Excited State Dynamics of Alizarin Red S Nanoparticles in Solution
Abstract
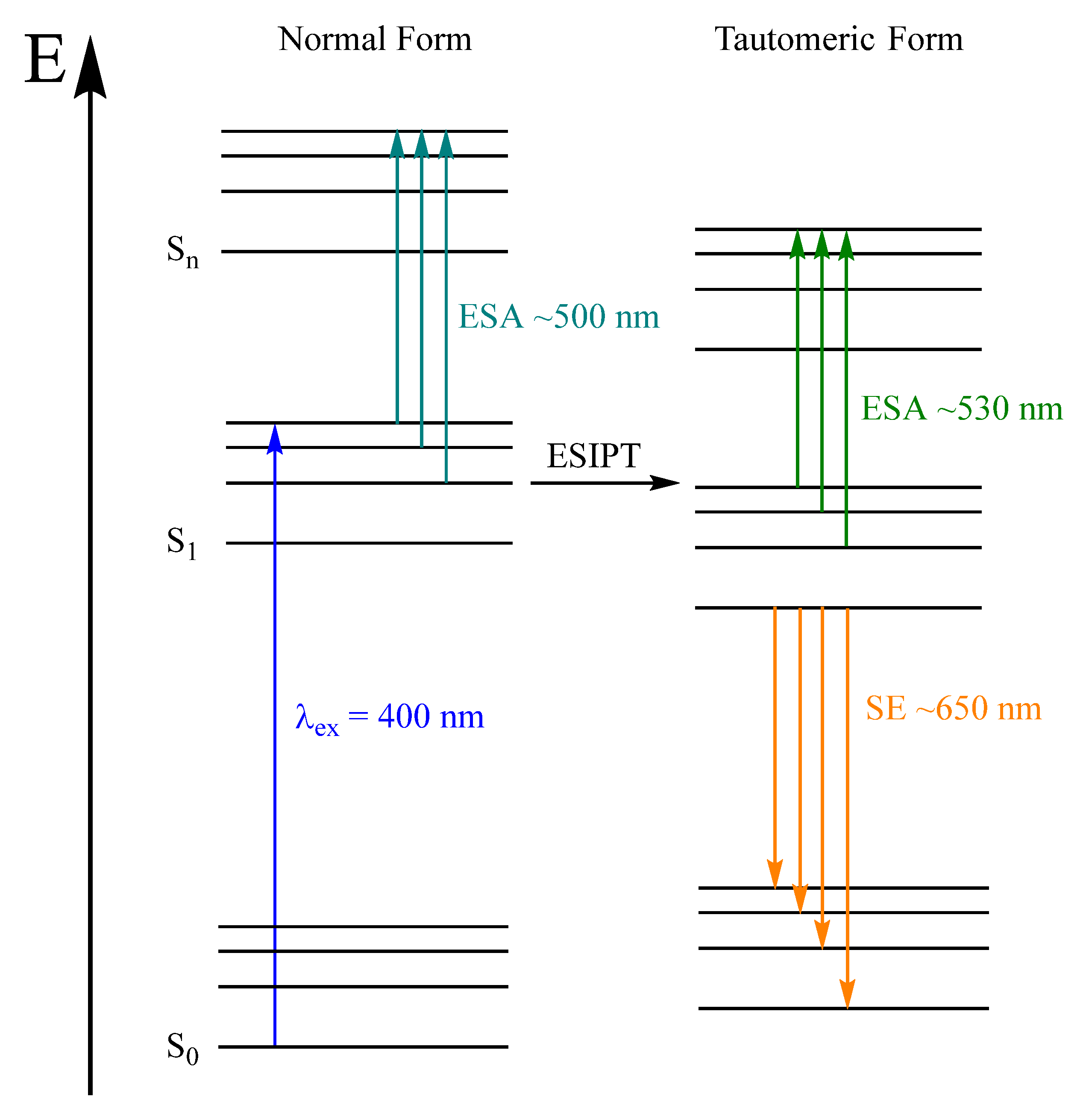
1. Introduction
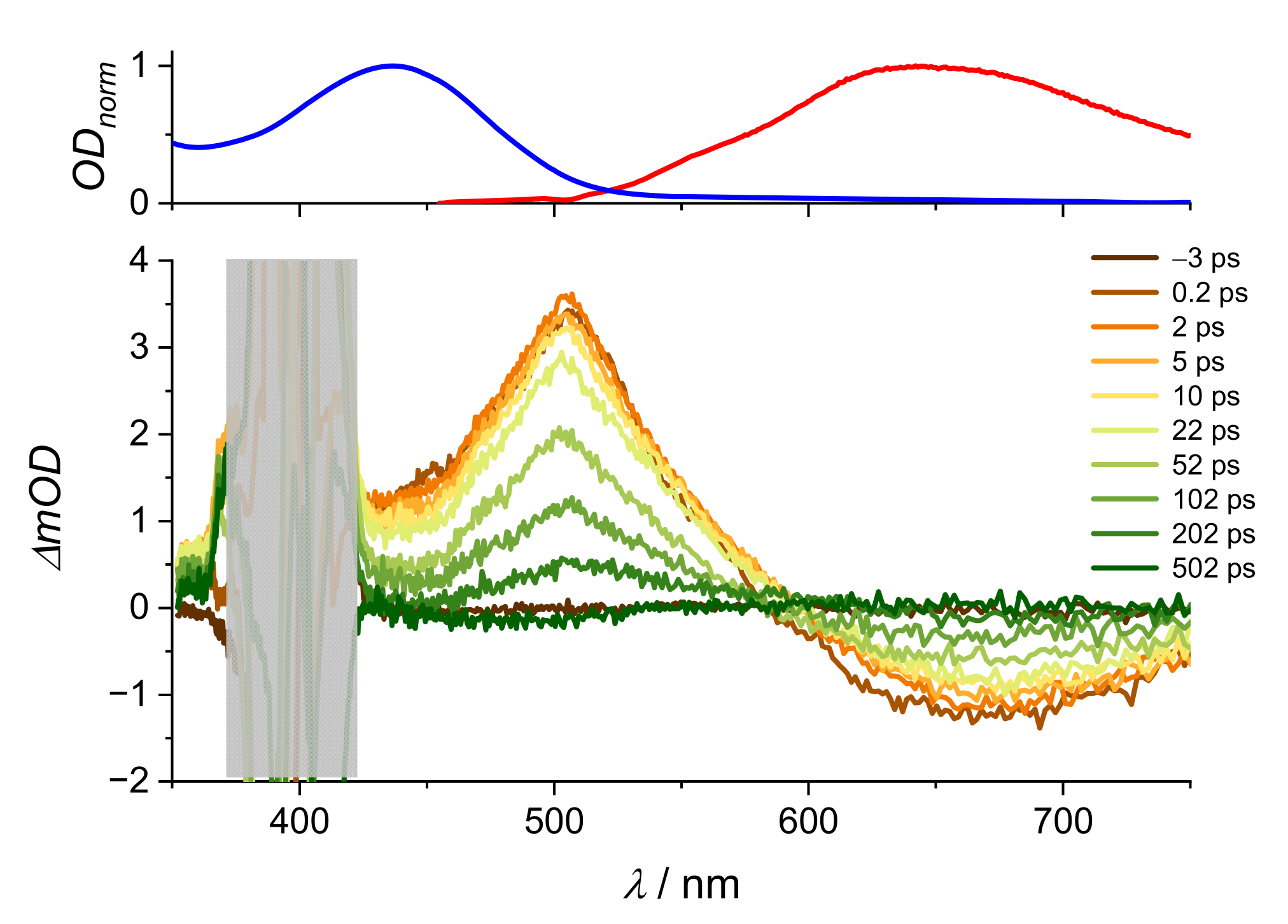
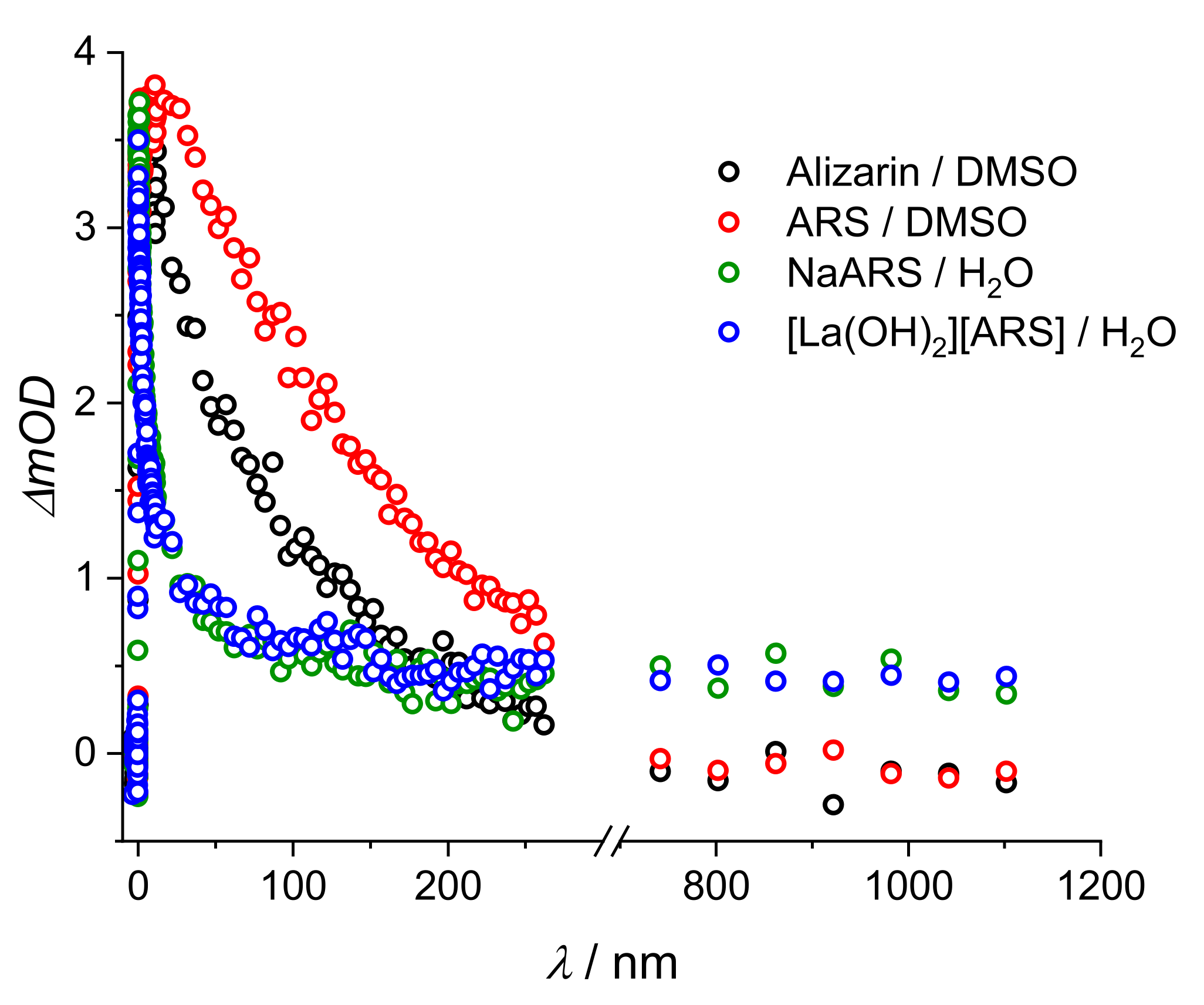
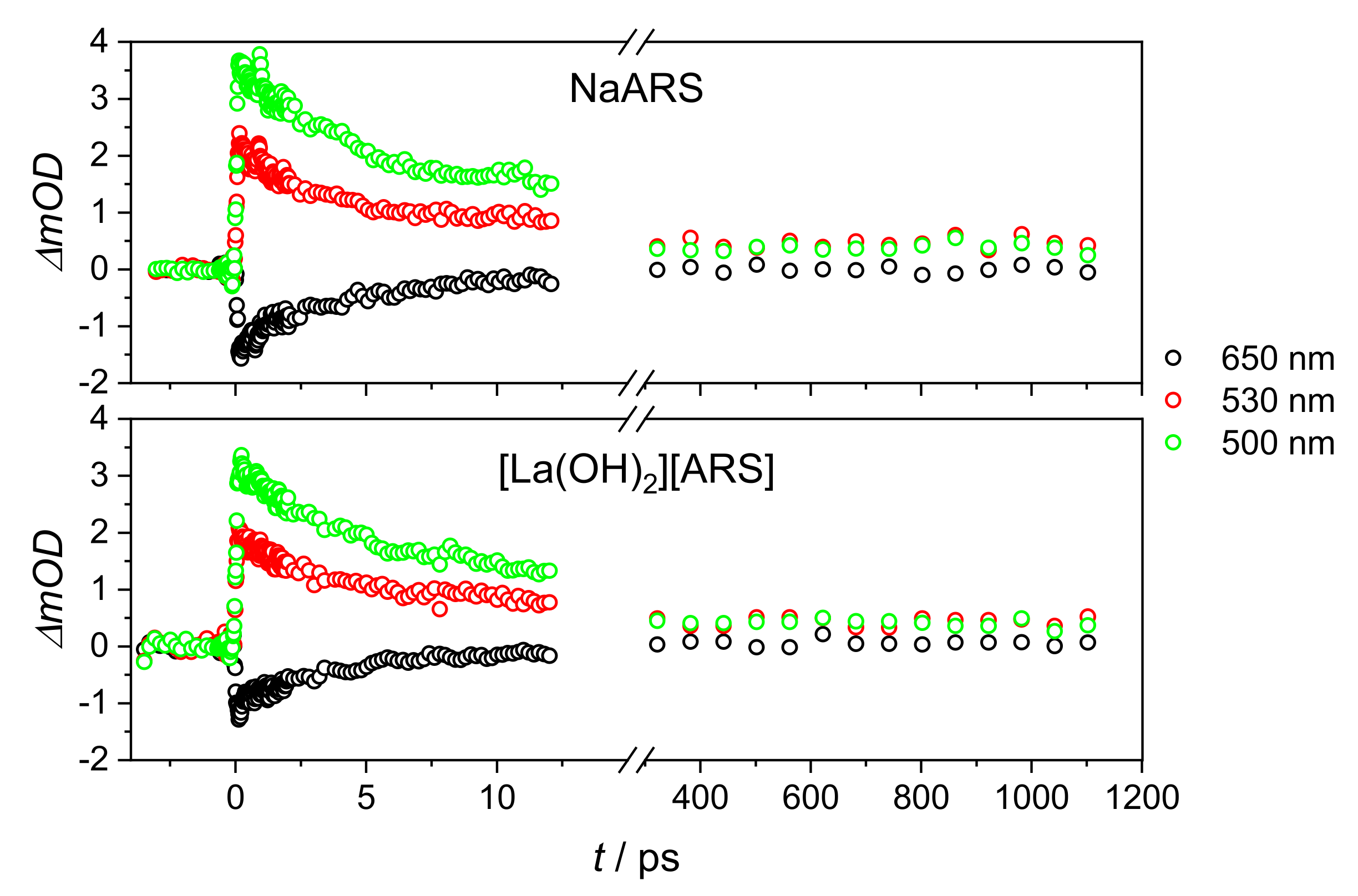
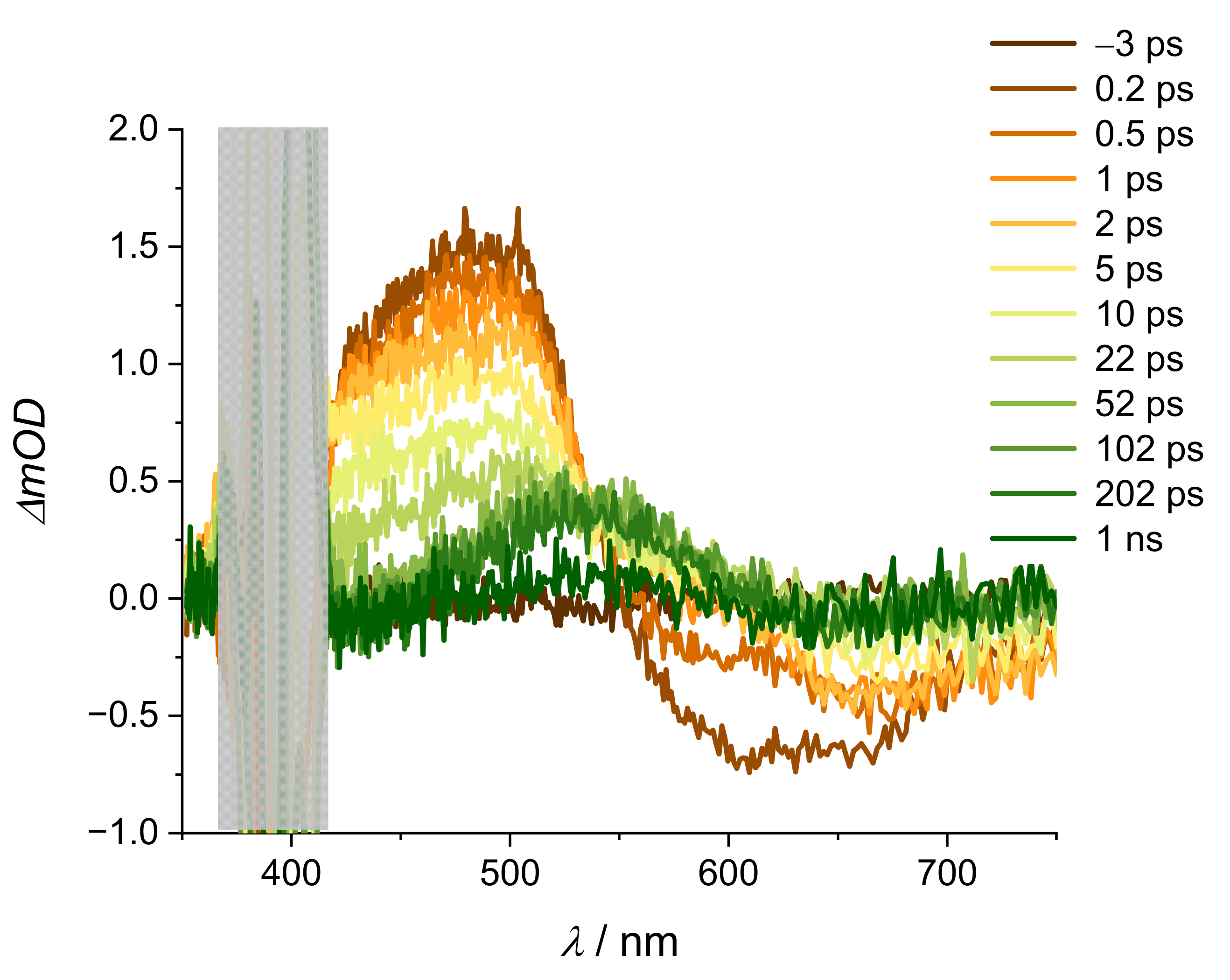
2. Results and Discussion
2.1. Nanoparticle Synthesis
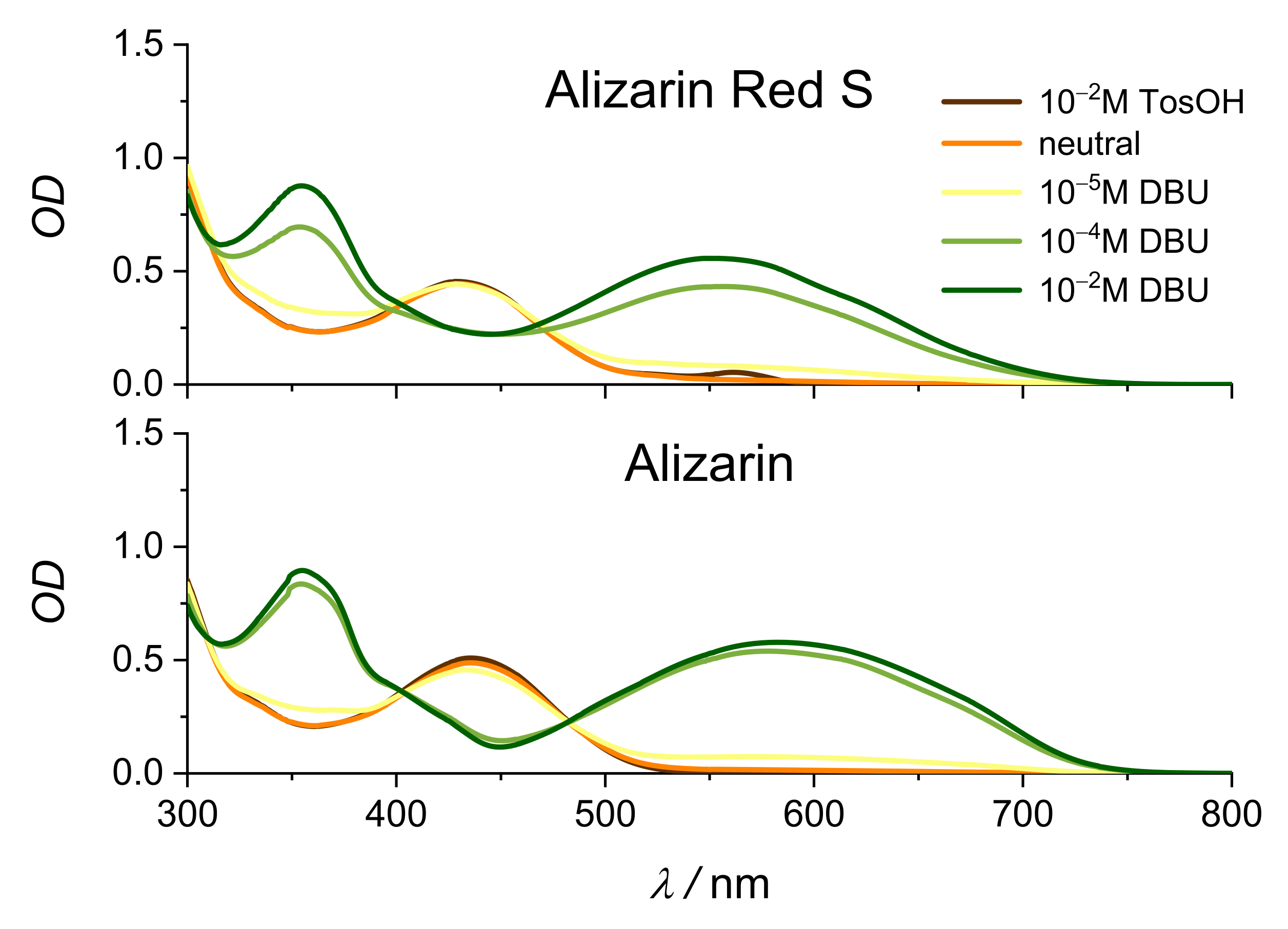
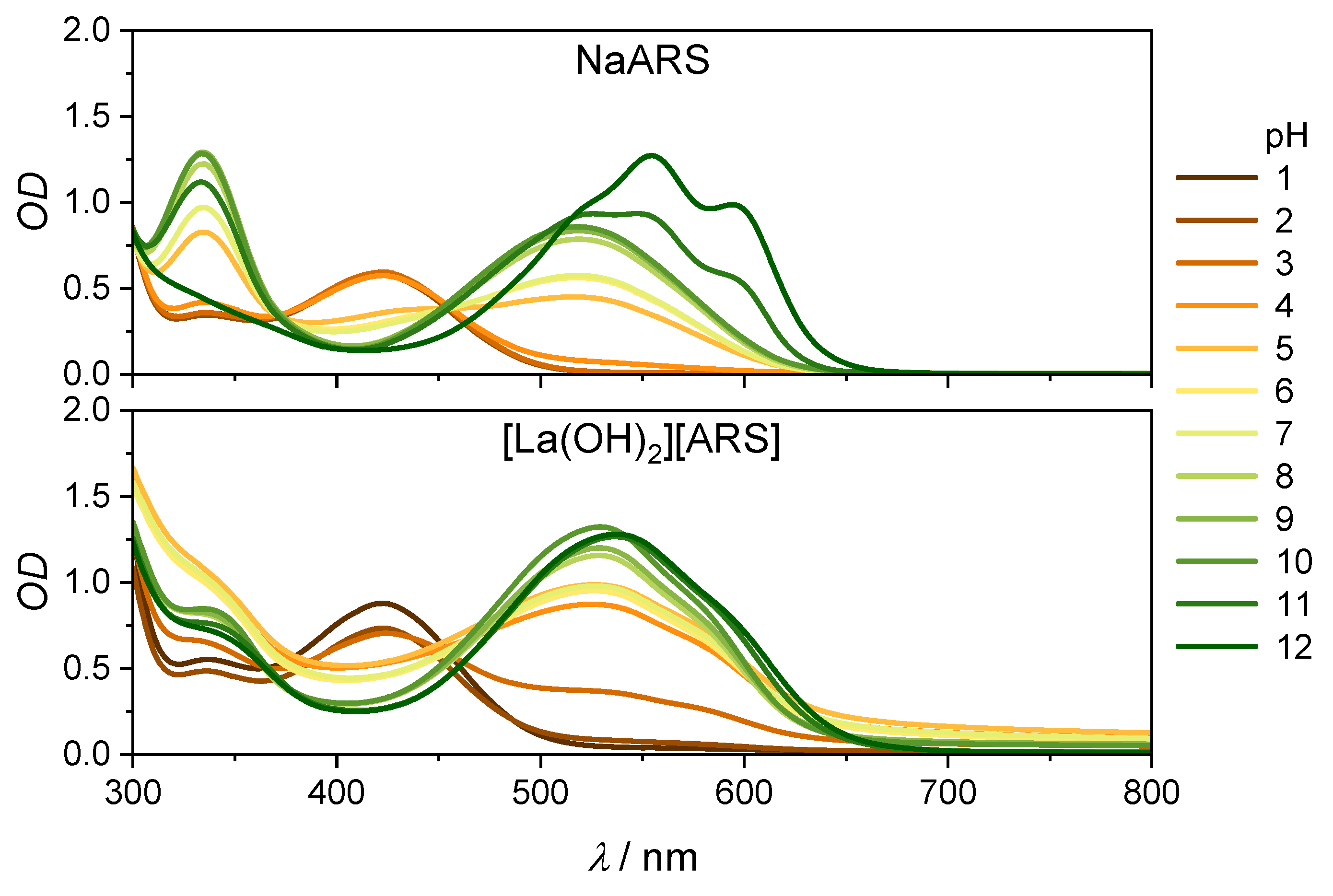
2.2. Stationary Absorption Spectra
3. Materials and Methods
3.1. Stationary Spectra
3.2. Transient Absorption Spectroscopy
3.3. Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
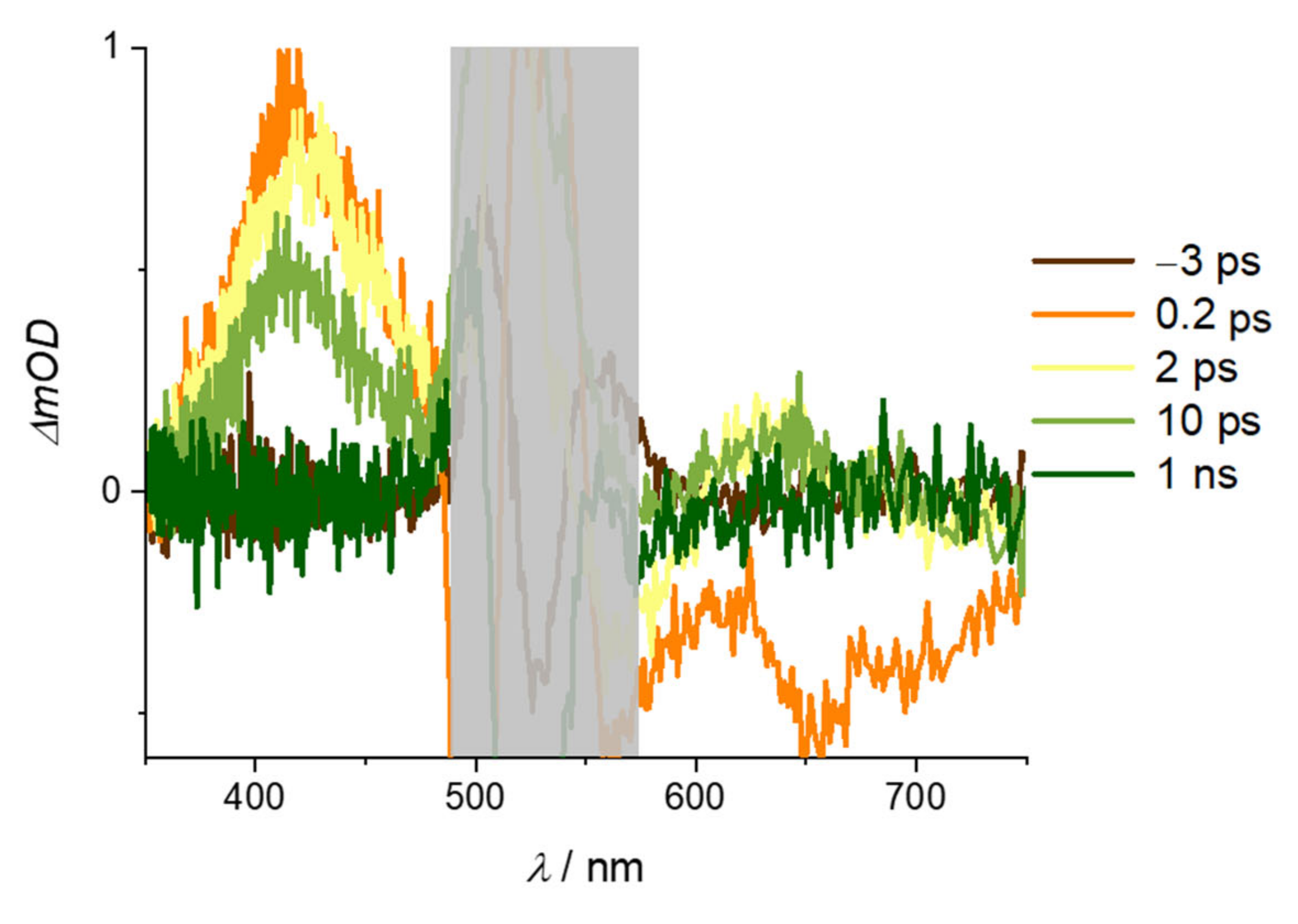
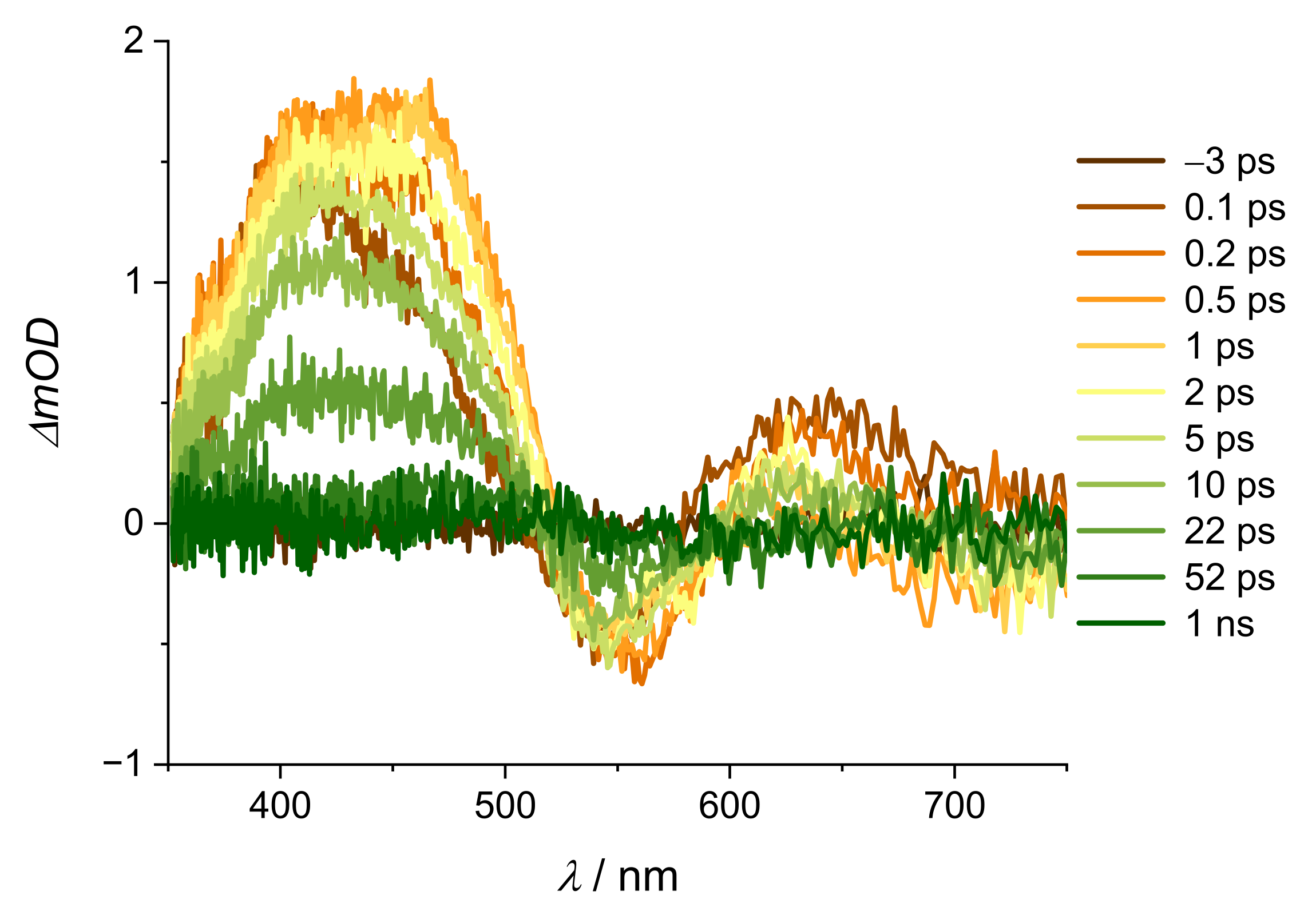
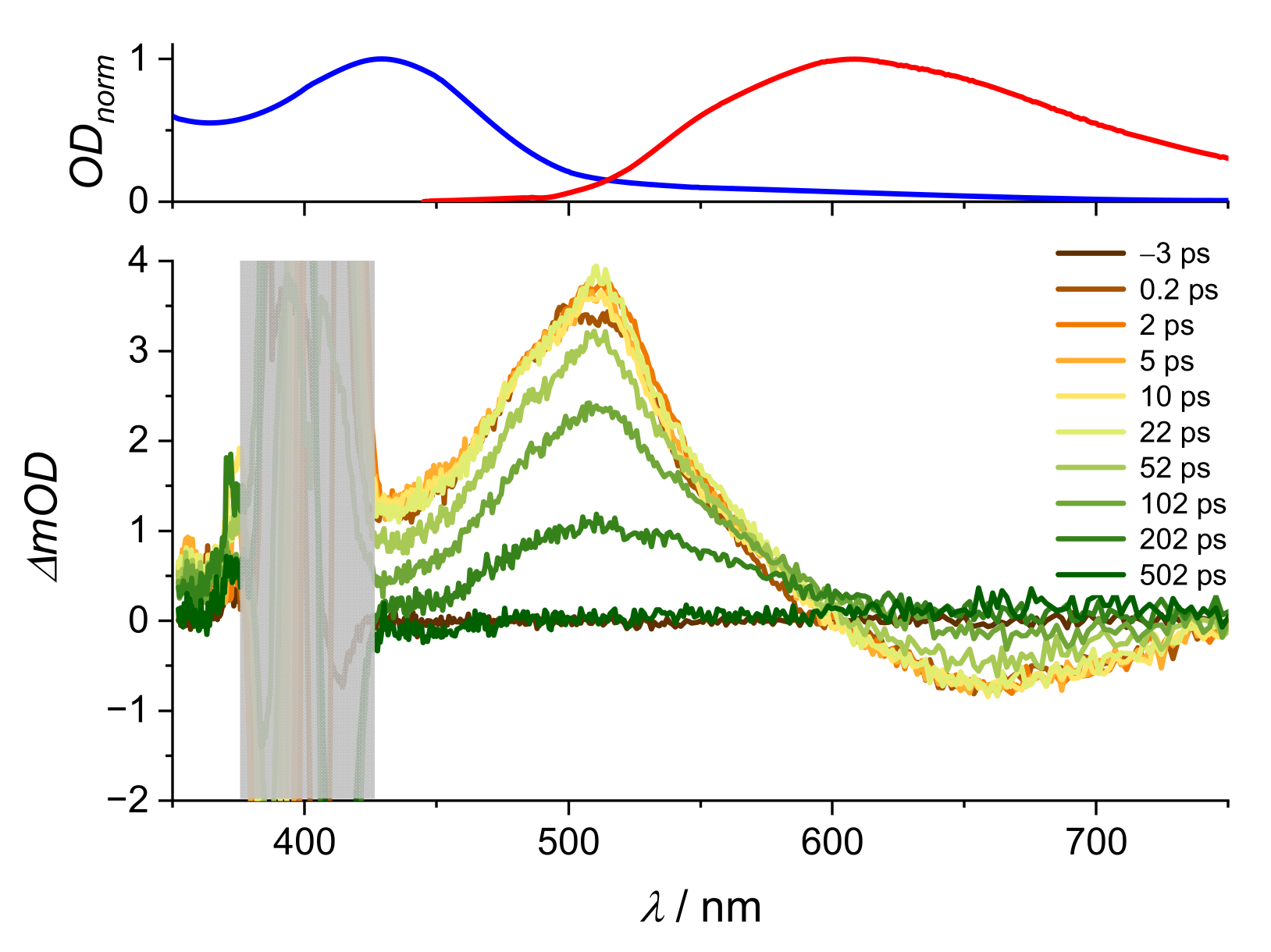



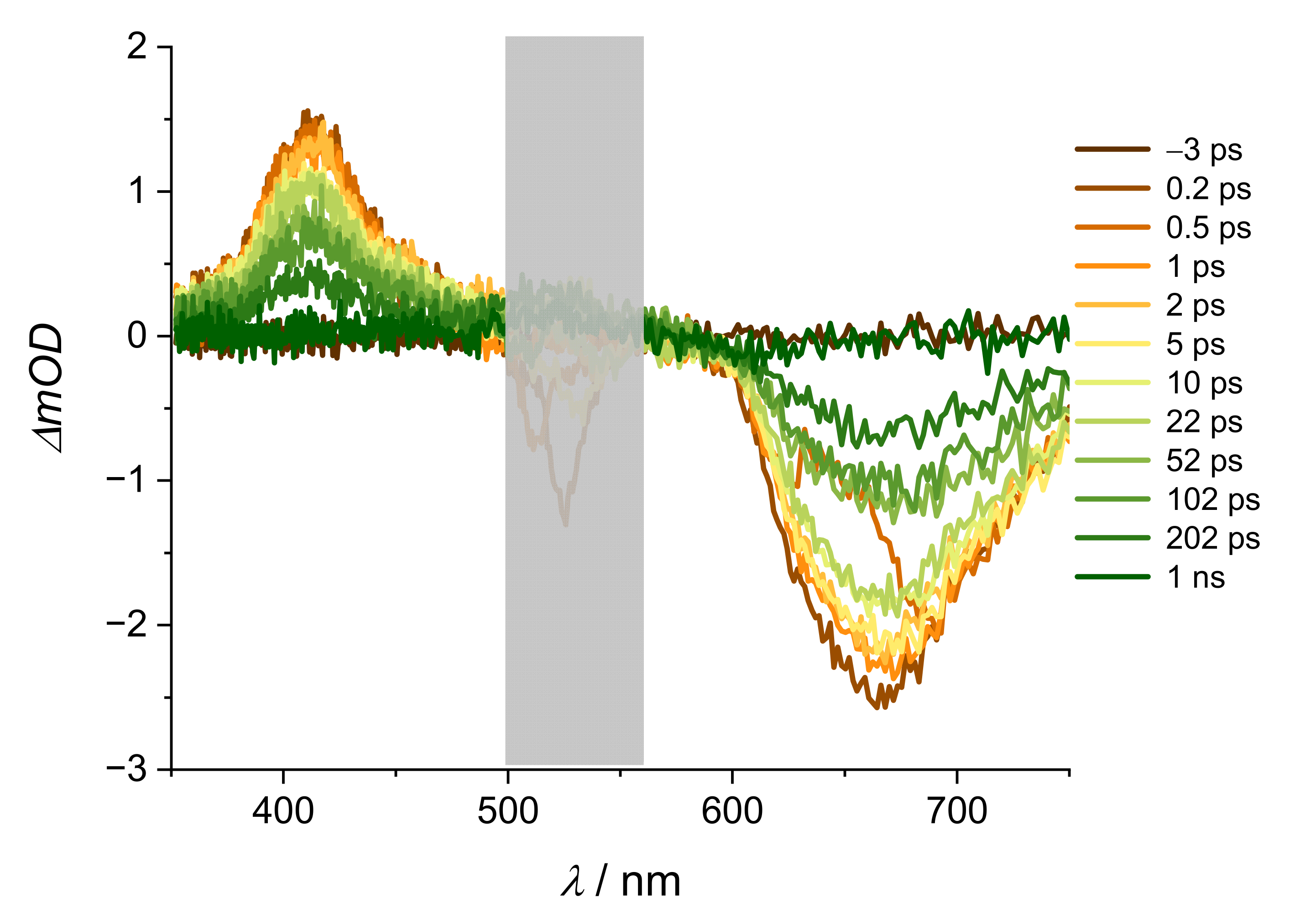
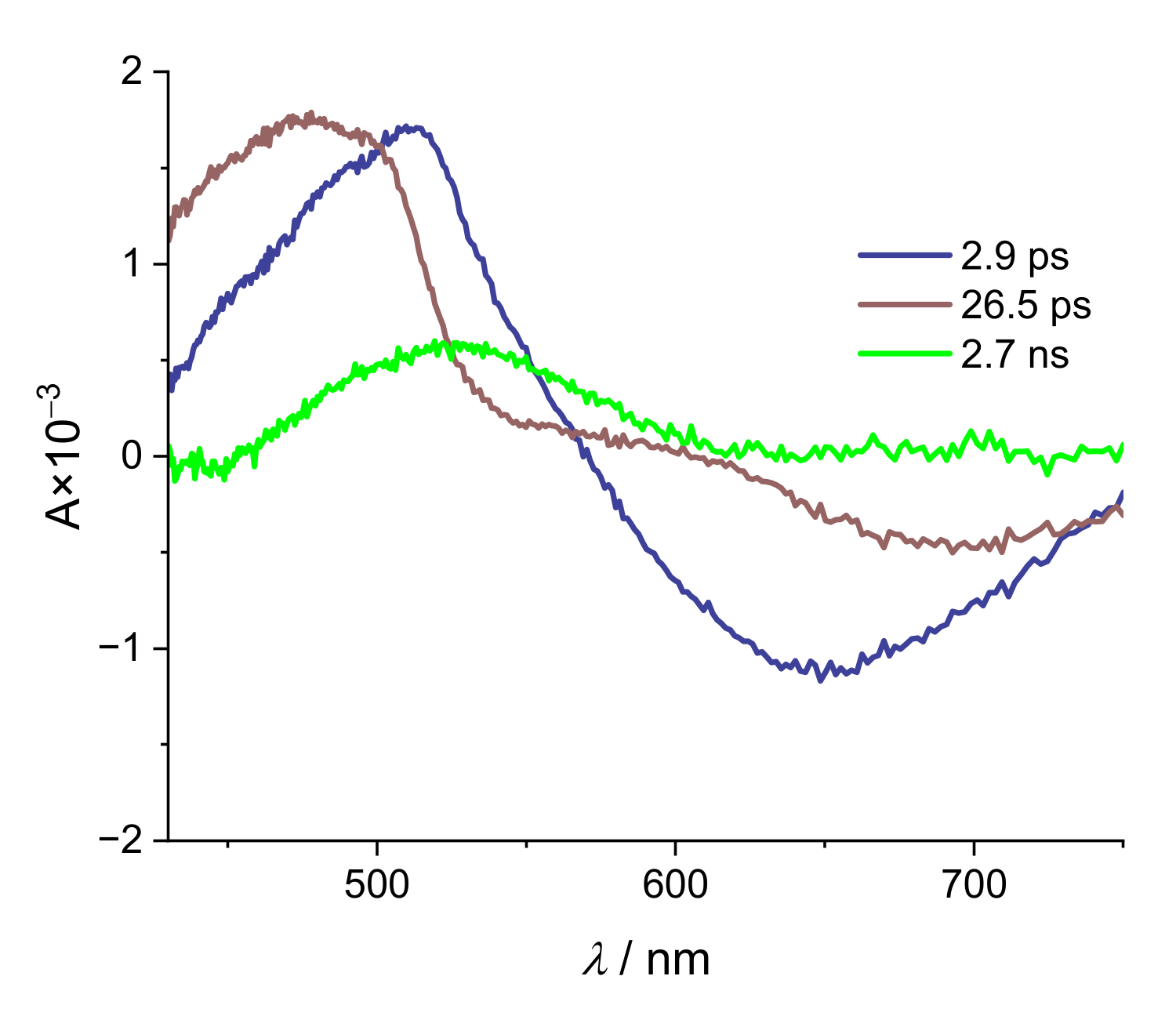
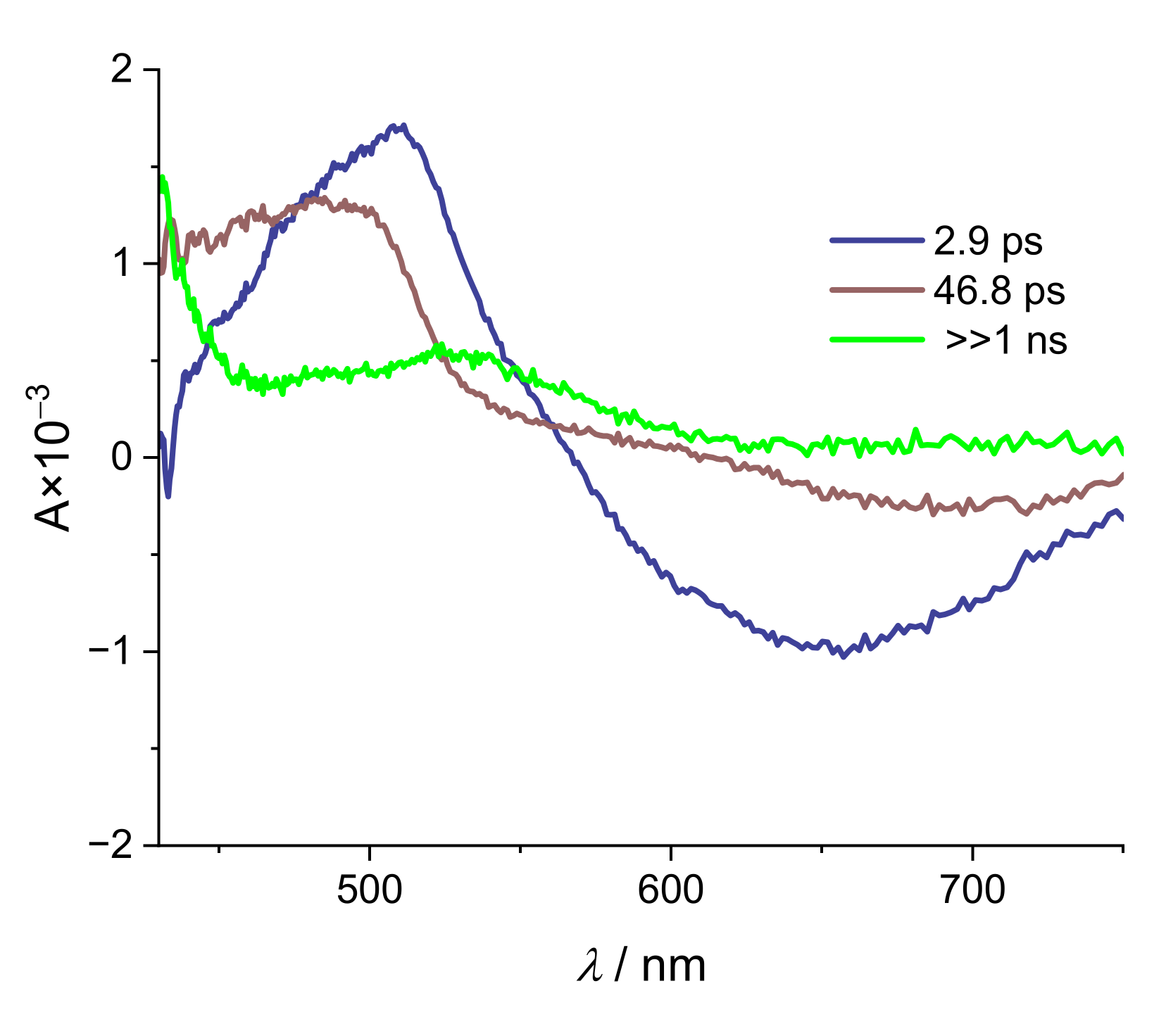
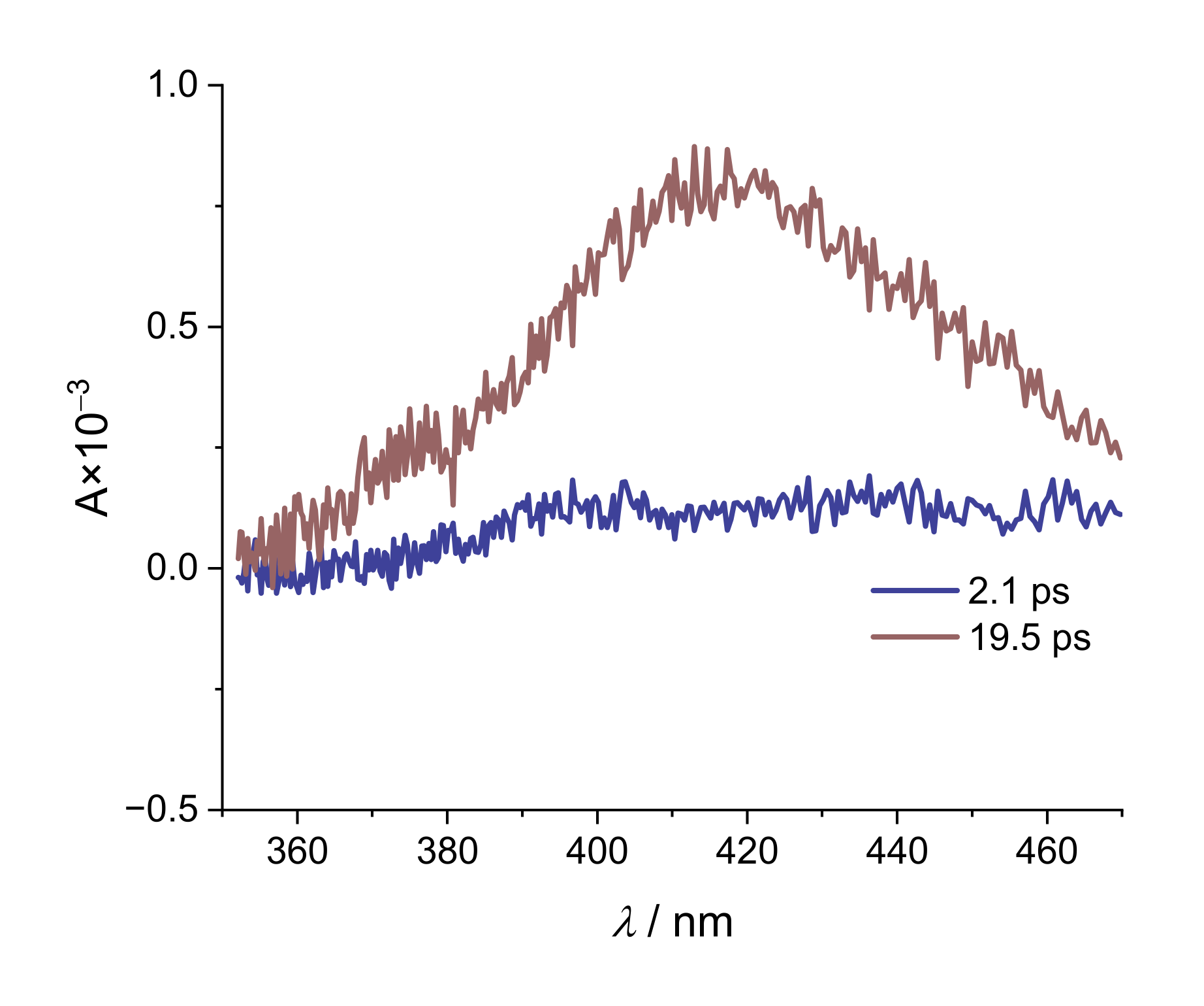
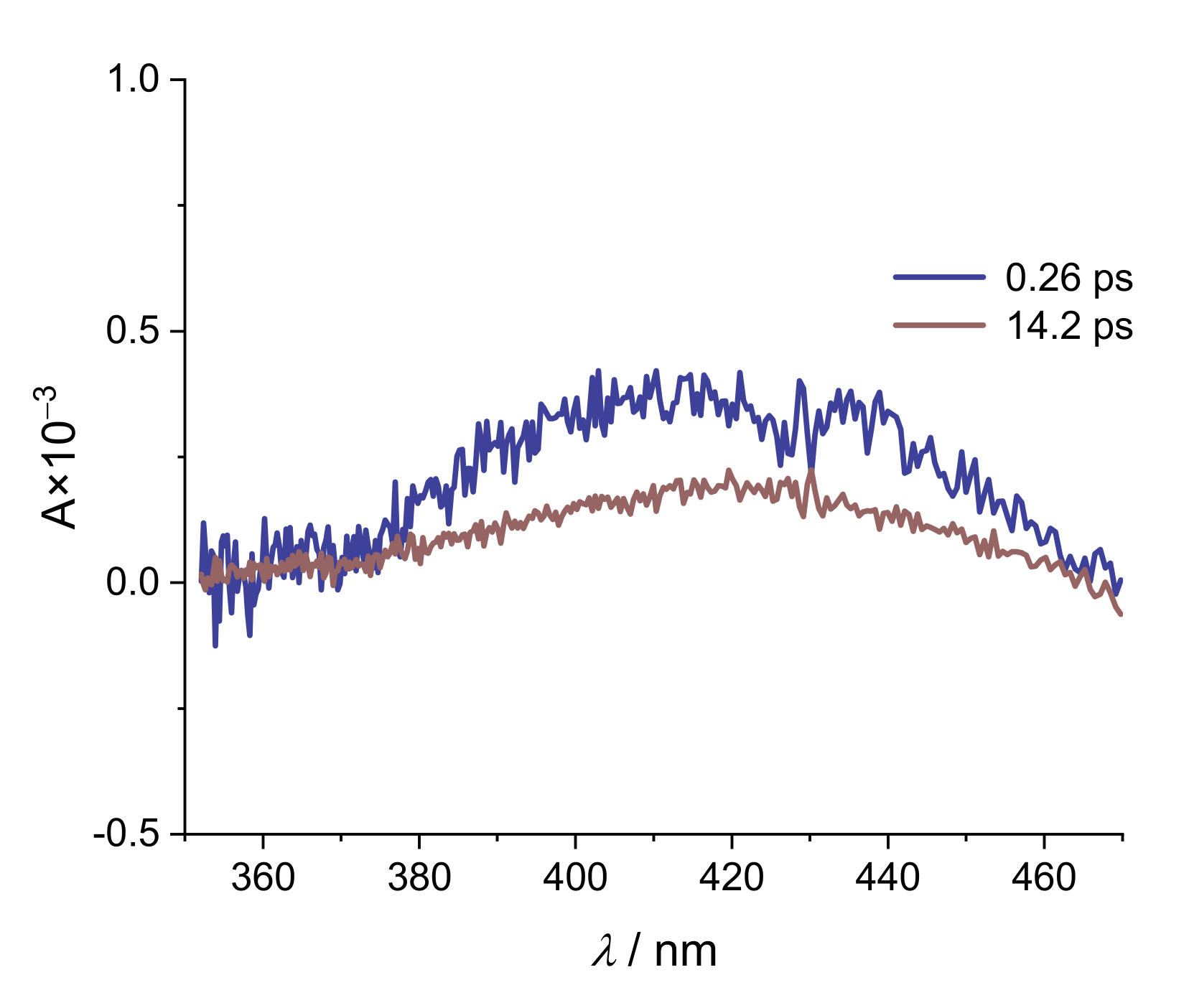
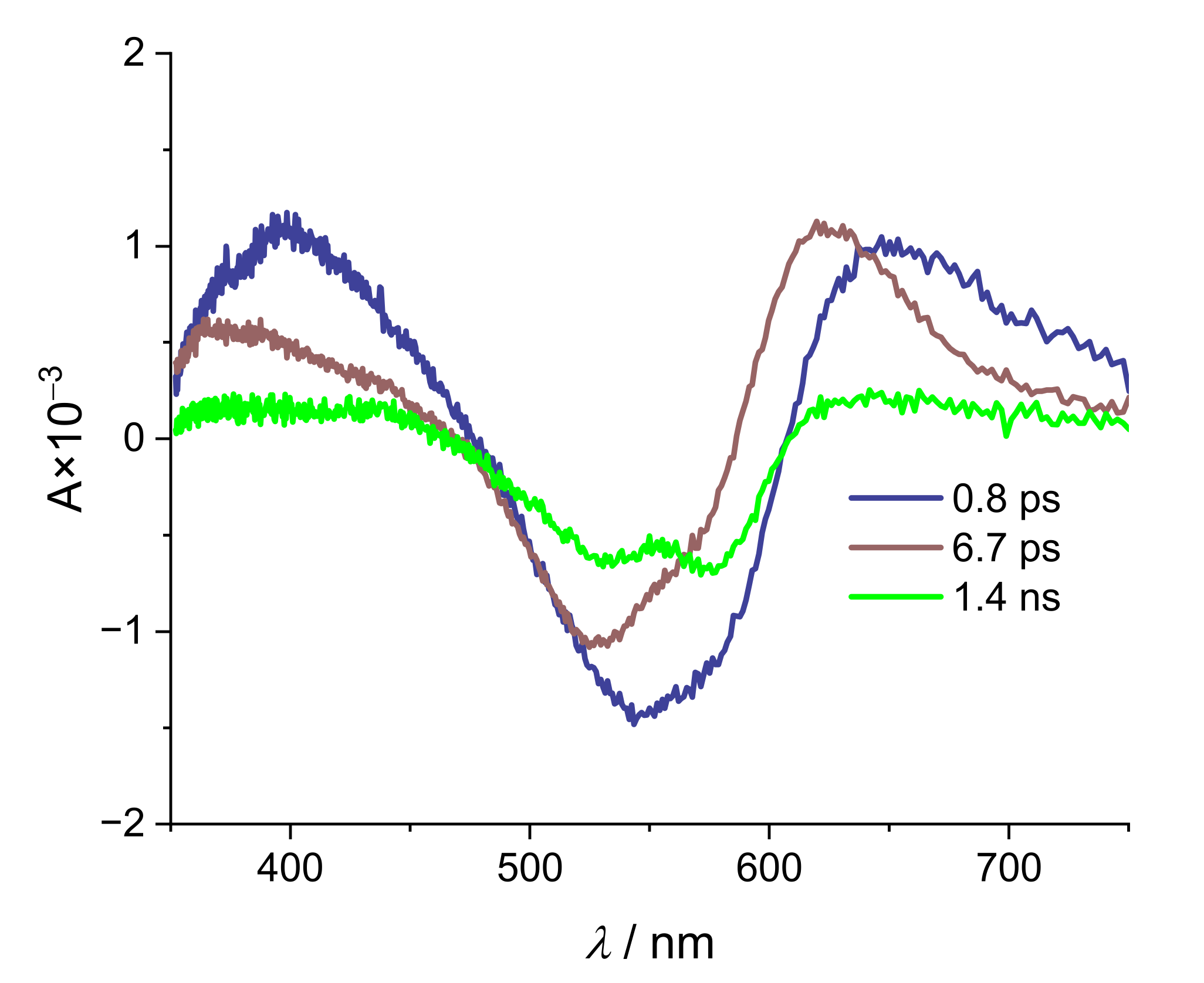
| Alizarin/DMSO | ARS/DMSO | |
|---|---|---|
| τ1/ps | 0.06 | 0.1 |
| τ2/ps | 89 | 169 |
| 1 μJ | 1.2 μJ | 2.4 μJ | |
|---|---|---|---|
| τ1/ps | 2.9 | 4.0 | 3.4 |
| τ2/ps | 26.5 | 18.7 | 24.4 |
References
- Cañamares, M.V.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Surface-enhanced Raman scattering study of the adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J. Raman Spectrosc. 2004, 35, 921–927. [Google Scholar] [CrossRef]
- Miliani, C.; Romani, A.; Favaro, G. Acidichromic effects in 1,2-di- and 1,2,4-tri- hydroxyanthraquinones. A spectrophotometric and fluorimetric study. J. Phys. Org. Chem. 2000, 13, 141–150. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Wang, B. Alizarin Red S. as a general optical reporter for studying the binding of boronic acids with carbohydrates. Chem. Commun. 2001, 1608–1609. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, B.L.; Heck, J.G.; Feldmann, C. Fluorescence-based histamine sensing with inorganic–organic hybrid nanoparticles. J. Mater. Chem. C 2019, 7, 3543–3552. [Google Scholar] [CrossRef]
- Rudolph, D.; Redinger, N.; Schaible, U.E.; Feldmann, C. Transport of Lipophilic Anti-Tuberculosis Drug Benzothiazone-043 in Ca3(PO4)2 Nanocontainers. ChemNanoMat 2021, 7, 7–16. [Google Scholar] [CrossRef]
- Heck, J.G.; Napp, J.; Simonato, S.; Möllmer, J.; Lange, M.; Reichardt, H.M.; Staudt, R.; Alves, F.; Feldmann, C. Multifunctional phosphate-based inorganic-organic hybrid nanoparticles. J. Am. Chem. Soc. 2015, 137, 7329–7336. [Google Scholar] [CrossRef]
- Neumeier, B.L.; Khorenko, M.; Alves, F.; Goldmann, O.; Napp, J.; Schepers, U.; Reichardt, H.M.; Feldmann, C. Fluorescent Inorganic-Organic Hybrid Nanoparticles. ChemNanoMat 2019, 5, 24–45. [Google Scholar] [CrossRef]
- Sabljo, K.; Napp, J.; Alves, F.; Feldmann, C. pH-Dependent fluorescence of La(OH)2+ARS- hybrid nanoparticles for intracellular pH-sensing. Chem. Commun. 2022, 58, 9417–9420. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Pang, Y. Excited state intramolecular proton transfer of 1,2-dihydroxyanthraquinone by femtosecond transient absorption spectroscopy. Curr. Appl. Phys. 2015, 15, 1492–1499. [Google Scholar] [CrossRef]
- Huber, R.; Spörlein, S.; Moser, J.E.; Grätzel, M.; Wachtveitl, J. The Role of Surface States in the Ultrafast Photoinduced Electron Transfer from Sensitizing Dye Molecules to Semiconductor Colloids. J. Phys. Chem. B 2000, 104, 8995–9003. [Google Scholar] [CrossRef]
- Duncan, W.R.; Stier, W.M.; Prezhdo, O.V. Ab initio nonadiabatic molecular dynamics of the ultrafast electron injection across the alizarin-TiO2 interface. J. Am. Chem. Soc. 2005, 127, 7941–7951. [Google Scholar] [CrossRef]
- Huber, R.; Moser, J.-E.; Grätzel, M.; Wachtveitl, J. Real-Time Observation of Photoinduced Adiabatic Electron Transfer in Strongly Coupled Dye/Semiconductor Colloidal Systems with a 6 fs Time Constant. J. Phys. Chem. B 2002, 106, 6494–6499. [Google Scholar] [CrossRef]
- Matylitsky, V.V.; Lenz, M.O.; Wachtveitl, J. Observation of pH-dependent back-electron-transfer dynamics in alizarin/TiO2 adsorbates: Importance of trap states. J. Phys. Chem. B 2006, 110, 8372–8379. [Google Scholar] [CrossRef]
- Dawson, A.B. A Note on the Staining of the Skeleton of Cleared Specimens with Alizarin Red S. Stain Technol. 1926, 1, 123–124. [Google Scholar] [CrossRef]
- Kubo, Y.; Ishida, T.; Kobayashi, A.; James, T.D. Fluorescent alizarin–phenylboronic acid ensembles: Design of self-organized molecular sensors for metal ions and anions. J. Mater. Chem. 2005, 15, 2889. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Huggins, D.J.; Sherman, W.; Tidor, B. Rational approaches to improving selectivity in drug design. J. Med. Chem. 2012, 55, 1424–1444. [Google Scholar] [CrossRef]
- Lica, J.J.; Wieczór, M.; Grabe, G.J.; Heldt, M.; Jancz, M.; Misiak, M.; Gucwa, K.; Brankiewicz, W.; Maciejewska, N.; Stupak, A.; et al. Effective Drug Concentration and Selectivity Depends on Fraction of Primitive Cells. Int. J. Mol. Sci. 2021, 22, 4931. [Google Scholar] [CrossRef]
- Pinelli, F.; Perale, G.; Rossi, F. Coating and Functionalization Strategies for Nanogels and Nanoparticles for Selective Drug Delivery. Gels 2020, 6, 6. [Google Scholar] [CrossRef]
- Potter, E.D.; Herek, J.L.; Pedersen, S.; Liu, Q.; Zewail, A.H. Femtosecond laser control of a chemical reaction. Nature 1992, 355, 66–68. [Google Scholar] [CrossRef]
- Guthrie, J.P. Hydrolysis of esters of oxy acids: PKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units. Can. J. Chem. 1978, 56, 2342–2354. [Google Scholar] [CrossRef]
- Jen, M.; Lee, S.; Jeon, K.; Hussain, S.; Pang, Y. Ultrafast Intramolecular Proton Transfer of Alizarin Investigated by Femtosecond Stimulated Raman Spectroscopy. J. Phys. Chem. B 2017, 121, 4129–4136. [Google Scholar] [CrossRef] [PubMed]
- Jen, M.; Jeon, K.; Lee, S.; Hwang, S.; Chung, W.-J.; Pang, Y. Ultrafast intramolecular proton transfer reactions and solvation dynamics of DMSO. Struct. Dyn. 2019, 6, 64901. [Google Scholar] [CrossRef]
- Kawashima, T.; Ogawa, H.; Hamaguchi, H. Spectrophotometric study of the complex of lanthanum and alizarin Red S. Talanta 1961, 8, 552–556. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sugaya, T.; Iwatsuki, S.; Inamo, M.; Takagi, H.D.; Odani, A.; Ishihara, K. Detailed Reaction Mechanism of Phenylboronic Acid with Alizarin Red S in Aqueous Solution: Re-Investigation with Spectrophotometry and Fluorometry. ChemistrySelect 2017, 2, 2956–2964. [Google Scholar] [CrossRef]
- Shalaby, A.A.; Mohamed, A.A. Determination of acid dissociation constants of Alizarin Red S, Methyl Orange, Bromothymol Blue and Bromophenol Blue using a digital camera. RSC Adv. 2020, 10, 11311–11316. [Google Scholar] [CrossRef]
- Gomez, T.; Hermann, G.; Zarate, X.; Pérez-Torres, J.F.; Tremblay, J.C. Imaging the Ultrafast Photoelectron Transfer Process in Alizarin-TiO2. Molecules 2015, 20, 13830–13853. [Google Scholar] [CrossRef]
- Kaniyankandy, S.; Verma, S.; Mondal, J.A.; Palit, D.K.; Ghosh, H.N. Evidence of Multiple Electron Injection and Slow Back Electron Transfer in Alizarin-Sensitized Ultrasmall TiO2 Particles. J. Phys. Chem. C 2009, 113, 3593–3599. [Google Scholar] [CrossRef]
- Supkowski, R.M.; Bolender, J.P.; Smith, W.D.; Reynolds, L.E.; Horrocks, W.D., Jr. Lanthanide ions as redox probes of long-range electron transfer in proteins. Coord. Chem. Rev. 1999, 185–186, 307–319. [Google Scholar] [CrossRef]
- Akbar, R.; Baral, M.; Kanungo, B.K. Experimental and theoretical approach of photophysical properties of lanthanum(III) and erbium(III) complexes of tris(methoxymethyl)-5-oxine podant. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 365–376. [Google Scholar] [CrossRef]
- Zollinger, H. Mesomeric effect of the sulphonic acid group. Nature 1953, 172, 257–258. [Google Scholar] [CrossRef]
- Imaizumi, H.; Koyanagi, T.; Zhao, D. Reactivity of sulfonic acid group and estimation of its substituent-effect in T-for-H exchange reaction. J. Radioanalyt. Nucl. Chem. 2002, 252, 467–472. [Google Scholar] [CrossRef]
- Fehér, P.P.; Purgel, M.; Joó, F. Performance of exchange–correlation functionals on describing ground state geometries and excitations of Alizarin Red S: Effect of complexation and degree of deprotonation. Comput. Theor. Chem. 2014, 1045, 113–122. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, H.; Zhou, P. Effects of solvents on the excited state intramolecular proton transfer and hydrogen bond mechanisms of alizarin and its isomers. J. Mol. Liquids 2020, 301, 112415. [Google Scholar] [CrossRef]
- Amat, A.; Miliani, C.; Romani, A.; Fantacci, S. DFT/TDDFT investigation on the UV-vis absorption and fluorescence properties of alizarin dye. Phys. Chem. Chem. Phys. 2015, 17, 6374–6382. [Google Scholar] [CrossRef]
- Le Person, A.; Cornard, J.-P.; Say-Liang-Fat, S. Studies of the tautomeric forms of alizarin in the ground state by electronic spectroscopy combined with quantum chemical calculations. Chem. Phys. Lett. 2011, 517, 41–45. [Google Scholar] [CrossRef]
- Lv, G.; Li, C. A theoretical study of solvent effect on the excited state intramolecular proton transfer of 3-hydroxyflavone. J. Phys. Org. Chem. 2023, 36, e4476. [Google Scholar] [CrossRef]
- Tao, M.; Wen, L.; Huo, D.; Kuang, Z.; Di Song; Wan, Y.; Zhao, H.; Yan, J.; Xia, A. Solvent Effect on Excited-State Intramolecular Proton-Coupled Charge Transfer Reaction in Two Seven-Membered Ring Pyrrole-Indole Hydrogen Bond Systems. J. Phys. Chem. B 2021, 125, 11275–11284. [Google Scholar] [CrossRef]
- Schedlbauer, J.; Streicher, S.; Forster, M.; Scherf, U.; Vogelsang, J.; Lupton, J.M. Tracking Exciton Diffusion and Exciton Annihilation in Single Nanoparticles of Conjugated Polymers by Photon Correlation Spectroscopy. Adv. Opt. Mater. 2022, 10, 2200092. [Google Scholar] [CrossRef]
- Michenfelder, N.C.; Gienger, C.; Dilanas, M.; Schnepf, A.; Unterreiner, A.-N. Photoexcitation of Ge9- Clusters in THF: New Insights into the Ultrafast Relaxation Dynamics and the Influence of the Cation. Molecules 2020, 25, 2639. [Google Scholar] [CrossRef] [PubMed]


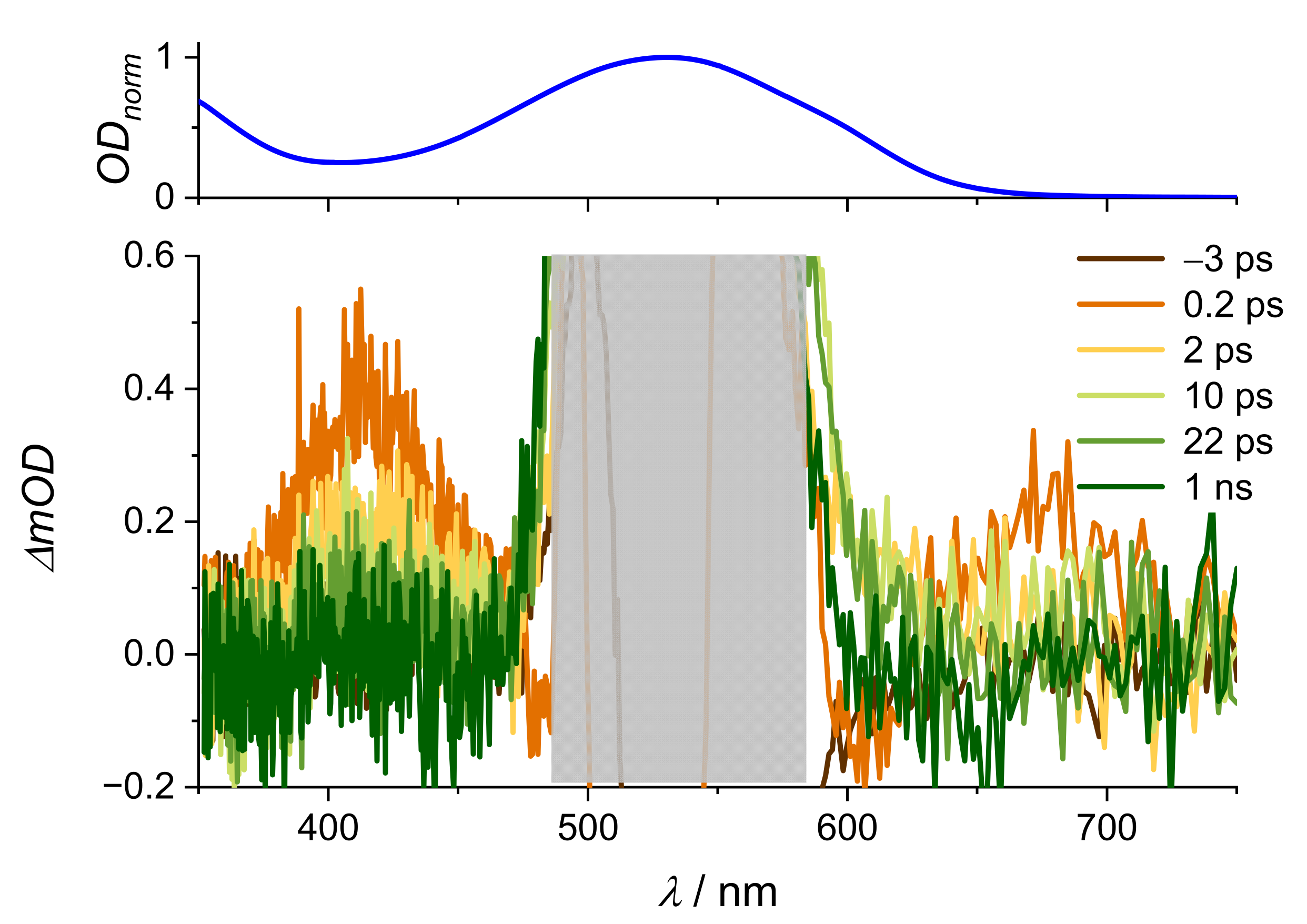
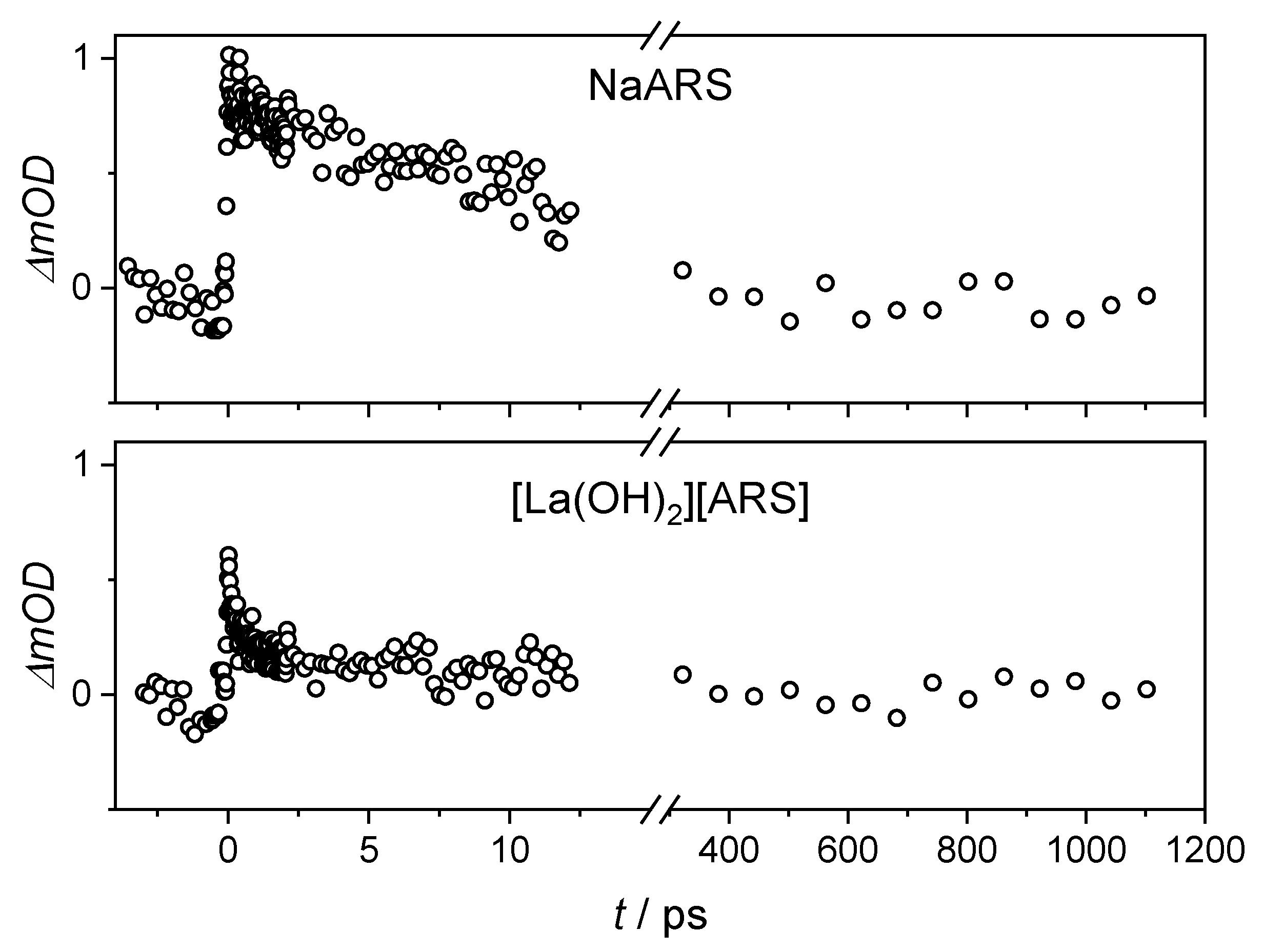

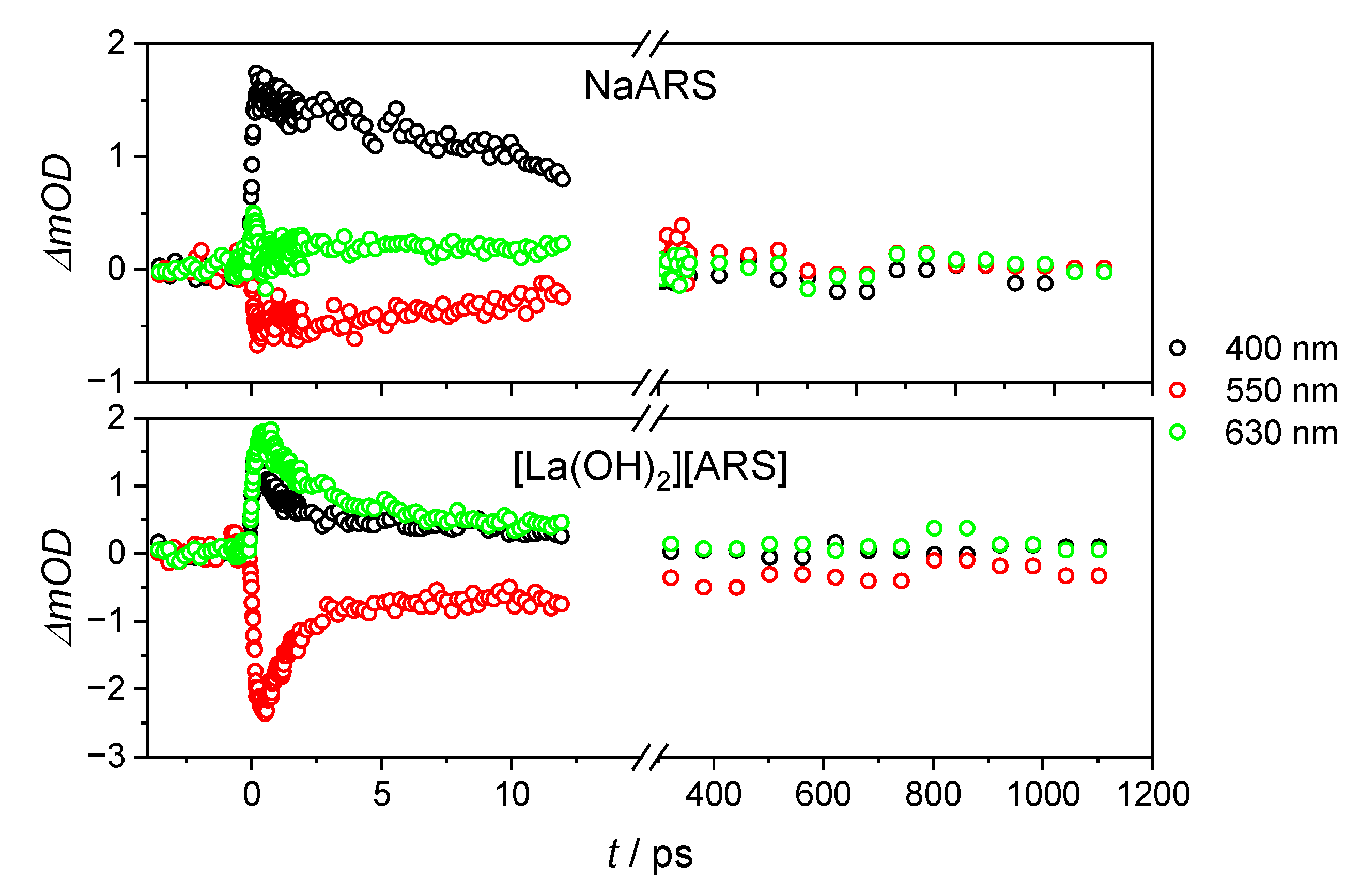
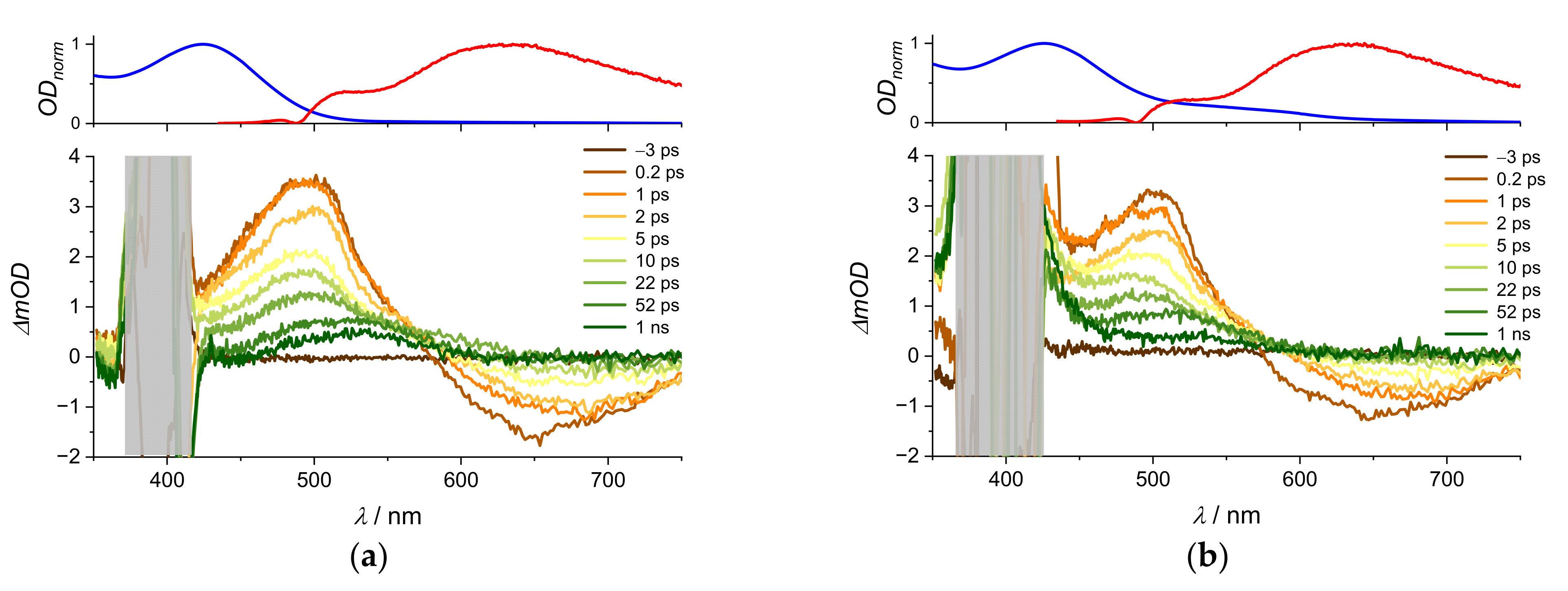
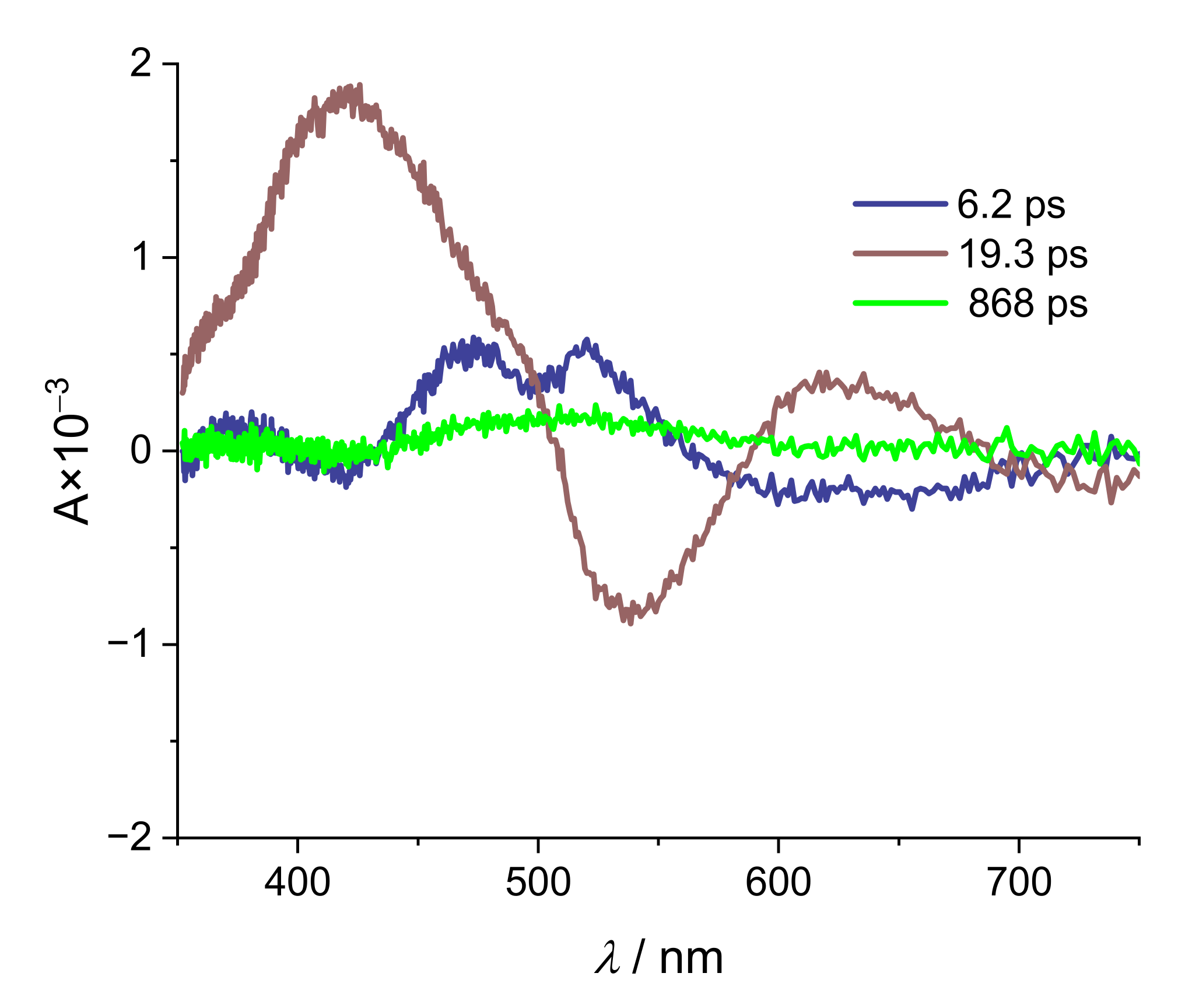
| Na(ARS) | [La(OH)2][ARS] | |
|---|---|---|
| τ1/ps | 2.1 | 0.26 |
| τ2/ps | 19.5 | 14.2 |
| Na(ARS) | [La(OH)2][ARS] | |
|---|---|---|
| τ1/ps | 6.2 | 0.8 |
| τ2/ps | 19.3 | 6.7 |
| τ3/ps | 868 | 1390 |
| Na(ARS) | [La(OH)2][ARS] | |
|---|---|---|
| τ1/ps | 2.9 | 2.9 |
| τ2/ps | 26.5 | 46.8 |
| τ3/ps | >>1000 | >>1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauthe, P.; Sabljo, K.; Vogelbacher, M.K.; Feldmann, C.; Unterreiner, A.-N. Excited State Dynamics of Alizarin Red S Nanoparticles in Solution. Molecules 2023, 28, 5633. https://doi.org/10.3390/molecules28155633
Rauthe P, Sabljo K, Vogelbacher MK, Feldmann C, Unterreiner A-N. Excited State Dynamics of Alizarin Red S Nanoparticles in Solution. Molecules. 2023; 28(15):5633. https://doi.org/10.3390/molecules28155633
Chicago/Turabian StyleRauthe, Pascal, Kristina Sabljo, Maike Kristin Vogelbacher, Claus Feldmann, and Andreas-Neil Unterreiner. 2023. "Excited State Dynamics of Alizarin Red S Nanoparticles in Solution" Molecules 28, no. 15: 5633. https://doi.org/10.3390/molecules28155633
APA StyleRauthe, P., Sabljo, K., Vogelbacher, M. K., Feldmann, C., & Unterreiner, A.-N. (2023). Excited State Dynamics of Alizarin Red S Nanoparticles in Solution. Molecules, 28(15), 5633. https://doi.org/10.3390/molecules28155633






