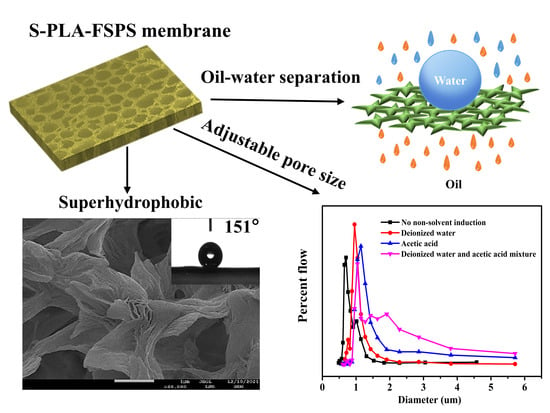
The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation
Abstract
1. Introduction
2. Results and Discussion
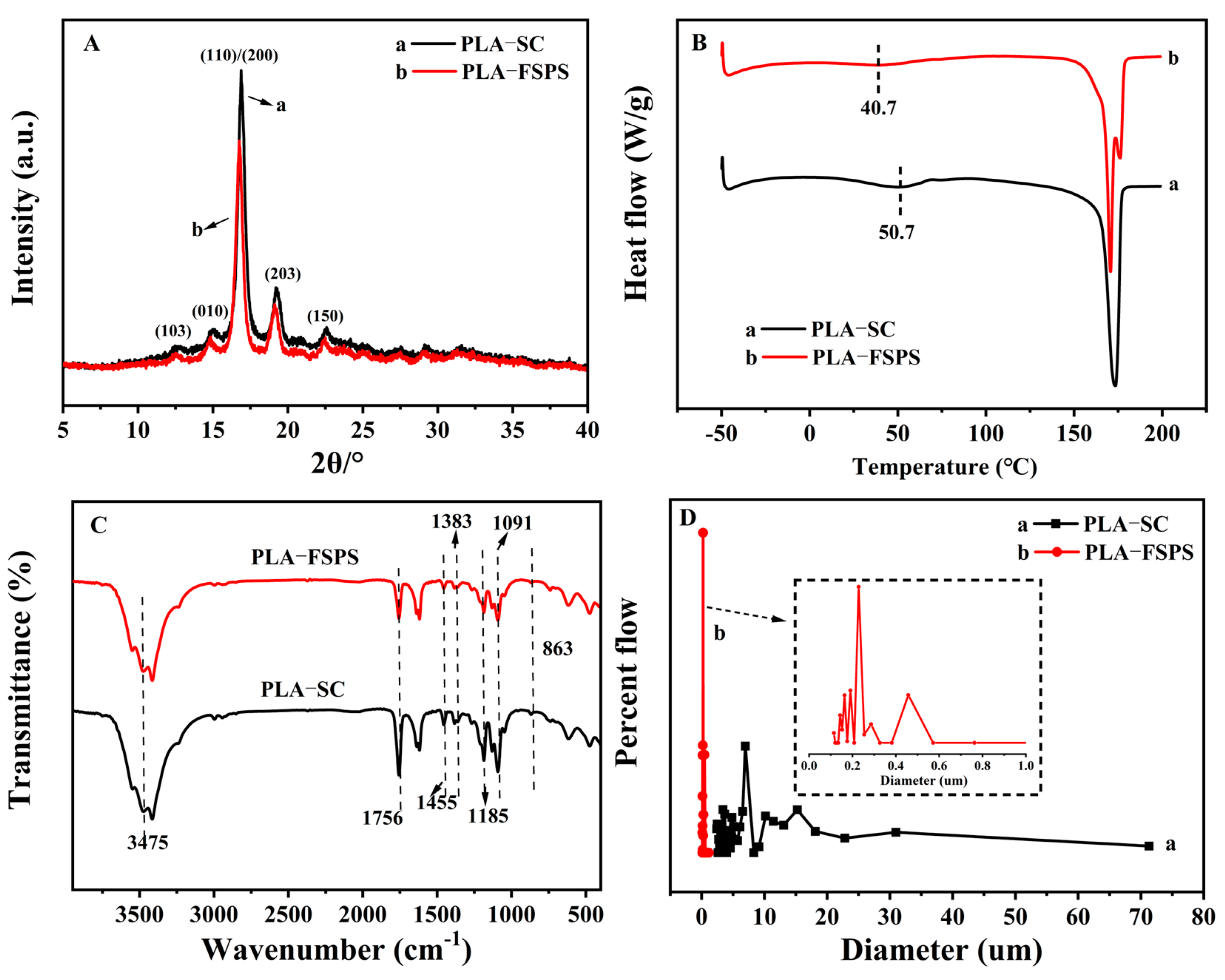
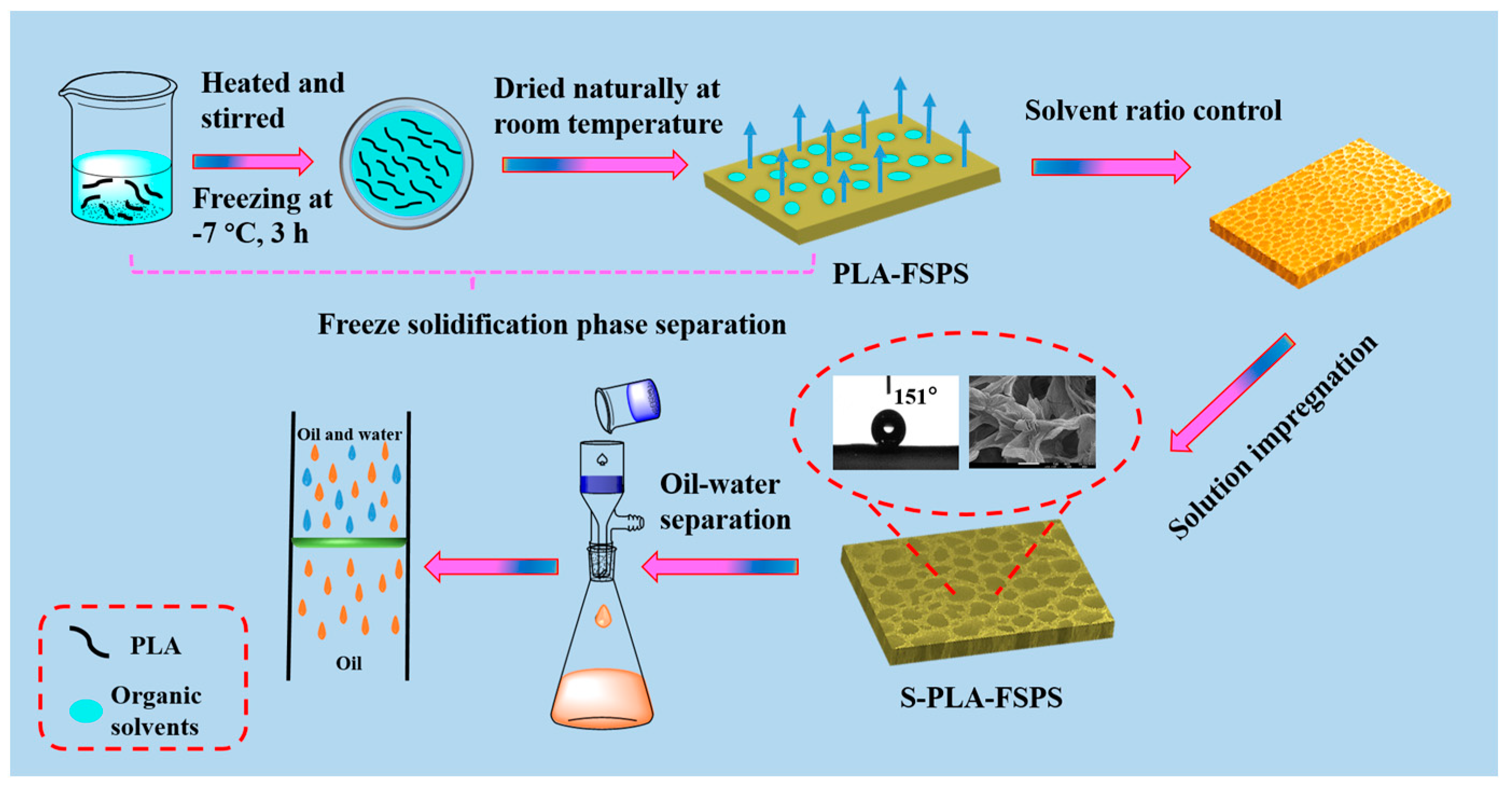
2.1. Optimization of Preparation Method
2.2. Control of the Pore and Surface Structure
2.2.1. Effect of Different Solvent Ratios
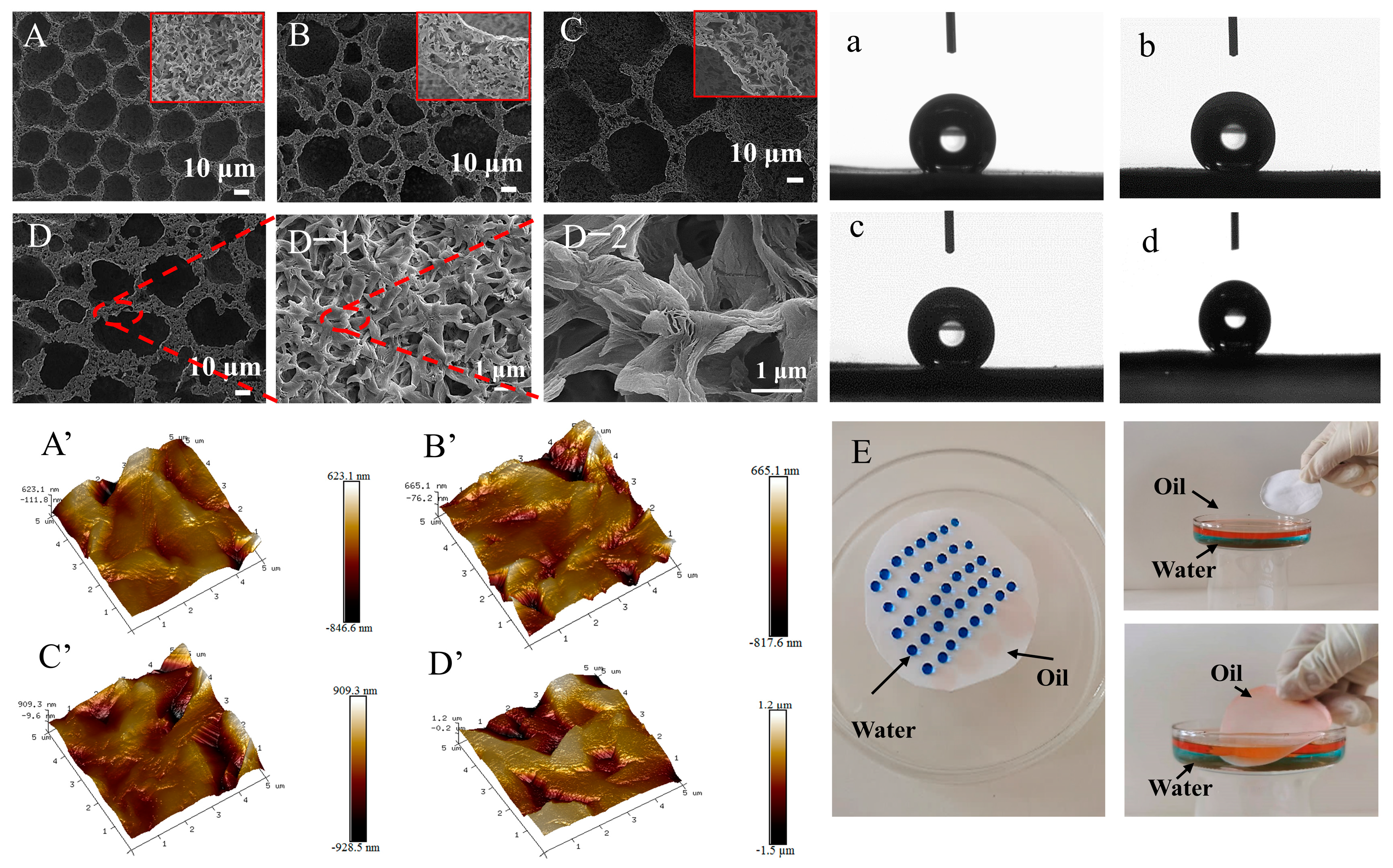
2.2.2. Effect of the Impregnating Solution
2.3. Oil–Water Separation Performance
2.4. Adsorption Performance
3. Experimental
3.1. Materials
3.2. Preparation of PLA Membranes
3.3. Oil–Water Separation Experiments
3.4. Oil Adsorption Experiment
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lu, H.; Pan, Z.; Wang, H.; Liu, Y.; Dai, P.; Yang, Q. Fiber coalescence treatment of oily wastewater: A new theory and application. J. Hazard. Mater. 2021, 412, 125188. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Mou, X.; Cai, Z. Textile-inspired methodology toward asymmetric fabric based on weft-backed weave for oil/water separation. J. Mater. Sci. 2018, 53, 4683–4692. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, G.; Bai, R.; Shen, S.; Zhou, X.; Wyman, I. Fabrication of superhydrophilic and underwater superoleophobic membranes via an in situ crosslinking blend strategy for highly efficient oil/water emulsion separation. J. Membr. Sci. 2019, 569, 60–70. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Wang, J.; Zhang, F.; Li, J.; Jin, J. Layer-by-layer construction of Cu2+/alginate multilayer modified ultrafiltration membrane with bioinspired superwetting property for high-efficient crude-oil-in-water emulsion separation. Adv. Funct. Mater. 2018, 28, 1801944. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, H.; Zhong, Y.; Qin, Y.; Li, T.; Liu, F. Robust superhydrophilic polylactide (PLA) membrane with TiO2 nano-particles inlayed surface for oil/water separation. J. Mater. Chem. A 2017, 5, 6538–6545. [Google Scholar] [CrossRef]
- Wei, Y.; Qi, H.; Gong, X.; Zhao, S. Specially wettable membranes for oil–water separation. Adv. Mater. Interfaces 2018, 5, 1800576. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, W.; Wu, S.; Tang, G.; Cui, J.; Zhang, Q.; Chen, F.; Xiong, R.; Huang, C. Electrospun frogspawn structured membrane for gravity-driven oil-water separation. J. Colloid Interface Sci. 2019, 547, 136–144. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Nataraj, S.K.; Gogda, A.; Meena, R. Bio-based superhydrophilic foam membranes for sustainable oil–water separation. Green Chem. 2014, 16, 4552–4558. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, C.; Zhao, T.; Liu, M.; Jiang, L. Recent advances in bioinspired gel surfaces with superwettability and special adhesion. Adv. Sci. 2019, 6, 1900996. [Google Scholar] [CrossRef]
- Haleem, A.; Pan, J.M.; Shah, A.; Hussain, H.; He, W.-d. A systematic review on new advancement and assessment of emerging polymeric cryogels for environmental sustainability and energy production. Sep. Purif. Technol. 2023, 316, 123678. [Google Scholar] [CrossRef]
- Ejeta, D.D.; Wang, C.-F.; Kuo, S.-W.; Chen, J.-K.; Tsai, H.-C.; Hung, W.-S.; Hu, C.-C.; Lai, J.-Y. Preparation of superhydrophobic and superoleophilic cotton-based material for extremely high flux water-in-oil emulsion separation. Chem. Eng. J. 2020, 402, 126289. [Google Scholar] [CrossRef]
- Obaid, M.; Mohamed, H.O.; Alayande, A.B.; Kang, Y.; Ghaffour, N.; Kim, I.S. Facile fabrication of superhydrophilic and underwater superoleophobic nanofiber membranes for highly efficient separation of oil-in-water emulsion. Sep. Purif. Technol. 2021, 272, 118954. [Google Scholar] [CrossRef]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef]
- Ruidas, S.; Das, A.; Kumar, S.; Dalapati, S.; Manna, U.; Bhaumik, A. Non-fluorinated and robust superhydrophobic modification on covalent organic framework for crude-oil-in-water emulsion separation. Angew. Chem. Int. Ed. 2022, 61, e202210507. [Google Scholar] [CrossRef]
- Wu, M.; Liu, W.; Mu, P.; Wang, Q.; Li, J. Sacrifice template strategy to the fabrication of a self-cleaning nanofibrous membrane for efficient crude oil-in-water emulsion separation with high flux. ACS Appl. Mater. Interfaces 2020, 12, 53484–53493. [Google Scholar] [CrossRef]
- Gao, N.; Wang, L.; Zhang, Y.; Liang, F.; Fan, Y. Modified ceramic membrane with pH/ethanol induced switchable superwettability for antifouling separation of oil-in-acidic water emulsions. Sep. Purif. Technol. 2022, 293, 121022. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Wang, C.; Rana, M.; Ma, P.C. Surface roughness induced superhydrophobicity of graphene foam for oil-water separation. J. Colloid Interface Sci. 2017, 508, 254–262. [Google Scholar] [CrossRef]
- Haleem, A.; Chen, J.; Guo, X.X.; Hou, S.C.; Chen, S.Q.; Siddiq, M.; He, W.D. Radiation-induced synthesis of hydrophobic cryogels with rapid and high absorption of organic solvents and oils. Microporous Mesoporous Mater. 2022, 330, 111486. [Google Scholar] [CrossRef]
- Haleem, A.; Li, H.J.; Li, P.Y.; Hu, C.S.; Li, X.C.; Wang, J.Y.; Chen, S.Q.; He, W.D. Rapid UV-radiation synthesis of polyacrylate cryogel oil-sorbents with adaptable structure and performance. Environ. Res. 2020, 187, 109488. [Google Scholar] [CrossRef]
- Wong, W.S. Surface chemistry enhancements for the tunable super-liquid repellency of low-surface-tension liquids. Nano Lett. 2019, 19, 1892–1901. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Zhao, X.L.; Weng, Y.X.; Li, Y.D.; Zeng, J.B. Fully sustainable, nanoparticle-free, fluorine-free, and robust superhydrophobic cotton fabric fabricated via an eco-friendly method for efficient oil/water separation. ACS Sustain. Chem. Eng. 2019, 7, 15696–15705. [Google Scholar] [CrossRef]
- Kong, W.; Li, F.; Pan, Y.; Zhao, X. Hygro-responsive, photo-decomposed superoleophobic/superhydrophilic coating for on-demand oil-water separation. ACS Appl. Mater. Interfaces 2021, 13, 35142–35152. [Google Scholar] [CrossRef]
- Shu, D.; Xi, P.; Cheng, B.; Wang, Y.; Yang, L.; Wang, X.; Yan, X. One-step electrospinning cellulose nanofibers with superhydrophilicity and superoleophobicity underwater for high-efficiency oil-water separation. Int. J. Biol. Macromol. 2020, 162, 1536–1545. [Google Scholar] [CrossRef]
- Sun, X.; Bai, L.; Li, J.; Huang, L.; Gao, X. Robust preparation of flexibly super-hydrophobic carbon fiber membrane by electrospinning for efficient oil-water separation in harsh environments. Carbon 2021, 182, 11–22. [Google Scholar] [CrossRef]
- Saini, H.; Kallem, P.; Otyepkova, E.; Geyer, F.; Schneemann, A.; Ranc, V.; Banat, F.; Zboril, R.; Otyepka, M.; Fischer, R.A. Two-dimensional MOF-based liquid marbles: Surface energy calculations and efficient oil-water separation using a ZIF-9-III@PVDF membrane. J. Mater. Chem. A 2021, 9, 23651–23659. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, H.; Su, M.; Shao, C.; Liu, C.; Guo, Z.; Shen, C.; Liu, X. Simple fabrication of superhydrophobic PLA with honeycomb-like structures for high-efficiency oil-water separation. Chin. Chem. Lett. 2020, 31, 365–368. [Google Scholar] [CrossRef]
- Zheng, L.; Su, X.; Lai, X.; Chen, W.; Zeng, X. Conductive superhydrophobic cotton fabrics via layer-by-layer assembly of carbon nanotubes for oil-water separation and human motion detection. Mater. Lett. 2019, 253, 230–233. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Han, B.; Sun, Z.; Zhang, J.; Sun, D. Phase-separation-induced micropatterned polymer surfaces and their applications. Adv. Funct. Mater. 2005, 15, 655–663. [Google Scholar] [CrossRef]
- Ruan, C.; Ai, K.; Li, X.; Lu, L. A superhydrophobic sponge with excellent absorbency and flame retardancy. Angew. Chem. Int. Ed. 2014, 126, 5662–5666. [Google Scholar] [CrossRef]
- Tsuji, H. Poly (lactic acid) stereocomplexes: A decade of progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly (lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Rajeshkumar, G.; Seshadri, S.A.; Devnani, G.; Sanjay, M.; Siengchin, S.; Maran, J.P.; Al-Dhabi, N.A.; Karuppiah, P.; Mariadhas, V.A.; Sivarajasekar, N. Environment friendly, renewable and sustainable poly lactic acid (PLA) based natural fiber reinforced composites–A comprehensive review. J. Clean. Prod. 2021, 310, 127483. [Google Scholar] [CrossRef]
- Benavides, P.T.; Lee, U.; Zarè-Mehrjerdi, O. Life cycle greenhouse gas emissions and energy use of polylactic acid, bio-derived polyethylene, and fossil-derived polyethylene. J. Clean. Prod. 2020, 277, 124010. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Xue, B.; Huo, K.; Zheng, Q. Super-hydrophobic poly (lactic acid) by controlling the hierarchical structure and polymorphic transformation. Chem. Eng. J. 2020, 397, 125297. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Liu, X.; Liu, H.; Li, N.; Liu, C.; Schubert, D.W.; Shen, C. Facile fabrication of superhydrophobic and eco-friendly poly(lactic acid) foam for oil-water separation via skin peeling. ACS Appl. Mater. Interfaces 2019, 11, 14362–14367. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly (L-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Bayattork, M.; Rajkhowa, R.; Allardyce, B.J.; Wang, X.; Li, J. Tuning the microstructure and mechanical properties of lyophilized silk scaffolds by pre-freezing treatment of silk hydrogel and silk solution. J. Colloid Interface Sci. 2023, 631, 46–55. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J. Effect of crystalline forms (α′ and α) of poly (lactic acid) on its mechanical, thermo-mechanical, heat deflection temperature and creep properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Li, B.; Zhao, G.; Wang, G.; Zhang, L.; Hou, J.; Gong, J. A green strategy to regulate cellular structure and crystallization of poly (lactic acid) foams based on pre-isothermal cold crystallization and CO2 foaming. Int. J. Biol. Macromol. 2019, 129, 171–180. [Google Scholar] [CrossRef]
- Geng, L.; Li, L.; Mi, H.; Chen, B.; Sharma, P.; Ma, H.; Hsiao, B.S.; Peng, X.; Kuang, T. Superior impact toughness and excellent storage modulus of poly (lactic acid) foams reinforced by shish-kebab nanoporous structure. ACS Appl. Mater. Interfaces 2017, 9, 21071–21076. [Google Scholar] [CrossRef]
- Wang, S.; Yang, W.; Li, X.; Hu, Z.; Wang, B.; Li, M.; Dong, W. Preparation of high-expansion open-cell polylactic acid foam with superior oil-water separation performance. Int. J. Biol. Macromol. 2021, 193, 1059–1067. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Chen, Z.; Chen, N.; Pang, X.; Zhang, L.; Minari, T.; Liu, X.; Liu, H.; Chen, J. Recyclable oil-absorption foams via secondary phase separation. ACS Sustain. Chem. Eng. 2018, 6, 13834–13843. [Google Scholar] [CrossRef]
- Gao, A.; Zhao, Y.; Yang, Q.; Fu, Y.; Xue, L. Facile preparation of patterned petal-like PLA surfaces with tunable water micro-droplet adhesion properties based on stereo-complex co-crystallization from non-solvent induced phase separation processes. J. Mater. Chem. A 2016, 4, 12058–12064. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Blackman, B.R.K.; Saiz, E. Mimicking nature to control bio-material surface wetting and adhesion. Int. Mater. Rev. 2021, 67, 658–681. [Google Scholar] [CrossRef]
- Wang, S.; Liu, K.; Yao, X.; Jiang, L. Bioinspired Surfaces with Superwettability: New Insight on Theory, Design, and Applications. Chem. Rev. 2015, 115, 8230–8293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Cao, Q.; Wang, C.; Yang, C.; Li, Y.; Zhou, J. Novel porous oil-water separation material with super-hydrophobicity and super-oleophilicity prepared from beeswax, lignin, and cotton. Sci. Total Environ. 2020, 706, 135807. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, G.; Gao, A.; Cui, J.; Zhao, S.; Yan, Y. Robust graphene/poly (vinyl alcohol) janus aerogels with a hierarchical architecture for highly efficient switchable separation of oil/water emulsions. ACS Appl. Mater. Interfaces 2019, 11, 36638–36648. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, M.; Liu, Z.; Huang, C.; Fu, G. Nature-inspired creation of a robust free-standing electrospun nanofibrous membrane for efficient oil–water separation. Environ. Sci. Nano 2018, 5, 2909–2920. [Google Scholar] [CrossRef]
- Wei, C.; Lin, L.; Zhao, Y.; Zhang, X.; Yang, N.; Chen, L.; Huang, X. Fabrication of pH-sensitive superhydrophilic/underwater superoleophobic poly (vinylidene fluoride)-graft-(SiO2 nanoparticles and PAMAM dendrimers) membranes for oil–water separation. ACS Appl. Mater. Interfaces 2020, 12, 19130–19139. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Xu, Y.; Lin, K.-H.; Kobayashi, M.; Kajiyama, M.; Enomae, T. Fabrication of cellulose nanofiber-deposited cellulose sponge as an oil-water separation membrane. Sep. Purif. Technol. 2019, 224, 322–331. [Google Scholar] [CrossRef]
- Prabunathan, P.; Elumalai, P.; Dinesh Kumar, G.; Manoj, M.; Hariharan, A.; Rathika, G.; Alagar, M. Antiwetting and low-surface-energy behavior of cardanol-based polybenzoxazine-coated cotton fabrics for oil–water separation. J. Coat. Technol. Res. 2020, 17, 1455–1469. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Liu, X.; Wang, J.; Zhang, C.; Wen, Y. A durable and high-flux composite coating nylon membrane for oil-water separation. J. Clean. Prod. 2018, 193, 702–708. [Google Scholar] [CrossRef]
- Yang, S.; Sha, S.; Lu, H.; Wu, J.; Sheng, Z. Graphene oxide and reduced graphene oxide coated cotton fabrics with opposite wettability for continuous oil/water separation. Sep. Purif. Technol. 2020, 259, 118095. [Google Scholar] [CrossRef]
- Kim, B.S.; Harriott, P. Critical entry pressure for liquids in hydrophobic membranes. J. Colloid Interface Sci. 1987, 115, 1–8. [Google Scholar] [CrossRef]
- Zhou, C.; Cheng, J.; Hou, K.; Zhu, Z.; Zheng, Y. Preparation of CuWO4@ Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem. Eng. J. 2017, 307, 803–811. [Google Scholar] [CrossRef]

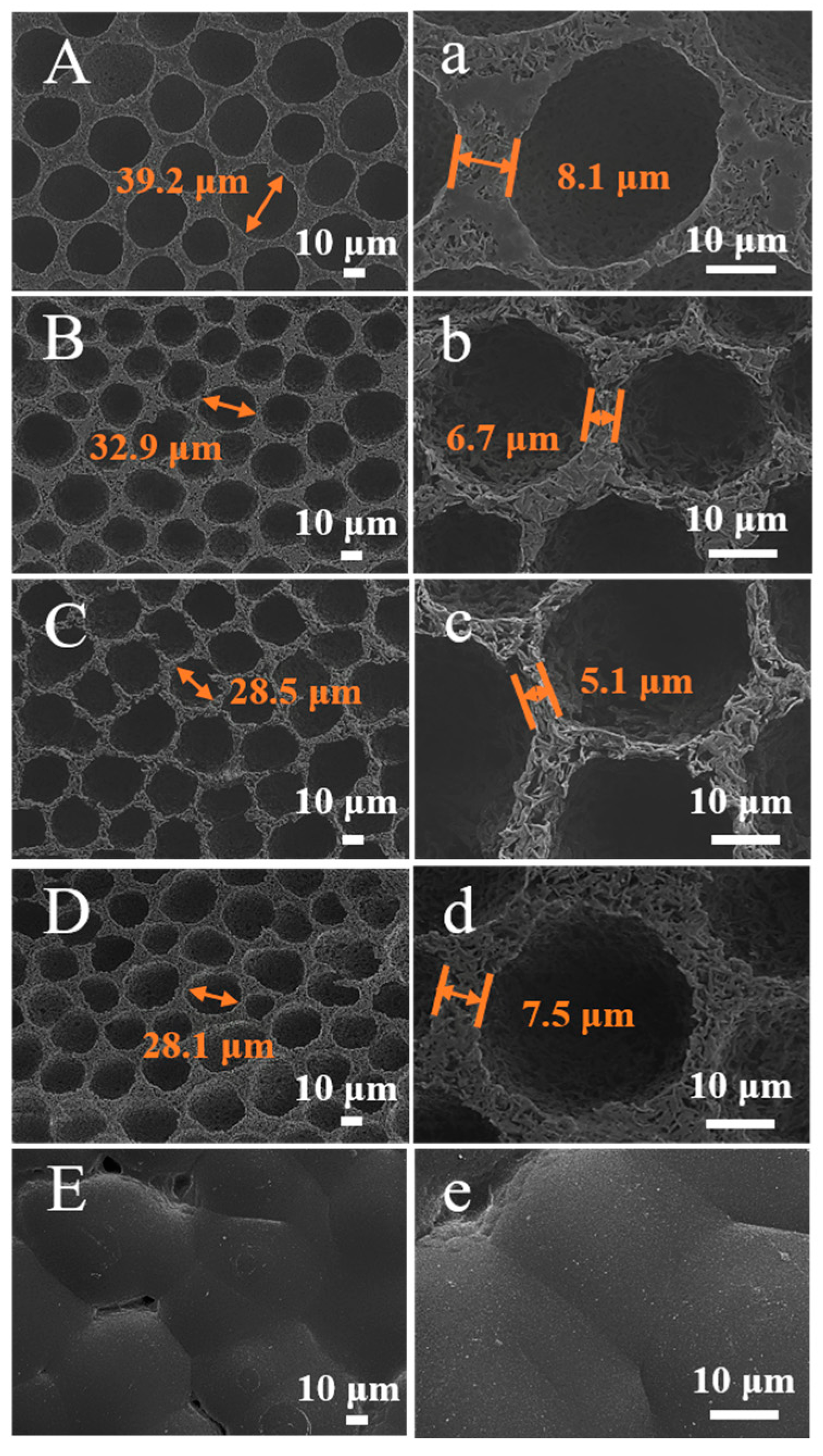




| DiOX:DCM | 10:0 | 9:1 | 4:1 | 1:1 | 0:10 |
|---|---|---|---|---|---|
| Maximum aperture/μm | 0.46 | 6.10 | 6.10 | 2.29 | -- |
| Average aperture/μm | 0.54 | 0.98 | 1.32 | 0.35 | -- |
| Minimum aperture/μm | 0.25 | 0.68 | 0.51 | 0.29 | -- |
| Material | Drive | Membrane Flux | Separation Efficiency | Reference |
|---|---|---|---|---|
| TiO2-PLA | 1 bar | 963 L·m−2·h−1 | 99% | [5] |
| PDMS/SNPs-PI | Gravity | 4400 L·m−2·h−1 | 99.55% | [7] |
| Grphene/Poly(vinyl alcohol) janus aerogels | Gravity | 1306 L·m−2·h−1 | 99.7% | [47] |
| Fe3+-PA/OTMS/PI | Gravity | 8424 L·m−2·h−1 | 99% | [48] |
| PVDF-g-SiO2NPs/PAMAM membrane | 0.9 bar | >3100 L·m−2·h−1 | >99% | [49] |
| MCNF-membrane | Gravity | 3730 L·m−2·h−1 | 99% | [50] |
| Polybenzoxazine-coated cotton fabric | Gravity | 7200 L·m−2·h−1 | 99% | [51] |
| Cellulose-starch silica composite coating nylon membrane | 1 bar | 31,847 L·m−2·h−1 | 99.8% | [52] |
| GO and rGO coated cotton fabric | Gravity | 7120 L·m−2·h−1 | 98.5% | [53] |
| S-PLA-FSPS membrane | Gravity | 16,084 L·m−2·h−1 | 99.7% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Sun, T.; Zhang, D.; Sun, S.; Liu, J.; Li, B.; Shi, Z. The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation. Molecules 2023, 28, 5590. https://doi.org/10.3390/molecules28145590
Zhang Y, Sun T, Zhang D, Sun S, Liu J, Li B, Shi Z. The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation. Molecules. 2023; 28(14):5590. https://doi.org/10.3390/molecules28145590
Chicago/Turabian StyleZhang, Yan, Tianyi Sun, Dashuai Zhang, Shishu Sun, Jinrui Liu, Bangsen Li, and Zaifeng Shi. 2023. "The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation" Molecules 28, no. 14: 5590. https://doi.org/10.3390/molecules28145590
APA StyleZhang, Y., Sun, T., Zhang, D., Sun, S., Liu, J., Li, B., & Shi, Z. (2023). The Preparation of Superhydrophobic Polylactic Acid Membrane with Adjustable Pore Size by Freeze Solidification Phase Separation Method for Oil–Water Separation. Molecules, 28(14), 5590. https://doi.org/10.3390/molecules28145590