A Novel Bromophenol Compound from Leathesia nana Inhibits Breast Cancer in a Direct Tumor Killing and Immunotherapy Manner
Abstract
1. Introduction
2. Results and Discussion
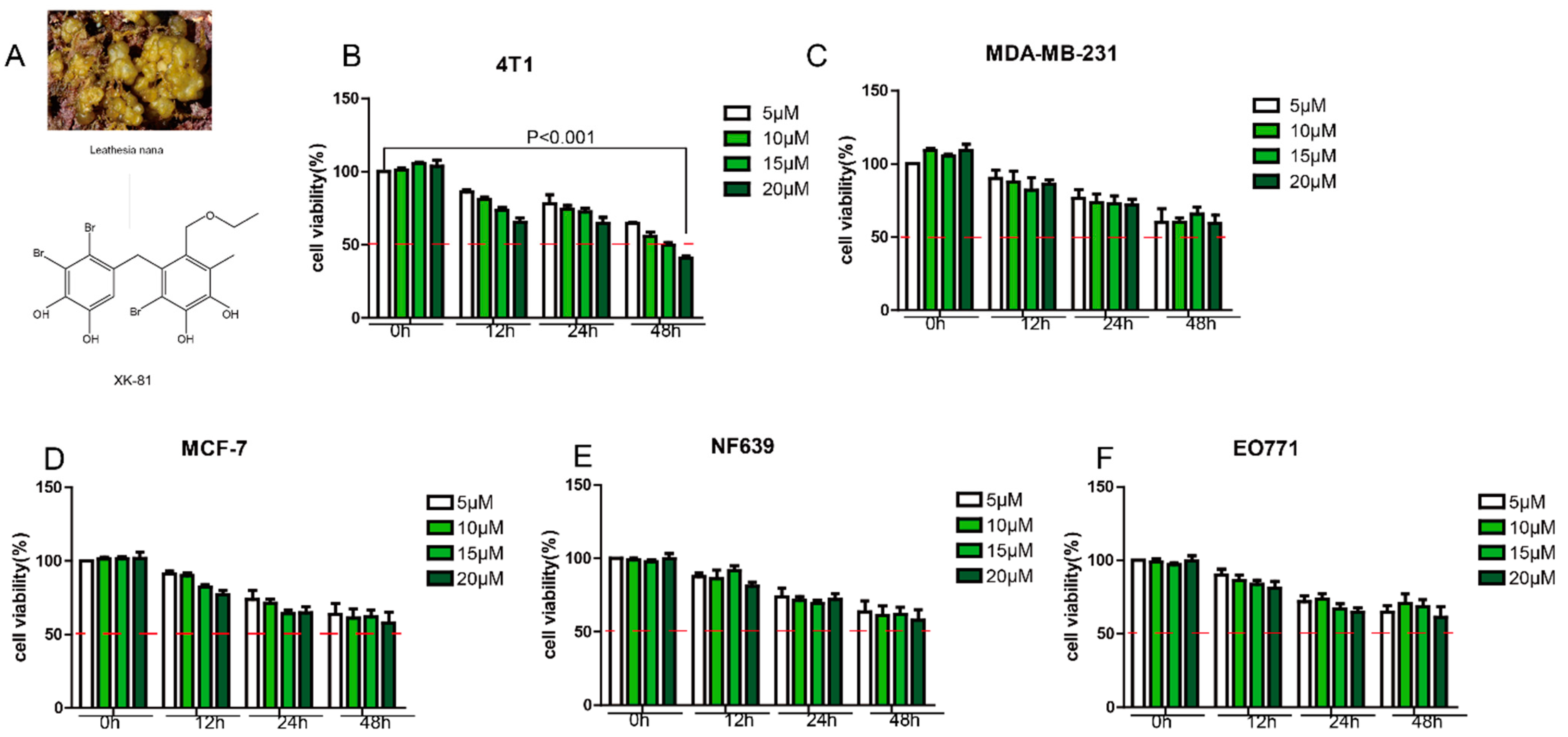
2.1. XK-81 Selectively Inhibited 4T-1 Cell Proliferation In Vitro
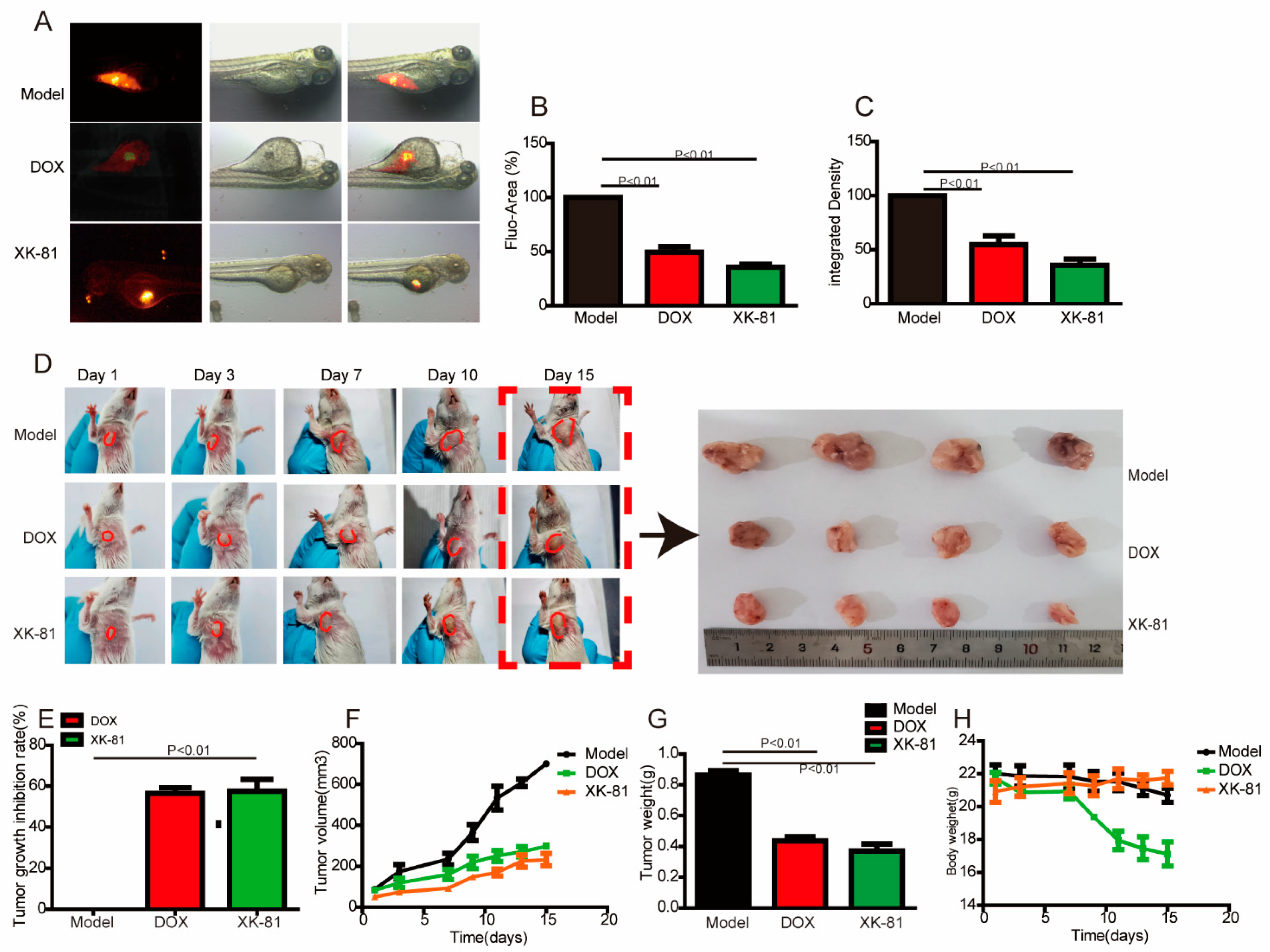
2.2. XK-81 Treatment Inhibited Tumor Growth In Vivo
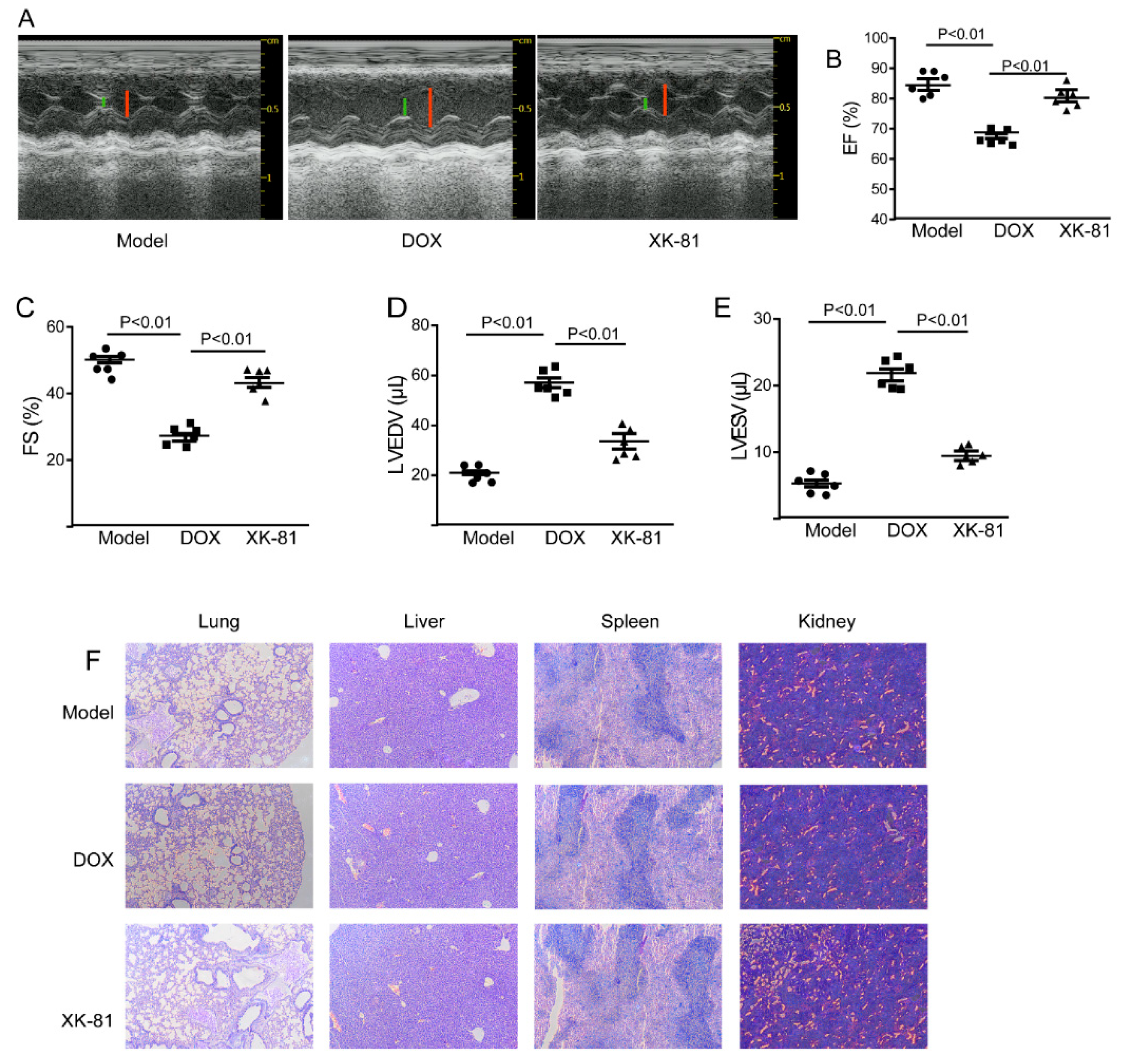
2.3. XK-81 Had Little Cardiotoxicity in Tumor-Bearing Mice
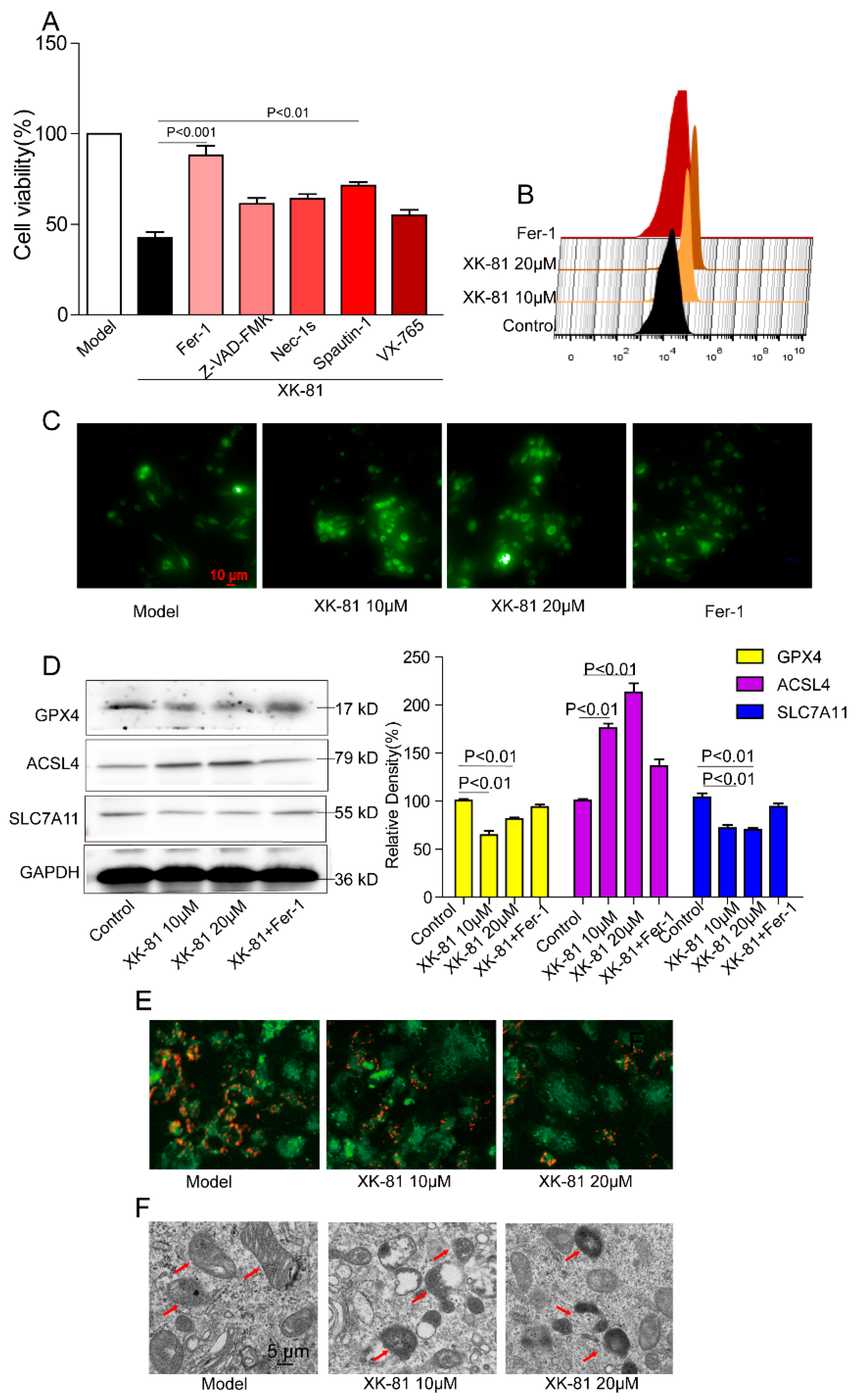
2.4. XK-81 Promoted 4T-1 Cells Death via Ferroptosis
2.5. XK-81 Promoted Reactive Oxygen Species (ROS) Generation in 4T-1 Cells
2.6. Ferroptosis Occurred in a XK-81-Treated Mouse Tumor
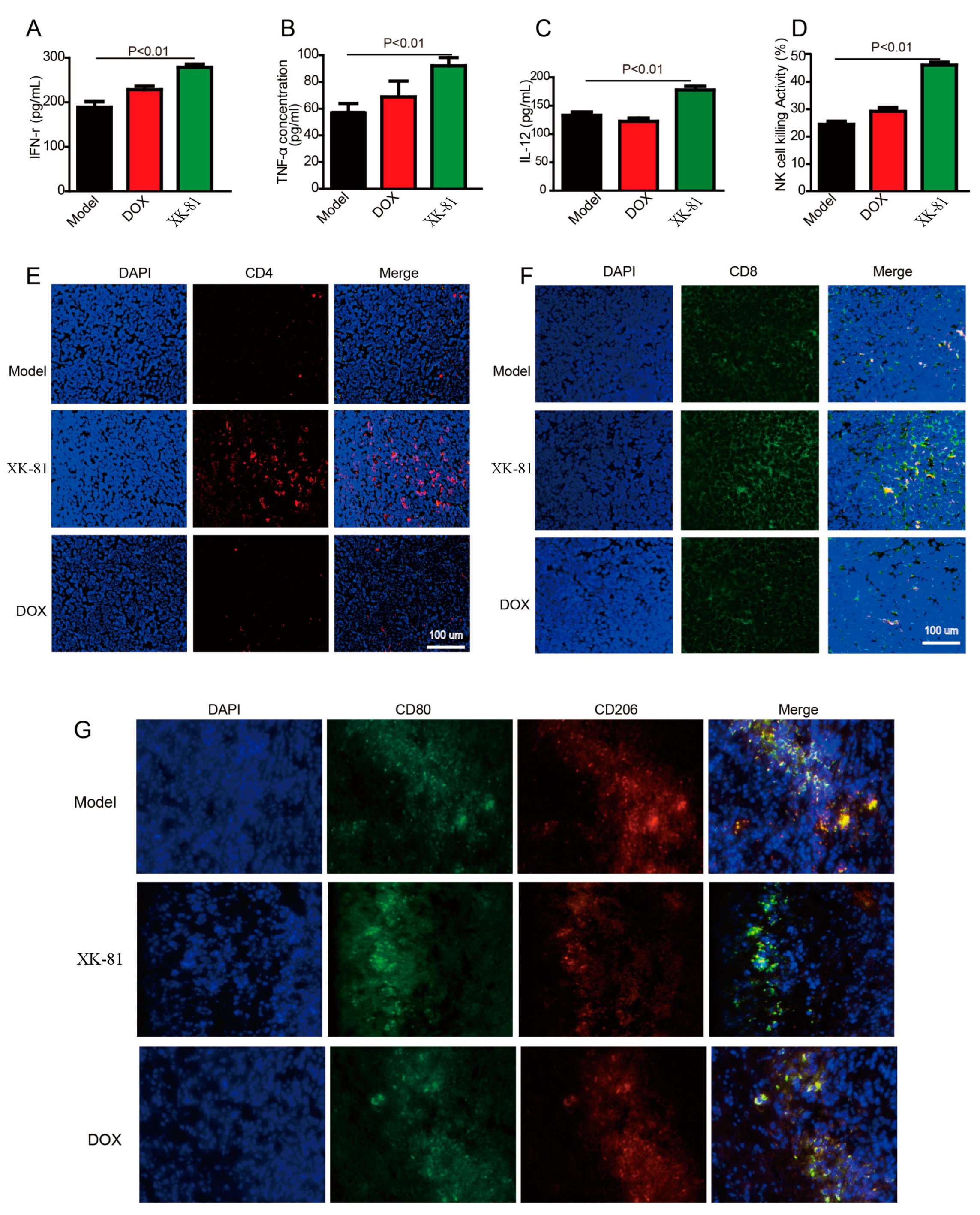
2.7. Immunomodulatory Activity of XK-81 in the 4T-1 Mouse Tumor Model
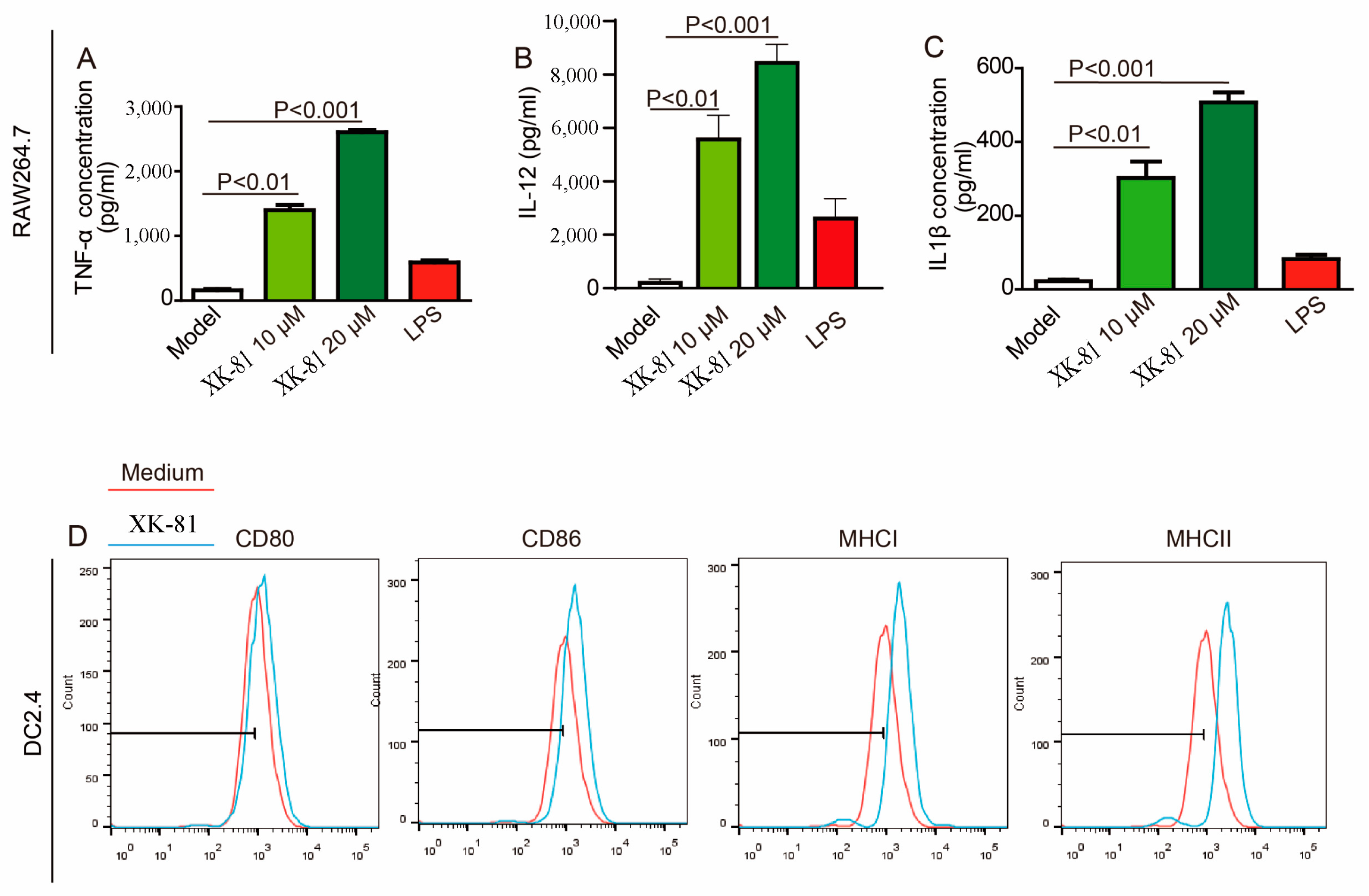
2.8. XK-81 Activated APCs In Vitro
3. Materials and Methods
3.1. Materials and Chemical Reagents
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Ethics Statement
3.5. Establishment of Breast Cancer Zebrafish Model
3.6. Animal Model and Treatment Protocols
3.7. Evaluation of Blood Parameters
3.8. Preparation and Activity Assay of NK Cells from Spleen
3.9. Echocardiography
3.10. Identification of Hallmarks of Ferroptosis
3.11. Clinical Samples
3.12. Determination of Reactive Oxygen Species (ROS) Generation
3.13. Immunohistochemistry Analysis
3.14. Western Blotting Analysis
3.15. Flow Cytometry Analysis
3.16. Immunofluorescence Analysis
3.17. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thurlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef] [PubMed]
- Loveless, R.; Bloomquist, R.; Teng, Y. Pyroptosis at the forefront of anticancer immunity. J. Exp. Clin. Cancer Res. 2021, 40, 264. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Ding, X.; Qiao, Y.; Li, B. Ubiquitin-specific protease 35 (USP35) mediates cisplatin-induced apoptosis by stabilizing BIRC3 in non-small cell lung cancer. Lab. Investig. 2022, 102, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Skubnik, J.; Svobodova Pavlickova, V.; Ruml, T.; Rimpelova, S. Autophagy in cancer resistance to paclitaxel: Development of combination strategies. Biomed. Pharmacother. 2023, 161, 114458. [Google Scholar] [CrossRef]
- Su, L.; Chen, Y.; Huang, C.; Wu, S.; Wang, X.; Zhao, X.; Xu, Q.; Sun, R.; Kong, X.; Jiang, X.; et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci. Transl. Med. 2023, 15, eabl7895. [Google Scholar] [CrossRef]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2021, 268, 120537. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, X.; Yang, P.; Zhao, J.; Zhang, W.; Feng, N.; Yang, W.; Tang, J. Defect self-assembly of metal-organic framework triggers ferroptosis to overcome resistance. Bioact. Mater. 2023, 19, 1–11. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Guo, J.; Xu, B.; Han, Q.; Zhou, H.; Xia, Y.; Gong, C.; Dai, X.; Li, Z.; Wu, G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, H.; Li, S.; Qin, T.; Shi, H.; Ma, J.; Li, L.; Yu, G.; Jiang, T.; Li, C. Dihydroartemisinin enhances the inhibitory effect of sorafenib on HepG2 cells by inducing ferroptosis and inhibiting energy metabolism. J. Pharmacol. Sci. 2022, 148, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target Ther. 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; ME, L.L. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- Tan, W.; Pan, T.; Wang, S.; Li, P.; Men, Y.; Tan, R.; Zhong, Z.; Wang, Y. Immunometabolism modulation, a new trick of edible and medicinal plants in cancer treatment. Food Chem. 2021, 376, 131860. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Yin, S.; To, K.K.W.; Fu, L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol. Cancer 2023, 22, 44. [Google Scholar] [CrossRef]
- Song, J.; Cheng, M.; Xie, Y.; Li, K.; Zang, X. Efficient tumor synergistic chemoimmunotherapy by self-augmented ROS-responsive immunomodulatory polymeric nanodrug. J. Nanobiotechnol. 2023, 21, 93. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Aspects Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Zang, X.; Song, J.; Yi, X.; Piyu, J. Polymeric indoximod based prodrug nanoparticles with doxorubicin entrapment for inducing immunogenic cell death and improving the immunotherapy of breast cancer. J. Mater. Chem. B 2022, 10, 2019–2027. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schroder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 2023, 186, 1432–1447.e1417. [Google Scholar] [CrossRef]
- Onkar, S.S.; Carleton, N.M.; Lucas, P.C.; Bruno, T.C.; Lee, A.V.; Vignali, D.A.A.; Oesterreich, S. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023, 13, 23–40. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e1005. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, L.; Li, Y.; Hu, Z.; He, F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: Perspectives for immunotherapy of SCLC. Semin. Cancer Biol. 2022, 86, 273–285. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Y.; Ding, J.H.; Jin, X.; Ma, D.; Li, D.Q.; Shi, J.X.; Huang, W.; Wang, Y.P.; Jiang, Y.Z.; et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 2023, 35, 84–100.e108. [Google Scholar] [CrossRef]
- Xu, H.; Ye, D.; Ren, M.; Zhang, H.; Bi, F. Ferroptosis in the tumor microenvironment: Perspectives for immunotherapy. Trends Mol. Med. 2021, 27, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, X.; Zhou, Y.; Wang, X.; Yu, Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target Ther 2022, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Duan, X.; Ni, K.; Li, Y.; Chan, C.; Lin, W. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials 2022, 280, 121315. [Google Scholar] [CrossRef]
- Harding, J.J.; Khalil, D.N.; Fabris, L.; Abou-Alfa, G.K. Rational development of combination therapies for biliary tract cancers. J. Hepatol. 2023, 78, 217–228. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Papon, N.; Copp, B.R.; Courdavault, V. Marine drugs: Biology, pipelines, current and future prospects for production. Biotechnol. Adv. 2022, 54, 107871. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Goncalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Mahfoudhi, G.; Ameur, W.B.; Malysheva, S.V.; Szternfeld, P.; Touil, S.; Driss, M.R.; Joly, L. First study of bromophenols and hexabromocyclododecanes in seafood from North Africa (case of Bizerte Lagoon, Tunisia): Occurrence and human health risk. Environ. Sci. Pollut. Res. Int. 2023, 30, 64499–64516. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Perez-Correa, J.R.; Dominguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Paudel, P.; Park, S.E.; Seong, S.H.; Fauzi, F.M.; Jung, H.A.; Choi, J.S. Bromophenols from Symphyocladia latiuscula (Harvey) Yamada as Novel Cholecystokinin 2 Receptor Antagonists. J. Integr. Neurosci. 2023, 22, 10. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Z.; Wang, L.; Liu, Y.; Stagos, D.; Lin, X.; Liu, M. Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Mar. Drugs 2021, 19, 641. [Google Scholar] [CrossRef]
- Deng, R.; Zhang, H.L.; Huang, J.H.; Cai, R.Z.; Wang, Y.; Chen, Y.H.; Hu, B.X.; Ye, Z.P.; Li, Z.L.; Mai, J.; et al. MAPK1/3 kinase-dependent ULK1 degradation attenuates mitophagy and promotes breast cancer bone metastasis. Autophagy 2021, 17, 3011–3029. [Google Scholar] [CrossRef]
- Kang, M.W.C.; Liu, H.; Kah, J.C.Y. Innate immune activation by conditioned medium of cancer cells following combined phototherapy with photosensitizer-loaded gold nanorods. J. Mater. Chem. B 2020, 8, 10812–10824. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Qiang, W.A.; Wang, C.M.; Tan, W.; Wang, Y.T. Furanodiene enhances the anti-cancer effects of doxorubicin on ERalpha-negative breast cancer cells in vitro. Eur. J. Pharmacol. 2016, 774, 10–19. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Zang, X.; Xie, Y.; Song, J.; Chen, X. ROS-responsive Galactosylated-nanoparticles with Doxorubicin Entrapment for Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2023, 18, 1381–1397. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, S.; Zheng, Y.; Wang, N.; Yang, B.; Wang, D.; Yang, D.; Mei, W.; Zhao, Z.; Wang, Z. Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-kappaB/c-Myc pathway. Biochem. Pharmacol. 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, L.L.; Yang, S.L.; Wang, R.Q.; Gao, H.; Lin, Z.Y.; Zhao, Y.Y.; Tang, W.W.; Han, R.; Wang, W.J.; et al. Andrographolide suppresses breast cancer progression by modulating tumor-associated macrophage polarization through the Wnt/beta-catenin pathway. Phytother. Res. 2022, 36, 4587–4603. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Le Menn, G.; Tuusa, J.; Chen, Z.J.; Tasanen, K.; Kokkonen, N. Absence of NC14A Domain of COLXVII/BP180 in Mice Results in IL-17–Associated Skin Inflammation. J. Investig. Dermatol. 2023, 143, 48–56.e47. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Gong, Z.; Wang, Z.; Yang, W.; Liu, W.; Hou, L.; Liu, X.; Hua, J.; Wang, B.; Li, N. The Degradation of TMEM166 by Autophagy Promotes AMPK Activation to Protect SH-SY5Y Cells Exposed to MPP+. Cells 2022, 11, 2706. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, W.; Sun, M.; He, P.; Lv, H.; Wang, Q.; Zhang, S.; Wu, Q.; Ling, P.; Chen, S.; et al. Novel bi-layered dressing patches constructed with radially-oriented nanofibrous pattern and herbal compound-loaded hydrogel for accelerated diabetic wound healing. Appl. Mater. Today 2022, 28, 101542. [Google Scholar] [CrossRef]
- Guan, X.; Zheng, X.; Vong, C.T.; Zhao, J.; Xiao, J.; Wang, Y.; Zhong, Z. Combined effects of berberine and evodiamine on colorectal cancer cells and cardiomyocytes in vitro. Eur. J. Pharmacol. 2020, 875, 173031. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, D.; Ji, Q.; Li, Y.; Cai, Z.; Fang, L.; Huo, H.; Zhou, G.; Yan, X.; Shen, L.; et al. Jujuboside A attenuates sepsis-induced cardiomyopathy by inhibiting inflammation and regulating autophagy. Eur. J. Pharmacol. 2022, 947, 175451. [Google Scholar] [CrossRef]
- Xu, Q.; Liao, Z.; Gong, Z.; Liu, X.; Yang, Y.; Wang, Z.; Yang, W.; Hou, L.; Yang, J.; Song, J.; et al. Down-regulation of EVA1A by miR-103a-3p promotes hepatocellular carcinoma cells proliferation and migration. Cell Mol. Biol. Lett. 2022, 27, 93. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, R.; Zhang, M.; Li, B.; Jiang, S.; Yu, W.; Yang, L.; Han, Y.; Zhong, Z.; Zhao, W. A Novel Bromophenol Compound from Leathesia nana Inhibits Breast Cancer in a Direct Tumor Killing and Immunotherapy Manner. Molecules 2023, 28, 5349. https://doi.org/10.3390/molecules28145349
Sun R, Zhang M, Li B, Jiang S, Yu W, Yang L, Han Y, Zhong Z, Zhao W. A Novel Bromophenol Compound from Leathesia nana Inhibits Breast Cancer in a Direct Tumor Killing and Immunotherapy Manner. Molecules. 2023; 28(14):5349. https://doi.org/10.3390/molecules28145349
Chicago/Turabian StyleSun, Ruochen, Mi Zhang, Bufan Li, Shan Jiang, Wanpeng Yu, Lina Yang, Yantao Han, Zhangfeng Zhong, and Wenwen Zhao. 2023. "A Novel Bromophenol Compound from Leathesia nana Inhibits Breast Cancer in a Direct Tumor Killing and Immunotherapy Manner" Molecules 28, no. 14: 5349. https://doi.org/10.3390/molecules28145349
APA StyleSun, R., Zhang, M., Li, B., Jiang, S., Yu, W., Yang, L., Han, Y., Zhong, Z., & Zhao, W. (2023). A Novel Bromophenol Compound from Leathesia nana Inhibits Breast Cancer in a Direct Tumor Killing and Immunotherapy Manner. Molecules, 28(14), 5349. https://doi.org/10.3390/molecules28145349







