Photocatalytic CO2 Conversion into Solar Fuels Using Carbon-Based Materials—A Review
Abstract
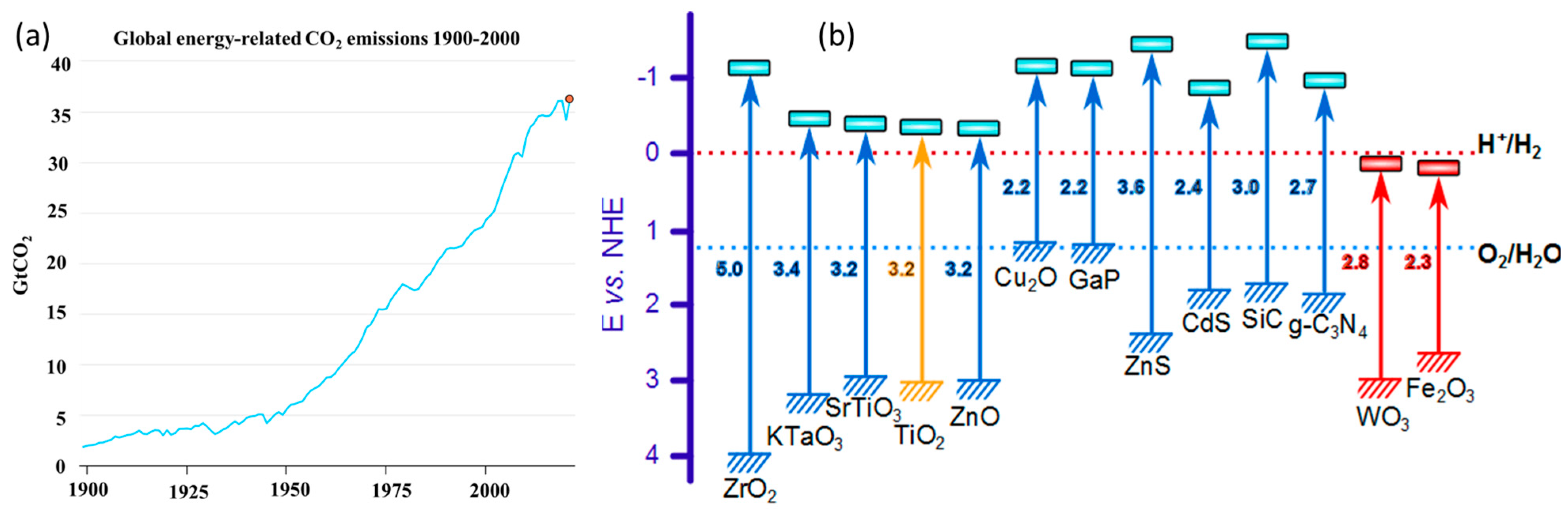
1. Introduction
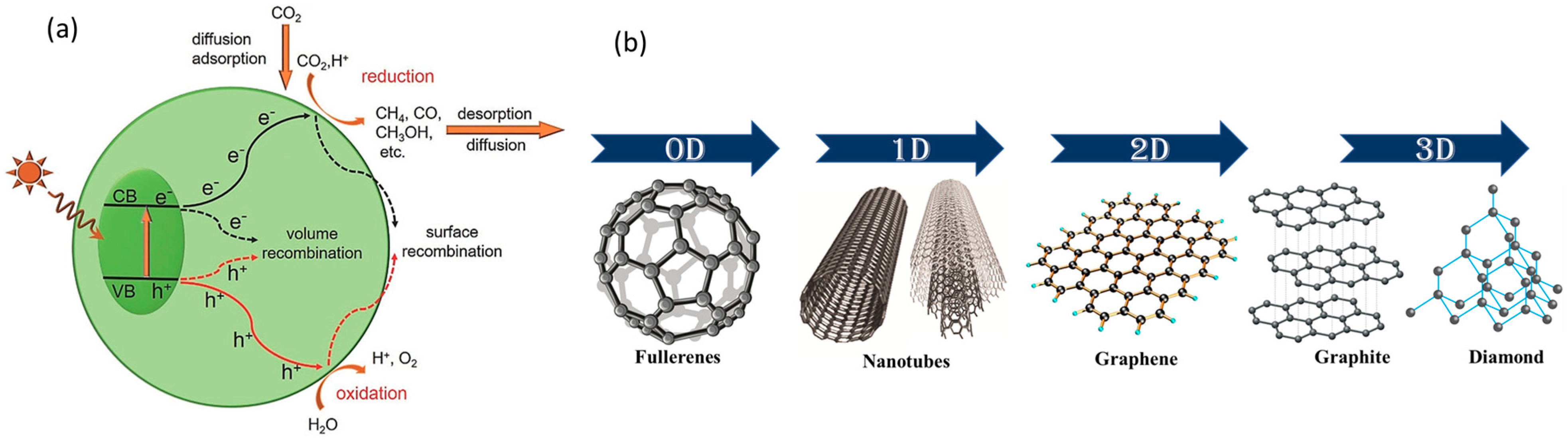
2. Principles of Photocatalytic CO2 Reduction
3. Multi-Dimensional Carbon-Based Materials for Photocatalytic CO2 Reduction
4. Advantages of Utilizing Carbon Materials for CO2 Reduction
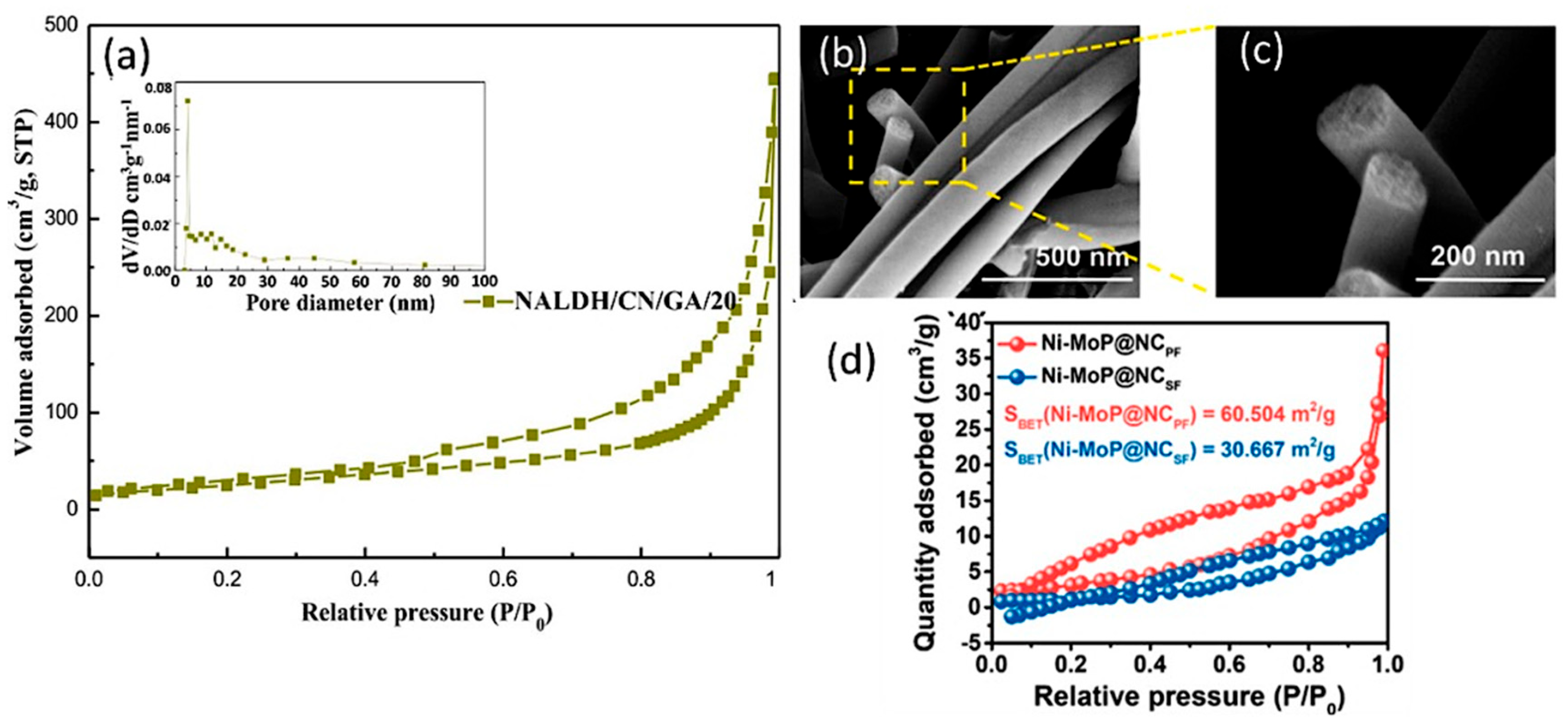
4.1. Enlarging the Specific Surface Area
4.2. Increasing the CO2 Adsorption and Activation
4.3. Separation of Photogenerated Electron–Hole Pairs
4.4. Increasing Light Absorption
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aminabhavi, T.M.; Aggarwal, M.; Basu, S.; Shetti, N.P.; Nadagouda, M.N. Photocatalytic conversion of CO2 into valuable products using emerging two-dimensional graphene-based nanomaterials: A step towards sustainability. Chem. Eng. J. 2021, 425, 131401. [Google Scholar]
- Rajni, K.S.; Raguram, T.; Renganathan, V.; Chen, S. Type II heterojunction photocatalysts for environmental applications. In Heterojunction Photocatalytic Materials, Advances, and Applications in Energy and the Environment; Jenny Stanford Publishing: Singapore, 2022; pp. 56–79. [Google Scholar]
- Azmat, A.K.; Muhammad, T. Recent advancements in engineering approach towards the design of photo-reactors for selective photocatalytic CO2 reduction to renewable fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar]
- Sheoran, K.; Vijay Kumar, T.; Samarjeet, S.S. Synthesis and overview of carbon-based materials for high-performance energy storage application: A review. Mater. Today Proc. 2022, 56, 9–17. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; In, S. Development of graphene-based photocatalysts for CO2 reduction to C1 chemicals: A brief overview. Catal. Today 2019, 335, 39–54. [Google Scholar] [CrossRef]
- Kuang, Y.; Shang, J.; Zhu, T. Photoactivated Graphene Oxide to Enhance Photocatalytic Reduction of CO2. ACS Appl. Mater. Interfaces 2020, 12, 3580–3591. [Google Scholar] [CrossRef]
- Fatima, R.; Warsi, M.F.; Zulfiqar, S.; Ragab, S.A.; Shakir, I.; Sarwar, M.I. Nanocrystalline transition metal oxides and their composites with reduced graphene oxide and carbon nanotubes for photocatalytic applications. Ceram. Int. 2020, 40, 16480–16492. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Yoshino, S.; Takayama, T.; Yamaguchi, Y.; Iwahe, A.; Kudo, A. CO2 Reduction Using Water as an Electron Donor over Heterogeneous Photocatalysts Aiming at Artificial Photosynthesis. Acc. Chem. Res. 2022, 55, 966–977. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Wang, H.; Huo, P. Integration of 3D macroscopic reduced graphene oxide aerogel with DUT-67 for selective CO2 photoreduction to CO in Gas-Solid reaction. Chem. Eng. J. 2022, 446, 137034. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. [Google Scholar] [CrossRef]
- Karamian, E.; Sharifnia, S. On the general mechanism of photocatalytic reduction of CO2. J. CO2 Util. 2016, 16, 194–203. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 170019. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, F.; Lin, X.; Yang, J. Visible-light-responsive photocatalysts xBiOBr–(1–x)BiOI. Catal. Commun. 2008, 9, 8–12. [Google Scholar] [CrossRef]
- Kandy, M.M. Carbon-based photocatalysts for enhanced photocatalytic reduction of CO2 to solar fuels. Sustain. Energy Fuels 2020, 4, 469–484. [Google Scholar] [CrossRef]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal center of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef]
- Cao, J.; Alsharif, S.; El-Shafay, A.S. Preparation suppressed the charge carrier recombination, and improved photocatalytic performance of g-C3N4/MoS2 p-n heterojunction photocatalyst for tetracycline and dyes degradation upon visible light. Mater. Sci. Semicond. Process. 2022, 144, 106569. [Google Scholar] [CrossRef]
- Pal, A.; Ghosh, U.; Majumdar, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures; Recent progress and perspects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar]
- Jiang, Z.; Zhang, X.; Yuan, Z.; Chen, J.; Huang, B.; Dionysios, D.D.; Yang, G. Enhanced photocatalytic CO2 reduction via the synergistic effect between Ag and activated carbon in TiO2/AC-Ag ternary composite. J. Chem. Eng. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, P.; Zhan, X.; Xu, L.; Fu, X.; Zheng, T.; Yang, X.; Xu, Q.; Wang, D.; Qi, D.; et al. Mass production of a single-atom cobalt photocatalyst for high-performance visible-light photocatalytic CO2 reduction. J. Mater. Chem. A. 2021, 9, 26286–26297. [Google Scholar]
- Zhang, Z.; Shu, M.; Jiang, Y.; Xu, J. Fullerene modified CsPbBr3 perovskite nanocrystals for efficient charge separation and photocatalytic CO2 reduction. J. Chem. Eng. 2021, 414, 128889. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Cheng, C.; Li, L.; Liu, J.; Zhang, B. C60-Stabilized Cu+ sites boost electrocatalytic reduction of CO2 to C2+ products. Adv. Energy Mater. 2023, 13, 2204346. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, R.K.; Abishek, K.; Park, N.J.; Kim, J.Y.; Baeg, J.O. Fullerene polymer film as a highly efficient photocatalyst for selective solar fuel production from CO2. J. Appl. Polym. Sci. 2020, 137, 48536. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. Smart Utilization of Carbon Dots in Semiconductor Photocatalysis. Adv. Mater. 2016, 28, 9454–9477. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Shi, T.; Liang, T.; Liang, Q.; Cheng, Z. Hierarchical hollow-microsphere cadmium sulfide-carbon dots composites with enhancing charge transfer efficiency for photocatalytic CO2 reduction. J. Alloys Compd. 2023, 936, 168286. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, X.; Jin, H.; Du, R.; Wang, Y.; Zhou, Y.; Chong, R.; Liu, X.; Huang, Q. Integrating carbon vacancy modified carbon quantum dots with carbon nitride for efficient photocatalytic CO2 reduction to syngas with tunable hydrogen to carbon monoxide ratio. Carbon 2023, 203, 671–685. [Google Scholar] [CrossRef]
- Chen, P.; Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic Water splitting and CO2 Reduction. ACS Nano. 2018, 12, 3523–3532. [Google Scholar]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002, 41, 11. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal Nanoparticles and Related Materials supported on carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar] [CrossRef]
- Quinonera, D.; Frontera, A.; Deya, P.M. Feasibility of single-walled carbon nanotubes as materials for CO2 adsorption: A DFT study. Phys. Chem. C 2012, 116, 21083–21092. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. 2012, 429–430, 31–38. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Wang, Y.; Li, Z. Rational design of metal halide perovskite nanocrystals for photocatalytic CO2 reduction: Recent advances, challenges, and prospects. ACS Energy Lett. 2022, 7, 2043–2059. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Mao, J.; Liu, J.; Zhou, Y.; Guay, D.; Qiao, J. Electrochemical reduction of CO2 by SnOx nanosheets anchored on multiwalled carbon nanotubes with tunable functional groups. ChemSusChem 2019, 12, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Beenish, T.; Nawawi, M.G.M.; Hussain, M.; Muhammad, A. Cu-NPs embedded 1D/2D CNTs/pCN heterojunction composite towards enhanced and continuous photocatalytic CO2 reduction to fuels. Appl. Surf. Sci. 2019, 485, 450–461. [Google Scholar]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, T.; Li, Z.; You, B.; Xia, B.Y. Engineering 2D photocatalysts toward carbon dioxide reduction. Adv. Energy Mater. 2021, 11, 2003159. [Google Scholar] [CrossRef]
- Ong, W.J.; Putri, L.K. Mohamed A R. Rational design of carbon-based 2D nanostructures for enhanced photocatalytic CO2 reduction: A dimensionality perspective. Chem. Eur. J. 2020, 26, 9710–9748. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Ho, W. Graphene-based photocatalysts for CO2 reduction to solar fuel. J. Phys. Chem. Lett. 2015, 6, 4244–4251. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, X.; Zeng, J.; Li, S.; Wang, X.; Zhang, B. 0D/2D/3D Au/Ti3C2/TiO2 photocatalyst based on accelerating charge transfer and enhanced stability for efficiently hydrogen production. Appl. Surf. Sci. 2023, 615, 156397. [Google Scholar] [CrossRef]
- Cheng, Y.Z.; Ding, X.; Han, B.H. Porous organic polymers for photocatalytic carbon dioxide reduction. ChemPhotoChem 2021, 5, 406–417. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Ji, H. Zinc porphyrin and rhenium complex-based donor-acceptor conjugated porous polymer for visible-light-driven conversion of CO2 to CO. J. CO2 Util. 2022, 60, 101972. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Deng, X.; Li, Z. Metal–organic frameworks (MOFs) for photocatalytic CO2 reduction. Catal. Sci. Technol. 2017, 7, 4893–4904. [Google Scholar] [CrossRef]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO2 reduction over NH2-Uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal–organic frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, X.S.; Zhao, H.; Lin, H.; Huang, Y.B.; Cao, R. Rhenium-modified porous covalent triazine framework for highly efficient photocatalytic carbon dioxide reduction in a solid–gas system. Catal. Sci. Technol. 2018, 8, 2224–2230. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, Y.; Jiang, B. Encapsulation of Pd nanoparticles in covalent triazine frameworks for enhanced photocatalytic CO2 conversion. ACS Sustain. Chem. Eng. 2021, 9, 12646–12654. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, N.; Xue, Y.; Zuo, Z.; Liu, H.; Li, Y. Progress in research into 2D graphdiyne-based materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, F.; Long, G.; Zhang, T.; Leng, K.; Zhang, Y.; Huang, Y.; Ma, Y.; Zhang, M.; et al. Controlling the effective surface area and pore size distribution of sp2 carbon materials and their impact on the capacitance performance of these materials. J. Am. Chem. Soc. 2013, 135, 5921–5929. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Cui, J.; Yang, Q.; Liang, S. High-index faceted metal oxide micro-/nanostructures: A review on characterization, synthesis, and applications. Nanoscale 2019, 11, 15739–15762. [Google Scholar] [CrossRef]
- Yang, M.; Wang, P.; Li, Y.; Tang, S.; Lin, X.; Zhang, H.; Zhu, Z.; Chen, F. Graphene aerogel-based NiAl-LDH/g-C3N4 with ultratight sheet-sheet heterojunction for excellent visible-light photocatalytic activity of CO2 reduction. Appl. Catal. B Environ. 2022, 306, 121065. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Basca, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Gong, S.; Hou, M.; Niu, N.; Teng, X.; Liu, X.; Xu, M.; Xu, C.; Vonika, K.M.A.; Chen, Z. Molybdenum phosphide coupled with highly dispersed nickel confined in porous carbon nanofibers for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2022, 427, 131717. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Lu, A. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef]
- Gao, X.; Yang, S.; Hu, L.; Cai, S.; Wu, L.; Kawi, S. Carbonaceous materials as adsorbents for CO2 capture: Synthesis and modification. Carbon Capture Sci. Technol. 2022, 3, 100039. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Zuo, C.; Li, R.; Zhou, Y.; Zhang, Y.; Bo, W. Few-layer porous carbon nitride anchoring Co and Ni with charge transfer mechanism for photocatalytic CO2 reduction. COCIS 2022, 625, 722–733. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol. 2016, 27, 72–99. [Google Scholar] [CrossRef]
- Jaroniec, M.; Xu, Q.; Zhang, L.; Yu, J.; Wagen, S.; Al-Ghamdi, A.A. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 10. [Google Scholar]
- Zhao, D.; Wang, Y.; Dong, C.; Huang, Y.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy. 2021, 6, 388–397. [Google Scholar] [CrossRef]
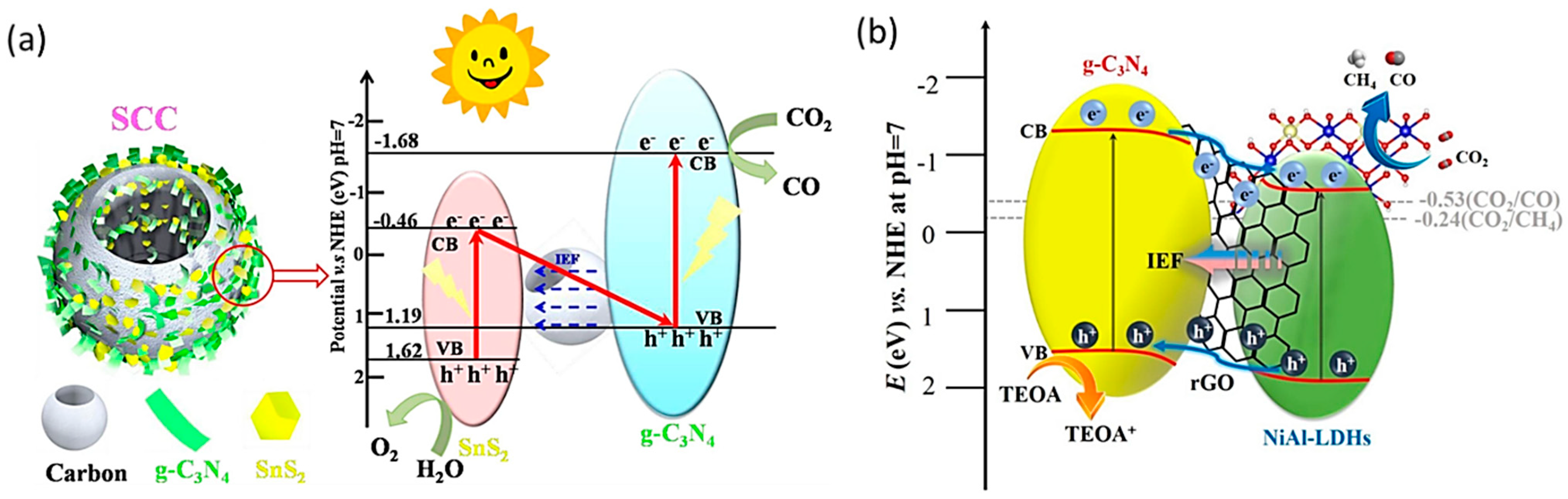
- Li, Y.; Yin, Q.; Zeng, Y.; Liu, Z. Hollow spherical biomass derived-carbon dotted with SnS2/g-C3N4 Z-scheme heterojunction for efficient CO2 photoreduction into CO. Chem. Eng. J. 2022, 438, 135652. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, J.; Jin, Z.; Di, T.; Wang, T. Reduced graphene oxide assisted g-C3N4/rGO/NiAl-LDHs type II heterostructure with high-performance photocatalytic CO2 reduction. Chem. Eng. J. 2022, 450, 138108. [Google Scholar] [CrossRef]
- Jian, W.; Hengming, H.; Zhongzi, X.; Jiahui, K.; Chunhua, L. The Potential of Carbon-based Materials for Photocatalytic Application. Curr. Org. Chem. 2014, 18, 1346–1364. [Google Scholar]
- Nolan, M.; Iwaszuk, A.; Lucid, A.K.; Carey, J.J.; Fronzi, M. Design of Novel Visible Light Active Photocatalyst Materials: Surface Modified TiO2. Adv. Mater. 2016, 28, 5425–5446. [Google Scholar] [CrossRef]
- Rodriquez, V.; Camarillo, R.; Martinez, F.; Jimenez, C.; Rincon, J. CO2 photocatalytic reduction with CNT/TiO2 based nanocomposites prepared by high-pressure technology. J. Supercrit. Fluids 2020, 163, 104876. [Google Scholar] [CrossRef]
- Paul, R.; Hou, J.; Yang, M.; Dou, Q.; Chen, Q.; Wang, X.; Hu, C. Defect engineering in polymeric carbon nitride with the accordion structure for efficient photocatalytic CO2 reduction and H2 production. Chem. Eng. J. 2022, 450, 138425. [Google Scholar]
- Sonowal, K.; Nandal, N.; Basyach, P.; Kalita, L.; Jain, S.L.; Saikia, L. Photocatalytic reduction of CO2 to methanol using Zr (IV)-based MOF composite with g-C3N4 quantum dots under visible light irradiation. J. CO2 Util. 2022, 57, 101905. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, Y.; Yu, T. Synthesis of g-C3N4/NiO-carbon microsphere composites for Co-reduction of CO2 by photocatalytic hydrogen production from water decomposition. J. Clean. Prod. 2022, 357, 13180. [Google Scholar] [CrossRef]
- Sahoo, R.C.; Lu, H.; Garg, D.; Yin, Z.; Matte, H.S.S.M. Bandgap-engineered g-C3N4 and its graphene composites for stable photoreduction of CO2 to methanol. Carbon 2022, 192, 101–108. [Google Scholar] [CrossRef]
- Ahmad, K.N.; Isahak, W.N.R.W.; Rosli, M.I.; Yusop, M.R.; Kassim, M.R.; Yarmo, M.A. Rare earth metal doped nickel catalysts supported on exfoliated graphitic carbon nitride for highly selective CO and CO2 methanation. Appl. Surf. Sci. 2022, 571, 151321. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, P.; Wen, Q.; Fan, J.; Xiang, Q. Copper and platinum dual-single-atoms supported on crystalline graphitic carbon nitride for enhanced photocatalytic CO2 reduction. Chinese J. Catal. 2022, 43, 451–460. [Google Scholar] [CrossRef]
- Andrade, O.R.; Rodriguez, V.; Camarillo, R.; Martinez, F.; Jimenez, C.; Rincon, J. Photocatalytic reduction of CO2 with N-doped TiO2-based photocatalysts obtained in one-pot supercritical synthesis. Nanomaterials 2022, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, S.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nano architectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Lv, X.; Niu, X.; Lin, X.; Zhong, S.; Wang, D.; Lin, H.; Chen, J.; Bai, S. Stacking engineering of semiconductor heterojunctions on hollow carbon spheres for boosting photocatalytic CO2 reduction. ACS Catal. 2022, 12, 569–2580. [Google Scholar] [CrossRef]
- Muhammad, T.; Beenish, T. Constructing S-scheme 2D/0D g-C3N4/TiO2 NPs/MPs heterojunction with 2D-Ti3AlC2 MAX cocatalyst for photocatalytic CO2 reduction to CO/CH4 in fixed-bed and monolith photoreactors. J. Mater. Sci. Technol. 2022, 106, 195–210. [Google Scholar]
- Zhao, X.; Han, D.; Dai, M.; Fan, Y.; Wang, Z.; Han, D.; Niu, L. Direct Z-scheme FeV2O4/g-C3N4 binary catalyst for highly selective reduction of carbon dioxide. Chem. Eng. J. 2022, 436, 132051. [Google Scholar] [CrossRef]

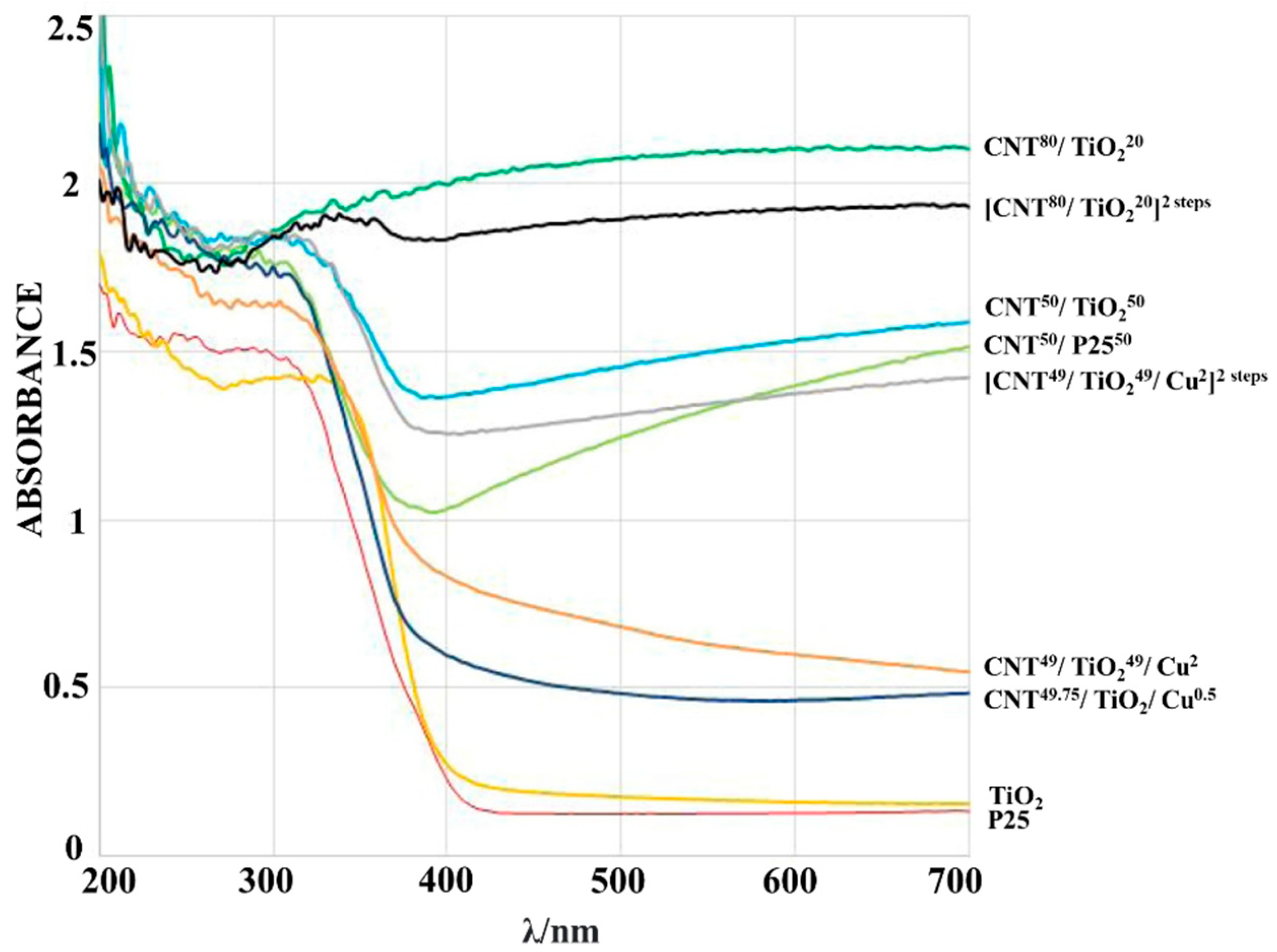
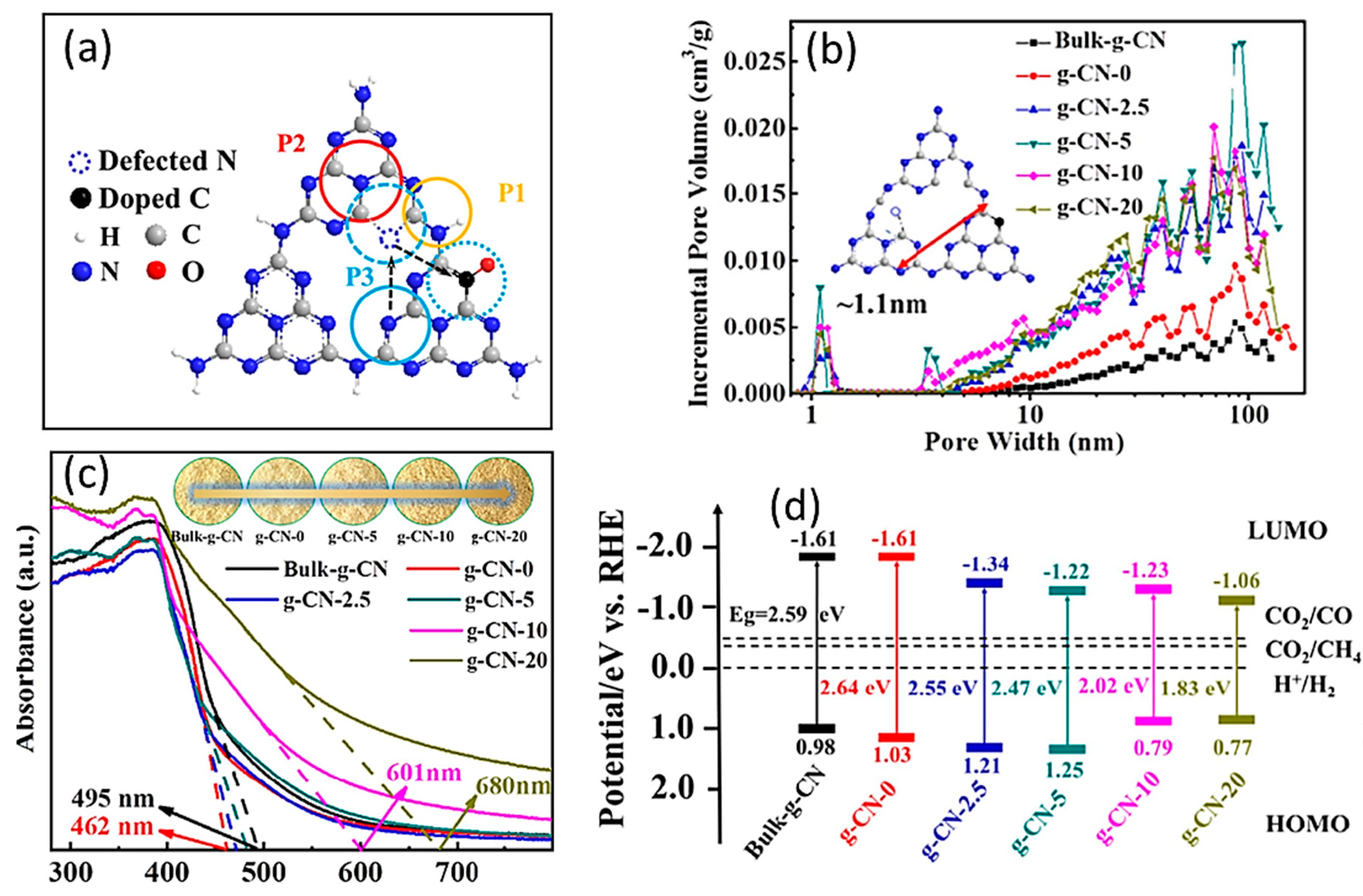
| S. No | Carbon Materials as Supporters | Advantages | Findings | Ref. No |
|---|---|---|---|---|
| 1 | g-CNQDs@MOF | Increased surface area | The addition of g-CNQDs enhanced the specific surface area of the binary composite from 338.6 m2/g to 828.3 m2/g | [67] |
| 2 | NiO/g–C3N4–carbon microsphere composites | Increased surface area | g-C3N4 and carbon microsphere were added as templates for a larger surface area. | [68] |
| 3 | Band-gap-engineered g-C3N4/rGO | charge separation and conductivity | The copolymerization technique highly helped for reducing the bandgap. The formation of heterostructure aided in increasing the charge separation | [69] |
| 4 | Ni-Ce/eg-C3N4 | Promoted CO2 adsorption | eg-C3N4 created abundant mesopores in the catalytic surface for CO2 adsorption | [70] |
| 5 | PtCu-crCN | Widespread metal loading | Carbon nitride provided the ligand for the dispersion of isolated Pt and Cu single atoms | [71] |
| 6 | N-doped TiO2/CNT and N-doped TiO2/rGO | Increased the charge recombination time | The addition of rGO doubled the CO2 conversion | [72] |
| 7 | MXene-based heterojunction photocatalysts | electron/hole reservoirs provided more active sites for CO2 adsorption | An optimum amount of Ti3C2 was used to avoid the stacking effect | [73] |
| 8 | C/CdS@ZnIn2S4 heterojunction photocatalysts | Increased light harvesting | A multi-layer reflection occurred within the stacked structured hollow nanostructure and preserved the metal CdS from corrosion | [74] |
| 9 | g-C3N4/TiO2/Ti3AlC2 2D/0D/2D composite | Enhanced selectivity | The 2D Ti3AlC2 MAX acted as a conductive substrate for the 2D/0D S-scheme heterojunction of g-C3N4, g-C3N4 increased the selectivity toward CO2 to CH4 | [75] |
| 10 | Z-scheme FeV2O4/g-C3N4 | Increased charge separation and active sites | The ion exchange liquid chemical.The method combined with the post-annealing technique helped in activity and selectivity | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar, D.; Liu, C.-H.; Anandan, S.; Wu, J.J. Photocatalytic CO2 Conversion into Solar Fuels Using Carbon-Based Materials—A Review. Molecules 2023, 28, 5383. https://doi.org/10.3390/molecules28145383
Sundar D, Liu C-H, Anandan S, Wu JJ. Photocatalytic CO2 Conversion into Solar Fuels Using Carbon-Based Materials—A Review. Molecules. 2023; 28(14):5383. https://doi.org/10.3390/molecules28145383
Chicago/Turabian StyleSundar, Dhivya, Cheng-Hua Liu, Sambandam Anandan, and Jerry J. Wu. 2023. "Photocatalytic CO2 Conversion into Solar Fuels Using Carbon-Based Materials—A Review" Molecules 28, no. 14: 5383. https://doi.org/10.3390/molecules28145383
APA StyleSundar, D., Liu, C.-H., Anandan, S., & Wu, J. J. (2023). Photocatalytic CO2 Conversion into Solar Fuels Using Carbon-Based Materials—A Review. Molecules, 28(14), 5383. https://doi.org/10.3390/molecules28145383








