The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Hydrogen Bond Donors (HBDs) and Hydrogen Bond Acceptors (HBAs) in BNDESs
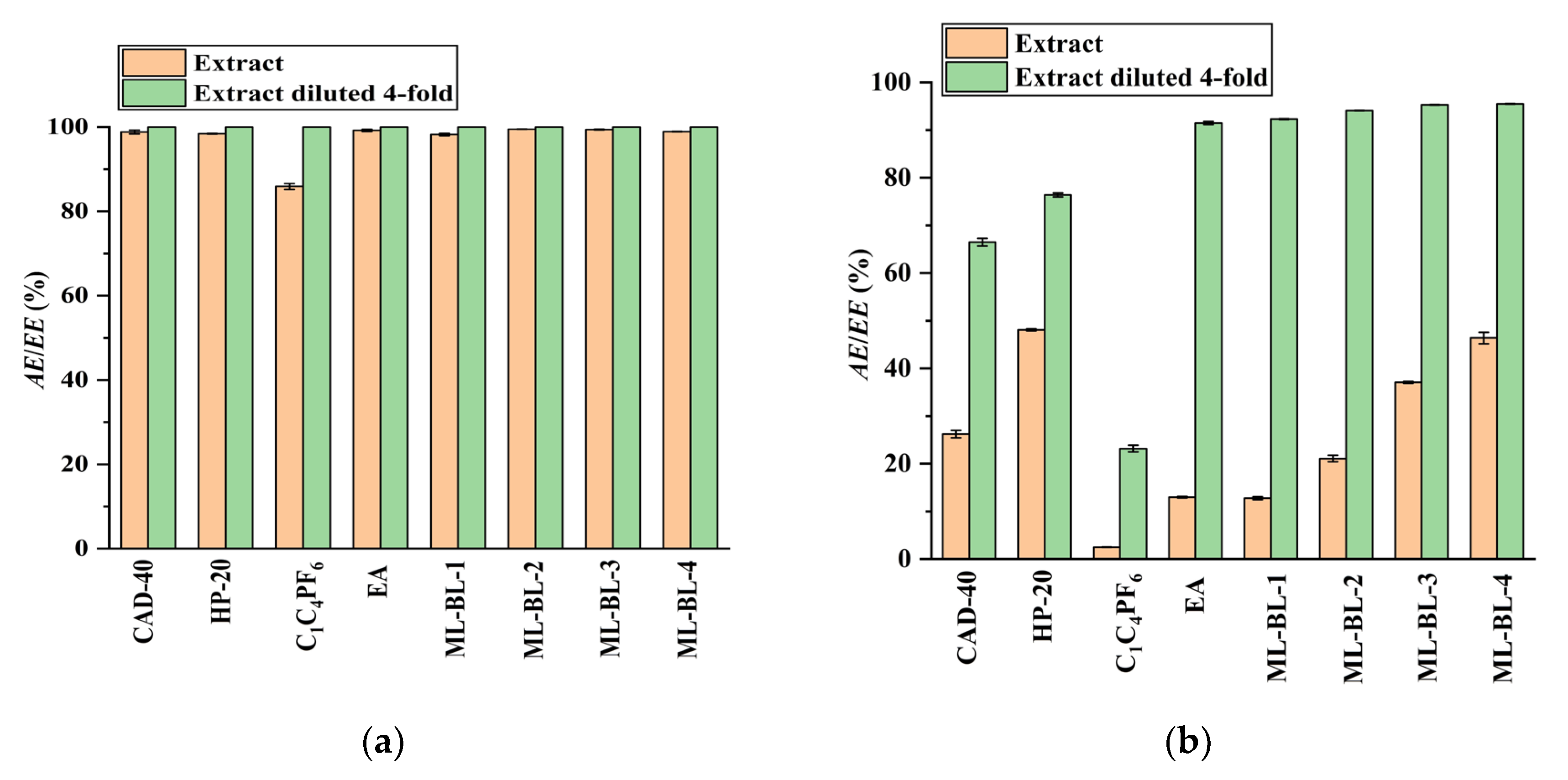
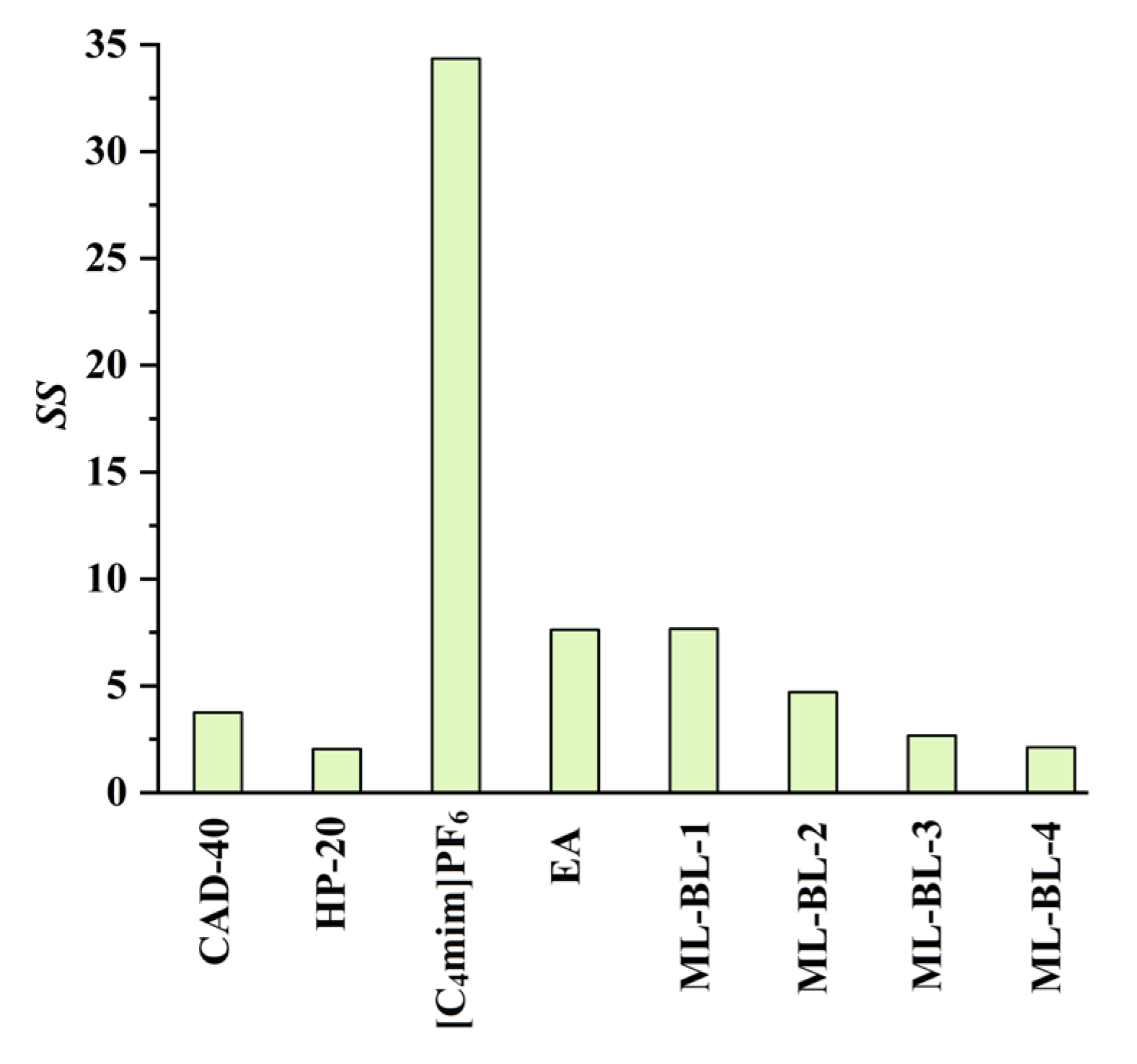
2.2. Separation and Purification of CA and RA from the PEG-400 Extract
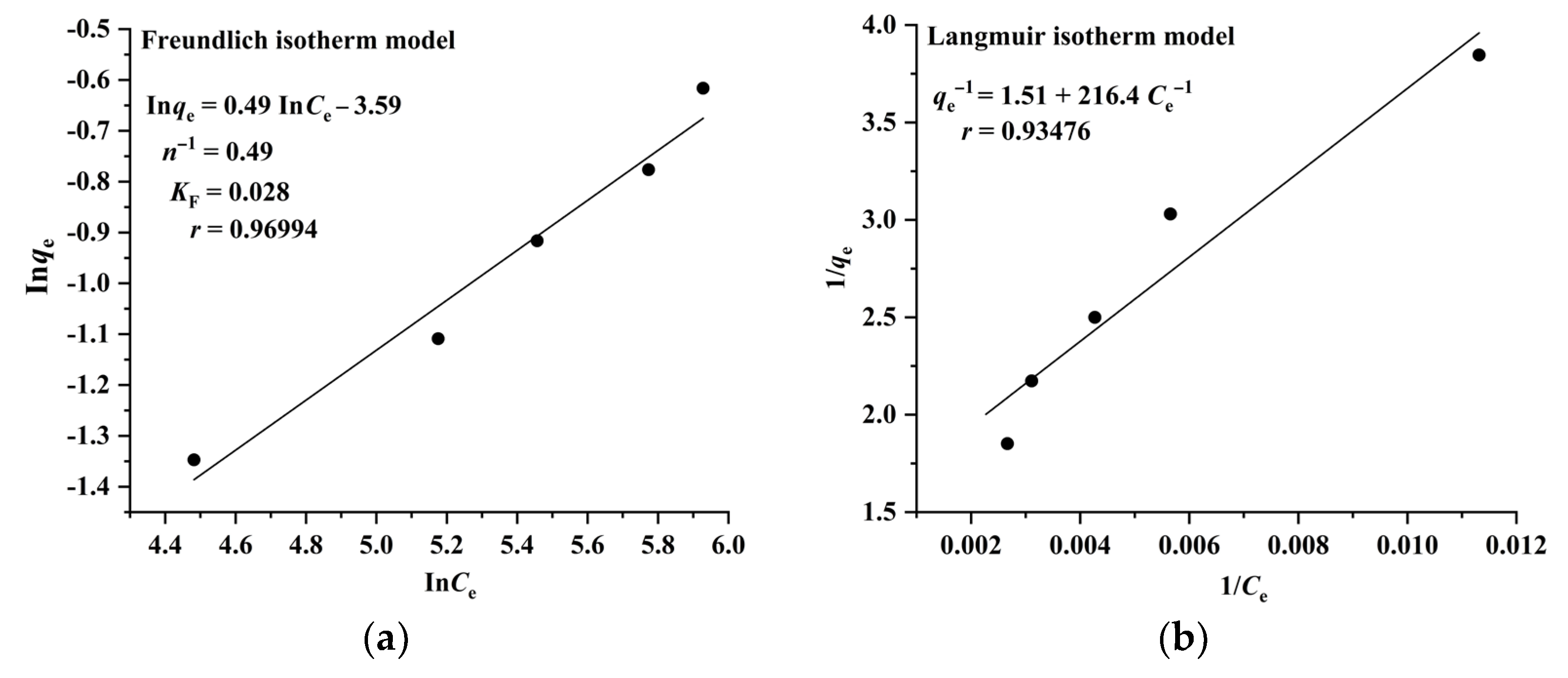
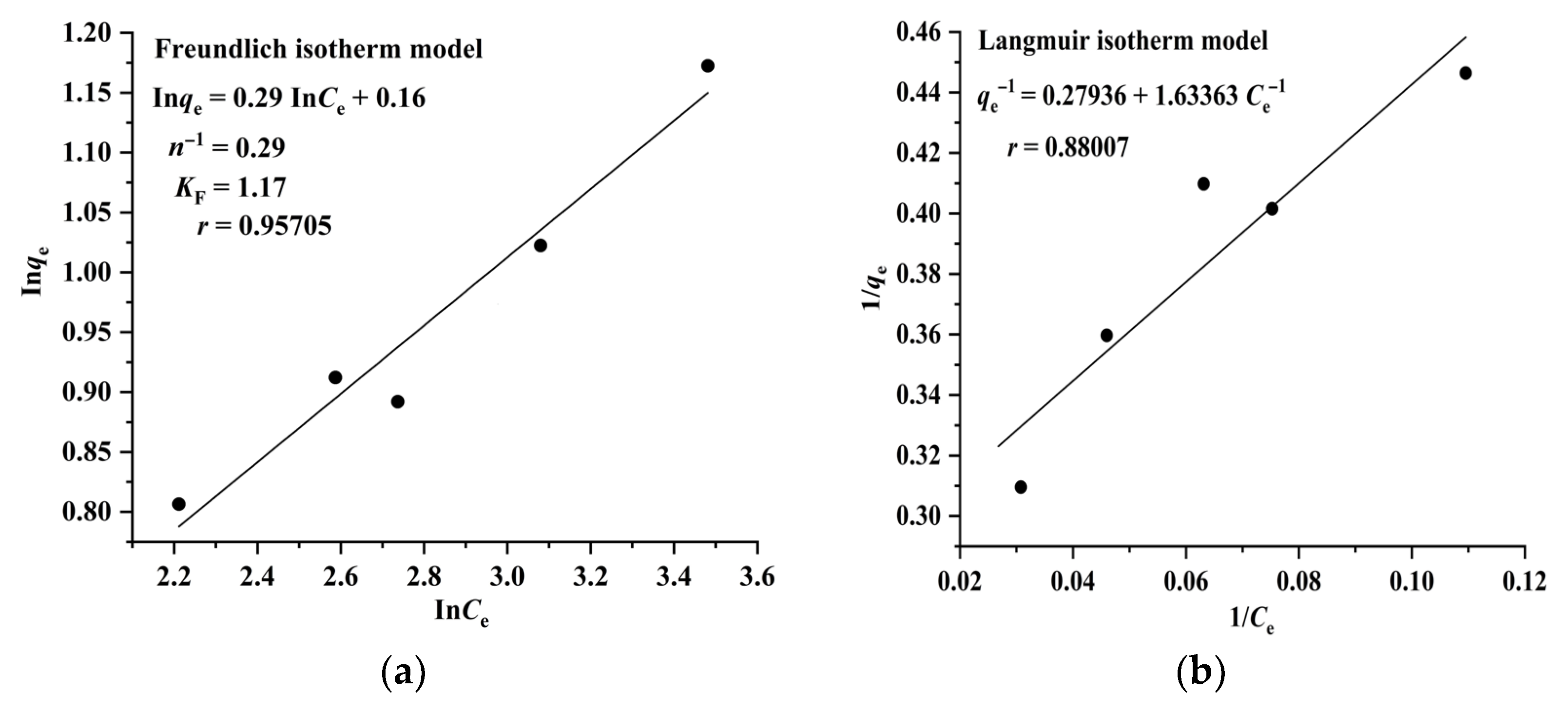
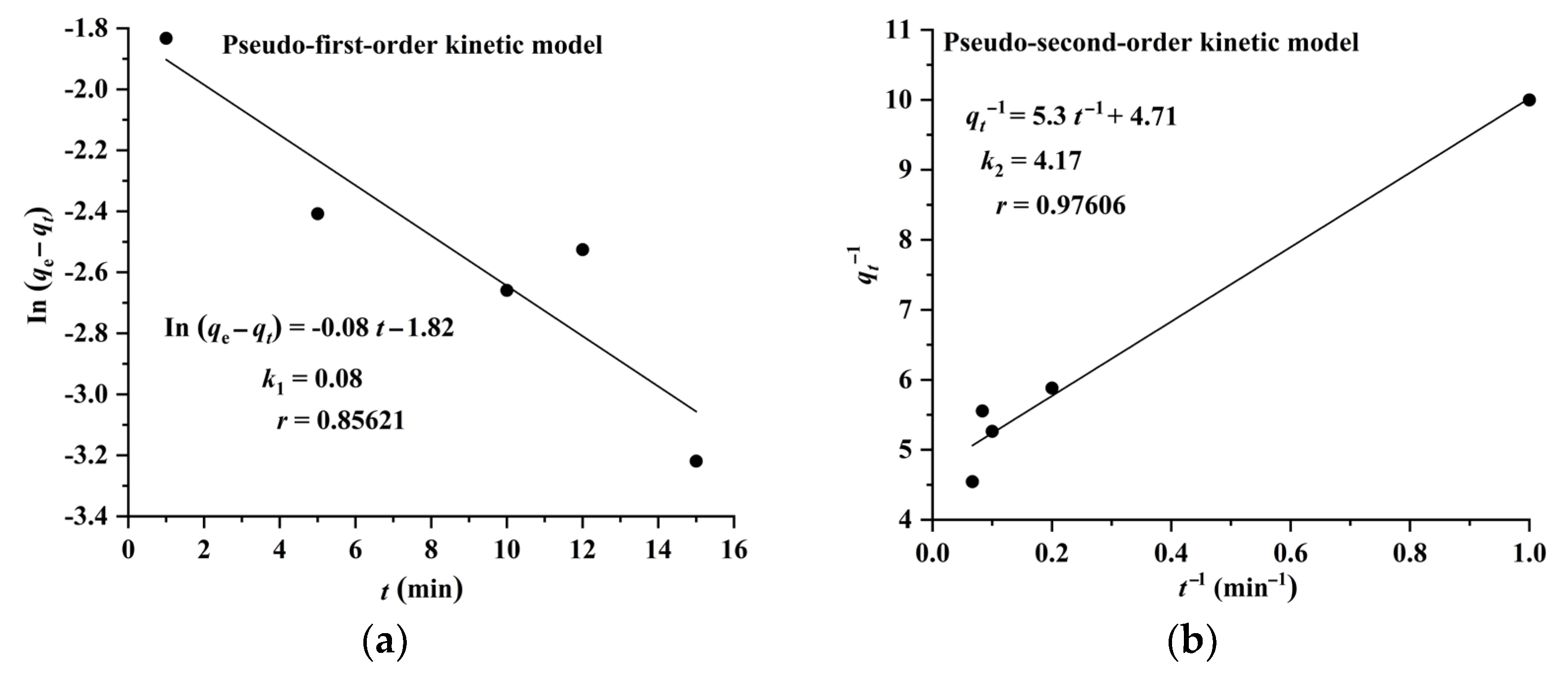
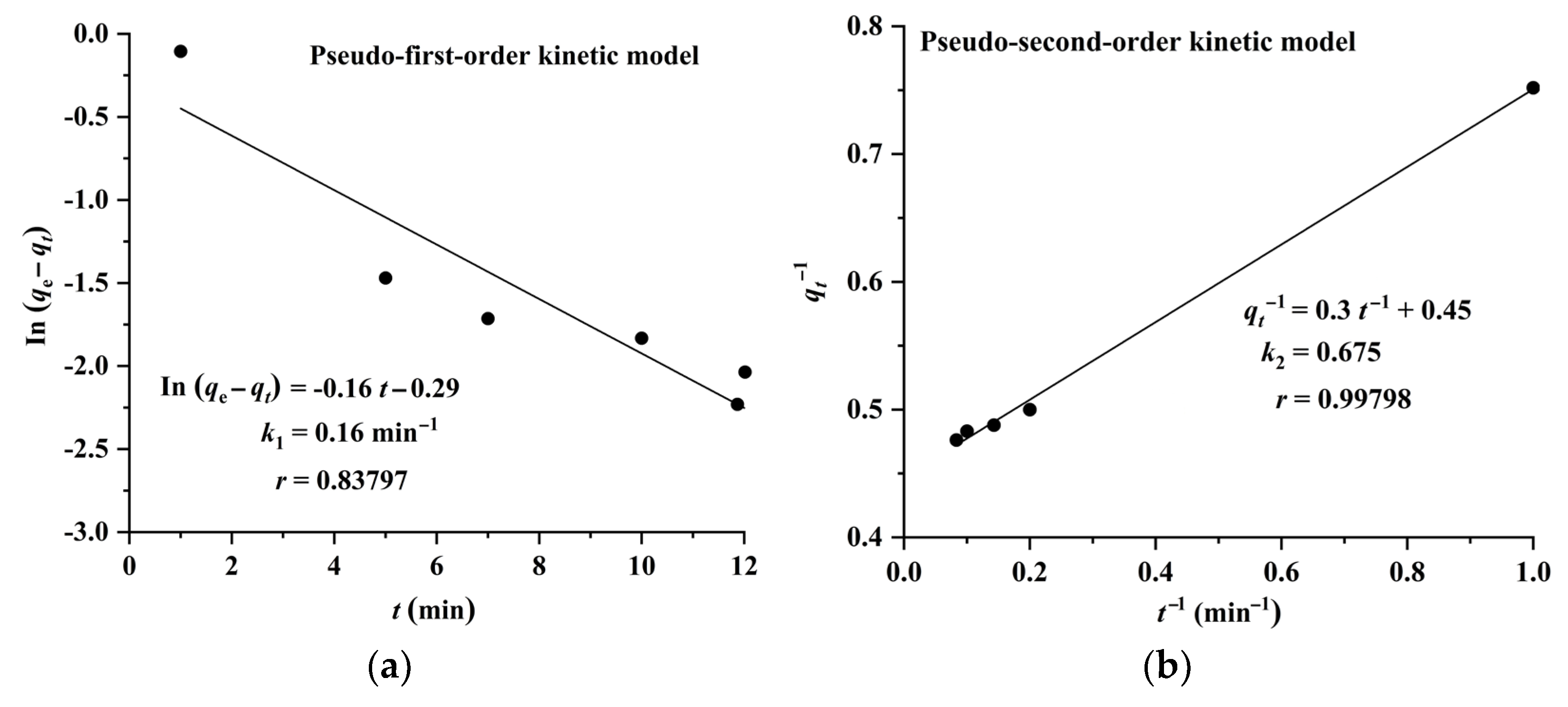
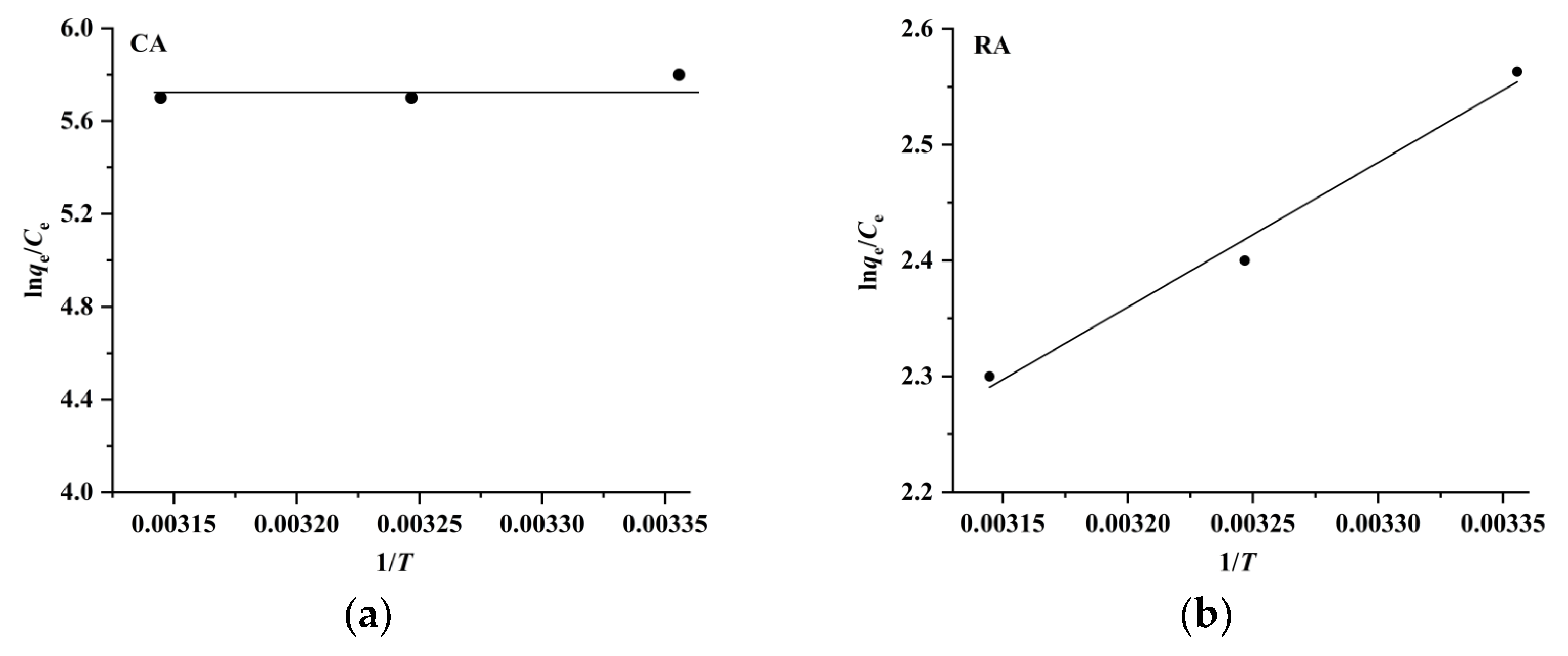
2.3. Adsorption Isotherms, Kinetics, and Thermodynamics of CA and RA on CAD-40
2.4. Comparison with the Reported Methods
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of BNDESs
3.3. Measurements of the Viscosities and Solubilities of the Synthesized BNDESs
3.4. Determination of the Kamlet–Taft Polarity Parameters of the Synthesized BNDESs
3.5. Determination of CA and RA
3.6. Adsorption of CA and RA on Macroporous Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jordán, M.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rho, S.J.; Kim, Y.R. Enhancing antioxidant and antimicrobial activity of carnosic acid in rosemary (Rosmarinus officinalis L.) extract by complexation with cyclic glucans. Food Chem. 2019, 299, 125119. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fons, L.; Garzón, M.; Micol, V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J. Agric. Food Chem. 2010, 58, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef]
- Mo, C.; Xu, Y.; Feng, Y.; Chen, A.J.; Yang, C.W.; Ni, H. Simultaneous preparation of water- and lipid-soluble antioxidant and antibacterial activity of purified carnosic acid from Rosmarinus offoccnalis L. Ind. Crops Prod. 2022, 187, 115448. [Google Scholar] [CrossRef]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Q.; Li, P.; Yang, H. Deep-eutectic solvents/ionic liquids/water mixture as a novel type of green thermo-switchable solvent system for selective extraction and separation of natural products from Rosmarinus officinalis leaves. Food Chem. 2022, 390, 133225. [Google Scholar] [CrossRef]
- Kim, S.; Yun, E.J.; Bak, J.S.; Lee, H.; Lee, S.J.; Kim, C.T.; Lee, J.H.; Kim, K.H. Response surface optimised extraction and chromatographic purification of rosmarinic acid from Melissa officinalis leaves. Food Chem. 2010, 121, 521–526. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, M.; Bai, B.Q.; Jia, H.W.; Fan, S.H. Studies on adsorption kinetics and thermodynamics of macroporous resin for rosmarinic acid. J. Oleo Sci. 2021, 70, 439–451. [Google Scholar] [CrossRef]
- Yu, M.; Wang, S.; Zhu, H.; Wang, H.; Yao, R.; Li, F.; Bian, X. In-situ reactive heat breaking cell wall by SO3 hydration: Innovative cell-wall breaking technique to enhance extraction of cinnamaldehyde from cinnamon. Prep. Biochem. Biotechnol. 2021, 51, 833–841. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Yu, W.; Jin, H.; Shen, A.; Deng, L.; Shi, J.; Xue, X.; Guo, Y.; Liu, Y.; Liang, X. Purification of high-purity glycyrrhizin from licorice using hydrophilic interaction solid phase extraction coupled with preparative reversed-phase liquid chromatography. J. Chromatogr. B 2017, 1040, 47–52. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, Y.; Bai, X. A green and effective polyethylene glycols-based microwave-assisted extraction of carnosic and rosmarinic acids from Rosmarinus officinalis leaves. Foods 2023, 12, 1761. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Browne, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM fragrance ingredient safety assessment, l-menthyl lactate, CAS registry number 59259-38-0. Food Chem. Toxicol. 2018, 115, S61–S71. [Google Scholar] [CrossRef] [PubMed]
- Bowmer, C.T.; Hooftman, R.N.; Hanstveit, A.O.; Venderbosch, P.W.M.; van der Hoeven, N. The ecotoxicity and the biodegradability of lactic acid, alkyl lactate esters and lactate salts. Chemosphere 1998, 37, 1317–1333. [Google Scholar] [CrossRef]
- Bernet, B.; Vasella, A. 1H-NMR analysis of intra- and intermolecular H-bonds of alcohols in DMSO: Chemical shift of hydroxylgroups and aspects of conformational analysis of selected monosaccharides, inositols, and ginkgolides. Helv. Chim. Acta 2000, 83, 995–1021. [Google Scholar] [CrossRef]
- Gowda, C.M.; Vasconcelos, F.; Schwartz, E.; van Eck, E.R.H.; Marsman, M.; Cornelissen, J.J.L.M.; Rowan, A.E.; de Wijs, G.A.; Kentgens, A.P.M. Hydrogen bonding and chemical shift assignments in carbazole functionalized isocyanides from solid-state NMR and firstprinciples calculations. Phys. Chem. Chem. Phys. 2011, 13, 13082–13095. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Fu, Y.; Yuan, Z.; Qin, M. Saccharide separation from wood prehydrolysis liquor: Comparison of selectivity toward non-saccharide compounds with separate techniques. RSC Adv. 2015, 5, 28925–28931. [Google Scholar] [CrossRef]
- Kovač, M.J.; Pavić, V.; Huđ, A.; Cindrić, I.; Molnar, M. Determination of suitable macroporous resins and desorbents for carnosol and carnosicacid from deep eutectic solvent sage (Salvia officinalis) extract with assessment of antiradical and antibacterial activity. Antioxidants 2021, 10, 556. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Lee, M.H.; Kim, H.T.; Seo, W.D.; Kim, J.Y.; Baek, I.Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds inthe antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cao, Y.; Chen, N.; Feng, P. Synthesis and characterization of cobalt metal organic frameworks prepared by ultrasonic wave-assisted ball milling for adsorptive removal of congo red dye from aqueous solutions. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1231–1240. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.A.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue andmalachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Micropor. Mesopor. Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Parvin, S.; Biswas, B.K.; Rahman, M.A.; Rahman, M.H.; Anik, M.S.; Uddin, M.R. Study on adsorption of congo red onto chemically modified egg shell membrane. Chemosphere 2019, 236, 124326. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ren, X.; Chen, L. Synthesis and characterization of magnetic metal–organic framework for the adsorptive removal of Rhodamine B from aqueous solution. J. Ind. Eng. Chem. 2016, 34, 278–285. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Jiang, L.; Zeng, G.; Wang, H.; Liu, Z.; Li, Y. Highly efficient adsorption of Congo red in single and binary water with cationic dyes by reduced graphene oxide decorated NH2-MIL-68(Al). J. Mol. Liq. 2017, 247, 215–229. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Wei, F.; Chen, N.; Liang, Z.; Luo, Y. Removal of Congo red dye from aqueous solution with nickel-based metal-organic framework/graphene oxide composites prepared by ultrasonic wave-assisted ball milling. Ultrason. Sonochemistry 2017, 39, 845–852. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; King, A.; Kilpeläinen, I.; Sixta, H. Role of solvent parameters in the regeneration of cellulose from ionic liquid solutions. Biomacromolecules 2012, 13, 2896–2905. [Google Scholar] [CrossRef]
- Doherty, T.V.; Mora-Pale, M.; Foley, S.E.; Linhardt, R.J.; Dordick, J.S. Ionic liquid solvent properties as predictors of lignocellulose pretreatment efficacy. Green Chem. 2010, 12, 1967–1975. [Google Scholar] [CrossRef]


| BNDES | Viscosity (mPa⋅s) | Solubility in Water (mg (100 mL)−1) | α | β | αβ |
|---|---|---|---|---|---|
| ML-BL-1 | 23.4 ± 0.21 | 908.3 ± 1.3 | 0.69 ± 0.011 | 0.64 ± 0.0086 | 0.44 ± 0.013 |
| ML-BL-2 | 12.4 ± 0.071 | 1142.7 ± 0.26 | 0.72 ± 0.0039 | 0.64 ± 0.00 | 0.46 ± 0.0025 |
| ML-BL-3 | 6.2 ± 0.014 | 1603.9 ± 0.064 | 0.74 ± 0.010 | 0.64 ± 0.017 | 0.47 ± 0.019 |
| ML-BL-4 | 5.0 ± 0.0071 | 1674.1 ± 0.064 | 0.74 ± 0.0052 | 0.65 ± 0.0086 | 0.48 ± 0.0097 |
| Target Compound | T (K) | ∆G (kJ mol–1) | ∆H (kJ mol–1) | ∆S (J mol–1 K–1) |
|---|---|---|---|---|
| CA | 288 | −14.1 | 0 | 47.4 |
| 298 | −14.6 | |||
| 308 | −15.1 | |||
| RA | 288 | −6.3 | −10.4 | −13.6 |
| 298 | −6.2 | |||
| 308 | −6.1 |
| Separation Method | Extraction/Adsorption Efficiency | Purity | Reference |
|---|---|---|---|
| CCE | 96% for CA and 94% for RA | 6.84% for RA; 31.18% for CA | [5] |
| Supramolecular formation-LLE | – a | 58.4–67.4% for RA | [6] |
| LLE composed of ChCl-LA/[C4mim]PF6/H2O | 97.46% for CA; 88.97% for RA | – | [7] |
| CC with Sephadex LH-20 as the stationary phase | – | 38.8% for RA | [8] |
| CC with NK-109 macroporous resin as the stationary phase | 68.3% for RA | 42.1% for RA | [9] |
| SPE with CAD-40 macroporous resin as the adsorbent coupled with LLE, using EA as the extraction solvent | 98.8% for CA; 91.5% for RA | 76.8% for CA; 56.3% for RA | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Fan, Y.; Wu, H. The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study. Molecules 2023, 28, 5493. https://doi.org/10.3390/molecules28145493
Zhu C, Fan Y, Wu H. The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study. Molecules. 2023; 28(14):5493. https://doi.org/10.3390/molecules28145493
Chicago/Turabian StyleZhu, Chunyan, Yunchang Fan, and Hongwei Wu. 2023. "The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study" Molecules 28, no. 14: 5493. https://doi.org/10.3390/molecules28145493
APA StyleZhu, C., Fan, Y., & Wu, H. (2023). The Selective Separation of Carnosic Acid and Rosmarinic Acid by Solid-Phase Extraction and Liquid–Liquid Extraction: A Comparative Study. Molecules, 28(14), 5493. https://doi.org/10.3390/molecules28145493






