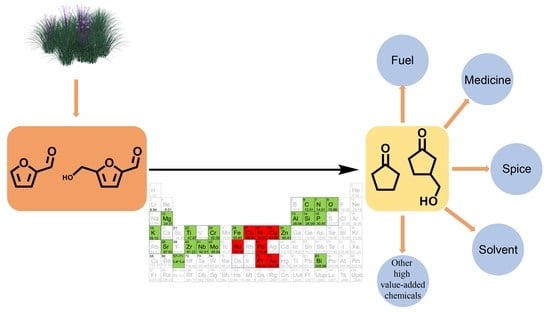
A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF
Abstract
1. Introduction
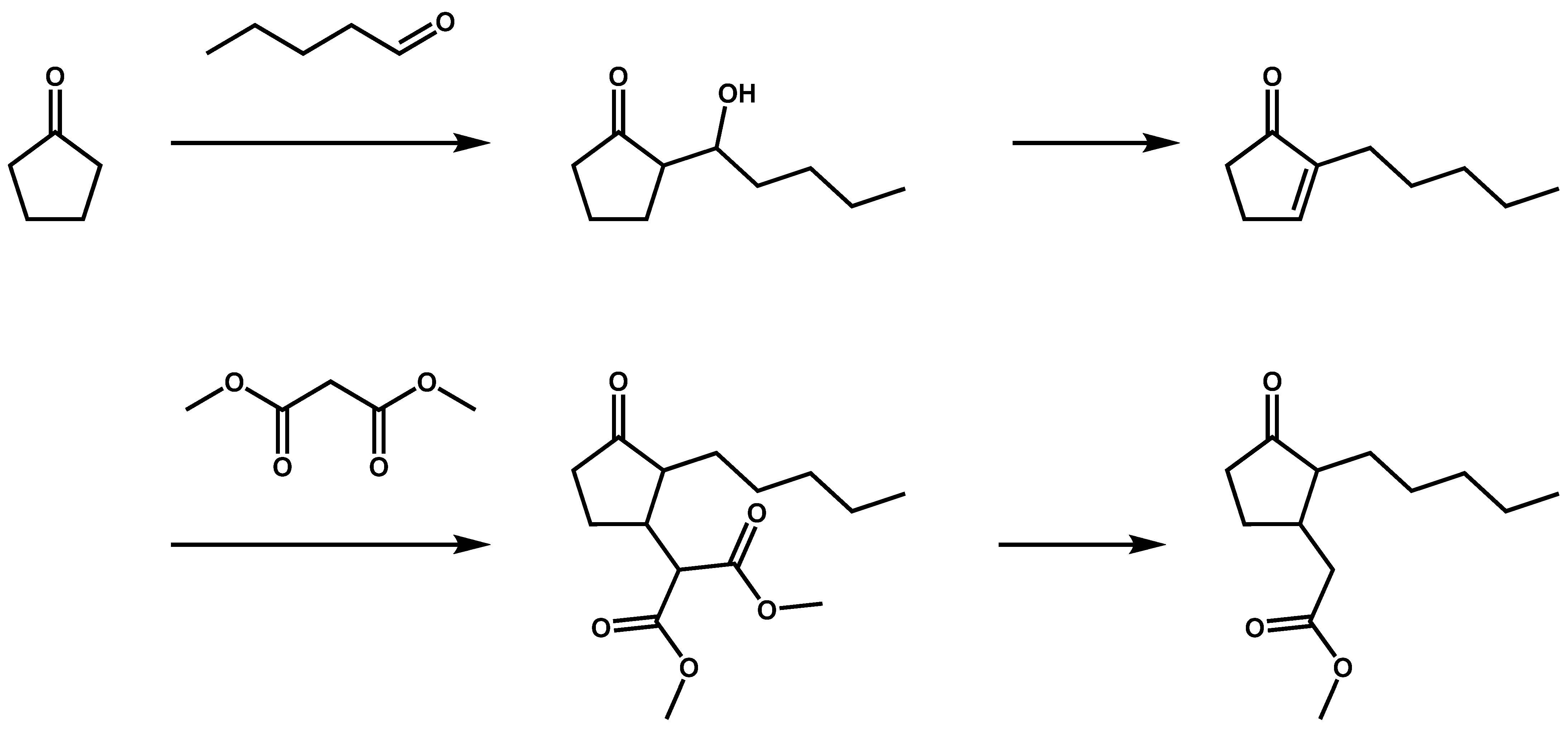
2. Application of Cyclopentanone Derivatives
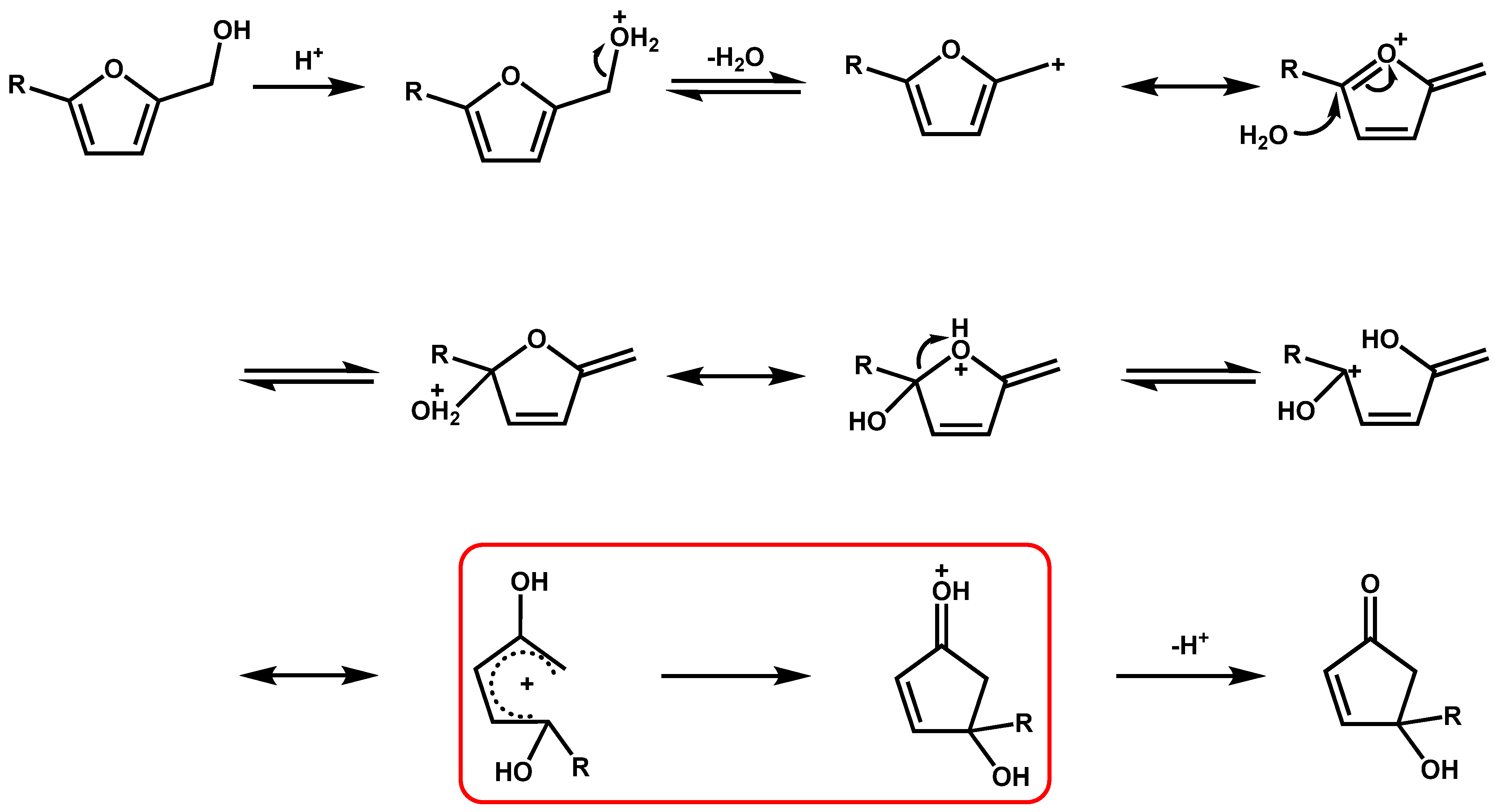
3. Mechanism for the Production of Cyclopentanone Derivatives
4. Reaction Conditions
5. Production of CPO from Furfural
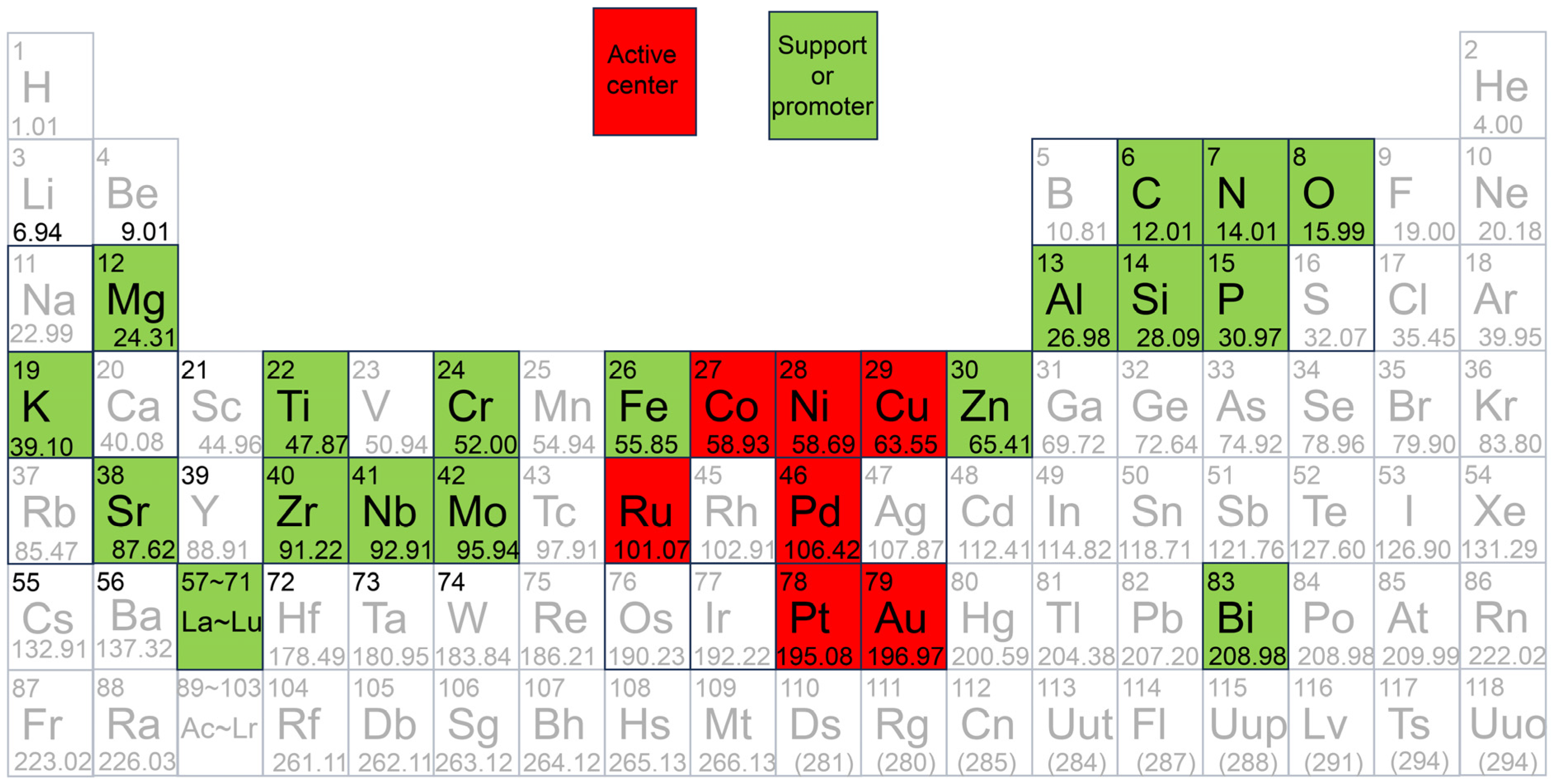
5.1. Single Non-Noble Metal Catalyst
5.1.1. Cu
5.1.2. Ni
5.1.3. Co
| Entry | Catalyst | Concn. a (wt.%) | T (°C) | t (h) | P (MPa) | Conv. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | CuZnAl-500-0.5 | 3.1 | 150 | 6 | 4 | >90 | 62 | [64] |
| 2 | Cu-Mg-Al | 5.5 | 140 | 10 | 4 | 98.5 | (93.4) | [65] |
| 3 | 20wt.%Ni/HY-0.018 | 5.0 | 150 | 9 | 4 | 96.5 | 86.5 | [72] |
| 4 | RANEY® Ni | 8.8 | 180 | 4 | 1 N2 | 94.4 | 75.4 | [86] |
| 5 | Cu/ZrO2 | 3.1 | 150 | 4 | 1.5 | 100 | 91.3 | [67] |
| 6 | 10%Cu/Fe3O4 | 1.0 | 170 | 4 | 3 | 100 | 91 | [68] |
| 7 | 50%Cu/Fe3O4 | 1.0 | 170 | 3 | 3 | 100 | (82) | [68] |
| 8 | Cu0.4Mg5.6Al2 | 4.6 | 180 | 5 | 0.2 | 100 | 98.1 | [66] |
| 9 | Cu0.4Mg5.6Al2 | 4.6 | 190 | 12 | 2 | 100 | (98.6) | [66] |
| 10 | Ni-NiO/TiO2-Re450 | 1.0 | 140 | 6 | 1 | 100 | 87.4 | [73] |
| 11 | Ni/CNTox1 | 2.2 | 200 | 4 | 2 | 94 | 64.9 | [75] |
| 12 | Ni/CNTox | 2.2 | 200 | 1 | 2 | 35 | 25 | [74] |
| 13 | Ni/SiC-CrCl3 | 2.3 | 160 | 2 | 3 | 99.9 | 88.1 | [77] |
| 14 | Cu0-Zn(Al)(Zr)O-2 | 4.8 | 160 | 3 | 2.5 | 100 | 92 | [69] |
| 15 | NiMo/CNT | 4.8 | 140 | 2 | 4 | 94 | (56.3) | [87] |
| 16 | Ni–P/γ-Al2O3 | 1.0 | 190 | 2 | 3 | 97.6 | 93.8 | [88] |
| 17 | 15%Ni + 10%P | 1.0 | 150 | 2 | 3 | 96.7 | 90.1 | [88] |
| 18 | Ni/CNTox | 2.2 | 200 | 2 | 10 | 6.3 | [76] | |
| 19 | 0.8%Ni/SiO2 | 1 | 160 | 2 | 3 | 34.2 | 33.6 | [81] |
| 20 | 2.0%Ni/SiO2 | 1 | 160 | 2 | 3 | 98.8 | 80.3 | [81] |
| 21 | Cu/ZnO-Al3 | 3.7 | 160 | 4 | 4 | >99 | (60.7) | [70] |
| 22 | Cu–K | 1.4 | 200 | 4 | 1 | 100 | 54.8 | [71] |
| 23 | Ni@HCS | 1 | 150 | 2 | 100 | 99.1 | [82] | |
| 24 | Ni@NP-C | 1.9 | 130 | 2 | 1.5 | 100 | 86.7 | [78] |
| 25 | Co@Co-NC | 4.1 | 150 | 6 | 4 | 99.9 | (95.1) | [83] |
| 26 | CI0.25-700 | 0.7 | 130 | 10 | 1.5 | 100 | 79.4 (20.4) | [84] |
| 27 | Ni3P/γ-Al2O3 | 4.8 | 180 | 1 | 4 | 99.9 | 74.6 | [79] |
| 28 | (Sr2P2O7)0.40/Ni2P | 1 | 150 | 2.2 | 0.1 | - | 96 | [80] |
| 29 | CoOx/Nb2O5 | 2.0 | 160 | 6 | 2 | 100 | 61 (21) | [85] |
5.2. Bimetallic Non-Noble Metal Catalyst
5.2.1. NiCu
5.2.2. Cu-Co
5.2.3. NiCo
5.2.4. Other Bimetallic Catalyst
| Entry | Catalyst | Concn. a (wt.%) | T (°C) | t (h) | P (MPa) | Conv. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | NiCu/SBA-15 | 5 | 160 | 4 | 4 | 99.9 | 62 | [57] |
| 2 | Cu-Ni-Al | 5.3 | 140 | 8 | 4 | 100 | 95.8 | [90] |
| 3 | Cu-Co-CP-500 | 1.9 | 170 | 1 | 2 | >99 | 67 | [95] |
| 4 | Cu-Co-OG-500 | 1.9 | 170 | 1 | 2 | >99 | (68) | [95] |
| 5 | CuNi0.5@C | 5 | 130 | 5 | 5 | 99.3 | 96.9 | [91] |
| 6 | Co@NCNTs | 0.9 | 140 | 5 | 4 | 100 | 75.3 | [101] |
| 7 | CuCo0.8@C-500 | 0.6 | 150 | 9 | 0.5 | 100 | 90.2 | [97] |
| 8 | 10%Co-10%Ni/TiO2 | 4.8 | 150 | 4 | 4 | 100 | 53.3 (16.3) | [98] |
| 9 | NiFe/SBA-15 | 5.7 | 160 | 2 | 3.4 | 99.8 | 90 | [62] |
| 10 | Ni2Cu1/Al2O3 | 3.2 | 140 | 1 | 1 | 100 | 89.5 | [94] |
| 11 | Co-Ni alloy | 1.6 | 150 | 6 | 1.5 | 100 | 92.5 | [99] |
| 12 | Cu-Ni/Al-MCM-41 | 1.2 | 160 | 5 | 2 | 99.0 | 96.7 | [89] |
| 13 | 1.5Co-1.5Fe-1.0Al | 2.5 | 170 | 1 | 3 | 100 | 53.6 (37.9) | [100] |
| 14 | Ni5Cu15/m-SiO2 | 3.2 | 140 | 4 | 3 | 99.9 | 89.6 | [92] |
| 15 | NiCu/SiO2-AE-450 | 3.2 | 150 | 6 | 2 | 100 | 95.4 | [93] |
| 16 | CuZn/CNT | 5.0 | 140 | 10 | 4 | 95 | 85 | [96] |
5.3. Single Noble Metal Catalyst
5.3.1. Pd
5.3.2. Pt
5.3.3. Au
5.3.4. Ru
| Entry | Catalyst | Concn. a (wt.%) | T (°C) | t (h) | P (MPa) | Conv. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5% Pt/C | 4.8 | 160 | 0.5 | 8 | 100 | 76.5 | [39] |
| 2 | 3 wt% Ru/MIL-101 | 9.0 | 160 | 2.5 | 4 | >99 | 96 | [116] |
| 3 | Au/TiO2-A | 4.8 | 160 | 4 | 4 | >99 | 99 | [115] |
| 4 | Pt/NC-BS-800 | 2.3 | 150 | 4 | 3 | >99 | 76 | [113] |
| 5 | Ru/CNTs | 1.1 | 160 | 3 | 1 | 99 | 87.4 | [118] |
| 6 | 4%Pd/f-SiO2 | 5 | 165 | 5 | 500 (psi) | 98 | 87.2 | [105] |
| 7 | Pt/C + Al11.6PO23.7 | 3.7 | 160 | 8 | 4 | 90 | 81.0 | [117] |
| 8 | Pd-Bi/SiO2 | 4.8 | 150 | 2.3 | 5 | - | 57 | [102] |
| 9 | Pd/Fe-MIL-100 | 2.4 | 150 | 6 | 4 | 99 | 92.2 | [106] |
| 10 | Pd/CNTs | 5 | 150 | 1 | 3 | 98.0 | 48.4 | [104] |
| 11 | Pd/TiO2 | 2.4 | 170 | 4 | 2 | >99 | 55.5 | [119] |
| 12 | Pd/Y2(Sn0.7Ce0.3)2O7-δ | 2.4 | 150 | 6 | 4 | 99.9 | 95.0 | [109] |
| 13 | Pd/CNTs | 4.4 | 150 | 1 | 3 | >99 | 43 | [103] |
| 14 | Pd/Cu-BTC | 2.4 | 150 | 6 | 4 | 96.4 | 93.0 | [120] |
| 15 | Pt/TiO2 | 2.5 | 170 | 2 | 2 | 98 | 49.4 | [114] |
| 16 | Y2(Sn0.65Al0.35)2O7-δ/Al2O3 | 2.3 | 150 | 6 | 4 | 99.9 | 98.1 | [110] |
| 17 | Pd/La2Ti2O7 | 2.4 | 150 | 6 | 4 | 99.5 | 82 | [111] |
| 18 | Pd/UiO-66-NO2 | 1.0 | 150 | 5 | 1 | 98.9 | 95.5 | [107] |
| 19 | 2% Pd/H–ZSM–5(25) | 2.3 | 160 | 3 | 93.7 | 91.8 | [108] | |
| 20 | Pd/CeO2/SiO2-10 | 2.5 | 150 | 3 | 2 | 93 | 78.1 | [112] |
5.4. Bimetallic Noble-Containing Catalyst
| Entry | Catalyst | Concn. a (wt.%) | T (°C) | t (h) | P (MPa) | Conv. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5% Pd + 10%Cu/C | 4.8 | 160 | 1 | 3 | 98 | 92.1 | [121] |
| 2 | Pt(3)Co(3)/C | 1 | 180 | 5 | 1 | 100 | 75 | [124] |
| 3 | Pd/FeZn-15 | 2.4 | 150 | 6 | 4 | 99.9 | 96.5 | [126] |
| 4 | RuMo/CNT | 4.8 | 160 | 4 | 4 | 100 | 9 (74) | [125] |
| 5 | Pd-Co@UiO-66 | 0.9 | 120 | 12 | 3 | 99 | 95.0 | [122] |
| 6 | Pd/NiMoO4-Cl | 2.4 | 150 | 6 | 4 | >99 | 85.3 | [123] |
| 7 | Pd-NiMoO4-AC | 2.4 | 150 | 6 | 4 | >99 | (85.2) | [123] |
6. Production of HCPN from HMF
6.1. Noble Metal Catalyst
6.2. Non-Noble Metal Catalyst
| Entry | Catalyst | Concn. a (wt.%) | T (°C) | t (h) | P (MPa) | Conv. (%) | Yield b (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Au/Nb2O5 | 0.8 | 140 | 12 | 8 | >99 | 86 | [40] |
| 2 | Pt/Nb2O5 | 0.8 | 140 | 12 | 8 | >99 | 28 | [40] |
| 3 | Pd/Nb2O5 | 0.8 | 140 | 12 | 8 | >99 | 43 | [40] |
| 4 | Ru/Nb2O5 | 0.8 | 140 | 12 | 8 | >99 | 66 | [40] |
| 5 | Pt/SiO2 + Ta2O5 | 0.8 | 140 | 12 | 3 | 100 | 82 | [127] |
| 6 | Pt/SiO2 + Nd2O3 | 0.8 | 140 | 30 | 3 | 100 | 88 | [128] |
| 7 | Cu-Al2O3 | 0.5 | 180 | 6 | 2 | 100 | 86 | [129] |
| 8 | Co-Al2O3 | 0.5 | 140 | 48 | 2 | 100 | (94) | [129] |
| 9 | Pd/Cu-BTC | 3.2 | 150 | 24 | 4 | 99.5 | 90.4 | [120] |
| 10 | Pd/Fe-MIL-100 | 3.2 | 150 | 24 | 4 | 99.9 | 85.4 | [106] |
| 11 | Pd/FeZn-15 | 3.2 | 150 | 24 | 4 | 99.9 | 87.4 | [126] |
| 12 | Ni-Cu/C | 4.8 | 140 | 5 | 2 | >99 | 70.3 | [131] |
| 13 | Pd/Y2(Sn0.7Ce0.3)2O7-δ | 3.2 | 150 | 12 | 4 | 99.9 | 92.4 | [109] |
| 14 | Ni-Fe/Al2O3 | 2.1 | 160 | 4 | 4 | 100 | 86 | [130] |
| 15 | Pd/La2Ti2O7 | 3.2 | 150 | 6 | 4 | 99.8 | 82.3 | [111] |
| 16 | Ni0.5Co0.5@C | 1.2 | 140 | 7 | 2 | 99 | 92 | [132] |
7. Conclusions
- (1)
- Non-noble metals Cu, Ni, and Co showed different catalytic performances in the reaction. Cu has high selectivity for the reaction and generates no furan ring hydrogenation and carbon–oxygen bond cleavage by-products, but the catalytic activity of Cu for the reaction was low. Ni and Co had higher activity but were prone to furan ring hydrogenation and other side reactions. Moreover, Co was difficult to stay at the cyclopentanone derivative stage, and the products were usually further hydrogenated to form cyclopentanol derivatives.
- (2)
- Since the furan rearrangement reaction requires a high temperature, high temperature is still needed for noble metal catalysts. The most studied catalyst for this reaction is the noble metal Pd. Pd catalysts have high catalytic hydrogenation activity for the aldehyde groups in HMF, and furfural and can easily obtain cyclopentanone derivatives with high yields. However, Pd catalysts still need to be carefully designed to avoid the production of furan ring hydrogenation products. Compared with Pd, Au catalysts have lower activity, but are less sensitive to furan ring hydrogenation and carbon–oxygen bond cleavage and can easily obtain higher yields of cyclopentanone derivatives.
- (3)
- Bimetallic catalysts combine the advantages of different metal catalytic centers, suppress unfavorable factors, and obtain higher catalytic performance than monometallic catalysts by adjusting the electronic structure of the active center through the interaction between the metals. On the other hand, the addition of a second metal component can reduce the amount of noble metal used and is conducive to reducing the reaction cost.
- (4)
- The support plays an important role in the metal hydrogenation of catalysts. In addition to being able to disperse and stabilize the metal active centers, more importantly, it can provide the acidic sites required for the rearrangement reaction. The Lewis acid site is more favorable for the reaction than the Bronsted acid.
8. Prospection
- (1)
- The role of each component of the catalyst in the furfural and HMF conversion reaction needs to be further studied in detail. What other roles does the catalytic hydrogenation metal active center have in addition to providing the hydrogenation site? Different hydrogenation centers often have great selectivity differences in addition to the different hydrogenation activities. It indicates that the metal catalyst still has other roles in the reaction process. Uncovering these roles will guide the design of high-performance catalysts. Recent studies have found some factors, such as the generation of acidic sites through the hydrogen spillover effect.
- (2)
- The Piancatelli rearrangement, a key step of the furfural-prepared cyclopentanone derivative reaction, is a ring opening followed by the electrocyclic reaction. Combining traditional thermal catalysis with microwave-assisted reactions, photocatalysis/electrocatalysis, etc., may promote the rearrangement reaction at lower temperatures, thus inhibiting the occurrence of side reactions and obtaining cyclopentanone more efficiently.
- (3)
- The concentration of reactants is one of the crucial indicators for industrial applications. Most of the catalytic systems reported up to now have a reactant concentration of no more than 5%. Few studies have been conducted under high-concentration conditions. Under high-concentration conditions, it is necessary to accurately design the catalyst to ensure catalytic activity and avoid catalyst deactivation, and more attention should be paid to selective regulation. In addition, it is necessary to solve the problem that intermediate products are more prone to aggregation under high-concentration conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviation
| CPL | cyclopentanol |
| CPO | cyclopentanone |
| FOL | furfuryl alcohol |
| HCPL | 3-(hydroxymethyl)cyclopentanol |
| HCPN | 3-hydroxymethylcyclopentanone |
| HMF | 5-hydroxymethylfurfural |
| MOF | metal–organic framework |
| THFA | tetrahydrofurfuryl alcohol |
| THFDM | 2,5-dihydroxymethyl tetrahydrofurfuryl alcohol |
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic transformations of lignocellulosic biomass into chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. BRJ 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef]
- Endot, N.A.; Junid, R.; Jamil, M.S.S. Insight into biomass upgrade: A review on hydrogenation of 5-hydroxymethylfurfural (hmf) to 2,5-dimethylfuran (DMF). Molecules 2021, 26, 6848. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-Lukasik, E.; Bogel-Lukasik, R. Ionic liquid-mediated formation of 5-hydroxymethylfurfural-A promising biomass-derived building block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sadaba, I.; Lopez Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuels Bioprod. Biorefin. 2008, 2, 438–454. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfuralu a promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, K. Recent advances in the catalytic synthesis of 2,5-furandicarboxylic acid and its derivatives. ACS Catal. 2015, 5, 6529–6544. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid Using O2 and a photocatalyst of Co-thioporphyrazine bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef]
- Sajid, M.; Zhao, X.; Liu, D. Production of 2,5-furandicarboxylic acid (FDCA) from 5-hydroxymethylfurfural (HMF): Recent progress focusing on the chemical-catalytic routes. Green Chem. 2018, 20, 5427–5453. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Daniel, R.; Ghafourian, A.; Martin Herreros, J.; Shuai, S.; Ma, X. Combustion characteristics and emissions of 2-methylfuran compared to 2,5-dimethylfuran, gasoline and ethanol in a DISI engine. Fuel 2013, 103, 200–211. [Google Scholar] [CrossRef]
- Thananatthanachon, T.; Rauchfuss, T.B. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from Fructose Using Formic Acid as a Reagent. Angew. Chem. Int. Ed. 2010, 49, 6616–6618. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Rackemann, D.W.; Doherty, W.O.S. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefin. 2011, 5, 198–214. [Google Scholar] [CrossRef]
- Pileidis, F.D.; Titirici, M.M. Levulinic acid biorefineries: New challenges for efficient utilization of biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wu, X.-P.; Tong, T.; Shao, Z.-J.; Wang, Y.; Liu, X.; Xia, Q.; Gong, X.-Q. The critical role of water in the ring opening of furfural alcohol to 1,2-pentanediol. ACS Catal. 2016, 7, 333–337. [Google Scholar] [CrossRef]
- Date, N.S.; Chikate, R.C.; Roh, H.S.; Rode, C.V. Bifunctional role of Pd/MMT-K 10 catalyst in direct transformation of furfural to 1,2-pentanediol. Catal. Today 2018, 309, 195–201. [Google Scholar] [CrossRef]
- Fu, X.M.; Ren, X.Q.; Shen, J.C.; Jiang, Y.; Wang, Y.H.; Orooji, Y.; Xu, W.L.; Liang, J.H. Synergistic catalytic hydrogenation of furfural to 1,2-pentanediol and 1,5-pentanediol with LDO derived from CuMgAl hydrotalcite. Mol. Catal. 2021, 499, 111298. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Lv, X.; Gao, X.; Duan, Y.; Sui, D.; Yang, Y. Catalytic hydrogenation of 5-hydroxymethylfurfural to hexanetriol. ChemistrySelect 2022, 7, e202103797. [Google Scholar] [CrossRef]
- Yao, S.; Wang, X.; Jiang, Y.; Wu, F.; Chen, X.; Mu, X. One-step conversion of biomass-derived 5-hydroxymethylfurfural to 1,2,6-hexanetriol over Ni–Co–Al mixed oxide catalysts under mild conditions. ACS Sustain. Chem. Eng. 2013, 2, 173–180. [Google Scholar] [CrossRef]
- Buntara, T.; Noel, S.; Phua, P.H.; Melian-Cabrera, I.; de Vries, J.G.; Heeres, H.J. From 5-Hydroxymethylfurfural (HMF) to polymer precursors: Catalyst screening studies on the conversion of 1,2,6-hexanetriol to 1,6-hexanediol. Top. Catal. 2012, 55, 612–619. [Google Scholar] [CrossRef]
- Ren, D.; Song, Z.; Li, L.; Liu, Y.; Jin, F.; Huo, Z. Production of 2,5-hexanedione and 3-methyl-2-cyclopenten-1-one from 5-hydroxymethylfurfural. Green Chem. 2016, 18, 3075–3081. [Google Scholar] [CrossRef]
- Liu, F.; Audemar, M.; De Oliveira Vigier, K.; Clacens, J.-M.; De Campo, F.; Jérôme, F. Combination of Pd/C and Amberlyst-15 in a single reactor for the acid/hydrogenating catalytic conversion of carbohydrates to 5-hydroxy-2,5-hexanedione. Green Chem. 2014, 16, 4110–4114. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, D.; Zhang, C.; Zheng, M.; Duan, Y. Preparation of 1-hydroxy-2,5-hexanedione from HMF by the combination of commercial Pd/C and acetic acid. Molecules 2020, 25, 2475. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, C.; Deng, D.; Sui, D.; Gao, X.; Yang, Y. Hydrogenation of BHMF with controllable selectivity to tetrahydropyranone and 1-hydroxy-2,5-hexanedione under atmospheric H2 pressure. Green Chem. 2023, 25, 1823–1834. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, M.; Li, D.; Deng, D.; Ma, L.-F.; Yang, Y. Conversion of HMF to methyl cyclopentenolone by the Pd/Nb2O5 and Ca-Al catalysts via two-steps procedure. Green Chem. 2017, 19, 5103–5113. [Google Scholar] [CrossRef]
- Liang, L.X.; Guo, L.D.; Tong, R.B. Achmatowicz rearrangement-inspired development of green chemistry, organic methodology, and total synthesis of natural products. Acc. Chem. Res. 2022, 55, 2326–2340. [Google Scholar] [CrossRef]
- Zhao, G.D.; Liang, L.X.; Wang, E.Y.; Tong, R.B. Fenton chemistry for achmatowicz rearrangement. ACS Catal. 2021, 11, 3740–3748. [Google Scholar] [CrossRef]
- Kim, S.; Oiler, J.; Xing, Y.L.; O’Doherty, G.A. De novo asymmetric Achmatowicz approach to oligosaccharide natural products. Chem. Commun. 2022, 58, 12913–12926. [Google Scholar] [CrossRef] [PubMed]
- Bielski, R.; Grynkiewicz, G. Half a century with Achmatowicz rearrangement. Tetrahedron 2021, 85, 132058. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Ohyama, J.; Kanao, R.; Esaki, A.; Satsuma, A. Conversion of 5-hydroxymethylfurfural to a cyclopentanone derivative by ring rearrangement over supported Au nanoparticles. Chem. Commun. 2014, 50, 5633–5636. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic transformation of biomass-derived furfurals to cyclopentanones and their derivatives: A review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef] [PubMed]
- Hronec, M.; Fulajtarova, K.; Liptaj, T.; Stolcova, M.; Pronayova, N.; Sotak, T. Cyclopentanone: A raw material for production of C-15 and C-17 fuel precursors. Biomass Bioenergy 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Li, G.Y.; Li, N.; Wang, X.K.; Sheng, X.R.; Li, S.S.; Wang, A.Q.; Cong, Y.; Wang, X.D.; Zhang, T. Synthesis of diesel or jet fuel range cycloalkanes with 2-methylfuran and cyclopentanone from lignocellulose. Energy Fuels 2014, 28, 5112–5118. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K.; Liptaj, T.; Pronayova, N.; Sotak, T. Bio-derived fuel additives from furfural and cyclopentanone. Fuel Process. Technol. 2015, 138, 564–569. [Google Scholar] [CrossRef]
- Deng, Q.; Xu, J.S.; Han, P.J.; Pan, L.; Wang, L.; Zhang, X.W.; Zou, J.J. Efficient synthesis of high-density aviation biofuel via solvent-free aldol condensation of cyclic ketones and furanic aldehydes. Fuel Process. Technol. 2016, 148, 361–366. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K.; Liptaj, T.; Sotak, T.; Pronayova, N. Nickel catalysed hydrogenation of aldol condensation product of furfural with cyclopentanone to C-15 cyclic ethers. ChemistrySelect 2016, 1, 331–336. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Diaz, E.; Ordonez, S. Cyclopentanone as an alternative linking reactant for heterogeneously catalyzed furfural aldol condensation. ChemCatChem 2017, 9, 1765–1770. [Google Scholar] [CrossRef]
- Wang, W.; Ji, X.H.; Ge, H.G.; Li, Z.Z.; Tian, G.H.; Shao, X.Z.; Zhang, Q. Synthesis of C-15 and C-10 fuel precursors with cyclopentanone and furfural derived from hemicellulose. RSC Adv. 2017, 7, 16901–16907. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Diaz, E.; Ordonez, S. Enhancement of furfural-cyclopentanone aldol condensation using binary water-ethanol mixtures as solvent. J. Chem. Technol. Biotechnol. 2018, 93, 1563–1571. [Google Scholar] [CrossRef]
- Jing, Y.X.; Xia, Q.N.; Xie, J.J.; Liu, X.H.; Guo, Y.; Zou, J.J.; Wang, Y.Q. Robinson annulation-directed synthesis of jet-fuel-ranged alkylcyclohexanes from biomass-derived chemicals. ACS Catal. 2018, 8, 3280–3285. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kadlec, D.; Velvarska, R.; Kubicka, D. Using Mg-Al mixed oxide and reconstructed hydrotalcite as basic catalysts for aldol condensation of furfural and cyclohexanone. ChemCatChem 2018, 10, 1464–1475. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhang, C.H.; Shi, N.; Zhang, X.H.; Wang, C.G.; Ma, L.L. Production of renewable long-chained cycloalkanes from biomass-derived furfurals and cyclic ketones. RSC Adv. 2018, 8, 13686–13696. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, W.; Guan, Y.S.; Wang, D.K.; Liu, K.S.; Guo, T.T.; Fan, X.; Wei, X.Y. Efficient synthesis of C-15 fuel precursor by heterogeneously catalyzed aldol-condensation of furfural with cyclopentanone. RSC Adv. 2019, 9, 3661–3668. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, S.Y.; Han, F.G.; Li, G.Y.; Shao, X.Z.; Li, N. Synthesis of diesel and jet fuel range cycloalkanes with cyclopentanone and furfural. Catalysts 2019, 9, 886. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Diaz, E.; Ordonez, S. Optimization of the process conditions for minimizing the deactivation in the furfural-cyclopentanone aldol condensation in a continuous reactor. Appl. Catal. B Environ. 2020, 263, 118341. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.H.; Zhang, Q.; Liu, Q.Y.; Wang, C.G.; Ma, L.L. Synthesis of Jet Fuel Range Cycloalkanes with Cyclopentanone and Furfural. Energy Fuels 2020, 34, 7149–7159. [Google Scholar] [CrossRef]
- Yang, Y.L.; Du, Z.T.; Huang, Y.Z.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni-Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Piancatelli, G.; Scettri, A. A simple conversion of 4-substituted 5-hydroxy-3-oxocyclopentenes into the 2-substituted analogs. Synthesis 1977, 1977, 116–117. [Google Scholar] [CrossRef]
- Zhong, S.; Xu, L.; Cai, Y. Recent advances on Piancatelli reactions and related cascade processes. Synthesis 2022, 54, 589–599. [Google Scholar] [CrossRef]
- Verrier, C.; Moebs-Sanchez, S.; Queneau, Y.; Popowycz, F. The Piancatelli reaction and its variants: Recent applications to high added-value chemicals and biomass valorization. Org. Biomol. Chem. 2018, 16, 676–687. [Google Scholar] [CrossRef]
- Piutti, C.; Quartieri, F. The Piancatelli rearrangement: New applications for an intriguing reaction. Molecules 2013, 18, 12290. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.C.; Li, X.D.; Wang, T.F. Highly selective hydrogenation of furfural to cyclopentanone over a NiFe bimetallic catalyst in a methanol/water solution with a solvent effect. ACS Sustain. Chem. Eng. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
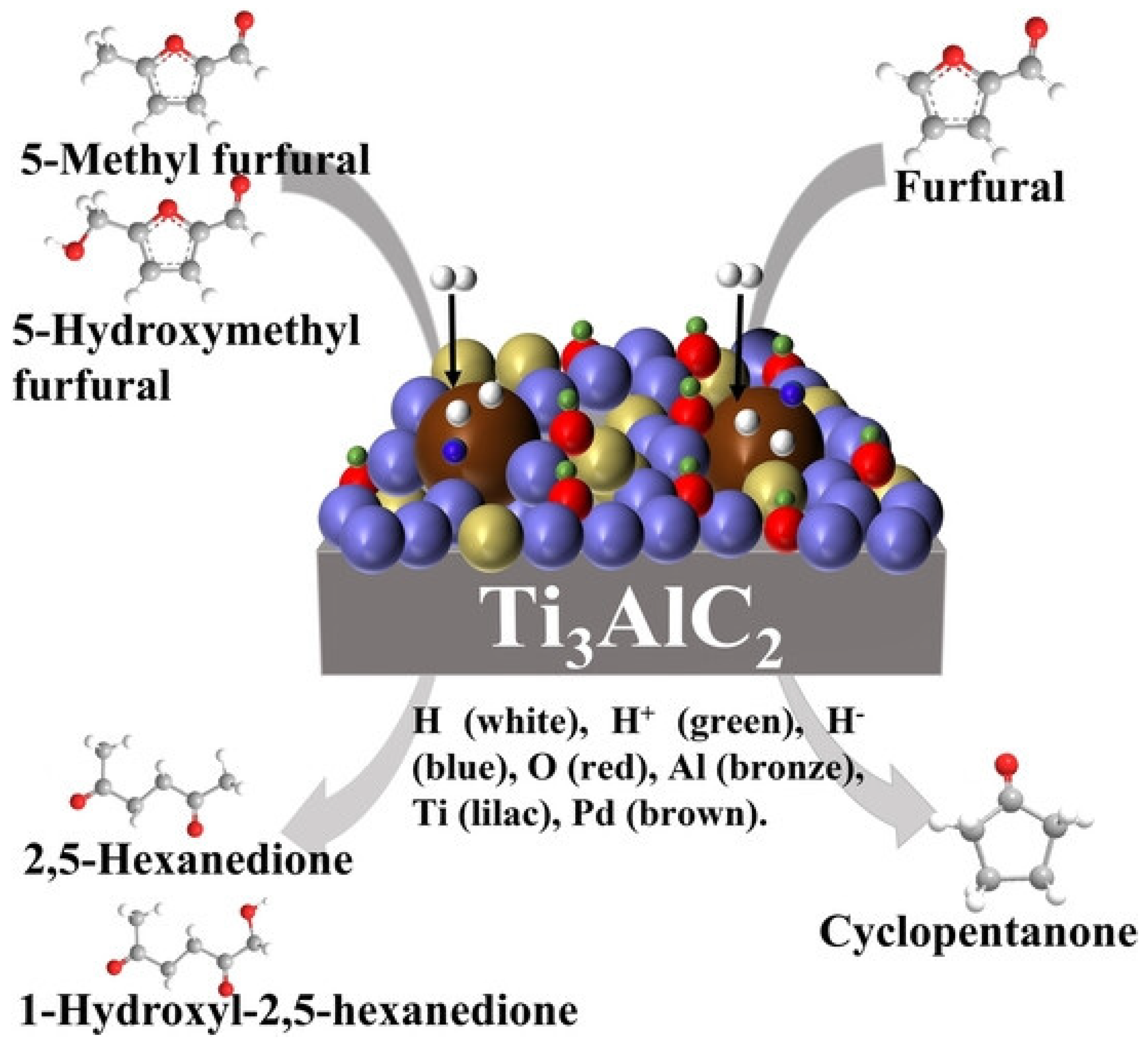
- Deng, Q.; Zhou, R.; Zhang, Y.C.; Li, X.; Li, J.H.; Tu, S.B.; Sheng, G.; Wang, J.; Zeng, Z.L.; Yoskamtorn, T.; et al. H+-H- pairs in partially oxidized max phases for bifunctional catalytic conversion of furfurals into linear ketones. Angew. Chem. Int. Ed. 2023, 62, e202211461. [Google Scholar] [CrossRef]
- Guo, J.H.; Xu, G.Y.; Han, Z.; Zhang, Y.; Fu, Y.; Guo, Q.X. Selective conversion of furfural to cyclopentanone with CuZnAl catalysts. ACS Sustain. Chem. Eng. 2014, 2, 2259–2266. [Google Scholar] [CrossRef]
- Zhou, M.H.; Zeng, Z.; Zhu, H.Y.; Xiao, G.M.; Xiao, R. Aqueous-phase catalytic hydrogenation of furfural to cyclopentanol over Cu-Mg-Al hydrotalcites derived catalysts: Model reaction for upgrading of bio-oil. J. Energy Chem. 2014, 23, 91–96. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Feng, Z.P.; Guo, W.W.; Liu, J.M.; Li, R.Y.; Chen, R.Z.; Huang, J. Hydrogenation and hydrolysis of furfural to furfuryl alcohol, cyclopentanone, and cyclopentanol with a heterogeneous copper catalyst in water. Ind. Eng. Chem. Res. 2019, 58, 3988–3993. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Fan, G.L.; Yang, L.; Li, F. Efficient conversion of furfural into cyclopentanone over high performing and stable Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2018, 561, 117–126. [Google Scholar] [CrossRef]
- Pan, P.; Xu, W.Y.; Pu, T.J.; Wang, X.D.; Pei, X.J.; Tang, F.; Feng, Y.S. Selective conversion of furfural to cyclopentanone and cyclopentanol by magnetic Cu-Fe3O4 NPs catalyst. ChemistrySelect 2019, 4, 5845–5852. [Google Scholar] [CrossRef]
- Zhu, Y.R.; Zhang, J.; Ma, X.D.; An, Z.; Guo, S.W.; Shu, X.; Song, H.Y.; Xiang, X.; He, J. A gradient reduction strategy to produce defects-rich nano-twin Cu particles for targeting activation of carbon-carbon or carbon-oxygen in furfural conversion. J. Catal. 2020, 389, 78–86. [Google Scholar] [CrossRef]
- Niu, H.Y.; Cheng, Y.; Li, C.A.; Li, S.J.; Luo, J.J.; Liang, C.H. Construction of Cu-M-O-x (M = Zn or Al) Interface in Cu Catalysts for Hydrogenation Rearrangement of Furfural. Ind. Eng. Chem. Res. 2021, 60, 16939–16950. [Google Scholar] [CrossRef]
- Li, D.S.; Tian, Z.Y.; Cai, X.C.; Li, Z.Q.; Zhang, C.; Zhang, W.; Song, Y.J.; Wang, H.; Li, C.Q. Nature of polymeric condensates during furfural rearrangement to cyclopentanone and cyclopentanol over Cu-based catalysts. New J. Chem. 2021, 45, 22767–22777. [Google Scholar] [CrossRef]
- Liu, C.Y.; Wei, R.P.; Geng, G.L.; Zhou, M.H.; Gao, L.J.; Xiao, G.M. Aqueous-phase catalytic hydrogenation of furfural over Ni-bearing hierarchical Y zeolite catalysts synthesized by a facile route. Fuel Process. Technol. 2015, 134, 168–174. [Google Scholar] [CrossRef]
- Chen, S.; Qian, T.T.; Ling, L.L.; Zhang, W.H.; Gong, B.B.; Jiang, H. Hydrogenation of furfural to cyclopentanone under mild conditions by a structure-optimized Ni-NiO/TiO(2) heterojunction catalyst. ChemSusChem 2020, 13, 5507–5515. [Google Scholar] [CrossRef]
- Herrera, C.; Fuentealba, D.; Ghampson, I.T.; Sepulveda, C.; Garcia-Fierro, J.L.; Canales, R.I.; Escalona, N. Selective conversion of biomass-derived furfural to cyclopentanone over carbon nanotube-supported Ni catalyst in Pickering emulsions. Catal. Commun. 2020, 144, 106092. [Google Scholar] [CrossRef]
- Herrera, C.; Barrientos, L.; Rosenkranz, A.; Sepulveda, C.; Garcia-Fierro, J.L.; Laguna-Bercero, M.A.; Escalona, N. Tuning amphiphilic properties of Ni/Carbon nanotubes functionalized catalysts and their effect as emulsion stabilizer for biomass-derived furfural upgrading. Fuel 2020, 276, 118032. [Google Scholar] [CrossRef]
- Herrera, C.; Pinto-Neira, J.; Fuentealba, D.; Sepulveda, C.; Rosenkranz, A.; Garcia-Fierro, J.L.; Gonzalez, M.; Escalona, N. Effect of Ni metal content on emulsifying properties of Ni/CNTox catalysts for catalytic conversion of furfural in Pickering emulsions. ChemCatChem 2021, 13, 682–694. [Google Scholar] [CrossRef]
- Yu, Z.J.; Tian, H.L.; Sun, K.; Shao, Y.W.; Zhang, L.J.; Zhang, S.; Duan, P.G.; Liu, Q.; Niu, S.L.; Dong, D.H.; et al. Impacts of externally added Bronsted and Lewis acid on conversion of furfural to cyclopentanone over Ni/SiC catalyst. Mol. Catal. 2020, 496, 111187. [Google Scholar] [CrossRef]
- Hu, Z.; Xie, A.D.; Chen, C.; Zou, Z.D.; Shen, Y.; Fu, Z.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J.; Wang, G.Z. Facile synthesis of N, P co-doped carbon encapsulated Ni catalyst for green production of cyclopentanone from biomass derivative furfural. Fuel 2022, 319, 123815. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Li, Y.; Yao, Y.L.; Wang, Y.; Liu, Y.Y.; Sun, Z.C.; Shi, C.; Wang, W.; Wang, A.J. Highly selective hydrogenative ring-rearrangement of furfural to cyclopentanone over a bifunctional Ni3P/gamma-Al2O3 catalyst. Mol. Catal. 2022, 522, 112239. [Google Scholar] [CrossRef]
- Cheng, C.; Zhao, C.S.; Zhao, D.; Ding, S.M.; Chen, C. The importance of constructing Triple-functional Sr2P2O7/Ni2P catalysts for smoothing hydrogenation ring-rearrangement of biomass-derived furfural compounds in water. J. Catal. 2023, 421, 117–133. [Google Scholar] [CrossRef]
- Tian, H.L.; Gao, G.M.; Xu, Q.; Gao, Z.R.; Zhang, S.; Hu, G.Z.; Xu, L.L.; Hu, X. Facilitating selective conversion of furfural to cyclopentanone via reducing availability of metallic nickel sites. Mol. Catal. 2021, 510, 111697. [Google Scholar] [CrossRef]
- Hu, Z.; Han, M.M.; Chen, C.; Zou, Z.D.; Shen, Y.; Fu, Z.; Zhu, X.G.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J.; et al. Hollow carbon sphere encapsulated nickel nanoreactor for aqueous-phase hydrogenation-rearrangement tandem reaction with enhanced catalytic performance. Appl. Catal. B Environ. 2022, 306, 121140. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.K.; Zhou, R.; Chen, S.X.; Wang, J.; Zeng, Z.L.; Zou, J.J.; Deng, S.G.; Deng, Q. Bifunctional role of hydrogen in aqueous hydrogenative ring rearrangement of furfurals over Co@Co-NC. ACS Sustain. Chem. Eng. 2022, 10, 7321–7329. [Google Scholar] [CrossRef]
- Ranaware, V.; Kurniawan, R.G.; Verma, D.; Kwak, S.K.; Ryu, B.C.; Kang, J.W.; Kim, J. Solvent-mediated selectivity control of furfural hydrogenation over a N-doped carbon-nanotube-supported Co/CoOx catalyst. Appl. Catal. B Environ. 2022, 318, 121838. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, B.L.; Yu, Z.D.; Huang, R.J.; Yan, G.H.; Li, Z.; Sun, Y.; Yang, S.L.; Tang, X.; Lin, L.; et al. Efficient catalytic hydrogenation of furfural over cobalt-based catalysts with adjustable acidity. Chem. Eng. Sci. 2023, 270, 118527. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, S.B.; Long, J.X.; Wang, C.G.; Chang, J.M.; Tan, J.; Liu, Q.Y.; Ma, L.L.; Wang, T.J.; Zhang, Q. In situ hydrogenation of furfural with additives over a RANEY (R) Ni catalyst. RSC Adv. 2015, 5, 91190–91195. [Google Scholar] [CrossRef]
- Chen, C.Z.; Zhou, M.H.; Jiang, J.C. Selective aqueous-phase hydrogenation of furfural to cyclopentanol over Ni-based catalyst under mild conditions. J. Chin. Chem. Soc. 2021, 68, 1177–1180. [Google Scholar] [CrossRef]
- Gao, G.M.; Shao, Y.W.; Gao, Y.; Wei, T.; Gao, G.G.; Zhang, S.; Wang, Y.; Chen, Q.F.; Hu, X. Synergetic effects of hydrogenation and acidic sites in phosphorus-modified nickel catalysts for the selective conversion of furfural to cyclopentanone. Catal. Sci. Technol. 2021, 11, 575–593. [Google Scholar] [CrossRef]
- Zhang, S.J.; Ma, H.; Sun, Y.X.; Liu, X.; Zhang, M.Y.; Luo, Y.; Gao, J.; Xu, J. Selective tandem hydrogenation and rearrangement of furfural to cyclopentanone over CuNi bimetallic catalyst in water. Chin. J. Catal. 2021, 42, 2216–2224. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Zhou, M.H.; Zeng, Z.; Xiao, G.M.; Xiao, R. Selective hydrogenation of furfural to cyclopentanone over Cu-Ni-Al hydrotalcite-based catalysts. Korean J. Chem. Eng. 2014, 31, 593–597. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.Y.; Zhu, W.; Gao, L.J.; Xiao, G.M. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Balaga, R.; Balla, P.; Zhang, X.Q.; Ramineni, K.; Du, H.; Lingalwar, S.; Perupogu, V.; Zhang, Z.C. Enhanced cyclopentanone yield from furfural hydrogenation: Promotional effect of surface silanols on Ni-Cu/m-silica catalyst. Catalysts 2023, 13, 580. [Google Scholar] [CrossRef]
- Fan, Z.L.; Zhang, J.X.; Wu, D.F. Highly Efficient NiCu/SiO2 Catalyst Induced by Ni(Cu)-Silica Interaction for Aqueous-Phase Furfural Hydrogenation. Catal. Lett. 2023, 153, 1543–1555. [Google Scholar] [CrossRef]
- Liu, M.R.; Yuan, L.Y.; Fan, G.L.; Zheng, L.R.; Yang, L.; Li, F. NiCu nanoparticles for catalytic hydrogenation of biomass-derived carbonyl compounds. ACS Appl. Nano Mater. 2020, 3, 9226–9237. [Google Scholar] [CrossRef]
- Li, X.L.; Deng, J.; Shi, J.; Pan, T.; Yu, C.G.; Xu, H.J.; Fu, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using different preparation methods of Cu-Co catalysts. Green Chem. 2015, 17, 1038–1046. [Google Scholar] [CrossRef]
- Zhou, M.H.; Li, J.; Wang, K.; Xia, H.H.; Xu, J.M.; Jiang, J.C. Selective conversion of furfural to cyclopentanone over CNT-supported Cu based catalysts: Model reaction for upgrading of bio-oil. Fuel 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Gong, W.B.; Chen, C.; Zhang, H.M.; Wang, G.Z.; Zhao, H.J. In situ synthesis of highly dispersed cu-co bimetallic nanoparticles for tandem hydrogenation/rearrangement of bioderived furfural in aqueous-phase. ACS Sustain. Chem. Eng. 2018, 6, 14919–14925. [Google Scholar] [CrossRef]
- Li, Y.R.; Guo, X.C.; Liu, D.S.; Mu, X.D.; Chen, X.F.; Shi, Y. Selective conversion of furfural to cyclopentanone or cyclopentanol using Co-Ni catalyst in water. Catalysts 2018, 8, 193. [Google Scholar] [CrossRef]
- Huang, L.; Hao, F.; Lv, Y.; Liu, Y.; Liu, P.L.; Xiong, W.; Luo, H.A. MOF-derived well-structured bimetallic catalyst for highly selective conversion of furfural. Fuel 2021, 289, 119910. [Google Scholar] [CrossRef]
- Shao, Y.W.; Wu, J.; Zheng, Z.Y.; Fan, M.J.; Sun, K.; Kontchouo, F.M.B.; Zhang, L.J.; Zhang, S.; Hu, G.Z.; Hu, X. Alloying cobalt in Co-Fe-Al catalyst for achieving the selective conversion of furfural to cyclopentanone. Renew. Energy 2022, 195, 957–971. [Google Scholar] [CrossRef]
- Gong, W.B.; Chen, C.; Zhang, H.M.; Wang, G.Z.; Zhao, H.J. Highly dispersed Co and Ni nanoparticles encapsulated in N-doped carbon nanotubes as efficient catalysts for the reduction of unsaturated oxygen compounds in aqueous phase. Catal. Sci. Technol. 2018, 8, 5506–5514. [Google Scholar] [CrossRef]
- Cherkasov, N.; Exposito, A.J.; Aw, M.S.; Fernandez-Garcia, J.; Huband, S.; Sloan, J.; Paniwnyk, L.; Rebrov, E.V. Active site isolation in bismuth-poisoned Pd/SiO2 catalysts for selective hydrogenation of furfural. Appl. Catal. A Gen. 2019, 570, 183–191. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Talsi, V.P.; Likholobov, V.A. Mechanism of Pd/C-catalyzed hydrogenation of furfural under hydrothermal conditions. J. Catal. 2020, 389, 721–734. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Talsi, V.P.; Gulyaeva, T.I.; Trenikhin, M.V.; Belskaya, O.B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: Effect of the support on the reaction routes. React. Kinet. Mech. Catal. 2019, 126, 811–827. [Google Scholar] [CrossRef]
- Date, N.S.; Kondawar, S.E.; Chikate, R.C.; Rode, C.V. Single-pot reductive rearrangement of furfural to cyclopentanone over silica-supported Pd catalysts. ACS Omega 2018, 3, 9860–9871. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhang, L.K.; Wang, J.; Wang, R.; Zeng, Z.L.; Deng, S.G. Highly efficient hydrogenative ring-rearrangement of furanic aldehydes to cyclopentanone compounds catalyzed by noble metals/MIL-MOFs. Appl. Catal. A Gen. 2019, 575, 152–158. [Google Scholar] [CrossRef]
- Wang, C.H.; Yu, Z.Q.; Yang, Y.H.; Sun, Z.C.; Wang, Y.; Shi, C.; Liu, Y.Y.; Wang, A.J.; Leus, K.; Van der Voort, P. Hydrogenative ring-rearrangement of furfural to cyclopentanone over Pd/UiO-66-NO2 with tunable missing-linker defects. Molecules 2021, 26, 5736. [Google Scholar] [CrossRef]
- Gao, X.; Ding, Y.Y.; Peng, L.L.; Yang, D.; Wan, X.Y.; Zhou, C.M.; Liu, W.; Dai, Y.H.; Yang, Y.H. On the effect of zeolite acid property and reaction pathway in Pd-catalyzed hydrogenation of furfural to cyclopentanone. Fuel 2022, 314, 123074. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, R.; Li, X.; Wang, J.; Zeng, Z.L.; Zou, J.J.; Deng, S.G. Hydrogenative ring-rearrangement of biobased furanic aldehydes to cyclopentanone compounds over Pd/pyrochlore by introducing oxygen vacancies. ACS Catal. 2020, 10, 7355–7366. [Google Scholar] [CrossRef]
- Gao, R.; Li, X.; Guo, L.Y.; Tong, Z.K.; Deng, Q.; Wang, J.; Zeng, Z.L.; Zou, J.J.; Deng, S.G. Pyrochlore/Al2O3 composites supported Pd for the selective synthesis of cyclopentanones from biobased furfurals. Appl. Catal. A Gen. 2021, 612, 117985. [Google Scholar] [CrossRef]
- Tong, Z.K.; Gao, R.; Li, X.; Guo, L.Y.; Wang, J.; Zeng, Z.L.; Deng, Q.; Deng, S.G. Highly controllable hydrogenative ring rearrangement and complete hydrogenation of biobased furfurals over Pd/La2B2O7 (B = Ti, Zr, Ce). ChemCatChem 2021, 13, 4549–4556. [Google Scholar] [CrossRef]
- Yuan, E.X.; Wang, C.L.; Wu, C.; Shi, G.J.; Jian, P.M.; Hou, X. Constructing hierarchical structures of Pd catalysts to realize reaction pathway regulation of furfural hydroconversion. J. Catal. 2023, 421, 30–44. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, B.; Fei, B.H.; Chen, X.F.; Zhang, J.Y.; Mu, X.D. Tunable and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Pt supported on biomass-derived porous heteroatom doped carbon. Faraday Discuss. 2017, 202, 79–98. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, Y.E.; Baek, J.H.; Jae, J.; Lee, M.S. Effect of surface properties of TiO2 on the performance of Pt/TiO2 catalysts for furfural hydrogenation. RSC Adv. 2021, 12, 860–868. [Google Scholar] [CrossRef]
- Zhang, G.S.; Zhu, M.M.; Zhang, Q.; Liu, Y.M.; He, H.Y.; Cao, Y. Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem. 2016, 18, 2155–2164. [Google Scholar] [CrossRef]
- Fang, R.Q.; Liu, H.L.; Luque, R.; Li, Y.W. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 17, 4183–4188. [Google Scholar] [CrossRef]
- Shen, T.; Hu, R.J.; Zhu, C.J.; Li, M.; Zhuang, W.; Tang, C.L.; Ying, H.J. Production of cyclopentanone from furfural over Ru/C with Al11.6PO23.7 and application in the synthesis of diesel range alkanes. RSC Adv. 2018, 8, 37993–38001. [Google Scholar] [CrossRef]
- Liu, Y.H.; Chen, Z.H.; Wang, X.F.; Liang, Y.; Yang, X.M.; Wang, Z.C. Highly selective and efficient rearrangement of biomass-derived furfural to cyclopentanone over interface-active Ru/Carbon nanotubes catalyst in water. ACS Sustain. Chem. Eng. 2017, 5, 744–751. [Google Scholar] [CrossRef]
- Byun, M.Y.; Park, D.W.; Lee, M.S. Effect of oxide supports on the activity of Pd based catalysts for furfural hydrogenation. Catalysts 2020, 10, 837. [Google Scholar] [CrossRef]
- Deng, Q.; Wen, X.H.; Zhang, P. Pd/Cu-MOF as a highly efficient catalyst for synthesis of cyclopentanone compounds from biomass-derived furanic aldehydes. Catal. Commun. 2019, 126, 5–9. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarova, K.; Vavra, I.; Sotak, T.; Dobrocka, E.; Micusik, M. Carbon supported Pd-Cu catalysts for highly selective rearrangement of furfural to cyclopentanone. Appl. Catal. B Environ. 2016, 181, 210–219. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, C.; Zhang, X.F. One-step encapsulation of bimetallic Pd-Co nanoparticles within UiO-66 for selective conversion of furfural to cyclopentanone. Catal. Lett. 2020, 150, 2158–2166. [Google Scholar] [CrossRef]
- Li, X.; Tong, Z.K.; Zhu, S.; Deng, Q.; Chen, S.X.; Wang, J.; Zeng, Z.L.; Zhang, Y.L.; Zou, J.J.; Deng, S.G. Water-mediated hydrogen spillover accelerates hydrogenative ring-rearrangement of furfurals to cyclic compounds. J. Catal. 2022, 405, 363–372. [Google Scholar] [CrossRef]
- Dohade, M.; Dhepe, P.L. Efficient method for cyclopentanone synthesis from furfural: Understanding the role of solvents and solubility in a bimetallic catalytic system. Catal. Sci. Technol. 2018, 8, 5259–5269. [Google Scholar] [CrossRef]
- Wang, L.L.; Weng, Y.J.; Wang, X.L.; Yin, H.X.; Wang, F.C.; Xue, X.X.; Liu, X.Y.; Wang, F.; Duan, P.G.; Zhang, Y.L. Synergistic bimetallic RuMo catalysts for selective rearrangement of furfural to cyclopentanol in aqueous phase. Catal. Commun. 2019, 129, 105745. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Zhou, S.H.; Zou, J.D.; Wang, J.; Wang, R.; Zeng, Z.L.; Deng, S.G. Double-metal cyanide-supported Pd catalysts for highly efficient hydrogenative ring-rearrangement of biomass-derived furanic aldehydes to cyclopentanone compounds. J. Catal. 2019, 378, 201–208. [Google Scholar] [CrossRef]
- Ohyama, J.; Kanao, R.; Ohira, Y.; Satsuma, A. The effect of heterogeneous acid-base catalysis on conversion of 5-hydroxymethylfurfural into a cyclopentanone derivative. Green Chem. 2016, 18, 676–680. [Google Scholar] [CrossRef]
- Ohyama, J.; Ohira, Y.; Satsuma, A. Hydrogenative ring-rearrangement of biomass derived 5-(hydroxymethyl) furfural to 3-(hydroxymethyl) cyclopentanol using combination catalyst systems of Pt/SiO2 and lanthanoid oxides. Catal. Sci. Technol. 2017, 7, 2947–2953. [Google Scholar] [CrossRef]
- Ramos, R.; Grigoropoulos, A.; Perret, N.; Zanella, M.; Katsoulidis, A.P.; Manning, T.D.; Claridge, J.B.; Rosseinsky, M.J. Selective conversion of 5-hydroxymethylfurfural to cyclopentanone derivatives over Cu-Al2O3 and Co-Al2O3 catalysts in water. Green Chem. 2017, 19, 1701–1713. [Google Scholar] [CrossRef]
- Li, J.C.; Feng, Y.C.; Wang, H.Q.; Tang, X.; Sun, Y.; Zeng, X.H.; Lin, L. Highly selective ring rearrangement of 5-hydroxymethylfurfural to 3-hydroxymethylcyclopentanon catalyzed by non-noble Ni-Fe/Al2O3. Mol. Catal. 2021, 505, 111505. [Google Scholar] [CrossRef]
- Zhang, S.J.; Ma, H.; Sun, Y.X.; Luo, Y.; Liu, X.; Zhang, M.Y.; Gao, J.; Xu, J. Catalytic selective hydrogenation and rearrangement of 5-hydroxymethylfurfural to 3-hydroxymethyl-cyclopentone over a bimetallic nickel-copper catalyst in water. Green Chem. 2019, 21, 1702–1709. [Google Scholar] [CrossRef]
- Hurtado, B.; Arias, K.S.; Climent, M.J.; Concepcion, P.; Corma, A.; Iborra, S. Selective conversion of HMF into 3-hydroxymethylcyclopentylamine through a one-pot cascade process in aqueous phase over bimetallic NiCo nanoparticles as catalyst. ChemSusChem 2022, 15, e202200194. [Google Scholar] [CrossRef] [PubMed]



| Entry | Volume Ratio (Methanol/Water) | Conv. (%) | Selectivity (%) | ||
|---|---|---|---|---|---|
| FOL | THFA | CPO | |||
| 1 | 100/0 | 99.8 | 88.3 | 1.7 | 0 |
| 2 | 75/25 | 99.8 | 1.9 | 95.8 | 2.3 |
| 3 | 50/50 | 79.9 | 58.7 | 17.2 | 24.1 |
| 4 | 25/75 | 67.8 | 27.8 | 13.5 | 58.6 |
| 5 | 0/100 | 53.5 | 12.5 | 4.3 | 75.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Cheng, Y.; Hu, Z.; Wang, C.; Sui, D.; Yang, Y.; Lu, T. A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF. Molecules 2023, 28, 5397. https://doi.org/10.3390/molecules28145397
Duan Y, Cheng Y, Hu Z, Wang C, Sui D, Yang Y, Lu T. A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF. Molecules. 2023; 28(14):5397. https://doi.org/10.3390/molecules28145397
Chicago/Turabian StyleDuan, Ying, Yiyi Cheng, Zhi Hu, Chenxu Wang, Dong Sui, Yanliang Yang, and Tianliang Lu. 2023. "A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF" Molecules 28, no. 14: 5397. https://doi.org/10.3390/molecules28145397
APA StyleDuan, Y., Cheng, Y., Hu, Z., Wang, C., Sui, D., Yang, Y., & Lu, T. (2023). A Comprehensive Review on Metal Catalysts for the Production of Cyclopentanone Derivatives from Furfural and HMF. Molecules, 28(14), 5397. https://doi.org/10.3390/molecules28145397