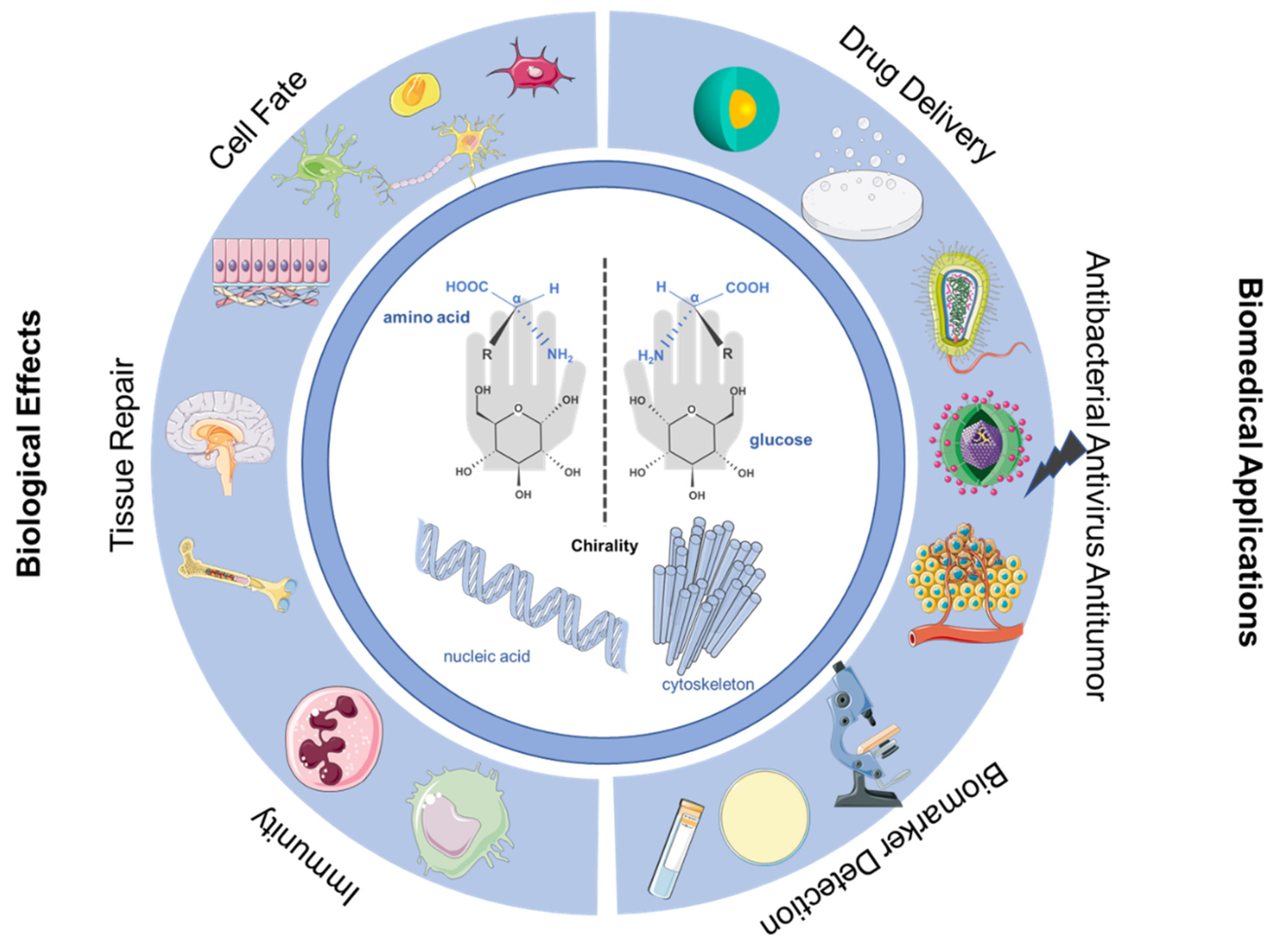
Interface Chirality: From Biological Effects to Biomedical Applications
Abstract
1. Introduction
2. Chiral Interface for Controlling the Cell Fate
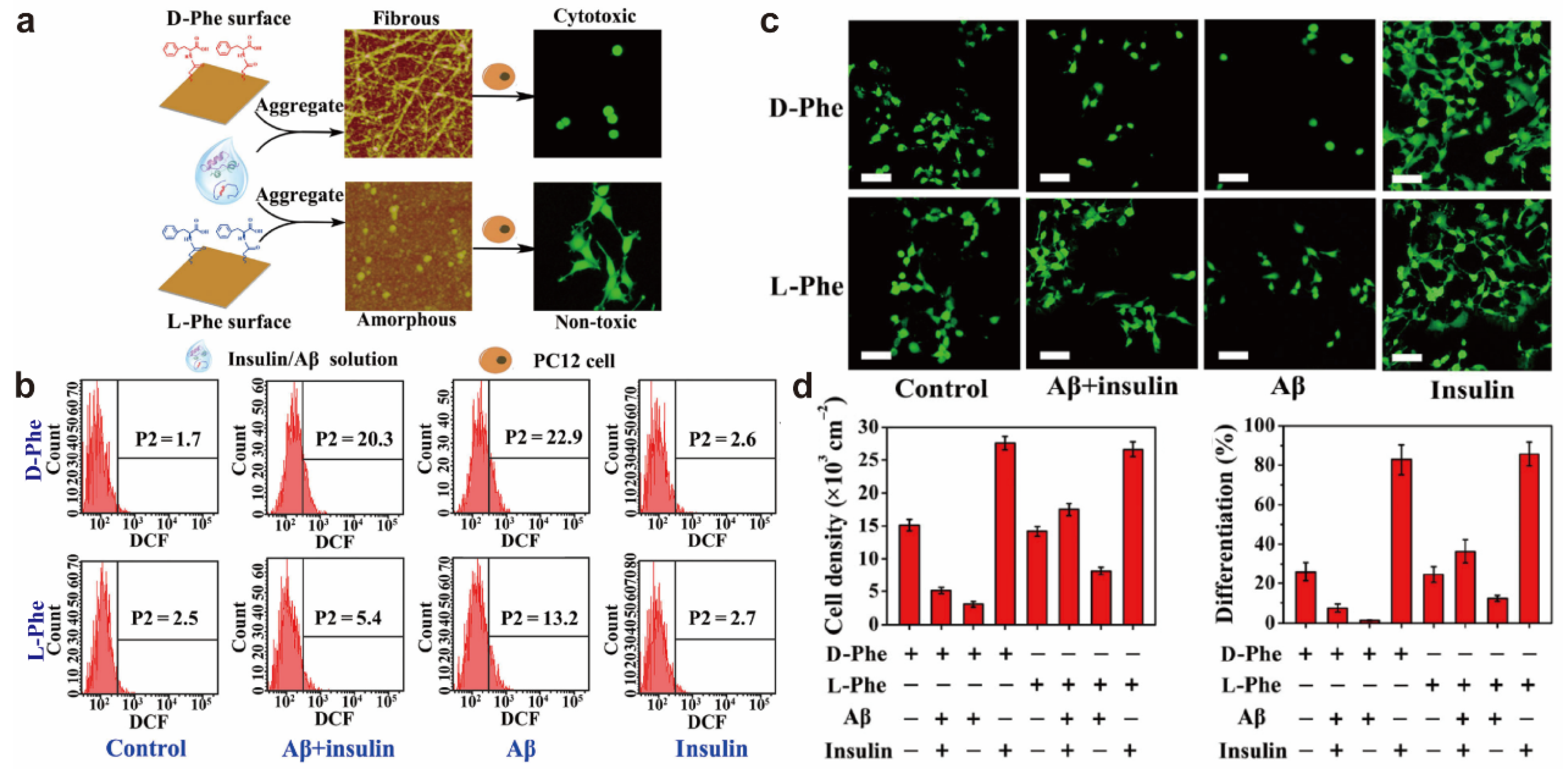
2.1. Chiral Effects on Cell Toxicity or Viability
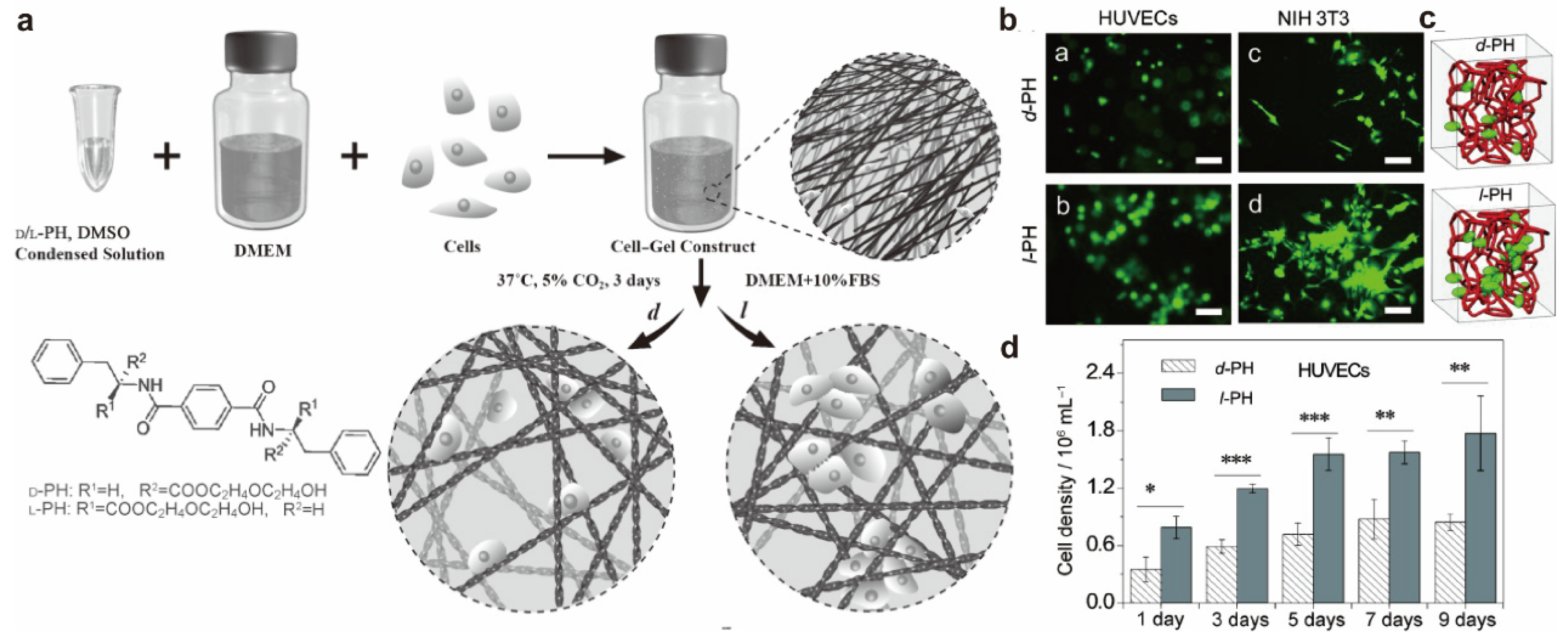
2.2. Chiral Effects on Cell Adhesion
2.3. Chiral Effects on Stem Cell Differentiation
3. Chiral Effects on Tissue Repair
4. Chiral Effects on Immunity
5. Application of Chiral Interface
5.1. Chiral Effects on Drug Delivery
5.2. Chiral Effects on Antibacterial, Antivirus and Antitumor
5.3. Chiral Effects on Biomarker Detection
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, W.; Zhu, B.; Fang, K.; Li, X.Y.; Hansen, T.W.; Ou, Y.; Yang, H.; Wagner, J.B.; Gao, Y.; Wang, Y.; et al. In situ manipulation of the active Au-TiO2 interface with atomic precision during CO oxidation. Science 2021, 371, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, J.; Wang, X.; Xiao, M.; Chandrasekaran, A.R.; Li, L.; Yi, C.; Pei, H. Biointerface Engineering with Nucleic Acid Materials for Biosensing Applications. Adv. Funct. Mater. 2022, 32, 2201069. [Google Scholar] [CrossRef]
- Ding, P.; Wang, Z.; Wu, Z.; Zhou, Y.; Sun, N.; Pei, R. Natural Biointerface Based on Cancer Cell Membranes for Specific Capture and Release of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2020, 12, 20263–20270. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Guo, X.; Xu, L.; Kuang, H.; Xu, C. Chirality at nanoscale for bioscience. Chem. Sci. 2022, 13, 3069–3081. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, L.; Yue, H.; Gao, X. Recognition at chiral interfaces: From molecules to cells. Colloids Surf B Biointerfaces 2020, 195, 111268. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-F.; Zhang, D.; Feng, C.-L. Control of Three-Dimensional Cell Adhesion by the Chirality of Nanofibers in Hydrogels. Angew. Chem. Int. Ed. 2014, 53, 7789–7793. [Google Scholar] [CrossRef]
- Sun, N.; Dou, X.; Tang, Z.; Zhang, D.; Ni, N.; Wang, J.; Gao, H.; Ju, Y.; Dai, X.; Zhao, C.; et al. Bio-inspired chiral self-assemblies promoted neuronal differentiation of retinal progenitor cells through activation of metabolic pathway. Bioact. Mater. 2021, 6, 990–997. [Google Scholar] [CrossRef] [PubMed]
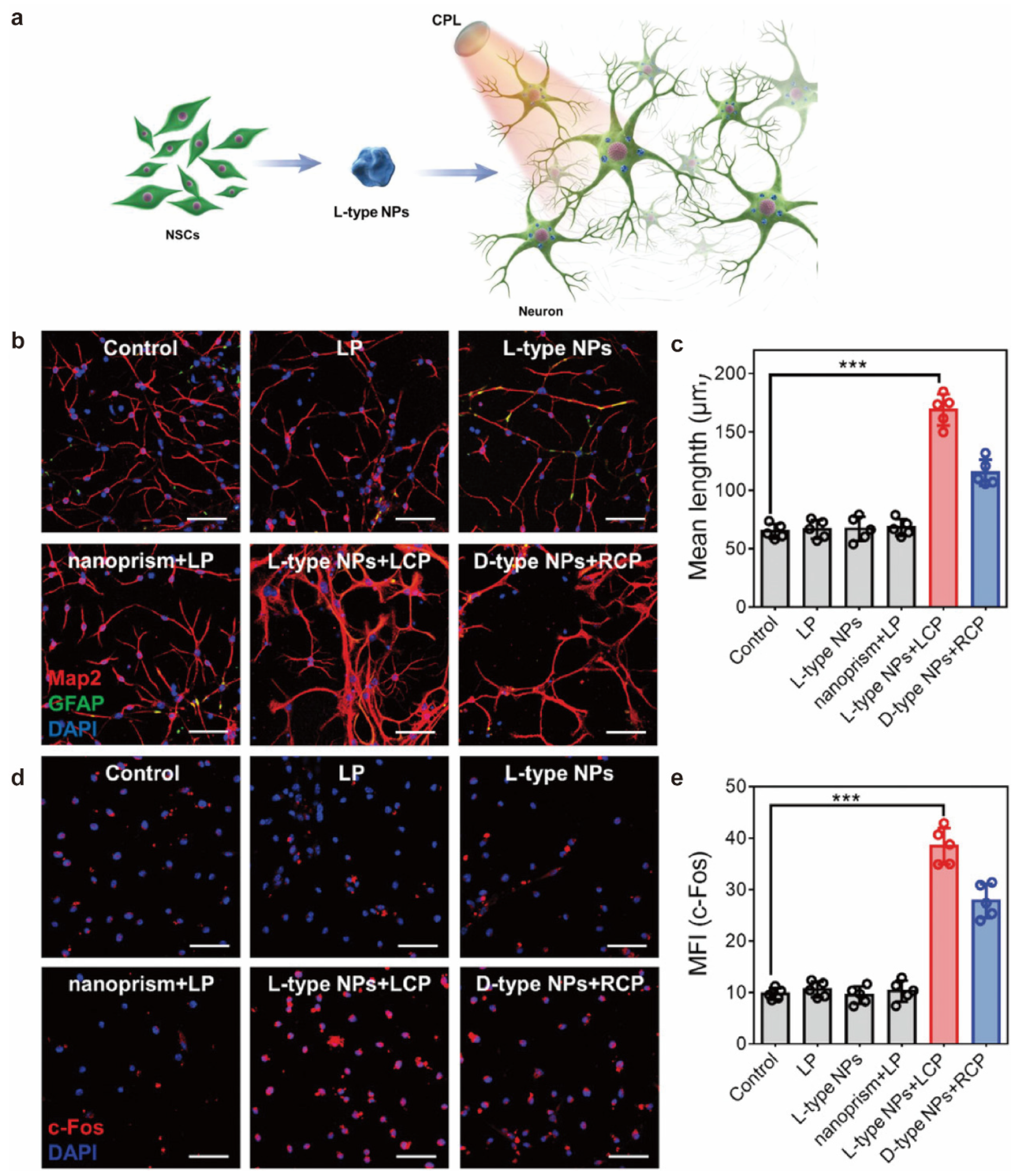
- Shi, B.; Zhao, J.; Xu, Z.; Chen, C.; Xu, L.; Xu, C.; Sun, M.; Kuang, H. Chiral Nanoparticles Force Neural Stem Cell Differentiation to Alleviate Alzheimer’s Disease. Adv. Sci. 2022, 9, e2202475. [Google Scholar] [CrossRef] [PubMed]
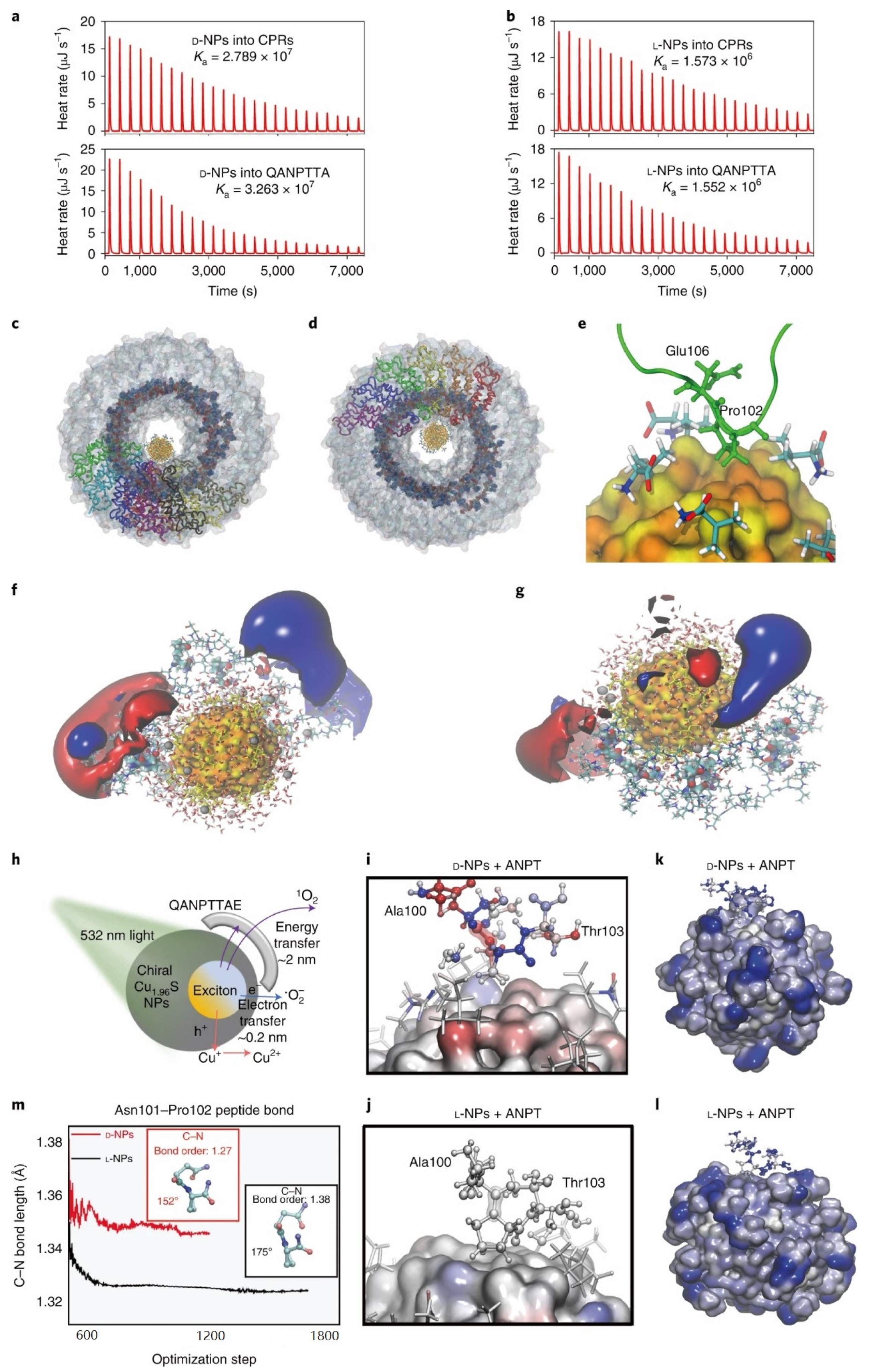
- Xu, L.; Wang, X.; Wang, W.; Sun, M.; Choi, W.J.; Kim, J.-Y.; Hao, C.; Li, S.; Qu, A.; Lu, M.; et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef]
- Green, D.W.; Lee, J.M.; Kim, E.J.; Lee, D.J.; Jung, H.S. Chiral Biomaterials: From Molecular Design to Regenerative Medicine. Adv. Mater. Interfaces 2016, 3, 1500411. [Google Scholar] [CrossRef]
- Tee, Y.H.; Goh, W.J.; Yong, X.; Ong, H.T.; Hu, J.; Tay, I.Y.Y.; Shi, S.; Jalal, S.; Barnett, S.F.H.; Kanchanawong, P.; et al. Actin polymerisation and crosslinking drive left-right asymmetry in single cell and cell collectives. Nat. Commun. 2023, 14, 776. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, C.; Sun, M.; Xu, L.; Wu, X.; Xu, C.; Kuang, H. Peptide Mediated Chiral Inorganic Nanomaterials for Combating Gram-Negative Bacteria. Adv. Funct. Mater. 2018, 28, 05112. [Google Scholar] [CrossRef]
- Gao, R.; Xu, L.; Sun, M.; Xu, M.; Hao, C.; Guo, X.; Colombari, F.M.; Zheng, X.; Král, P.; de Moura, A.F.; et al. Site-selective proteolytic cleavage of plant viruses by photoactive chiral nanoparticles. Nat. Catal. 2022, 5, 694–707. [Google Scholar] [CrossRef]
- Chen, B.; Song, L.; Yuan, Y.; Liu, X.; Guo, Z.; Gu, Y.; Lou, Z.; Liu, Y.; Zhang, C.; Li, C.; et al. Chirality-Dependent Tumor Phototherapy Using Amino Acid-Engineered Chiral Phosphorene. ACS Appl. Mater. Interfaces 2023, 15, 651–661. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Maclin, J.M.; Wan, L.Q. The Actin Crosslinker Fascin Regulates Cell Chirality. Adv. Biol. 2023, 7, e2200240. [Google Scholar] [CrossRef]
- El-Gindi, J.; Benson, K.; De Cola, L.; Galla, H.-J.; Kehr, N.S. Cell Adhesion Behavior on Enantiomerically Functionalized Zeolite L Monolayers. Angew. Chem. Int. Ed. 2012, 51, 3716–3720. [Google Scholar] [CrossRef]
- Yi, Q.; Wen, X.; Li, L.; He, B.; Nie, Y.; Wu, Y.; Zhang, Z.; Gu, Z. The chiral effects on the responses of osteoblastic cells to the polymeric substrates. Eur. Polym. J. 2009, 45, 1970–1978. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Gao, N.; Ren, J.; Qu, X. Opposing enantiomers of tartaric acid anchored on a surface generate different insulin assemblies and hence contrasting cellular responses. Chem. Sci. 2014, 5, 4367–4374. [Google Scholar] [CrossRef]
- Deng, J.; Wu, S.; Yao, M.; Gao, C. Surface-anchored poly(acryloyl-L(D)-valine) with enhanced chirality-selective effect on cellular uptake of gold nanoparticles. Sci. Rep. 2016, 6, 31595. [Google Scholar] [CrossRef]
- Zhang, M.; Qing, G.; Sun, T. Chiral biointerface materials. Chem. Soc. Rev. 2011, 41, 1972–1984. [Google Scholar] [CrossRef]
- He, H.; Zhao, K.; Xiao, L.; Zhang, Y.; Cheng, Y.; Wan, S.; Chen, S.; Zhang, L.; Zhou, X.; Liu, K.; et al. Detection and Chiral Recognition of α-Hydroxyl Acid through 1H and CEST NMR Spectroscopy Using a Ytterbium Macrocyclic Complex. Angew. Chem. Int. Ed. 2019, 58, 18286–18289. [Google Scholar] [CrossRef]
- Luo, C.; Hu, G.; Huang, M.; Zou, J.; Jiang, Y. Prediction on separation factor of chiral arylhydantoin compounds and recognition mechanism between chiral stationary phase and the enantiomers. J. Mol. Graph. Model. 2020, 94, 107479. [Google Scholar] [CrossRef]
- Mauro, G.; Black, N.; Billiot, E.; Billiot, F.; Morris, K.F.; Fang, Y. Chiral Recognition of Dansyl Derivatives with an Amino Acid-Based Molecular Micelle: A Molecular Dynamics Investigation. Open J. Phys. Chem. 2021, 11, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wang, H.Y.; Perrett, S.; Zhao, Y.; Tang, Z.; Nie, G. Chirality of glutathione surface coating affects the cytotoxicity of quantum dots. Angew. Chem. Int. Ed. 2011, 50, 5860–5864. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Wang, Y.C.; Elvati, P.; Qu, Z.B.; Kim, K.; Jiang, S.; Baumeister, E.; Lee, J.; Yeom, B.J.; Bahng, J.H.; et al. Chiral Graphene Quantum Dots. ACS Nano 2016, 10, 1744–1755. [Google Scholar] [CrossRef]
- Deng, J.; Yao, M.; Gao, C. Cytotoxicity of gold nanoparticles with different structures and surface-anchored chiral polymers. Acta Biomater. 2017, 53, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Guan, Y.; Ding, C.; Gao, N.; Ren, J.; Qu, X. Cross-fibrillation of insulin and amyloid β on chiral surfaces: Chirality affects aggregation kinetics and cytotoxicity. Nano Res. 2018, 11, 4102–4110. [Google Scholar] [CrossRef]
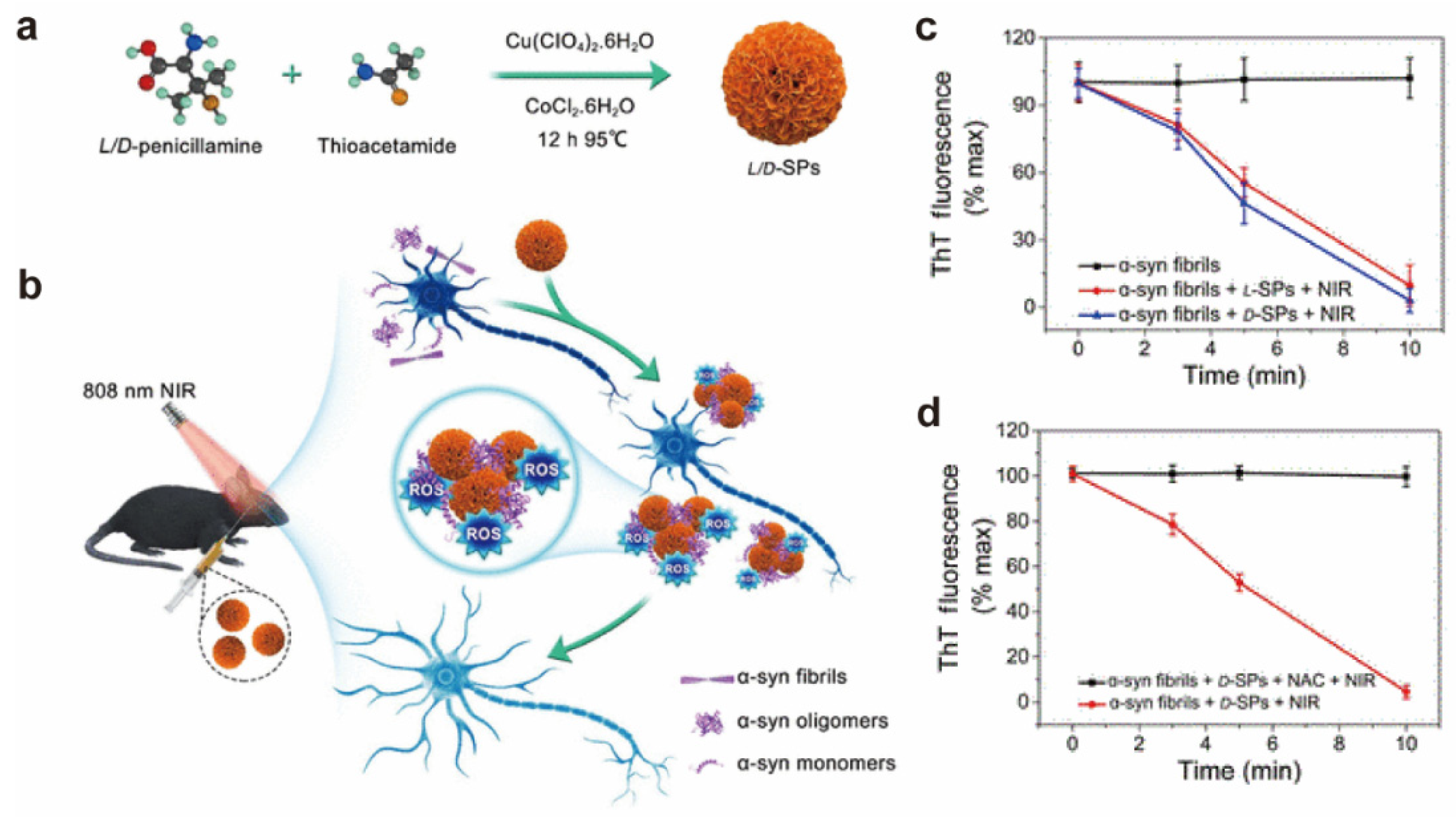
- Shi, B.; Qu, A.; Wang, W.; Lu, M.; Xu, Z.; Chen, C.; Hao, C.; Sun, M.; Xu, L.; Xu, C.; et al. Chiral CuxCoyS Supraparticles Ameliorate Parkinson’s Disease. CCS Chem. 2022, 4, 2440–2451. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, C.; Yan, F.; Sheng, Z. Synthesis of Chiral Penicillamine-Coated Gold Nanoparticles and Effect on PC12 Cells for the Treatment of Alzheimer’s Disease. J. Clust. Sci. 2020, 31, 1071–1075. [Google Scholar] [CrossRef]
- Deng, J.; Li, Z.; Yao, M.; Gao, C. Influence of Albumin Configuration by the Chiral Polymer-Grafted Gold Nanoparticles. Langmuir 2016, 32, 5608–5616. [Google Scholar] [CrossRef]
- Qing, G.; Zhao, S.; Xiong, Y.; Lv, Z.; Jiang, F.; Liu, Y.; Chen, H.; Zhang, M.; Sun, T. Chiral Effect at Protein/Graphene Interface: A Bioinspired Perspective To Understand Amyloid Formation. J. Am. Chem. Soc. 2014, 136, 10736–10742. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Zhang, L.; Lu, W.; Liu, M. Control of supramolecular chirality of nanofibers and its effect on protein adhesion. ACS Appl. Mater. Interfaces 2014, 6, 18878. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zheng, H.; Gao, C. Influence of protein adsorption on the cellular uptake of AuNPs conjugated with chiral oligomers. Mater. Chem. Front. 2016, 1, 542–549. [Google Scholar] [CrossRef]
- Wang, X.; Gan, H.; Sun, T. Chiral Design for Polymeric Biointerface: The Influence of Surface Chirality on Protein Adsorption. Adv. Funct. Mater. 2011, 21, 3276–3281. [Google Scholar] [CrossRef]
- Sun, T.; Han, D.; Rhemann, K.; Chi, L.; Fuchs, H. Stereospecific Interaction between Immune Cells and Chiral Surfaces. J. Am. Chem. Soc. 2007, 129, 1496–1497. [Google Scholar] [CrossRef]
- Wang, X.; Gan, H.; Sun, T.; Su, B.; Fuchs, H.; Vestweber, D.; Butz, S. Stereochemistry triggered differential cell behaviours on chiral polymer surfaces. Soft Matter 2010, 6, 3851–3855. [Google Scholar] [CrossRef]
- Baranes, K.; Moshe, H.; Alon, N.; Schwartz, S.; Shefi, O. Neuronal Growth on l- and d-Cysteine Self-Assembled Monolayers Reveals Neuronal Chiral Sensitivity. ACS Chem. Neurosci. 2014, 5, 370–376. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, L.; Sun, M.; Ma, W.; Wu, X.; Xu, C.; Kuang, H. Tuning the interactions between chiral plasmonic films and living cells. Nat. Commun. 2017, 8, 2007. [Google Scholar] [CrossRef]
- Sun, N.; Wang, J.; Dou, X.; Wang, Y.; Yang, Y.; Xiao, D.; Zhao, P.; Li, J.; Wang, S.; Gu, P.; et al. A chiral microenvironment promotes retinal progenitor cell proliferation by activating the Akt and ERK pathways. Biomater. Sci. 2022, 10, 5938–5946. [Google Scholar] [CrossRef]
- Zheng, H.; Yoshitomi, T.; Yoshimoto, K. Analysis of Chirality Effects on Stem Cell Fate Using Three-dimensional Fibrous Peptide Hydrogels. ACS Appl. Bio Mater. 2018, 1, 538–543. [Google Scholar] [CrossRef]
- Yoshitomi, T.; Zheng, H.; Yoshimoto, K. Investigations of Chirality Effects on Undifferentiated State of Mesenchymal Stem Cells Using Soft Nanofibrous Oligopeptide Hydrogels. Anal. Sci. 2020, 37, 539–543. [Google Scholar] [CrossRef]
- Dou, X.; Wu, B.; Liu, J.; Zhao, C.; Qin, M.; Wang, Z.; Schönherr, H.; Feng, C.-L. Effect of Chirality on Cell Spreading and Differentiation: From Chiral Molecules to Chiral Self-Assembly. ACS Appl. Mater. Interfaces 2019, 11, 38568–38577. [Google Scholar] [CrossRef]
- Peng, W.; Ding, F. Enantioselective recognition of an isomeric ligand by a biomolecule: Mechanistic insights into static and dynamic enantiomeric behavior and structural flexibility. Mol. Biosyst. 2017, 13, 2226–2234. [Google Scholar] [CrossRef]
- Kang, X.; Wang, Y.; Cai, X.-L.; Hua, Y.; Shao, Z.-H.; Chen, X.; Zhao, X.; Zang, S.-Q. Chiral gold clusters functionalized two-dimensional nanoparticle films to regulate the adhesion and differentiation of stem cells. J. Colloid Interface Sci. 2022, 625, 831–838. [Google Scholar] [CrossRef]
- Kim, T.; Kwak, S.; Hwang, M.; Hong, J.; Choi, J.; Yeom, B.; Kim, Y. Recognition of 3D Chiral Microenvironments for Myoblast Differentiation. ACS Biomater. Sci. Eng. 2022, 8, 4230–4235. [Google Scholar] [CrossRef]
- Hao, C.; Qu, A.; Xu, L.; Sun, M.; Zhang, H.; Xu, C.; Kuang, H. Chiral Molecule-mediated Porous Cu xO Nanoparticle Clusters with Antioxidation Activity for Ameliorating Parkinson’s Disease. J. Am. Chem. Soc. 2019, 141, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
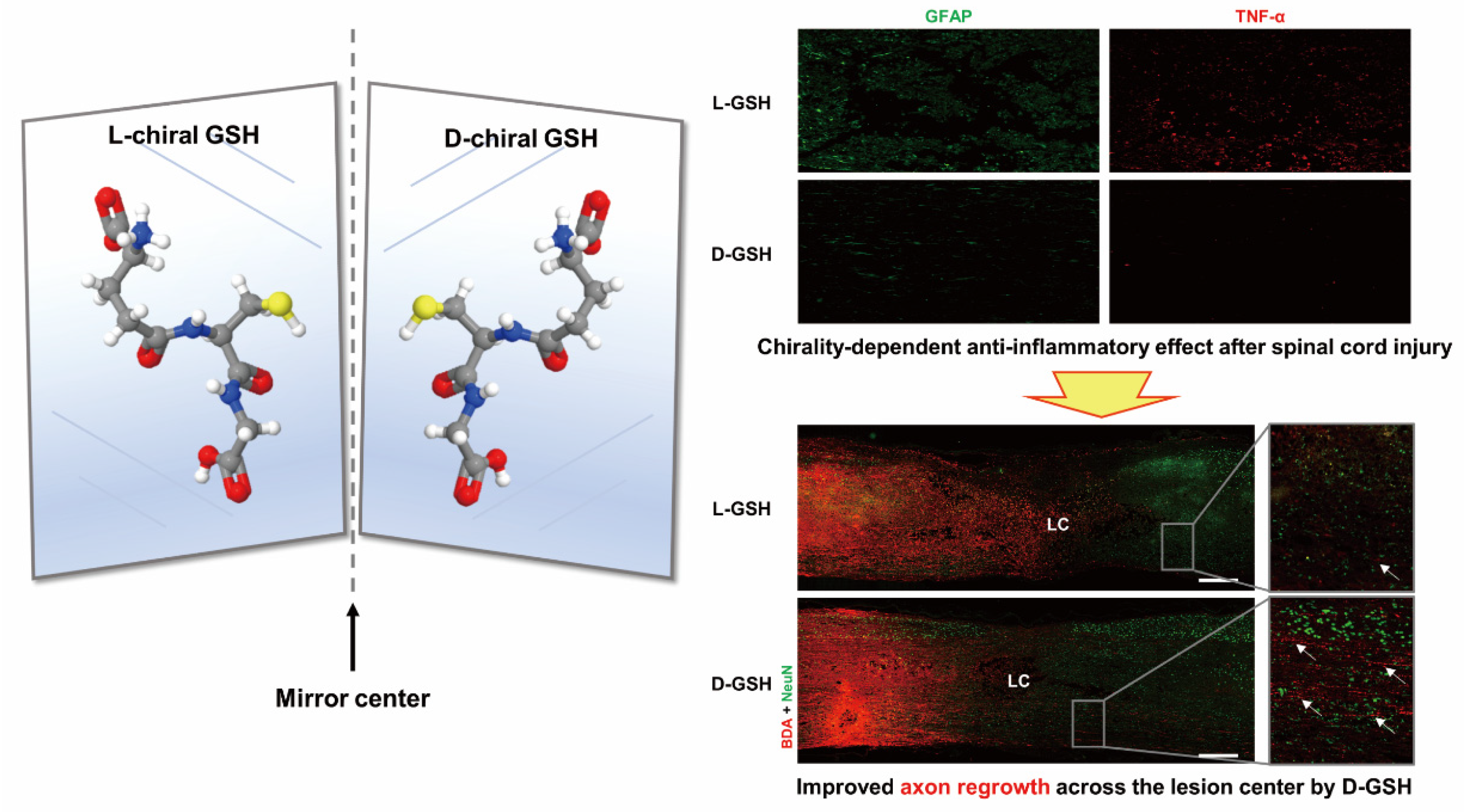
- Kim, S.-J.; Ko, W.-K.; Han, G.-H.; Lee, D.; Lee, Y.; Sheen, S.-H.; Hong, J.-B.; Sohn, S. Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model. Pharmaceuticals 2021, 14, 792. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.Y.; Yeon, B.; Moon, H.J.; Jeong, B. PEG-l-PAF and PEG-d-PAF: Comparative Study on Thermogellation and Biodegradation. Macromolecules 2012, 45, 2007–2013. [Google Scholar] [CrossRef]
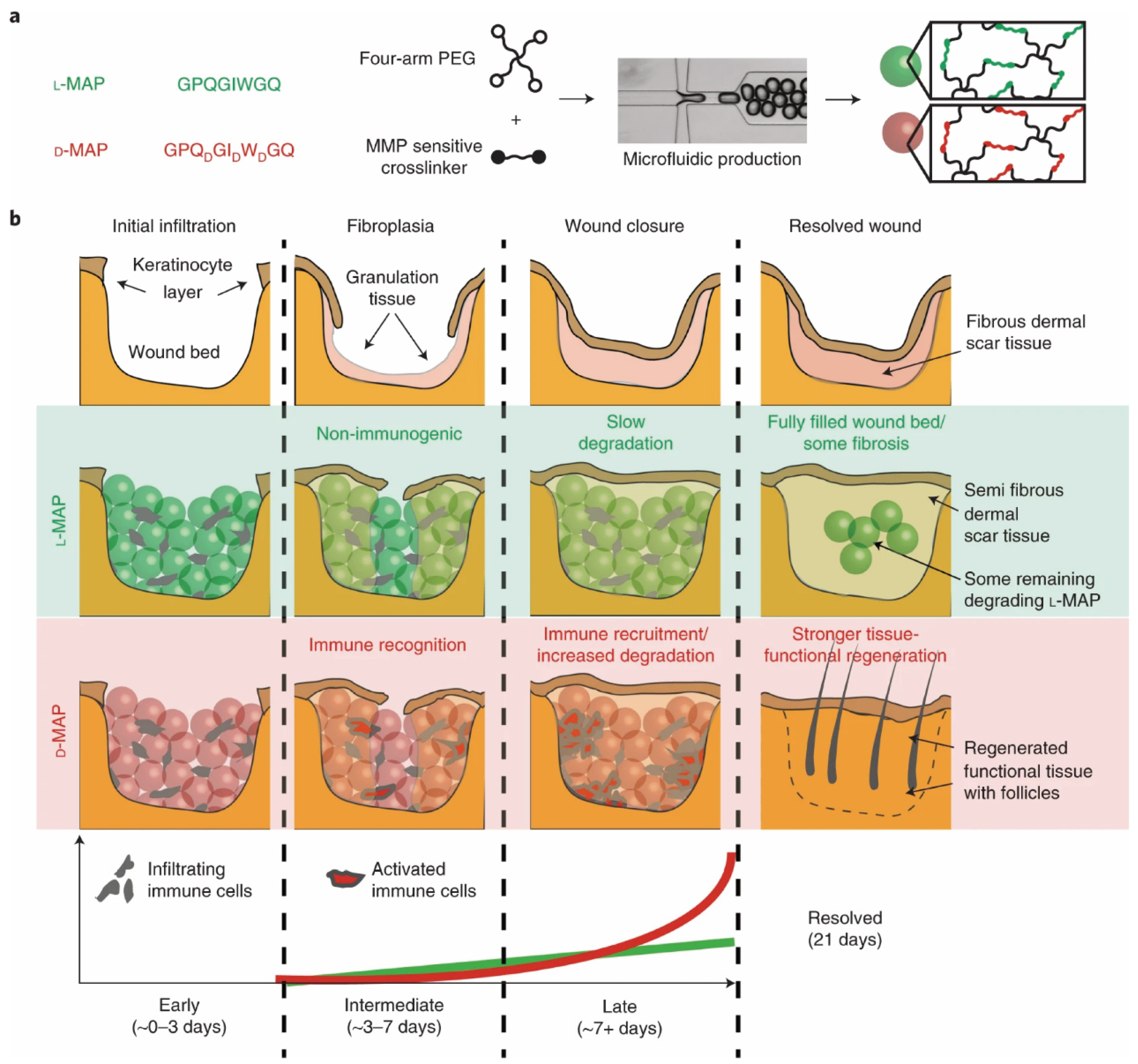
- Griffin, D.R.; Archang, M.M.; Kuan, C.-H.; Weaver, W.M.; Weinstein, J.S.; Feng, A.C.; Ruccia, A.; Sideris, E.; Ragkousis, V.; Koh, J.; et al. Activating an adaptive immune response from a hydrogel scaffold imparts regenerative wound healing. Nat. Mater. 2021, 20, 560–569. [Google Scholar] [CrossRef]
- Wang, F.; Yue, X.; Ding, Q.; Lin, H.; Xu, C.; Li, S. Chiral inorganic nanomaterials for biological applications. Nanoscale 2023, 15, 2541–2552. [Google Scholar] [CrossRef]
- Guo, Z.; Du, Y.; Liu, X.; Ng, S.-C.; Chen, Y.; Yang, Y. Enantioselectively controlled release of chiral drug (metoprolol) using chiral mesoporous silica materials. Nanotechnology 2010, 21, 165103. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, A.; Dorri, P.; Kehr, N.S. Self-assembled monolayers of chiral periodic mesoporous organosilica as a stimuli responsive local drug delivery system. J. Mater. Chem. B 2019, 7, 2362–2371. [Google Scholar] [CrossRef]
- Fan, N.; Liu, R.; Ma, P.; Wang, X.; Li, C.; Li, J. The On-Off chiral mesoporous silica nanoparticles for delivering achiral drug in chiral environment. Colloids Surf. B Biointerfaces 2018, 176, 122–129. [Google Scholar] [CrossRef] [PubMed]
- D’acquarica, I.; Agranat, I. The Quest for Secondary Pharmaceuticals: Drug Repurposing/Chiral-Switches Combination Strategy. ACS Pharmacol. Transl. Sci. 2023, 6, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial Adhesion of Borneol-Based Polymer via Surface Chiral Stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, H.; Zhang, M.; Zhang, J.; Yan, W. Plasmon-Enhanced Antibacterial Activity of Chiral Gold Nanoparticles and In Vivo Therapeutic Effect. Nanomaterials 2021, 11, 1621. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Feng, J.; Zhou, Y.; Wang, B. Synergistic chemotherapy, physiotherapy and photothermal therapy against bacterial and biofilms infections through construction of chiral glutamic acid functionalized gold nanobipyramids. Chem. Eng. J. 2020, 393, 124778. [Google Scholar] [CrossRef]
- Chen, P.; Wang, G.; Hao, C.; Ma, W.; Xu, L.; Kuang, H.; Xu, C.; Sun, M. Peptide-directed synthesis of chiral nano-bipyramids for controllable antibacterial application. Chem. Sci. 2022, 13, 10281–10290. [Google Scholar] [CrossRef]
- Baddi, S.; Dang-I, A.Y.; Huang, T.; Xing, C.; Lin, S.; Feng, C.-L. Chirality-influenced antibacterial activity of methylthiazole- and thiadiazole-based supramolecular biocompatible hydrogels. Acta Biomater. 2022, 141, 59–69. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, C.; Qu, A.; Sun, M.; Xu, L.; Xu, C.; Kuang, H. Light-Induced Chiral Iron Copper Selenide Nanoparticles Prevent beta-Amyloidopathy In Vivo. Angew. Chem. Int. Ed. 2020, 59, 7131–7138. [Google Scholar] [CrossRef]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D.; et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Eraña, H.; López-Martínez, E.; Claes, N.; Martín, V.F.; Solís, D.M.; Bals, S.; Cortajarena, A.L.; Castilla, J.; Liz-Marzán, L.M. Detection of amyloid fibrils in Parkinson’s disease using plasmonic chirality. Proc. Natl. Acad. Sci. USA 2018, 115, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ran, P.; Chen, C.; Chen, Y.; Xuan, C.; Fu, Y. Study on Chiral Nanocomposite for Recognition of DOPA Enantiomers via Square Wave Voltammetry. J. Electrochem. Soc. 2015, 162, B354–B359. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Oh, J.-W.; Kim, Y.-R.; Kim, J.S.; Kim, H. Chiral gold nanoparticle-based electrochemical sensor for enantioselective recognition of 3,4-dihydroxyphenylalanine. Chem. Commun. 2010, 46, 5665–5667. [Google Scholar] [CrossRef]
- Wei, B.; Liu, N.; Zhang, J.; Ou, X.; Duan, R.; Yang, Z.; Lou, X.; Xia, F. Regulation of DNA Self-Assembly and DNA Hybridization by Chiral Molecules with Corresponding Biosensor Applications. Anal. Chem. 2015, 87, 2058–2062. [Google Scholar] [CrossRef]
- Li, Z.; Mo, Z.; Meng, S.; Gao, H.; Niu, X.; Guo, R.; Wei, T. The construction of electrochemical chiral interfaces using hydroxypropyl chitosan. RSC Adv. 2017, 7, 8542–8549. [Google Scholar] [CrossRef]
- Su, H.; Zheng, Q.; Li, H. Colorimetric detection and separation of chiral tyrosine based on N-acetyl-l-cysteine modified gold nanoparticles. J. Mater. Chem. 2012, 22, 6546–6548. [Google Scholar] [CrossRef]
- Deng, J.; Zheng, H.; Zheng, X.; Yao, M.; Li, Z.; Gao, C. Gold nanoparticles with surface-anchored chiral poly(acryloyl-L(D)-valine) induce differential response on mesenchymal stem cell osteogenesis. Nano Res. 2016, 9, 3683–3694. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A.; Ullah, S.N.M.N.; Barkat, A.; Beg, S. Chiral-engineered supraparticles: Emerging tools for drug delivery. Drug Discov. Today 2023, 28, 103420. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Guo, Y.; Wang, R.; Feng, J.; Shao, N.; Zhou, X.; Zhou, Y. Interface Chirality: From Biological Effects to Biomedical Applications. Molecules 2023, 28, 5629. https://doi.org/10.3390/molecules28155629
Guo L, Guo Y, Wang R, Feng J, Shao N, Zhou X, Zhou Y. Interface Chirality: From Biological Effects to Biomedical Applications. Molecules. 2023; 28(15):5629. https://doi.org/10.3390/molecules28155629
Chicago/Turabian StyleGuo, Liting, Yanqiu Guo, Rui Wang, Jie Feng, Nannan Shao, Xiaolin Zhou, and Yunlong Zhou. 2023. "Interface Chirality: From Biological Effects to Biomedical Applications" Molecules 28, no. 15: 5629. https://doi.org/10.3390/molecules28155629
APA StyleGuo, L., Guo, Y., Wang, R., Feng, J., Shao, N., Zhou, X., & Zhou, Y. (2023). Interface Chirality: From Biological Effects to Biomedical Applications. Molecules, 28(15), 5629. https://doi.org/10.3390/molecules28155629




