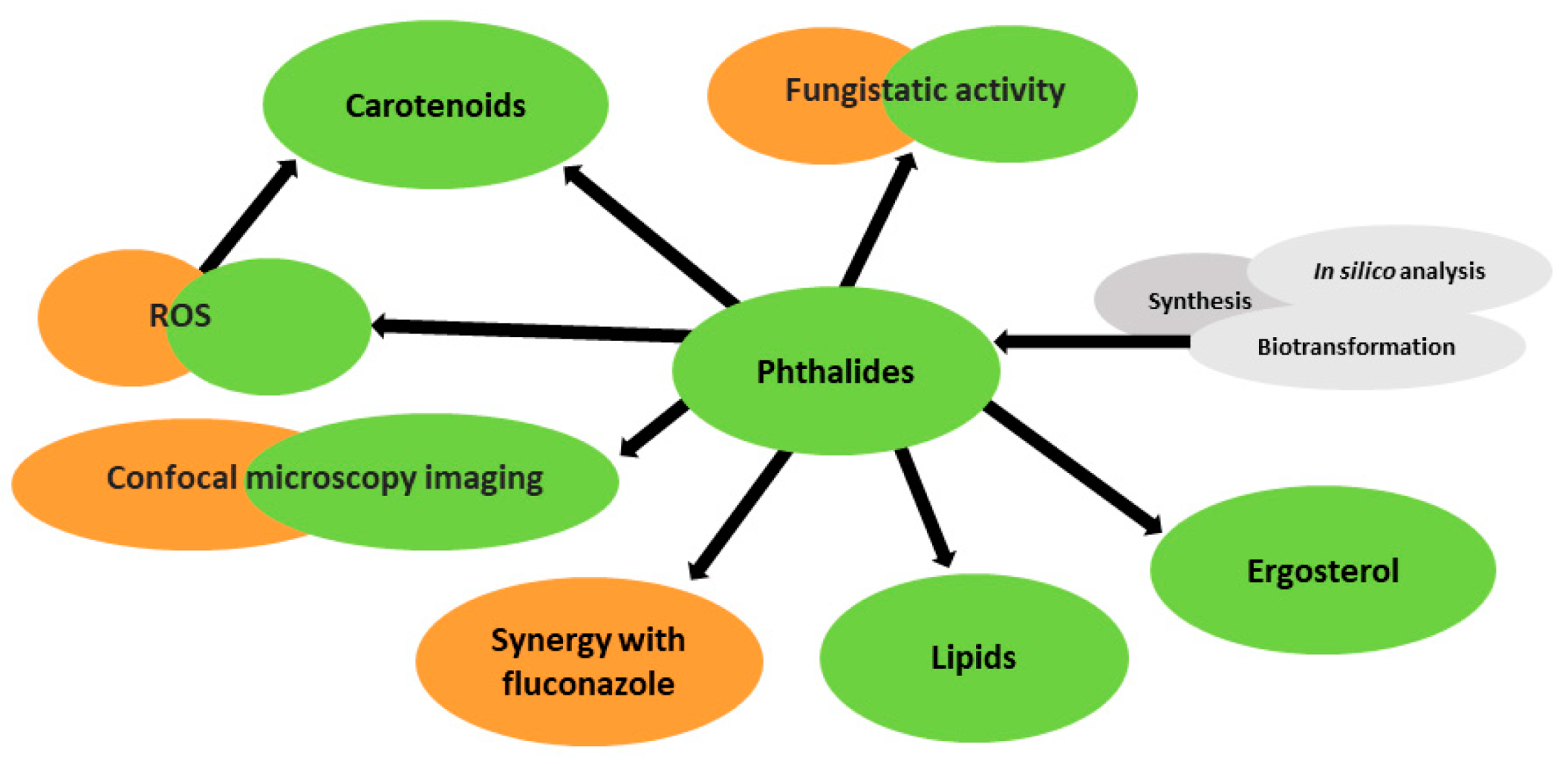
Fungistatic Effect of Phthalide Lactones on Rhodotorula mucilaginosa
Abstract
1. Introduction
2. Results and Discussion
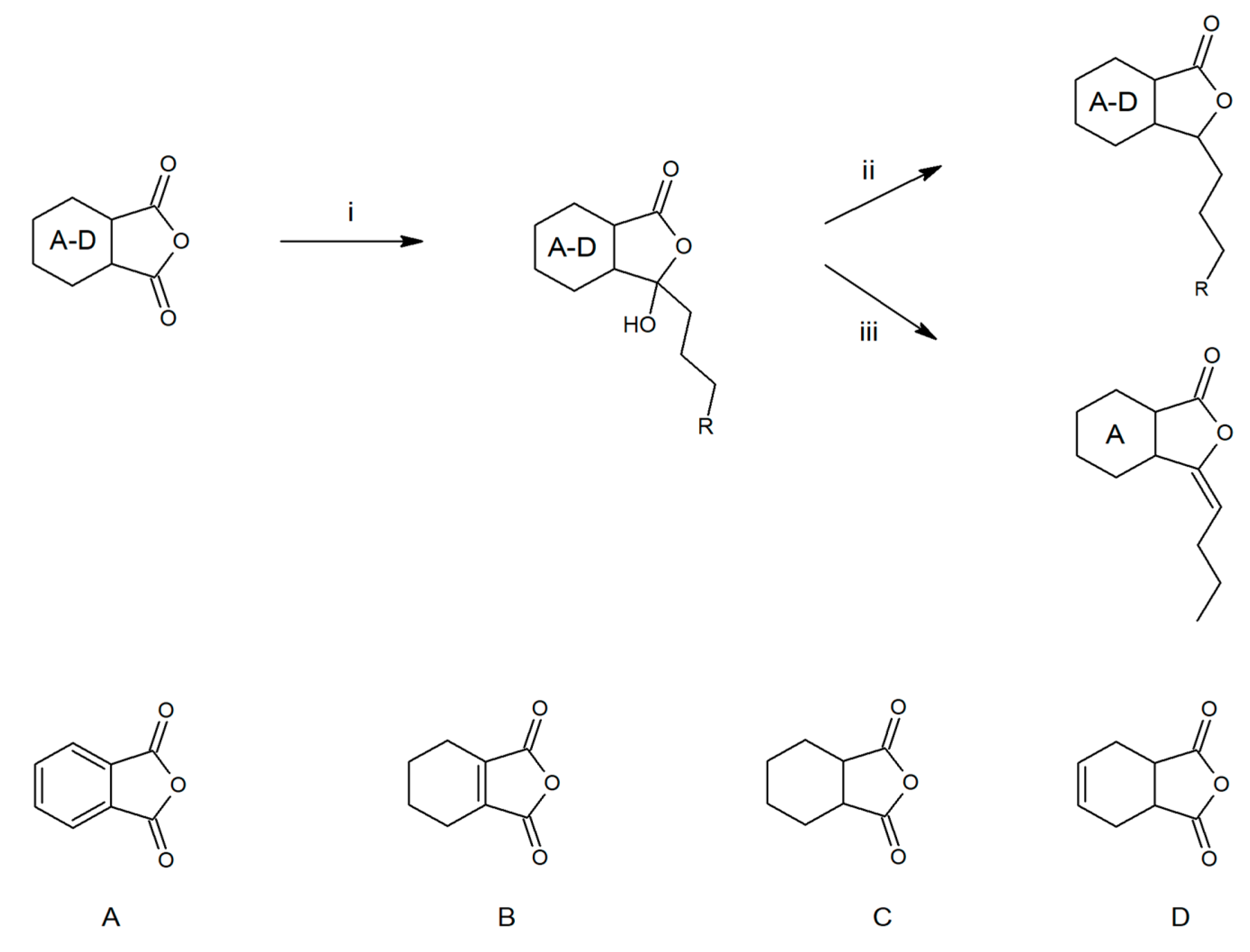
2.1. Synthesis of Phthalide Lactones
2.2. Fungistatic Activity against R. mucilaginosa IHEM 18459 and Correlation with Structure and In Silico Data
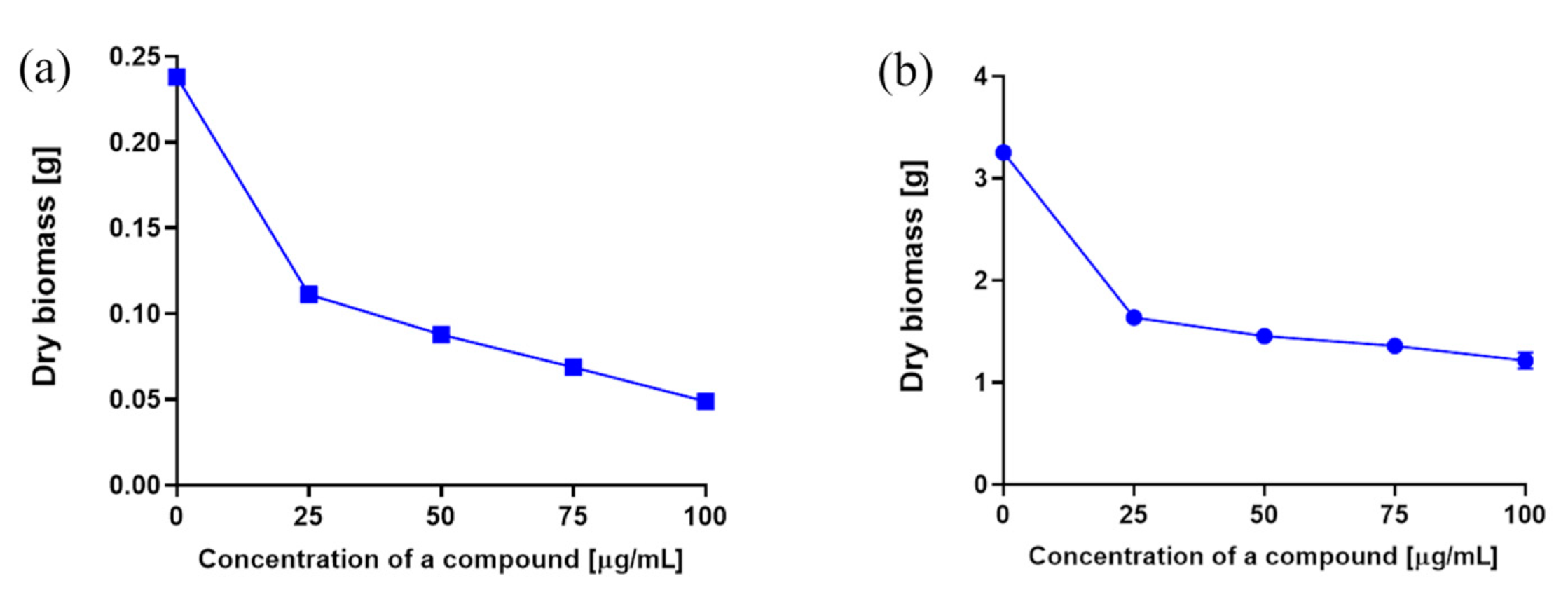
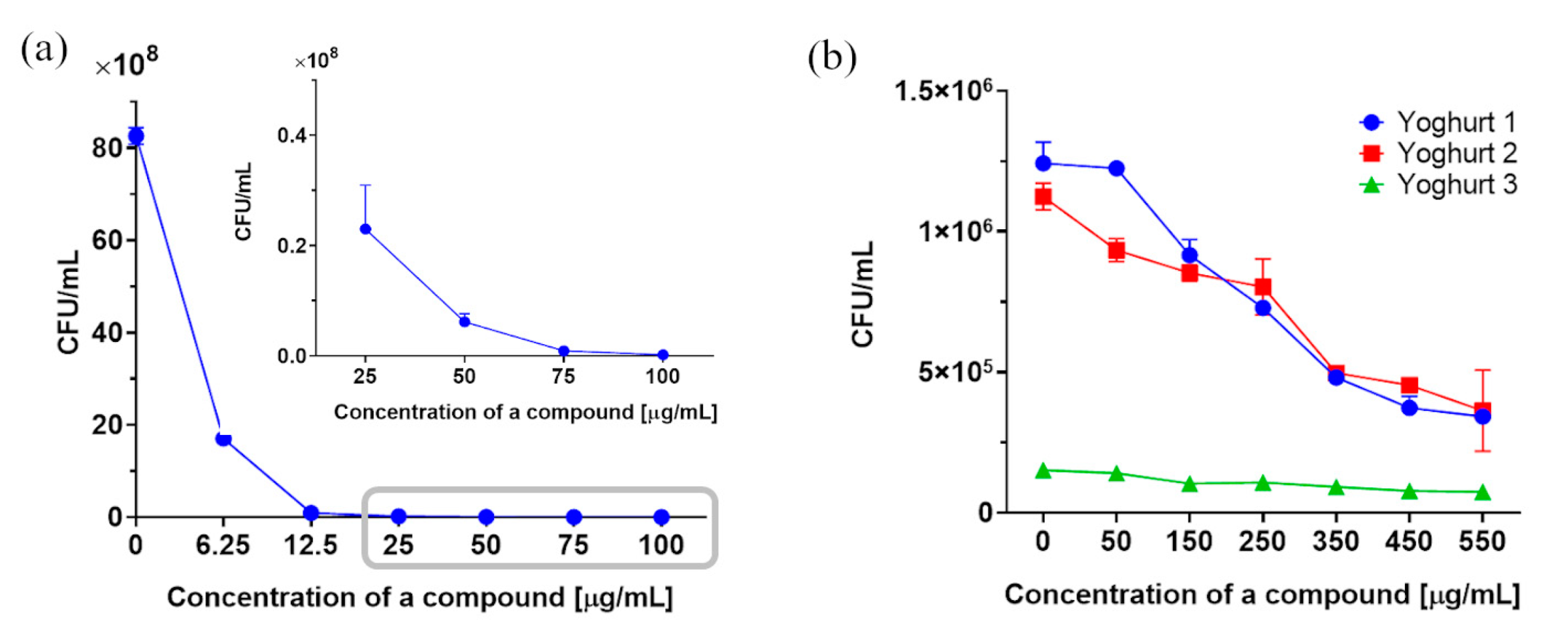
2.3. Determination of Fungistatic Activity for 3-n-Butylidenephthalide (1) against R. mucilaginosa IHEM 18459 In Vitro and in Food Matrices
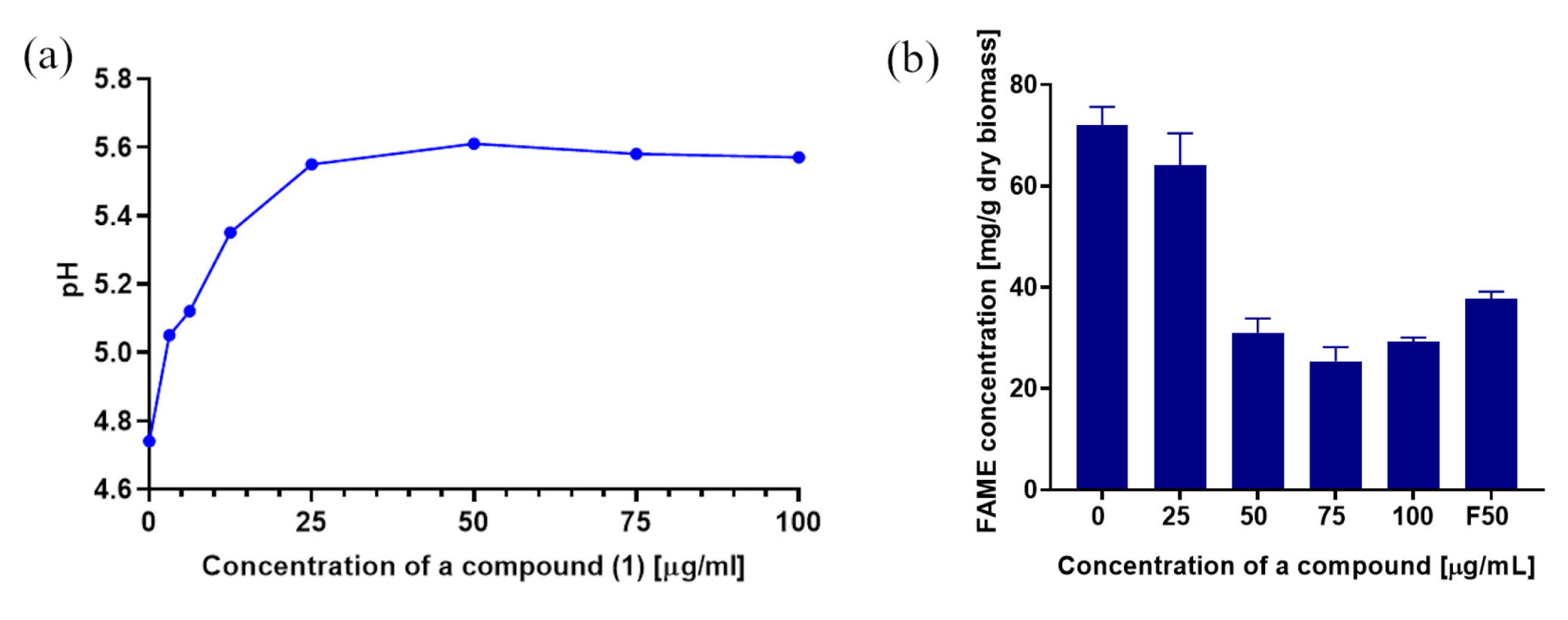
2.3.1. Determination of Dry Yeast Biomass
2.3.2. Plate Count Method In Vitro and in Food Matrices
2.4. Microbial Transformation 3-n-Butylidenephthalide (1) by R. mucilaginosa
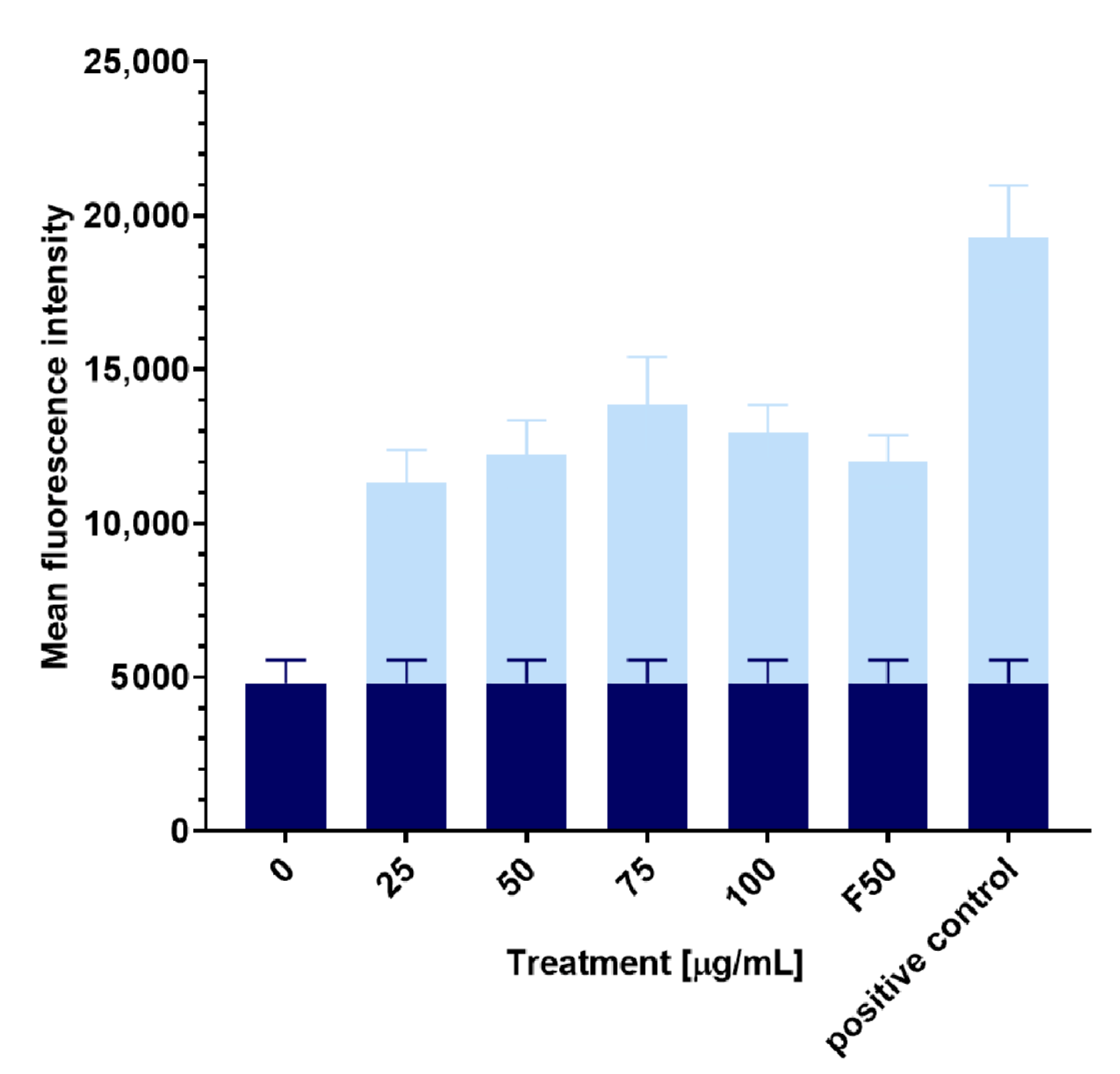
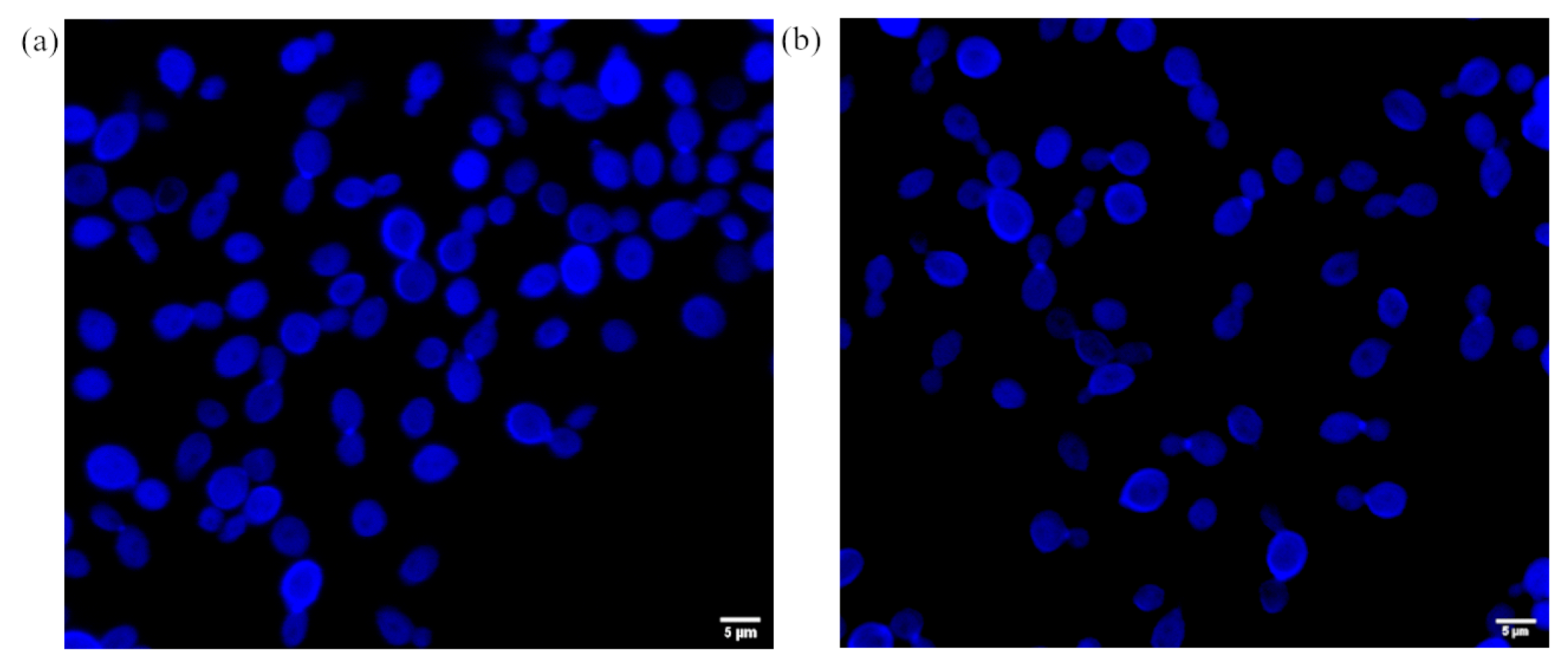
2.5. The Influence of the 3-n-Butylidenephthalide (1) on ROS Level
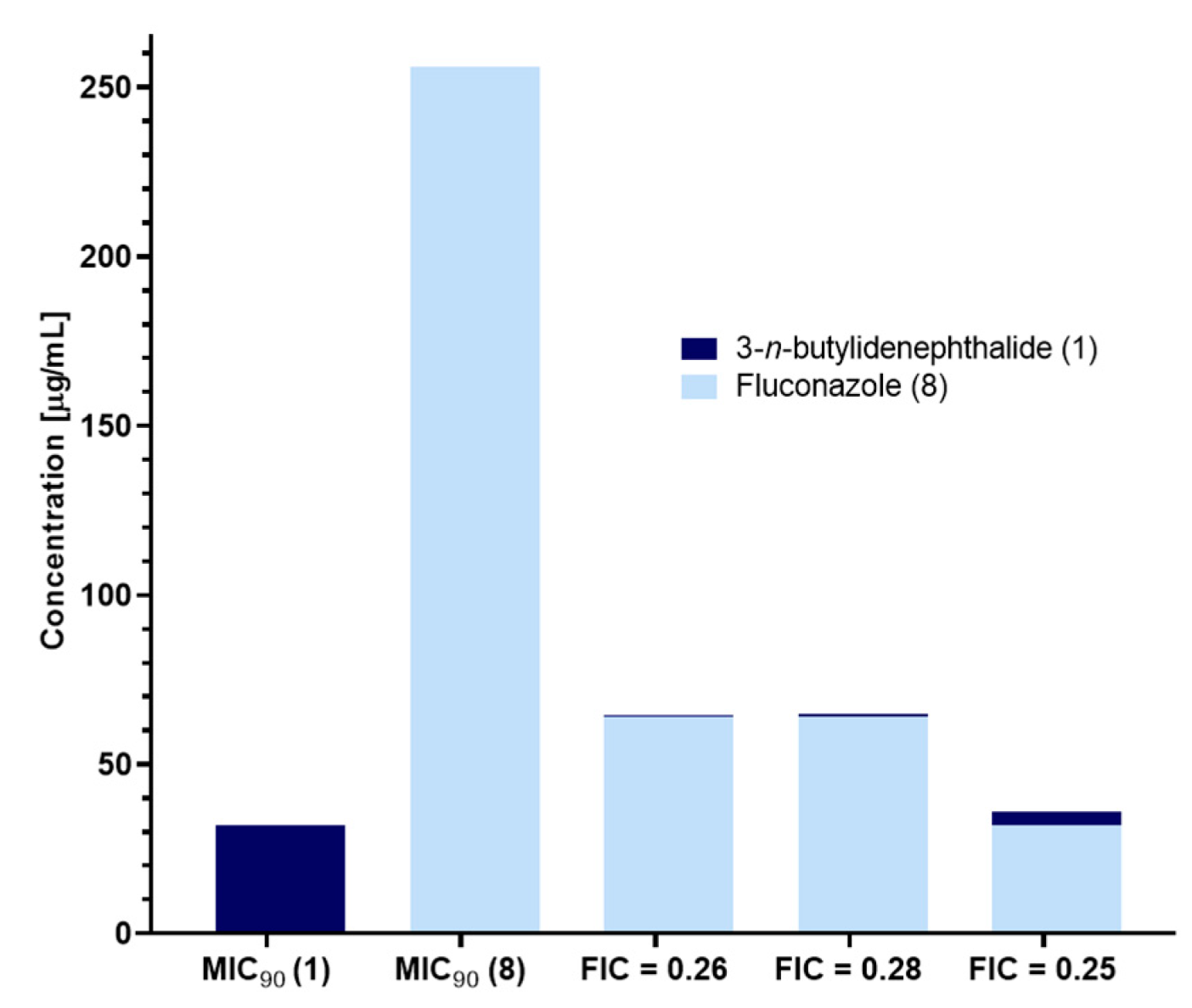
2.6. Synergistic Effect of 3-n-Butylidenephthalide (1) and Fluconazole (8)
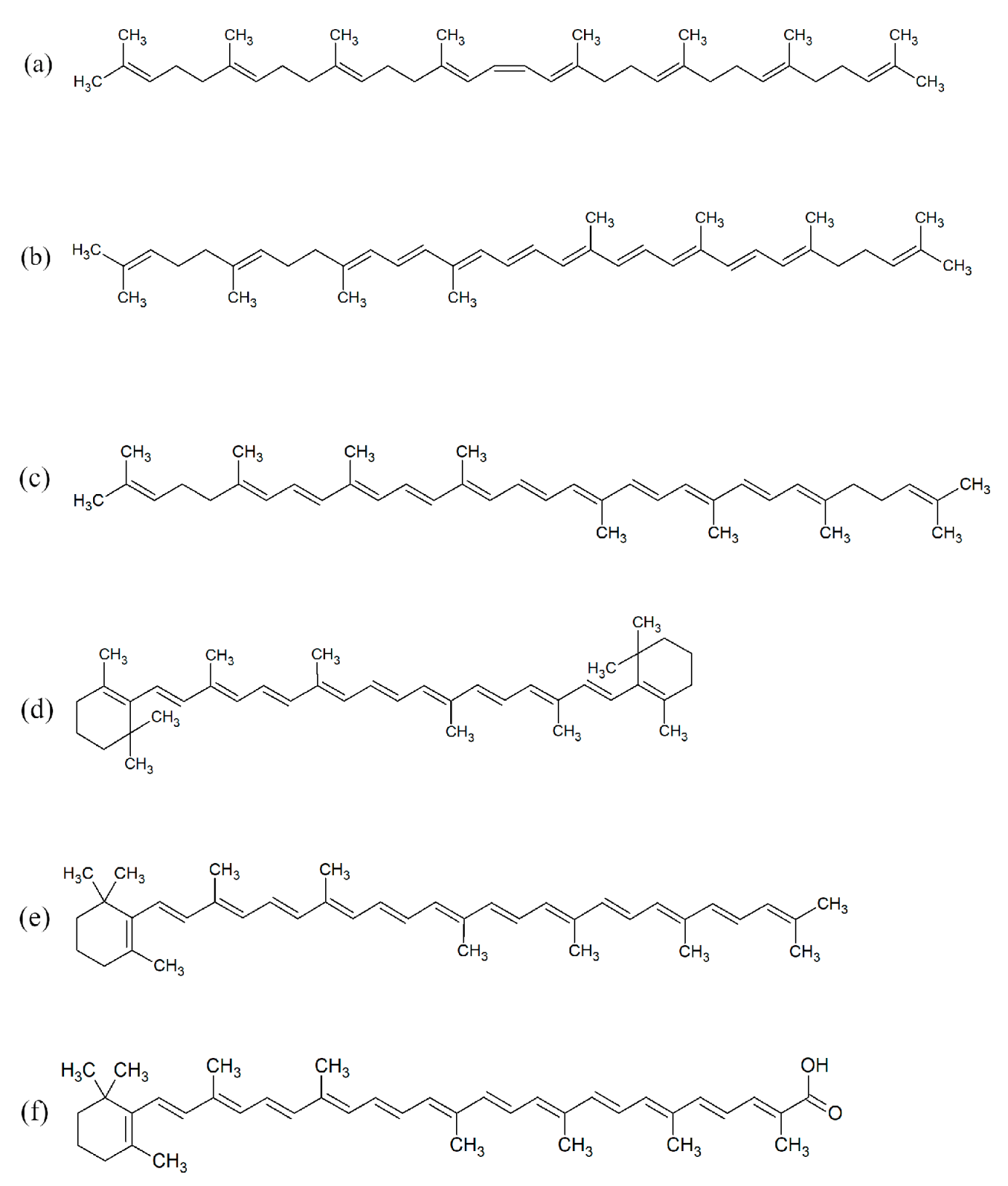
2.7. The Influence of the 3-n-Butylidenephthalide (1) on the Production of Carotenoids
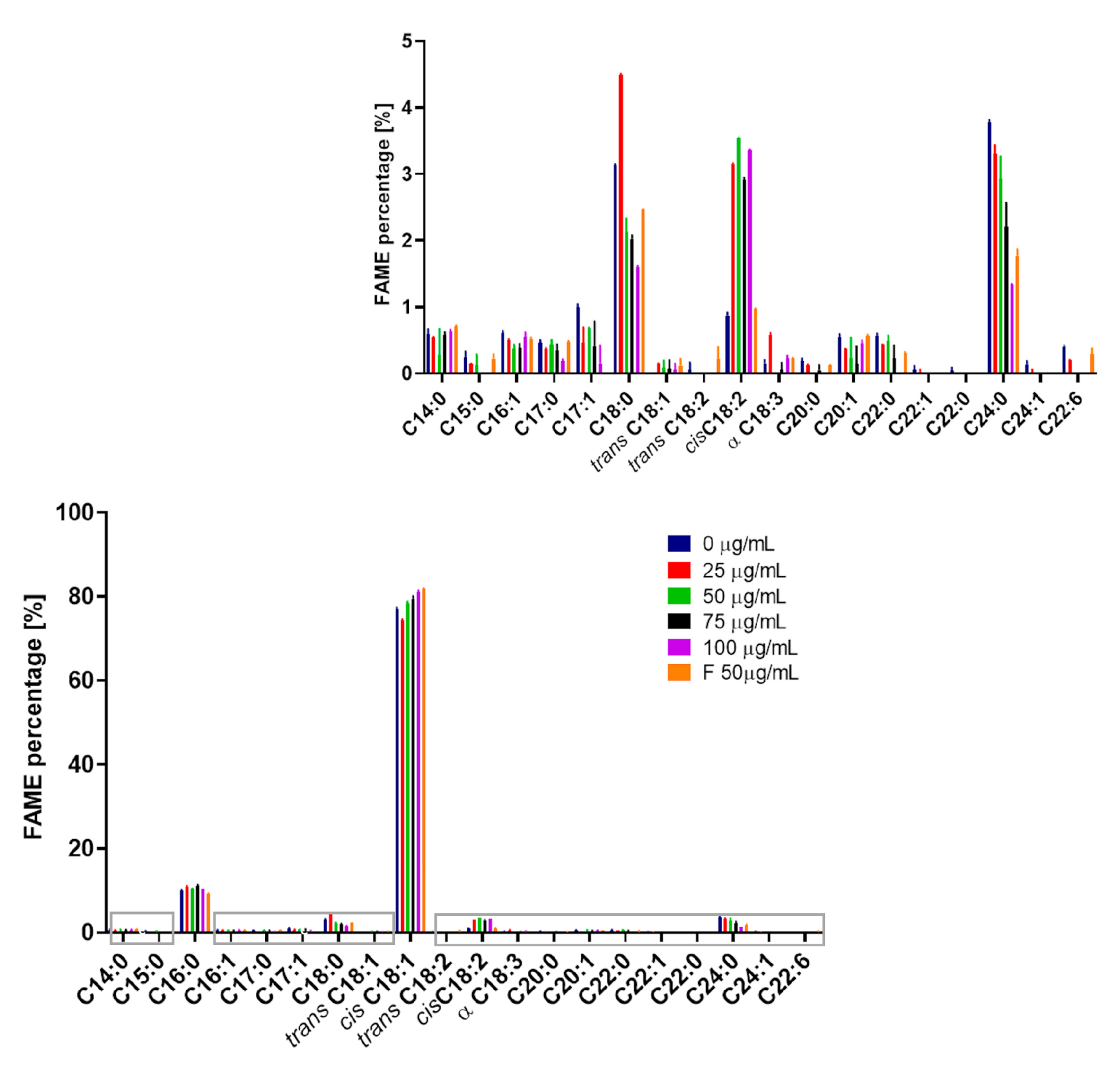
2.8. The Influence of the 3-n-Butylidenephthalide (1) on the Production of FAME
2.9. The Influence of the 3-n-Butylidenephthalide (1) on the Ergosterol Content
3. Materials and Methods
3.1. General
3.2. Synthesis of Phthalide Lactones
3.3. Fungistatic Activity against R. mucilaginosa IHEM 18459 and Correlation with Structure and In Silico Data
3.3.1. Broth Microdilution Method
3.3.2. In Silico ADME Studies
3.4. Determination of Fungistatic Activity for 3-n-Butylidenephthalide (1) against R. mucilaginosa IHEM 18459 In Vitro and Food Matrices
3.4.1. Dry Biomass Determination
3.4.2. Plate Count Method
3.5. Microbial Transformation of 3-n-Butylidenephthalide (1) by R. mucilaginosa IHEM 18459
3.6. The Influence of 3-n-Butylidenephthalide (1) on the ROS Level
3.7. Confocal Microscopy
3.8. Synergistic Effect—Determination of Fractional Inhibitory Concentration (FIC)
3.9. The Determination of Carotenoids in Biomass
3.9.1. Samples Preparation
3.9.2. HR-APCI–MS/MS Analyses
3.9.3. HPLC-DAD Analyses of β-Carotene Content
3.10. FAME Content
3.11. Ergosterol Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lyman, M.; Urbin, S.; Strout, C.; Rubinfeld, B. The Oleaginous Red Yeast Rhodotorula/Rhodosporidium: A Factory for Industrial Bioproducts. In Yeasts in Biotechnology; Peixoto Basso, T., Ed.; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative Sources of Natural Carotenoids, Lipids, and Enzymes for Industrial Use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef] [PubMed]
- Geronikou, A.; Larsen, N.; Lillevang, S.K.; Jespersen, L. Occurrence and Identification of Yeasts in Production of White-Brined Cheese. Microorganisms 2022, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeasts in fruit and fruit products. In Yeasts in Food. Beneficial and Detrimental Aspects; Boekhout, T., Robert, V., Eds.; CRC Press Woodhead Publishing: Cambridge, UK, 2003; Volume 28, pp. 219–316. [Google Scholar]
- Koutsoumanis, K.P.; Lianou, A.; Sofos, J.N. Food Safety: Emerging Pathogens. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 250–272. [Google Scholar]
- Tomsíková, A. Risk of fungal infection from foods, particularly in immunocompromised patients. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Epidemiol. Mikrobiol. Ceske Lek. Spol. JE Purkyne 2002, 51, 78–81. [Google Scholar]
- Sanna, C.; Marras, L.; Desogus, A.; Marras, B.; Montero, N.; Bertolino, G.; Schintu, M.; Coroneo, V. Evaluation of Rhodotorula spp. Contamination in Hospital Environments. Environ. Monit. Assess 2021, 193, 152. [Google Scholar] [CrossRef]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An Emerging Pathogen. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef]
- Spiliopoulou, A.; Anastassiou, E.D.; Christofidou, M. Rhodotorula Fungemia of an Intensive Care Unit Patient and Review of Published Cases. Mycopathologia 2012, 174, 301–309. [Google Scholar] [CrossRef]
- Neofytos, D.; Horn, D.; De Simone, J.A. Rhodotorula mucilaginosa catheter-related fungemia in a patient with sickle cell disease: Case presentation and literature review. South. Med. J. 2007, 100, 198–200. [Google Scholar] [CrossRef]
- Low, C.-Y.; Rotstein, C. Emerging Fungal Infections in Immunocompromised Patients. F1000 Med. Rep. 2011, 3, 14. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, W.; Huang, X.; Hao, L.; Li, Y.; Sun, S. Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole Against Candida albicans. Front. Microbiol. 2019, 10, 1461. [Google Scholar] [CrossRef]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of Pathogenic Candida Species to Evade the Host Complement Attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef]
- Krężel, P.; Olejniczak, T.; Tołoczko, A.; Gach, J.; Weselski, M.; Bronisz, R. Synergic Effect of Phthalide Lactones and Fluconazole and Its New Analogues as a Factor Limiting the Use of Azole Drugs against Candidiasis. Antibiotics 2022, 11, 1500. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in Nature, Chemical Reactivity, Synthesis, and Biological Activity. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 104, pp. 127–246. [Google Scholar]
- Pannek, J.; Gach, J.; Boratyński, F.; Olejniczak, T. Antimicrobial Activity of Extracts and Phthalides Occurring in Apiaceae Plants: Antimicrobial Activity of Phthalides. Phytother. Res. 2018, 32, 1459–1487. [Google Scholar] [CrossRef]
- Miran, M.; Monsef Esfahani, H.R.; Jung, J.H.; Aliahmadi, A.; Skropeta, D.; Abbas-Mohammadi, M.; Ebrahimi, S.; Moridi Farimani, M. Characterization and Antibacterial Activity of Phthalides from the Roots of the Medicinal Herb Levisticum officinale WDJ Koch. IJPR 2020, 19, 182. [Google Scholar] [CrossRef]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of Carotenoids Production from Rhodotorula Sp. Strain ATL72 for Enhancing Its Biotechnological Applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef]
- Zadorozhnii, P.V.; Kiselev, V.V.; Kharchenko, A.V. In Silico ADME Profiling of Salubrinal and Its Analogues. Future Pharmacol. 2022, 2, 160–197. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempińska-Kupczyk, D.; Kot-Wasik, A.; Namieśnik, J.; Mazerska, Z. The Impact of Lipophilicity on Environmental Processes, Drug Delivery and Bioavailability of Food Components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Druzhilovskiy, D.S.; Rudik, A.V.; Filimonov, D.A.; Gloriozova, T.A.; Lagunin, A.A.; Dmitriev, A.V.; Pogodin, P.V.; Dubovskaya, V.I.; Ivanov, S.M.; Tarasova, O.A.; et al. Computational Platform Way2Drug: From the Prediction of Biological Activity to Drug Repurposing. Russ. Chem. Bull. 2017, 66, 1832–1841. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Yuan, Y.; Gui, L.; Wang, J.; Gao, P.; Qin, B.; Sima, D.; Wang, Q.; Pan, W. Use of L-3-n-Butylphthalide within 24 h after Intravenous Thrombolysis for Acute Cerebral Infarction. Complement. Ther. Med. 2020, 52, 102442. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, S.; Zang, Y.; Gu, F.; Mao, S.; Feng, S.; Hu, L.; Zhang, C. The Efficacy and Safety of Dl-3n-Butylphthalide on Progressive Cerebral Infarction: A Randomized Controlled STROBE Study. Medicine 2017, 96, e7257. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Dhiman, S.; Kumar, V.; Gour, A.; Manhas, D.; Sharma, K.; Ojha, P.K.; Nandi, U. Assessment of the CYP1A2 Inhibition-Mediated Drug Interaction Potential for Pinocembrin Using In Silico, In Vitro, and In Vivo Approaches. ACS Omega 2022, 7, 20321–20331. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J. Clinical Application of CYP2C19 Pharmacogenetics toward More Personalized Medicine. Front. Gene 2013, 3, 318. [Google Scholar] [CrossRef] [PubMed]
- Benkova, M.; Soukup, O.; Marek, J. Antimicrobial Susceptibility Testing: Currently Used Methods and Devices and the near Future in Clinical Practice. J. Appl. Microbiol. 2020, 129, 806–822. [Google Scholar] [CrossRef]
- Cruz, R.C.; Werneck, S.M.C.; Oliveira, C.S.; Santos, P.C.; Soares, B.M.; Santos, D.A.; Cisalpino, P.S. Influence of Different Media, Incubation Times, and Temperatures for Determining the MICs of Seven Antifungal Agents against Paracoccidioides Brasiliensis by Microdilution. J. Clin. Microbiol. 2013, 51, 436–443. [Google Scholar] [CrossRef]
- Smith, K.P.; Kirby, J.E. The Inoculum Effect in the Era of Multidrug Resistance: Minor Differences in Inoculum Have Dramatic Effect on MIC Determination. Antimicrob. Agents Chemother. 2018, 62, e00433-18. [Google Scholar] [CrossRef]
- Afzali, S.; Edalatian Dovom, M.R.; Habibi Najafi, M.B.; Mazaheri Tehrani, M. Determination of the Anti-Yeast Activity of Lactobacillus Spp. Isolated from Traditional Iranian Cheeses in Vitro and in Yogurt Drink (Doogh). Sci. Rep. 2020, 10, 6291. [Google Scholar] [CrossRef]
- Lima, R.C.; de Carvalho, A.P.A.; Vieira, C.P.; Moreira, R.V.; Conte-Junior, C.A. Green and Healthier Alternatives to Chemical Additives as Cheese Preservative: Natural Antimicrobials in Active Nanopackaging/Coatings. Polymers 2021, 13, 2675. [Google Scholar] [CrossRef]
- Gach, J.; Olejniczak, T.; Krężel, P.; Boratyński, F. Microbial Synthesis and Evaluation of Fungistatic Activity of 3-Butyl-3-Hydroxyphthalide, the Mammalian Metabolite of 3-n-Butylidenephthalide. Int. J. Mol. Sci. 2021, 22, 7600. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food Antimicrobials Nanocarriers. Sci. World J. 2014, 2014, 837215. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kumar, S.; Choi, E.-H.; Chaudhary, S.; Kim, M.-H. Molecular Dynamic Simulations of Oxidized Skin Lipid Bilayer and Permeability of Reactive Oxygen Species. Sci. Rep. 2019, 9, 4496. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, J.; Srivastava, V.K.; Kaushik, S.; Singh, H.; Abo-EL-Sooud, K.; Abdel-Daim, M.M.; Jyoti, A.; Saluja, R. The Interplay of Oxidative Stress and ROS Scavenging: Antioxidants as a Therapeutic Potential in Sepsis. Vaccines 2022, 10, 1575. [Google Scholar] [CrossRef]
- Dbouk, N.H.; Covington, M.B.; Nguyen, K.; Chandrasekaran, S. Increase of Reactive Oxygen Species Contributes to Growth Inhibition by Fluconazole in Cryptococcus neoformans. BMC Microbiol. 2019, 19, 243. [Google Scholar] [CrossRef]
- Peng, C.A.; Gaertner, A.A.E.; Henriquez, S.A.; Fang, D.; Colon-Reyes, R.J.; Brumaghim, J.L.; Kozubowski, L. Fluconazole Induces ROS in Cryptococcus neoformans and Contributes to DNA Damage in Vitro. PLoS ONE 2018, 13, e0208471. [Google Scholar] [CrossRef]
- Dagher, Z.; Xu, S.; Negoro, P.E.; Khan, N.S.; Feldman, M.B.; Reedy, J.L.; Tam, J.M.; Sykes, D.B.; Mansour, M.K. Fluorescent Tracking of Yeast Division Clarifies the Essential Role of Spleen Tyrosine Kinase in the Intracellular Control of Candida glabrata in Macrophages. Front. Immunol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antiadhesive Properties of Imidazolium Ionic Liquids Based on (−)-Menthol Against Candida spp. Int. J. Mol. Sci. 2021, 22, 7543. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial Combinations. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 365–440. [Google Scholar]
- Sharma, R.; Ghoshal, G. Optimization of Carotenoids Production by Rhodotorula mucilaginosa (MTCC-1403) Using Agro-Industrial Waste in Bioreactor: A Statistical Approach. Biotechnol. Rep. 2020, 25, e00407. [Google Scholar] [CrossRef]
- Irazusta, V.; Nieto-Peñalver, C.G.; Cabral, M.E.; Amoroso, M.J.; de Figueroa, L.I.C. Relationship among Carotenoid Production, Copper Bioremediation and Oxidative Stress in Rhodotorula mucilaginosa RCL-11. Process Biochem. 2013, 48, 803–809. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula Glutinis—Potential Source of Lipids, Carotenoids, and Enzymes for Use in Industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef]
- Moliné, M.; Libkind, D.; van Broock, M. Production of Torularhodin, Torulene, and β-Carotene by Rhodotorula Yeasts. In Microbial Carotenoids from Fungi; Barredo, J.-L., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 898, pp. 275–283. ISBN 9781617799174. [Google Scholar]
- Moț, A.C.; Pârvu, M.; Pârvu, A.E.; Roşca-Casian, O.; Dina, N.E.; Leopold, N.; Silaghi-Dumitrescu, R.; Mircea, C. Reversible Naftifine-Induced Carotenoid Depigmentation in Rhodotorula mucilaginosa (A. Jörg.) F.C. Harrison Causing Onychomycosis. Sci. Rep. 2017, 7, 11125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, D.; Yang, Q.; Wang, P. Enhancing Carotenoid Production in Rhodotorula mucilaginosa KC8 by Combining Mutation and Metabolic Engineering. Ann. Microbiol. 2017, 67, 425–431. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Pan, X.; Li, Z.; Lu, Y.; He, N.; Meng, T.; Yao, C.; Chen, C.; Ling, X. The Role of Fluconazole in the Regulation of Fatty Acid and Unsaponifiable Matter Biosynthesis in Schizochytrium sp. MYA 1381. BMC Microbiol. 2019, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Nicaud, J.-M.; Gaillardin, C. An Overview of Lipid Metabolism in Yeasts and Its Impact on Biotechnological Processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical Steps in Carbon Metabolism Affecting Lipid Accumulation and Their Regulation in Oleaginous Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Khot, M.; Ghosh, D. Lipids of Rhodotorula mucilaginosa IIPL32 with Biodiesel Potential: Oil Yield, Fatty Acid Profile, Fuel Properties. J. Basic Microbiol. 2017, 57, 345–352. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast Lipid Metabolism at a Glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef]
- Liang, C.-M.; Yang, C.-F.; Du, J.-S. Lipid Production and Waste Reutilization Combination Using Yeast Isolate Rhodotorula mucilaginosa LP-2. Bioenerg. Res. 2021, 14, 1184–1195. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Bhattacharya, A.; Nain, L.; Prasanna, R.; Khare, S.K. Valorization of Agro-Starchy Wastes as Substrates for Oleaginous Microbes. Biomass Bioenergy 2019, 127, 105294. [Google Scholar] [CrossRef]
- Adel, A.; El-Baz, A.; Shetaia, Y.; Sorour, N.M. Biosynthesis of Polyunsaturated Fatty Acids by Two Newly Cold-Adapted Egyptian Marine Yeast. 3 Biotech 2021, 11, 461. [Google Scholar] [CrossRef]
- Karatay, S.E.; Dönmez, G. Improving the Lipid Accumulation Properties of the Yeast Cells for Biodiesel Production Using Molasses. Bioresour. Technol. 2010, 101, 7988–7990. [Google Scholar] [CrossRef]
- Sorgo, A.G.; Heilmann, C.J.; Dekker, H.L.; Bekker, M.; Brul, S.; de Koster, C.G.; de Koning, L.J.; Klis, F.M. Effects of Fluconazole on the Secretome, the Wall Proteome, and Wall Integrity of the Clinical Fungus Candida albicans. Eukaryot. Cell 2011, 10, 1071–1081. [Google Scholar] [CrossRef]
- Dasgupta, D.; Sharma, T.; Bhatt, A.; Bandhu, S.; Ghosh, D. Cultivation of Oleaginous Yeast Rhodotorula mucilaginosa IIPL32 in Split Column Airlift Reactor and Its Influence on Fuel Properties. Biocatal. Agric. Biotechnol. 2017, 10, 308–316. [Google Scholar] [CrossRef]
- Park, Y.; Ledesma-Amaro, R.; Nicaud, J.-M. De Novo Biosynthesis of Odd-Chain Fatty Acids in Yarrowia lipolytica Enabled by Modular Pathway Engineering. Front. Bioeng. Biotechnol. 2020, 7, 484. [Google Scholar] [CrossRef]
- Ayadi, I.; Belghith, H.; Gargouri, A.; Guerfali, M. Utilization of Wheat Bran Acid Hydrolysate by Rhodotorula mucilaginosa Y-MG1 for Microbial Lipid Production as Feedstock for Biodiesel Synthesis. BioMed Res. Int. 2019, 2019, 3213521. [Google Scholar] [CrossRef]
- Ballweg, S.; Ernst, R. Control of Membrane Fluidity: The OLE Pathway in Focus. Biol. Chem. 2017, 398, 215–228. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of Membrane Lipids Enhances the Tolerance of Saccharomyces cerevisiae to Freezing and Salt Stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef]
- Liu, Y.; Koh, C.M.J.; Yap, S.A.; Cai, L.; Ji, L. Understanding and Exploiting the Fatty Acid Desaturation System in Rhodotorula toruloides. Biotechnol. Biofuels 2021, 14, 73. [Google Scholar] [CrossRef]
- Tsai, Y.-Y.; Ohashi, T.; Wu, C.-C.; Bataa, D.; Misaki, R.; Limtong, S.; Fujiyama, K. Delta-9 Fatty Acid Desaturase Overexpression Enhanced Lipid Production and Oleic Acid Content in Rhodosporidium toruloides for Preferable Yeast Lipid Production. J. Biosci. Bioeng. 2019, 127, 430–440. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive Oxygen Species-Inducing Antifungal Agents and Their Activity against Fungal Biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Walczak, P.; Pannek, J.; Boratyński, F.; Janik-Polanowicz, A.; Olejniczak, T. Synthesis and Fungistatic Activity of Bicyclic Lactones and Lactams against Botrytis Cinerea, Penicillium Citrinum, and Aspergillus glaucus. J. Agric. Food Chem. 2014, 62, 8571–8578. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Second Edition; NCCLS Document M27-A2; National Committee for Clinical Laboratory Standards: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Arthington-Skaggs, B.A.; Lee-Yang, W.; Ciblak, M.A.; Frade, J.P.; Brandt, M.E.; Hajjeh, R.A.; Harrison, L.H.; Sofair, A.N.; Warnock, D.W. Comparison of Visual and Spectrophotometric Methods of Broth Microdilution MIC End Point Determination and Evaluation of a Sterol Quantitation Method for In Vitro Susceptibility Testing of Fluconazole and Itraconazole against Trailing and Nontrailing Candida Isolates. Antimicrob. Agents Chemother. 2002, 46, 2477–2481. [Google Scholar] [CrossRef] [PubMed]

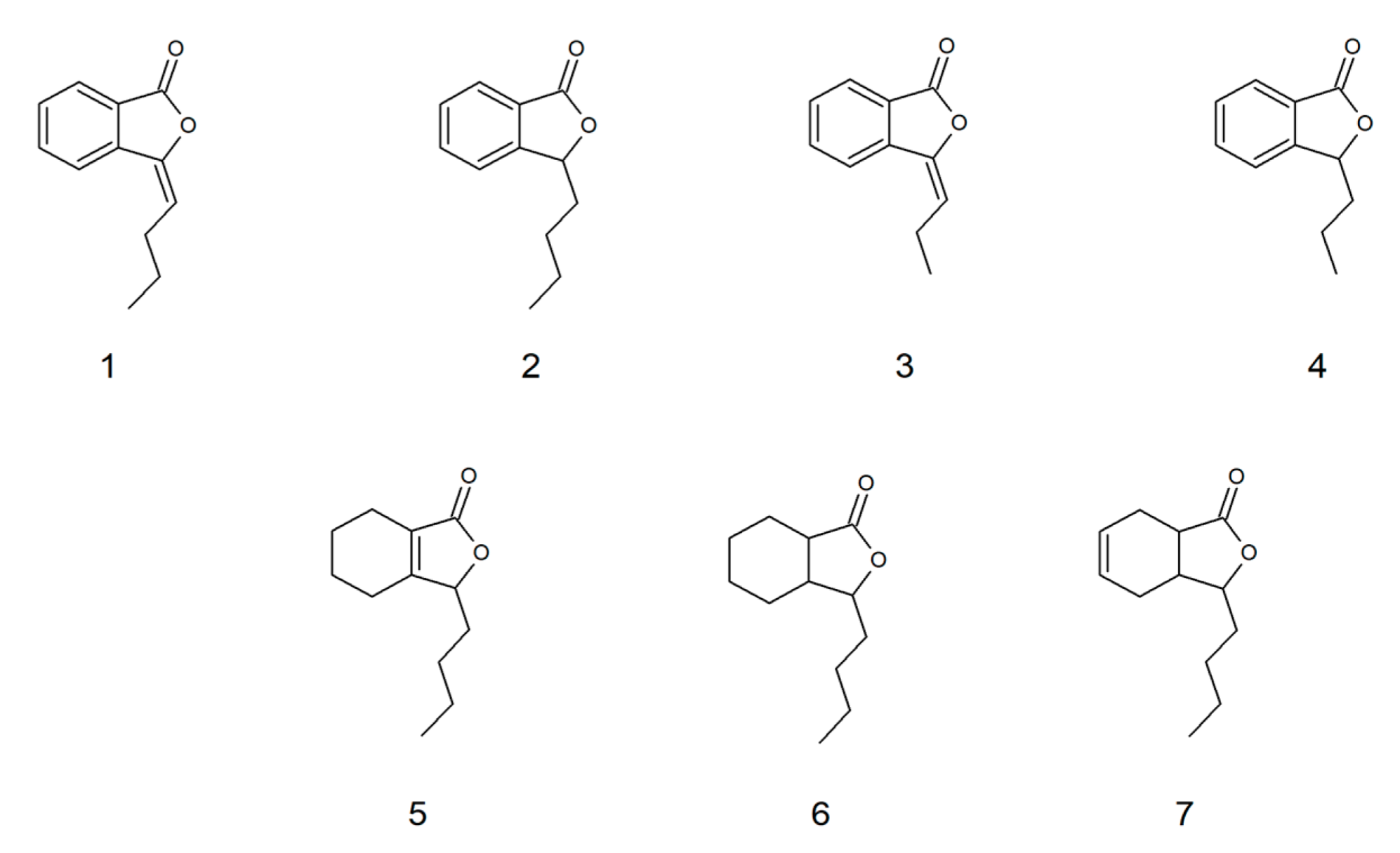

| Compound | IC50 at 25 °C [µg/mL] | IC50 at 30 °C [µg/mL] | Log P | Log S | LC50 [mg/kg] | Inhibition of: |
|---|---|---|---|---|---|---|
| 1 | 13 | 13 | 2.93 | −3.30 | 1865 | CYP1A2 |
| 2 | 20 | 23 | 2.81 | −3.01 | 4872 | CYP1A2 CYP2C9 |
| 3 | 38 | 32 | 2.78 | −2.43 | 1895 | CYP1A2 |
| 4 | 130 | 109 | 2.44 | −2.45 | 4061 | CYP1A2 |
| 5 | 65 | 66 | 2.93 | −3.20 | 5184 | - |
| 6 | 42 | 43 | 2.96 | −3.79 | 4702 | CYP2C9 |
| 7 | 54 | 57 | 2.93 | −3.20 | 4186 | CYP2C9 |
| 8 | 199 | 207 | 0.88 | −1.63 | 584 | CYP2C19 |
| Carotenoid | APCI-LC-HR-MS [M + H]+ | 0 | 25 | 50 | 75 | 100 | F50 |
|---|---|---|---|---|---|---|---|
| Phytoene | 545.5081 | 1 | 50.92 | 70.61 | 95.83 | 35.33 | 3.52 |
| Neurosporene | 539.4611 | n.d. | n.d. | n.d. | d. | n.d. | n.d. |
| β-carotene + lycopene | 537.4445 | 1 | 4.45 a | 1.10 a | 1.72 a | 0.20 | 2.30 a |
| Torulene | 535.4280 | 1 | 0.88 | 0.21 | 0.35 | 0.07 | 1.66 |
| Torularhodin | 565.4063 | 1 | 0.41 | 0.01 | 0.05 | n.d. | 1.63 |
| Concentration of Compounds [µg/mL] | β-Carotene Content [mg/g d.m.] |
|---|---|
| 0 | 0.2108 ± 0.0055 |
| 25 | 0.4433 ± 0.0282 |
| 50 | 0.2305 ± 0.0108 |
| 75 | 0.0262 ± 0.0042 |
| 100 | n.d. |
| F50 | 0.4792 ± 0.0055 |
| Concentration of a Compound [µg/mL] | Saturated [%] | Unsaturated [%] | Unsaturated Including: | |
|---|---|---|---|---|
| Monounsaturated (MUFA) [%] | Polyunsaturated (PUFA) [%] | |||
| 0 | 19.02 ± 0.08 | 80.98 ± 0.08 | 98.16 ± 0.14 | 1.84 ± 0.14 |
| 25 | 20.31 ± 0.05 | 79.69 ± 0.05 | 95.06 ± 0.02 | 4.94 ± 0.02 |
| 50 | 16.77 ± 0.01 | 83.23 ± 0.01 | 95.74 ± 0.02 | 4.26 ± 0.02 |
| 75 | 16.54 ± 0.04 | 83.46 ± 0.04 | 96.43 ± 0.09 | 3.57 ± 0.09 |
| 100 | 14.12 ± 0.18 | 85.88 ± 0.18 | 95.82 ± 0.05 | 4.18 ± 0.05 |
| F50 | 15.27 ± 0.14 | 84.73 ± 0.14 | 98.00 ± 0.12 | 2.00 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gach, J.; Olejniczak, T.; Pannek, J.; Boratyński, F. Fungistatic Effect of Phthalide Lactones on Rhodotorula mucilaginosa. Molecules 2023, 28, 5423. https://doi.org/10.3390/molecules28145423
Gach J, Olejniczak T, Pannek J, Boratyński F. Fungistatic Effect of Phthalide Lactones on Rhodotorula mucilaginosa. Molecules. 2023; 28(14):5423. https://doi.org/10.3390/molecules28145423
Chicago/Turabian StyleGach, Joanna, Teresa Olejniczak, Jakub Pannek, and Filip Boratyński. 2023. "Fungistatic Effect of Phthalide Lactones on Rhodotorula mucilaginosa" Molecules 28, no. 14: 5423. https://doi.org/10.3390/molecules28145423
APA StyleGach, J., Olejniczak, T., Pannek, J., & Boratyński, F. (2023). Fungistatic Effect of Phthalide Lactones on Rhodotorula mucilaginosa. Molecules, 28(14), 5423. https://doi.org/10.3390/molecules28145423







