Abstract
d-pantolactone is an intermediate in the synthesis of d-pantothenic acid, which is known as vitamin B5. The commercial synthesis of d-pantolactone is carried out through the selective resolution of dl-pantolactone catalyzed by lactone hydrolase. In contrast to a kinetic resolution approach, the deracemization of dl-pantolactone is a simpler, greener, and more sustainable way to obtain d-pantolactone with high optical purity. Herein, an efficient three-enzyme cascade was developed for the deracemization of dl-pantolactone, using l-pantolactone dehydrogenase from Amycolatopsis methanolica (AmeLPLDH), conjugated polyketone reductase from Zygosaccharomyces parabailii (ZpaCPR), and glucose dehydrogenase from Bacillus subtilis (BsGDH). The AmeLPLDH was used to catalyze the dehydrogenated l-pantolactone into ketopantolactone; the ZpaCPR was used to further catalyze the ketopantolactone into d-pantolactone; and glucose dehydrogenase together with glucose fulfilled the function of coenzyme regeneration. All three enzymes were co-expressed in E. coli strain BL21(DE3), which served as the whole-cell biocatalyst. Under optimized conditions, 36 h deracemization of 1.25 M dl-pantolactone d-pantolactone led to an e.e.p value of 98.6%, corresponding to productivity of 107.7 g/(l·d).
1. Introduction
As a chiral alcohol, d-pantolactone serves as an important intermediate in the synthesis of d-pantothenic acid (vitamin B5), which is widely used as a pharmaceutical and cosmetic ingredient as well as a food and feed additive [1,2]. In commercial production, d-pantothenic acid is synthesized through the condensation reaction between d-pantolactone and β-alanine, and d-pantolactone is mainly prepared through the lactonase-catalyzed resolution of racemic dl-pantolactone [3,4,5,6]. In the process of enzymatic resolution, the generated enantiopure d-pantothenic acid is separated from the remaining l-pantolactone and further converted into d-pantolactone by a chemical lactonization step, requiring the consumption of energy, solvents, and acids/alkalis [7]. In efforts to develop a simpler and greener process, the oxidoreductase-based asymmetric synthesis of d-pantolactone has received increasing attention in recent years [7,8,9,10,11,12,13]. These efforts have mainly focused on the enzymatic reduction of ketopantolactone to d-pantolactone with the assistance of a glucose dehydrogenase/glucose coenzyme regeneration system. For instance, an E. coli strain co-expressing conjugated polyketone reductase from Saccharomyces cerevisiae (SceCPR1) and glucose dehydrogenase from Exiguobacterium sibiricum (EsGDH) was applied for the asymmetric reduction of ketopantolactone, providing 458 mM d-pantolactone with an e.e.p value of 99.9% and a yield of 91.6% after 6 h [8]. Ketopantolactone is prone to spontaneous hydrolysis into ketopantoic acid at neutral pH, which is unfavorable for the direct reduction of ketopantolactone to d-pantolactone. Thus, the deracemization of dl-pantolactone as a more stable substrate is more advantageous for practical purposes. More importantly, the deracemization process via an oxidation–reduction sequence allows us to transform a cheap racemate into a single enantiomer (e.g., d-pantolactone), while reducing byproduct formation and circumventing the isolation of intermediates [14,15,16,17].
In the process of enzymatic deracemization, dl-pantolactone is catalyzed by three oxidoreductases for efficient coenzyme regeneration: l-pantolactone dehydrogenase (LPLDH), ketopantolactone reductase (KPLR), and glucose dehydrogenase (GDH). Enzymatic deracemization consists of the stereoselective dehydrogenation of l-enantiomer and the subsequent asymmetric reduction of ketopantolactone to d-enantiomer. It is crucial that both l-pantolactone dehydrogenase and ketopantolactone reductase possess high activity and strict enantioselectivity. However, enzyme resources for the deracemization of dl-pantolactone are relatively limited; thus, lactonase-catalyzed kinetic resolution is still the leading approach. Specifically, known l-pantolactone dehydrogenases are membrane-bound enzymes, and ascertaining their heterologous expression remains challenging [13,18]. Mining novel enzymes or engineering known enzymes is essential to meet the requirements of activity and enantioselectivity for the efficient deracemization of dl-pantolactone [19,20].
For multi-enzymatic reactions, enzyme cascades combine the advantages of biocatalysis and one-pot multi-step reactions [21]. In the case of dl-pantolactone deracemization, FMN-dependent l-pantolactone dehydrogenases oxidize the substrate with flavin reduction, and electrons from the reduced flavin are captured by electron acceptors to regenerate the oxidized flavin. Thus, whole-cell biocatalysts outperform free enzymes in the deracemization of dl-pantolactone [22]. In addition, the use of whole-cell biocatalysts co-expressing multiple enzymes can improve the mass transfer efficiency between enzymes and the cell envelope can provide protection for intracellular enzymes [23]. In multi-enzymatic reactions, the fusion of multiple enzymes can be a viable method for generating proteins with improved catalytic activity [24,25,26]. It must be noted that, often, not every enzymatic reaction in an enzyme cascade will reach the optimal conditions, and balancing different catalytic properties is necessary to account for synergistic effects [27,28].
We previously developed a three-enzyme cascade approach to mediate the one-pot, two-step reduction of (E/Z)-citral to (S)-citronellol, a chiral alcohol with a rose fragrance [29]. Inspired by this work, a new three-enzyme system using LPLDH, conjugated polyketone reductase (CPR), and GDH was constructed to achieve deracemization of dl-pantolactone into d-pantolactone (Scheme 1). Each enzyme involved in the process was carefully selected, and the enzymatic properties of newly mined l-pantolactone dehydrogenase and ketopantolactone reductase were characterized. In addition, genetic fusion of ketopantolactone reductase and glucose dehydrogenase was attempted to increase the catalytic efficiency. Finally, the optimization of whole-cell biotransformation enabled efficient deracemization of dl-pantolactone at high concentrations.
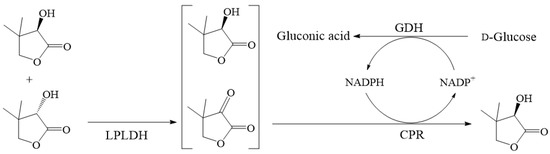
Scheme 1.
One-pot deracemization of dl-pantolactone to its d-enantiomer through a biocatalysis cascade using E. coli cells co-expressing l-pantolactone dehydrogenase (LPLDH), conjugated polyketone reductase (CPR), and glucose dehydrogenase (GDH).
2. Results and Discussion
2.1. Three-Enzyme Cascade for Deracemization of dl-Pantolactone
A whole-cell biocatalyst co-expressing SceCPR1 and EsGDH in E. coli was previously constructed to catalyze the asymmetric reduction of ketopantolactone to d-pantolactone. To upgrade the asymmetric reduction to the deracemization process, it is necessary to use l-pantolactone dehydrogenase with strict l-enantioselectivity to initiate the dehydrogenation of l-pantolactone and keep the d-pantolactone unreacted. l-pantolactone dehydrogenase from Nocardia asteroides (NasLPLDH) was purified from the wild-type strain and was able to catalyze the dehydrogenation of l-pantolactone into ketopantolactone [30]. The amino acid sequence encoding NasLPLDH was chosen to perform a homology search in the NCBI database. Four putative l-pantolactone dehydrogenases were selected from Nocardia cyriacigeorgica GUH-2 (91% sequence identity), Nocardia farcinica IFM 10152 (88% sequence identity), Amycolatopsis methanolica 239 (76% sequence identity), and Cnuibacter physcomitrellae strain XA(T) (71% sequence identity) and named NcyLPLDH, NfaLPLDH, AmeLPLDH, and CphLPLDH, respectively. All of the genes were synthesized onto the pET28a plasmid and overexpressed in E. coli BL21(DE3) (Figure S1). When the substrate specificity was investigated, all five LPLDHs showed the capacity to catalyze the dehydrogenation of l-pantolactone but not d-pantolactone, indicating their strict l-enantioselectivity.
To further screen l-pantolactone dehydrogenase with high catalytic activity, each recombinant plasmid harboring the LPLDH-encoding gene was individually introduced into competent cells containing the pACYCDuet-1-SceCPR1-EsGDH plasmid, resulting in the recombinant strains co-expressing different l-pantolactone dehydrogenases, SceCPR1 and EsGDH. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis showed good expression levels of all tested enzymes (Figure S2). As whole-cell biocatalysts, the strains co-expressing LPLDH, SceCPR1 and EsGDH, increased the e.e.p value from 10.5% to 24.5%, demonstrating that they were feasible for enzymatic deracemization of dl-pantolactone (Figure 1). Among them, AmeLPLDH had the highest e.e.p value (24.5%) and was selected for further studies.
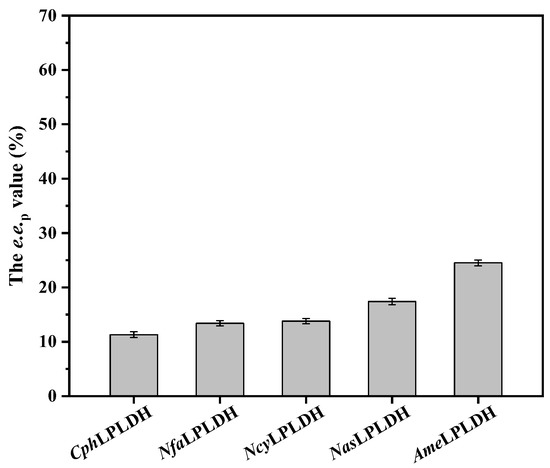
Figure 1.
Catalytic activity screening of l-pantolactone dehydrogenases. Reaction mixture (10 mL) consisted of 200 g/L wet cells co-expressing LPLDH, SceCPR1 and EsGDH, 1 M dl-pantolactone, 1.5 M glucose and 200 mM PBS buffer (pH 7.0). Constant pH was maintained through titration with 1 M NaOH. Reaction was run at 30 °C and 600 rpm for 24 h. Data represent mean values ± SD from three independent experiments.
Similar to l-pantolactone dehydrogenase, the high activity and strict d-enantioselectivity of conjugated polyketone reductase facilitate the efficient reduction of ketopantolactone to d-pantolactone. Unlike the limited resources of LPLDH, the number of characterized conjugated polyketone reductases rapidly increases [7,8,9,10,11,12,13]. The conjugated polyketone reductase from Candida dubliniensis CD36 (CduCPR) and a newly mined enzyme from Zygosaccharomyces parabailii (ZpaCPR) were individually co-expressed with EsGDH through the construction of recombinant plasmids pACYCDuet-1-CduCPR-EsGDH and pACYCDuet-1-ZpaCPR-EsGDH, respectively [9]. E. coli cells harboring these recombinant plasmids were used to catalyze the reduction of ketopantolactone, affording the single d-enantiomer. The recombinant plasmids were individually introduced into competent cells possessing pET28a-AmeLPADH, resulting in the recombinant strains co-expressing AmeLPLDH, conjugated polyketone reductase (SceCPR1, CduCPR or ZpaCPR), and EsGDH (Figure S3). ZpaCPR had the best catalytic performance in the deracemization of dl-pantolactone, increasing the e.e.p value from 24.5% to 44.6% (Figure 2a). In addition to LPLDH and CPR, glucose dehydrogenase, which is responsible for coenzyme regeneration, is required for the efficient deracemization of dl-pantolactone. Glucose dehydrogenases from Bacillus megaterium (BmGDH) and Bacillus subtilis (BsGDH) have been well characterized, and their catalytic performance was compared with that of EsGDH [27,31,32]. E. coli strains co-expressing AmeLPLDH, ZpaCPR, and different GDHs were constructed and used as whole-cell biocatalysts (Figure S4). Among the tested GDHs, BsGDH increased the e.e.p value up to 66.3% (Figure 2b). Therefore, the subsequent whole-cell biocatalysts consisted of AmeLPLDH, ZpaCPR, and BsGDH.
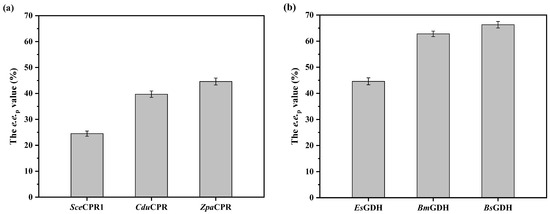
Figure 2.
Catalytic activity screening of (a) conjugated polyketone reductases and (b) glucose dehydrogenases. Reaction mixture (10 mL) consisted of 200 g/L wet cells co-expressing LPLDH, CPR and GDH, 1 M dl-pantolactone, 1.5 M glucose, and 200 mM PBS buffer (pH 7.0). Constant pH was maintained through titration with 1 M NaOH. Reaction was run at 30 °C and 600 rpm for 24 h. Data represent mean values ± SD from three independent experiments.
2.2. Purification and Characterization of Key Enzymes AmeLPLDH and ZpaCPR
As newly mined enzymes, AmeLPLDH and ZpaCPR were purified and then characterized to gain insights into their enzymatic and catalytic properties. The purification was conducted by affinity chromatography (Figure S5). Various factors, including temperature, pH, metal ions, and organic solvents, were investigated. The influence of temperature was determined over a range of 20–50 °C, and the highest activity for AmeLPLDH and ZpaCPR was observed at 30 and 45 °C, respectively (Figure 3a). To determine the effect of pH on activity, an enzyme assay was carried out at pH ranging from 5.0 to 10.0. The optimal pH for ZpaCPR was 5.5, and an increase in pH from 5.5 to 10.0 decreased the activity. In contrast, AmeLPLDH adapted to a much wider pH range (Figure 3b). Various metal ions were investigated for their effect on enzyme activity, and only Ca2+ slightly enhanced the enzyme activity of AmeLPLDH. None of the tested metal ions had a beneficial effect on the enzyme activity of ZpaCPR (Figure 4a). The use of organic solvents may have a profound effect on the activity of AmeLPLDH and ZpaCPR; thus, various readily available and cost-effective organic solvents (chloroform, ethyl acetate, isopropanol, acetone, octanol, and DMSO) were tested in this regard. Except for acetone, the tested organic solvents inhibited enzyme activity; octanol was the most harmful (Figure 4b). The kinetic parameters were determined, and the kcat/Km values of AmeLPLDH and ZpaCPR were 63.85 and 11.60 mM−1s−1, respectively (Table 1). Overall, the difference in enzymatic properties between AmeLPLDH and ZpaCPR indicated that it was necessary to subsequently orchestrate their catalytic performance with BsGDH.
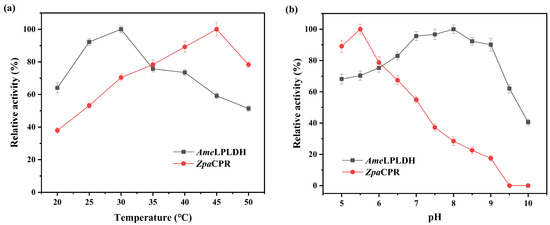
Figure 3.
Effect of (a) temperature and (b) pH on AmeLPLDH and ZpaCPR activity. (a) Relative activity of 100% for AmeLPLDH and ZpaCPR represents 1.47 U/mg (assayed at 30 °C, pH 6.5) and 4.73 U/mg (assayed at 45 °C, pH 6.0). (b) The relative activity of 100% for AmeLPLDH and ZpaCPR represents 1.81 U/mg (assayed at 30 °C, pH 8.0) and 5.63 U/mg (assayed at 45 °C, pH 5.5). Data represent mean values ± SD from three independent experiments.
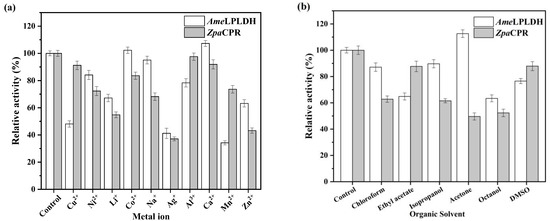
Figure 4.
Effect of (a) metal ions and (b) organic reagents on AmeLPLDH and ZpaCPR activity. Relative activity of 100% represents 1.81 U/mg (assayed at 30 °C, pH 8.0) and 5.63 U/mg (assayed at 45 °C, pH 5.5), respectively.

Table 1.
Kinetic parameters of AmeLPLDH and ZpaCPR.
2.3. Advanced Engineering of Biocatalyst Co-Expressing AmeLPLDH, ZpaCPR, and BsGDH
In cases of multi-enzymatic co-expression, fusion between two or more enzymes could outperform combinations of individual enzymes for enhanced catalytic activity [24,25,26]. With the aim of facilitating coenzyme regeneration, the enzymes ZpaCPR and BsGDH were ligated with the linker GSG, resulting in E. coli strain BL21 (DE3)/pET28a-AmeLPLDH/pACYCDuet-1-ZpaCPR-(GSG)-BsGDH. Indeed, introducing the fusion protein ZpaCPR-(GSG)-BsGDH increased the e.e.p value to 77.5%, indicating increased catalytic efficiency. Although the pET28a and pACYCDuet-1 vectors were compatible, their vector types might have affected the expression level of each gene product. The genes encoding AmeLPLDH and ZpaCPR-GSG-BsGDH exchanged vector type, resulting in E. coli strain BL21 (DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCRR-(GSG)-BsGDH. The newly constructed whole-cell biocatalyst further increased the e.e.p value to 88.1% (Figure 5). Thus, E. coli strain BL21 (DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCPR-(GSG)-BsGDH was used for subsequent deracemization of dl-pantolactone at high concentrations.
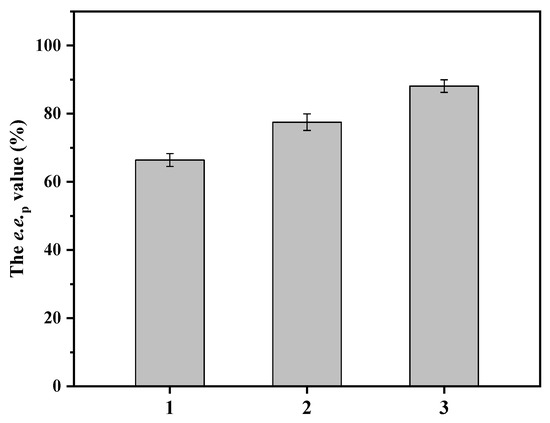
Figure 5.
Catalytic performance of whole-cell biocatalysts based on different methods of construction. 1: E. coli strain BL21 (DE3)/pET28a-AmeLPLDH/pACYCDuet-1-ZpaCPR-BsGDH; 2: E. coli strain BL21 (DE3)/pET28a-AmeLPLDH/pACYCDuet-1-ZpaCPR-(GSG)-BsGDH; 3: E. coli strain BL21 (DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCPR-(GSG)-BsGDH. Reaction mixture (10 mL) consisted of 200 g/L wet cells, 1 M dl-pantolactone, 1.5 M glucose, and 200 mM PBS buffer (pH 7.0). Constant pH was maintained through titration with 1 M NaOH. Reaction was run at 30 °C and 600 rpm for 24 h. Data represent mean values ± SD from three independent experiments.
2.4. Deracemization of dl-Pantolactone at High Concentrations
The enzymes AmeLPLDH, ZpaCPR, and BsGDH have different catalytic properties, and the deracemization process must be optimized to achieve adequate productivity and product concentrations [28]. AmeLPLDH, ZpaCPR, and BsGDH require FMN or NADPH as a coenzyme. However, neither FMN nor NADPH was exogenously supplied to the reaction mixture, so that the developed deracemization process would be cost-effective.
To achieve synergistic effects, factors including temperature, pH, agitation, glucose concentration, and substrate loading were investigated. The substrate concentration was set as 1000 mM and the pH was kept constant through titration with 1 M NaOH. The optimal temperature was 30 °C, and the e.e.p value remained >30% between 20 and 40 °C (Figure S7a). The effect of pH on catalytic efficiency was determined within the range of 5.0–10.0 (Figure S7b). The optimal pH was 6.0, and the e.e.p value decreased as the pH rose to 10.0, indicating that the enzymatic stability was more adapted to a weak acidic environment. Agitation was optimized to 400 rpm, although the e.e.p value remained >80% between 300 and 600 rpm (Figure S7c). As a co-substrate for coenzyme regeneration, glucose in various concentrations was used to test the effect on catalytic efficiency. When no glucose was added, the e.e.p value was 24.2%, suggesting the presence of an intracellular proton supply. The e.e.p value rose when the glucose concentration increased from 0 to 2000 mM, and concentrations higher than 2000 mM caused no further improvement. Thus, the ratio of glucose to substrate was set as 2:1. Under optimized conditions, substrate loading was increased stepwise from 500 to 1750 mM in order to investigate the effect on catalytic efficiency (Figure 6). When the substrate concentration was not greater than 1000 mM, the e.e.p value remained at >98%. When the substrate concentration was 1250 mM, the yield of both l-pantolactone and ketopantolactone began to increase, resulting in a lower e.e.p value (81.4%). When the substrate concentration was 1750 mM, the yield of l-pantolactone and ketopantolactone was 14.4 and 16.3%, respectively, indicating that the catalytic efficiency of the enzymes was insufficient after 24 h reaction. To identify the rate-limiting enzyme, E. coli cells expressing AmeLPLDH, ZpaCPR, or BsGDH were added to the reaction mixture (Figure 7). The addition of AmeLPLDH or ZpaCPR resulted in similar catalytic performance to the control, whereas supplementation with BsGDH dramatically increased the e.e.p value to 98.6%. A high proportion of d-pantolactone simplified the subsequent product separation procedure, and crude product was obtained through solvent extraction and solvent evaporation. Finally, the crude product was verified to be d-pantolactone through a combination of chiral GC, GC-MS, 1 H NMR, and 13C NMR analysis.
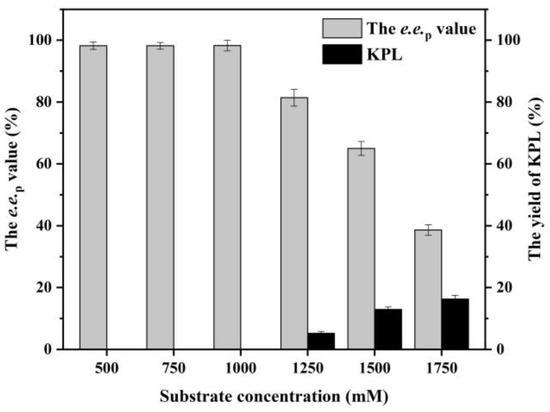
Figure 6.
Influence of substrate concentration on three-enzyme cascade catalytic system. Reaction mixture (10 mL) consisted of 200 g/L wet cells co-expressing three enzymes, 500–1750 mM dl-pantolactone, glucose/substrate ratio of 2:1 and 200 mM PBS buffer (pH 6.0). Constant pH was maintained through titration with 1 M NaOH. Reaction was run at 30 °C and 400 rpm for 24 h. Data represent mean values ± SD from three independent experiments.
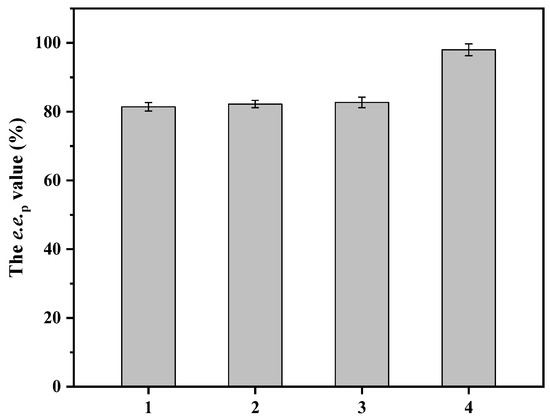
Figure 7.
Comparison of catalytic performance with added AmeLPLDH, ZpaCPR and BsGDH after 24 h. 1: Control without enzyme supplementation; 2: supplementation with ZpaCPR; 3: supplementation with AmeLPLDH; 4: supplementation with BsGDH. Initial reaction mixture (10 mL) consisted of 200 g/L wet cells, 1.25 M dl-pantolactone, 2.5 M glucose, and 200 mM PBS buffer (pH 7.0). Constant pH was maintained through titration with 1 M NaOH. Reaction was run at 30 °C and 400 rpm for 36 h. Data represent mean values ± SD from three independent experiments.
3. Materials and Methods
3.1. Chemicals, Genes, Plasmids, and Organisms
dl-pantolactone, ketopantolactone, and other chemicals and reagents were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Kits and enzymes for genetic manipulation were purchased from Takara Biomedical Technology Co., Ltd. (Beijing, China). Chromatography columns for protein purification were purchased from GE Healthcare Life Sciences (Shanghai, China). The genes encoding l-pantolactone dehydrogenase, ketopantolactone reductase and glucose dehydrogenase were codon-optimized and then synthesized by Qingke Biotechnology Co., Ltd. (Hangzhou, China). The pET28a and pACYCDuet-1 vectors were used for co-expression of l-pantolactone dehydrogenase, ketopantolactone reductase, and glucose dehydrogenase, and E. coli strain BL21(DE3) was used as the host. E. coli cells were grown routinely in LB medium at 37 °C for 12 h.
3.2. Construction of Toolbox through Homologous Protein-Search Analysis
The BLAST tool of the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 20 May 2020) was used to search potential l-pantolactone dehydrogenase and ketopantolactone reductase with the NasLPLDH amino acid sequence (GenBank accession no. GAD85679.1) and SceCPR1 amino acid sequence (no. NP_010159.1) as a probe. In the study, l-pantolactone dehydrogenase sequences included CphLPLDH (no. CP020715.1), NfaLPLDH (no. AP006618.1), NcyLPLDH (no. AP026975.1), and AmeLPLDH (no. WP_017981470.1), and ketopantolactone reductase sequences included CduCPR (no. XP_002421511.1) and ZpaCPR (no. AQZ16881.1). Based on the literature [27,31,32], the selected glucose dehydrogenases included EsGDH (no. KM817194.1), BmGDH (no. WP_033578120.1), and BsGDH (no. AFQ56330.1). Clustal X 2.0 software and ESPript 3.0 were used for multiple sequence alignment.
3.3. Overexpression of Single LPLDH, CPR, or GDH in E. coli BL(DE3)
The genes encoding LPLDH (CphLPLDH, NfaLPLDH, NcyLPLDH, NasLPLDH, or AmeLPLDH), CPR (SceCPR1, CduCPR, or ZpaCPR) and GDH (EsGDH, BmGDH, or BsGDH) were separately inserted into the EcoR I/Hind III sites of the pET28a vector, and the resulting plasmids were individually transferred into E. coli BL21(DE3), yielding recombinant strains E. coli BL21(DE)/pET28a-CphLPLDH, E. coli BL21(DE)/pET28a-NfaLPLDH, E. coli BL21(DE)/pET28a-NcyLPLDH, E. coli BL21(DE)/pET28a-NasLPLDH, E. coli BL21(DE)/pET28a-AmeLPLDH, E. coli BL21(DE)/pET28a-SceCRP1, E. coli BL21(DE)/pET28a-CduCPR, E. coli BL21(DE)/pET28a-ZpaCPR, E. coli BL21(DE)/pET28a-EsGDH, E. coli BL21(DE)/pET28a-BmGDH, and E. coli BL21(DE)/pET28a-BsGDH. The recombinant strains were initially grown at 37 °C in LB medium containing 50 µg/mL kanamycin. When the OD600 value reached 0.6, 0.2 mM isopropyl-β-D-thiolactopyranoside (IPTG) was added to initiate induction at 24 °C for 14 h. After induction, the cells were harvested by centrifugation at 4 °C for 10 min.
3.4. Screening of LPLDH, CPR, and GDH Co-Expressed in E. coli BL21 (DE3)
E. coli BL21 (DE3)/pACYCDuet-1-SceCPR1-EsGDH was successfully constructed, as described previously [8]. The pET28a recombinant plasmid with the gene encoding l-pantolactone dehydrogenase was transferred into E. coli BL21(DE3) competent cells with the recombinant plasmid pACYCDuet-1-SceCPR1-EsGDH, resulting in the strains co-expressing different LPLDH, SceCPR1 and EsGDH.
The genes encoding CduCPR and ZpaCPR were amplified from the plasmids pET28a-CduCPR and pET28a-ZpaCPR, respectively. The PCR program consisted of the following steps: 5 min at 98 °C, 30 cycles of 98 °C (10 s), 59 °C (15 s), and 72 °C (30 s), with a final extension at 72 °C for 5 min. The linear pACYCDuet-1-EsGDH fragment was amplified from the plasmid pACYCDuet-1-SceCPR1-EsGDH by inverse PCR. The corresponding PCR program was as follows: 5 min at 98 °C, 30 cycles of 98 °C (10 s), 58 °C (15 s), and 72 °C (90 s), with a final extension at 72 °C for 5 min. According to the instructions for the ClonExpress® II One Step Cloning Kit, the genes encoding CduCPR and ZpaCPR were ligated with linear pACYCDuet-1-EsGDH fragments via Exnase II-derived homologous recombination to form recombinant plasmids pACYCDuet-1-CduCPR-EsGDH and pACYCDuet-1-ZpaCPR-EsGDH. Recombinant plasmids pACYCDuet-1-SceCPR1-EsGDH, pACYCDuet-1-CduCPR-EsGDH, and pACYCDuet-1-ZpaCPR-EsGDH were transferred into E. coli BL21(DE3) competent cells with recombinant plasmid pET28a-AmeLPLDH, resulting in the strains co-expressing AmeLPLDH, different CPR, and EsGDH.
Similarly, the gene encoding EsGDH on the plasmid pACYCDuet-1-ZpaCPR-EsGDH was replaced with the gene encoding BmGDH or BsGDH, yielding the recombinant plasmids pACYCDuet-1-ZpaCPR-BmGDH and pACYCDuet-1-ZpaCPR-BsGDH. The recombinant plasmids pACYCDuet-1-ZpaCPR-EsGDH, pACYCDuet-1-ZpaCPR-BmGDH, and pACYCDuet-1-ZpaCPR-BsGDH were transferred into E. coli BL21(DE3) competent cells with the recombinant plasmid pET28a-AmeLPLDH, resulting in the strains co-expressing AmeLPLDH, ZpaCPR, and different GDH. The primers for the co-expression of LPLDH, CPR, and GDH are listed in Table S1.
For each recombinant strain co-expressing LPLDH, CPR, and GDH, single colonies were picked and transferred to 50 mL tubes containing 100 μg/mL kanamycin and 100 μg/mL chloramphenicol in LB medium. The cultures were incubated at 37 °C and 200 rpm for 12 h. When the OD600 reached 0.6–0.8, 0.2 mM IPTG was added to initiate induction at 24 °C and 150 rpm for 14 h. Cells were washed twice using 50 mM Tris–HCl buffer (pH 8.0), then harvested by centrifuging at 8000 × g at 4 °C for 10 min. A 12% (w/v) SDS-PAGE was used to visualize the co-expression of LPLDH, CPR, and GDH [33].
To select LPLDH, CPR, and GDH, recombinant E. coli cells as whole-cell biocatalyst were used to catalyze the deracemization of dl-pantolactone into d-pantolactone. The reaction mixture (10 mL) contained 1.0 M dl-pantolactone, 1.5 M d-glucose, 200 g/L wet cells, and 200 mM PBS buffer (pH 7.0). The reaction was conducted at 30 °C and 600 rpm for 24 h, and was terminated by adding an equal volume of 3 M HCl. After that, the mixture was centrifuged and the supernatant was extracted with 5 equivalent volumes of ethyl acetate. The organic phase was harvested by centrifugation at 8000× g for 10 min at room temperature and subsequently dehydrated with anhydrous sodium sulfate. Finally, 200 µL of the resulting dehydrated sample was subjected to GC analysis, as described in Section 3.10.
3.5. Purification of AmeLPLDH and ZpaCPR
Cells expressing AmeLPLDH and ZpaCPR were resuspended in 50 mM Tris-HCl buffer (pH 8.0) at a concentration of 50 g/L, then sonicated for cell disruption. Brij 35 was added to the AmeLPLDH cell homogenate to give a final concentration of 1%. After gentle stirring for 2.5 h, the cell debris was removed by centrifugation. The cell-free extracts of AmeLPLDH and ZpaCPR were then analyzed by Ni-NTA chelating affinity chromatography. Unbound proteins were washed off by applying a binding buffer (5 mM imidazole and 300 mM NaCl dissolved in 50 mM Tris-HCl, pH 8.0). AmeLPLDH was eluted out by applying an elution buffer (300 mM imidazole and 300 mM NaCl dissolved in 50 mM Tris-HCl, pH 8.0), and ZpaCPR was eluted out by applying an elution buffer (200 mM imidazole and 300 mM NaCl dissolved in 50 mM Tris-HCl, pH 8.0). The purity of the purified AmeLPLDH and ZpaCPR was verified using SDS-PAGE. The purified AmeLPLDH and ZpaCPR enzymes were desalted with 50 mM Tris-HCl buffer (pH 8.0) and stored at −20 °C for further investigation.
3.6. Activity Assay of AmeLPLDH and ZpaCPR
The activity of AmeLPLDH was measured at 30 °C by monitoring changes in absorbance at 450 nm. The 1 mL assay mixture of AmeLPLDH consisted of 1 µg purified enzyme, 10 mM l-pantolactone, 0.15 mM FMN, and 200 mM PBS buffer (pH 8.0). The enzyme assays began with the addition of the coenzyme FMN. One unit of activity represents the reduction of 1 µmol FMN per minute. The pure enzyme activity of ZpaCPR was measured at 45 °C by monitoring changes in absorbance at 340 nm. The 1 mL assay mixture of ZpaCPR consisted of 1 µg purified enzyme, 10 mM ketopantolactone, 0.15 mM NADPH, and 200 mM PBS buffer (pH 5.5). The enzyme assay began with the addition of the coenzyme NADPH. One unit of activity represented the oxidation of 1 µmol NADPH per minute. The BCA method was used to determine the protein concentration of all samples, with bovine serum albumin used as the standard protein. All enzyme assays were performed in triplicate [34].
In the characterization of AmeLPLDH and ZpaCPR, the effects of temperature and pH on the activity were investigated at 20–50 °C and pH 5.0–10.0. The metal ions (Cu2+, Ni2+, Li+, Co2+, Na+, Ag+, Al3+, Ca2+, Mn2+, Zn2+) and organic solvents (chloroform, ethyl acetate, isopropanol, acetone, octanol, DMSO) were individually added to the reaction mixture to determine their effects on the activity. In the determination of kinetic parameters, the tested substrate concentrations included 1, 2, 4, 6, 8, 10, 20, 30, and 40 mM. According to Michaelis–Menten kinetics, the parameters Km and Vmax were calculated through curve fitting using Origin Pro software (version 8.5).
3.7. Co-Expression of AmeLPLDH and Fusion Enzyme ZpaCPR-(GSG)-BsGDH
The fusion genes encoding ZpaCPR and BsGDH were constructed by multiple overlap extension PCR. To assemble the ZpaCPR-(linker)-BsGDH fusion gene, the stop codon of the ZpaCPR gene was removed and the linker GSG was introduced between the open reading frames of the genes encoding ZpaCPR and BsGDH via two rounds of PCR. The first round of PCR introduced the linker GSG into the ZpaCPR gene using one pair of primers (Table S2). Simultaneously, the complementary linker GSG was introduced into the BsGDH gene using the other pair of primers (Table S2). Each PCR product was purified and served as a template in the second round of PCR. The PCR program was as follows: 3 min at 98 °C, 30 cycles at 98 °C (10 s), 58 °C (15 s), and 72 °C (30 s), and a final 10 min extension at 72 °C. The PCR products of the ZpaCPR and BsGDH genes were joined by overlap extension PCR. The PCR program was as follows: 3 min at 98 °C, 30 cycles at 98 °C (10 s), 58 °C (15 s), and 72 °C (40 s), and a final 5 min extension at 72 °C. The purified PCR products were ligated into a pACYCDuet-1 vector. The fusion gene was confirmed by sequencing. Finally, the fusion gene was ligated into pACYCDuet-1 between EcoR I and Hind III sites, yielding pACYCDuet-1-ZpaCPR-(GSG)-BsGDH. Using the construction of pACYCDuet-1-ZpaCPR-(GSG)-BsGDH plasmid as an example, pET28a-ZpaCPR-(GSG)-BsGDH was also constructed (Figure S8). Plasmids pACYCDuet-1-ZpaCPR-(GSG)-BsGDH and pET28a-ZpaCPR-(GSG)-BsGDH were transformed into competent cells with pET28a-AmeLPLDH and pACYCDuet-1-AmeLPLDH, respectively, resulting in E. coli strains BL21 (DE3)/pET28a-AmeLPLDH/pACYCDuet-1-ZpaCPR-(GSG)-BsGDH and BL21 (DE3)/pET28a-ZpaCPR-(GSG)-BsGDH/pACYCDuet-1-AmeLPLDH. Following the procedure in Section 3.4, the cells were induced and harvested; then, the catalytic performance in the deracemization of dl-pantolactone was determined.
3.8. Optimization of Multi-Enzymatic Deracemization of dl-Pantolactone
E. coli strain BL21 (DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCPR-(GSG)-BsGDH was used as whole-cell catalyst. The initial reaction mixture (10 mL) contained 1 M dl-pantolactone, 200 g/L wet cells, 1.5 M glucose, and 200 mM PBS buffer (pH 6.0). The reaction was carried out at 30 °C and 600 rpm for 24 h. The pH was kept constant during the reaction by stream addition of 1 M NaOH solution.
The optimal conditions for the process were investigated using a single factor method. Temperature varied from 20 to 40 °C, and pH values ranged from 5.0 to 10.0. Agitation between 0 and 600 rpm and the concentration of glucose as co-substrate within the range of 0 to 2500 mm were tested. In addition, the effects of substrate concentration (500 to 1750 mM) on the deracemization of dl-pantolactone were also determined. After 24 h reaction, the samples were treated as described in Section 3.4, then subjected to GC analysis as described in Section 3.10.
3.9. Deracemization of 1.25 mM dl-Pantolactone through Supplementation with BsGDH
After optimization, the reaction mixture (10 mL) contained 1.25 M dl-pantolactone, 2.5 M glucose, 200 g/L wet E. coli BL21 (DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCPR-(GSG)-BsGDH cells, and 50 mM PBS buffer (pH 6.0). After 24 h reaction at 30 °C and 400 rpm, 100 g/L wet E. coli BL21 (DE3)/pET28a-BsGDH cells were added to the reaction mixture. Then, the reaction proceeded at 30 °C and 400 rpm for another 12 h. For the whole reaction, pH was kept constant using an auto-titration system. When the reaction was terminated at 36 h, the samples were treated as described in Section 3.4, then subjected to GC analysis as described in Section 3.10.
3.10. GC, GC-MS and NMR Analysis
The details of the method for analyzing l-pantolactone, d-pantolactone, and ketopantolactone by gas chromatography (Agilent 7890A, Agilent Technologies, Inc., Santa Clara, CA, USA) are as follows: detector: FID; chiral capillary column: BGB-174 (30 m × 250 µm × 0.25 µm; BGB Analytik, Böckten, Switzerland); carrier gas: N2; flow rate: 30.0 mL/min; split ratio: 30:1; injection volume: 1 µL; injector and detector temperature: 250 °C [8]. The column temperature was kept constant at 175 °C for 10 min. The retention times for d-pantolactone, l-pantolactone, and ketopantolactone were 6.19, 6.51, and 6.78 min, respectively (Figure S9).
Upon completion of the catalytic reaction, the reaction mixture was treated with ethyl acetate; then, the product in the resulting organic phase was collected through evaporation of the solvent. The resulting crude product was validated by GC-MS (Agilent 7890A/5975C, Agilent Technologies Inc., Santa Clara, CA, USA) using previously reported parameters (Figure S10) [8]. The crude product was dissolved in CDCl3 for NMR analysis (Avance NEO, Bruker, Switzerland) to further verify the remaining product, d-pantolactone. The NMR spectroscopy was operated at 600 MHz for 1H and 151 MHz for 13C detection (Figure S11).
4. Conclusions
In summary, a toolbox consisting of five LPLDHs, three CPRs, and three GDHs was developed for carrying out multi-enzymatic deracemization of dl-pantolactone. By screening catalytic activity, the enzymes AmeLPLDH, ZpaCPR, and BsGDH were selected, and the newly mined enzymes AmeLPLDH and ZpaCPR were purified and then characterized. The kcat/Km values of AmeLPLDH and ZpaCPR were 63.85 and 11.60 mM−1s−1, respectively. Through genetic fusion and vector selection, E. coli strain BL21(DE3)/pACYCDuet-1-AmeLPLDH/pET28a-ZpaCPR-(GSG)-BsGDH was obtained and used as whole-cell catalyst, providing a competitive catalytic process to synthesize d-pantolactone with high optical purity (>98% e.e.p). Under the optimized conditions, the process enabled efficient deracemization of 1 M dl-pantolactone at 24 h, and supplementation with BsGDH further yielded nearly complete deracemization of 1.25 M dl-pantolactone to d-pantolactone after 36 h.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules28145308/s1: Table S1: Primers used for co-expression of LPLDH, CPR and GDH; Table S2: Primers used for genetic fusion of BsGDH and ZpaCPR genes; Figure S1: SDS-PAGE analysis of l-pantolactone dehydrogenases (LPLDHs); Figure S2: SDS-PAGE analysis of co-expression of LPLDH, SceCPR1 and EsGDH; Figure S3: SDS-PAGE analysis of co-expression of AmeLPLDH, different CPRs and EsGDH; Figure S4: SDS-PAGE analysis of co-expression of AmeLPLDH, ZpaCPR and different GDHs; Figure S5: SDS-PAGE (12%) analysis of (a) ZpaAR and (b) AmeLPLDH; Figure S6: SDS-PAGE analysis of E. coli cells co-expressing AmeLPLDH and fusion enzyme ZpaCPR-(GSG)-BsGDH; Figure S7: Effects of (a) temperature, (b) pH, (c) agitation and (d) glucose concentration on catalytic performance; Figure S8: Schematic diagram of ZpaCPR-(GSG)-BsGDH fusion enzyme construction; Figure S9: GC chromatogram of standards of substrate and product; Figure S10: GC-MS chromgatogram of d-pantolactone; Figure S11: (a) 1H NMR and (b) 13C NMR analysis of product.
Author Contributions
Conceptualization, J.S. and X.Y.; methodology, software, validation, formal analysis, investigation, resources, data curation, L.J., X.L., T.W., Y.W., X.Z., W.M., Y.Z., Z.W., J.S. and X.Y.; writing—original draft preparation, L.J. and X.Y.; writing—review and editing, L.J., X.L., T.W., Y.W., X.Z., W.M., Y.Z., Z.W., J.S. and X.Y.; visualization, X.Y.; supervision, J.S. and X.Y.; project administration, J.S. and X.Y.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Zhejiang Province, China (No. LY18B020021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors upon request.
References
- Pscheidt, B.; Avi, M.; Gaisberger, R.; Hartner, F.S.; Skranc, W.; Glieder, A. Screening hydroxynitrile lyases for (R)-pantolactone synthesis. J. Mol. Catal. B Enzym. 2008, 52–53, 183–188. [Google Scholar] [CrossRef]
- Pscheidt, B.; Liu, Z.; Gaisberger, R.; Avi, M.; Skranc, W.; Gruber, K.; Griengl, H.; Glieder, A. Efficient Biocatalytic Synthesis of (R)-Pantolactone. Adv. Synth. Catal. 2008, 350, 1943–1948. [Google Scholar] [CrossRef]
- Heidlindemann, M.; Hammel, M.; Scheffler, U.; Mahrwald, R.; Hummel, W.; Berkessel, A.; Gröger, H. Chemoenzymatic Synthesis of Vitamin B5-Intermediate (R)-Pantolactone via Combined Asymmetric Organo- and Biocatalysis. J. Org. Chem. 2015, 80, 3387–3396. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Fang, Y.; Luo, W.F.; Huang, L.N. Biocatalytic kinetic resolution of d,l-pantolactone by using a novel recombinant d-lactonase. RSC Adv. 2020, 11, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Yang, L.; Tang, Y.B.; Huang, L.N.; Luo, W.F. Industrial kinetic resolution of d,l-pantolactone by an immobilized whole-cell biocatalyst. RSC Adv. 2021, 11, 30373–30376. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zheng, P.; Wu, D.; Chen, P.; Bai, Y.; Wang, J. Biocatalysis of heterogenously-expressed d-lactonohydrolases and its efficient preparation of desirable d-pantoic acid. Enzyme Microb. Technol. 2022, 155, 109981. [Google Scholar] [CrossRef]
- Zhu, F.-Y.; Zhong, J.; Shen, Q.; Jia, D.-X.; Ma, S.-J.; Du, J.; Wu, H.; Yang, Q.; Cao, M.; Liu, Z.-Q.; et al. Development of an Escherichia coli whole cell catalyst harboring conjugated polyketone reductase from Candida glabrata for synthesis of d-(−)-pantolactone. Process Biochem. 2022, 112, 223–233. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, L.; Zhang, L.; Bai, Y.; Chen, L.; Yu, M.; Cheng, F.; Sun, J.; Wang, Z.; Ying, X. Asymmetric reduction of ketopantolactone using a strictly (R)-stereoselective carbonyl reductase through efficient NADPH regeneration and the substrate constant-feeding strategy. Biotechnol. Lett. 2017, 39, 1741–1746. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Wu, Y.; Jiang, X.; Pei, X.; Su, W. Recombinant expression and molecular insights into the catalytic mechanism of an NADPH-dependent conjugated polyketone reductase for the asymmetric synthesis of (R)-pantolactone. Enzyme Microb. Technol. 2019, 126, 77–85. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, P.; Wu, Y.; Wang, A.; Liu, F.; Pei, X. Discovery of a new NADPH-dependent aldo-keto reductase from Candida orthopsilosis catalyzing the stereospecific synthesis of (R)-pantolactone by genome mining. J. Biotechnol. 2019, 291, 26–34. [Google Scholar] [CrossRef]
- Cheng, P.; Tang, M.; Chen, Z.; Liu, W.; Jiang, X.; Pei, X.; Su, W. Dual-enzyme and NADPH co-embedded organic-inorganic hybrid nanoflowers prepared using biomimetic mineralization for the asymmetric synthesis of (R)-(-)-pantolactone. React. Chem. Eng. 2020, 5, 1973–1980. [Google Scholar] [CrossRef]
- Pei, X.; Wang, J.; Zheng, H.; Cheng, P.; Wu, Y.; Wang, A.; Su, W. Highly efficient asymmetric reduction of ketopantolactone to d-(−)-pantolactone by Escherichia coli cells expressing recombinant conjugated polyketone reductase and glucose dehydrogenase in a fed-batch biphasic reaction system. React. Chem. Eng. 2020, 5, 531–538. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Yang, Q.; Cao, M.; Zheng, K.; Zhang, X.J.; Shen, Q.; Cai, X.; Liu, Z.Q.; Zheng, Y.G. Tuning an efficient Escherichia coli whole-cell catalyst expressing l-pantolactone dehydrogenase for the biosynthesis of d-(-)-pantolactone. J. Biotechnol. 2023, 367, 1–10. [Google Scholar] [CrossRef]
- Voss, C.V.; Gruber, C.C.; Faber, K.; Knaus, T.; Macheroux, P.; Kroutil, W. Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemisation of sec-alcohols. J. Am. Chem. Soc. 2008, 130, 13969–13972. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Nie, Y.; Mu, X.Q.; Xu, Y. De novo construction of multi-enzyme system for one-pot deracemization of (R,S)-1-phenyl-1,2-ethanediol by stereoinversion of (S)-enantiomer to the corresponding counterpart. J. Mol. Catal. B-Enzym. 2016, 129, 21–28. [Google Scholar] [CrossRef]
- Xue, Y.P.; Zeng, H.; Jin, X.L.; Liu, Z.Q.; Zheng, Y.G. Enantioselective cascade biocatalysis for deracemization of 2-hydroxy acids using a three-enzyme system. Microb. Cell. Fact. 2016, 15, 162. [Google Scholar] [CrossRef]
- Cao, C.H.; Gong, H.; Dong, Y.; Li, J.M.; Cheng, F.; Xue, Y.P.; Zheng, Y.G. Enzyme cascade for biocatalytic deracemization of d,l-phosphinothricin. J. Biotechnol. 2021, 325, 372–379. [Google Scholar] [CrossRef]
- Si, D.; Urano, N.; Nozaki, S.; Honda, K.; Shimizu, S.; Kataoka, M. L-pantoyl lactone dehydrogenase from Rhodococcus erythropolis: Genetic analyses and application to the stereospecific oxidation of L-pantoyl lactone. Appl. Microbiol. Biotechnol. 2012, 95, 431–440. [Google Scholar] [CrossRef]
- Zheng, G.-W.; Liu, Y.-Y.; Chen, Q.; Huang, L.; Yu, H.-L.; Lou, W.-Y.; Li, C.-X.; Bai, Y.-P.; Li, A.-T.; Xu, J.-H. Preparation of Structurally Diverse Chiral Alcohols by Engineering Ketoreductase CgKR1. ACS Catal. 2017, 7, 7174–7181. [Google Scholar] [CrossRef]
- Liang, C.; Nie, Y.; Mu, X.; Xu, Y. Gene mining-based identification of aldo–keto reductases for highly stereoselective reduction of bulky ketones. Bioresour. Bioprocess. 2018, 5, 33. [Google Scholar] [CrossRef]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-Enzymatic Cascade Reactions: Overview and Perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Weber, D.; de Souza Bastos, L.; Winkler, M.; Ni, Y.; Aliev, A.E.; Hailes, H.C.; Rother, D. Multi-enzyme catalysed processes using purified and whole-cell biocatalysts towards a 1,3,4-substituted tetrahydroisoquinoline. RSC Adv. 2023, 13, 10097–10109. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, Q.; Qiao, J.; Feng, B.; Zhou, X.; Jin, L.; Feng, Y.; Yang, D.; Lu, C.; Ying, X. Cascading Old Yellow Enzyme, Alcohol Dehydrogenase and Glucose Dehydrogenase for Selective Reduction of (E/Z)-Citral to (S)-Citronellol. Catalysts. 2021, 11, 931. [Google Scholar] [CrossRef]
- Aalbers, F.S.; Fraaije, M.W. Enzyme Fusions in Biocatalysis: Coupling Reactions by Pairing Enzymes. Chembiochem. 2019, 20, 20–28. [Google Scholar] [CrossRef]
- Ying, X.; Wang, C.; Shao, S.; Wang, Q.; Zhou, X.; Bai, Y.; Chen, L.; Lu, C.; Zhao, M.; Wang, Z. Efficient Oxidation of Methyl Glycolate to Methyl Glyoxylate Using a Fusion Enzyme of Glycolate Oxidase, Catalase and Hemoglobin. Catalysts 2020, 10, 943. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, C.; Zeng, Y.; Wang, T.; Qiao, J.; Lu, C.; Wang, Z.; Ying, X. Efficient whole-cell oxidation of α,β-unsaturated alcohols to α,β-unsaturated aldehydes through the cascade biocatalysis of alcohol dehydrogenase, NADPH oxidase and hemoglobin. Microb. Cell. Fact. 2021, 20, 17. [Google Scholar] [CrossRef]
- Siedentop, R.; Claassen, C.; Rother, D.; Luetz, S.; Rosenthal, K. Getting the Most Out of Enzyme Cascades: Strategies to Optimize In Vitro Multi-Enzymatic Reactions. Catalysts 2021, 11, 1183. [Google Scholar] [CrossRef]
- Siedentop, R.; Siska, M.; Moeller, N.; Lanzrath, H.; von Lieres, E.; Luetz, S.; Rosenthal, K. Bayesian Optimization for an ATP-Regenerating In Vitro Enzyme Cascade. Catalysts 2023, 13, 468. [Google Scholar] [CrossRef]
- Feng, B.; Li, X.; Jin, L.; Wang, Y.; Tang, Y.; Hua, Y.; Lu, C.; Sun, J.; Zhang, Y.; Ying, X. Engineering the Activity of Old Yellow Enzyme NemR-PS for Efficient Reduction of (E/Z)-Citral to (S)-Citronellol. Catalysts 2022, 12, 631. [Google Scholar] [CrossRef]
- Kataoka, M.; Shimizu, S.; Yamada, H. Purification and characterization of a novel FMN-dependent enzyme Membrane-bound l-(+)-pantoyl lactone dehydrogenase from Nocardia asteroides. Eur. J. Biochem. 1992, 204, 799–806. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Ye, J.-J.; Shen, Z.-Y.; Hong, H.-B.; Yan, J.-B.; Lin, Y.; Chen, Z.-X.; Zheng, Y.-G.; Shen, Y.-C. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl. Microbiol. Biotechnol. 2015, 99, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.Z.; Ou, L.; Li, C.X.; Pan, J.; Xu, J.H.; Chen, Q.; Zheng, G.W. Evolution of Glucose Dehydrogenase for Cofactor Regeneration in Bioredox Processes with Denaturing Agents. Chembiochem 2020, 21, 2680–2688. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.L.; Vinuela, E.; Maizel, J.V., Jr. Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 1967, 28, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).