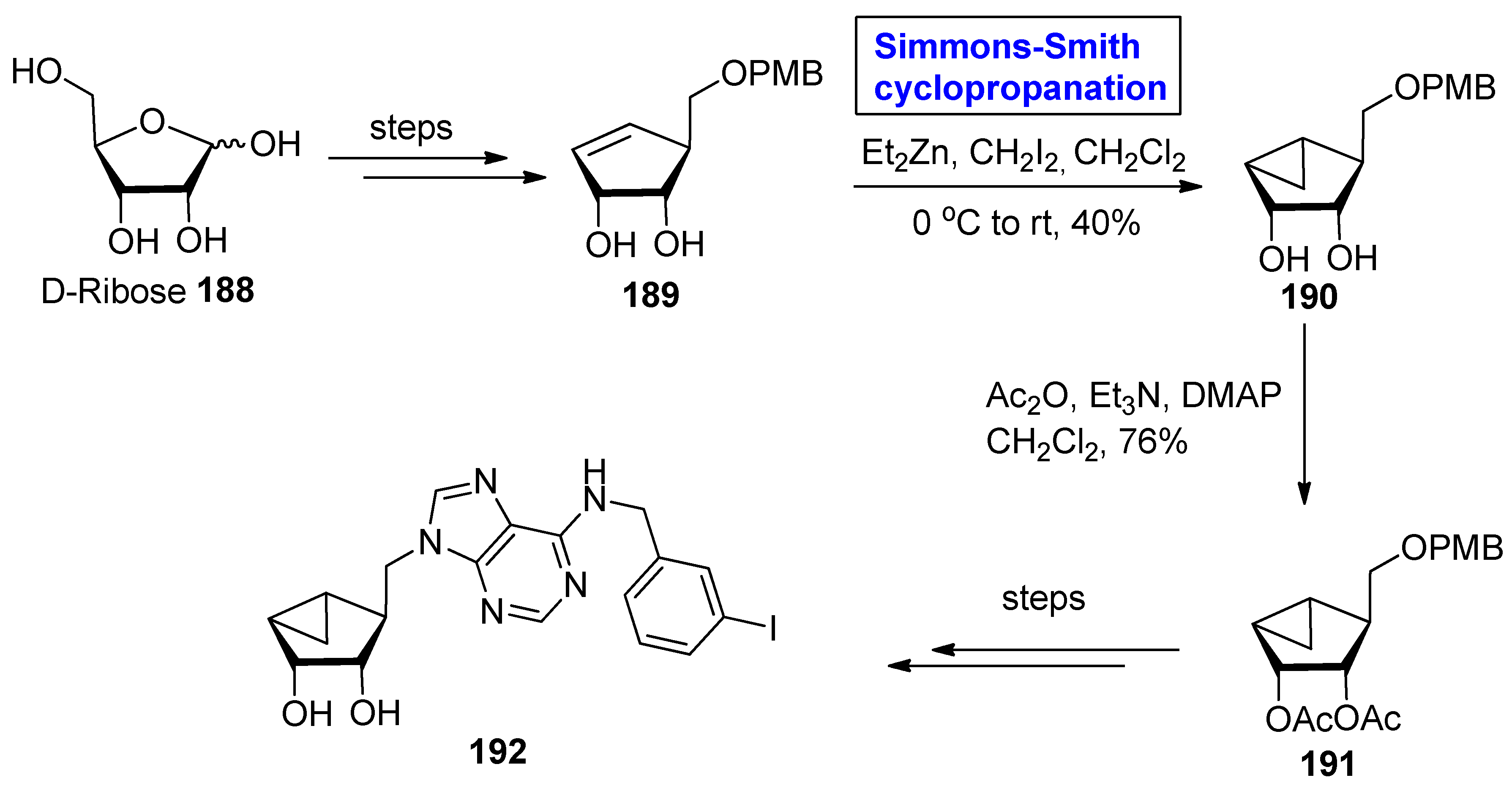
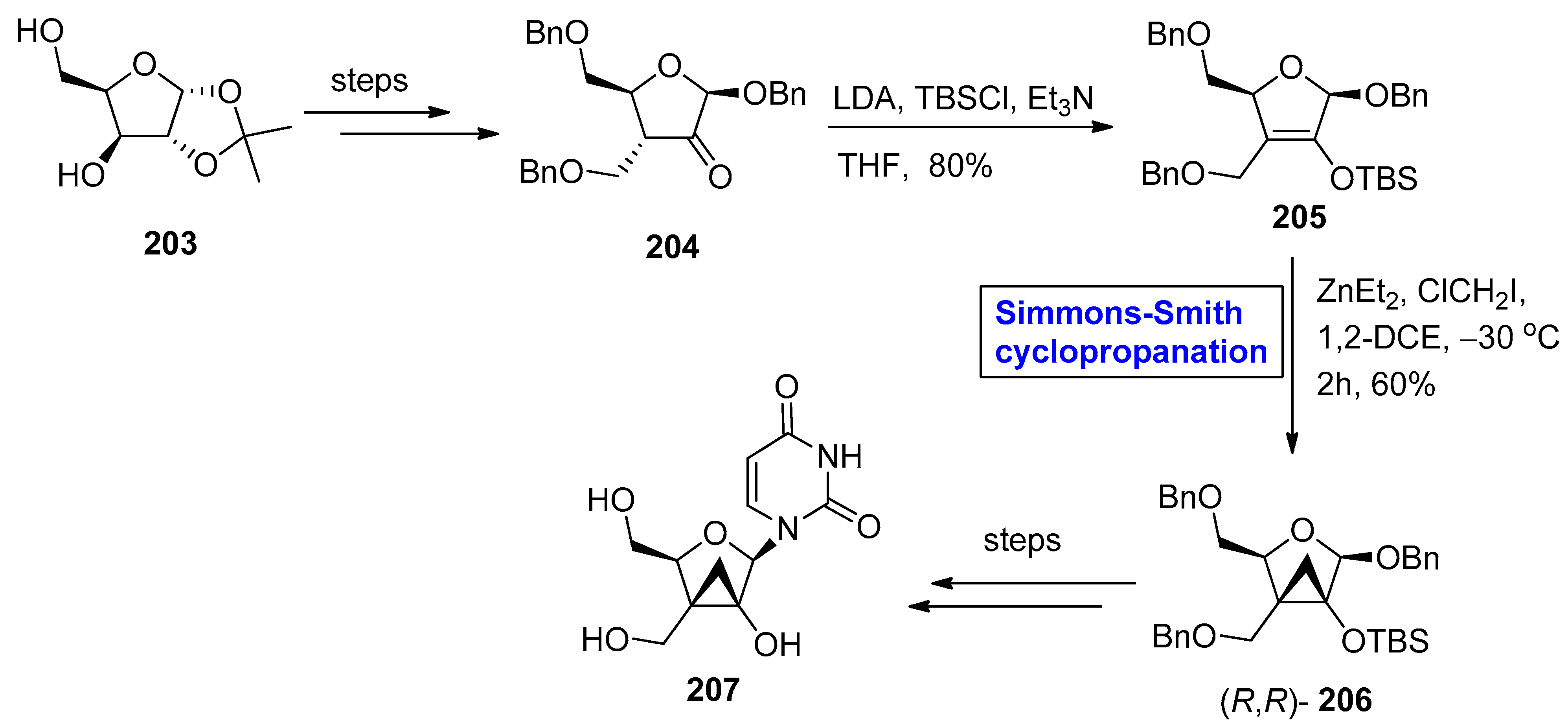
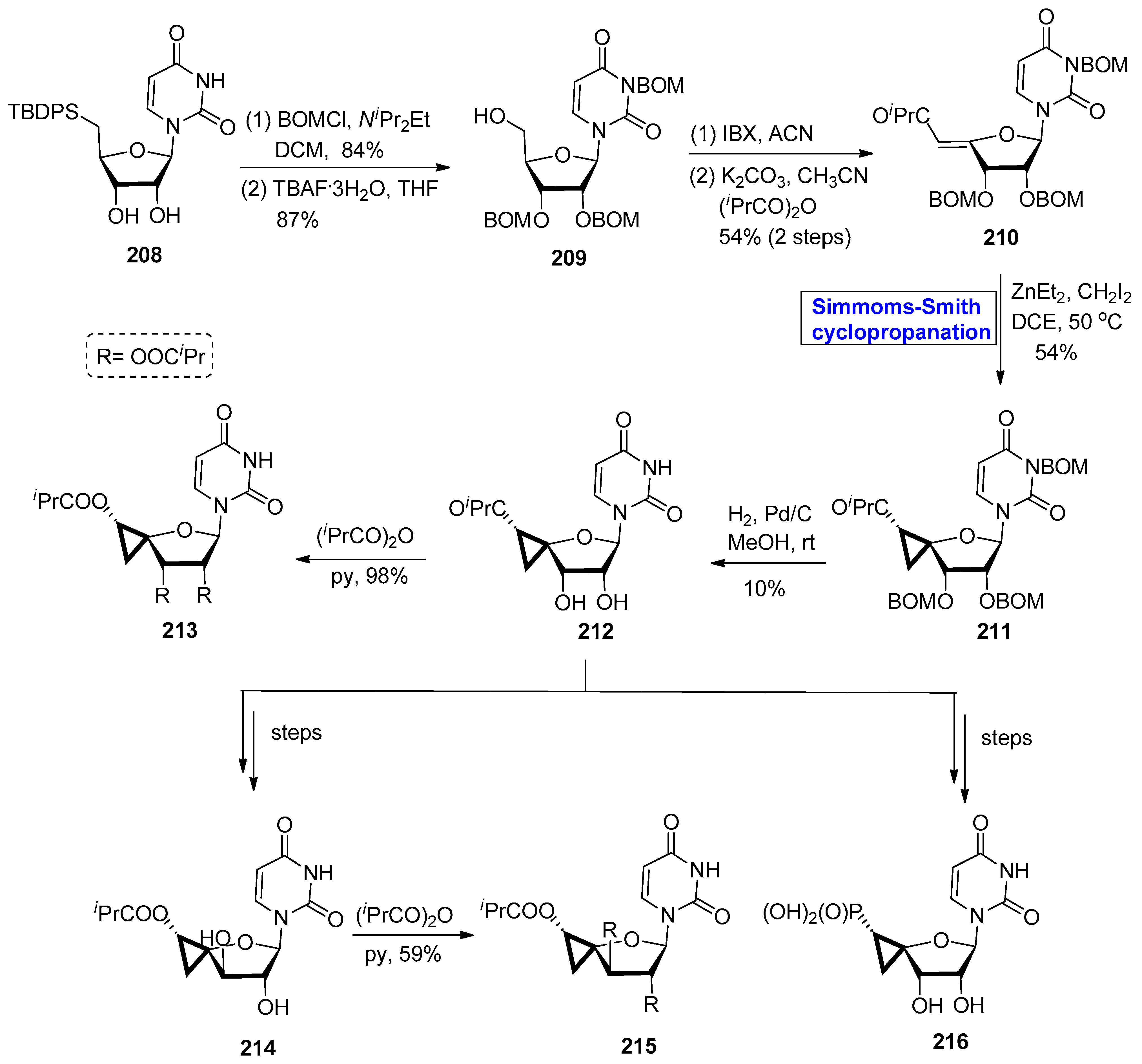
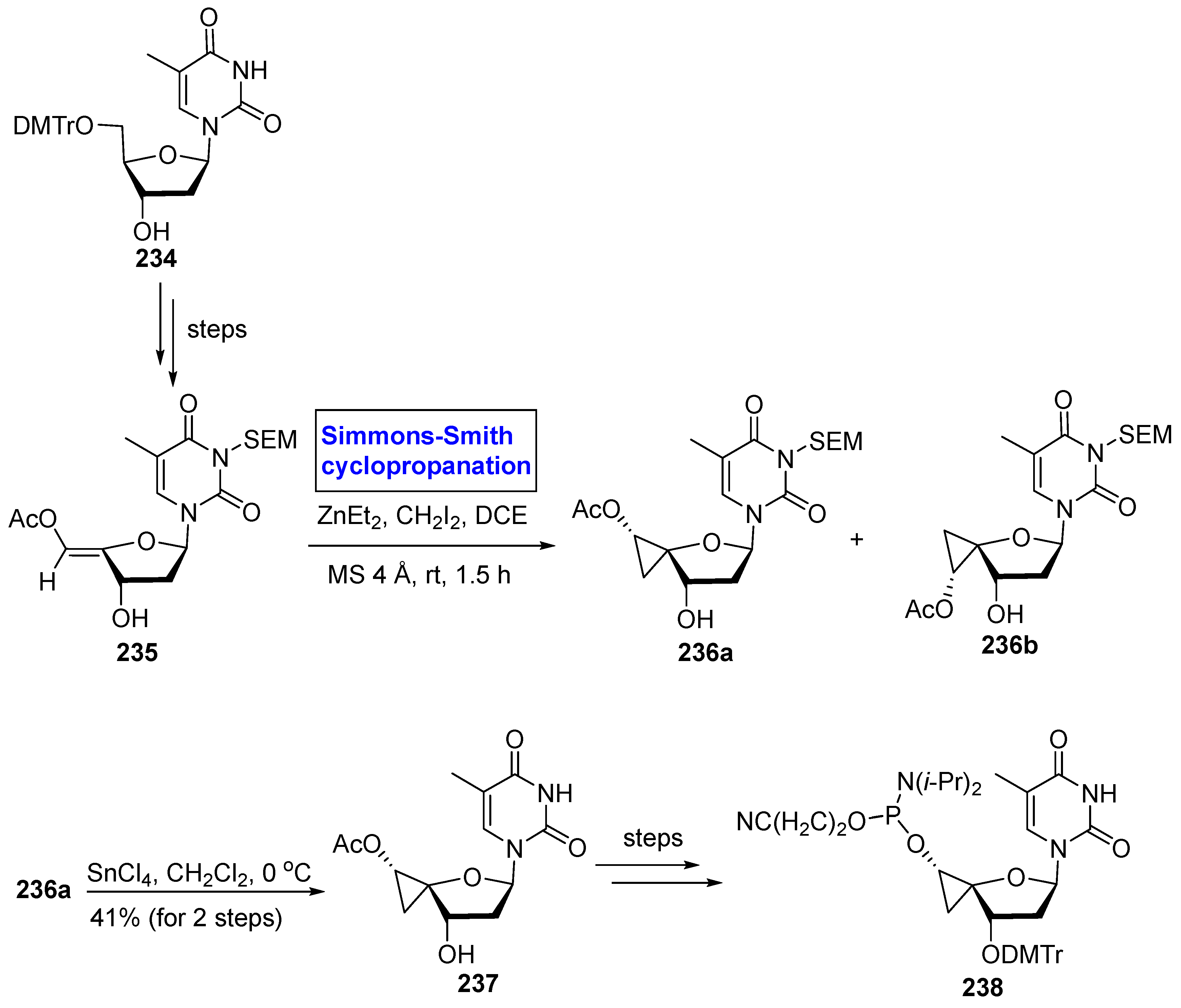
2.4. Synthesis of Nucleosides
2.5. Synthesis of γ-Pyrone-Based Natural Product
2.6. Synthesis of Polyketide-Based Natural Product
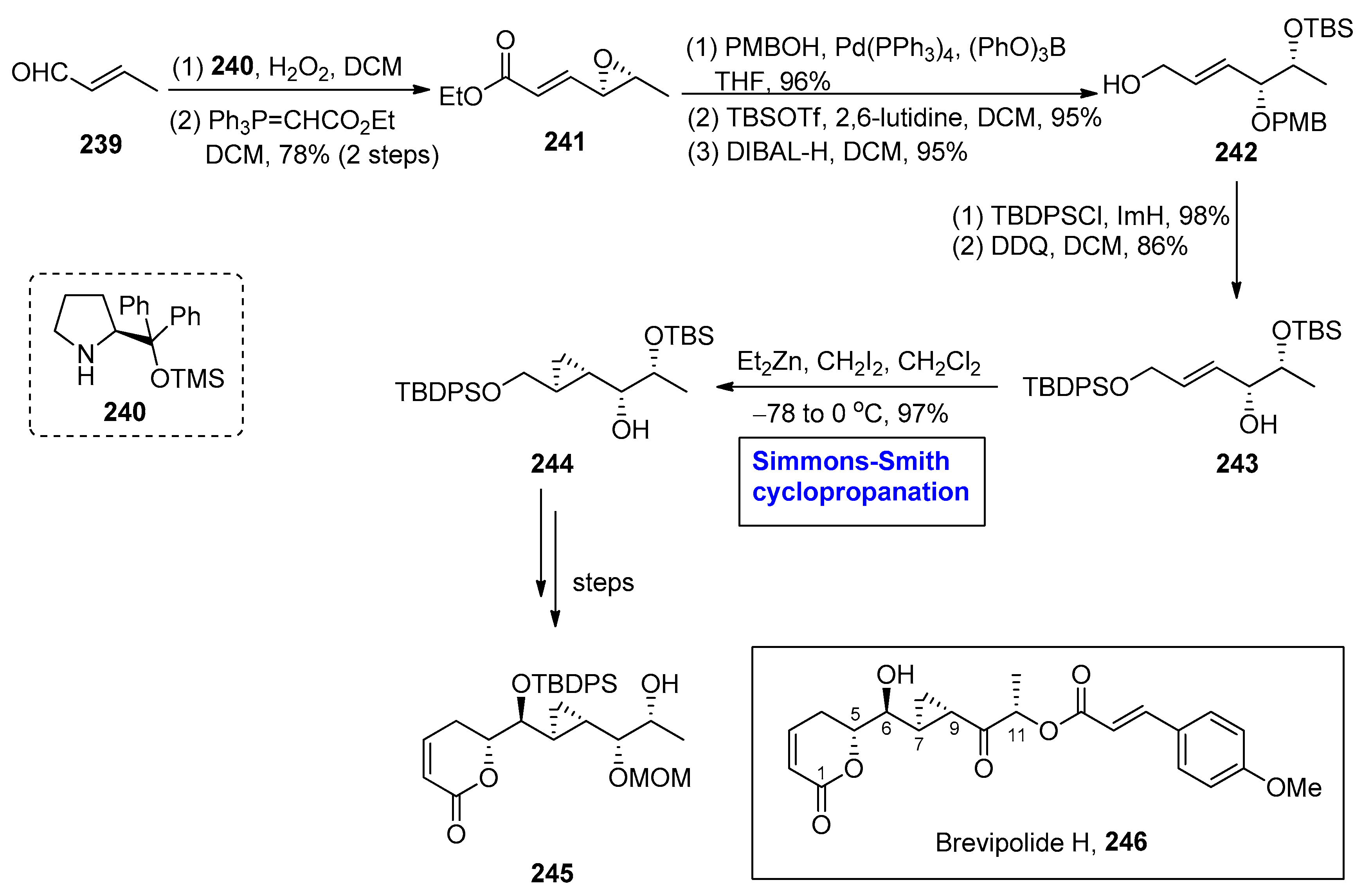
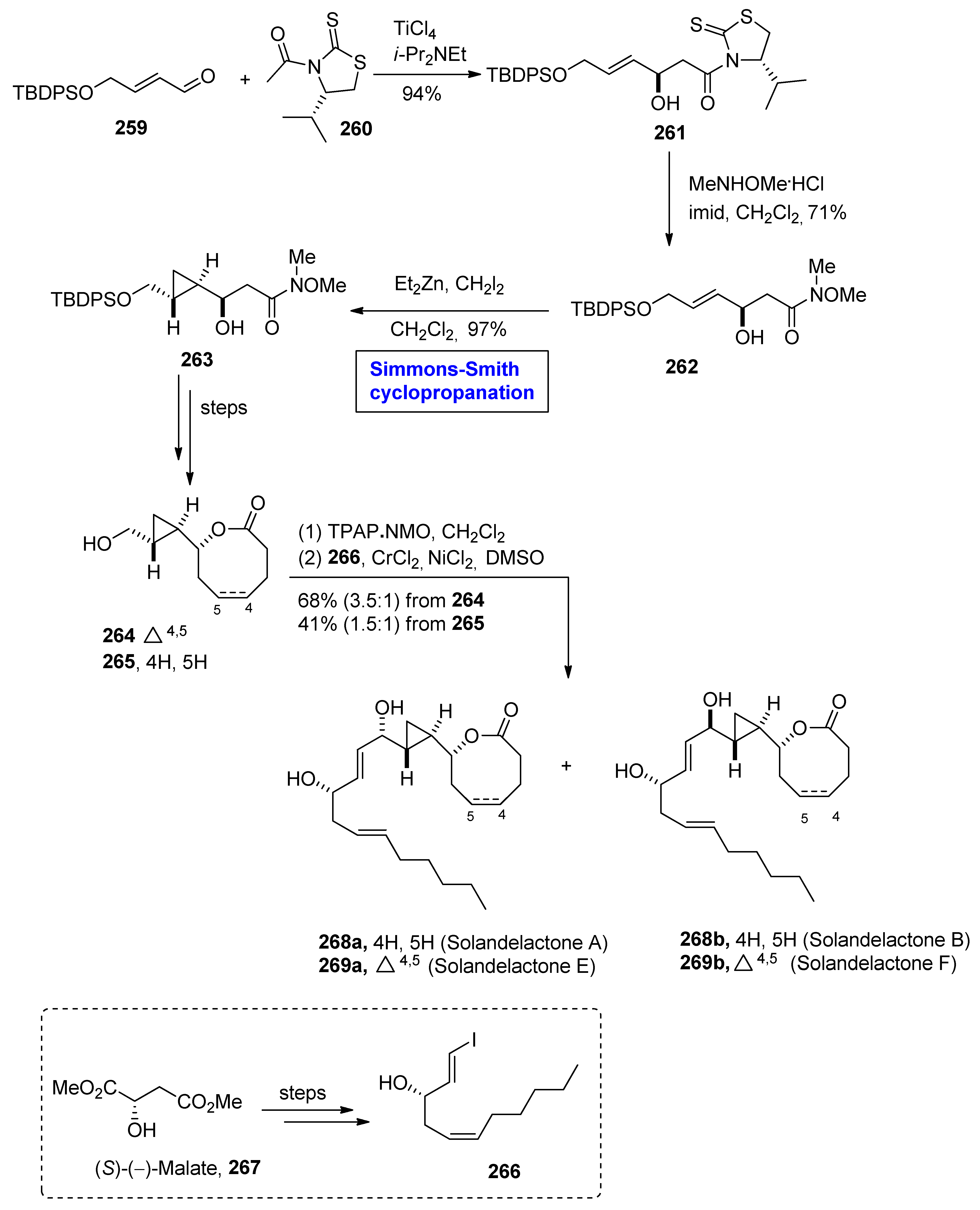
2.7. Synthesis of Fatty-Acid-Based Natural Products
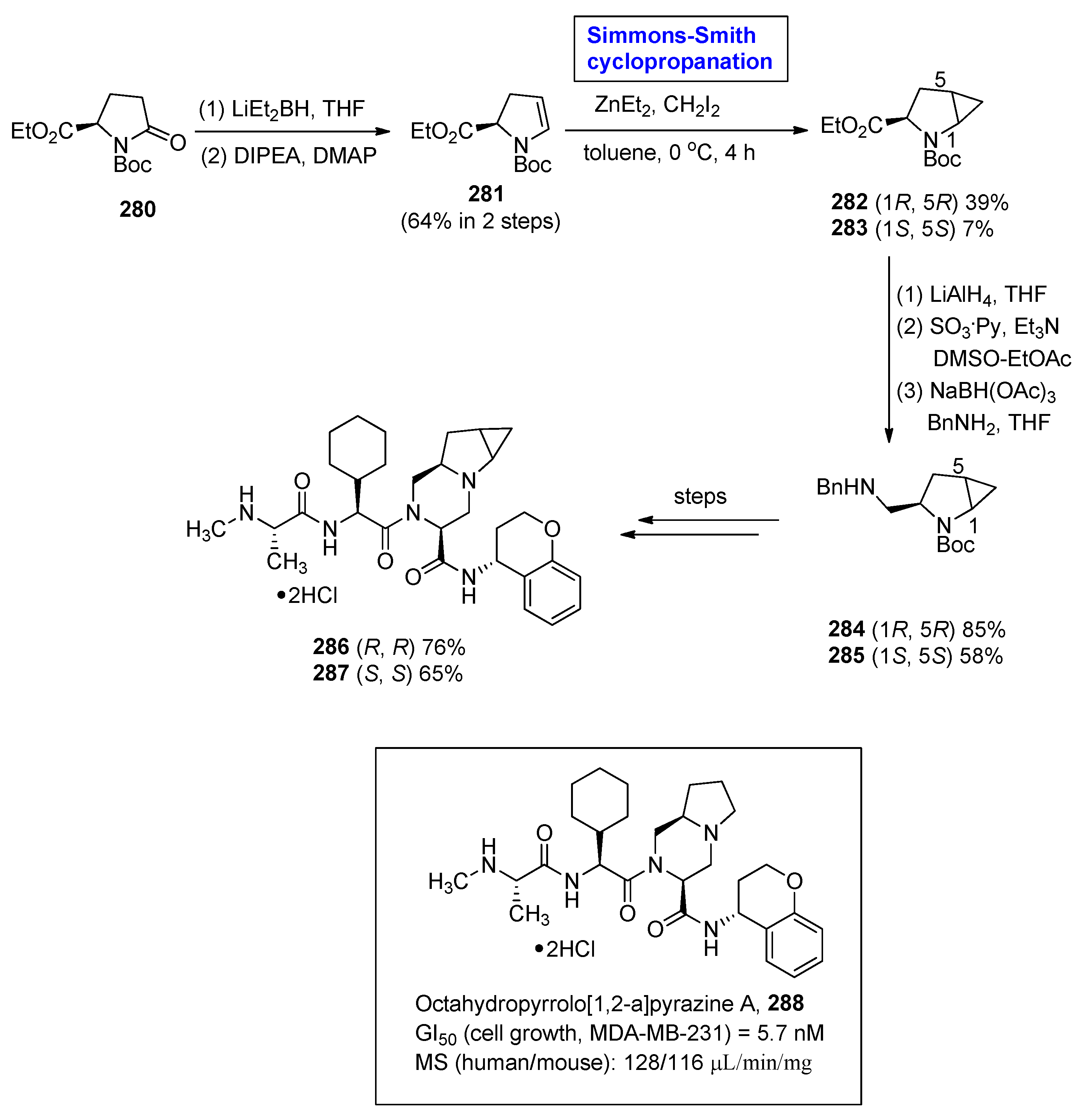
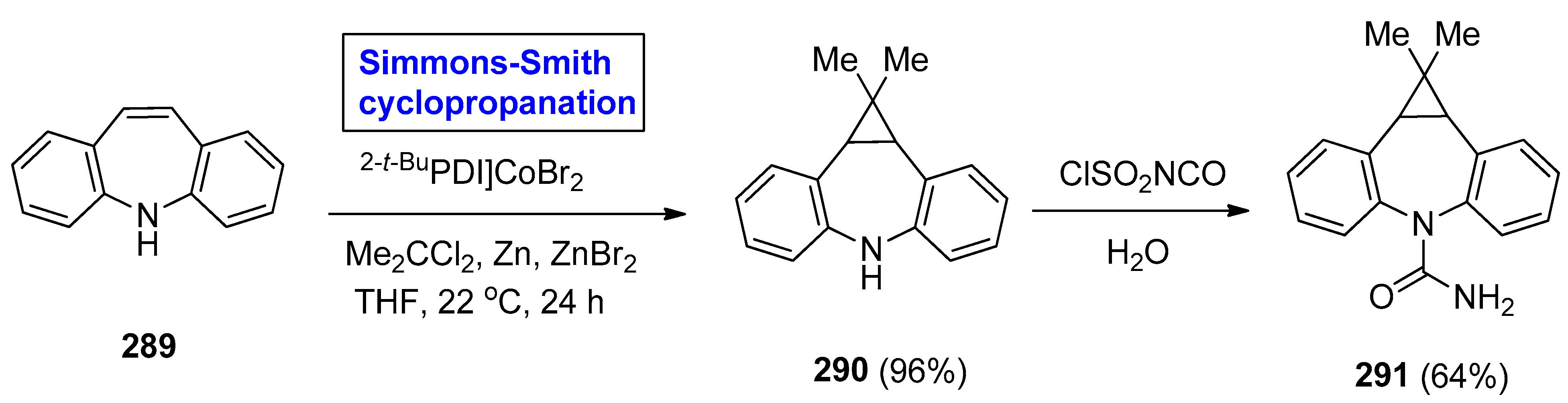
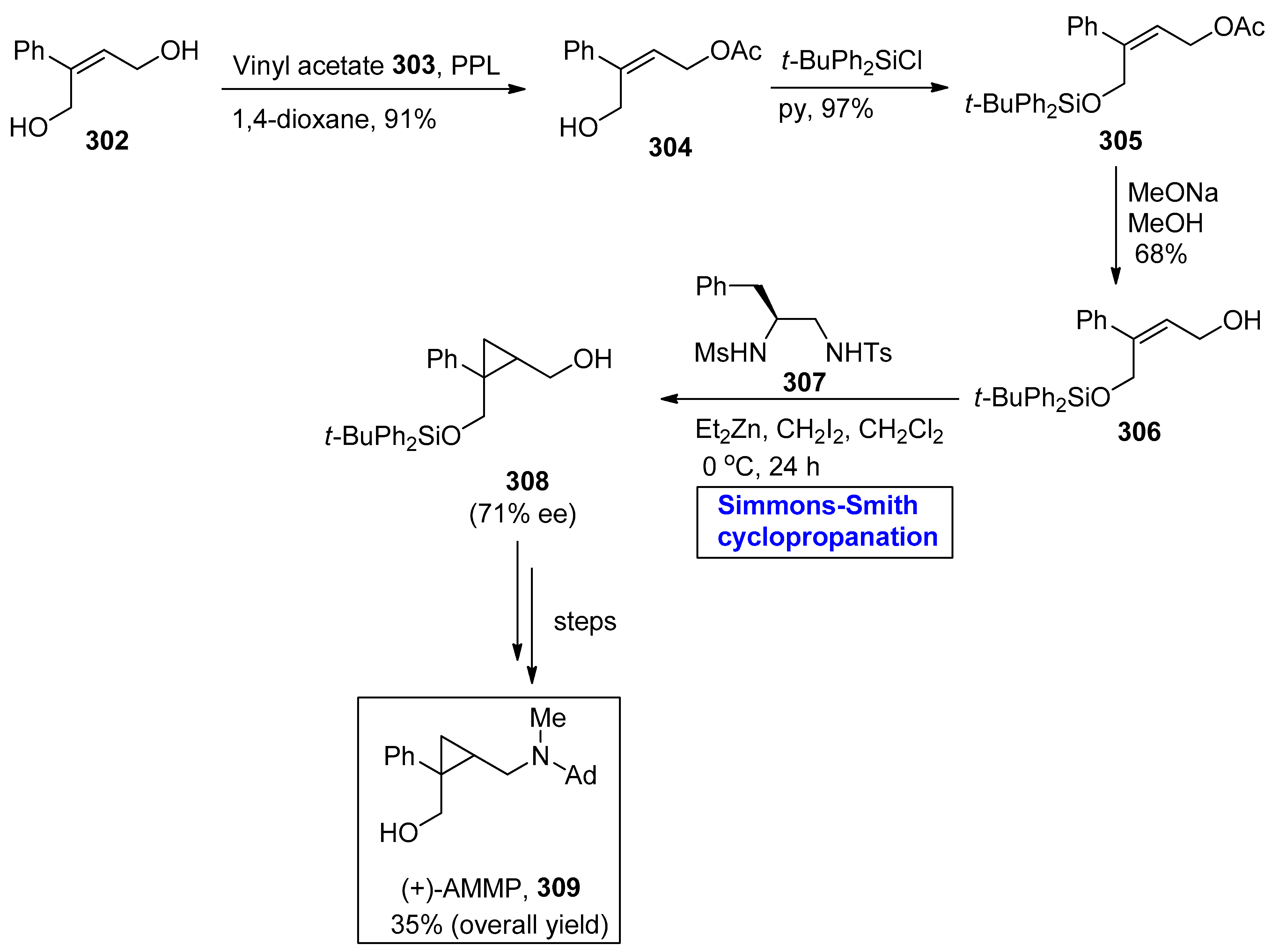
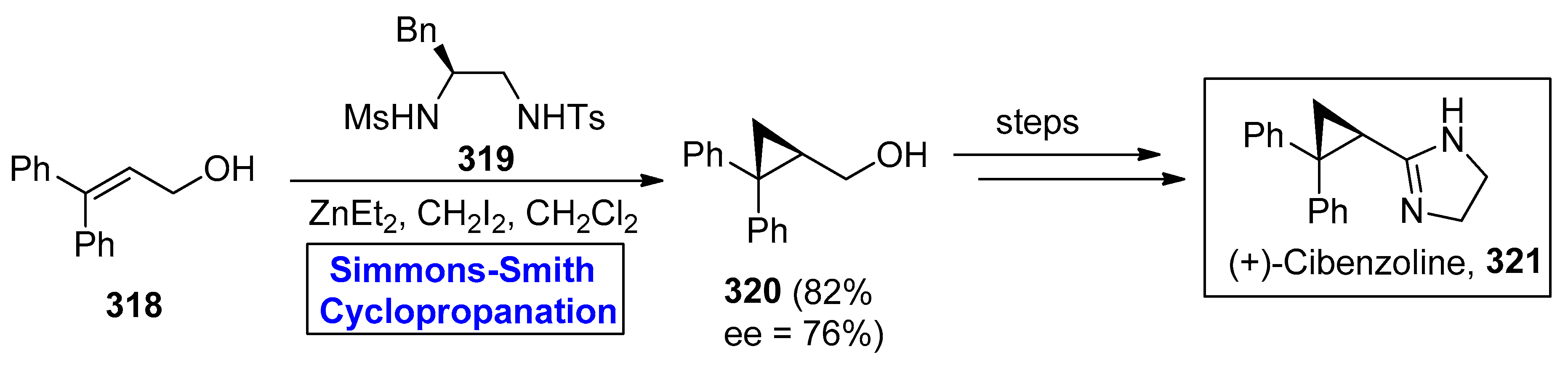
2.8. Synthesis of Drugs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakamura, M.; Hirai, A.; Nakamura, E. Reaction pathways of the Simmons−Smith reaction. J. Am. Chem. Soc. 2003, 125, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Edwards, J.P.; Wilson, S.R. Solution and solid-state structure of the “Wittig-Furukawa” cyclopropanation reagent. J. Am. Chem. Soc. 1991, 113, 723–725. [Google Scholar] [CrossRef]
- Charette, A.B.; Juteau, H. Design of amphoteric bifunctional ligands: Application to the enantioselective Simmons-Smith cyclopropanation of allylic alcohols. J. Am. Chem. Soc. 1994, 116, 2651–2652. [Google Scholar] [CrossRef]
- Ukaji, Y.; Sada, K.; Inomata, K. Synthesis of silicon substituted cyclopropylmethyl alcohols in optically active form via asymmetric Simmons-Smith reaction of γ-silicon substituted allylic alcohols. Chem. Lett. 1993, 22, 1227–1230. [Google Scholar] [CrossRef]
- Gonzalez, J.; Gonzalez, J.; Perez-Calleja, C.; Lopez, L.A.; Vicente, R. Zinc-Catalyzed Synthesis of Functionalized Furans and Triarylmethanes from Enynones and Alcohols or Azoles: Dual X–H Bond Activation by Zinc. Angew. Chem. Int. Ed. 2013, 52, 5853–5857. [Google Scholar] [CrossRef]
- Simmons, H.E.; Smith, R.D. A new synthesis of cyclopropanes from olefins. J. Am. Chem. Soc. 1958, 80, 5323–5324. [Google Scholar] [CrossRef]
- Simmons, H.E.; Smith, R.D. A new synthesis of cyclopropanes1. J. Am. Chem. Soc. 1959, 81, 4256–4264. [Google Scholar] [CrossRef]
- Furukawa, J.; Kawabata, N.; Nishimura, J. Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide. Tetrahedron 1968, 24, 53–58. [Google Scholar] [CrossRef]
- Long, J.; Du, H.; Li, K.; Shi, Y. Catalytic asymmetric Simmons–Smith cyclopropanation of unfunctionalized olefins. Tetrahedron Lett. 2005, 46, 2737–2740. [Google Scholar] [CrossRef]
- Sawada, S.; Oda, J.; Inouye, Y. Partial asymmetric synthesis in the Simmons-Smith reaction. II. J. Org. Chem. 1968, 33, 2141–2143. [Google Scholar] [CrossRef]
- Fujii, K.; Misaki, T.; Sugimura, T. Another Role of Copper in the Simmons–Smith Reaction: Copper-catalyzed Nucleophilic Michael-type Cyclopropanation of α, β-Unsaturated Ketones. Chem. Lett. 2014, 43, 634–636. [Google Scholar] [CrossRef]
- Simmons, H.E.; Blanchard, E.P.; Smith, R.D. Cyclopropane synthesis from methylene iodide, zinc-copper couple, and olefins. III. The methylene-transfer reaction. J. Am. Chem. Soc. 1964, 86, 1347–1356. [Google Scholar] [CrossRef]
- Imamoto, T.; Takeyama, T.; Koto, H. The reaction of carbonyl compounds with diiodomethane in the presence of samarium: Novel syntheses of iodohydrins and cyclopropanols. Tetrahedron Lett. 1985, 27, 3243–3246. [Google Scholar] [CrossRef]
- Friedrich, E.C.; Biresaw, G. Zinc dust-cuprous chloride promoted cyclopropanations of allylic alcohols using ethylidene iodide. J. Org. Chem. 1982, 47, 1615–1618. [Google Scholar] [CrossRef]
- Fujii, K.; Shiine, K.; Misaki, T.; Sugimura, T. Efficient Simmons–Smith cyclopropanation with Zn/Cu and CH2I2. Appl. Organomet. Chem. 2013, 27, 69–72. [Google Scholar] [CrossRef]
- Denmark, S.E.; Edwards, J.P.; Wilson, S.R. Solution-and solid-state structural studies of (halomethyl) zinc reagents. J. Am. Chem. Soc. 1992, 114, 2592–2602. [Google Scholar] [CrossRef]
- Donaldson, W. Synthesis of cyclopropane containing natural products. Tetrahedron 2011, 27, 8589–8627. [Google Scholar] [CrossRef]
- Lu, T.; Hayashi, R.; Hsung, R.P.; DeKorver, K.A.; Lohse, A.G.; Song, Z.; Tang, Y. Synthesis of amido-spiro [2.2] pentanes via Simmons–Smith cyclopropanation of allenamides. Org. Biomol. Chem. 2009, 7, 3331–3337. [Google Scholar] [CrossRef]
- Mali, M.; Sharma, G.V.; Ghosh, S.; Roisnel, T.; Carboni, B.; Berree, F. Simmons–Smith Cyclopropanation of Alkenyl 1, 2-Bis (boronates): Stereoselective Access to Functionalized Cyclopropyl Derivatives. J. Org. Chem. 2022, 87, 7649–7657. [Google Scholar] [CrossRef] [PubMed]
- Pietruszka, J. Synthesis and properties of oligocyclopropyl-containing natural products and model compounds. Chem. Rev. 2003, 103, 1051–1070. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Brandt, W.; Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev. 2003, 103, 1625–1648. [Google Scholar] [CrossRef]
- Salaun, J. Cyclopropane derivatives and their diverse biological activities. In Small Ring Compounds in Organic Synthesis; Springer: Berlin/Heidelberg, Germany, 2000; Volume 207, ISBN 978-3-540-48255-0. [Google Scholar] [CrossRef]
- Kim, H.Y.; Walsh, P.J. Tandem catalytic enantio-and diastereoselective synthesis of cyclopropyl alcohols using aryl aldehydes. J. Phys. Org. Chem. 2012, 25, 933–938. [Google Scholar] [CrossRef]
- Long, J.; Yuan, Y.; Shi, Y. Asymmetric Simmons−Smith Cyclopropanation of Unfunctionalized Olefins. J. Am. Chem. Soc. 2003, 125, 13632–13633. [Google Scholar] [CrossRef] [PubMed]
- Arai, I.; Mori, A.; Yamamoto, H. An asymmetric Simmons-Smith reaction. J. Am. Chem. Soc. 1985, 107, 8254–8256. [Google Scholar] [CrossRef]
- Verpoorte, R.; van der Heijden, R.; Moreno, P.R. Biosynthesis of terpenoid indole alkaloids in Catharanthus roseus cells. In The Alkaloids: Chemistry and Pharmacology; Academic Press: Cambridge, MA, USA, 1997; Volume 49, pp. 221–299. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, A.F.; Asrar, M.; Selamoglu, Z.; Ji, X.Y.; Adem, S.; Sarker, S.D. 6-Phosphogluconate dehydrogenase fuels multiple aspects of cancer cells: From cancer initiation to metastasis and chemoresistance. BioFactors 2020, 46, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Zahoor, A.F.; Parveen, B.; Rasul, A.; Raza, Z.; Ahmad, S.; Irfan, A.; El-Hiti, G.A. Acefylline derivatives as a new class of anticancer agents: Synthesis, molecular docking, and anticancer, hemolytic, and thrombolytic activities of acefylline-triazole hybrids. J. Chem. 2022, 2022, 3502872. [Google Scholar] [CrossRef]
- Sears, J.E.; Boger, D.L. Total synthesis of vinblastine, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure–function properties. Acc. Chem. Res. 2015, 48, 653–662. [Google Scholar] [CrossRef]
- Keglevich, P.; Hazai, L.; Kalaus, G.; Szántay, C. Cyclopropanation of some alkaloids. Period. Polytech. Chem. Eng. 2015, 59, 3–15. [Google Scholar] [CrossRef]
- Keglevich, A.; Mayer, S.; Pspai, R.; Szigetvsri, A.; Santa, Z.; Dekany, M.; Szantay, C.; Keglevich, P.; Hazai, L. Attempted synthesis of vinca alkaloids condensed with three-membered rings. Molecules 2018, 23, 2574. [Google Scholar] [CrossRef]
- Kam, T.S.; Yoganathan, K.; Chuah, C.H. Lundurines A, B and C, new indole alkaloids with a novel carbon skeleton containing a cyclopropyl moiety. Tetrahedron Lett. 1995, 36, 759–762. [Google Scholar] [CrossRef]
- Kam, T.S.; Lim, K.H.; Yoganathan, K.; Hayashi, M.; Komiyama, K. Lundurines A–D, cytotoxic indole alkaloids incorporating a cyclopropyl moiety from Kopsia tenuis and revision of the structures of tenuisines A–C. Tetrahedron 2004, 60, 10739–10745. [Google Scholar] [CrossRef]
- Jin, S.; Gong, J.; Qin, Y. Total Synthesis of (−)-Lundurine A and Determination of its Absolute Configuration. Angew. Chem. Int. Ed. 2015, 127, 2256–2259. [Google Scholar] [CrossRef]
- Zhou, Z.F.; Liu, H.L.; Zhang, W.; Kurtan, T.; Mandi, A.; Benyei, A.; Li, J.; Taglialatela-Scafati, O.; Guo, Y.W. Bioactive rearranged limonoids from the Chinese mangrove Xylocarpus granatum Koenig. Tetrahedron 2014, 70, 6444–6449. [Google Scholar] [CrossRef]
- Pan, J.Y.; Chen, S.L.; Li, M.Y.; Li, J.; Yang, M.H.; Wu, J. Limonoids from the Seeds of a Hainan Mangrove, Xylocarpus granatum. J. Nat. Prod. 2010, 73, 1672–1679. [Google Scholar] [CrossRef]
- Schuppe, A.W.; Huang, D.; Chen, Y.; Newhouse, T.R. Total synthesis of (−)-Xylogranatopyridine B via a Palladium-catalyzed oxidative stannylation of enones. J. Am. Chem. Soc. 2018, 140, 2062–2066. [Google Scholar] [CrossRef]
- Munir, I.; Zahoor, A.F.; Rasool, N.; Naqvi, S.A.R.; Zia, K.M.; Ahmad, R. Synthetic applications and methodology development of Chan–Lam coupling: A review. Mol. Divers. 2019, 23, 215–259. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, K.; Qiu, Y.; He, H.; Gao, S. A unified strategy to construct the tetracyclic ring of calyciphylline A alkaloids: Total synthesis of himalensine A. Org. Lett. 2019, 21, 3741–3745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Guo, L.D.; Tian, P.; Xu, T.; Xu, J. Synthesis of the core structure of Daphnimacropodines. Org. Lett. 2019, 21, 4309–4312. [Google Scholar] [CrossRef]
- Hanessian, S.; Dorich, S.; Menz, H. Concise and stereocontrolled synthesis of the tetracyclic core of daphniglaucin C. Org. Lett. 2013, 15, 4134–4137. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.D.; Xu, J. Synthesis of the tricyclic skeleton of Daphniphyllum alkaloids daphnimacropodines. Tetrahedron Lett. 2021, 71, 153030. [Google Scholar] [CrossRef]
- Nakazawa, J.; Yajima, J.; Usui, T.; Ueki, M.; Takatsuki, A.; Imoto, M.; Toyoshima, Y.Y.; Osada, H. A novel action of terpendole E on the motor activity of mitotic Kinesin Eg5. Chem. Biol. 2003, 10, 131–137. [Google Scholar] [CrossRef]
- Tarui, Y.; Chinen, T.; Nagumo, Y.; Motoyama, T.; Hayashi, T.; Hirota, H.; Muroi, M.; Ishii, Y.; Kondo, H.; Osada, H.; et al. Terpendole E and its Derivative Inhibit STLC-and GSK-1-Resistant Eg5. ChemBioChem 2014, 15, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, T.; Murokawa, T.; Enomoto, M.; Kuwahara, S. Synthesis of (±)-terpendole E. Biosci. Biotechnol. Biochem. 2015, 79, 11–15. [Google Scholar] [CrossRef]
- Ogata, M.; Ueda, J.Y.; Hoshi, M.; Hashimoto, J.; Nakashima, T.; Anzai, K.; Takagi, M.; Shin-ya, K. A novel indole-diterpenoid, JBIR-03 with anti-MRSA activity from Dichotomomyces cejpii var. cejpii NBRC 103559. J. Antibiot. 2007, 60, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.F.; Ji, N.Y.; Liu, X.H.; Li, K.; Zhu, Q.M.; Xue, Q.Z. Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg. Med. Chem. Lett. 2010, 20, 5677–5680. [Google Scholar] [CrossRef] [PubMed]
- Tetsuro, M.; Masaru, E.; Takaaki, T.; Yusuke, O.; Shigefumi, K. Total synthesis of JBIR-03 and asporyzin C. Tetrahedron Lett. 2018, 59, 4107–4109. [Google Scholar] [CrossRef]
- Liu, G.; Romo, D. Cover Picture: Total Synthesis of (+)-Omphadiol. Angew. Chem. Int. Ed. 2011, 50, 7449. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, Y.; Xu, W.; Liang, G. Total syntheses of (±)-omphadiol and (±)-pyxidatol C through a cis-fused 5, 7-carbocyclic common intermediate. J. Org. Chem. 2014, 79, 5345–5350. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Eggert, U.; Kalesse, M. Synthesis of (+)-Omphadiol and (+)-Pyxidatol C. Org. Lett. 2016, 18, 2320–2322. [Google Scholar] [CrossRef]
- McMorris, T.C.; Lira, R.; Gantzel, P.K.; Kelner, M.J.; Dawe, R. Sesquiterpenes from the Basidiomycete Omphalotus i lludens. J. Nat. Prod. 2000, 63, 1557–1559. [Google Scholar] [CrossRef]
- Zheng, Y.B.; Lu, C.H.; Zheng, Z.H.; Lin, X.J.; Su, W.J.; Shen, Y.M. New sesquiterpenes from edible fungus Clavicorona pyxidata. Helv. Chim. Acta 2008, 91, 2174–2180. [Google Scholar] [CrossRef]
- Osler, J.D.; Unsworth, W.P.; Taylor, R.J. Synthetic Studies towards the Africanane Sesquiterpenes via the Cope Rearrangement of gem-Dimethyl-Substituted Divinyl Cyclopropanes. Synlett 2016, 27, 70–74. [Google Scholar] [CrossRef]
- Singh, V.; Thomas, B. Recent developments in general methodologies for the synthesis of linear triquinanest. Tetrahedron 1998, 54, 3647–3692. [Google Scholar] [CrossRef]
- Mehta, G.; Srikrishna, A. Synthesis of polyquinane natural products: An update. Chem. Rev. 1997, 97, 671–720. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Yuan, C.; Yu, Z.X. Tandem Rh (I)-catalyzed [(5+ 2)+ 1] cycloaddition/aldol reaction for the construction of linear triquinane skeleton: Total syntheses of (±)-hirsutene and (±)-1-desoxyhypnophilin. J. Am. Chem. Soc. 2008, 130, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Gao, Z.B.; Wang, F.D.; Liao, S.G.; Chen, H.D.; Zhang, C.R.; Hu, G.Y.; Yue, J.M. Chlorahololides A and B, two potent and selective blockers of the potassium channel isolated from Chloranthus holostegius. Org. Lett. 2007, 9, 903–906. [Google Scholar] [CrossRef]
- Liu, Y.; Nan, F.J. Synthetic studies towards Chlorahololides A: Practical synthesis of a lindenane-type sesquiterpenoid core framework with a 5, 6-double bond. Tetrahedron Lett. 2010, 51, 1374–1376. [Google Scholar] [CrossRef]
- Qian, S.; Zhao, G. Synthetic studies toward the total synthesis of Chlorahololide A. Synlett 2011, 722–724. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Ying, Y.M.; Ma, L.F.; Shan, W.G. Natural disesquiterpenoids. Nat. Prod. Rep. 2011, 28, 594–629. [Google Scholar] [CrossRef]
- Takeda, Y.; Yamashita, H.; Matsumoto, T.; Terao, H. Chloranthalactone F, a sesquiterpenoid from the leaves of Chloranthus glaber. Phytochemistry 1993, 33, 713–715. [Google Scholar] [CrossRef]
- Qian, S.; Zhao, G. Total synthesis of (+)-chloranthalactone F. Chem. Commun. 2012, 48, 3530–3532. [Google Scholar] [CrossRef] [PubMed]
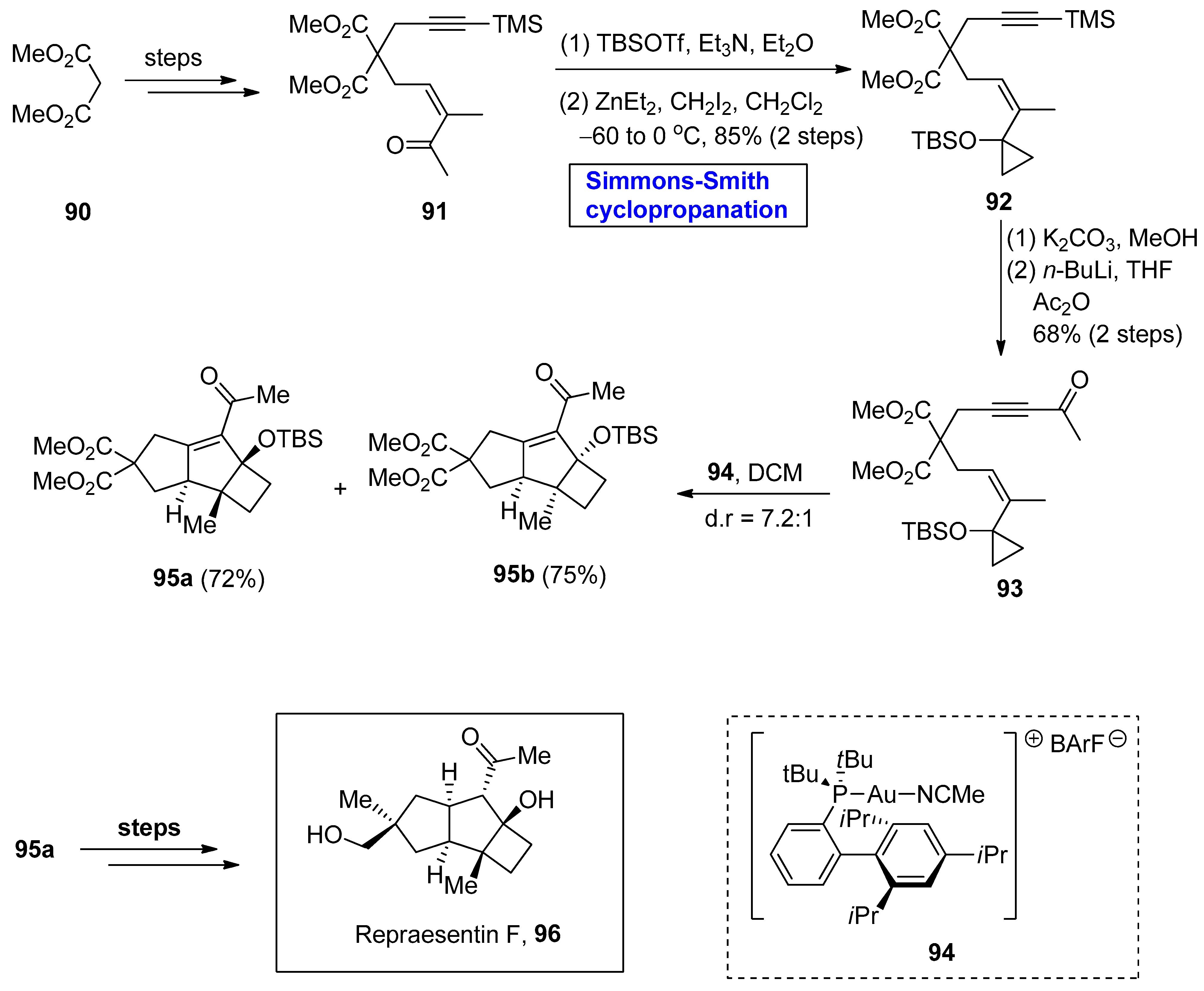
- Kashiwabara, M.; Kamo, T.; Makabe, H.; Shibata, H.; Hirota, M. Repraesentins D, E and F, new plant growth promoters from Lactarius repraesentaneus. Biosci. Biotechnol. Biochem. 2006, 70, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Shimizu, Y.; Kamo, T.; Makabe, H.; Shibata, H. New plant growth promoters, repraesentins A, B and C, from Lactarius repraesentaneus. Biosci. Biotechnol. Biochem. 2003, 67, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, S.; Echavarren, A.M. Total synthesis of repraesentin F and configuration reassignment by a gold (I)-catalyzed cyclization cascade. Org. Lett. 2018, 20, 5784–5788. [Google Scholar] [CrossRef]
- Khatri Chhetri, B.; Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Raghavan, V.; Gagaring, K.; Dale, B.; McNamara, C.W.; Soapi, K.; Quave, C.L.; et al. Peyssonnosides A–B, unusual diterpene glycosides with a sterically encumbered cyclopropane motif: Structure elucidation using an integrated spectroscopic and computational workflow. J. Org. Chem. 2019, 84, 8531–8541. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation strategies in recent total syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef] [PubMed]
- Chesnokov, G.A.; Gademann, K. Concise Total Synthesis of Peyssonnoside A. J. Am. Chem. Soc. 2021, 143, 14083–14088. [Google Scholar] [CrossRef]
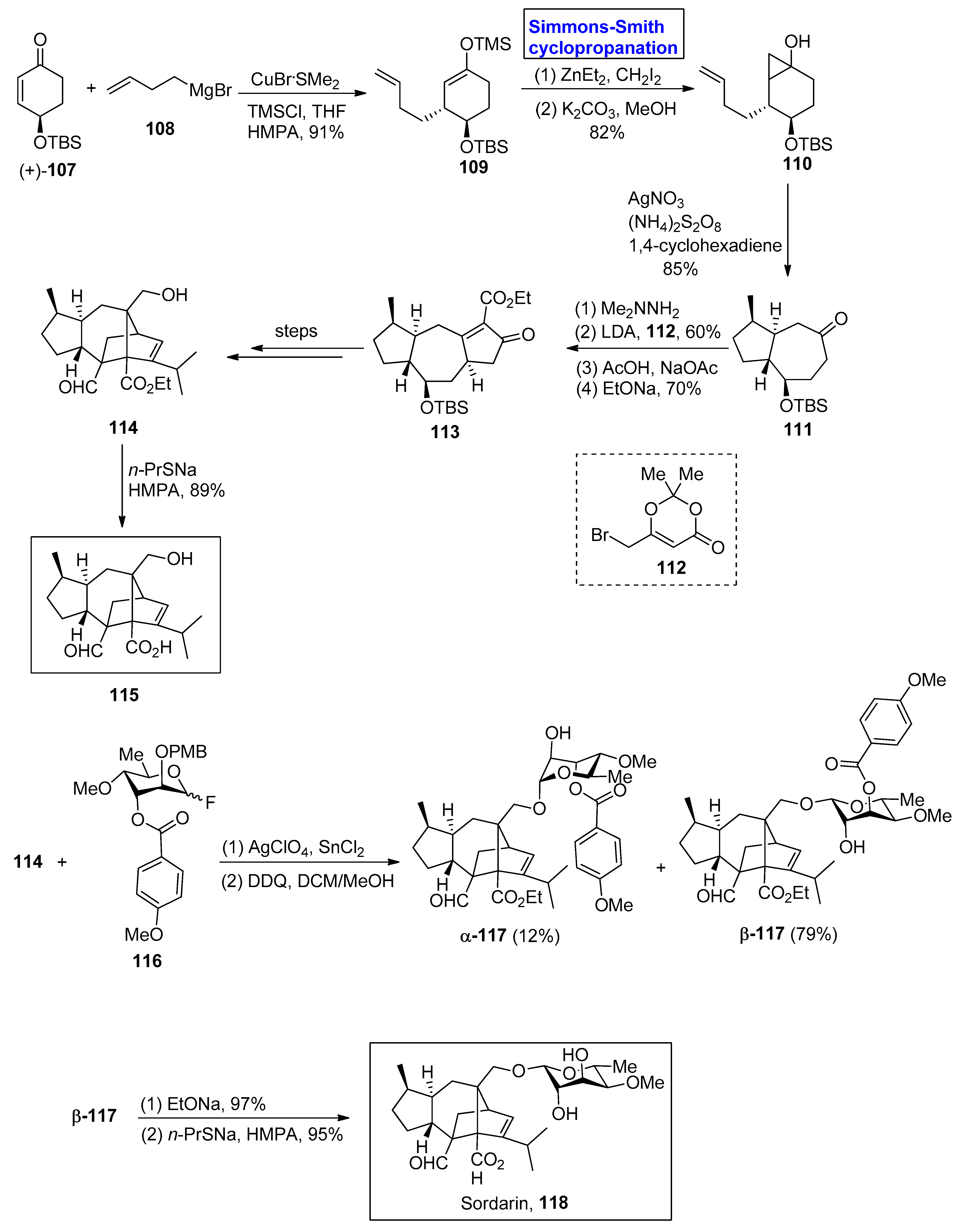
- Justice, M.C.; Hsu, M.J.; Tse, B.; Ku, T.; Balkovec, J.; Schmatz, D.; Nielsen, J. Elongation factor 2 as a novel target for selective inhibition of fungal protein synthesis. J. Biol. Chem. 1998, 273, 3148–3151. [Google Scholar] [CrossRef]
- Quesnelle, C.A.; Gill, P.; Dodier, M.; Laurent, D.S.; Serrano-Wu, M.; Marinier, A.; Martel, A.; Mazzucco, C.E.; Stickle, T.M.; Barrett, J.F.; et al. Sordaricin antifungal agents. Bioorg. Med. Chem. Lett. 2003, 13, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Kitamura, M.; Narasaka, K. Synthesis of (−)-sordarin. J. Am. Chem. Soc. 2006, 128, 6931–6937. [Google Scholar] [CrossRef]
- Block, S.; Gerkens, P.; Peulen, O.; Jolois, O.; Mingeot-Leclercq, M.P.; De Pauw-gillet, M.C.; Quetin-Leclercq, J. Induction of apoptosis in human promyelocytic leukemia cells by a natural trachylobane diterpene. Anticancer Res. 2005, 25, 363–368. [Google Scholar] [PubMed]
- Block, S.; Baccelli, C.; Tinant, B.; Van Meervelt, L.; Rozenberg, R.; Jiwan, J.L.H.; Llabres, G.; Pauw-Gillet, M.C.D.; Quetin-Leclercq, J. Diterpenes from the leaves of Croton zambesicus. Phytochemistry 2004, 65, 1165–1171. [Google Scholar] [CrossRef]
- Abad, A.; Agullo, C.; Cunat, A.C.; de Alfonso Marzal, I.; Navarro, I.; Gris, A. A unified synthetic approach to trachylobane-, beyerane-, atisane-and kaurane-type diterpenes. Tetrahedron 2006, 62, 3266–3283. [Google Scholar] [CrossRef]
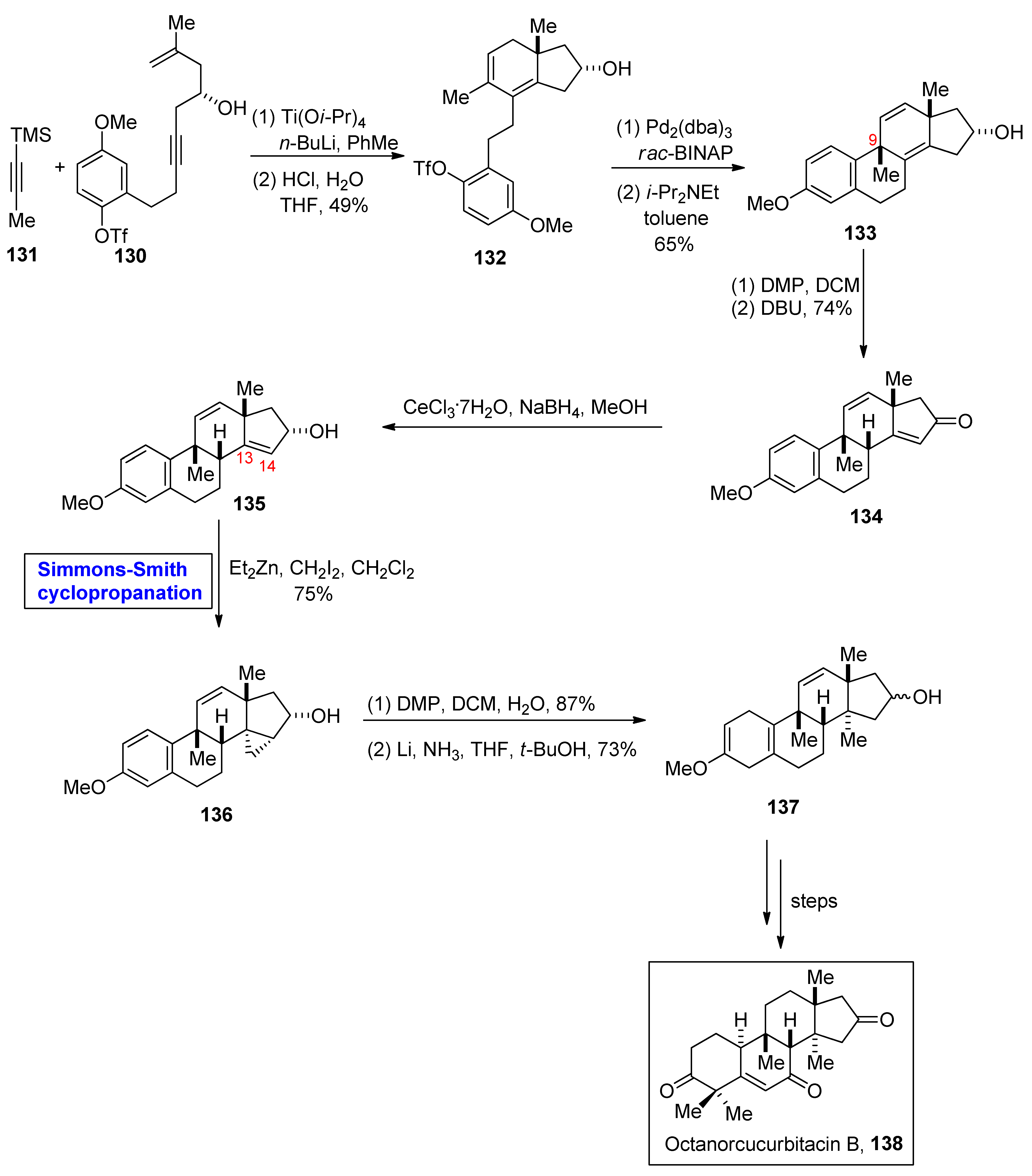
- Eddy, N.A.; Fenteany, G. Model studies directed to the synthesis of cucurbitacin IC/D rings. Tetrahedron Lett. 2015, 56, 5079–5081. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, C.; Molnar, J.; Mulhovo, S.; Rosario, V.E.; Ferreira, M.J.U. New potent P-glycoprotein modulators with the cucurbitane scaffold and their synergistic interaction with doxorubicin on resistant cancer cells. Bioorg. Med. Chem. 2009, 17, 6942–6951. [Google Scholar] [CrossRef] [PubMed]
- Bucknam, A.R.; Micalizio, G.C. Asymmetric de novo synthesis of a cucurbitane triterpenoid: Total synthesis of octanorcucurbitacin B. J. Am. Chem. Soc. 2022, 144, 8493–8497. [Google Scholar] [CrossRef] [PubMed]
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology 2009, 49, 1335–1374. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, F.R.; Levinskas, G.J. Subchronic toxicity of tetramethylsuccinonitrile. Fundam. Appl. Toxicol. 1986, 7, 41–48. [Google Scholar] [CrossRef]
- Young, I.S.; Qiu, Y.; Smith, M.J.; Hay, M.B.; Doubleday, W.W. Preparation of a tricyclopropylamino acid derivative via Simmons–Smith cyclopropanation with downstream intramolecular aminoacetoxylation for impurity control. Org. Process Res. Dev. 2016, 20, 2108–2115. [Google Scholar] [CrossRef]
- Tandon, M.; Wu, M.; Begley, T.P.; Myllyharju, J.; Pirskanen, A.; Kivirikko, K. Substrate specificity of human prolyl-4-hydroxylase. Bioorg. Med. Chem. Lett. 1998, 8, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
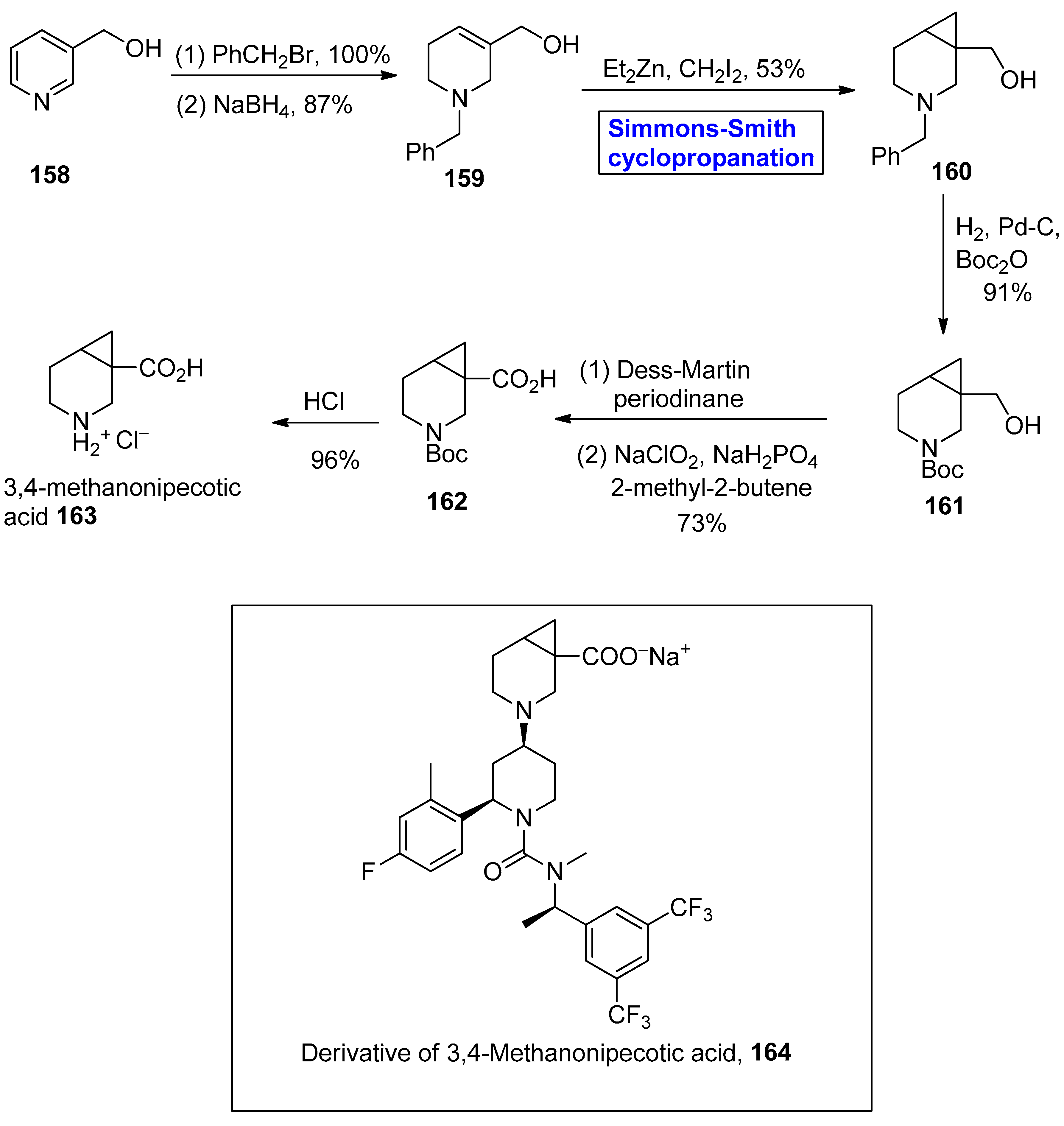
- Grygorenko, O.O.; Radchenko, D.S.; Volochnyuk, D.M.; Tolmachev, A.A.; Komarov, I.V. Bicyclic conformationally restricted diamines. Chem. Rev. 2011, 111, 5506–5568. [Google Scholar] [CrossRef] [PubMed]
- Tymtsunik, A.V.; Bilenko, V.A.; Ivon, Y.M.; Grygorenko, O.O.; Komarov, I.V. Synthesis of a novel Boc-protected cyclopropane-modified proline analogue. Tetrahedron Lett. 2012, 53, 3847–3849. [Google Scholar] [CrossRef]
- Savage, S.A.; Jones, G.S.; Kolotuchin, S.; Ramrattan, S.A.; Vu, T.; Waltermire, R.E. Preparation of saxagliptin, a novel DPP-IV inhibitor. Org. Process Res. Dev. 2009, 13, 1169–1176. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Miranda, P.C.; Correia, C.R. Efficient and expeditious protocols for the synthesis of racemic and enantiomerically pure endocyclic enecarbamates from N-acyl lactams and N-acyl pyrrolidines. J. Org. Chem. 1999, 64, 6646–6652. [Google Scholar] [CrossRef]
- Tymtsunik, A.V.; Ivon, Y.M.; Komarov, I.V.; Grygorenko, O.O. Synthesis of Boc-protected 4, 5-methano-β-proline. Tetrahedron Lett. 2014, 55, 3312–3315. [Google Scholar] [CrossRef]
- Rizzo, S.; Waldmann, H. Development of a Natural-Product-Derived Chemical Toolbox for Modulation of Protein Function. Chem. Rev. 2014, 114, 4621–4639. [Google Scholar] [CrossRef]
- Spiteller, P.; Von Nussbaum, F. β-Amino Acids in Natural Products. In Enantioselective Synthesis of β-Amino Acids; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 19–91. [Google Scholar] [CrossRef]
- Tymtsunik, A.V.; Ivon, Y.M.; Komarov, I.V.; Grygorenko, O.O. Synthesis of racemic and enantiopure 3, 4-methanonipecotic acid. Tetrahedron Asymmetry 2015, 26, 1268–1272. [Google Scholar] [CrossRef]
- Frantz, M.C.; Wipf, P. Mitochondria as a target in treatment. Environ. Mol. Mutagen. 2010, 51, 462–475. [Google Scholar] [CrossRef]
- Epperly, M.W.; Goff, J.P.; Li, S.; Gao, X.; Wipf, P.; Dixon, T.; Wang, H.; Franicola, D.; Shen, H.; Rwigema, J.C.M.; et al. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo 2010, 24, 811–819. [Google Scholar]
- Frantz, M.C.; Pierce, J.G.; Pierce, J.M.; Kangying, L.; Qingwei, W.; Johnson, M.; Wipf, P. Large-scale asymmetric synthesis of the bioprotective agent JP4-039 and analogs. Org. Lett. 2011, 13, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.N.; Radchenko, D.S.; Mykhailiuk, P.K.; Grygorenko, O.O.; Komarov, I.V. 4-Fluoro-2, 4-methanoproline. Org. Lett. 2009, 11, 5674–5676. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Nettles, R.E.; Belema, M.; Snyder, L.B.; Nguyen, V.N.; Fridell, R.A.; Serrano-Wu, M.H.; Langley, D.R.; Sun, J.H.; O’Boyle, D.R.; et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 2010, 465, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; James, C.A.; Meanwell, N.A.; Hamann, L.G.; Belema, M. A scalable synthesis of (1R, 3S, 5R)-2-(tert-butoxycarbonyl)-2-azabicyclo [3.1. 0] hexane-3-carboxylic acid. Tetrahedron Lett. 2013, 54, 6722–6724. [Google Scholar] [CrossRef]
- Saenger, W. Defining terms for the nucleic acids. In Principles of Nucleic Acid Structure; Springer: New York, NY, USA, 1984; pp. 9–28. [Google Scholar] [CrossRef]
- Gagneron, J.; Gosselin, G.; Mathe, C. Synthesis of nucleoside analogues bearing the five naturally occurring nucleic acid bases built on a 2-oxabicylo [3.1. 0] hexane scaffold. J. Org. Chem. 2005, 70, 6891–6897. [Google Scholar] [CrossRef]
- Toti, K.S.; Osborne, D.; Ciancetta, A.; Boison, D.; Jacobson, K.A. South (S)-and North (N)-methanocarba-7-deazaadenosine analogues as inhibitors of human adenosine kinase. J. Med. Chem. 2016, 59, 6860–6877. [Google Scholar] [CrossRef]
- An, S.; Kim, G.; Kim, H.J.; Ahn, S.; Kim, H.Y.; Ko, H.; Hyun, Y.E.; Nguyen, M.; Joeng, J.; Liu, Z.; et al. Discovery and structure–activity relationships of novel template, truncated 1′-homologated adenosine derivatives as pure dual PPARγ/δ modulators. J. Med. Chem. 2020, 63, 16012–16027. [Google Scholar] [CrossRef]
- Rasool, I.; Ahmad, M.; Khan, Z.A.; Mansha, A.; Maqbool, T.; Zahoor, A.F.; Aslam, S. Recent advancements in oxadiazole-based anticancer agents. Trop. J. Pharm. Res. 2017, 16, 723–733. [Google Scholar] [CrossRef]
- Hyun, Y.E.; Kim, H.R.; Jeong, L.S. Stereoselective Synthesis of (S)-and (N)-Cyclopropyl-Fused Carbocyclic Nucleosides using stereoselective cyclopropanation. J. Org. Chem. 2021, 86, 9828–9837. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Ali, S.; Javed, S.; Irfan, M.; Irfan, A.; Mojzych, K.K.; Mojzych, M. Mitsunobu Reaction: A Powerful Tool for the Synthesis of Natural Products: A Review. Molecules 2022, 27, 6953. [Google Scholar] [CrossRef]
- Bessieres, M.; Chevrier, F.; Roy, V.; Agrofoglio, L.A. Recent progress for the synthesis of selected carbocyclic nucleosides. Future Med. Chem. 2015, 7, 1809–1828. [Google Scholar] [CrossRef]
- Singh, U.S.; Mulamoottil, V.A.; Chu, C.K. 2′-Fluoro-6′-methylene carbocyclic adenosine and its phosphoramidate prodrug: A novel anti-HBV agent, active against drug-resistant HBV mutants. Med. Res. Rev. 2018, 38, 977–1002. [Google Scholar] [CrossRef]
- Singh, U.S.; Chu, C.K. Synthesis of 2′-deoxy-2′-fluoro-2′-C-methyl spiro cyclopentyl carbocyclic uridine analog as potential inhibitors of HCV NS5B polymerase. Nucleosides Nucleotides Nucleic Acids 2020, 39, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Tong, C.Y.W.; Wong, T.; Wilkinson, M. New therapies for chronic hepatitis C infection: A systematic review of evidence from clinical trials. Int. J. Clin. Pract. 2012, 66, 342–355. [Google Scholar] [CrossRef]
- Suhara, Y.; Nihei, K.I.; Kurihara, M.; Kittaka, A.; Yamaguchi, K.; Fujishima, T.; Kommo, K.; Miyata, N.; Takayama, H. Efficient and versatile synthesis of novel 2α-substituted 1α, 25-dihydroxyvitamin D3 analogues and their docking to vitamin D receptors. J. Org. Chem. 2001, 66, 8760–8771. [Google Scholar] [CrossRef] [PubMed]
- Komsta, Z.; Mayes, B.A.; Moussa, A.; Shelbourne, M.; Stewart, A.; Tyrrell, A.J.; Wallis, L.L.; Weymouth-Wilson, A.C.; Yurek-George, A. Stereoselective Cyclopropanation in the Synthesis of 3′-Deoxy-3′-C-hydroxymethyl-2′, 3′-methylene-uridine. Org. Lett. 2014, 16, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Symons, J.A.; Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 2018, 155, 76–88. [Google Scholar] [CrossRef]
- Panayides, J.L.; Mathieu, V.; Banuls, L.M.Y.; Apostolellis, H.; Dahan-Farkas, N.; Davids, H.; Harmse, L.; Rey, M.E.C.; Green, I.R.; Pelly, S.C.; et al. Synthesis and in vitro growth inhibitory activity of novel silyl-and trityl-modified nucleosides. Bioorg. Med. Chem. 2016, 24, 2716–2724. [Google Scholar] [CrossRef]
- Kollmann, C.; Wiechert, S.M.; Jones, P.G.; Pietschmann, T.; Werz, D.B. Synthesis of 4′/5′-Spirocyclopropanated Uridine and d-Xylouridine Derivatives and Their Activity against the Human Respiratory Syncytial Virus. Org. Lett. 2019, 21, 6966–6971. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Ksebati, M.B.; Ptak, R.G.; Fan, B.Y.; Breitenbach, J.M.; Lin, J.S.; Cheng, Y.C.; Kern, E.R.; Drach, J.C.; Zemlicka, J. (Z)-and (E)-2-((hydroxymethyl) cyclopropylidene) methyladenine and-guanine. New nucleoside analogues with a broad-spectrum antiviral activity. J. Med. Chem. 1998, 41, 10–23. [Google Scholar] [CrossRef]
- Kim, A.; Hong, J.H. Synthesis and antiviral activity of C-fluoro-branched cyclopropyl nucleosides. Eur. J. Med. Chem. 2007, 42, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, R.D.; Salinas, J.C.; Ostergaard, M.E.; Swayze, E.E.; Seth, P.P.; Hanessian, S. Design, synthesis, and duplex-stabilizing properties of conformationally constrained tricyclic analogues of LNA. Org. Biomol. Chem. 2016, 14, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Hari, Y.; Osawa, T.; Obika, S. Synthesis and duplex-forming ability of oligonucleotides containing 4′-carboxythymidine analogs. Org. Biomol. Chem. 2012, 10, 9639–9649. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamamoto, C.; Horiba, M.; Aoyama, H.; Obika, S. Synthesis and duplex-forming ability of oligonucleotides modified with 4′-C, 5′-C-methylene-bridged nucleic acid (4′, 5′-BNA). Bioorg. Med. Chem. 2021, 46, 116359. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Guguloth, N.; Yadav, J.S. The first asymmetric total syntheses of 3 ((1R, 2R)-and 3 ((1S, 2R)-2-(12-methyltridecyl) cyclopropyl) propanoic acid. Tetrahedron Lett. 2012, 53, 6718–6720. [Google Scholar] [CrossRef]
- Hegde, V.R.; Pu, H.; Patel, M.; Das, P.R.; Strizki, J.; Gullo, V.P.; Chou, C.C.; Buevich, A.V.; Chan, T.M. Three new compounds from the plant Lippia alva as inhibitors of chemokine receptor 5 (CCR5). Bioorg. Med. Chem. Lett. 2004, 14, 5339–5342. [Google Scholar] [CrossRef]
- Mohapatra, D.K.; Kanikarapu, S.; Naidu, P.R.; Yadav, J.S. Toward the synthesis of brevipolide H. Tetrahedron Lett. 2016, 56, 1041–1044. [Google Scholar] [CrossRef]
- Gesinski, M.R.; Rychnovsky, S.D. Total synthesis of the cyanolide A aglycon. J. Am. Chem. Soc. 2011, 133, 9727–9729. [Google Scholar] [CrossRef]
- Trost, B.M.; Quintard, A. Asymmetric Catalytic Alkynylation of Acetaldehyde: Application to the Synthesis of (+)-Tetrahydropyrenophorol. Angew. Chem. Int. Ed. 2012, 124, 6808–6812. [Google Scholar] [CrossRef]
- Ralston, K.J.; Ramstadius, H.C.; Brewster, R.C.; Niblock, H.S.; Hulme, A.N. Self-Assembly of Disorazole C1 through a One-Pot Alkyne Metathesis Homodimerization Strategy. Angew. Chem. Int. Ed. 2015, 127, 7192–7196. [Google Scholar] [CrossRef][Green Version]
- Haydl, A.M.; Breit, B. Atom-Economical Dimerization Strategy by the Rhodium-Catalyzed Addition of Carboxylic Acids to Allenes: Protecting-Group-Free Synthesis of Clavosolide A and Late-Stage Modification. Angew. Chem. Int Ed. 2015, 127, 15750–15754. [Google Scholar] [CrossRef]
- Salim, H.; Piva, O. Sequential cross-metathesis/cyclopropanation: Short syntheses of (+/−)-cascarillic acid and (+/−)-grenadamide. Tetrahedron Lett. 2007, 48, 2059–2062. [Google Scholar] [CrossRef]
- Seo, Y.; Cho, K.W.; Rho, J.R.; Shin, J.; Kwon, B.M.; Bok, S.H.; Song, J.I. Solandelactones AI, lactonized cyclopropyl oxylipins isolated from the hydroid Solanderia secunda. Tetrahedron 1996, 52, 10583–10596. [Google Scholar] [CrossRef]
- White, J.D.; Lincoln, C.M.; Yang, J.; Martin, W.H.; Chan, D.B. Total Synthesis of Solandelactones A, B, E, and F Exploiting a Tandem Petasis− Claisen Lactonization Strategy. J. Org. Chem. 2008, 73, 4139–4150. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Montano, N.; Vicente, J.; Rodriguez, A.D. Novel cyclopropane fatty acids from the phospholipids of the Caribbean sponge Pseudospongosorites suberitoides. Lipids 2007, 42, 519–524. [Google Scholar] [CrossRef]
- Carballeira, N.M.; Montano, N.; Reguera, R.M.; Balana-Fouce, R. The first total synthesis of the (±)-17-methyl-trans-4, 5-methyleneoctadecanoic acid and related analogs with antileishmanial activity. Tetrahedron Lett. 2010, 51, 6153–6155. [Google Scholar] [CrossRef][Green Version]
- Deveraux, Q.L.; Reed, J.C. IAP family proteins—Suppressors of apoptosis. Genes Dev. 1999, 13, 239–252. [Google Scholar] [CrossRef]
- Erdogan, M.; Daştan, A. Synthesis of N-substituted dibenzoazepine–pyridazine derivatives as potential neurologically active drugs. Synth. Commun. 2020, 50, 3845–3853. [Google Scholar] [CrossRef]
- Asano, M.; Hashimoto, K.; Saito, B.; Shiokawa, Z.; Sumi, H.; Yabuki, M.; Yoshimatsu, M.; Aoyama, K.; Hamada, T.; Morishita, N.; et al. Design, stereoselective synthesis, and biological evaluation of novel tri-cyclic compounds as inhibitor of apoptosis proteins (IAP) antagonists. Bioorg. Med. Chem. 2013, 21, 5725–5737. [Google Scholar] [CrossRef]
- Singh, H.; Gupta, N.; Kumar, P.; Dubey, S.K.; Sharma, P.K. A new industrial process for 10-methoxyiminostilbene: Key intermediate for the synthesis of oxcarbazepine. Org. Process Res. Dev. 2009, 13, 870–874. [Google Scholar] [CrossRef]
- Tian, M.; Abdelrahman, A.; Weinhausen, S.; Hinz, S.; Weyer, S.; Dosa, S.; El-Tayeb, A.; Muller, C.E. Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorg. Med. Chem. 2014, 22, 1077–1088. [Google Scholar] [CrossRef]
- Werth, J.; Uyeda, C. Cobalt-catalyzed Reductive Dimethylcyclopropanation of 1, 3-dienes. Angew. Chem. Int. Ed. 2018, 57, 13902–13906. [Google Scholar] [CrossRef] [PubMed]
- Bali, M.; Akabas, M.H. Defining the propofol binding site location on the GABAA receptor. Mol. Pharm. 2004, 65, 68–76. [Google Scholar] [CrossRef]
- White, P.F.; Warner, D.S. Propofol: Its role in changing the practice of anesthesia. Anesthesiologists 2008, 109, 1132–1136. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Liu, Z.; Long, Y.; Zhao, J.; Xu, W.; Zhang, H. Development of a Kilogram-Scale Route for Clinical Sample Production of the Intravenous Anesthetic Cipepofol. Org. Process Res. Dev. 2022, 26, 1054–1062. [Google Scholar] [CrossRef]
- Majounie, E.; Abramzon, Y.; Renton, A.E.; Perry, R.; Bassett, S.S.; Pletnikova, O.; Traynor, B.J. Repeat expansion in C9ORF72 in Alzheimer’s disease. N. Engl. J. Med. 2012, 366, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, A.; Prezzavento, O.; Pappalardo, M.S.; Bousquet, E.; Iadanza, M.; Pike, V.W.; Ronsisvalle, G. Synthesis of (+)-and (−)-cis-2-[(1-adamantylamino)-methyl]-1-phenylcyclopropane derivatives as high affinity probes for σ1 and σ2 binding sites. Il Farm. 2002, 57, 45–53. [Google Scholar] [CrossRef]
- Kawashima, Y.; Ezawa, T.; Yamamura, M.; Harada, T.; Noguchi, T.; Imai, N. Convenient synthesis of (+)-cis-4-(N-adamantyl-N-methylamino)-2, 3-methano-2-phenylbutan-1-ol as a candidate of anti-Alzheimer’s medicine via catalytic enantioselective Simmons–Smith reaction using l-phenylalanine-derived disulfonamide. Tetrahedron Lett. 2016, 57, 668–671. [Google Scholar] [CrossRef]
- Dilmaç, A.M.; Wezeman, T.; Bsr, R.M.; Brase, S. Occurrence, synthesis and applications of natural and designed [3.3. 3] propellanes. Nat. Prod. Rep. 2020, 37, 224–245. [Google Scholar] [CrossRef]
- Belzner, J.; Gareib, B.; Polborn, K.; Schmid, W.; Semmler, K.; Szeimies, G. Synthesen substituierter [1.1. 1] Propellane. Chem. Berichte 1989, 122, 1509–1529. [Google Scholar] [CrossRef]
- Nassar, Y.; Piva, O. A short route to access oxaspiro [n, 3, 3] propellanes. Org. Biomol. Chem. 2020, 18, 5811–5815. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Quinke, K.; Spahn, H.; Mutschler, E. (−)-(S)-Flunoxaprofen and (−)-(S)-naproxen isocyanate: Two new fluorescent chiral derivatizing agents for an enantiospecific determination of primary and secondary amines. Chirality 1989, 1, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Murakami, Y.; Imai, N. Syntheses of (R)-(+)-cibenzoline and analogues via catalytic enantioselective cyclopropanation using (S)-phenylalanine-derived disulfonamide. Tetrahedron Asymmetry 2006, 17, 3067–3069. [Google Scholar] [CrossRef]
- Burger, A.; Yost, W.L. Arylcycloalkylamines. I. 2-phenylcyclopropylamine. J. Am. Chem. Soc. 1948, 70, 2198–2201. [Google Scholar] [CrossRef]
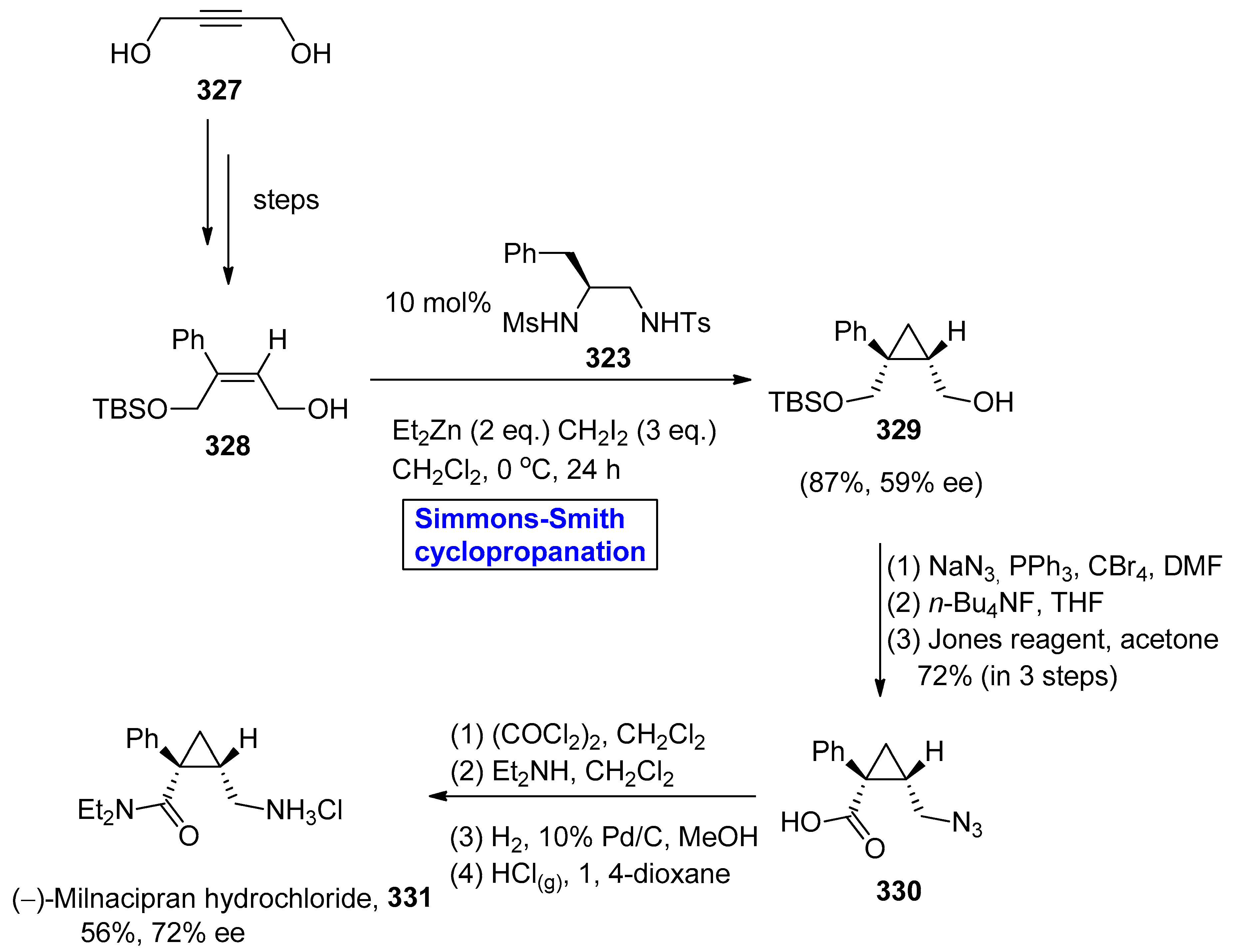
- Alliot, J.; Gravel, E.; Pillon, F.; Buisson, D.A.; Nicolas, M.; Doris, E. Enantioselective synthesis of levomilnacipran. Chem. Commun. 2012, 48, 8111–8113. [Google Scholar] [CrossRef]
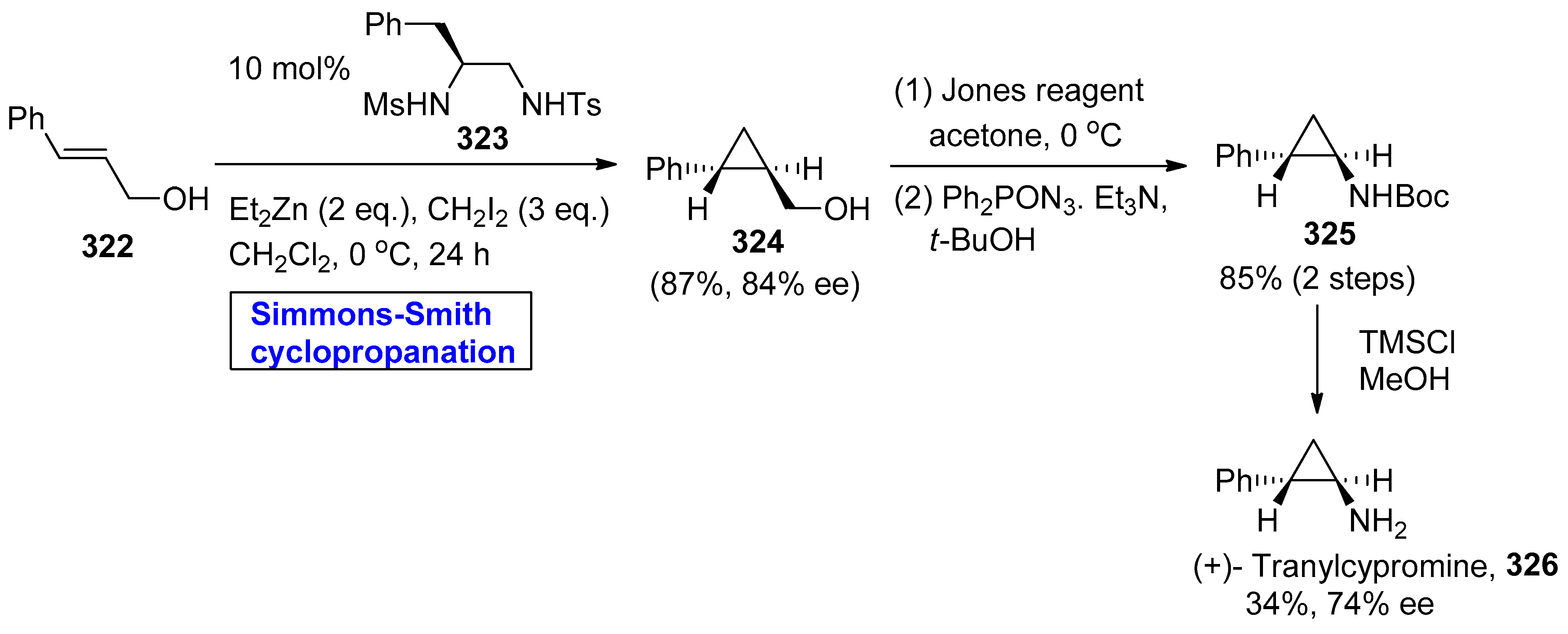
- Ishizuka, Y.; Fujimori, H.; Noguchi, T.; Kawasaki, M.; Kishida, M.; Nagai, T.; Imai, N.; Kirihara, M. Asymmetric Syntheses of Pharmaceuticals Containing a Cyclopropane Moiety Using Catalytic Asymmetric Simmons–Smith Reactions of Allylalcohols: Syntheses of Optically Active Tranylcypromine and Milnacipran. Chem. Lett. 2013, 42, 1311–1313. [Google Scholar] [CrossRef]
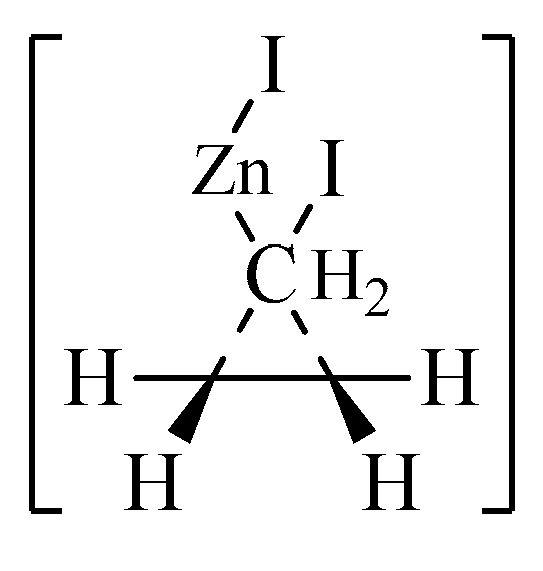




















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, R.; Zahoor, A.F.; Javed, S.; Parveen, B.; Mansha, A.; Irfan, A.; Khan, S.G.; Irfan, A.; Kotwica-Mojzych, K.; Mojzych, M. Simmons–Smith Cyclopropanation: A Multifaceted Synthetic Protocol toward the Synthesis of Natural Products and Drugs: A Review. Molecules 2023, 28, 5651. https://doi.org/10.3390/molecules28155651
Munir R, Zahoor AF, Javed S, Parveen B, Mansha A, Irfan A, Khan SG, Irfan A, Kotwica-Mojzych K, Mojzych M. Simmons–Smith Cyclopropanation: A Multifaceted Synthetic Protocol toward the Synthesis of Natural Products and Drugs: A Review. Molecules. 2023; 28(15):5651. https://doi.org/10.3390/molecules28155651
Chicago/Turabian StyleMunir, Ramsha, Ameer Fawad Zahoor, Sadia Javed, Bushra Parveen, Asim Mansha, Ahmad Irfan, Samreen Gul Khan, Ali Irfan, Katarzyna Kotwica-Mojzych, and Mariusz Mojzych. 2023. "Simmons–Smith Cyclopropanation: A Multifaceted Synthetic Protocol toward the Synthesis of Natural Products and Drugs: A Review" Molecules 28, no. 15: 5651. https://doi.org/10.3390/molecules28155651
APA StyleMunir, R., Zahoor, A. F., Javed, S., Parveen, B., Mansha, A., Irfan, A., Khan, S. G., Irfan, A., Kotwica-Mojzych, K., & Mojzych, M. (2023). Simmons–Smith Cyclopropanation: A Multifaceted Synthetic Protocol toward the Synthesis of Natural Products and Drugs: A Review. Molecules, 28(15), 5651. https://doi.org/10.3390/molecules28155651