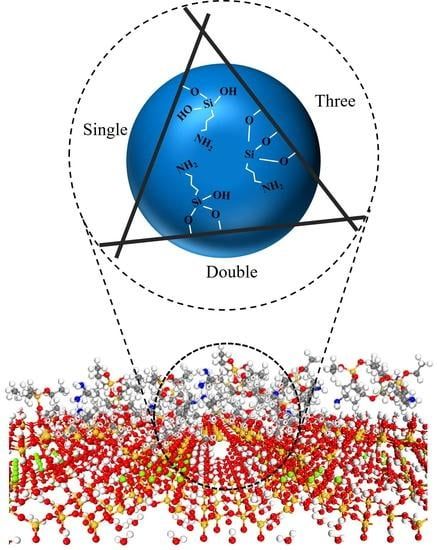
Investigation on the Mechanism of PAL (100) Surface Modified by APTES
Abstract
1. Introduction
2. Results and Discussion
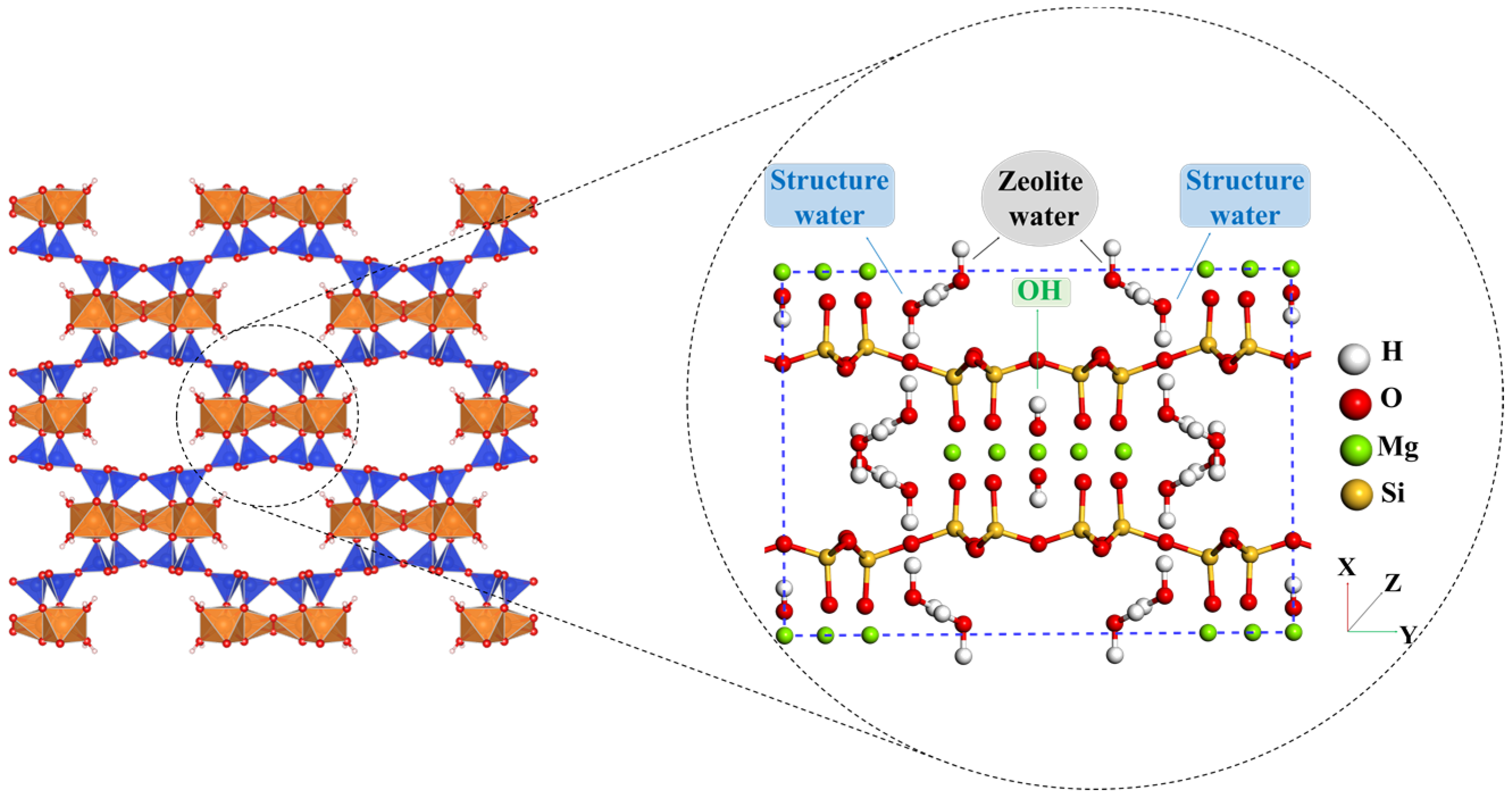
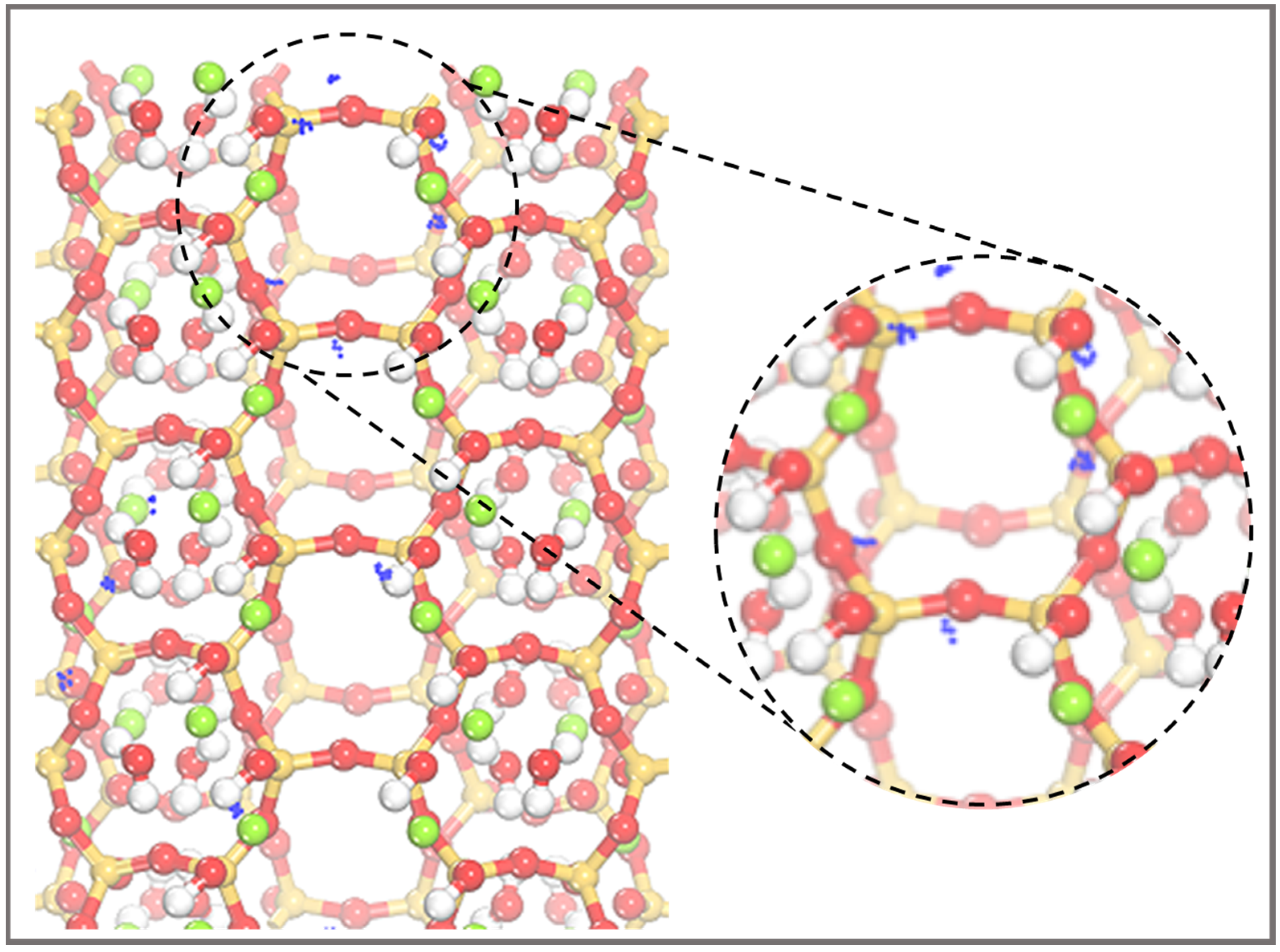
2.1. Surface Models
2.2. The Performance of Surface Modification
2.3. Modification Mechanism
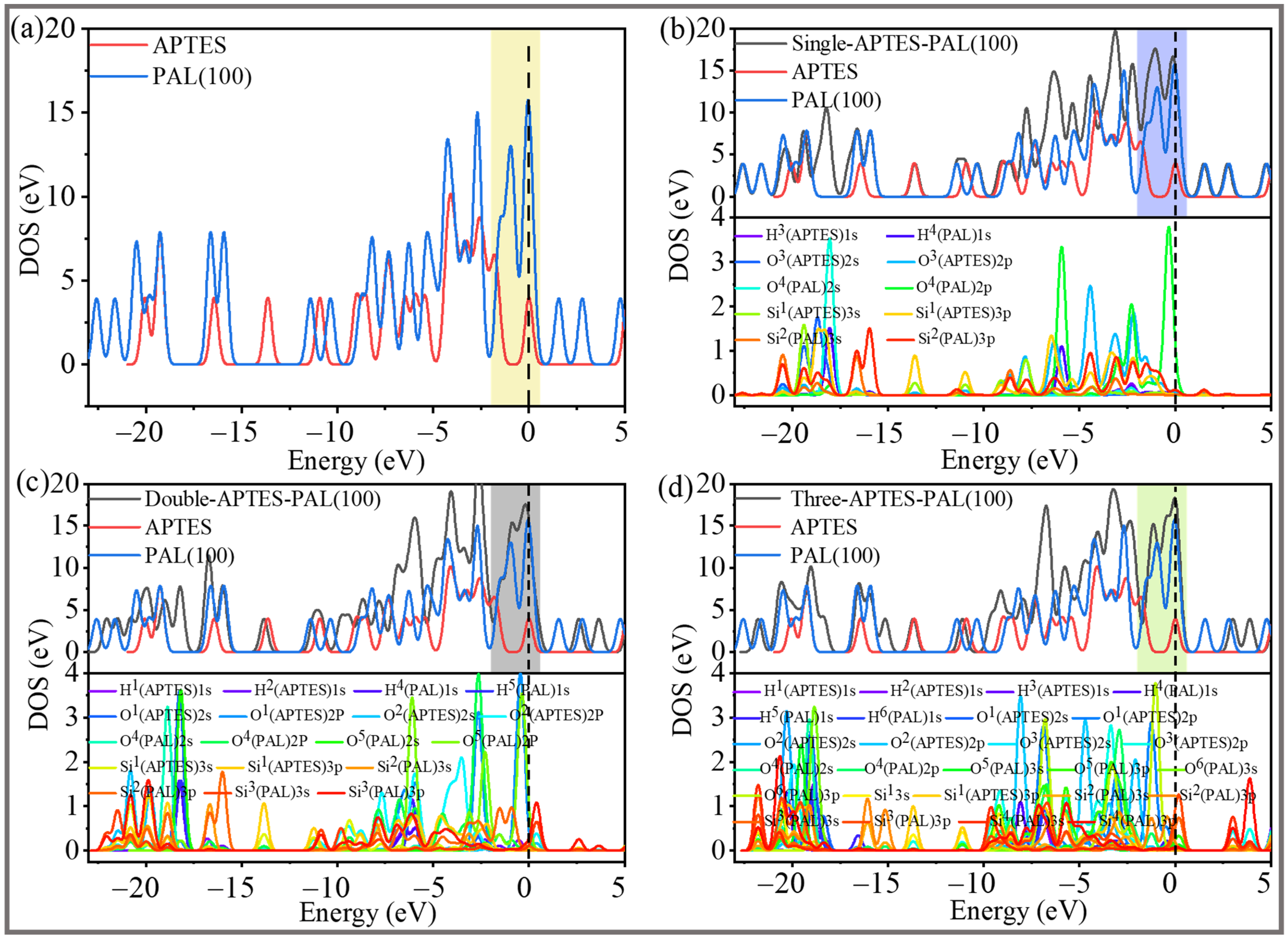
2.4. Electronic Structures
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ogorodova, L.; Vigasina, M.; Melchakova, L.; Krupskaya, V.; Kiseleva, I. Thermochemical study of natural magnesium aluminum phyllosilicate: Palygorskite. J. Chem. Thermodyn. 2015, 89, 205–211. [Google Scholar] [CrossRef]
- Zhuang, G.Z.; Li, L.; Li, M.Y.; Yuan, P. Influences of micropores and water molecules in the palygorskite structure on the color and stability of Maya blue pigment. Microporous Mesoporous Mater. 2022, 330, 111615. [Google Scholar] [CrossRef]
- Mo, X.X.; Takahashi, Y.; Siebecker, M.; Gou, W.X.; Wang, Z.; Lu, X.C.; Li, W. In situ/operando XAFS investigation of the sorption/precipitation of Zn (II) on palygorskite surface at the molecular scale: Implications for Zn stable isotope fractionation. Geochim. Cosmochim. Acta 2023, 349, 64–80. [Google Scholar] [CrossRef]
- Su, L.; Shen, Y.; Liu, Z.; Sheng, X.; Zhu, Y.; Wang, J.; Liu, J.; Wang, M. Effects of palygorskite on steroid-transformation in mycobacterium neoaurum. Appl. Clay Sci. 2023, 233, 106839. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhang, H.R.; Peng, F.; Wang, C.; Li, H.L.; Xiong, L.; Guo, H.J.; Chen, X.D. Monoethanolamine-modified attapulgite-based amorphous silica for the selective adsorption of CO2 from simulated biogas. Energ. Fuel. 2020, 34, 2097–2106. [Google Scholar] [CrossRef]
- Giustetto, R.; Wahyudi, O. Sorption of red dyes on palygorskite: Synthesis and stability of red/purple mayan nanocomposites. Microporous Mesoporous Mater. 2011, 142, 221–235. [Google Scholar] [CrossRef]
- Su, Q.; Jia, W.; Lu, Z.; Qi, B.; Wang, Y.; Wang, C.; Nian, J.; Ren, F.; Zhao, J.; Liang, J. Evolution mechanism of SO2 adsorption on palygorskite (100) surface modified by APTES: Effect of different grafting modification. Comput. Theor. Chem. 2023, 1224, 114130. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Tan, X.; Jiang, L.; Zeng, G.; Liu, S.; Tian, S.; Liu, S.; Liu, N.; Li, M. Adsorption of 17β-estradiol by a novel attapulgite/biochar nanocomposite: Characteristics and influencing factors. Process Saf. Environ. Prot. 2019, 121, 155–164. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, P.; Zhao, S. Magnetic ATP/FA/Poly (AA-co-AM) ternary nanocomposite microgel as selective adsorbent for removal of heavy metals from wastewater. Colloid. Surface. A 2015, 470, 31–38. [Google Scholar] [CrossRef]
- Rafiq, M.K.; Joseph, S.D.; Li, F.; Bai, Y.; Shang, Z.; Rawal, A.; Hook, J.M.; Munroe, P.R.; Donne, S.; Taherymoosavi, D.R.; et al. Pyrolysis of attapulgite clay blended with yak dung enhances pasture growth and soil health: Characterization and initial field trials. Sci. Total Environ. 2017, 607, 184–194. [Google Scholar] [CrossRef]
- Otunola, B.O.; Ololade, O.O. A review on the application of clay minerals as heavy metal adsorbents for remediation purposes. Environ. Technol. Innov. 2020, 18, 100692. [Google Scholar] [CrossRef]
- Antosik, A.K.; Kucharska, E.; Mozelewska, K. Study of applyingnaturally occurring mineral materials for silicone pressure-sensitive adhesives. Materials 2023, 16, 2092. [Google Scholar] [CrossRef]
- Pan, C.G.; Liu, P. Surface modification of attapulgite nanorods with nitrile butadiene rubber via thiolene interfacial click reaction: Grafting or crosslinking. Ind. Eng. Chem. Res. 2018, 57, 4949–4954. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Iqbal, S.; Nadeem, S.; Bano, R.; Bahadur, A.; Ahmad, Z.; Javed, M.; Al-Anazy, M.M.; Qasier, A.A.; Laref, A.; Shoaib, M.; et al. Green synthesis of biodegradable terpolymer modified starch nanocomposite with carbon nanoparticles for food packaging application. J. Appl. Polym. Sci. 2021, 138, 50604. [Google Scholar] [CrossRef]
- Iqbal, S.; Nadeem, S.; Bahadur, A.; Javed, M.; Ahmad, Z.; Ahmad, M.N.; Shoaib, M.; Liu, G.; Mohyuddin, A.; Raheel, M. The effect of Ni-doped ZnO NPs on the antibacterial activity and degradation rate of polyacrylic acid-modified starch nanocomposite. JOM 2021, 73, 380–386. [Google Scholar] [CrossRef]
- Xu, J.K.; Wang, C.X.; Zhou, S.C.; Zhang, R.B.; Tian, Y.H. Low-temperature direct bonding of Si and quartz glass using the APTES modification. Ceram. Int. 2019, 45, 16670–16675. [Google Scholar] [CrossRef]
- Wang, R.G.; Li, Z.; Wang, Y.M.; Liu, W.B.; Deng, L.B.; Jiao, W.C.; Yang, F. Effects of modified attapulgite on the properties of attapulgite/epoxy nanocomposites. Polym. Compos. 2013, 34, 22–31. [Google Scholar] [CrossRef]
- Xue, A.L.; Zhou, S.Y.; Zhao, Y.J.; Lu, X.P.; Han, P.G. Effective NH2-grafting on attapulgite surfaces for adsorption of reactive dyes. J. Hazard Mater. 2011, 194, 7–14. [Google Scholar] [CrossRef]
- Jesionowski, T.; Krysztafkiewicz, A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. 2001, 172, 18–32. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, J.; Chu, H.Q.; Zhou, X.F.; Wei, Y. Effect of modified attapulgite addition on the performance of a PVDF ultrafiltration membrane. Desalination 2014, 344, 71–78. [Google Scholar] [CrossRef]
- Moreira, M.A.; Ciuffi, K.J.; Rives, V.; Vicente, M.A.; Trujillano, R.; Gil, A.; Korili, S.A.; De Faria, E.H. Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl. Surf. Sci. 2017, 135, 394–404. [Google Scholar] [CrossRef]
- Tadiello, L.; D’Arienzo, M.; Di Credico, B.; Hanel, T.; Matejka, L.; Mauri, M.; Morazzoni, F.; Simonutti, R.; Spirkova, M.; Scotti, R. The filler-rubber interface in styrene butadiene nanocomposites with anisotropic silica particles: Morphology and dynamic properties. Soft Matter 2015, 11, 4022–4033. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Wang, Y.; Wu, H.C.; Cai, J.B.; Xu, S.S. Molecular dynamics simulation on the interaction between palygorskite coating and linear chain alkane base lubricant. Coatings 2021, 11, 286. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Cheng, X.C.; Jin, Y.L.; Wu, J.; Zhao, D.S. Molecular dynamics simulation on interacting and mechanical properties of polylactic acid and attapulgite (100) surface. J. Appl. Polym. Sci. 2013, 128, 3043–3049. [Google Scholar] [CrossRef]
- Henderson, M. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Guan, K.S. Relationship between photocatalytic activity. Surf. Coat. Tech. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, A.; Pan, R. The Crystallography and Mineralogy; Geological Press: Beijing, China, 1998. [Google Scholar]
- Zhou, J.; Lu, X.; Boek, E.S. Confined water in tunnel nanopores of sepiolite: Insights from molecular simulations. Am. Mineral. 2016, 101, 713–718. [Google Scholar] [CrossRef]
- Peng, J.; Sun, H.; Wang, J.; Qiu, F.; Zhang, P.; Ning, W.; Zhang, D.; Li, W.; Wei, C.; Miao, S. Highly stable and recyclable sequestration of CO2 using supported melamine on layered-chain clay mineral. ACS Appl. Mater. Interfaces 2021, 13, 10933–10941. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, B.; Peng, Y.; Luo, X.; Huang, Y.; Jin, B.; Gao, H.; Liang, Z.; Hu, X.; Zhou, Y. CO2 adsorption behavior of 3-aminopropyltrimethoxysilane-functionalized attapulgite with the grafting modification method. Ind. Eng. Chem. Res. 2021, 60, 17150–17161. [Google Scholar] [CrossRef]
- Benková, Z.; Natália, D. Molecular dynamics study of poly (ethylene oxide) chains densely grafted on siloxane surface in dry conditions. J. Phys. Chem. C. 2012, 116, 3576–3584. [Google Scholar] [CrossRef]
- Kasprzhitskii, A.; Lazorenko, G.; Kruglikov, A.; Kuchkina, I.; Gorodov, V. Effect of silane functionalization on properties of poly (lactic acid)/palygorskite nanocomposites. Inorganics 2021, 9, 3. [Google Scholar] [CrossRef]
- Dkhissi, A.; Estève, A.; Jeloaica, L.; Estève, D.; Rouhani, M.D. Grafting of chains organo-silane on silica surface: A quantum chemical investigation. Chem. Phys. Lett. 2004, 400, 353–356. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, X. Theoretical study on the interactions between silica and the products of 3-mercaptopropyltriethoxysilane (MPTS) with different hydrolysis degrees. Appl. Surf. Sci. 2020, 502, 143853. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Adolph, H.G. The relationship of Impact Sensitivity with structure of organic high explosives. II. polynitroaromatic explosives. Propell. Explos. Pyrot. 1979, 4, 30–34. [Google Scholar] [CrossRef]
- Shoaf, A.L.; Bayse, C.A. Trigger bond analysis of nitroaromatic energetic materials using wiberg bond indices. J. Comput. Chem. 2018, 39, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Xing, J.D.; Ding, S.F.; Su, W. Stability, electronic and mechanical properties of Fe2B. Phys. B 2008, 403, 1723–1730. [Google Scholar] [CrossRef]
- Hug, G. Electronic structures of and composition gaps among the ternary carbides Ti2MC. Phys. Rev. B 2006, 74, 3840–3845. [Google Scholar] [CrossRef]
- Agarwal, A.; Das, S.; Rao, S.; Sen, D. Erratum: Enhancement of tunneling density of states at a junction of three luttinger liquid wires. Phys. Rev. Lett. 2009, 103, 026401. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Antosik, A.K.; Makuch, E.; Gziut, K. Influence of modified attapulgite on silicone pressure-sensitive adhesives properties. J. Polym. Res. 2022, 29, 135. [Google Scholar] [CrossRef]
- Zhu, J.X.; Zhang, P.; Wang, Y.B.; Wen, K.; Su, X.L.; Zhu, R.L.; He, H.P.; Xi, Y.F. Effect of acid activation of palygorskite on theirtoluene adsorption behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Hernández, D.; Quiñones, L.; Lazo, L.; Charnay, C.; Velázquez, M.; Altshuler, E.; Rivera, A. Removal of an emergent contaminant by a palygorskite from pontezuela/cuban region. J. Porous Mater. 2022, 32, 1149–1161. [Google Scholar] [CrossRef]
- Pardo-Canales, L.; Essih, S.; Cecilia, J.A.; Domínguez-Maqueda, M.; Olmo-Sánchez, M.I.; Pozo-Rodríguez, M.; Franco, F. Modification of the textural properties of palygorskite through microwave assisted acid treatment. Influence of the octahedral sheet composition. Appl. Clay Sci. 2020, 196, 105745. [Google Scholar] [CrossRef]
- Peng, J.; Wei, C.; Li, X.; Zhang, P.; Sun, Y.; Guo, R.; Li, W.; Gao, Q.; Miao, S. Insight into catalyst of tetramethylguanidine decorated palygorskite for CO2 conversion assisted with zinc halides. Appl. Clay Sci. 2022, 228, 106626. [Google Scholar] [CrossRef]
- Liu, Y.L.; Deng, Y.; Zheng, J.L.; Wang, H.; Wu, F.Z.; Lu, J.; Sun, S.Y. Micro mechanism of latent heat enhancement of polyethylene glycol/aminated modified palygorskite composite phase change materials. Appl. Surf. Sci. 2022, 228, 106641. [Google Scholar] [CrossRef]
- Karamanis, I.; Daouli, A.; Monnier, H.; Dziurla, M.A.; Maurin, G.; Badawi, M. A systematic DFT screening of cationic faujasite-type zeolites for the adsorption of NO, NO2 and H2O. Mol. Syst. Des. Eng. 2023. [Google Scholar] [CrossRef]
- Fan, H.; Frank, E.S.; Lakey, P.J.; Shiraiwa, M.; Tobias, D.J.; Grassian, V.H. Heterogeneous interactions between carvone and hydroxylated SiO2. J. Phys. Chem. C 2022, 126, 6267–6279. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Y.; Wu, F.Z.; Dai, X.Y.; Wang, W.H.; Mai, Y.; Gu, Y.J.; Liu, Y.L. Effect of dopamine-modified expanded vermiculite on phase change behavior and heat storage characteristic of polyethylene glycol. Chem. Eng. J. 2021, 415, 128992. [Google Scholar] [CrossRef]
- Saputera, W.; Tahini, H.; Lovell, E.; Tan, T.; Rawal, A.; Aguey-Zinsou, K.; Friedmann, D.; Smith, S.; Amal, R.; Scott, J. Cooperative defect-enriched SiO2 for oxygen activation and organic dehydrogenation. J. Catal. 2019, 376, 168–179. [Google Scholar] [CrossRef]
- Gosiamemang, T.; Heng, J.Y. Sodium hydroxide catalysed silica solgel synthesis:physicochemical properties of silica nanoparticles and their post-grafting using C8 and C18 alkyl-organosilanes. Powder Technol. 2023, 112, 118237. [Google Scholar] [CrossRef]
- Delley, B.J. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zeng, J.P.; Zhang, J.Y.; Gong, X.D. Molecular dynamics simulation of interaction between benzotriazoles and cuprous oxide crystal. Comput. Theor. Chem. 2011, 963, 110–114. [Google Scholar] [CrossRef]
- Zeng, J.P.; Wang, F.H.; Zhou, C.; Gong, X.D. Molecular dynamics simulation on scale inhibition mechanism of polyepoxysuccinic acid to calcium sulphate. Chin. J. Chem. Phys. 2012, 25, 219. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
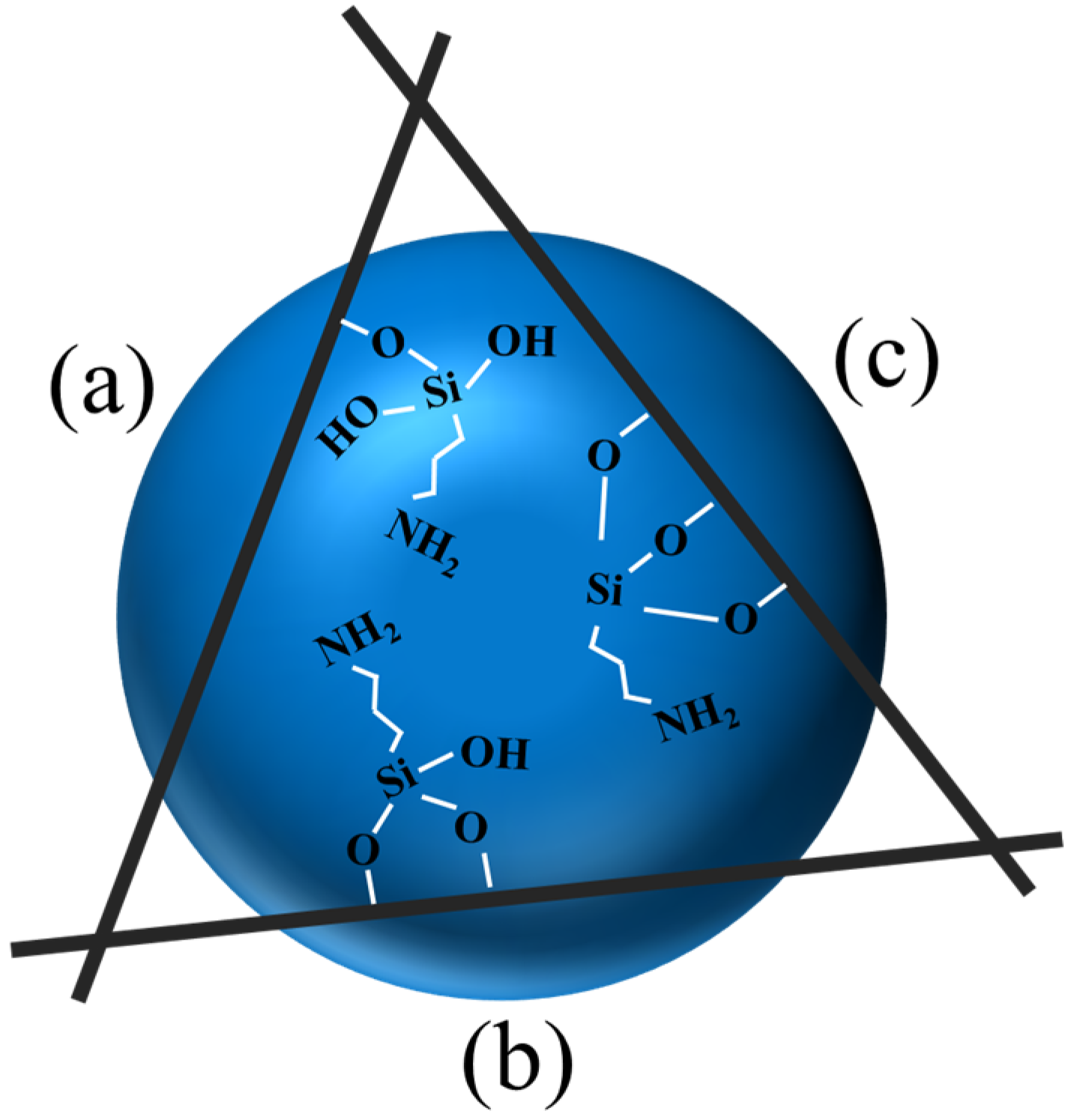

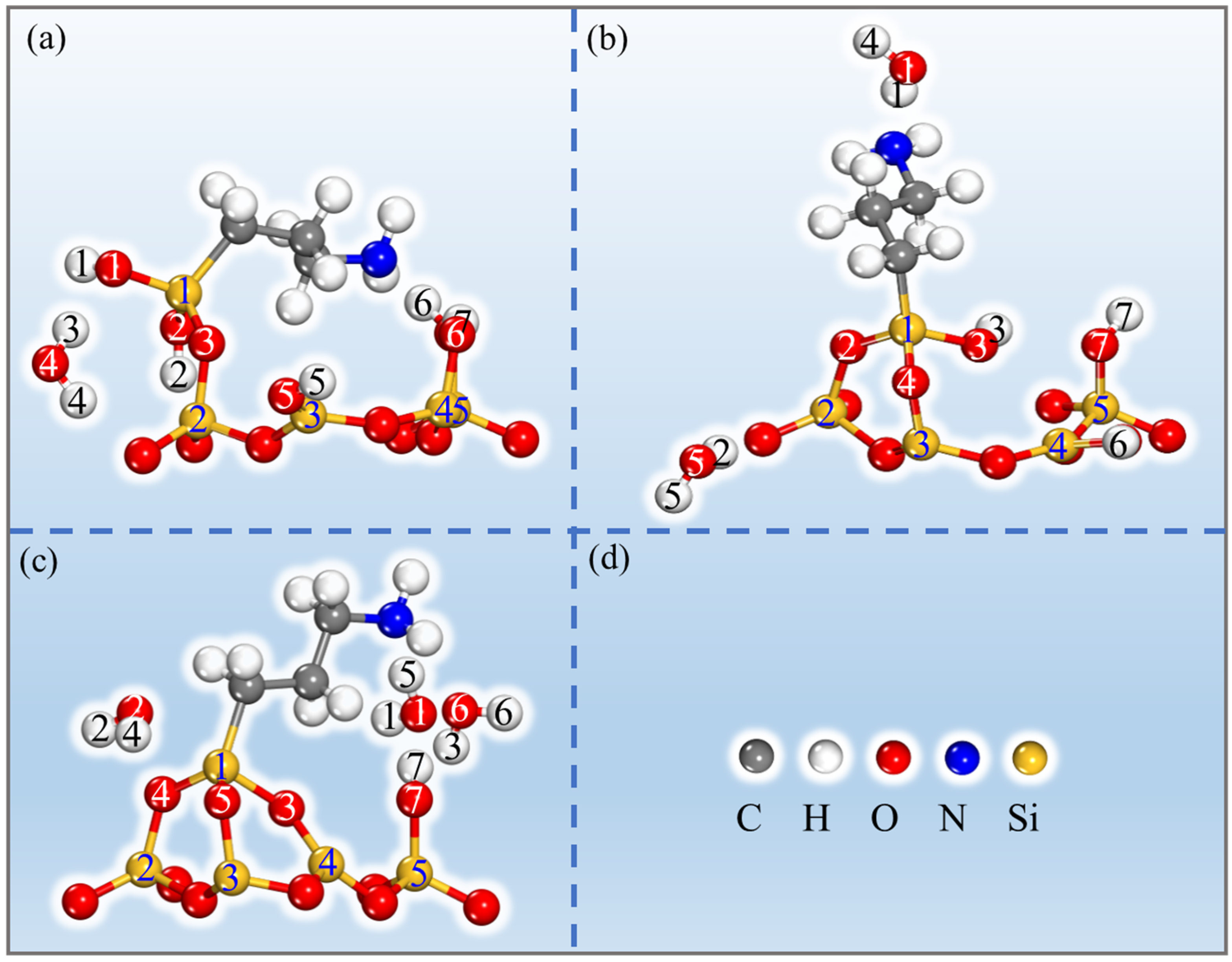
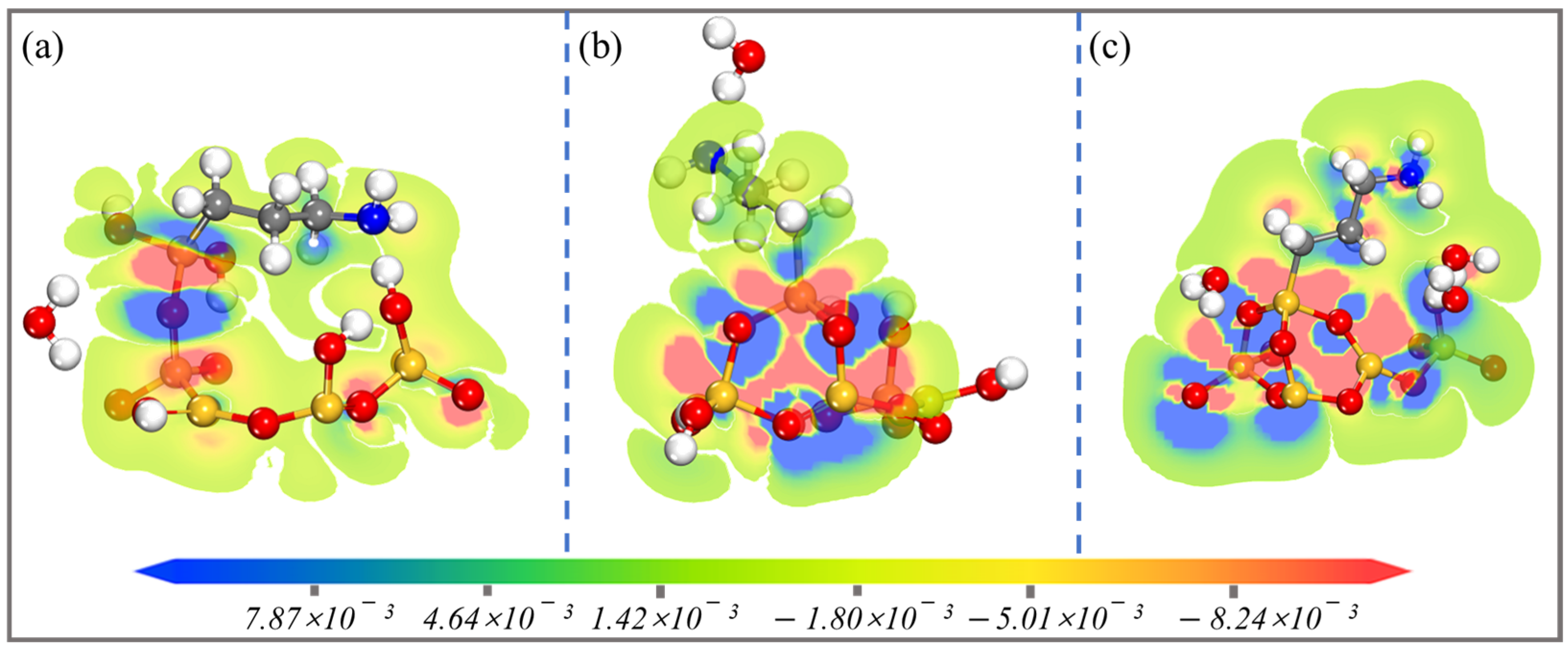
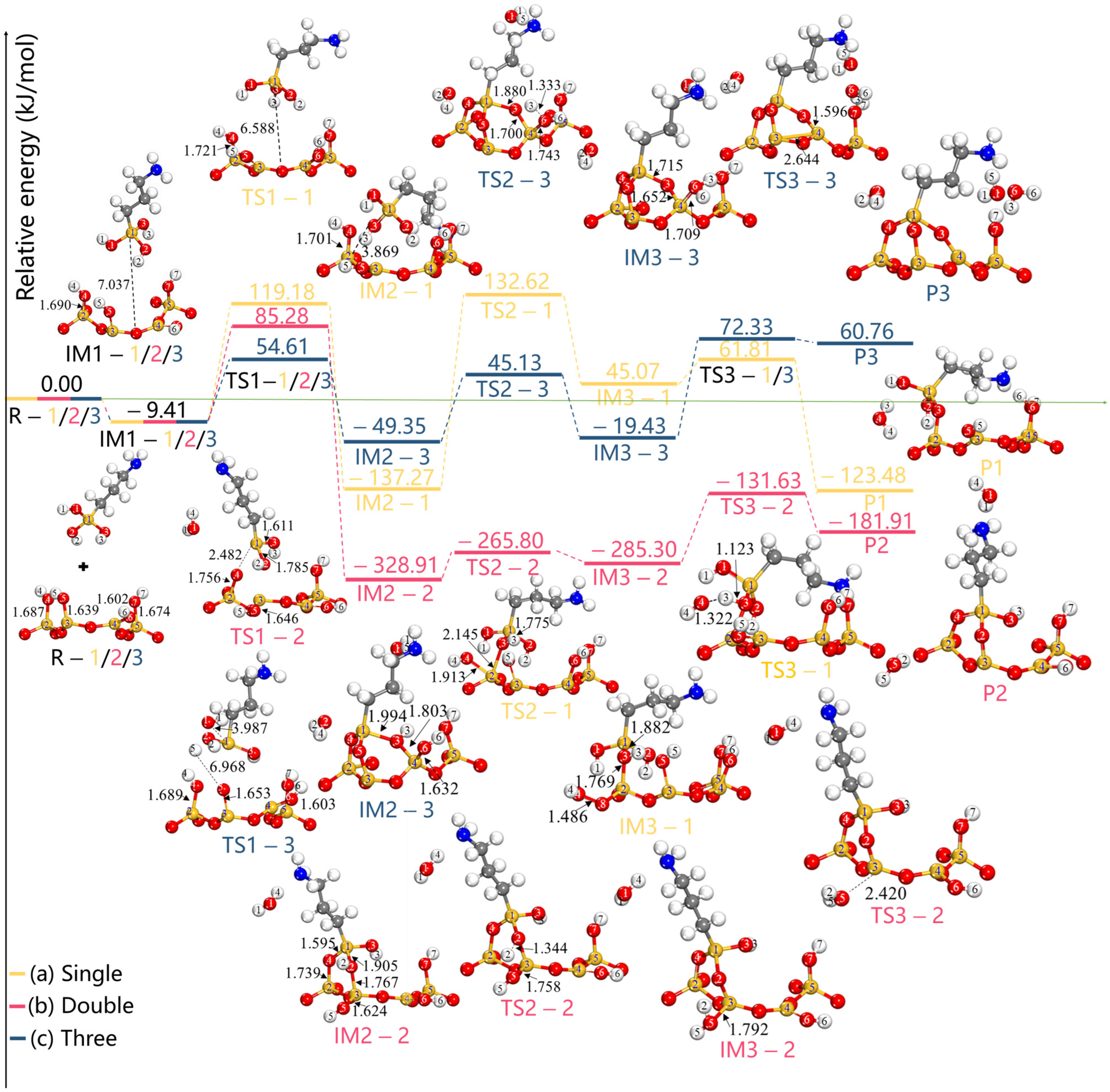
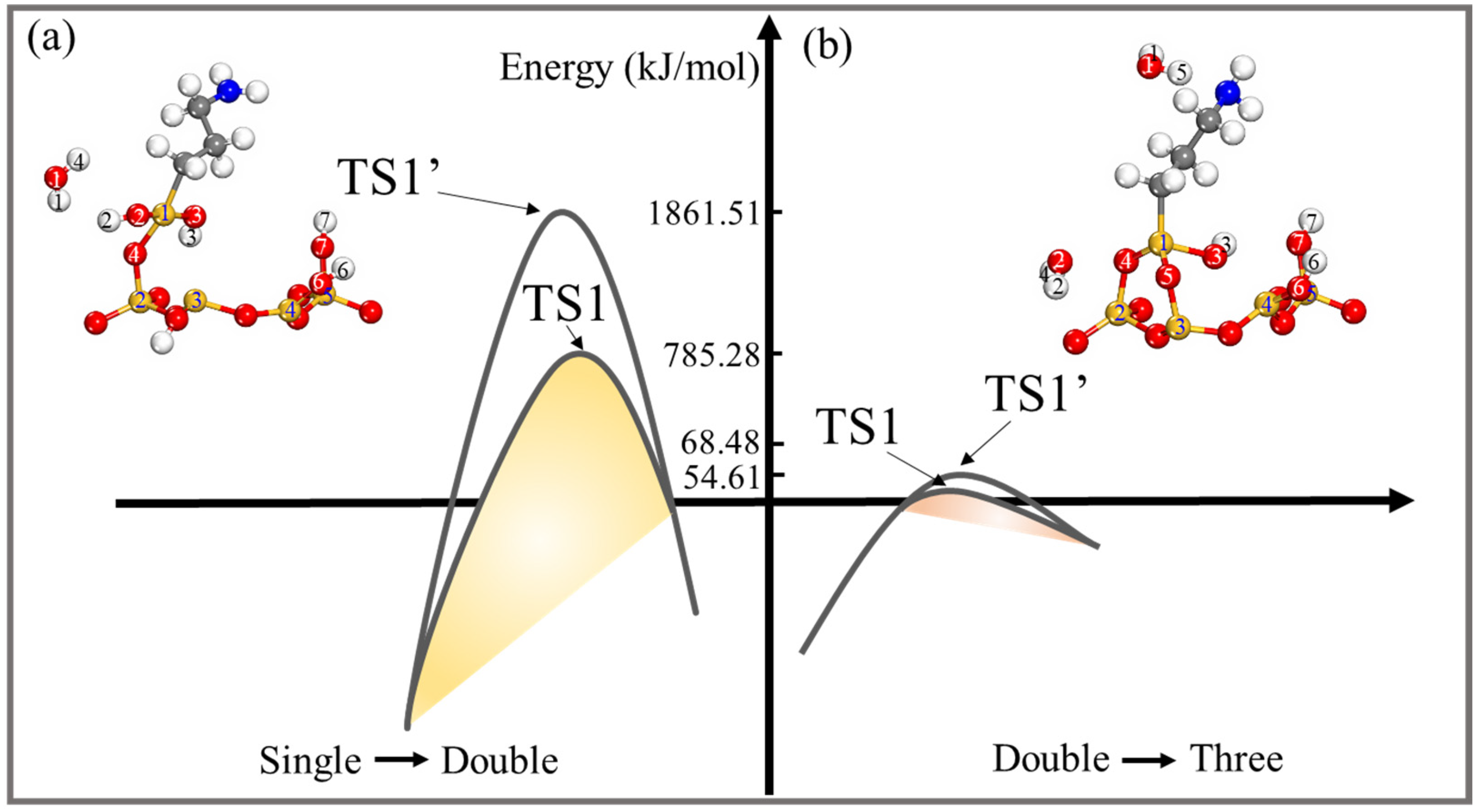
| Configurations | Ebind (kJ/mol) | Total Charge Transfer Qt (e) | Bond Type | Length (Å) |
|---|---|---|---|---|
| Single | −123.48 | 0.68 | Si1(APTES)-O3(APTES) | 1.679 |
| O3(APTES)-Si2(PAL) | 1.670 | |||
| Double | −181.91 | 1.02 | Si1(APTES)-O4(PAL) | 1.632 |
| Si1(APTES)-O2(APTES) | 1.723 | |||
| O4(PAL)-Si2(PAL) | 1.715 | |||
| O2(APTES)-Si3(PAL) | 1.622 | |||
| Three | 60.76 | 0.77 | Si1(APTES)-O3(PAL) | 1.802 |
| Si1(APTES)-O4(PAL) | 1.662 | |||
| Si1(APTES)-O5(PAL) | 1.690 | |||
| O3(APTES)-Si4(PAL) | 1.587 | |||
| O4(PAL)-Si2(PAL) | 1.683 | |||
| O5(PAL)-Si3(PAL) | 1.675 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Qi, B.; Wang, Y.; Lu, Z.; Wang, J.; Su, Q.; Nian, J.; Liang, J. Investigation on the Mechanism of PAL (100) Surface Modified by APTES. Molecules 2023, 28, 5417. https://doi.org/10.3390/molecules28145417
Jia W, Qi B, Wang Y, Lu Z, Wang J, Su Q, Nian J, Liang J. Investigation on the Mechanism of PAL (100) Surface Modified by APTES. Molecules. 2023; 28(14):5417. https://doi.org/10.3390/molecules28145417
Chicago/Turabian StyleJia, Weimin, Bomiao Qi, Yanbin Wang, Zhibin Lu, Jiqian Wang, Qiong Su, Jingyan Nian, and Junxi Liang. 2023. "Investigation on the Mechanism of PAL (100) Surface Modified by APTES" Molecules 28, no. 14: 5417. https://doi.org/10.3390/molecules28145417
APA StyleJia, W., Qi, B., Wang, Y., Lu, Z., Wang, J., Su, Q., Nian, J., & Liang, J. (2023). Investigation on the Mechanism of PAL (100) Surface Modified by APTES. Molecules, 28(14), 5417. https://doi.org/10.3390/molecules28145417