Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent
Abstract
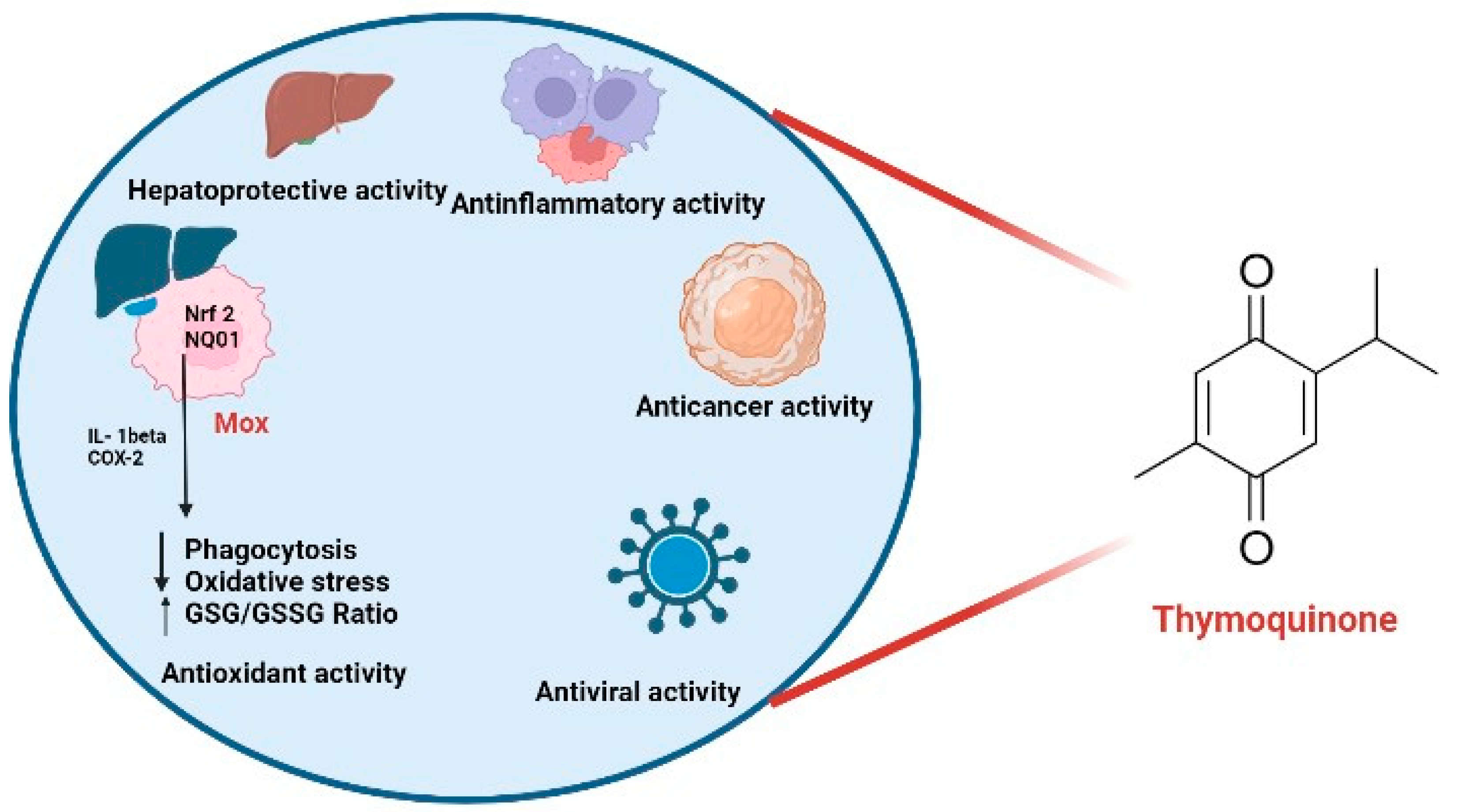
1. An Introduction to Thymoquinone and Viral Diseases
2. The Immune Mechanism in Viral Disease
3. Antiviral Properties and Mechanisms of Action of Thymoquinone in Cellular and Molecular Aspects
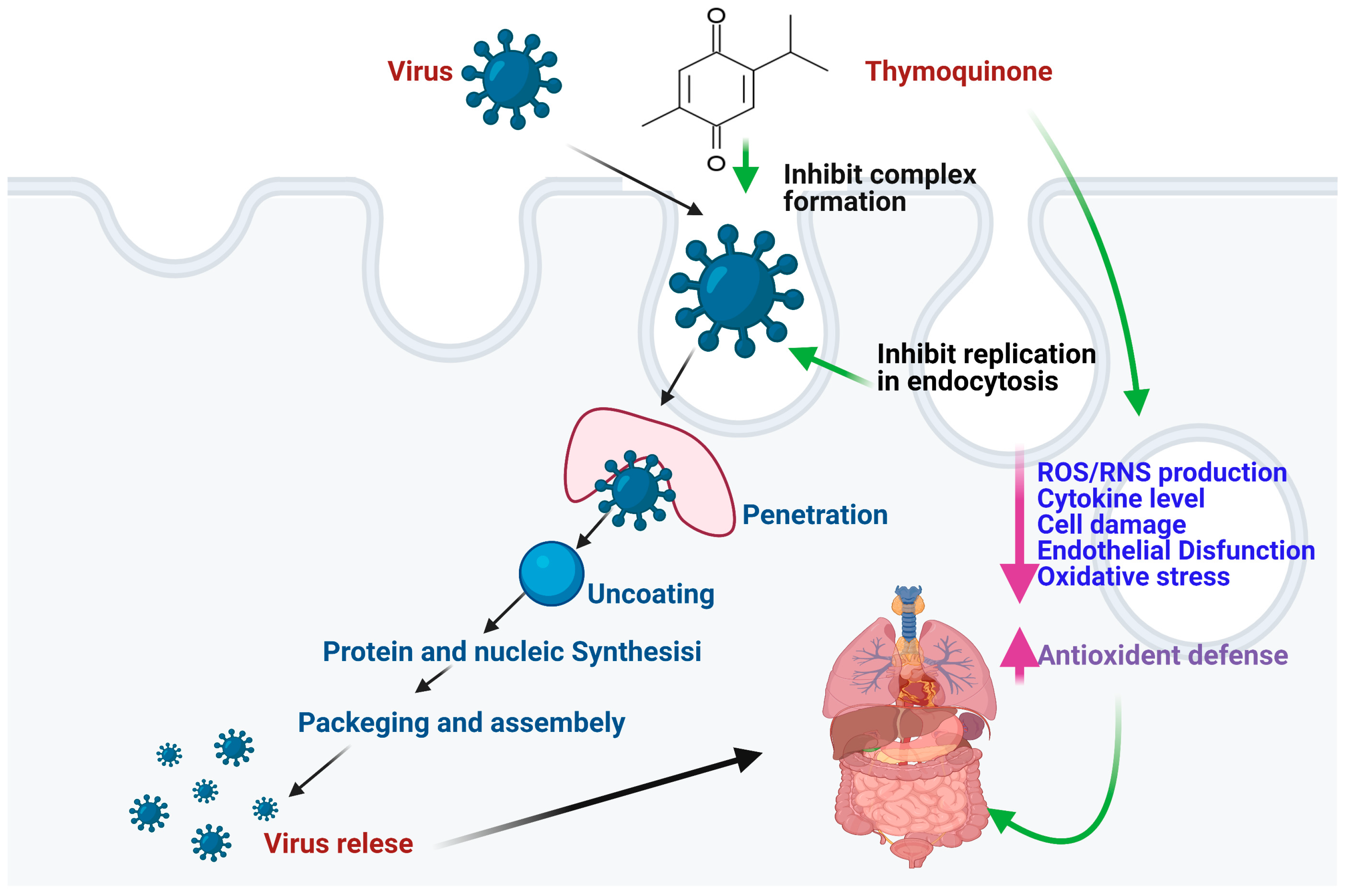
3.1. Antiviral Properties
3.2. Mechanisms of Action
4. Pharmacological Applications of Thymoquinone as an Antiviral Agent
5. Biopharmaceutical Problems of Thymoquinone
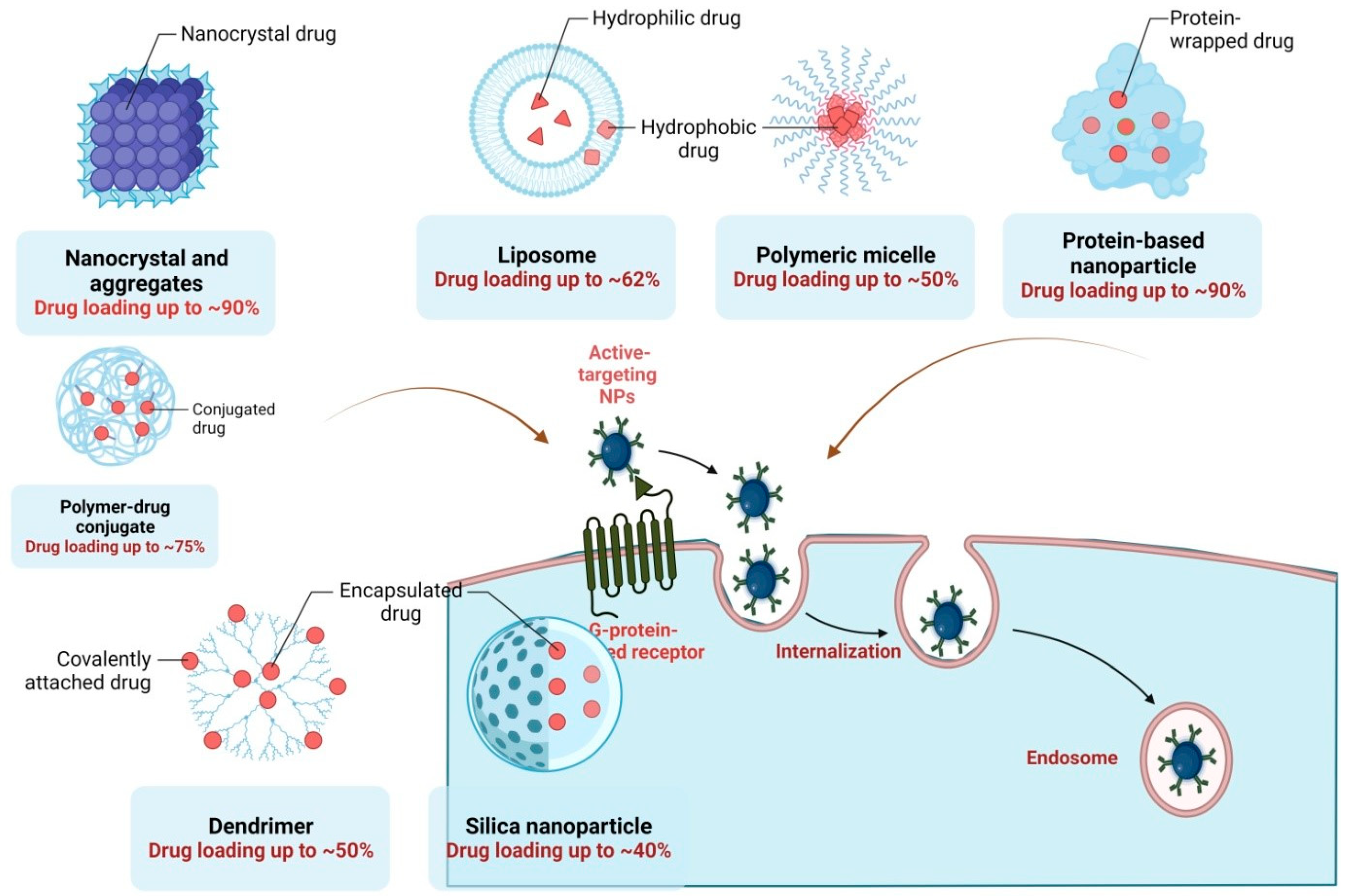
6. Nanotechnological Approaches of Thymoquinone for Effective Antiviral Therapy
7. Current Clinical Trials and Patents of Thymoquinone with Antiviral Potency
8. Advancements in the Field
9. Future Prospects
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Bibi, S. Heavy metal, waste, COVID-19, and rapid industrialization in this modern era—Fit for sustainable future. Sustainability 2022, 14, 4746. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The logic of virus evolution. Cell Host Microbe 2022, 30, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Dessie, Z.G.; Noreddin, A.; El Zowalaty, M.E. Systematic review of important viral diseases in Africa in light of the ‘one health’concept. Pathogens 2020, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaraj, G.; Vaithyasubramanian, S. A study on contagious viruses based on mortality of statistical data of pandemic. AIP Conf. Proc. 2022, 2516, 340013. [Google Scholar]
- Amber, R.; Adnan, M.; Tariq, A.; Mussarat, S. A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. J. Pharm. Pharmacol. 2017, 69, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.L.; Hossain, M.S. Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. Int. J. Immunopharmacol. 2000, 22, 729–740. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Goharshadi, K.; Moghayedi, M. The use of nanotechnology in the fight against viruses: A critical review. Coord. Chem. Rev. 2022, 464, 214559. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z. Prevention and treatment of viral respiratory infections by traditional Chinese herbs. Chin. Med. J. 2014, 127, 1344–1350. [Google Scholar]
- Adamson, C.S.; Chibale, K.; Goss, R.J.; Jaspars, M.; Newman, D.J.; Dorrington, R.A. Antiviral drug discovery: Preparing for the next pandemic. Chem. Soc. Rev. 2021, 50, 3647–3655. [Google Scholar] [CrossRef]
- Friedrich, M. WHO’s top health threats for 2019. JAMA 2019, 321, 1041. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Wahab, S.; Ahmad, F.A.; Alam, M.I.; Ather, H.; Siddiqua, A.; Ashraf, S.A.; Shaphe, M.A.; Khan, M.I.; Beg, R.A. A novel perspective approach to explore pros and cons of face mask in prevention the spread of SARS-CoV-2 and other pathogens. Saudi Pharm. J. 2021, 29, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Azmi, L.; Shukla, I.; Alqahtani, S.S.; Alsarra, I.A.; Shakeel, F. Properties of ethnomedicinal plants and their bioactive compounds: Possible use for COVID-19 prevention and treatment. Curr. Pharm. Des. 2021, 27, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Beshbishy, A.M.; Kifayatullah, M.; Olukanni, A.; Zahoor, M.; Naeem, M.; Amin, M.; Shah, M.; Abdelaziz, A.S.; Ullah, R.; et al. Chemotherapeutic Potential of Carthamus Oxycantha Root Extract as Antidiarrheal and In Vitro Antibacterial Activities. Antibiotics 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.A.; Bajwa, N.; Baldi, A. Possible role of traditional systems of medicine to manage COVID-19: A review. Isr. J. Plant Sci. 2021, 68, 3–28. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Azad, Z.; Ahmad, A.; Alam, M.I.; Ansari, J.A.; Panda, B.P. Edible mushrooms as health promoting agent. Adv. Sci. Focus 2013, 1, 189–196. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Fayyad, M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015, 28, 295–304. [Google Scholar] [CrossRef]
- Barrett, B.; Kiefer, D.; Rabago, D. Assessing the risks and benefits of herbal medicine: An overview of scientific evidence. Altern. Ther. Health Med. 1999, 5, 40. [Google Scholar]
- Odeh, F.; Ismail, S. Liposomal Formulations Comprising Thymoquinone and Taxane, and Method of Treating Cancer Using Same. WO2016005786A1, 14 January 2016. [Google Scholar]
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An updated knowledge of Black seed (Nigella sativa Linn.): Review of phytochemical constituents and pharmacological properties. J. Herb. Med. 2021, 25, 100404. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (black cumin): A promising natural remedy for wide range of illnesses. Evid.-Based Complement. Altern. Med. 2019, 2019, 1528635. [Google Scholar] [CrossRef]
- Tavakkoli, A.; Ahmadi, A.; Razavi, B.M.; Hosseinzadeh, H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran. J. Pharm. Res. IJPR 2017, 16, 2. [Google Scholar]
- Barakat, E.M.F.; El Wakeel, L.M.; Hagag, R.S. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J. Gastroenterol. WJG 2013, 19, 2529. [Google Scholar] [CrossRef] [PubMed]
- Onifade, A.A.; Jewell, A.P.; Adedeji, W.A. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Onifade, A.A.; Jewell, A.; Okesina, A. Seronegative conversion of an HIV positive subject treated with Nigella sativa and honey. Afr. J. Infect. Dis. 2015, 9, 47–50. [Google Scholar] [CrossRef]
- Dajani, E.; Shahwan, T.; Dajani, N. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): A complementary drug with historical and clinical significance. J. Physiol. Pharm. 2016, 67, 801–817. [Google Scholar]
- Ahmed, S.I.; Jamil, S.; Ismatullah, H.; Hussain, R.; Bibi, S.; Khandaker, M.U.; Naveed, A.; Idris, A.M.; Emran, T.B. A Comprehensive Perspective of Traditional Arabic or Islamic Medicinal Plants as an Adjuvant Therapy against COVID-19. Saudi J. Biol. Sci. 2023, 30, 103561. [Google Scholar] [CrossRef]
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (α/β) in immunity and autoimmunity. Annu. Rev. Immunol. 2005, 23, 307–335. [Google Scholar] [CrossRef]
- Tal, M.C.; Sasai, M.; Lee, H.K.; Yordy, B.; Shadel, G.S.; Iwasaki, A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 2770–2775. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef]
- Schulz, O.; Diebold, S.S.; Chen, M.; Näslund, T.I.; Nolte, M.A.; Alexopoulou, L.; Azuma, Y.-T.; Flavell, R.A.; Liljeström, P.; Reis e Sousa, C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J. Clin. Investig. 2009, 119, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Catalfamo, M.; Karpova, T.; McNally, J.; Costes, S.V.; Lockett, S.J.; Bos, E.; Peters, P.J.; Henkart, P.A. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity 2004, 20, 219–230. [Google Scholar] [CrossRef]
- Favre, D.; Lederer, S.; Kanwar, B.; Ma, Z.-M.; Proll, S.; Kasakow, Z.; Mold, J.; Swainson, L.; Barbour, J.D.; Baskin, C.R. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009, 5, e1000295. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, H.; Zheng, X.; Chen, J.; Zhang, S.; Wu, J. Recent advances and emerging trends in antiviral defense networking in rice. Crop J. 2021, 9, 553–563. [Google Scholar] [CrossRef]
- Ryan, P.M.; Caplice, N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef]
- Farzi, R.; Aghbash, P.S.; Eslami, N.; Azadi, A.; Shamekh, A.; Hemmat, N.; Entezari-Maleki, T.; Baghi, H.B. The role of antigen-presenting cells in the pathogenesis of COVID-19. Pathol. Res. Pract. 2022, 233, 153848. [Google Scholar] [CrossRef]
- Ravetch, J. In vivo veritas: The surprising roles of Fc receptors in immunity. Nat. Immunol. 2010, 11, 183–185. [Google Scholar] [CrossRef]
- Brandstadter, J.D.; Yang, Y. Natural killer cell responses to viral infection. J. Innate Immun. 2011, 3, 274–279. [Google Scholar] [CrossRef]
- Lee, S.-H.; Miyagi, T.; Biron, C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007, 28, 252–259. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Dalet, A.; Gatti, E.; Pierre, P. Integration of PKR-dependent translation inhibition with innate immunity is required for a coordinated anti-viral response. FEBS Lett. 2015, 589, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.G.; Trifilo, M.J.; Edelmann, K.H.; Teyton, L.; McGavern, D.B.; Oldstone, M.B. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 2006, 12, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Zaher, K.S.; Ahmed, W.; Zerizer, S.N. Observations on the biological effects of black cumin seed (Nigella sativa) and green tea (Camellia sinensis). Glob. Vet. 2008, 2, 198–204. [Google Scholar]
- Fröhlich, T.; Reiter, C.; Saeed, M.E.; Hutterer, C.; Hahn, F.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Marschall, M.; Efferth, T. Synthesis of thymoquinone–artemisinin hybrids: New potent antileukemia, antiviral, and antimalarial agents. ACS Med. Chem. Lett. 2017, 9, 534–539. [Google Scholar]
- Karagöz, A.Ç.; Reiter, C.; Seo, E.-J.; Gruber, L.; Hahn, F.; Leidenberger, M.; Klein, V.; Hampel, F.; Friedrich, O.; Marschall, M. Access to new highly potent antileukemia, antiviral and antimalarial agents via hybridization of natural products (homo) egonol, thymoquinone and artemisinin. Bioorg. Med. Chem. 2018, 26, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Shah, M.; Munir, M.; Yaqoob, M.; Fiaz, M.; Anjum, S.; Kaboudi, K.; Bouzouaia, M.; Younus, M.; Nisa, Q. RETRACTED: Synergistic effects of thymoquinone and curcumin on immune response and anti-viral activity against avian influenza virus (H9N2) in turkeys. Poult. Sci. 2016, 95, 1513–1520. [Google Scholar] [CrossRef]
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Mol. Biol. Rep. 2014, 41, 1703–1711. [Google Scholar] [CrossRef]
- Ahmad, S.; Abbasi, H.W.; Shahid, S.; Gul, S.; Abbasi, S.W. Molecular docking, simulation and MM-PBSA studies of nigella sativa compounds: A computational quest to identify potential natural antiviral for COVID-19 treatment. J. Biomol. Struct. Dyn. 2021, 39, 4225–4233. [Google Scholar] [CrossRef]
- Elfiky, A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2021, 39, 3194–3203. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Mozafari, M. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Ahmad, A.; Jan, B.L.; Raish, M. Thymoquinone reduces mortality and suppresses early acute inflammatory markers of sepsis in a mouse model. Biomed. Pharmacother. 2018, 98, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, I.; Irfan, S.; Ahmad, M.F.; Usmani, S.; Shoaib, A.; Ahmad, W. Hydrogel: An Encouraging Nanocarrier System for the Delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 17, 797–807. [Google Scholar] [CrossRef]
- Sommer, A.P.; Försterling, H.-D.; Naber, K.G. Thymoquinone: Shield and sword against SARS-CoV-2. Precis. Nanomed. 2020, 3, 541–548. [Google Scholar] [CrossRef]
- Liu, S.F.; Malik, A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef]
- Bouchentouf, S.; Missoum, N. Identification of Compounds from Nigella Sativa as New Potential Inhibitors of 2019 Novel Coronasvirus (COVID-19): Molecular Docking Study. Available online: https://www.preprints.org/manuscript/202004.0079/v1 (accessed on 9 July 2023).
- Omar, S.; Bouziane, I.; Bouslama, Z.; Djemel, A. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: Quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Feng, Z.; Diao, B.; Wang, R.; Wang, G.; Wang, C.; Tan, Y.; Liu, L.; Wang, C.; Liu, Y.; Liu, Y. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv 2020. [Google Scholar] [CrossRef]
- Peterhans, E. Oxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulation. J. Nutr. 1997, 127, 962S–965S. [Google Scholar] [CrossRef]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Chu, D.T.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef]
- Aqil, K.; Aslam, A.; Javeed, A.; Qayyum, R.; Yousaf, F.; Yasmeen, F.; Sohail, M.L.; Umar, S. In vitro antiviral activity of Nigella sativa against Peste des Petits Ruminants (PPR) virus. Pak. J. Zool. 2018, 50, 2223. [Google Scholar] [CrossRef]
- Seadawy, M.G.; Gad, A.F.; Elhoseny, M.; ELharty, B.E.; Shamel, M.; Elfiky, A.A.; Ahmed, A.; Zekri, A.R.N. In vitro: Natural compounds (Thymol, Carvacrol, Hesperidine, and Thymoquinone) against Sars-Cov2 strain isolated from Egyptian patients. bioRxiv 2020. [Google Scholar] [CrossRef]
- Baghdadi, H.B.; Al-Mathal, E.M. Anti-coccidial activity of Nigella sativa L. J. Food Agric. Environ. 2011, 9, 10–17. [Google Scholar]
- Kadil, Y.; Mouhcine, M.; Filali, H. In silico investigation of the SARS CoV2 protease with thymoquinone, the Major Constituent of Nigella Sativa. Curr. Drug Discov. Technol. 2021, 18, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, W.; Alhazmi, H.A.; Patel, K.S.; Mangla, B.; Al Bratty, M.; Javed, S.; Najmi, A.; Sultan, M.H.; Makeen, H.A.; Khalid, A. Recent Advancements in the diagnosis, prevention, and prospective drug therapy of COVID-19. Front. Public Health 2020, 8, 384. [Google Scholar] [CrossRef]
- Goyal, S.N.; Prajapati, C.P.; Gore, P.R.; Patil, C.R.; Mahajan, U.B.; Sharma, C.; Talla, S.P.; Ojha, S.K. Therapeutic potential and pharmaceutical development of thymoquinone: A multitargeted molecule of natural origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef]
- Negi, P.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O.P. Thymoquinone a potential therapeutic molecule from the plant Nigella sativa: Role of colloidal carriers in its effective delivery. Recent Pat. Drug Deliv. Formul. 2018, 12, 3–22. [Google Scholar] [CrossRef]
- Rathore, C.; Rathbone, M.J.; Chellappan, D.K.; Tambuwala, M.M.; Pinto, T.D.J.A.; Dureja, H.; Hemrajani, C.; Gupta, G.; Dua, K.; Negi, P. Nanocarriers: More than tour de force for thymoquinone. Expert Opin. Drug Deliv. 2020, 17, 479–494. [Google Scholar] [CrossRef]
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-lamellar liquid crystalline nanocarriers for thymoquinone encapsulation. Molecules 2019, 25, 16. [Google Scholar] [CrossRef]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef]
- Gurunathan, S.; Qasim, M.; Choi, Y.; Do, J.T.; Park, C.; Hong, K.; Kim, J.-H.; Song, H. Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials 2020, 10, 1645. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- Kerry, R.G.; Malik, S.; Redda, Y.T.; Sahoo, S.; Patra, J.K.; Majhi, S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 196–220. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.T.N.; Nam, V.N.; Nhan, T.T.; Ngoc, T.T.B.; Minh, L.Q.; Nga, B.T.T.; Quang, D.V. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express 2020, 6, 1250g1259. [Google Scholar]
- Qin, T.; Ma, R.; Yin, Y.; Miao, X.; Chen, S.; Fan, K.; Xi, J.; Liu, Q.; Gu, Y.; Yin, Y. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 2019, 9, 6920–6935. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Maduray, K.; Parboosing, R. Metal nanoparticles: A promising treatment for viral and arboviral infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, M.; Alrobaian, M.M.; Alghamdi, S.A.; Warsi, M.H.; Sultana, S.; Khan, R.A. Brain targeted Polysorbate-80 coated PLGA thymoquinone nanoparticles for the treatment of Alzheimer’s disease, with biomechanistic insights. J. Drug Deliv. Sci. Technol. 2021, 61, 102214. [Google Scholar] [CrossRef]
- El-far, A.H.; Al Jaouni, S.K.; Li, W.; Mousa, S.A. Protective roles of thymoquinone nanoformulations: Potential nanonutraceuticals in human diseases. Nutrients 2018, 10, 1369. [Google Scholar] [CrossRef]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-based antidiabetic nanoformulations: The emerging paradigm for effective therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef]
- Shende, P.; Mallick, C. Nanonutraceuticals: A way towards modern therapeutics in healthcare. J. Drug Deliv. Sci. Technol. 2020, 58, 101838. [Google Scholar] [CrossRef]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS Pharmscitech 2016, 17, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Raish, M.; Ahmed, A.; Alkharfy, K.M.; Mohsin, K.; Alshamsan, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Shakeel, F. Oral bioavailability enhancement and hepatoprotective effects of thymoquinone by self-nanoemulsifying drug delivery system. Mater. Sci. Eng. C 2017, 76, 319–329. [Google Scholar] [CrossRef]
- Vignesh Kumar, S.K.; Renuka Devi, P.; Harish, S.; Hemananthan, E. Synthesis and characterisation of peg modified chitosan nanocapsules loaded with thymoquinone. IET Nanobiotechnol. 2017, 11, 104–112. [Google Scholar] [CrossRef]
- Goleva, T.; Rogov, A.; Korshunova, G.; Trendeleva, T.; Mamaev, D.; Aliverdieva, D.; Zvyagilskaya, R. SkQThy, a novel and promising mitochondria-targeted antioxidant. Mitochondrion 2019, 49, 206–216. [Google Scholar] [CrossRef]
- Al-Qubaisi, M.S.; Rasedee, A.; Flaifel, M.H.; Eid, E.E.; Hussein-Al-Ali, S.; Alhassan, F.H.; Salih, A.M.; Hussein, M.Z.; Zainal, Z.; Sani, D. Characterization of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex: Application to anti-allergy properties. Eur. J. Pharm. Sci. 2019, 133, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Zakarial Ansar, F.H.; Latifah, S.Y.; Wan Kamal, W.H.B.; Khong, K.C.; Ng, Y.; Foong, J.N.; Gopalsamy, B.; Ng, W.K.; How, C.W.; Ong, Y.S. Pharmacokinetics and biodistribution of thymoquinone-loaded nanostructured lipid carrier after oral and intravenous administration into rats. Int. J. Nanomed. 2020, 2020, 7703–7717. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Vasarri, M.; Marroncini, G.; Barletta, E.; Degl’Innocenti, D. Thymoquinone-loaded soluplus®-solutol® HS15 mixed micelles: Preparation, in vitro characterization, and effect on the SH-SY5Y cell migration. Molecules 2020, 25, 4707. [Google Scholar] [CrossRef]
- AbouAitah, K.; Lojkowski, W. Delivery of natural agents by means of mesoporous silica nanospheres as a promising anticancer strategy. Pharmaceutics 2021, 13, 143. [Google Scholar] [CrossRef]
- AbouAitah, K.; Swiderska-Sroda, A.; Kandeil, A.; Salman, A.M.; Wojnarowicz, J.; Ali, M.A.; Opalinska, A.; Gierlotka, S.; Ciach, T.; Lojkowski, W. Virucidal action against avian influenza H5N1 virus and immunomodulatory effects of nanoformulations consisting of mesoporous silica nanoparticles loaded with natural prodrugs. Int. J. Nanomed. 2020, 15, 5181–5202. [Google Scholar] [CrossRef]
- Rathore, C.; Upadhyay, N.; Kaundal, R.; Dwivedi, R.; Rahatekar, S.; John, A.; Dua, K.; Tambuwala, M.M.; Jain, S.; Chaudari, D. Enhanced oral bioavailability and hepatoprotective activity of thymoquinone in the form of phospholipidic nano-constructs. Expert Opin. Drug Deliv. 2020, 17, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Zameer, S.; Najmi, A.K.; Ahmad, F.J.; Imam, S.S.; Akhtar, M. Thymoquinone loaded solid lipid nanoparticles demonstrated antidepressant-like activity in rats via indoleamine 2, 3-dioxygenase pathway. Drug Res. 2020, 70, 206–213. [Google Scholar] [CrossRef]
- Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.-R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.; Rasti, B. Nanoliposomes and tocosomes as multifunctional nanocarriers for the encapsulation of nutraceutical and dietary molecules. Molecules 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Sarieddine, R.; Alwattar, J.K.; Chouaib, R.; Gali-Muhtasib, H. Anticancer activity of thymoquinone cubic phase nanoparticles against human breast cancer: Formulation, cytotoxicity and subcellular localization. Int. J. Nanomed. 2020, 15, 9557–9570. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, S.; Ansari, M.A.; Kandasamy, G.; Vasudevan, R.; Hani, U.; Chauhan, W.; Alhumaidi, M.S.; Altammar, K.A.; Azmi, S.; Ahmad, W. An Attention towards the Prophylactic and Therapeutic Options of Phytochemicals for SARS-CoV-2: A Molecular Insight. Molecules 2023, 28, 795. [Google Scholar] [CrossRef]
- Gamaleldin, M.; Nashat, S. Impact of Different Treatment Modalities on Immunity against COVID-19. 2020. Available online: https://osf.io/u56fc/ (accessed on 9 July 2023).
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef]
- Banerjee, S.; Kaseb, A.O.; Wang, Z.; Kong, D.; Mohammad, M.; Padhye, S.; Sarkar, F.H.; Mohammad, R.M. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009, 69, 5575–5583. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Akhondian, J.; Kianifar, H.; Raoofziaee, M.; Moayedpour, A.; Toosi, M.B.; Khajedaluee, M. The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Res. 2011, 93, 39–43. [Google Scholar] [CrossRef]
- Halwani, M.A.; Balkhy, H.H. Nano-Liposomal Aminoglycoside-Thymoquinone Formulations. WO2016061117, 21 April 2016.
- Greaves, J.A.; Narasimhamoorthy, B.; Srivastava, V.; Cloud, N.; Clark, D. Method for Production of Thymoquinone. US9745242B1, 29 August 2017. [Google Scholar]
- Kim, M.; Park, M.; Kim, T.; Lee, H. Pharmaceutical Composition for Treating or Preventing Neurological Disorders Caused by Alcohol Exposure during Pregnancy, Containing Metformin and/or Thymoquinone. WO2013172537A1, 21 November 2013. [Google Scholar]
- Available online: https://patents.google.com/patent/KR101493139B1/en (accessed on 9 July 2023).
- Available online: https://patents.google.com/patent/CN111000836B/en (accessed on 9 July 2023).
- Alkharfy, K.M.; Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S. Protective Effect of Thymoquinone in Sepsis. US8895625B2, 25 November 2014. Available online: https://patents.google.com/patent/US8895625B2/en (accessed on 9 July 2023).
- Crede, P. Compositions Comprising Thymoquinone for the Treatment of Inflammatory Diseases. 2013. Available online: https://patents.google.com/patent/WO2013030669A4/en?q=(Compositions+comprising+thymoquinone+for+the+treatment+of+inflammatory+diseases)&oq=Compositions+comprising+thymoquinone+for+the+treatment+of+inflammatory+diseases (accessed on 9 July 2023).
- Fahmy, H.M.; Khadrawy, Y.A.; Abd-El Daim, T.M.; Elfeky, A.S.; Abd Rabo, A.A.; Mustafa, A.B.; Mostafa, I.T. Thymoquinone-encapsulated chitosan nanoparticles coated with polysorbate 80 as a novel treatment agent in a reserpine-induced depression animal model. Physiol. Behav. 2020, 222, 112934. [Google Scholar] [CrossRef]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Decalf, J.; Tarbell, K.V.; Casrouge, A.; Price, J.D.; Linder, G.; Mottez, E.; Sultanik, P.; Mallet, V.; Pol, S.; Duffy, D. Inhibition of DPP 4 activity in humans establishes its in vivo role in CXCL 10 post-translational modification: Prospective placebo-controlled clinical studies. EMBO Mol. Med. 2016, 8, 679–683. [Google Scholar] [CrossRef] [PubMed]
| S.No | Microorganism | Treatment | Experimental Model/Assay | Results | Ref. |
|---|---|---|---|---|---|
| 1. | Peste des petits ruminant’s virus (PPRV) | Vero cell lines infected with PPRV were treated with six dilutions of N. sativa | MTT assay and Plaque reduction assay | All 3 dilutions (50, 25, 12.5 µg/mL) exhibited significant antiviral activity with a significant reduction in plaques count. | [62] |
| 2. | Murine Cytomegalovirus | N. sativa oil (100 µg/100 µL/mouse for 7 successive days) | The Murine Cytomegalovirus model was used for viral plaque forming assay, flow cytometry, cytolytic activity, ELISA assay, suppressor function assay, cytolytic T lymphocyte assay | Treatment with oil enhances the production of interferons-γ and causes augmentation in CD4+ helper T cell number, suppressor function, and facts of macrophages. | [6] |
| 3. | H9N2 avian influenza viruses | 4-week-old mixed-sex turkey poults | Characterization of cytokine gene expression, as well as the determination of whether or not virus particles are being shed and serological examination. | Improved cytokine gene expression and thus suppressing the pathogenesis of H9N2 avian influenza viruses | [48] |
| 4. | Hepatitis C virus | N. sativa at 450 mg thrice a day | Liver markers enzymes, hepatitis B surface antigen, hepatitis B core immunoglobulin G, and Hepatitis C virus antibody. | Enhanced oxidative stress and diminished viral load. | [24] |
| 5. | Coronavirus | Thymoquinone dissolved in Dulbecco’s Modified Eagle’s Medium | In-silico, cytotoxicity, and plaque reduction assay | Enhances the binding opportunity of thymoquinone with the main protease (Mpro). | [63] |
| 6. | Plasmodium yoelii | Methanolic extract of N. sativa seeds (1.25 g/kg) | in vivo model of malaria on Swiss albino mice | Mice that had been infected had their oxidative state in their red blood cells and hepatocytes illuminated. | [23] |
| 8. | Eimeria stiedae | 400 mg/kg N. sativa aqueous and oil emulsions | Eimeria stiedae infection in rabbit | Shows anti-coccidial effects with a rapid antiparasitic consequence | [64] |
| 9. | Coronavirus (SARS-CoV2) | In-silico inhibition of the replication by thymoquinone | In- silico model | SARS-CoV2 protease-inhibiting properties | [65] |
| 10. | Human cytomegalovirus | Thymoquinone bis artesunic acid hybrid | In-vitro inhibitory activity against Human cytomegalovirus in human fibroblasts cells. | The hybrid compound is more potent than ganciclovir and artesunic acid (EC50 0.63/0.56/0.69 M). | [46] |
| Clinical Trial.Gov Identifier | Study Title | Purpose | Status | Sponsor | Study Type | No of Participants | Allocation | Outcomes |
|---|---|---|---|---|---|---|---|---|
| NCT03208790 | Clinical and immunohistochemical evaluation of the chemopreventive effect of thymoquinone on oral potentially malignant lesions. | Treatment | Completed | Cairo University | Interventional | 30 | Randomized | The results of the study have not been posted until now. |
| NCT04686461 | Effect of thymoquinone extracted from Nigella sativa in the treatment of arsenical keratosis. | Treatment | Recruiting | Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh | Interventional | 34 | Allocation: N/A | Keratotic nodular size |
| NCT03776448 | The effect of 2 g daily supplementation of N. sativa oil on blood glucose levels of adults. | Treatment | Unknown | Sulaiman AlRajhi Colleges Al Bukairiyah, Qassim, Saudi Arabia | Interventional | 30 | Randomized | Fasting venous blood glucose, blood pressure, gastrointestinal symptoms |
| NCT04553705 | Omega-3, Nigella sativa, Indian costus, quinine, anise seed, deglycyrrhizinated licorice, artemisinin, and febrifugine on the immunity of patients with SARS-COV-2 | Treatment | Recruiting | Maternity and Children’s hospital Mecca, Makkah, Saudi Arabia | Interventional | 200 | Randomized | Clinical improvement, The recovery rate from positive to negative swabs, and fever to normal temperature in days. |
| NCT04292314 | Hydroxyurea, omega 3, Nigella sativa, and honey on oxidative stress and iron chelation in pediatric major thalassemia | Treatment | Completed | Beni-Suef University | Interventional | 350 | Randomized | F 2-isoprostanes pg/mL, HDL cholesterol Mg/dL. |
| Patent Number | Invention Title | Description of the Invention | Pharmaceutical Advantages | Ref. |
|---|---|---|---|---|
| WO2016005786 | Liposomal formulations comprising thymoquinone and taxane, and methods of treating cancer using the same. | This disclosure relates to liposomal pharmaceutical compositions comprising taxane and thymoquinone. | Significantly enhances the stability of the liposomes and provides more consistent taxane release patterns. | [20] |
| WO2016061117 | Nano-liposomal aminoglycoside–thymoquinone formulations | The liposome-encapsulated aminoglycoside–thymoquinone (TQ) formulations can be administered to a subject in need. | The liposome-encapsulated aminoglycoside–thymoquinone formulations enhanced the efficacy of thymoquinone | [103] |
| US9745242B1 | A method for the production of thymoquinone | A method for producing thymoquinone and thymohydroquinone in Monarda. | Monarda with elevated levels of carvacrol and thymol in the fresh plant tissue and vigorous growth. | [104] |
| WO2013172537A1 | Pharmaceutical composition for treating or preventing neurological disorders caused by alcohol exposure during pregnancy, containing metformin and/or thymoquinone | This invention relates to a pharmaceutical composition for treating or preventing neurological disorders caused by fetal alcohol exposure | The combination is effective in preventing or treating neurological disorders caused by alcohol exposure of a fetus. | [105] |
| KR101493139B1 | Composition for protecting nerve cells which comprise thymoquinone and vitamin C as active ingredients | This invention relates to a composition for protecting nerve cells, a composition for preventing or treating nerve disorders, and a healthy functional food for preventing or improving nerve disorders. | The composition of the present invention, which simultaneously comprises thymoquinone and vitamin C, has the effect of protecting nerve cells. | [106] |
| CN111000836B | Application of thymoquinone and combined use of thymoquinone and autophagy inhibitor ATG7-siRNA in the preparation of drugs treating esophageal cancer | The invention relates to the application of thymoquinone TQ in preparation for a composition treating esophageal cancer. | The inventor also found that the combined use of an esophageal cancer cell autophagy promoter siRNA and TQ had the characteristic of synergistic inhibition of esophageal cancer cell proliferation. | [107] |
| US8895625B2 | Protective effect of thymoquinone in sepsis | This invention refers to thymoquinone, a primary constituent of the volatile oil of Nigella sativa, and its protective effect against sepsis syndrome morbidity, mortality, and associated organ dysfunctions. | This invention refers to thymoquinone for use in the prevention and/or treatment of sepsis syndrome. This invention further refers to a pharmaceutical composition and a kit. | [108] |
| WO2013030669A4 | Compositions comprising thymoquinone for the treatment of inflammatory diseases | The invention provides a method of treating at least one symptom of an inflammatory disease or disorder in an individual in need of such treatment; the method comprises administering an effective amount of thymoquinone to the individual. | Thymoquinone and at least one physiologically acceptable carrier, wherein an effective amount of thymoquinone could reduce or prevent at least one symptom of the inflammatory disease or disorder. | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoaib, A.; Javed, S.; Wahab, S.; Azmi, L.; Tabish, M.; Sultan, M.H.; Abdelsalam, K.; Alqahtani, S.S.; Ahmad, M.F. Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent. Molecules 2023, 28, 5435. https://doi.org/10.3390/molecules28145435
Shoaib A, Javed S, Wahab S, Azmi L, Tabish M, Sultan MH, Abdelsalam K, Alqahtani SS, Ahmad MF. Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent. Molecules. 2023; 28(14):5435. https://doi.org/10.3390/molecules28145435
Chicago/Turabian StyleShoaib, Ambreen, Shamama Javed, Shadma Wahab, Lubna Azmi, Mohammad Tabish, Muhammad H. Sultan, Karim Abdelsalam, Saad S. Alqahtani, and Md Faruque Ahmad. 2023. "Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent" Molecules 28, no. 14: 5435. https://doi.org/10.3390/molecules28145435
APA StyleShoaib, A., Javed, S., Wahab, S., Azmi, L., Tabish, M., Sultan, M. H., Abdelsalam, K., Alqahtani, S. S., & Ahmad, M. F. (2023). Cellular, Molecular, Pharmacological, and Nano-Formulation Aspects of Thymoquinone—A Potent Natural Antiviral Agent. Molecules, 28(14), 5435. https://doi.org/10.3390/molecules28145435






