The Morphologically Controlled Synthesis and Application of Mesoporous Alumina Spheres
Abstract
1. Introduction
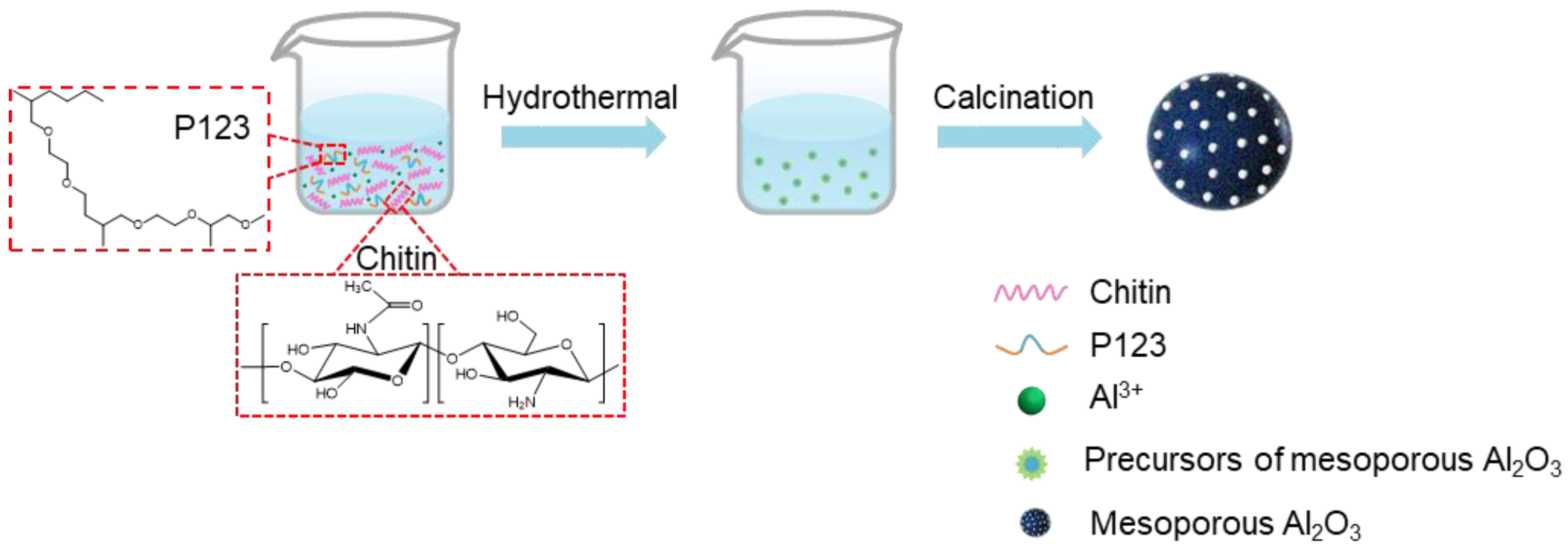
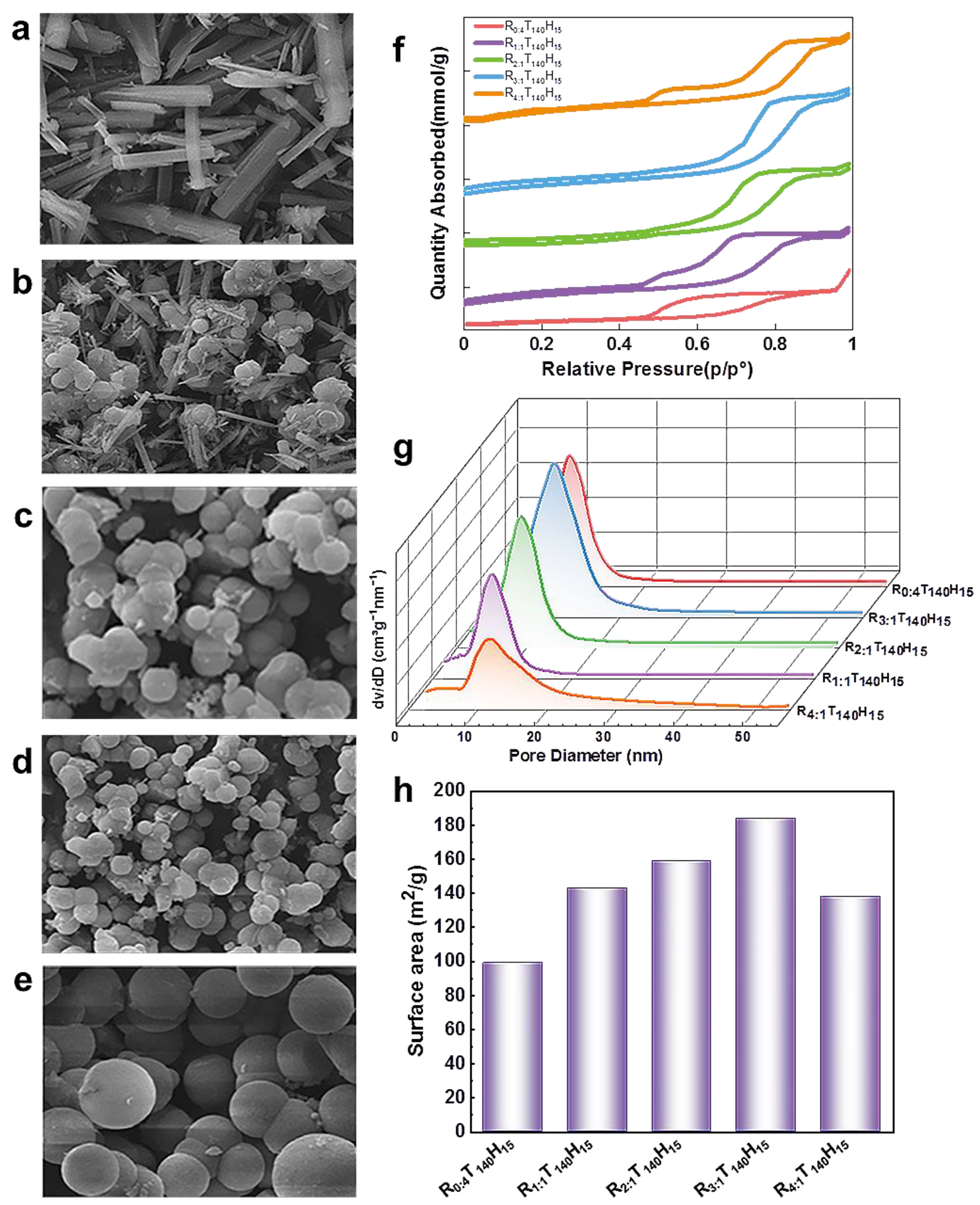
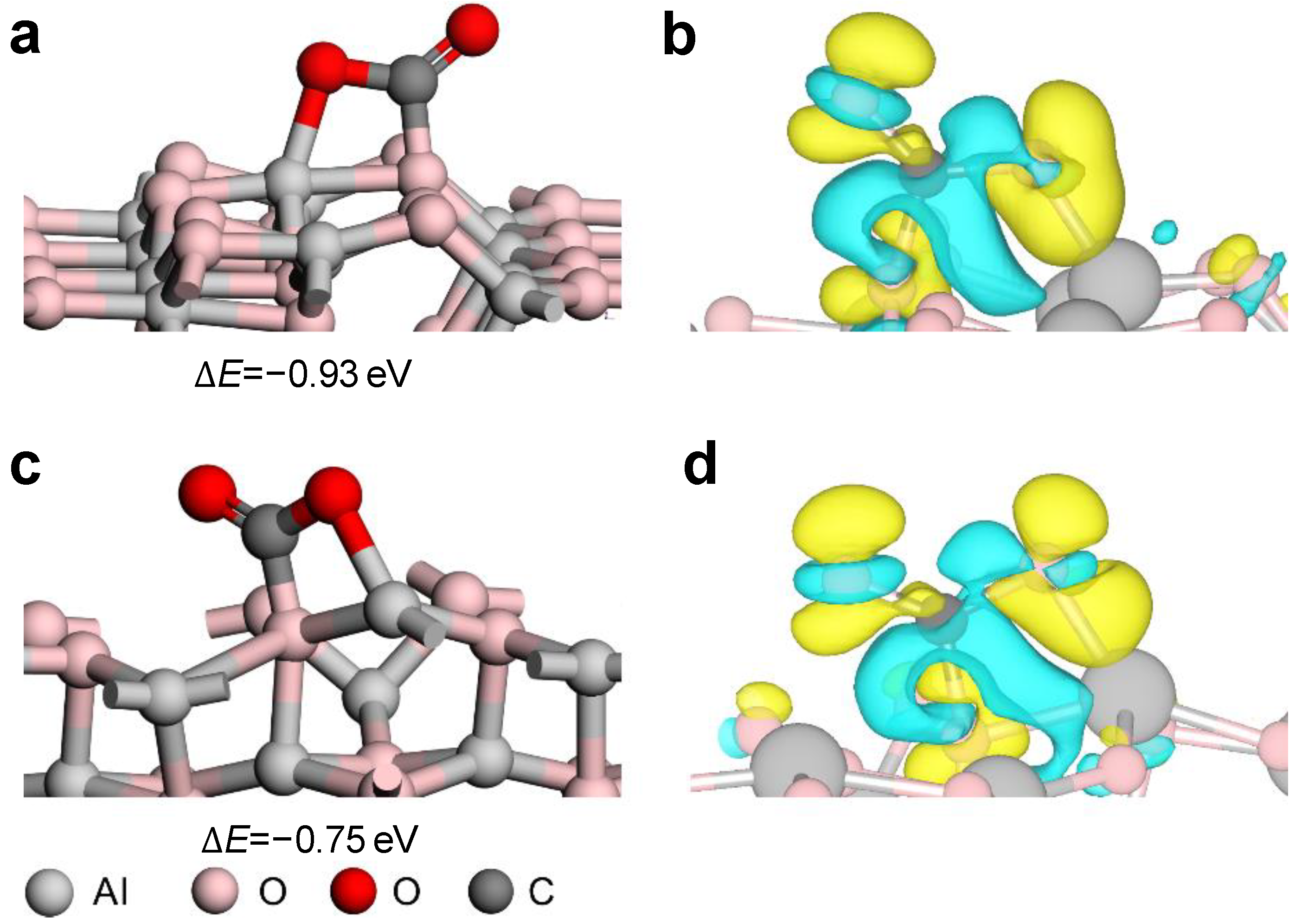
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Mesoporous Alumina Spheres
3.2. Physical Characterization
3.3. Electrochemistry Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Shan, G.; Liu, H.; Xing, J. Preparation of (Ni/W)-gamma-Al2O3 microspheres and their application in adsorption desulfurization for model gasoline. Chem. Eng. Commun. 2007, 194, 938–945. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Khan, M.; Malik, A.; Khraisheh, M.; Hijazi, D.; Mohamed, S.; Alsorour, S.; Eltayeb, R.; Al Mahmoud, F.; Alahmad, J. Development of novel nano-γ-Al2O3 adsorbent from waste aluminum foil for the removal of boron and bromide from aqueous solution. J. Water Process. Eng. 2022, 50, 103312. [Google Scholar] [CrossRef]
- Shen, X.; Yan, F.; Li, C.; Qu, F.; Wang, Y.; Zhang, Z. Biogas upgrading via cyclic CO2 adsorption: Application of highly regenerable PEI@ nano-Al2O3 adsorbents with anti-urea properties. Environ. Sci. Technol. 2021, 55, 5236–5247. [Google Scholar] [CrossRef]
- Bartsch, M.; Saruhan, B.; Schmücker, M.; Schneider, H. Novel low-temperature processing route of dense mullite ceramics by reaction sintering of amorphous SiO2-coated gamma-Al2O3 particle nanocomposites. J. Am. Ceram. Soc. 2004, 82, 1388–1392. [Google Scholar] [CrossRef]
- Liu, X.; Zou, B.; Xing, H.; Huang, C. The preparation of ZrO2-Al2O3 composite ceramic by SLA-3D printing and sintering processing. Ceram. Int. 2020, 46, 937–944. [Google Scholar] [CrossRef]
- Lietti, L.; Forzatti, P.; Nova, I.; Tronconi, E. NOx storage reduction over Pt-Ba/gamma-Al2O3 catalyst. J. Catal. 2001, 204, 175–191. [Google Scholar] [CrossRef]
- Shumilov, V.; Kirilin, A.; Tokarev, A.; Boden, S.; Schubert, M.; Hampel, U.; Hupa, L.; Salmi, T.; Murzin, D.Y. Preparation of γ-Al2O3/α-Al2O3 ceramic foams as catalyst carriers via the replica technique. Catal. Today 2022, 383, 64–73. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; Liu, H.; Shu, Q.; Zhang, Q. Improved charge transfer and morphology on Ti-modified Cu/γ-Al2O3/Al catalyst enhance the activity for methanol steam reforming. Int. J. Hydrogen Energy 2022, 47, 18294–18304. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Yan, B.; You, B.; Zheng, J.; Feng, L.; Zhang, C.; Jiang, S.; Chen, W.; He, S. Facet Engineering of Advanced Electrocatalysts Toward Hydrogen/Oxygen Evolution Reactions. Nano-Micro Lett. 2023, 15, 52. [Google Scholar] [CrossRef]
- Fereja, S.L.; Li, P.; Zhang, Z.; Guo, J.; Fang, Z.; Li, Z.; He, S.; Chen, W. W-doping induced abundant active sites in a 3D NiS2/MoO2 heterostructure as an efficient electrocatalyst for urea oxidation and hydrogen evolution reaction. Chem. Eng. J. 2022, 432, 134274–134280. [Google Scholar] [CrossRef]
- Du, C.; Li, P.; Zhuang, Z.; Fang, Z.; He, S.; Feng, L.; Chen, W. Highly porous nanostructures: Rational fabrication and promising application in energy electrocatalysis. Coord. Chem. Rev. 2022, 466, 214604. [Google Scholar] [CrossRef]
- Wang, C.; Yan, B.; Chen, Z.; You, B.; Liao, T.; Zhang, Q.; Lu, Y.; Jiang, S.; He, S. Recent advances in carbon substrate supported nonprecious nanoarrays for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 25773–25795. [Google Scholar] [CrossRef]
- Kim, K.T.; Dao, T.D.; Jeong, H.M.; Anjanapura, R.V.; Aminabhavi, T.M. Graphene coated with alumina and its utilization as a thermal conductivity enhancer for alumina sphere/thermoplastic polyurethane composite. Mater. Chem. Phys. 2015, 153, 291–300. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2015, 01, 23–27. [Google Scholar] [CrossRef]
- Dabbagh, H.A.; Shahraki, M. Mesoporous nano rod-like γ-alumina synthesis using phenol-formaldehyde resin as a template. Microporous Mesoporous Mater. 2013, 175, 8–15. [Google Scholar] [CrossRef]
- Feng, J.T.; Lin, Y.J.; Li, F.; Evans, D.G.; Li, D.Q. Preparation, structure and properties of micro-spherical alumina with magnetic spinel ferrite cores. Appl. Catal. A Gen. 2007, 329, 112–119. [Google Scholar] [CrossRef]
- Lv, Y.; Li, D.; Tang, P.; Feng, Y. A simple and promoter free way to synthesize spherical γ-alumina with high hydrothermal stability. Mater. Lett. 2015, 155, 75–77. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, F.; Yang, J.; Li, Q.; Tu, B.; Zhao, D. Synthesis of ordered mesoporous alumina with large pore sizes and hierarchical structure. Microporous Mesoporous Mater. 2011, 143, 406–412. [Google Scholar] [CrossRef]
- Tanaka, K.; Imai, T.; Murakami, Y.; Matsumoto, T.; Sugimoto, W.; Takasu, Y. Microporous structure of alumina prepared by a salt catalytic sol-gel process. Chem. Lett. 2002, 31, 110–111. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Zhang, Q.; Zhang, C.; Ding, Y.; Han, J.; Jiang, S.; He, S. Pore engineering: Structure-capacitance correlations for biomass-derived porous carbon materials. Mater. Des. 2023, 229, 111904. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Feng, L.; Yan, B.; Zheng, J.; Zhang, Q.; Wei, R.; Zhang, C.; Han, J.; Jiang, S.; He, S. Chemical foaming-assisted synthesis of N, O co-doped hierarchical porous carbon from soybean protein for high rate performance supercapacitors. Diam. Relat. Mater. 2023, 133, 109767. [Google Scholar] [CrossRef]
- Dahlan, I.; Marsih, N.; Panpranot, J. γ-Alumina Nanotubes Prepared by Hydrothermal Method as Support of Iron, Cobalt and Nickel for Fischer-Tropsch Catalysts. Chem. Mater. Res. 2012, 2, 31–38. [Google Scholar]
- Saeb, M.R.; Rabiee, N.; Seidi, F.; Far, B.F.; Bagherzadeh, M.; Lima, E.C.; Rabiee, M. Green CoNi2S4/porphyrin decorated carbon-based nanocomposites for genetic materials detection. J. Bioresour. Bioprod. 2021, 6, 215–222. [Google Scholar] [CrossRef]
- Deeksha, B.; Sadanand, V.; Hariram, N.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite fabrics with in situ generated silver nanoparticles by bioreduction method. J. Bioresour. Bioprod. 2021, 6, 75–81. [Google Scholar] [CrossRef]
- He, M.; Wang, X.; Wang, Z.; Chen, L.; Lu, Y.; Zhang, X.; Li, M.; Liu, Z.; Zhang, Y.; Xia, H.; et al. Biocompatible and biodegradable bioplastics constructed from chitin via a “green” pathway for bone repair. ACS Sustain. Chem. Eng. 2017, 5, 9126–9135. [Google Scholar] [CrossRef]
- Sun, G.; Zou, C.; Sun, W.; Fang, Y.; He, S.; Liu, Y.; Zhang, J.; Zhu, Y.; Wang, J. Structural reconstruction of BiPbO2 Br nanosheets for electrochemical CO2 reduction to formate. Mater. Chem. Front. 2023. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Wang, C.; Chen, D.; Zhang, Q.; Jiang, L.; Zhang, C.; Liu, K.; He, S. Capacitive properties of carbon nanofibers derived from blends of cellulose acetate and polyacrylonitrile. New J. Chem. 2023. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Han, J.; Zhang, C.; Yang, W.; Jiang, S.; He, S. Al Foil-Supported Carbon Nanosheets as Self-Supporting Electrodes for High Areal Capacitance Supercapacitors. Molecules 2023, 28, 1831. [Google Scholar] [CrossRef]
- Soni, S.S.; Brotons, G.; Bellour, M.; Narayanan, T.; Gibaud, A. Quantitative SAXS analysis of the P123/water/ethanol ternary phase diagram. J. Phys. Chem. B 2006, 110, 15157–15165. [Google Scholar] [CrossRef]
- Elma, M.; Wang, D.K.; Yacou, C.; da Costa, J.C.D. Interlayer-free P123 carbonised template silica membranes for desalination with reduced salt concentration polarisation. J. Membr. Sci. 2015, 475, 376–383. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, Y.; Kong, Q.; Wang, Q.; Chen, G.; Guam, H.; Dong, C. Soft-template synthesis of mesoporous NiFe2O4 for highly sensitive acetone detection. J. Mater. Sci. Mater. Electron. 2020, 31, 6000–6007. [Google Scholar] [CrossRef]
- Biriaei, R.; Nohair, B.; Kaliaguine, S. A facile route to synthesize mesoporous ZSM-5 with hexagonal arrays using P123 triblock copolymer. Microporous Mesoporous Mater. 2020, 298, 110067. [Google Scholar] [CrossRef]
- Cabrera, S.; Haskouri, J.E.; Alamo, J.; Beltrán, A.; Beltrán, D.; Mendioroz, S.; Marcos, M.D.; Amorós, P. Surfactant-Assisted Synthesis of Mesoporous Alumina Showing Continuously Adjustable Pore Sizes. Adv. Mater. 1999, 11, 379–381. [Google Scholar] [CrossRef]
- Zhi, C.; Shi, S.; Zhang, S.; Si, Y.; Yang, J.; Meng, S.; Fei, B.; Hu, J. Bioinspired All-Fibrous Directional Moisture-Wicking Electronic Skins for Biomechanical Energy Harvesting and All-Range Health Sensing. Nano-Micro Lett. 2023, 15, 60. [Google Scholar] [CrossRef]
- Sikora, A.; Bednarz, Ł.; Fałat, T.; Wałecki, M.; Adamowska, M. Investigation of the impact of simulated solar radiation on the micro- and nanoscale morphology and mechanical properties of a sheet moulded composite surface. Mater. Sci-Poland 2016, 34, 641–649. [Google Scholar] [CrossRef]
- Shkoda, O.A. Study of the effect of the size of the initial nickel powder on the structure formation of the mixture during mechanical activation of titanium and nickel. JPCS 2020, 1459, 012021. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Xie, L.; Tang, H.; Yan, F. Vertically-ordered mesoporous silica films grown on boron nitride-graphene composite modified electrodes for rapid and sensitive detection of carbendazim in real samples. Front. Chem. 2022, 10, 939510. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Vimala, K.; Thomas, V.; Varaprasad, K.; Sreedhar, B.; Bajpai, S.; Raju, K.M. Controlling of silver nanoparticles structure by hydrogel networks. J. Colloid Interface Sci. 2010, 342, 73–82. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, R.; Dou, Y.; Wu, Z.; Huang, T.; Feng, D.; Yang, J.; Yu, A.; Zhao, D. Synthesis of mesoporous carbon spheres with a hierarchical pore structure for the electrochemical double-layer capacitor. Carbon 2011, 49, 1248–1257. [Google Scholar] [CrossRef]
- Xin, Y.; Jiang, P.; Yu, M.; Gu, H.; Li, Q.; Zhang, Z. A universal route to fabricate hierarchically ordered macro/mesoporous oxides with enhanced intrinsic activity. J. Mater. Chem. A 2014, 2, 6419–6425. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B.; Feng, L.; Zheng, J.; You, B.; Chen, J.; Zhao, X.; Zhang, C.; Jiang, S.; He, S. Progress in the use of organic potassium salts for the synthesis of porous carbon nanomaterials: Microstructure engineering for advanced supercapacitors. Nanoscale 2022, 14, 8216–8244. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Wang, F.; Zhao, L.; Zhang, Q.; Xu, W.; He, S. Review on porous carbon materials engineered by ZnO templates: Design, synthesis and capacitance performance. Mater. Des. 2021, 201, 109518. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Use of DFT to achieve a rational understanding of acid–basic properties of γ-alumina surfaces. J. Catal. 2004, 226, 54–68. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Gutiérrez, G.; Taga, A.; Johansson, B. Theoretical structure determination of gamma-Al2O3. Phys. Rev. B 2001, 65, 012101. [Google Scholar] [CrossRef]
- Pinto, H.P.; Nieminen, R.M.; Elliott, S.D. Ab initio study of gamma-Al2O3 surfaces. Phys. Rev. B 2004, 70, 125402. [Google Scholar] [CrossRef]
- Krokidis, X.; Raybaud, P.; Gobichon, A.-E.; Rebours, B.; Euzen, P.; Toulhoat, H. Theoretical Study of the Dehydration Process of Boehmite to γ-Alumina. J. Phys. Chem. B 2001, 105, 5121–5130. [Google Scholar] [CrossRef]
- Menéndez-Proupin, E.; Gutiérrez, G. Electronic properties of bulk gamma-Al2O3. Phys. Rev. B 2005, 72, 035116. [Google Scholar] [CrossRef]
- Ching, W.Y.; Ouyang, L.; Rulis, P.; Yao, H. Ab initio study of the physical properties of γ-Al2O3: Lattice dynamics, bulk properties, electronic structure, bonding, optical properties, and ELNES/XANES spectra. Phys. Rev. B 2008, 78, 014106. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Martins, M.J.F.; Konstantinova, E.; Capaz, R.B.; Souza, W.F.; Chiaro, S.S.X.; Leitão, A.A. Direct comparison between two γ-alumina structural models by DFT calculations. J. Solid State Chem. 2011, 184, 1105–1111. [Google Scholar] [CrossRef]
- Dabbagh, H.A.; Taban, K.; Zamani, M. Effects of vacuum and calcination temperature on the structure, texture, reactivity, and selectivity of alumina: Experimental and DFT studies. J. Mol. Catal. A Chem. 2010, 326, 55–68. [Google Scholar] [CrossRef]
- Ionescu, A.; Allouche, A.; Aycard, J.-P.; Rajzmann, M.; Hutschka, F. Study of γ-Alumina Surface Reactivity: Adsorption of Water and Hydrogen Sulfide on Octahedral Aluminum Sites. J. Phys. Chem. B 2002, 106, 9359–9366. [Google Scholar] [CrossRef]
- Ionescu, A.; Allouche, A.; Aycard, J.-P.; Rajzmann, M.; Gall, R.L. Study of γ-Alumina-Supported Hydrotreating Catalyst: I. Adsorption of Bare MoS2 Sheets on γ-Alumina Surfaces. J. Phys. Chem. B 2003, 107, 8490–8497. [Google Scholar] [CrossRef]


| Sample | Weight Ratio (Chitin/P123) | Reaction Temperature (°C) | Reaction Time (h) |
|---|---|---|---|
| R2:1T120H3 | 2:1 | 120 | 3 |
| R0:1T120H9 | 0:1 | 120 | 9 |
| R4:1T120H15 | 4:1 | 120 | 15 |
| R1:2T140H3 | 1:2 | 140 | 3 |
| R3:1T140H9 | 3:1 | 140 | 9 |
| R1:1T140H15 | 1:1 | 140 | 15 |
| R0:4T160H3 | 0:4 | 160 | 3 |
| R2:3T160H9 | 2:3 | 160 | 9 |
| R3:1T160H15 | 3:1 | 160 | 15 |
| R3:1T180H3 | 3:1 | 180 | 3 |
| R1:2T180H9 | 1:2 | 180 | 9 |
| R0:4T180H15 | 0:4 | 180 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Gao, L.; Xue, M.; Hou, Y.; Yang, B.; Zhou, L.; Tong, X. The Morphologically Controlled Synthesis and Application of Mesoporous Alumina Spheres. Molecules 2023, 28, 5622. https://doi.org/10.3390/molecules28155622
Xie Y, Gao L, Xue M, Hou Y, Yang B, Zhou L, Tong X. The Morphologically Controlled Synthesis and Application of Mesoporous Alumina Spheres. Molecules. 2023; 28(15):5622. https://doi.org/10.3390/molecules28155622
Chicago/Turabian StyleXie, Yadian, Lanxing Gao, Miaoxuan Xue, Yanqing Hou, Bo Yang, Lingyun Zhou, and Xin Tong. 2023. "The Morphologically Controlled Synthesis and Application of Mesoporous Alumina Spheres" Molecules 28, no. 15: 5622. https://doi.org/10.3390/molecules28155622
APA StyleXie, Y., Gao, L., Xue, M., Hou, Y., Yang, B., Zhou, L., & Tong, X. (2023). The Morphologically Controlled Synthesis and Application of Mesoporous Alumina Spheres. Molecules, 28(15), 5622. https://doi.org/10.3390/molecules28155622








