Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins
Abstract
1. Introduction
2. Results
2.1. General Compositional Characterisation of Apple Pomaces
2.2. Phenolic Composition of Apple Pomaces
2.3. Measures of Antioxidant Potential in Apple Pomace Extracts
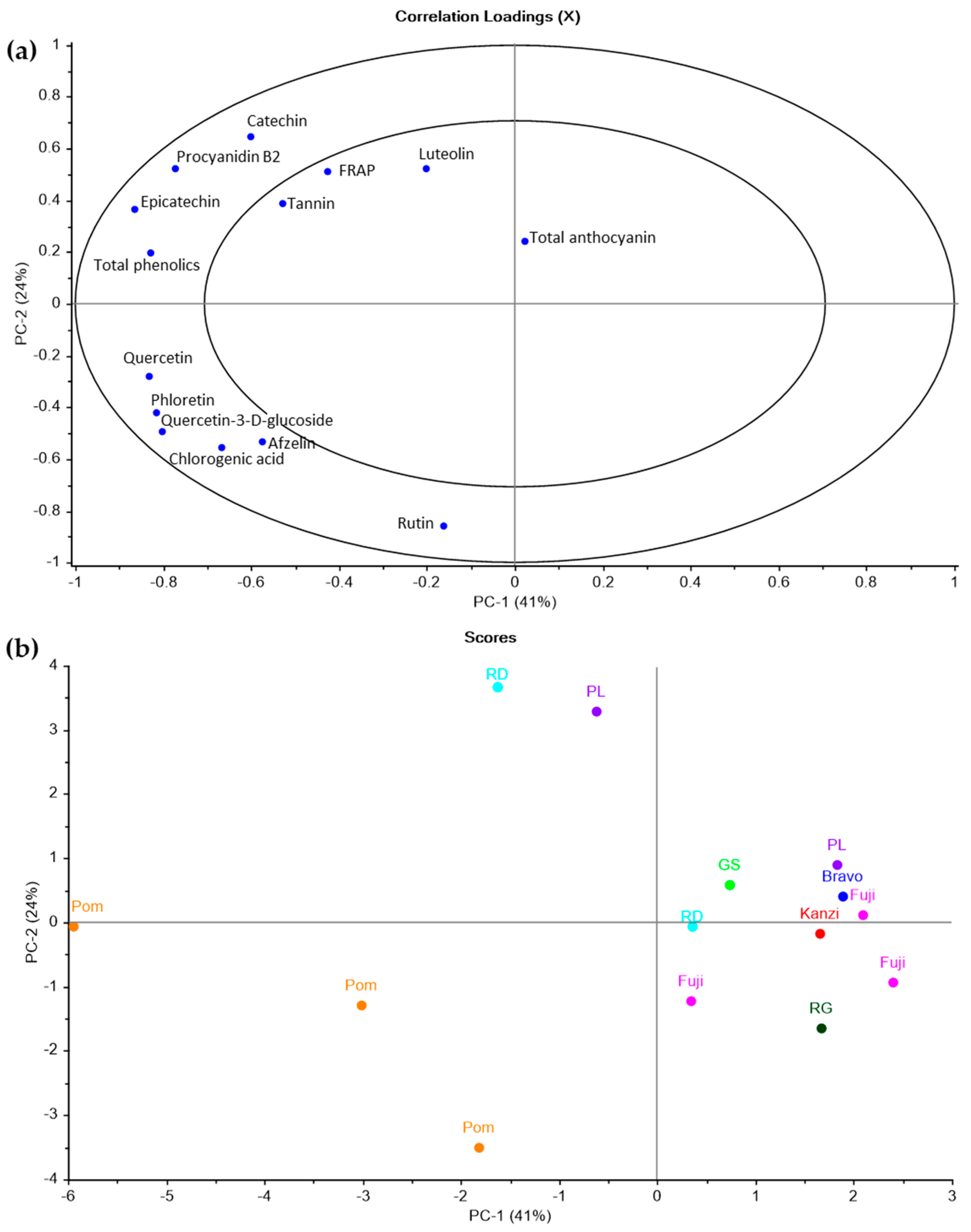
2.4. Multivariate Modeling of Apple Pomace Phenolic Composition
3. Discussion
4. Materials and Methods
4.1. Preparation of Apple Pomace Samples
4.2. Analysis of the Ethanol-Soluble Fraction of Apple Pomace
4.3. Analysis of the Ethanol-Insoluble Fraction of Apple Pomace
4.4. Protein Analysis
4.5. Non-Targeted Phenolic Profiling by LC-MS
4.6. Molecular Feature Extraction
4.7. Multivariate Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol.-Mysore 2016, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Solis, V.; Zamudio-Flores, P.B.; Tirado-Gallegos, J.M.; Ramirez-Mancinas, S.; Olivas-Orozco, G.I.; Espino-Diaz, M.; Hernandez-Gonzalez, M.; Garcia-Cano, V.G.; Sanchez-Ortiz, O.; Buenrostro-Figueroa, J.J.; et al. Evaluation of Cooking Quality, Nutritional and Texture Characteristics of Pasta Added with Oat Bran and Apple Flour. Foods 2019, 8, 299. [Google Scholar] [CrossRef]
- Villas-Boas, S.G.; Esposito, E.; de Mendonca, M.M. Bioconversion of apple pomace into a nutritionally enriched substrate by Candida utilis and Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2003, 19, 461–467. [Google Scholar] [CrossRef]
- Gorjanovic, S.; Micic, D.; Pastor, F.; Tosti, T.; Kalusevic, A.; Ristic, S.; Zlatanovic, S. Evaluation of apple pomace flour obtained industrially by dehydration as a source of biomolecules with antioxidant, antidiabetic and antiobesity effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Jovanovic, M.; Petrovic, M.; Miocinovic, J.; Zlatanovic, S.; Petronijevic, J.L.; Mitic-Culafic, D.; Gorjanovic, S. Bioactivity and sensory properties of probiotic yogurt fortified with apple pomace flour. Foods 2020, 9, 763. [Google Scholar] [CrossRef]
- Gumul, D.; Ziobro, R.; Korus, J.; Kruczek, M. Apple pomace as a source of bioactive polyphenol compounds in gluten-free breads. Antioxidants 2021, 10, 807. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Farr, J.; Wibisono, R.; Saleh, Z. Fruit-based functional foods I: Production of food-grade apple fibre ingredients. Int. J. Food Sci. Technol. 2008, 43, 2113–2122. [Google Scholar] [CrossRef]
- McDougall, G.J. Phenolic-enriched foods: Sources and processing for enhanced health benefits. Proc. Nutr. Soc. 2017, 76, 163–171. [Google Scholar] [CrossRef]
- Huber, G.M.; Rupasinghe, H.P.V. Phenolic profiles and antioxidant properties of apple skin extracts. J. Food Sci. 2009, 74, C693–C700. [Google Scholar] [CrossRef]
- Feng, S.H.; Yi, J.Y.; Li, X.; Wu, X.Y.; Zhao, Y.Y.; Ma, Y.C.; Bi, J.F. Systematic review of phenolic compounds in apple fruits: Compositions, distribution, absorption, metabolism, and processing stability. J. Agric. Food Chem. 2021, 69, 7–27. [Google Scholar] [CrossRef]
- Guyot, S.; Le Bourvellee, C.; Marnet, N.; Drilleau, J.F. Procyanidins are the most abundant polyphenols in dessert apples at maturity. Lebensm.-Wiss.-Technol.-Food Sci. Technol. 2002, 35, 289–291. [Google Scholar] [CrossRef]
- Guyot, S.; Doco, T.; Souquet, J.M.; Moutounet, M.; Drilleau, J.F. Characterization of highly polymerized procyanidins in cider apple (Malus sylvestris var kermerrien) skin and pulp. Phytochemistry 1997, 44, 351–357. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Drilleau, J.F. Thiolysis-HPLC characterization of apple procyanidins covering a large range of polymerization states. J. Agric. Food Chem. 2001, 49, 14–20. [Google Scholar] [CrossRef]
- Gosse, F.; Roussi, S.; Fischer, B.; Guyot, S.; Raul, F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of experimental carcinogenesis. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1920S. [Google Scholar]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Kobori, M.; Kanda, T.; Shinmoto, H.; Tsushida, T. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J. Agric. Food Chem. 2005, 53, 6105–6111. [Google Scholar] [CrossRef]
- Zessner, H.; Pan, L.; Will, F.; Klimo, K.; Knauft, J.; Niewohner, R.; Hummer, W.; Owen, R.; Richling, E.; Frank, N.; et al. Fractionation of polyphenol-enriched apple juice extracts to identify constituents with cancer chemopreventive potential. Mol. Nutr. Food Res. 2008, 52, S28–S44. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Deprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Schroeter, H.; Rechner, A.R.; Rice-Evans, C. Bioavailability of flavan-3-ols and procyanidins: Gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid. Redox Signal. 2001, 3, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, I.; Segliņa, D. Content of phenolic compounds and antioxidant activity in fresh apple, pomace and pomace water extract—Effect of cultivar. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2019, 73, 513–518. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Herderich, M.J.; Smith, P.A. High Throughput analysis of red wine and grape phenolics-adaptation and validation of methyl cellulose precipitable tannin assay and modified somers color assay to a rapid 96 well plate format. J. Agric. Food Chem. 2007, 55, 4651–4657. [Google Scholar] [CrossRef]
- Granucci, N.; Villas-Boas, S.G. Fruit residues: Low cost substrates for development of new food products. New Biotechnol. 2014, 31, S211. [Google Scholar] [CrossRef]
- Granucci, N.; Harris, P.; Villas-Boas, S.G. Functional food production by biocoversion of fruit/vegetable by-products using a food-grade non-Saccharomyces yeast. New Biotechnol. 2016, 33, S114. [Google Scholar] [CrossRef]
- Sethi, S.; Joshi, A.; Arora, B.; Bhowmik, A.; Sharma, R.R.; Kumar, P. Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. Eur. Food Res. Technol. 2020, 246, 591–598. [Google Scholar] [CrossRef]
- Kosmala, M.; Kolodziejczyk, K.; Markowski, J.; Mieszczakowska, M.; Ginies, C.; Renard, C. Co-products of black-currant and apple juice production: Hydration properties and polysaccharide composition. LWT-Food Sci. Technol. 2010, 43, 173–180. [Google Scholar] [CrossRef]
- Gosse, F.; Guyot, S.; Roussi, S.; Lobstein, A.; Fischer, B.; Seiler, N.; Raul, F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 2005, 26, 1291–1295. [Google Scholar] [CrossRef]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.F. Polyphenol profiles of French cider apple varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Birtic, S.; Regis, S.; Le Bourvellec, C.; Renard, C. Impact of air-drying on polyphenol extractability from apple pomace. Food Chem. 2019, 296, 142–149. [Google Scholar] [CrossRef]
- Heras-Ramirez, M.E.; Quintero-Ramos, A.; Camacho-Davila, A.A.; Barnard, J.; Talamas-Abbud, R.; Torres-Munoz, J.V.; Salas-Munoz, E. Effect of blanching and drying temperature on polyphenolic compound stability and antioxidant capacity of apple pomace. Food Bioprocess Technol. 2012, 5, 2201–2210. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; van Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Garcia, Y.D.; Valles, B.S.; Lobo, A.P. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. Optimisation of the phenolic content and antioxidant activity of apple pomace aqueous extracts. Cyta-J. Food 2015, 13, 293–299. [Google Scholar] [CrossRef]
- Hernandez-Carranza, P.; Avila-Sosa, R.; Guerrero-Beltran, J.A.; Navarro-Cruz, A.R.; Corona-Jimenez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit by-products: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Rana, S.; Gupta, S.; Rana, A.; Bhushan, S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum. Wellness 2015, 4, 180–187. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol.-Mysore 2021, 58, 166–174. [Google Scholar] [CrossRef]
- Khanizadeh, S.; Tsao, R.; Rekika, D.; Yang, R.; Charles, M.T.; Rupasinghe, H.P.V. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J. Food Compos. Anal. 2008, 21, 396–401. [Google Scholar] [CrossRef]
- Ran, J.J.; Sun, H.D.; Xu, Y.; Wang, T.T.; Zhao, R.X. Comparison of antioxidant activities and high-performance liquid chromatography analysis of polyphenol from different apple varieties. Int. J. Food Prop. 2016, 19, 2396–2407. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A.; van Westing, L.M. Flavonoid and chlorogenic acid levels in apple fruit: Characterisation of variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- Bai, X.L.; Zhang, H.W.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Gaillet, S.; Ordonez, J.L.; Mena, P.; Bresciani, L.; Bindon, K.A.; Del Rio, D.; Rouanet, J.M.; Moreno-Rojas, J.M.; Crozier, A. Bioavailability of red wine and grape seed proanthocyanidins in rats. Food Funct. 2020, 11, 3986–4001. [Google Scholar] [CrossRef]
- Dasiman, R.; Nor, N.M.; Eshak, Z.; Mutalip, S.S.M.; Suwandi, N.R.; Bidin, H. A review of procyanidin: Updates on current bioactivities and potential health benefits. Biointerface Res. Appl. Chem. 2022, 12, 5918–5940. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; Lopez-Giraldo, L.J.; Decker, E.A. Relationship between the physiochemical properties of cocoa procyanidins and their ability to inhibit lipid oxidation in liposomes. J. Agric. Food Chem. 2018, 66, 4490–4502. [Google Scholar] [CrossRef]
- Sato, M.F.; Vieira, R.G.; Zardo, D.M.; Falcao, L.D.; Nogueira, A.; Wosiacki, G. Apple pomace from eleven cultivars: An approach to identify sources of bioactive compounds. Acta Sci.-Agron. 2010, 32, 29–35. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Bekkour, K.; Wadhwa, S.S.; Waterhouse, G.I.N. Rheological and Chemical characterization of smoothie beverages containing high concentrations of fibre and polyphenols from apple. Food Bioprocess Technol. 2014, 7, 409–423. [Google Scholar] [CrossRef]
- Ishartati, E.; Sukardi, S.; Roeswitawati, D.; Zakia, A.; Ulfah, U. The study of Apple flour formulation for functional cookies. In Proceedings of the International Conference on Food Science and Technology (Foscitech), Yogyakarta, Indonesia, 14–15 November 2018. [Google Scholar]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef]
- de la Torre, A.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- McCleary, B.V.; Charmier, L.M.J.; McKie, V.A. Measurement of starch: Critical evaluation of current methodology. Starch-Starke 2019, 71, 1800146. [Google Scholar] [CrossRef]
- Kassara, S.; Li, S.J.; Smith, P.; Blando, F.; Bindon, K. Pectolytic enzyme reduces the concentration of colloidal particles in wine due to changes in polysaccharide structure and aggregation properties. Int. J. Biol. Macromol. 2019, 140, 546–555. [Google Scholar] [CrossRef]
| Apple Pomace | Source 2 | Sugars (mg/g D.wt) | Total Phenolics (mg/g D.wt) 3 | Anthocyanin (mg/g D.wt) | Polymeric Procyanidin (mg/g D.wt) 3 | Pectin (mg/g D.wt) | Hemicellulose (mg/g D.wt) | Cellulose (mg/g D.wt) | Lignin (mg/g D.wt) | Starch (mg/g D.wt) | Protein (mg/g D.wt) 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Red Delicious | Barossa Valley, SA | 524.7 ± 14 | 5.63 ± 0.03 | 0.13 ± 0.03 | 2.52 ± 0.17 | 66.89 ± 1.05 | 29.52 ± 0.16 | 14.72 ± 3.21 | 113.26 ± 2.00 | 5.59 ± 0.31 | |
| Red Delicious | Adelaide Hills, SA | 482.6 ± 7 | 12.02 ± 0.08 | 0.32 ± 0.02 | 7.04 ± 0.19 | 60.82 ± 0.11 | 30.14 ± 0.31 | 18.98 ± 3.97 | 164.71 ± 3.87 | 1.41 ± 0.08 | |
| Fuji | Barossa Valley, SA | 688.9 ± 13 | 4.98 ± 0.15 | 0.07 ± 0.01 | 2.78 ± 0.12 | 44.26 ± 4.05 | 19.76 ± 0.27 | 8.04 ± 1.09 | 165.17 ± 1.01 | 1.11 ± 0.30 | |
| Fuji | Barossa Valley, SA | 539.3 ± 6 | 3.31 ± 0.02 | 0.04 ± 0.02 | 0.94 ± 0.14 | 72.49 ± 1.22 | 28.25 ± 0.98 | 21.64 ± 3.96 | 111.59 ± 4.69 | 1.99 ± 0.70 | 19.0 |
| Fuji | McLaren Vale, SA | 557.4 ± 16 | 7.07 ± 0.07 | 0.15 ± 0.03 | 3.27 ± 0.15 | 64.98 ± 2.83 | 41.44 ± 0.84 | 6.99 ± 1.80 | 88.30 ± 2.07 | 21.13 ± 0.96 | |
| Pink Lady | Barossa Valley, SA | 464.8 ± 19 | 5.83 ± 0.02 | 0.10 ± 0.00 | 1.27 ± 0.05 | 82.53 ± 0.78 | 30.88 ± 0.15 | 19.87 ± 4.92 | 103.57 ± 9.72 | 5.53 ± 1.86 | 19.0 |
| Pink Lady | Adelaide Hills, SA | 543.9 ± 10 | 7.85 ± 0.45 | 0.25 ± 0.02 | 2.91 ± 0.19 | 55.81 ± 3.49 | 24.18 ± 2.84 | 9.94 ± 1.80 | 99.29 ± 10.09 | 2.10 ± 0.13 | |
| Royal Gala | Adelaide Hills, SA | 521.8 ± 1 | 5.77 ± 0.10 | 0.13 ± 0.01 | n.d. | 73.06 ± 1.22 | 31.80 ± 0.01 | 18.40 ± 4.19 | 159.52 ± 4.33 | 3.35 ± 0.46 | |
| Bravo | Adelaide Hills, SA | 517.9 ± 14 | 8.12 ± 0.19 | 0.88 ± 0.03 | 0.86 ± 0.00 | 67.77 ± 1.58 | 26.12 ± 0.12 | 10.02 ± 0.32 | 115.31 ± 2.68 | 4.24 ± 0.11 | |
| Kanzi | Adelaide Hills, SA | 498.3 ± 8 | 4.92 ± 0.05 | 0.07 ± 0.02 | 0.74 ± 0.05 | 69.27 ± 4.88 | 31.68 ± 1.62 | 32.75 ± 1.20 | 106.32 ± 11.25 | 1.42 ± 0.37 | |
| Granny Smith | McLaren Vale, SA | 460.3 ± 4 | 6.10 ± 0.21 | 0.05 ± 0.03 | 2.54 ± 0.14 | 86.76 ± 2.14 | 36.01 ± 0.97 | 11.16 ± 0.22 | 137.79 ± 0.00 | 2.75 ± 0.81 | |
| Pomace | Adelaide Hills, SA | 360.1 ± 1 | 8.47 ± 0.35 | 0.18 ± 0.02 | 3.51 ± 0.34 | 60.87 ± 3.10 | 41.06 ± 0.28 | 9.47 ± 1.01 | 208.00 ± 3.33 | 2.87 ± 0.06 | 43.0 |
| Pomace 1 | Packenham Upper, VIC | 334.7 ± 17 | 10.15 ± 0.22 | 0.15 ± 0.00 | 3.55 ± 0.02 | 89.63 ± 0.21 | 37.82 ± 0.62 | 11.24 ± 3.16 | 188.62 ± 15.81 | 2.88 ± 1.09 | 42.0 |
| Pomace 1 | Illinois, USA | 328.1 ± 13 | 11.82 ± 0.17 | 0.16 ± 0.00 | 2.61 ± 0.22 | 101.96 ± 1.12 | 26.85 ± 5.22 | 19.27 ± 1.29 | 133.42 ± 32.53 | 9.41 ± 2.46 | 32.0 |
| Apple Pomace | Source 2 | MM 3 (g/mol) | mDP 4 | EC-ext 5 (mol%) | EC-ter 5 (mol%) | CAT-ter 5 (mol%) |
|---|---|---|---|---|---|---|
| Red Delicious | Barossa Valley, SA | 1289 ± 23 | 4.44 ± 0.08 | 77.49 ± 0.39 | 18.21 ± 0.52 | 4.29 ± 0.13 |
| Red Delicious | Adelaide Hills, SA | 1179 ± 4 | 4.06 ± 0.02 | 75.40 ± 0.09 | 19.80 ± 0.08 | 4.79 ± 0.17 |
| Fuji | Barossa Valley, SA | 903 ± 109 | 3.11 ± 0.38 | 67.38 ± 3.94 | 24.31 ± 3.46 | 8.29 ± 3.94 |
| Fuji | Barossa Valley, SA | 970 ± 27 | 3.34 ± 0.09 | 70.06 ± 0.84 | 22.74 ± 0.93 | 7.18 ± 0.84 |
| Fuji | McLaren Vale, SA | 740 ± 15 | 2.54 ± 0.05 | 60.75 ± 0.80 | 22.00 ± 3.24 | 17.23 ± 0.80 |
| Pink Lady | Barossa Valley, SA | 1211 ± 59 | 4.17 ± 0.20 | 75.99 ± 1.17 | 21.06 ± 1.71 | 2.94 ± 1.17 |
| Pink Lady | Adelaide Hills, SA | 933 ± 3 | 3.21 ± 0.01 | 68.90 ± 0.09 | 24.38 ± 0.68 | 6.70 ± 0.09 |
| Royal Gala | Adelaide Hills, SA | n.d. | n.d. | n.d. | n.d. | n.d. |
| Bravo | Adelaide Hills, SA | 1484 ± 5 | 5.11 ± 0.02 | 80.44 ± 0.06 | 16.12 ± 0.27 | 3.43 ± 0.06 |
| Kanty | Adelaide Hills, SA | 933 ± 10 | 3.21 ± 0.04 | 68.91 ± 0.34 | 22.56 ± 0.17 | 8.52 ± 0.34 |
| Granny Smith | McLaren Vale, SA | 1487 ± 77 | 5.12 ± 0.27 | 80.43 ± 1.02 | 14.98 ± 0.66 | 4.57 ± 1.02 |
| Pomace | Adelaide Hills, SA | 1408 ± 167 | 4.75 ± 0.57 | 74.89 ± 2.61 | 17.46 ± 1.07 | 3.85 ± 2.61 |
| Pomace 1 | Packenham Upper, VIC | 1050 ± 37 | 3.61 ± 0.13 | 72.33 ± 0.98 | 25.33 ± 0.89 | 2.32 ± 0.08 |
| Pomace 1 | Illinois, USA | 816 ± 35 | 2.81 ± 0.12 | 64.38 ± 1.51 | 28.67 ± 0.21 | 6.94 ± 1.51 |
| ID 1 | Name | Formula | Molecular Weight (g·mol−1) | Retention Time (min) | Concentration Range (µg/g D.wt; Semi-Quantitative) |
|---|---|---|---|---|---|
| 27 | Catechin 2 | C15 H14 O6 | 290.08 | 13.05 | 0.08–3.01 |
| 45 | Chlorogenic acid | C16 H18 O9 | 354.10 | 17.07 | 0.60–14.35 |
| 63 | Procyanidin B2 2 | C30 H26 O12 | 578.14 | 18.93 | 0.44–21.20 |
| 73 | Phenethyl-primeveroside | C19 H28 O10 | 416.17 | 19.89 | 0.14–2.29 |
| 87 | Epicatechin | C15 H14 O6 | 290.08 | 21.30 | 1.70–19.09 |
| 156 | Quercetin-3-D-glucoside | C21 H20 O12 | 464.10 | 25.59 | 2.18–11.38 |
| 159 | Rutin | C27 H30 O16 | 610.15 | 25.67 | 0.08–2.96 |
| 170 | Phloretin | C15 H14 O5 | 274.08 | 26.22 | 0.02–2.12 |
| 190 | Afzelin | C21 H20 O10 | 432.11 | 27.17 | 0.04–1.30 |
| 192 | Quercetin | C15 H10 O7 | 302.04 | 27.87 | 0.03–6.93 |
| 195 | Luteolin | C15 H10 O6 | 286.05 | 28.84 | 0.14–0.43 |
| Apple Pomace | Source 2 | FRAP (µmol Trolox eq./g D.wt) 3 | FRAP (µmol FeSO4 eq./g D.wt) 4 |
|---|---|---|---|
| Red Delicious | Barossa Valley, SA | 34.36 ± 0.41 | 38.64 ± 0.46 |
| Red Delicious | Adelaide Hills, SA | 85.25 ± 0.57 | 95.87 ± 0.64 |
| Fuji | Barossa Valley, SA | 55.07 ± 0.89 | 61.93 ± 1.00 |
| Fuji | Barossa Valley, SA | 20.17 ± 0.25 | 22.68 ± 0.28 |
| Fuji | McLaren Vale, SA | 40.70 ± 0.42 | 45.78 ± 0.48 |
| Pink Lady | Barossa Valley, SA | 41.51 ± 1.37 | 46.68 ± 1.54 |
| Pink Lady | Adelaide Hills, SA | 53.78 ± 3.36 | 60.47 ± 3.78 |
| Royal Gala | Adelaide Hills, SA | 37.67 ± 3.73 | 42.38 ± 4.19 |
| Bravo | Adelaide Hills, SA | 48.46 ± 0.14 | 54.50 ± 0.16 |
| Kanzi | Adelaide Hills, SA | 36.90 ± 0.30 | 41.50 ± 0.33 |
| Granny Smith | McLaren Vale, SA | 53.63 ± 3.09 | 60.33 ± 3.47 |
| Pomace | Adelaide Hills, SA | 68.76 ± 2.21 | 77.33 ± 2.49 |
| Pomace 1 | Packenham Upper, VIC | 44.55 ± 0.50 | 50.11 ± 0.56 |
| Pomace 1 | Illinois, USA | 45.63 ± 0.91 | 51.32 ± 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bindon, K.; Qi, S.; Kassara, S.; Nicolotti, L.; Jouin, A.; Beer, M. Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins. Molecules 2023, 28, 5494. https://doi.org/10.3390/molecules28145494
Bindon K, Qi S, Kassara S, Nicolotti L, Jouin A, Beer M. Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins. Molecules. 2023; 28(14):5494. https://doi.org/10.3390/molecules28145494
Chicago/Turabian StyleBindon, Keren, Song Qi, Stella Kassara, Luca Nicolotti, Alicia Jouin, and Maggie Beer. 2023. "Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins" Molecules 28, no. 14: 5494. https://doi.org/10.3390/molecules28145494
APA StyleBindon, K., Qi, S., Kassara, S., Nicolotti, L., Jouin, A., & Beer, M. (2023). Apple Pomace Compositional Data Highlighting the Proportional Contribution of Polymeric Procyanidins. Molecules, 28(14), 5494. https://doi.org/10.3390/molecules28145494







