Synthesis and Optimization of Cs2B′B″X6 Double Perovskite for Efficient and Sustainable Solar Cells
Abstract
:1. Introduction
2. Crystal Synthesis of Cs2B′B″X6 Double Perovskite
| Compound | Synthesis Method | Synthesis Atmosphere | Heating Condition | Year and Reference |
|---|---|---|---|---|
| Cs2AgInCl6:Cr3+ | hydrothermal | 150 °C for 12 h | 2022 [37] | |
| Cs2AgInCl6:Cu2+ | hot injection | N2 | heating at 120 °C for 1 h, 220 °C for injection | 2020 [38] |
| Cs2AgInCl6:Mn2+ | hot injection | vacuum and N2 | dry under vacuum for 30 min at 40 °C, 105 °C for injection | 2018 [39] |
| Cs2AgInCl6 | hot injection | vacuum and N2 | 100 °C in vacuum for 2 h, 200 °C in N2 for injection | 2019 [40] |
| Cs2AgInCl6:Cr3+ | solid-state reaction | evacuated ampoules | 400 °C for 4 days | 2019 [41] |
| Cs2AgInCl6 | hot injection | 100 °C for injection | 2019 [42] | |
| Cs2AgInCl6:Mn2+ | solution processing | 72 °C for 20 min | 2018 [43] | |
| Cs2AgInCl6:Yb3+ | hot injection | vacuum and N2 | 100 °C in vacuum for 15 min, 105 °C in N2 for injection | 2019 [44] |
| Cs2Ag(SbxBi1−x)Br6 | solution processing | N2 | RT | 2020 [26] |
| Cs2AgSbBr6 | hot injection | vacuum and N2 | 110 °C in vacuum for 45 min, 180 °C in N2 for injection | 2018 [45] |
| Cs2AgSbBr6 | hydrothermal | 160 °C for 5 days | 2019 [46] | |
| Cs2AgSbBr6 | solid-state reaction | high-energy ball mill | 2019 [47] | |
| Cs2AgSbBr6 | solid-state reaction | planetary ball mill | 2022 [48] | |
| Cs2AgSbBr6 | solution processing | 383 K | 2022 [48] | |
| Cs2AgSbBr6 | hydrothermal | N2 | heat up to 473 K in 10 min with a pressure of 4.7 MPa | 2022 [48] |
| Cs2AgSbBr6 | solid-state reaction | inert loop system | spray-drying for 474 K | 2022 [48] |
| Cs2AgBiBr6 | solution processing | 120 °C for 3 h | 2021 [49] | |
| Cs2AgBiBr6 | solution processing | 100 °C | 2018 [50] | |
| Cs2AgBiBr6 | hydrothermal | 120 °C for 24 h | 2020 [51] | |
| Cs2AgBiBr6 | solution processing | 110 °C for 2 h | 2017 [52] | |
| Cs2AgBiBr6 | solution processing | RT for 2 h | 2021 [53] |

2.1. Solid-State Synthesis
2.2. Solution-Based Synthesis
3. Thin-Film Preparation of Cs2B′B″X6 Double Perovskite
3.1. Chemical Solution Deposition
3.2. Vacuum Deposition
4. Performance Enhancement of Cs2B′B″X6 Double Perovskite Solar Cells
4.1. Film Quality Optimization
4.1.1. Preheating
4.1.2. Post-Annealing
4.1.3. The Choice of Anti-Solvent
4.2. Band Gap Adjustment
4.2.1. Structural Transition
4.2.2. Functional Doping
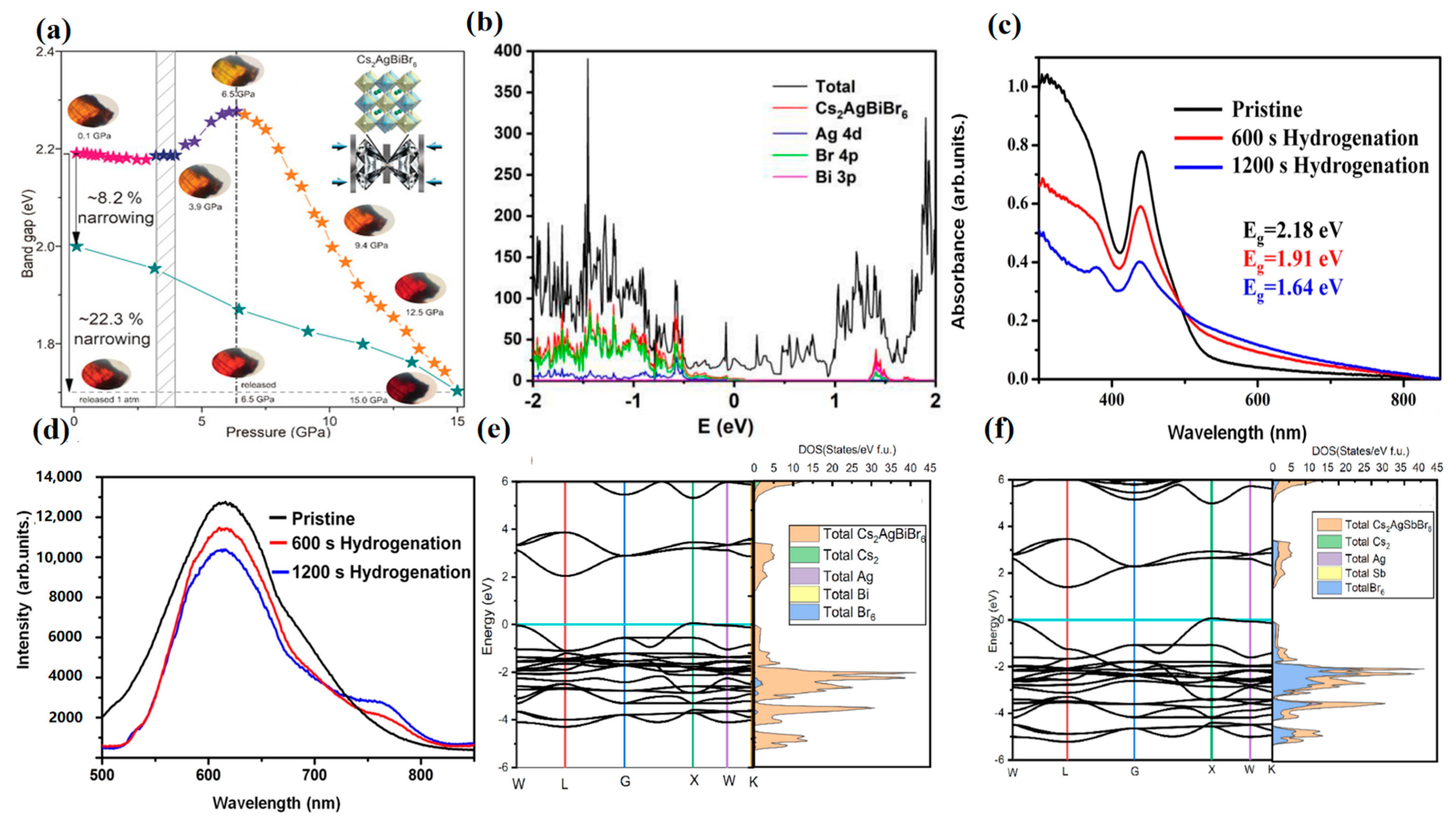
4.3. Interface Engineering
4.3.1. Band Gap Alignment
4.3.2. Surface Passivation
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Dunlop, E.D.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Siefer, G.; Hao, X. Solar cell efficiency tables (version 62). Prog. Photovolt. 2023, 31, 651–663. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.-B.; et al. Conformal quantum dot SnO2 layers as electron transporters for efficient perovskite solar cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Malik, R.; Raees, R.; Imran, M.; Wang, F.; Ali, S.; Khan, M.; Khan, Q.; Maqbool, M. Recent advancements and future insight of lead-free non-toxic perovskite solar cells for sustainable and clean energy production: A review. Sustain. Energy Technol. Assess. 2022, 53, 102433. [Google Scholar] [CrossRef]
- Ning, W.; Gao, F. Structural and functional diversity in lead-free halide perovskite materials. Adv. Mater. 2019, 31, e1900326. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Hutter, E.M. Halide Double-perovskite semiconductors beyond photovoltaics. ACS Energy Lett. 2022, 7, 2128–2135. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Khalfin, S.; Bekenstein, Y. Advances in lead-free double perovskite nanocrystals, engineering band-gaps and enhancing stability through composition tunability. Nanoscale 2019, 11, 8665–8679. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Q.; Liu, G.; Zhang, Z.; Wang, D.; Qu, B.; Chen, Z.; Xiao, L. From Pb to Bi: A promising family of Pb−free optoelectronic materials and devices. Adv. Energy Mater. 2019, 10, 1902496. [Google Scholar] [CrossRef]
- Connor, B.A.; Leppert, L.; Smith, M.D.; Neaton, J.B.; Karunadasa, H.I. Layered halide double perovskites: Dimensional reduction of Cs2AgBiBr6. J. Am. Chem. Soc. 2018, 140, 5235–5240. [Google Scholar] [CrossRef] [PubMed]
- Kung, P.-K.; Li, M.-H.; Lin, P.-Y.; Jhang, J.-Y.; Pantaler, M.; Lupascu, D.C.; Grancini, G.; Chen, P. Lead−free double perovskites for perovskite solar cells. Solar RRL 2019, 4, 1900306. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From lead halide perovskites to lead-free metal halide perovskites and perovskite derivatives. Adv. Mater. 2019, 31, e1803792. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Q.; Liu, Y.; Luo, W.; Guo, X.; Huang, Z.; Ting, H.; Sun, W.; Zhong, X.; Wei, S.; et al. The dawn of lead-free perovskite solar cell: Highly stable double perovskite Cs2AgBiBr6 film. Adv. Sci. 2018, 5, 1700759. [Google Scholar] [CrossRef]
- Rong, Y.; Ming, Y.; Ji, W.; Li, D.; Mei, A.; Hu, Y.; Han, H. Toward industrial-scale production of perovskite solar cells: Screen printing, slot-die coating, and emerging techniques. J. Phys. Chem. Lett. 2018, 9, 2707–2713. [Google Scholar] [CrossRef]
- Zheng, K.; Pullerits, T. Two dimensions are better for perovskites. J Phys. Chem. Lett. 2019, 10, 5881–5885. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, P.; Bai, S.; Gu, J.; Li, F.; Yang, Z.; Liu, M. High-quality sequential-vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells. Solar RRL 2018, 2, 1800217. [Google Scholar] [CrossRef]
- Lei, H.; Hardy, D.; Gao, F. Lead−free double perovskite Cs2AgBiBr6: Fundamentals, applications, and perspectives. Adv. Funct. Mater. 2021, 31, 2105898. [Google Scholar] [CrossRef]
- Nie, R.; Sumukam, R.R.; Reddy, S.H.; Banavoth, M.; Seok, S.I. Lead-free perovskite solar cells enabled by hetero-valent substitutes. Energy Environ. Sci. 2020, 13, 2363–2385. [Google Scholar] [CrossRef]
- Zhao, Y.; Cruse, K.; Abdelsamie, M.; Ceder, G.; Sutter-Fella, C.M. Synthetic approaches for thin-film halide double perovskites. Matter 2021, 4, 1801–1831. [Google Scholar] [CrossRef]
- Gao, W.; Ran, C.; Xi, J.; Jiao, B.; Zhang, W.; Wu, M.; Hou, X.; Wu, Z. High-quality Cs2AgBiBr6 double perovskite film for lead-free inverted planar heterojunction solar cells with 2.2% efficiency. ChemPhysChem 2018, 19, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Zhang, L.; Yun, J.-H.; Hao, M.; He, D.; Chen, P.; Bai, Y.; Lin, T.; Xiao, M.; Du, A.; et al. Dual-ion-diffusion induced degradation in lead-free Cs2AgBiBr6 double perovskite solar cells. Adv. Funct. Mater. 2020, 30, 2002342. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, H.; Yin, W.-J. Do chalcogenide double perovskites work as solar cell absorbers: A first-principles study. Chem. Mater. 2018, 31, 244–250. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Recent advances in Cs2AgBiBr6-based halide double perovskites as lead-free and inorganic light absorbers for perovskite solar cells. Energy Fuels 2020, 34, 10513–10528. [Google Scholar] [CrossRef]
- Slavney, A.H.; Leppert, L.; Bartesaghi, D.; Gold-Parker, A.; Toney, M.F.; Savenije, T.J.; Neaton, J.B.; Karunadasa, H.I. Defect-induced band-edge reconstruction of a bismuth-halide double perovskite for visible-light absorption. J. Am. Chem. Soc. 2017, 139, 5015–5018. [Google Scholar] [CrossRef]
- Li, Z.; Kavanagh, S.R.; Napari, M.; Palgrave, R.G.; Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Davies, D.W.; Laitinen, M.; Julin, J.; Isaacs, M.A.; et al. Bandgap lowering in mixed alloys of Cs2Ag(SbxBi1−x)Br6 double perovskite thin films. J. Mater. Chem. A 2020, 8, 21780–21788. [Google Scholar] [CrossRef]
- Lindquist, K.P.; Mack, S.A.; Slavney, A.H.; Leppert, L.; Gold-Parker, A.; Stebbins, J.F.; Salleo, A.; Toney, M.F.; Neaton, J.B.; Karunadasa, H.I. Tuning the bandgap of Cs2AgBiBr6 through dilute tin alloying. Chem. Sci. 2019, 10, 10620–10628. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Pan, W.; Yang, W.; Zou, B.; Tang, J.; Quan, Z. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite. Angew. Chem. Int. Ed. 2017, 56, 15969–15973. [Google Scholar] [CrossRef]
- Ji, F.; Klarbring, J.; Wang, F.; Ning, W.; Wang, L.; Yin, C.; Figueroa, J.S.M.; Christensen, C.K.; Etter, M.; Ederth, T.; et al. Lead-free halide double perovskite Cs2AgBiBr6 with decreased band gap. Angew. Chem. Int. Ed. 2020, 59, 15191–15194. [Google Scholar] [CrossRef]
- Abdelsamie, M.; Cruse, K.; Tamura, N.; Ceder, G.; Sutter-Fella, C.M. Impact of processing conditions on the film formation of lead-free halide double perovskite Cs2AgBiBr6. J. Mater. Chem. A 2022, 10, 19868–19880. [Google Scholar] [CrossRef]
- Tran, T.T.; Panella, J.R.; Chamorro, J.R.; Morey, J.R.; McQueen, T.M. Designing indirect–direct bandgap transitions in double perovskites. Mater. Horizons 2017, 4, 688–693. [Google Scholar] [CrossRef]
- Pan, W.; Kang, Y. Role of the microbiota in cancer growth and necrosis: The challenges and opportunities of bacteriotherapy for cancer and its complications. Rev. Med. Microbiol. 2018, 29, 20–23. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, R.; Wang, Z.-K.; Ma, X.-J.; Wang, Q.; Wang, K.-L.; Zhang, Y.; Liao, L.-S.; Yang, Y. Composition Stoichiometry of Cs2AgBiBr6 Films for Highly Efficient Lead-Free Perovskite Solar Cells. Nano Lett. 2019, 19, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2AgBiX6 (X = Br, Cl): New visible light absorbing, lead-free halide perovskite semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Volonakis, G.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Snaith, H.J.; Giustino, F. Lead-free halide double perovskites via heterovalent substitution of noble metals. J Phys. Chem. Lett. 2016, 7, 1254–1259. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A bismuth-halide double perovskite with long carrier recombination lifetime for photovoltaic applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef]
- Chen, D.W.; Zhang, X.G.; Wei, J.W.; Zhou, L.Y.; Chen, P.C.; Pang, Q.; Zhang, J.Z. Simultaneous enhancement of near infrared luminescence and stability of Cs2AgInCl6:Cr3+ double perovskite single crystals enabled by a Yb3+ dopant. Inorg. Chem. Front. 2022, 9, 4695–4704. [Google Scholar] [CrossRef]
- Liao, Q.; Chen, J.; Zhou, L.; Wei, T.; Zhang, L.; Chen, D.; Huang, F.; Pang, Q.; Zhang, J.Z. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgInCl6 Nanocrystals via Cu2+−Doping. J Phys. Chem. Lett. 2020, 11, 8392–8398. [Google Scholar] [CrossRef]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; et al. Colloidal synthesis of double perovskite Cs2AgInCl6 and Mn-doped Cs2AgInCl6 nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989–12995. [Google Scholar] [CrossRef]
- Lee, W.; Hong, S.; Kim, S. Colloidal synthesis of lead-free silver–indium double-perovskite Cs2AgInCl6 nanocrystals and their doping with lanthanide ions. J. Phys. Chem. C 2019, 123, 2665–2672. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Z.; Zhao, J.; Liu, Q. Double perovskite Cs2AgInCl6:Cr3+: Broadband and near-infrared luminescent materials. Inorg. Chem. Front. 2019, 6, 3621–3628. [Google Scholar] [CrossRef]
- Dahl, J.C.; Osowiecki, W.T.; Cai, Y.; Swabeck, J.K.; Bekenstein, Y.; Asta, M.; Chan, E.M.; Alivisatos, A.P. Probing the stability and band gaps of Cs2AgInCl6 and Cs2AgSbCl6 lead-free double perovskite nanocrystals. Chem. Mater. 2019, 31, 3134–3143. [Google Scholar] [CrossRef]
- Nila Nandha, K.; Nag, A. Synthesis and luminescence of Mn-doped Cs2AgInCl6 double perovskites. Chem. Commun. 2018, 54, 5205–5208. [Google Scholar] [CrossRef]
- Mahor, Y.; Mir, W.J.; Nag, A. Synthesis and near-infrared emission of Yb-doped Cs2AgInCl6 double perovskite microcrystals and nanocrystals. J. Phys. Chem. C 2019, 123, 15787–15793. [Google Scholar] [CrossRef]
- Yang, B.; Hong, F.; Chen, J.; Tang, Y.; Yang, L.; Sang, Y.; Xia, X.; Guo, J.; He, H.; Yang, S.; et al. Colloidal synthesis and charge-carrier dynamics of Cs2AgSb1-yBiyX6 (X: Br, Cl; 0 ≤ y ≤ 1) Double Perovskite Nanocrystals. Angew Chem. Int. Ed. Engl. 2019, 58, 2278–2283. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Hartono, N.T.P.; Seng, H.L.; Buonassisi, T.; Bristowe, P.D.; Cheetham, A.K. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6. Chem. Commun. 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- García-Espejo, G.; Rodríguez-Padrón, D.; Luque, R.; Camacho, L.; de Miguel, G. Mechanochemical synthesis of three double perovskites: Cs2AgBiBr6, (CH3NH3)2TlBiBr6 and Cs2AgSbBr6. Nanoscale 2019, 11, 16650–16657. [Google Scholar] [CrossRef]
- Yoon, S.; Fett, B.; Frebel, A.; Kroisl, S.; Herbig, B.; Widenmeyer, M.; Balke, B.; Sextl, G.; Mandel, K.; Weidenkaff, A. Sb−substituted Cs2AgBiBr6—as much as it could be?—influence of synthesis methods on Sb−substitution level in Cs2AgBiBr6. Energy Technol. 2022, 10, 2200197. [Google Scholar] [CrossRef]
- Zhang, H.; Debroye, E.; Zheng, W.; Fu, S.; Virgilio, L.D.; Kumar, P.; Bonn, M.; Wang, H.I. Highly mobile hot holes in Cs2AgBiBr6 double perovskite. Sci. Adv. 2021, 7, eabj9066. [Google Scholar] [CrossRef]
- Bartesaghi, D.; Slavney, A.H.; Gelvez-Rueda, M.C.; Connor, B.A.; Grozema, F.C.; Karunadasa, H.I.; Savenije, T.J. Charge carrier dynamics in Cs2AgBiBr6 double perovskite. J. Phys. Chem. C 2018, 122, 4809–4816. [Google Scholar] [CrossRef]
- Ji, F.; Huang, Y.; Wang, F.; Kobera, L.; Xie, F.; Klarbring, J.; Abbrent, S.; Brus, J.; Yin, C.; Simak, S.I.; et al. Near−infrared light−responsive Cu−doped Cs2AgBiBr6. Adv. Funct. Mater. 2020, 30, 2005521. [Google Scholar] [CrossRef]
- Pan, W.; Wu, H.; Luo, J.; Deng, Z.; Ge, C.; Chen, C.; Jiang, X.; Yin, W.-J.; Niu, G.; Zhu, L.; et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat. Photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Hou, P.; Yang, W.; Wan, N.; Fang, Z.; Zheng, J.; Shang, M.; Fu, D.; Yang, Z.; Yang, W. Precursor engineering for high-quality Cs2AgBiBr6 films toward efficient lead-free double perovskite solar cells. J. Mater. Chem. C 2021, 9, 9659–9669. [Google Scholar] [CrossRef]
- Fett, B.; Kabaklı, Ö.Ş.; Sierra, C.A.R.; Schulze, P.S.C.; Yoon, S.; Herbig, B.; Glunz, S.W.; Goldschmidt, J.C.; Sextl, G.; Mandel, K. In situ crystallization of the inorganic lead-free halide double perovskite Cs2AgBiBr6 via spray-drying. ACS Appl. Energy Mater. 2023, 6, 4372–4379. [Google Scholar] [CrossRef]
- Lei, L.-Z.; Shi, Z.-F.; Li, Y.; Ma, Z.-Z.; Zhang, F.; Xu, T.-T.; Tian, Y.-T.; Wu, D.; Li, X.-J.; Du, G.-T. High-efficiency and air-stable photodetectors based on lead-free double perovskite Cs2AgBiBr6 thin films. J. Mater. Chem. C 2018, 6, 7982–7988. [Google Scholar] [CrossRef]
- Ning, W.; Zhao, X.-G.; Klarbring, J.; Bai, S.; Ji, F.; Wang, F.; Simak, S.I.; Tao, Y.; Ren, X.-M.; Zhang, L.; et al. Thermochromic lead-free halide double perovskites. Adv. Funct. Mater. 2019, 29, 1807375. [Google Scholar] [CrossRef]
- Schmitz, A.; Schaberg, L.L.; Sirotinskaya, S.; Pantaler, M.; Lupascu, D.C.; Benson, N.; Bacher, G. Fine structure of the optical absorption resonance in Cs2AgBiBr6 double perovskite thin films. ACS Energy Lett. 2020, 5, 559–565. [Google Scholar] [CrossRef]
- Fang, F.; Li, H.; Fang, S.; Zhou, B.; Huang, F.; Ma, C.; Wan, Y.; Jiang, S.; Wang, Y.; Tian, B.; et al. 2D Cs2AgBiBr6 with boosted light–matter interaction for high-performance photodetectors. Adv. Opt. Mater. 2021, 9, 2001930. [Google Scholar] [CrossRef]
- Xiao, B.; Tan, Y.; Yi, Z.; Luo, Y.; Jiang, Q.; Yang, J. Band matching strategy for all-inorganic Cs2AgBiBr6 double perovskite solar cells with high photovoltage. ACS Appl. Mater. Interfaces 2021, 13, 37027–37034. [Google Scholar] [CrossRef]
- Shadabroo, M.S.; Abdizadeh, H.; Golobostanfard, M.R. Dimethyl sulfoxide vapor-assisted Cs2AgBiBr6 homogenous film deposition for solar cell application. ACS Appl. Energy Mater 2021, 4, 6797–6805. [Google Scholar] [CrossRef]
- Daem, N.; Dewalque, J.; Lang, F.; Maho, A.; Spronck, G.; Henrist, C.; Colson, P.; Stranks, S.D.; Cloots, R. Spray-coated lead-free Cs2AgBiBr6 double perovskite solar cells with high open-circuit voltage. Solar RRL 2021, 5, 2100422. [Google Scholar] [CrossRef]
- Han, Y.; Xie, H.; Lim, E.L.; Bi, D. Review of two−step method for lead halide perovskite solar cells. Solar RRL 2022, 6, 2101007. [Google Scholar] [CrossRef]
- Wu, H.; Erbing, A.; Johansson, M.B.; Wang, J.; Kamal, C.; Odelius, M.; Johansson, E.M.J. Mixed-halide double perovskite Cs2AgBiX6 (X=Br, I) with tunable optical properties via anion exchange. ChemSusChem 2021, 14, 4507–4515. [Google Scholar] [CrossRef]
- Greul, E.; Petrus, M.L.; Binek, A.; Docampo, P.; Bein, T. Highly stable, phase pure Cs2AgBiBr6 double perovskite thin films for optoelectronic applications. J. Mater. Chem. A 2017, 5, 19972–19981. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Yang, L.; Dall’Agnese, C.; Jena, A.K.; Miyasaka, T.; Wang, X.F. Organic dye/Cs2AgBiBr6 double perovskite heterojunction solar cells. J. Am. Chem. Soc. 2021, 143, 14877–14883. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, P.; Wang, Z.; Liu, M. Vapor-deposited Cs2AgBiCl6 double perovskite films toward highly selective and stable ultraviolet photodetector. Adv. Sci. 2020, 7, 1903662. [Google Scholar] [CrossRef]
- Rodkey, N.; Kaal, S.; Sebastia-Luna, P.; Birkholzer, Y.A.; Ledinsky, M.; Palazon, F.; Bolink, H.J.; Morales-Masis, M. Pulsed laser deposition of Cs2AgBiBr6: From mechanochemically synthesized powders to dry, single-step deposition. Chem. Mater. 2021, 33, 7417–7422. [Google Scholar] [CrossRef] [PubMed]
- Stroyuk, O.; Raievska, O.; Sebastia-Luna, P.; Huisman, B.A.H.; Kupfer, C.; Barabash, A.; Hauch, J.; Bolink, H.J.; Brabec, C.J. Highly luminescent transparent Cs2AgxNa1-xBiyIn1-yCl6 perovskite films produced by single-source vacuum deposition. ACS Mater. Lett. 2023, 5, 596–602. [Google Scholar] [CrossRef]
- Fang, F.; He, W.; Liu, Z.; Jiang, K.; Wang, Y.; Chen, F.; Li, H.; Shi, Y. Blade-coating of a highly crystallized lead-free silver-bismuth halide double perovskite thin film with improved stability for high-performance photodetection. J. Mater. Chem. C 2023, 11, 7048–7058. [Google Scholar] [CrossRef]
- Tang, H.; Xu, Y.; Hu, X.; Hu, Q.; Chen, T.; Jiang, W.; Wang, L.; Jiang, W. Lead-free halide double perovskite nanocrystals for light-emitting applications: Strategies for boosting efficiency and stability. Adv. Sci. 2021, 8, 2004118. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Lin, Y.; Liao, Y.; Wei, Q.; Chen, H.; Qiu, J.; Chen, Y.; Zheng, Y. Hot-substrate deposition of all-inorganic perovskite films for low-temperature processed high-efficiency solar cells. J. Mater. Chem. A 2019, 7, 2773–2779. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Z.; Xia, W.; Yuan, C.; Cheng, J.; Lu, Y. Uniform, stable, and efficient planar-heterojunction perovskite solar cells by facile low-pressure chemical vapor deposition under fully open-air conditions. ACS Appl. Mater. Interfaces 2015, 7, 2708–2714. [Google Scholar] [CrossRef]
- Li, Y.; Cooper, J.K.; Buonsanti, R.; Giannini, C.; Liu, Y.; Toma, F.M.; Sharp, I.D. Fabrication of planar heterojunction perovskite solar cells by controlled low-pressure vapor annealing. J. Phys. Chem. Lett. 2015, 6, 493–499. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, C.; Wang, D.; Liu, G.; Zhang, Q.; Luo, W.; Qi, X.; Guo, X.; Zhang, Y.; Lao, Y.; et al. Improvement of Cs2AgBiBr6 double perovskite solar cell by rubidium doping. Org. Electron. 2019, 74, 204–210. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, W.; Wang, J.; Yan, Y. Thermodynamic stability and defect chemistry of bismuth-based lead-free double perovskites. ChemSusChem 2016, 9, 2628–2633. [Google Scholar] [CrossRef]
- Maiti, A.; Pal, A.J. Effect of cation occupancy ordering in double perovskites to overcome hurdles in carrier transport: Cs2AgBiBr6 as a case study. J. Phys. Chem. C 2021, 125, 16324–16333. [Google Scholar] [CrossRef]
- Bruening, K.; Tassone, C.J. Antisolvent processing of lead halide perovskite thin films studied by in situ X-ray diffraction. J. Mater. Chem. A 2018, 6, 18865–18870. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, B.; Liang, C.; Liu, T.; Wei, Q.; Wang, S.; Wang, K.; Zhang, Z.; Li, X.; Peng, S.; et al. Facile deposition of high-quality Cs2AgBiBr6 films for efficient double perovskite solar cells. Sci. China Mater 2020, 63, 1518–1525. [Google Scholar] [CrossRef]
- Prochowicz, D.; Tavakoli, M.M.; Solanki, A.; Goh, T.W.; Pandey, K.; Sum, T.C.; Saliba, M.; Yadav, P. Understanding the effect of chlorobenzene and isopropanol anti-solvent treatments on the recombination and interfacial charge accumulation in efficient planar perovskite solar cells. J. Mater. Chem. A 2018, 6, 14307–14314. [Google Scholar] [CrossRef]
- Kangsabanik, J.; Sugathan, V.; Yadav, A.; Yella, A.; Alam, A. Double perovskites overtaking the single perovskites: A set of new solar harvesting materials with much higher stability and efficiency. Phys. Rev. Mater. 2018, 2, 055401. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, Z.K.; Liao, L.S. Progress of lead−free halide double perovskites. Adv. Energy Mater. 2019, 9, 1803150. [Google Scholar] [CrossRef]
- Wang, K.-q.; He, Y.; Zhang, M.; Shi, J.-j.; Cai, W.-w. Promising lead-free double-perovskite photovoltaic materials Cs2MM′Br6 (M = Cu, Ag, and Au; M′ = Ga, In, Sb, and Bi) with an ideal band gap and high power conversion efficiency. J. Phys. Chem. C 2021, 125, 21160–21168. [Google Scholar] [CrossRef]
- Wang, B.H.; Gao, B.; Zhang, J.R.; Chen, L.; Junkang, G.; Shen, S.; Au, C.T.; Li, K.; Cai, M.Q.; Yin, S.F. Thickness-induced band-gap engineering in lead-free double perovskite Cs2AgBiBr6 for highly efficient photocatalysis. Phys. Chem. Chem. Phys. 2021, 23, 12439–12448. [Google Scholar] [CrossRef] [PubMed]
- Caetano, C.; Guilhon, I.; Cappellini, G.; Marques, M.; Teles, L.K. First-principles study of electronic properties of cesium chloride double perovskites using a DFT-1/2 approach. J. Phys. Chem. C 2022, 126, 15065–15071. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Lu, Y.; Zhang, Z.; Wang, Y.; Yang, Y.; Dong, Q.; Yu, Y.; Qin, P.; Huang, F. Thermochromic Cs2AgBiBr6 single crystal with decreased band gap through order-disorder transition. Small 2022, 18, e2201943. [Google Scholar] [CrossRef]
- Lyu, M.; Yun, J.-H.; Chen, P.; Hao, M.; Wang, L. Addressing toxicity of lead: Progress and applications of low-toxic metal halide perovskites and their derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Yang, L.; Hou, P.; Wang, B.; Dall’Agnese, C.; Dall’Agnese, Y.; Chen, G.; Gogotsi, Y.; Meng, X.; Wang, X.-F. Performance improvement of dye-sensitized double perovskite solar cells by adding Ti3C2Tx MXene. Chem. Eng. J. 2022, 446, 136963. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Goddard, W.A., III. Schottky-barrier-free contacts with two-dimensional semiconductors by surface-engineered MXenes. J. Am. Chem. Soc. 2016, 138, 15853–15856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, Q.; Lu, Y.; Lu, F.; Mu, X.; Wei, S.H.; Sui, M. Hydrogenated Cs2AgBiBr6 for significantly improved efficiency of lead-free inorganic double perovskite solar cell. Nat. Commun. 2022, 13, 3397. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, M.W.; Ramzan, M.; Rashid, M.; Hussain, A.; Imran, M.; Fahim, F.; Neffati, R. Ab-initio study of lead-free double Perovskites Cs2AgZBr6 (Z = Bi, Sb) for Solar cells and other renewable energy applications. J. Am. Chem. Soc. 2022, 306, 122781. [Google Scholar] [CrossRef]
- Amraoui, S.; Feraoun, A.; Kerouad, M. Electronic and optical properties of the lead free halide double perovskites Cs2AgBiX6(X = F, Cl, Br and I) for the photovoltaic and optoelectronic applications. Inorg. Chem. Commun. 2022, 140, 109395. [Google Scholar] [CrossRef]
- Zelewski, S.J.; Urban, J.M.; Surrente, A.; Maude, D.K.; Kuc, A.; Schade, L.; Johnson, R.D.; Dollmann, M.; Nayak, P.K.; Snaith, H.J.; et al. Revealing the nature of photoluminescence emission in the metal-halide double perovskite Cs2AgBiBr6. J. Mater. Chem. C 2019, 7, 8350–8356. [Google Scholar] [CrossRef]
- Slavney, A.H.; Leppert, L.; Saldivar Valdes, A.; Bartesaghi, D.; Savenije, T.J.; Neaton, J.B.; Karunadasa, H.I. Small-band-gap halide double perovskites. Angew Chem Int Ed Engl. 2018, 57, 12765–12770. [Google Scholar] [CrossRef]
- Ghasemi, M.; Hao, M.; Xiao, M.; Chen, P.; He, D.; Zhang, Y.; Chen, W.; Fan, J.; Yun, J.H.; Jia, B.; et al. Lead-free metal-halide double perovskites: From optoelectronic properties to applications. Nanophotonics 2020, 10, 2181–2219. [Google Scholar] [CrossRef]
- Chand Yadav, S.; Srivastava, A.; Manjunath, V.; Kanwade, A.; Devan, R.S.; Shirage, P.M. Properties, performance and multidimensional applications of stable lead-free Cs2AgBiBr6 double perovskite. Mat. Today Phys. 2022, 26, 100731. [Google Scholar] [CrossRef]
- Zhao, D.; Liang, C.; Wang, B.; Liu, T.; Wei, Q.; Wang, K.; Gu, H.; Wang, S.; Mei, S.; Xing, G. Overcoming the limitation of Cs2AgBiBr6 double perovskite solar cells through using mesoporous TiO2 electron extraction layer. Energy Environ. Mater. 2022, 5, 1317–1322. [Google Scholar] [CrossRef]
- Pang, B.; Chen, X.; Bao, F.; Liu, Y.; Feng, T.; Dong, H.; Yu, L.; Dong, L. Improved charge extraction and atmospheric stability of all-inorganic Cs2AgBiBr6 perovskite solar cells by MoS2 nanoflakes. SSol. Energy Mater Sol. Cells 2022, 246, 111932. [Google Scholar] [CrossRef]
- Raj, A.; Kumar, M.; Kumar, A.; Laref, A.; Anshul, A. Investigating the potential of lead−free double perovskite Cs2AgBiBr6 material for solar cell applications: A theoretical study. Int. J. Energy Res. 2022, 46, 13801–13819. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Mustafa, A.N.; Chelvanathan, P.; Hossain, M.I.; Azam, M.A.; Amin, N. Improved performance of lead-free Perovskite solar cell incorporated with TiO2 ETL and CuI HTL using SCAPs. Appl. Phys. A 2023, 129, 132. [Google Scholar] [CrossRef]
- Mohandes, A.; Moradi, M.; Nadgaran, H. Numerical simulation of inorganic Cs2AgBiBr6 as a lead-free perovskite using device simulation SCAPS-1D. Opt. Quantum Electron 2021, 53, 319. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Yang, L.; Dall’Agnese, C.; Jena, A.K.; Sasaki, S.-i.; Miyasaka, T.; Tamiaki, H.; Wang, X.-F. Chlorophyll Derivative-Sensitized TiO2 electron transport layer for record efficiency of Cs2AgBiBr6 double perovskite solar cells. J. Am. Chem. Soc. 2021, 143, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, Y.; Liu, P.; Xiang, H.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Simultaneous power conversion efficiency and stability enhancement of Cs2AgBiBr6 lead-free inorganic perovskite solar cell through adopting a multifunctional dye interlayer. Adv. Funct. Mater. 2020, 30, 2001557. [Google Scholar] [CrossRef]

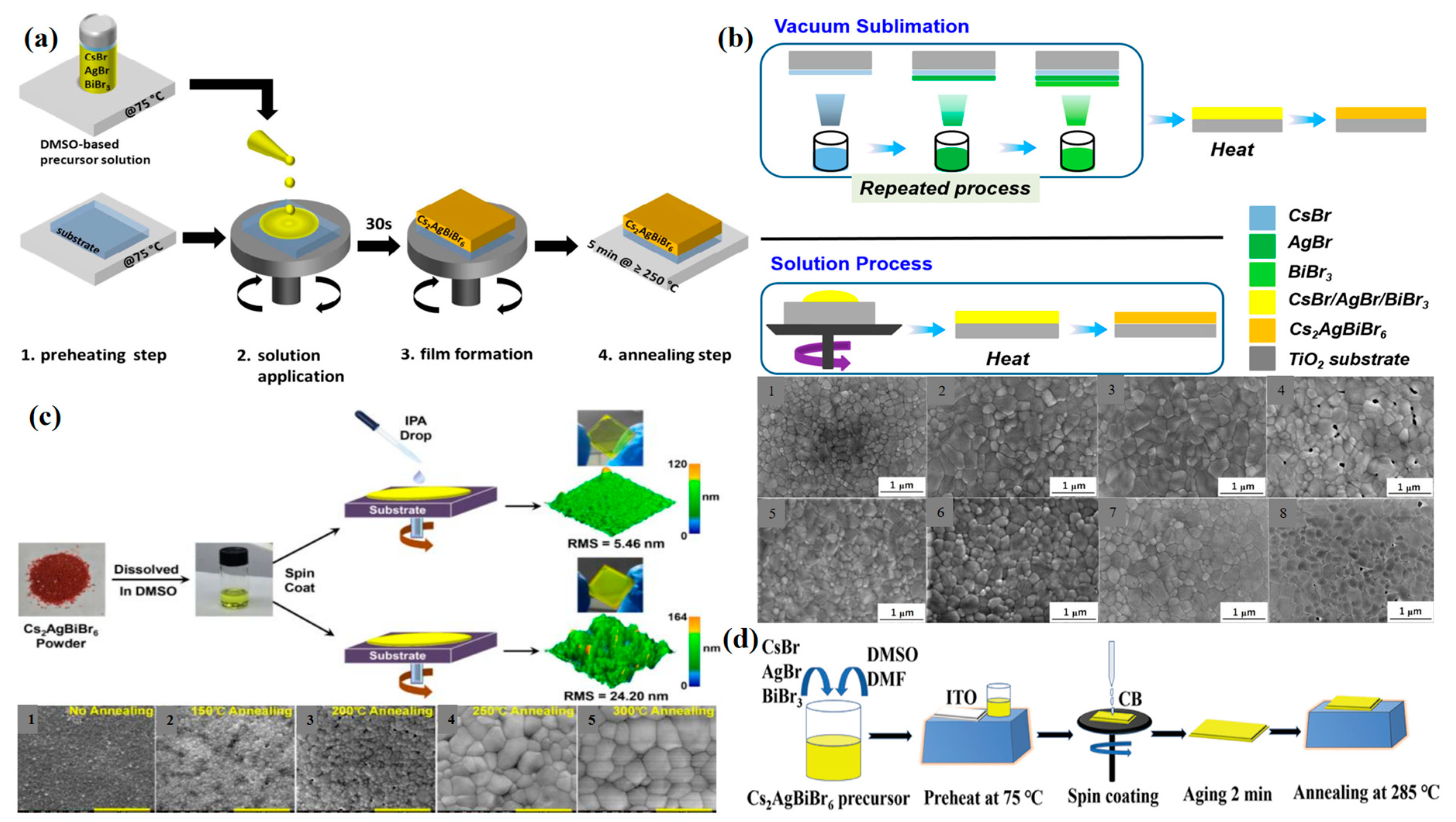

| Compound | Synthesis Method | Synthesis Atmosphere | Heating Condition | Film Thickness | Grain Size | Year and Reference |
|---|---|---|---|---|---|---|
| Cs2Ag(SbxBi1−x)Br6 | spin-coating | N2 | preheating at 75 °C, post-annealing 135 °C for 5 min | 200 nm | 80 nm | 2020 [26] |
| Cs2AgSbBr6 | spin-coating | preheating at 200 °C, post-annealing 150 °C for 30 min | 2019 [46] | |||
| Cs2AgBiBr6 | spin-coating | preheating at 75 °C, post-annealing 285 °C for 30 min | 2019 [46] | |||
| Cs2AgBiBr6 | spin-coating | preheating at 100 °C, post-annealing 200 °C for 5 min | 150 nm | 2018 [50] | ||
| 2D Cs2AgBiBr6 | space-confined | a pressure of 50 kPa | 70 °C for 24 h | 9.8 nm | 10μm | 2021 [58] |
| Cs2AgBiBr6 | sequential vapor deposition | post-annealing 250 °C for 30 min | 120 nm AgBr, 200 nm BiBr3, 200 nm CsBr | 500 nm | 2021 [59] | |
| Cs2AgBiBr6 | spin-coating | glovebox | post-annealing 250 °C for 10 min | 800 nm | 2021 [60] | |
| Cs2AgBiBr6 | spin-coating | post-annealing 280 °C for 5 min | 500 nm | 2021 [47] | ||
| Cs2AgBiBr6 | spin-coating | Ar | preheating at 75 °C, post-annealing 285 °C for 5 min | 200 nm | 28/34 nm | 2021 [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, R.; Zhou, T.; Ji, S.; Liu, W.; Li, X. Synthesis and Optimization of Cs2B′B″X6 Double Perovskite for Efficient and Sustainable Solar Cells. Molecules 2023, 28, 6601. https://doi.org/10.3390/molecules28186601
Yao R, Zhou T, Ji S, Liu W, Li X. Synthesis and Optimization of Cs2B′B″X6 Double Perovskite for Efficient and Sustainable Solar Cells. Molecules. 2023; 28(18):6601. https://doi.org/10.3390/molecules28186601
Chicago/Turabian StyleYao, Ruijia, Tingxue Zhou, Shilei Ji, Wei Liu, and Xing’ao Li. 2023. "Synthesis and Optimization of Cs2B′B″X6 Double Perovskite for Efficient and Sustainable Solar Cells" Molecules 28, no. 18: 6601. https://doi.org/10.3390/molecules28186601
APA StyleYao, R., Zhou, T., Ji, S., Liu, W., & Li, X. (2023). Synthesis and Optimization of Cs2B′B″X6 Double Perovskite for Efficient and Sustainable Solar Cells. Molecules, 28(18), 6601. https://doi.org/10.3390/molecules28186601






