Determination of Flavonoid Compounds in Shanxi Aged Vinegars Based on Hydrophobic Deep Eutectic Solvent VALLME-HPLC Method: Assessment of the Environmental Impact of the Developed Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Optimization of the Extraction Procedure
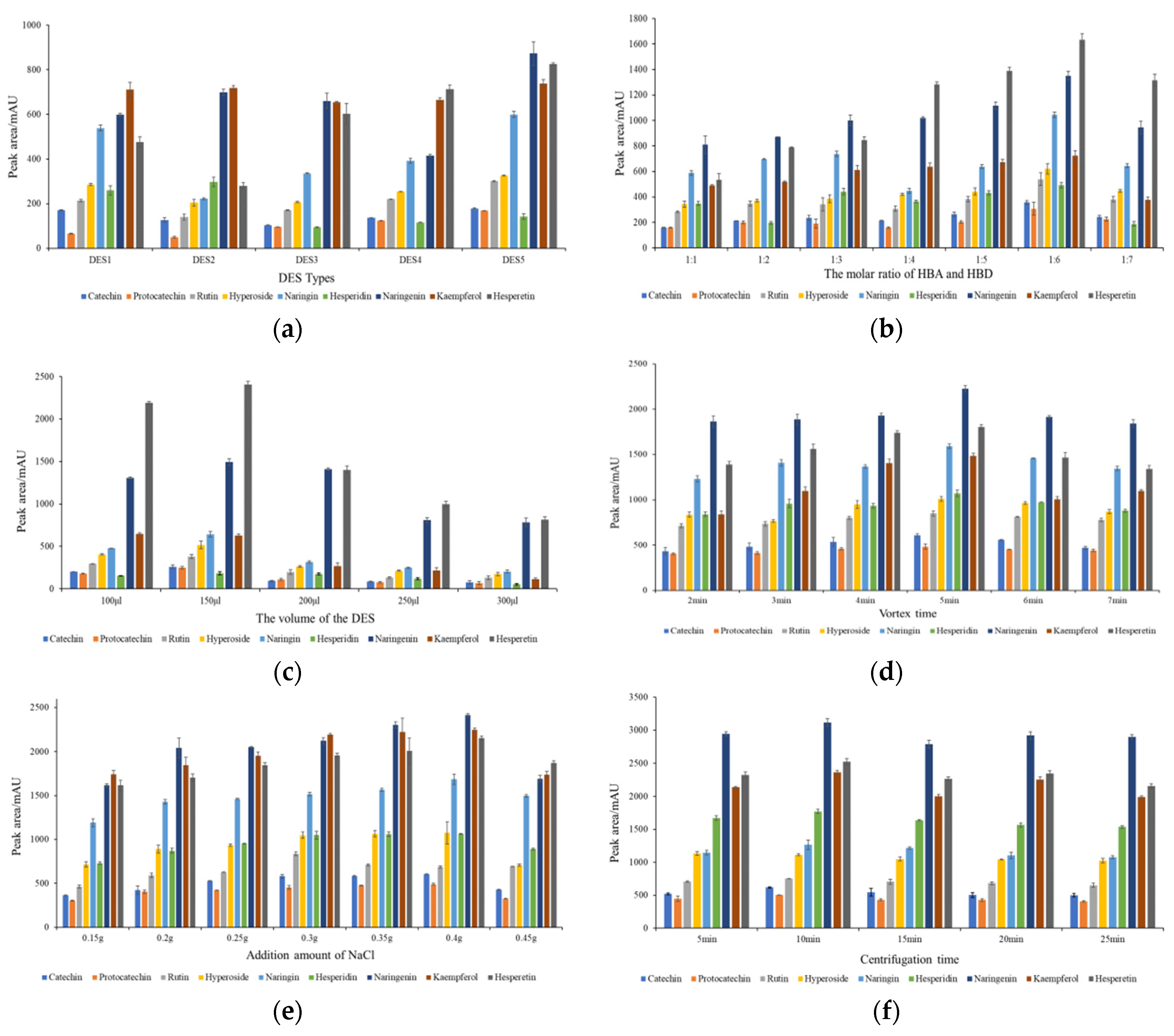
2.2.1. Effect of the Types of DES
2.2.2. Effect of the Molar Ratio of HBA and HBD
2.2.3. Effect of the Volume of the DES
2.2.4. Effect of Vortex Time
2.2.5. Effect of the Addition Amount of NaCl
2.2.6. Effect of Centrifugation Time
2.3. Analysis of Box-Behnken Design Results
2.4. Method Validation
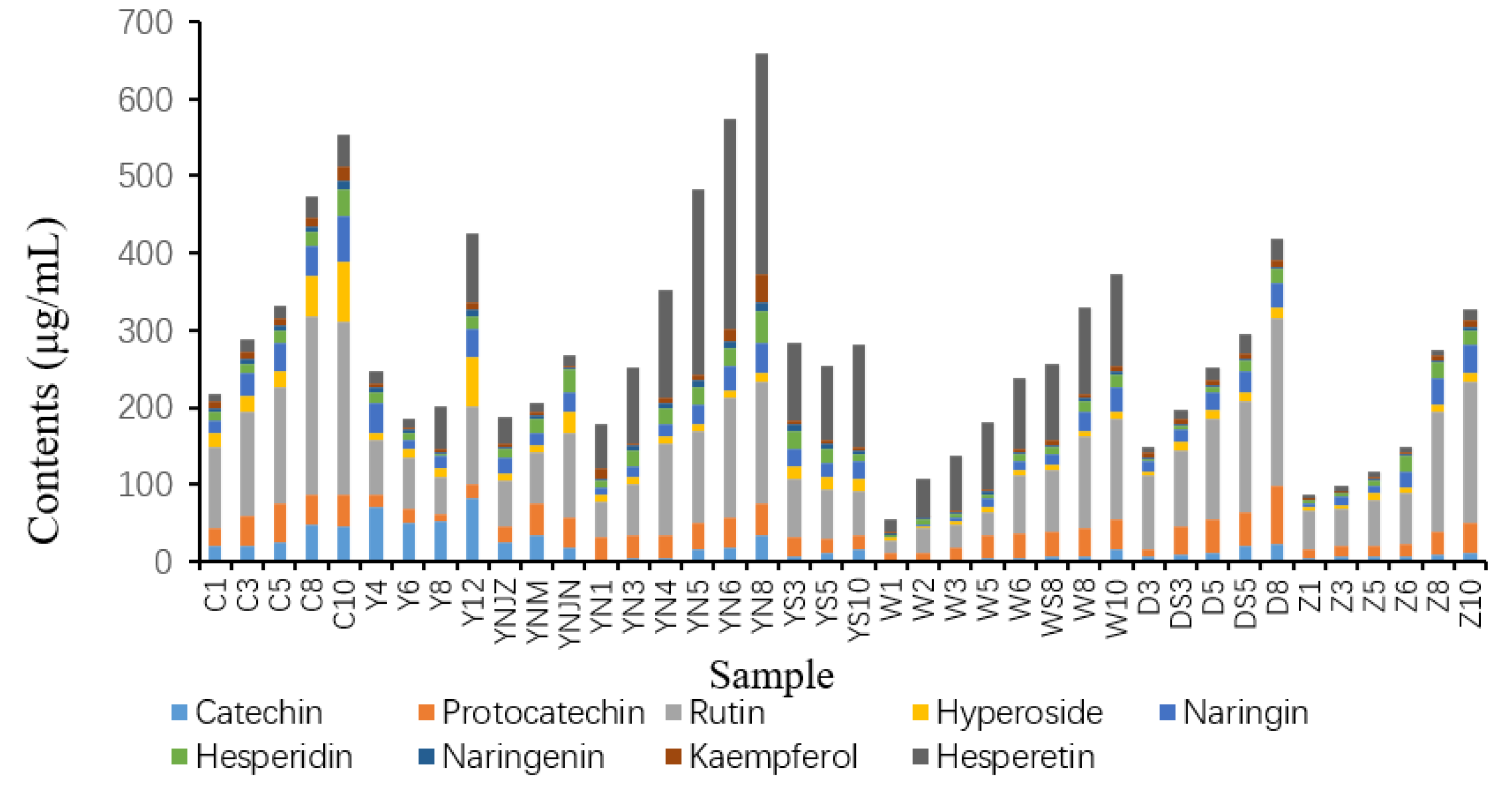
2.5. Targeted Metabolomic Analysis of Flavonoids in Actual Vinegar Samples
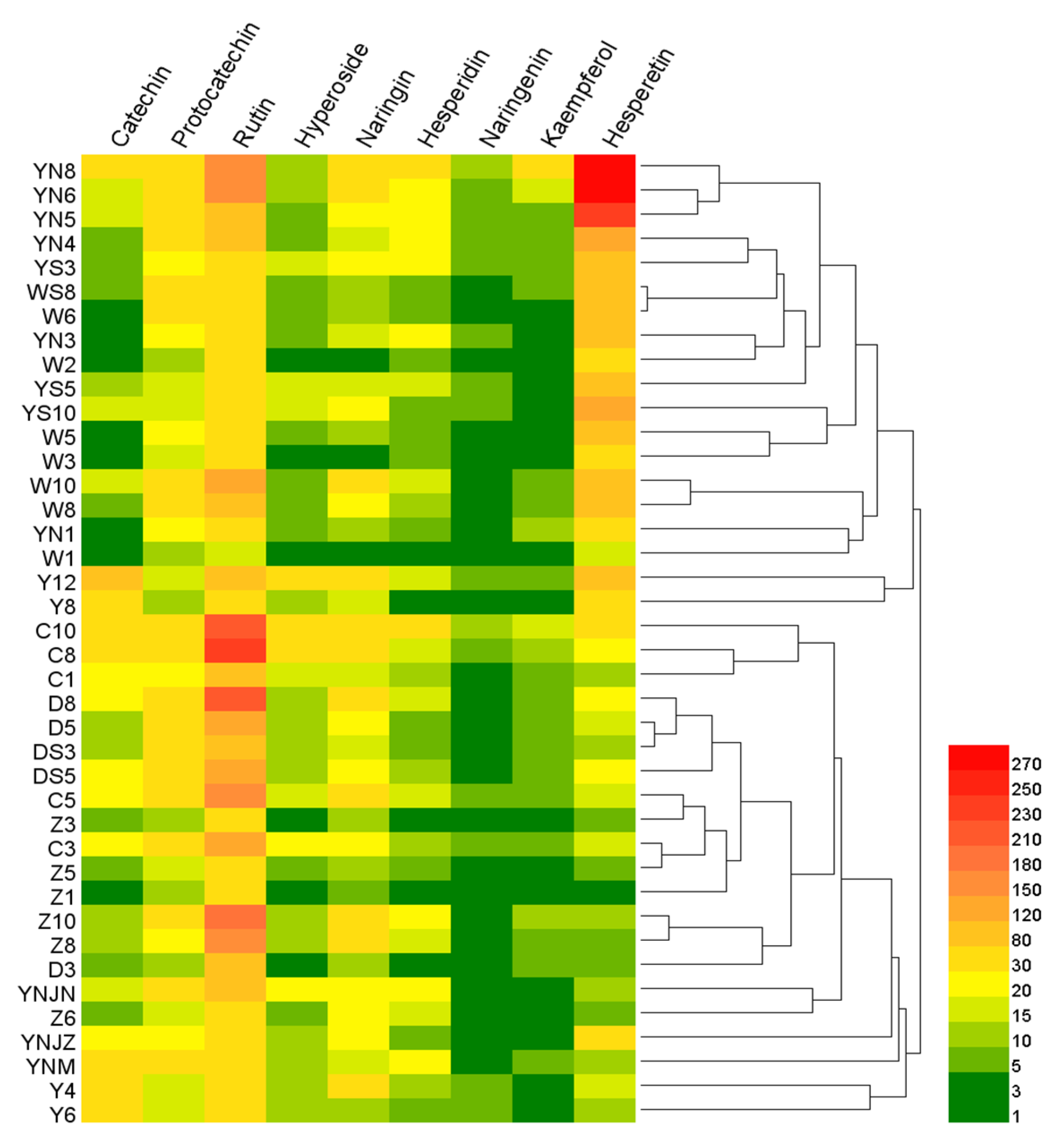
2.6. Heat Map Analysis
2.7. Assessment of Method’s Environmental Impact
2.8. Comparison of the Optimized Method with Other Procedures
2.9. Comprehensive Analysis of the Potential of DES-VALLME Based Programs
3. Experimental
3.1. Materials and Reagents
3.2. Instruments and Analytical Conditions
3.3. Real Samples Collection
3.4. Preparation of Hydrophobic Deep Eutectic Solvent
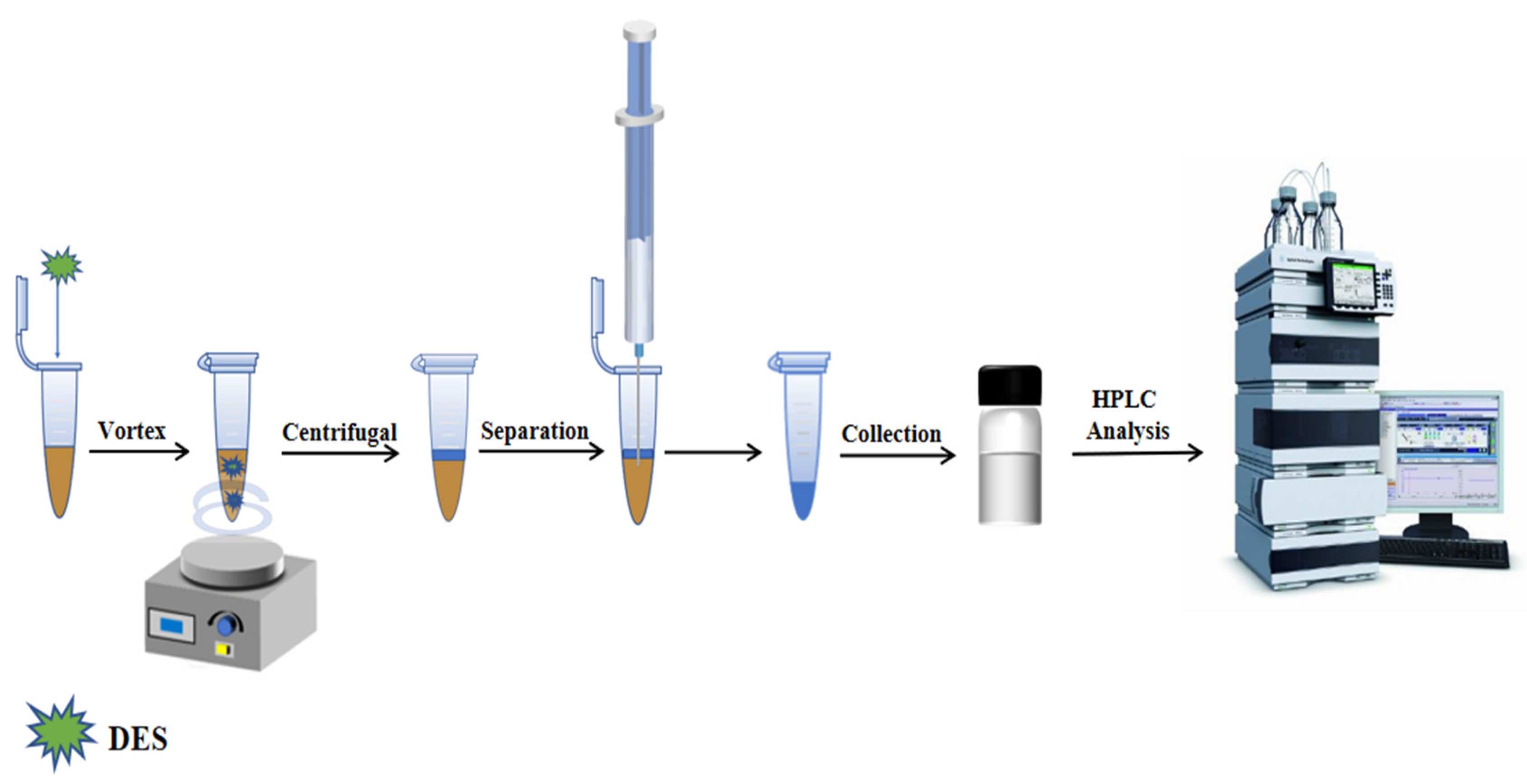
3.5. Vortex Assisted Liquid–liquid Microextraction Procedure
3.6. Optimization of Extraction Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hutchinson, U.F.; Jolly, N.P.; Chidi, B.S.; Ngongang, M.M.; Ntwampe, S.K.O. Vinegar Engineering: A Bioprocess Perspective. Food Eng. Rev. 2019, 11, 290–305. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, C.; Chen, H.; Wang, C.; Ren, X.; Fu, S.; Xu, N.; Li, P.; Song, J.; Wang, C. Characteristics and Discrimination of the Commercial Chinese Four Famous Vinegars Based on Flavor Compositions. Foods 2023, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Ávila, S.; Zalamanski, S.; Tanikawa, L.M.; Kruger, C.C.H.; Ferreira, S.M.R. Influence of Cooking Methods on in Vitro Bioaccessibility of Phenolics, Flavonoids, and Antioxidant Activity of Red Cabbage. Plant Foods Hum. Nutr. 2023, 78, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Ożarowski, M.; Karpiński, T.M. Extracts and Flavonoids of Passiflora Species as Promising Anti-Inflammatory and Antioxidant Substances. Curr. Pharm. Des. 2021, 27, 2582–2604. [Google Scholar] [CrossRef]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Oliveira, A.K.d.S.; de Oliveira e Silva, A.M.; Pereira, R.O.; Santos, A.S.; Barbosa Junior, E.V.; Bezerra, M.T.; Barreto, R.S.; Quintans-Junior, L.J.; Quintans, J.S. Anti-Obesity Properties and Mechanism of Action of Flavonoids: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7827–7848. [Google Scholar] [CrossRef]
- Shahidi, F.; McDonald, J.; Chandrasekara, A.; Zhong, Y. Phytochemicals of Foods, Beverages and Fruit Vinegars: Chemistry and Health Effects. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 380–382. [Google Scholar]
- Tošović, J.; Marković, S. Reproduction and Interpretation of the UV–Vis Spectra of Some Flavonoids. Chem. Pap. 2017, 71, 543–552. [Google Scholar] [CrossRef]
- Jasprica, I.; Smolčić-Bubalo, A.; Mornar, A.; Medić-Šarić, M. Investigation of the Flavonoids in Croatian Propolis by Thin-Layer Chromatography. JPC J. Planar Chromatogr. Mod. TLC 2004, 17, 95–101. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Primikyri, A.; Sakka, M.; Gerothanassis, I.P. Simultaneous Determination of Artemisinin and Its Analogs and Flavonoids in Artemisia Annua Crude Extracts with the Use of NMR Spectroscopy. Magn. Reson. Chem. 2020, 58, 232–244. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z. Application of Electrochemical Sensors Based on Carbon Nanomaterials for Detection of Flavonoids. Nanomaterials 2020, 10, 2020. [Google Scholar] [CrossRef]
- Sammani, M.S.; Clavijo, S.; Cerdà, V. Recent, Advanced Sample Pretreatments and Analytical Methods for Flavonoids Determination in Different Samples. TrAC Trends Anal. Chem. 2021, 138, 116220. [Google Scholar] [CrossRef]
- Tan, L.; Ji, T.; Jiang, G.; Hu, F. Simultaneous Identification and Quantification of Five Flavonoids in the Seeds of Rheum Palmatum L. by Using Accelerated Solvent Extraction and HPLC–PDA–ESI/MSn. Arab. J. Chem. 2019, 12, 1345–1352. [Google Scholar] [CrossRef]
- Hao, H.; Lin, L.; Liu, S.; Kang, Y.; Wang, Y.; Huang, J.; Weng, W. Deep Eutectic Solvent-Based Microwave-Assisted Extraction for the Chromatographic Analysis of Bioactive Flavonoids in Spirodela Polyrrhiza. J. Chromatogr. Sci. 2022, 60, 501–510. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, X.; Huang, G. Ultrasound-Assisted Extraction and Analysis of Maidenhairtree Polysaccharides. Ultrason. Sonochemistry 2023, 95, 106395. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Pérez, R.A. Ultrasound-Assisted Extraction of Organic Contaminants. TrAC Trends Anal. Chem. 2019, 118, 739–750. [Google Scholar] [CrossRef]
- Kavoura, D.; Kyriakopoulou, K.; Papaefstathiou, G.; Spanidi, E.; Gardikis, K.; Louli, V.; Aligiannis, N.; Krokida, M.; Magoulas, K. Supercritical CO2 Extraction of Salvia Fruticosa. J. Supercrit. Fluids 2019, 146, 159–164. [Google Scholar] [CrossRef]
- Tomai, P.; Dal Bosco, C.; D’Orazio, G.; Scuto, F.R.; Felli, N.; Gentili, A. Supercritical Fluid Chromatography for Vitamin and Carotenoid Analysis: An Update Covering 2011–2021. J. Chromatogr. Open 2022, 2, 100027. [Google Scholar] [CrossRef]
- He, X.; Guo, F.; Liu, B. Oolong Tea and LR-White Resin: A New Method of Plant Sample Preparation for Transmission Electron Microscopy. J. Microsc. 2018, 270, 244–251. [Google Scholar] [CrossRef]
- Jia, C.; Zhu, X.; Wang, J.; Zhao, E.; He, M.; Chen, L.; Yu, P. Extraction of Pesticides in Water Samples Using Vortex-Assisted Liquid–Liquid Microextraction. J. Chromatogr. A 2010, 1217, 5868–5871. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yang, L.; Chen, X.; Bai, X.-H.; Hu, S. Vortex-Assisted Dispersive Liquid-Phase Microextraction for the Analysis of Main Active Compounds from Zi-Cao-Cheng-Qi Decoction Based on a Hydrophobic Deep Eutectic Solvent. J. Sep. Sci. 2021, 44, 4376–4383. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and Sustainable Solvents of the Future: Deep Eutectic Solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; De la Guardia, M.; Andruch, V.; Vilková, M. Deep Eutectic Solvents vs Ionic Liquids: Similarities and Differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Aydin, F.; Yilmaz, E.; Soylak, M. Vortex Assisted Deep Eutectic Solvent (DES)-Emulsification Liquid-Liquid Microextraction of Trace Curcumin in Food and Herbal Tea Samples. Food Chem. 2018, 243, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhou, J.; Jia, H.; Zhang, H. Liquid–Liquid Microextraction of Synthetic Pigments in Beverages Using a Hydrophobic Deep Eutectic Solvent. Food Chem. 2018, 243, 351–356. [Google Scholar] [CrossRef]
- Surapong, N.; Pongpinyo, P.; Santaladchaiyakit, Y.; Burakham, R. A Biobased Magnetic Dual-Dummy-Template Molecularly Imprinted Polymer Using a Deep Eutectic Solvent as a Coporogen for Highly Selective Enrichment of Organophosphates. Food Chem. 2023, 418, 136045. [Google Scholar] [CrossRef]
- Tâme Parreira, R.L.; Galembeck, S.E.; Hobza, P. On the Origin of Red and Blue Shifts of X—H and C—H Stretching Vibrations in Formic Acid (Formate Ion) and Proton Donor Complexes. ChemPhysChem 2007, 8, 87–92. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable Deep Eutectic Solvents Preparation and Their Efficiency in Extraction and Enrichment of Main Bioactive Flavonoids from Sea Buckthorn Leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents as Green and Efficient Media for the Extraction of Artemisinin from Artemisia Annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Tailor-Made Hydrophobic Deep Eutectic Solvents for Cleaner Extraction of Polyprenyl Acetates from Ginkgo Biloba Leaves. J. Clean. Prod. 2017, 152, 399–405. [Google Scholar] [CrossRef]
- Hou, J.; Lin, S.; Zhang, M. Ionic-Liquid-Enhanced Solvent Extraction Mechanism: A Novel Concept. J. Environ. Chem. Eng. 2022, 10, 107899. [Google Scholar] [CrossRef]
- Fan, Y.; Luo, H.; Zhu, C.; Li, W.; Wu, D.; Wu, H. Hydrophobic Natural Alcohols Based Deep Eutectic Solvents: Effective Solvents for the Extraction of Quinine. Sep. Purif. Technol. 2021, 275, 119112. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.; Wang, J.; Wu, J.; Zhang, Z.; Jing, X.; Wang, X. Deep Eutectic Solvent-Based Dispersive Liquid–Liquid Microextraction Followed by Smartphone Digital Image Colorimetry for the Determination of Carbofuran in Water Samples. Anal. Methods 2023, 15, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Elencovan, V.; Joseph, J.; Yahaya, N.; Samad, N.A.; Raoov, M.; Lim, V.; Zain, N.N.M. Exploring a Novel Deep Eutectic Solvents Combined with Vortex Assisted Dispersive Liquid–Liquid Microextraction and Its Toxicity for Organophosphorus Pesticides Analysis from Honey and Fruit Samples. Food Chem. 2022, 368, 130835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Liu, S.; Chen, B.; Pan, C. Ionic Liquid-Based Vortex-Assisted Dispersive Liquid–Liquid Microextraction of Organophosphorus Pesticides in Apple and Pear. J. Sep. Sci. 2012, 35, 2514–2519. [Google Scholar] [CrossRef] [PubMed]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern Approaches in Dispersive Liquid-Liquid Microextraction (DLLME) Based on Ionic Liquids: A Review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Khoubnasabjafari, M.; Jouyban, A.; Hosseini, M.; Farajzadeh, M.A.; Saboohi, R.; Nemati, M.; Khosrowshahi, E.M.; Mogaddam, M.R.A. A Mixed Deep Eutectic Solvents-Based Air-Assisted Liquid–Liquid Microextraction of Surfactants from Exhaled Breath Condensate Samples Prior to HPLC-MS/MS Analysis. J. Chromatogr. B 2022, 1204, 123289. [Google Scholar] [CrossRef]
- Gila, A.; Aguilera, M.P.; Sánchez-Ortíz, A.; Jiménez, A.; Beltrán, G. Effect of Centrifugal Force (G) on Stability of Natural Emulsions (Water/Oil) Present in Fresh Virgin Olive Oils. J. Food Eng. 2022, 334, 111169. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for Assessing the Greenness of Analytical Procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A New Tool for the Evaluation of the Analytical Procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep–Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Shaaban, H. Sustainable Dispersive Liquid–Liquid Microextraction Method Utilizing a Natural Deep Eutectic Solvent for Determination of Chloramphenicol in Honey: Assessment of the Environmental Impact of the Developed Method. RSC Adv. 2023, 13, 5058–5069. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Qi, Z.; Xin, G.; Li, W. Response Surface Method Was Used to Optimize the Ultrasonic Assisted Extraction of Flavonoids from Crinum Asiaticum. Saudi J. Biol. Sci. 2019, 26, 2079–2084. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Rastrelli, L. Determination of Phenolic Compounds in Honey Using Dispersive Liquid–Liquid Microextraction. J. Chromatogr. A 2014, 1334, 9–15. [Google Scholar] [CrossRef]
- Campillo, N.; Viñas, P.; Férez-Melgarejo, G.; Hernández-Córdoba, M. Dispersive Liquid–Liquid Microextraction for the Determination of Flavonoid Aglycone Compounds in Honey Using Liquid Chromatography with Diode Array Detection and Time-of-Flight Mass Spectrometry. Talanta 2015, 131, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Xing, R.; Wang, R.; Chen, X.; Hu, S. A Switchable Deep Eutectic Solvent for the Homogeneous Liquid-Liquid Microextraction of Flavonoids from “Scutellariae Radix”. J. Chromatogr. A 2023, 1688, 463712. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, S.; Liu, D.; Yang, M.; Wei, J. Analysis of Flavonoids in Dalbergia Odorifera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Molecules 2020, 25, 389. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, L.; Lu, X.; Wang, S.; Guo, Y.; Liang, X. One-Step Fabrication of Hydrophobic Metal-Organic Framework@covalent Organic Framework Hybrid as Sorbent for High-Performance Solid-Phase Extraction of Flavonoids. J. Chromatogr. A 2023, 1691, 463814. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, L.; Hu, H.; Yang, Y.; Zhang, X.; Peng, Y.; Xiao, P. DPPH Radical Scavenging and Postprandial Hyperglycemia Inhibition Activities and Flavonoid Composition Analysis of Hawk Tea by UPLC-DAD and UPLC-Q/TOF MSE. Molecules 2017, 22, 1622. [Google Scholar] [CrossRef]
- Hai, X.; Shi, F.; Zhu, Y.; Ma, L.; Wang, L.; Yin, J.; Li, X.; Yang, Z.; Yuan, M.; Xiong, H.; et al. Development of Magnetic Dispersive Micro-Solid Phase Extraction of Four Phenolic Compounds from Food Samples Based on Magnetic Chitosan Nanoparticles and a Deep Eutectic Supramolecular Solvent. Food Chem. 2023, 410, 135338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, D.; Wang, Z.; Wang, R.; Yang, L.; Hu, S.; Du, L.; Chen, X. Crystal Film Accelerated Solvent Microextraction (CF-ASME) for Determination of Flavonoids in Natural Products Combined with High Performance Liquid Chromatography. J. Chromatogr. A 2022, 1676, 463286. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, L.; Yu, X.; Ruan, X.; Liu, B.; Wang, Q. Optimization of Supercritical Fluid Extraction of Total Flavonoids and Monomer Compositions from Taxus Remainder Extracts Free of Taxoids. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2016, 39, 2062–2067. [Google Scholar]
- Bernal-Millán, M.d.J.; Carrasco-Portugal, M.d.C.; Heredia, J.B.; Bastidas-Bastidas, P.d.J.; Gutiérrez-Grijalva, E.P.; León-Félix, J.; Angulo-Escalante, M.Á. Green Extracts and UPLC-TQS-MS/MS Profiling of Flavonoids from Mexican Oregano (Lippia Graveolens) Using Natural Deep Eutectic Solvents/Ultrasound-Assisted and Supercritical Fluids. Plants 2023, 12, 1692. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Xue, H.; Li, J.; Wang, G.; Zuo, W.; Zeng, Y.; Liu, L. Ultrasound-Assisted Extraction of Flavonoids from Potentilla Fruticosa L. Using Natural Deep Eutectic Solvents. Molecules 2022, 27, 5794. [Google Scholar] [CrossRef]

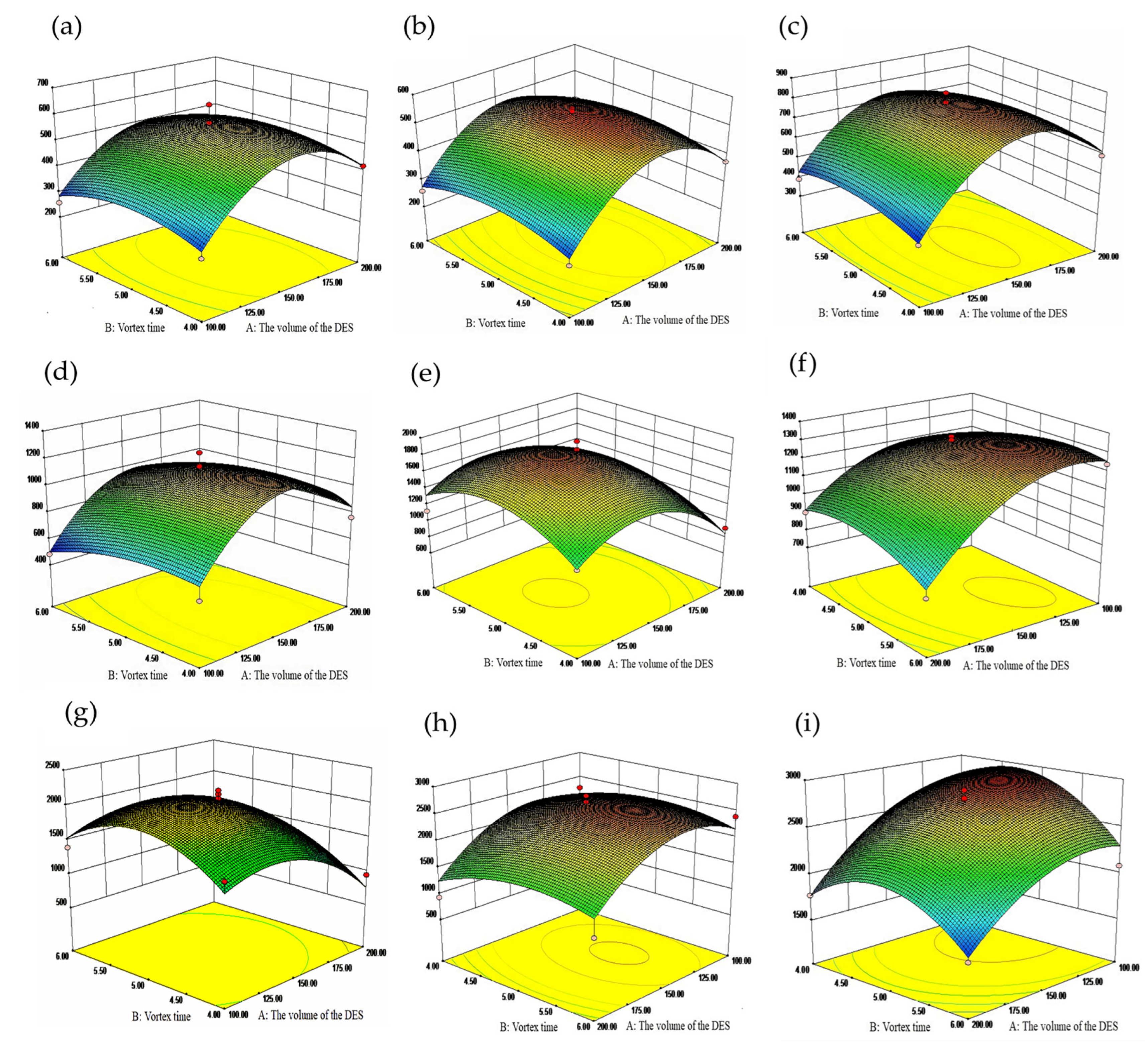
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| The volume of the DES/μL (A) | 100 | 150 | 200 |
| Vortex time/min (B) | 4 | 5 | 6 |
| Addition amount of NaCl/g (C) | 0.35 | 0.40 | 0.45 |
| Centrifugation time/min (D) | 5 | 10 | 15 |
| Catechin | Protocatechin | Rutin | Hyperoside | Naringin | ||||||
| F | p | F | p | F | p | F | p | F | p | |
| Model | 14.84 | <0.0001 | 28.71 | <0.0001 | 18.27 | <0.0001 | 9.94 | <0.0001 | 10.85 | <0.0001 |
| A | 28.77 | <0.0001 | 35.09 | <0.0001 | 28.89 | <0.0001 | 16.51 | 0.0012 | 16.54 | 0.0012 |
| B | 1.42 | 0.2538 | 1.64 | 0.2214 | 2.43 | 0.1414 | 6.61 | 0.0222 | 0.85 | 0.3710 |
| C | 0.29 | 0.6002 | 7.87 | 0.0140 | 2.33 | 0.1495 | 3.59 | 0.0790 | 0.0024 | 0.9613 |
| D | 1.42 | 0.2526 | 5.83 | 0.0300 | 1.99 | 0.1797 | 0.0001 | 0.9918 | 1.13 | 0.3063 |
| AB | 0.29 | 0.6016 | 0.31 | 0.5887 | 1.61 | 0.2258 | 0.010 | 0.9200 | 0.051 | 0.8239 |
| AC | 0.57 | 0.4611 | 3.45 | 0.0846 | 2.00 | 0.1795 | 0.32 | 0.5825 | 1.80 | 0.2016 |
| AD | 0.062 | 0.8068 | 0.56 | 0.4659 | 0.010 | 0.9215 | 0.11 | 0.7453 | 0.31 | 0.5851 |
| BC | 9.28 | 0.0087 | 4.27 | 0.0578 | 1.76 | 0.2061 | 3.65 | 0.0769 | 1.21 | 0.2904 |
| BD | 0.068 | 0.7981 | 0.083 | 0.7774 | 0.0002 | 0.9895 | 0.89 | 0.3611 | 0.028 | 0.8695 |
| CD | 0.0041 | 0.9498 | 1.81 | 0.2001 | 1.42 | 0.2538 | 0.034 | 0.8573 | 0.088 | 0.7708 |
| A2 | 152.62 | <0.0001 | 316.73 | <0.0001 | 207.56 | <0.0001 | 105.71 | <0.0001 | 46.63 | <0.0001 |
| B2 | 17.24 | 0.0010 | 30.15 | <0.0001 | 8.98 | 0.0096 | 4.82 | 0.0455 | 23.93 | 0.0002 |
| C2 | 10.68 | 0.0056 | 1.36 | 0.2628 | 0.20 | 0.6641 | 0.80 | 0.3857 | 21.86 | 0.0004 |
| D2 | 31.54 | <0.0001 | 46.11 | <0.0001 | 6.46 | 0.0235 | 1.55 | 0.2341 | 95.55 | <0.0001 |
| Lack of fit | 0.37 | 0.9105 | 1.63 | 0.3370 | 2.77 | 0.1689 | 0.59 | 0.7756 | 3.74 | 0.1078 |
| R2 | 0.9369 | 0.9663 | 0.9481 | 0.9086 | 0.9156 | |||||
| Adjusted R2 | 0.8738 | 0.9327 | 0.8962 | 0.8172 | 0.8312 | |||||
| Hesperidin | Naringenin | Kaempferol | Hesperetin | |||||||
| F | p | F | p | F | p | F | p | |||
| Model | 9.99 | <0.0001 | 11.09 | <0.0001 | 11.45 | <0.0001 | 9.29 | <0.0001 | ||
| A | 55.40 | <0.0001 | 71.28 | <0.0001 | 34.18 | <0.0001 | 11.96 | 0.0038 | ||
| B | 0.38 | 0.5477 | 9.54 | 0.0080 | 4.39 | 0.0548 | 0.77 | 0.3953 | ||
| C | 0.050 | 0.8259 | 0.30 | 0.5942 | 3.77 | 0.0727 | 16.18 | 0.0013 | ||
| D | 0.45 | 0.5124 | 6.58 | 0.0225 | 2.69 | 0.1232 | 0.030 | 0.8644 | ||
| AB | 2.72 | 0.1214 | 0.81 | 0.3833 | 0.17 | 0.6877 | 0.026 | 0.8748 | ||
| AC | 0.0010 | 0.9752 | 0.026 | 0.8744 | 1.27 | 0.2795 | 0.022 | 0.8834 | ||
| AD | 0.87 | 0.3668 | 1.57 | 0.2300 | 19.56 | 0.0006 | 0.25 | 0.6236 | ||
| BC | 2.81 | 0.1160 | 3.92 | 0.0678 | 0.0082 | 0.9293 | 1.69 | 0.2140 | ||
| BD | 0.028 | 0.8688 | 3.00 | 0.1054 | 0.49 | 0.4947 | 0.041 | 0.8419 | ||
| CD | 3.72 | 0.0744 | 15.23 | 0.0016 | 0.019 | 0.8921 | 0.14 | 0.7146 | ||
| A2 | 35.97 | <0.0001 | 15.81 | 0.0014 | 41.27 | <0.0001 | 14.85 | 0.0018 | ||
| B2 | 8.16 | 0.0127 | 33.89 | <0.0001 | 47.28 | <0.0001 | 3.90 | 0.0683 | ||
| C2 | 1.85 | 0.1957 | 4.01 | 0.0651 | 34.23 | <0.0001 | 10.43 | 0.0060 | ||
| D2 | 35.31 | <0.0001 | 4.73 | 0.0473 | 0.38 | 0.5455 | 93.35 | <0.0001 | ||
| Lack of fit | 1.67 | 0.3290 | 3.18 | 0.1383 | 2.62 | 0.1831 | 3.22 | 0.1356 | ||
| R2 | 0.9090 | 0.9173 | 0.9197 | 0.9028 | ||||||
| Adjusted R2 | 0.8180 | 0.8346 | 0.8393 | 0.8057 | ||||||
| Analytes | Standard Curve | LR a | R2 b | LOD c | LOQ d | EF e | RSD f (%) | |
|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||||
| Catechin | Y = 23.954x − 2.1598 | 0.2–50 | 0.9989 | 0.09 | 0.30 | 58 | 2.90 | 4.01 |
| Protocatechin | Y = 24.978x − 10.249 | 0.2–50 | 0.9990 | 0.09 | 0.30 | 156 | 2.49 | 3.04 |
| Rutin | Y = 34.627x + 20.352 | 0.2–50 | 0.9968 | 0.14 | 0.45 | 296 | 2.88 | 3.72 |
| Hyperoside | Y = 44.449x + 21.951 | 0.2–50 | 0.9973 | 0.12 | 0.40 | 54 | 2.99 | 4.24 |
| Naringin | Y = 47.103x + 52.2410 | 0.2–50 | 0.9946 | 0.14 | 0.45 | 62 | 3.77 | 4.53 |
| Hesperidin | Y = 50.146x + 43.7360 | 0.2–50 | 0.9944 | 0.18 | 0.60 | 87 | 2.34 | 3.11 |
| Naringenin | Y = 144.3x − 15.644 | 0.2–50 | 0.9964 | 0.15 | 0.50 | 83 | 3.30 | 3.98 |
| Kaempferol | Y = 145.1x − 13.07 | 0.2–50 | 0.9990 | 0.15 | 0.50 | 144 | 3.35 | 4.30 |
| Hesperetin | Y = 47.795x − 32.546 | 0.2–50 | 0.9984 | 0.12 | 0.40 | 43 | 2.55 | 4.96 |
| Analytes | Sample | Amount Added (μg/mL) | Recovery (%) | Analytes | Sample | Amount Added (μg/mL) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Catechin | W3 | 20 | 104.97 | Hesperidin | W3 | 20 | 103.88 |
| D3 | 20 | 103.71 | D3 | 20 | 98.26 | ||
| C2 | 20 | 99.54 | C2 | 20 | 99.34 | ||
| YN1 | 20 | 102.75 | YN1 | 20 | 90.73 | ||
| Z3 | 20 | 97.73 | Z3 | 20 | 89.10 | ||
| Protocatechin | W3 | 20 | 99.74 | Naringenin | W3 | 20 | 98.72 |
| D3 | 20 | 107.20 | D3 | 20 | 96.87 | ||
| C2 | 20 | 94.99 | C2 | 20 | 95.86 | ||
| YN1 | 20 | 98.28 | YN1 | 20 | 91.33 | ||
| Z3 | 20 | 108.11 | Z3 | 20 | 93.28 | ||
| Rutin | W3 | 20 | 93.48 | Kaempferol | W3 | 20 | 88.19 |
| D3 | 20 | 94.70 | D3 | 20 | 90.86 | ||
| C2 | 20 | 96.77 | C2 | 20 | 86.34 | ||
| YN1 | 20 | 98.60 | YN1 | 20 | 87.27 | ||
| Z3 | 20 | 104.15 | Z3 | 20 | 85.97 | ||
| Hyperoside | W3 | 20 | 96.19 | Hesperetin | W3 | 20 | 94.54 |
| D3 | 20 | 91.53 | D3 | 20 | 93.64 | ||
| C2 | 20 | 93.33 | C2 | 20 | 99.31 | ||
| YN1 | 20 | 97.02 | YN1 | 20 | 104.32 | ||
| Z3 | 20 | 101.46 | Z3 | 20 | 103.22 | ||
| Naringin | W3 | 20 | 93.29 | ||||
| D3 | 20 | 94.84 | |||||
| C2 | 20 | 89.10 | |||||
| YN1 | 20 | 91.84 | |||||
| Z3 | 20 | 95.61 |
| Items | PPs | ||
|---|---|---|---|
| 1. Reagent | |||
| Tricaprylylmethylammonium chloride | Amount | <10 mL | 1 |
| Hazard type | Signal word: warning | 1 | |
| Hazard amount | 1 pictogram | 1 | |
| Total PPs = 1 | |||
| 1,4-Butanediol | Amount | <10 mL | 1 |
| Hazard type | Signal word: warning | 1 | |
| Hazard amount | 1 pictogram | 1 | |
| Total PPs = 1 | |||
| methanol | Amount | <10 mL | 1 |
| Hazard type | Signal word: danger | 2 | |
| Hazard amount | 3 pictograms | 3 | |
| Total PPs = 6 | |||
| phosphoric acid | Amount | <10 mL | 1 |
| Hazard type | Signal word: danger | 2 | |
| Hazard amount | 1 pictogram | 1 | |
| Total PPs = 2 | |||
| acetonitrile | Amount | <10 mL | 1 |
| Hazard type | Signal word: danger | 2 | |
| Hazard amount | 2 pictograms | 2 | |
| Total PPs = 2 | |||
| 2. Instruments | |||
| 2.1. Energy (kW/h per sample) | HPLC | ≤0.1 kWh per sample | 0 |
| 2.2. Occupational hazard | Emission of vapors and gases to the air | 0 | |
| 3.Waste | |||
| 3.1. Waste amount | >10 mL | 5 | |
| 3.2. Waste treatment | No treatment | 3 | |
| Total PPs = 8 | |||
| Total penalty points = 20 | |||
| Eco-scale score | 100 − 20 = 80 | ||
| GAPI Pictograms | ||
|---|---|---|
| Sample preparation |  | |
| Collection (1) | Off line (red) | |
| Preservation (2) | None (green) | |
| Transport (3) | Required (yellow) | |
| Storage (4) | Simple treatment (yellow) | |
| Type of method | ||
| Direct or indirect (5) | Extraction required (red) | |
| Scale of extraction (6) | Micro-extraction (yellow) | |
| Solvents/reagents used (7) | Green solvents for extraction (yellow) | |
| Additional treatments (8) | No additional treatment (green) | |
| Reagent and solvents | ||
| Amount (9) | <10 mL (green) | |
| Health hazard (10) | NFPA = 1; slightly toxicity(green) | |
| Safety hazard (11) | NFPA = 3; high flammability (yellow) | |
| Instrumentation | ||
| Energy (12) | ≤0.1 kw h per sample (green) | |
| Occupational hazard (13) | Hermetic sealing of analytical procedure (green) | |
| Waste (14) | >10 mL (>10 g) | |
| Waste treatment (15) | No treatment (red) | |
| Reference | Actual Work | [44] | [45] | [46] |
|---|---|---|---|---|
| Sample Preparation | VALLME | UAE | DLLME | DLLME |
| Extraction solvent | TOMAC: BDO, 1:6 (150 μL) | 60% Ethanol (33.6 mL) | CHCl3 (450 μL) | chloroform (150 µL) |
| Sample (g/mL) | 3.5 mL | 2 g | 10 mL | 10 g |
| Detection | HPLC | UV | UHPLC–UV analysis | LC-DAD-ESI-ToFMS |
| AGREE plot | 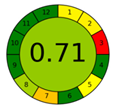 | 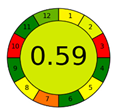 |  |  |
| Method | Sample | Analytes | Analytical Technique | LR | LOD | RSD (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | |||||||
| DES-HLLME | Scutellariae Radix | 6 kinds of flavonoids | HPLC-UV | 0.0022–8.65 mg/L | ≤8.0 g/L | 0.1–7.8 | 0.2–9.2 | [47] |
| Ultrasonic extraction | Dalbergia odorifera | 17 kinds of flavonoids | UHPLC-MS/MS | 0.516–5652 ng/mL | 0.085–1.790 g/mL | 0.45–3.51 | 1.26–4.94 | [48] |
| SPE | Chinese wolfberry, orange juice and wine samples | 4 kinds of flavonoids | HPLC | 1–500 ng/mL | 0.15–0.41 ng/mL | 2.64–4.56 | 3.64–4.20 | [49] |
| UAE | Hawk tea | 3 kinds of flavonoids | UPLC-DAD | 0.36–880 μg/mL | 0.086–0.308 μg/mL | 1.16–4.18 | 1.63–4.26 | [50] |
| MSPE-DES | orange, apple, onion and green tea | 4 kinds of flavonoids | HPLC-UV | 0.03–0.14 μg/L | 0.1–0.5 ng/mL | ≤5.4 | ≤5.6 | [51] |
| CF-ASME | Ginkgo biloba and Platycladus orientalis | 5 kinds of flavonoids | HPLC-UV | 0.01–5 μg/mL | 0.5–30 ng/mL | 1.8–12.6 | 3.3–12.8 | [52] |
| DES-VALLME | Shanxi aged vinegar | 9 kinds of flavonoids | HPLC | 0.20–50.00 μg/mL | 0.09–0.18 μg/mL | 2.34–3.77 | 3.04–4.96 | This work |
| DES | HBA | HBD | Molar Ratio |
|---|---|---|---|
| DES1 | Tricaprylylmethylammonium chloride | n-Caprylic acid | 1:2 |
| DES2 | Tricaprylylmethylammonium chloride | Ethylene glycol | 1:3 |
| DES3 | Tricaprylylmethylammonium chloride | 2,3-Butanediol | 1:2 |
| DES4 | Tricaprylylmethylammonium chloride | 1,3-Butanediol | 1:3 |
| DES5 | Tricaprylylmethylammonium chloride | 1,4-Butanediol | 1:6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, B.; Guo, Y.; Meng, S.; Chen, S.; Bo, T.; Zhang, J.; Shen, D.; Liu, Y.; Yang, Y.; Fan, S. Determination of Flavonoid Compounds in Shanxi Aged Vinegars Based on Hydrophobic Deep Eutectic Solvent VALLME-HPLC Method: Assessment of the Environmental Impact of the Developed Method. Molecules 2023, 28, 5619. https://doi.org/10.3390/molecules28145619
Bai B, Guo Y, Meng S, Chen S, Bo T, Zhang J, Shen D, Liu Y, Yang Y, Fan S. Determination of Flavonoid Compounds in Shanxi Aged Vinegars Based on Hydrophobic Deep Eutectic Solvent VALLME-HPLC Method: Assessment of the Environmental Impact of the Developed Method. Molecules. 2023; 28(14):5619. https://doi.org/10.3390/molecules28145619
Chicago/Turabian StyleBai, Baoqing, Yanli Guo, Siyuan Meng, Shujun Chen, Tao Bo, Jinhua Zhang, Dan Shen, Yifei Liu, Yukun Yang, and Sanhong Fan. 2023. "Determination of Flavonoid Compounds in Shanxi Aged Vinegars Based on Hydrophobic Deep Eutectic Solvent VALLME-HPLC Method: Assessment of the Environmental Impact of the Developed Method" Molecules 28, no. 14: 5619. https://doi.org/10.3390/molecules28145619
APA StyleBai, B., Guo, Y., Meng, S., Chen, S., Bo, T., Zhang, J., Shen, D., Liu, Y., Yang, Y., & Fan, S. (2023). Determination of Flavonoid Compounds in Shanxi Aged Vinegars Based on Hydrophobic Deep Eutectic Solvent VALLME-HPLC Method: Assessment of the Environmental Impact of the Developed Method. Molecules, 28(14), 5619. https://doi.org/10.3390/molecules28145619






