Determination of Lincomycin in Milk Using Cu-Based Metal-Organic Framework Adsorbent and Liquid Chromatography-Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
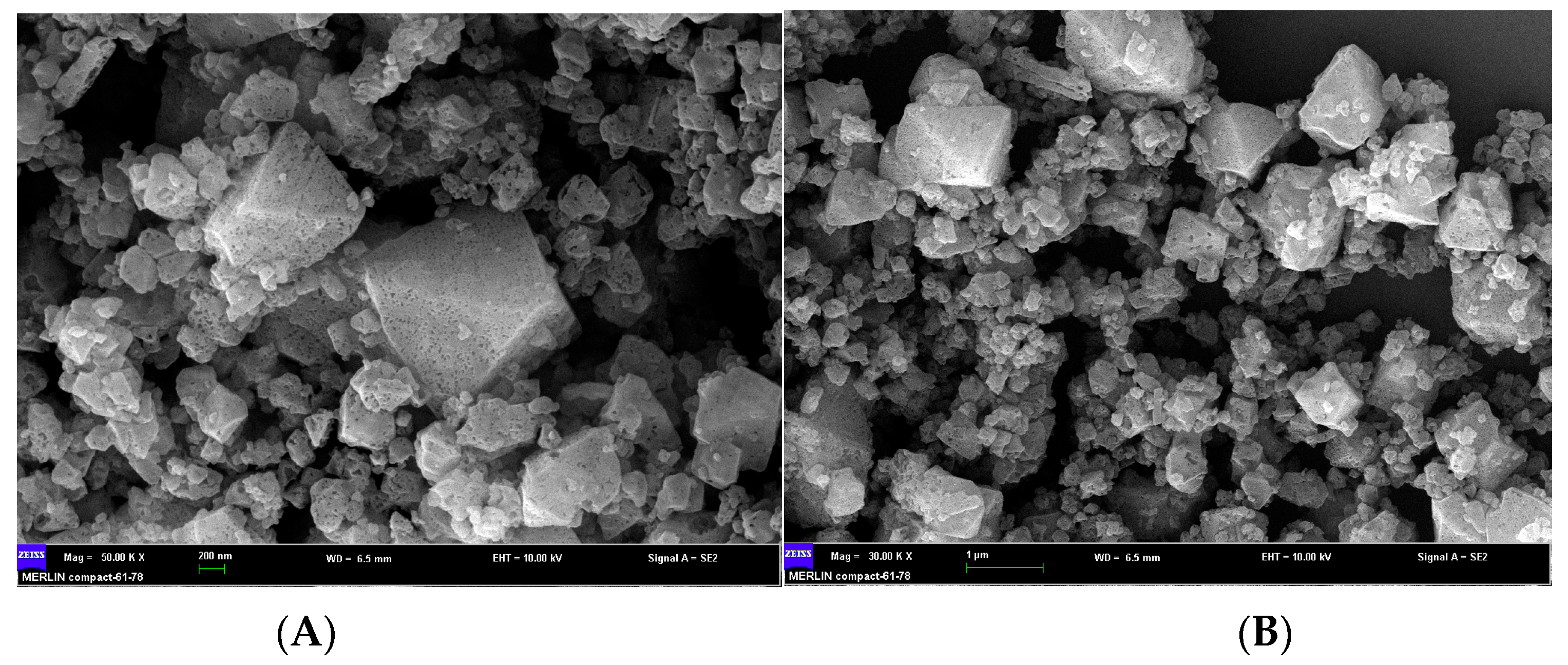
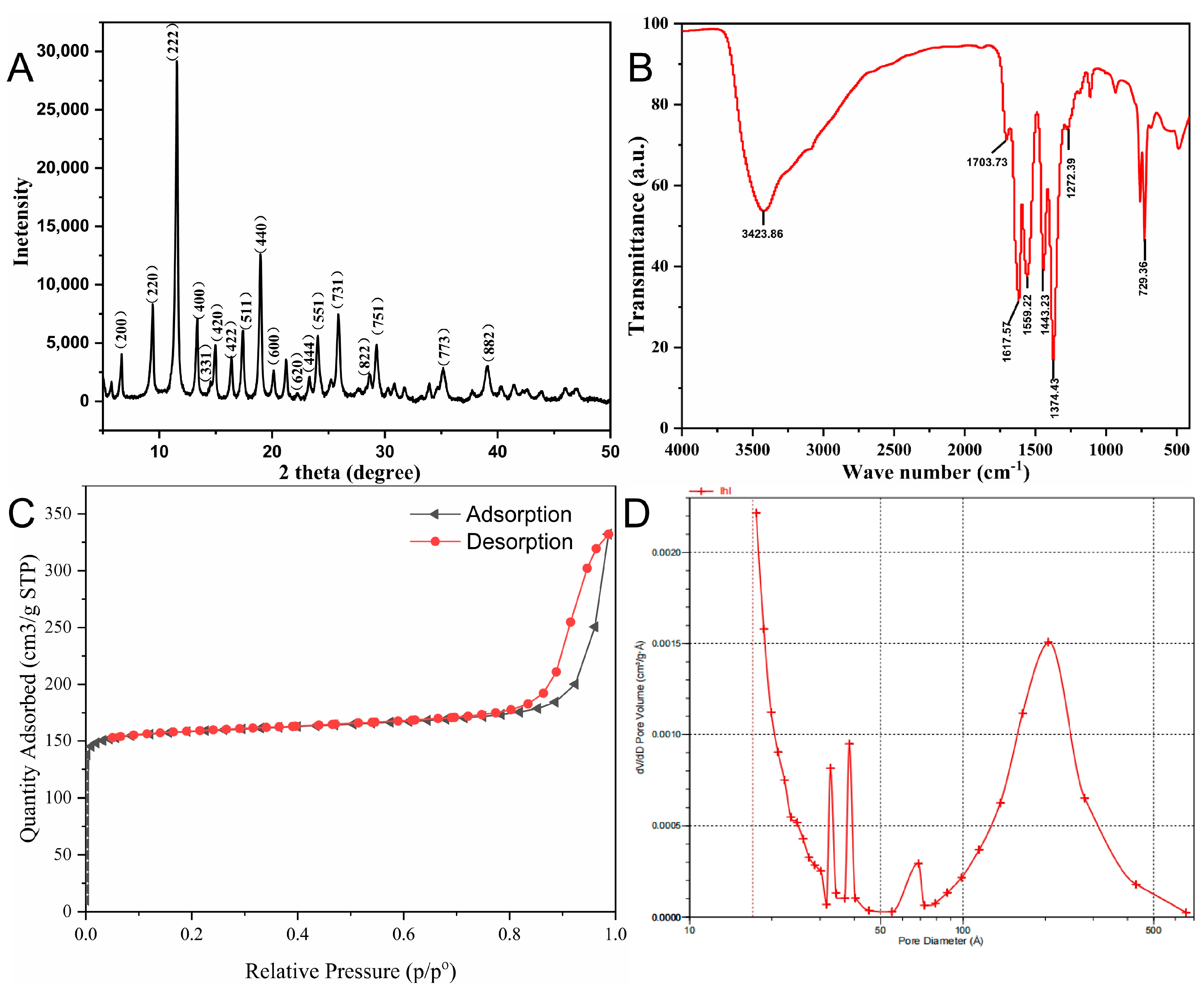
2.1. Characterization of Cu-MOFs
2.2. Adsorption Experiment of Cu-MOFs
2.2.1. Saturated Adsorption Capacity
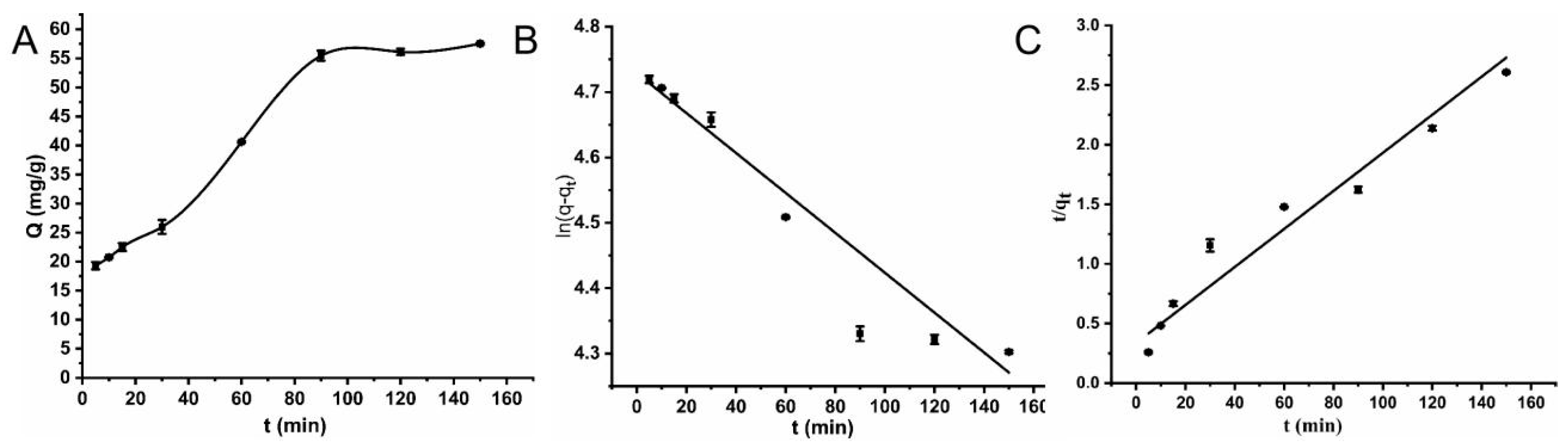
2.2.2. Adsorption Kinetics
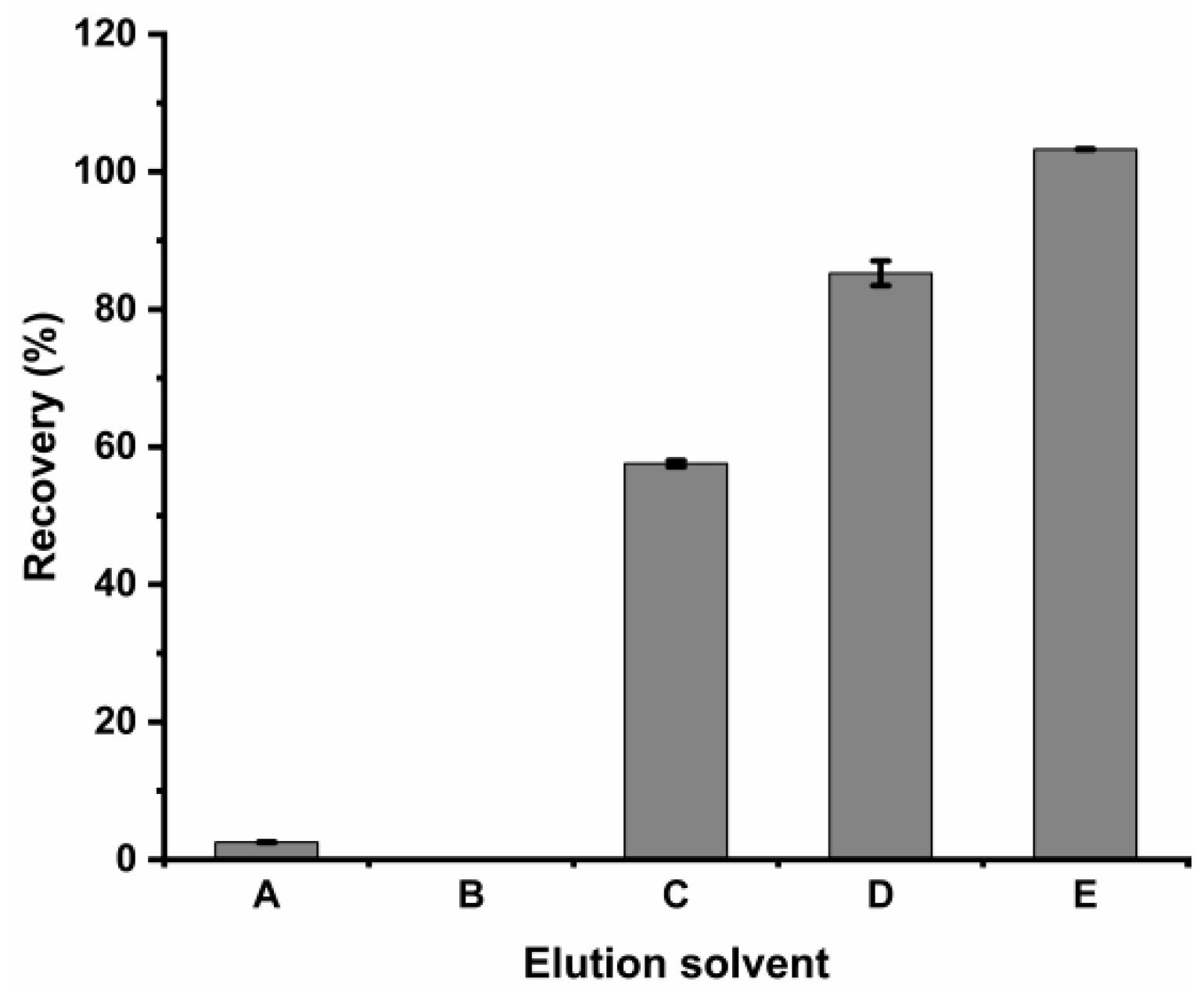
2.2.3. Optimization of the Eluent Solution
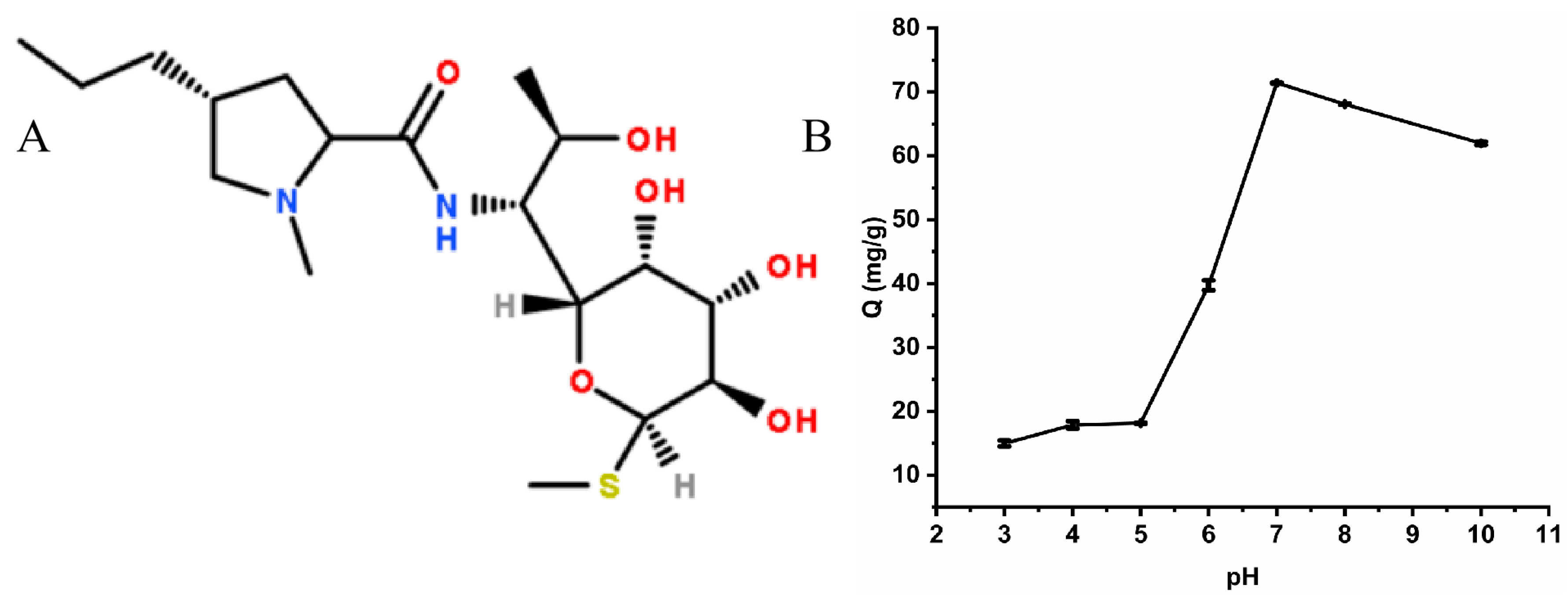
2.2.4. Effect of pH on the Adsorption Process
2.3. Method Validation
2.3.1. Linear Range and Detection Limit
2.3.2. Detection of Real Samples
2.3.3. Comparison of the Developed Method with Reported Methods
3. Experimental
3.1. Chemicals and Instruments
3.2. Instrument Conditions
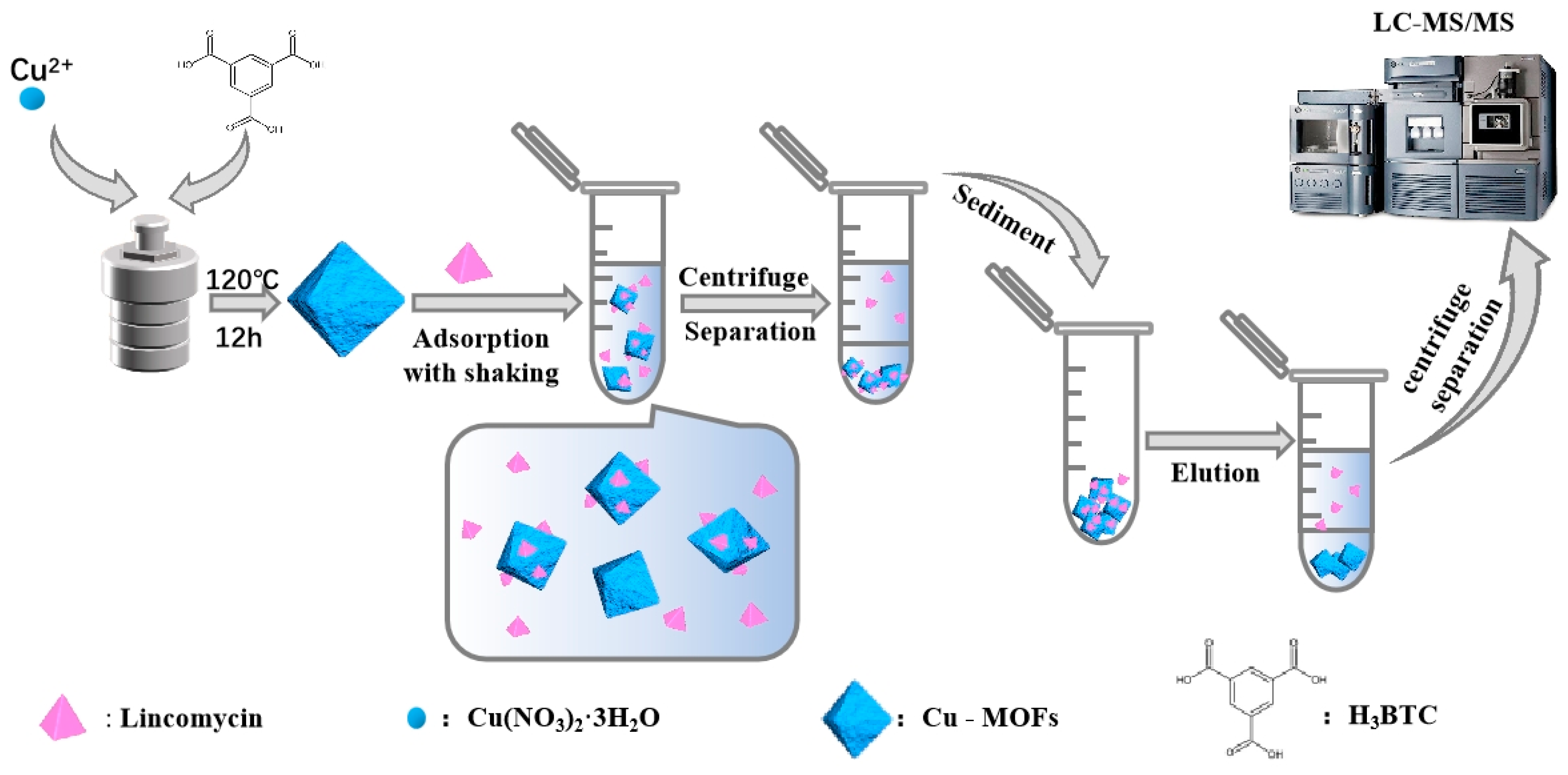
3.3. Synthesis of Cu-MOFs
3.4. Adsorption Equilibrium and Adsorption Kinetics Experiments
3.5. Optimization of the Eluent
3.6. Effect of pH on the Adsorption Process
3.7. Pretreatment of the Milk Samples and SPE-HPLC-MS/MS Method for Determination of Lincomycin in Cow Milk Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, G.; Gong, W.; Zhu, J.; Liu, L.; Zhu, L.; Liu, Z. Establishment and validation of the LC-MS/MS method for the determination of lincomycin in human blood: Application to an allergy case in forensic science. J. Forensic. Leg. Med. 2021, 77, 102094. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A. Development of a competitive indirect ELISA for the determination of lincomycin in milk, eggs, and honey. J. Agric. Food Chem. 2010, 58, 9893–9898. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, X.; Diao, Z.; Wang, Y.; Wang, B.; Xie, K.; Wang, X.; Zhang, P. Detection and determination of spectinomycin and lincomycin in poultry muscles and pork by ASE-SPE-GC-MS/MS. J. Food Compos. Anal. 2021, 101, 103979. [Google Scholar] [CrossRef]
- Mehrtens, A.; Licha, T.; Burke, V. Occurrence, effects and behaviour of the antibiotic lincomycin in the agricultural and aquatic environment—A review. Sci. Total Environ. 2021, 778, 146306. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.V.; Rao, A.; Kumar, P.; Ramakumar, K.L.; Malkhede, D.D. Studies on Complexation and Supercritical Fluid Extraction of Cd2+ with Calixarenes. Ind. Eng. Chem. Res. 2015, 54, 3933–3941. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Han, Y.; Luo, X.; Tang, W.; Yue, T.; Li, Z. Robust MOF film of self-rearranged UiO-66-NO2 anchored on gelatin hydrogel via simple thermal-treatment for efficient Pb(II) removal in water and apple juice. Food Control 2021, 130, 108409. [Google Scholar] [CrossRef]
- Mnyandu, H.M.; Mahlambi, P.N. Optimization and application of QuEChERS and SPE methods followed by LC-PDA for the determination of triazines residues in fruits and vegetables from Pietermaritzburg local supermarkets. Food Chem. 2021, 360, 129818. [Google Scholar] [CrossRef]
- Sammani, M.S.; Clavijo, S.; Cerdà, V. Recent, advanced sample pretreatments and analytical methods for flavonoids determination in different samples. TrAC Trends Anal. Chem. 2021, 138, 116220. [Google Scholar] [CrossRef]
- Liang, S.; Dai, H.; Wang, C.; Zhang, H.; Li, J.; Xu, Q.; Zhang, Q. Application of polydopamine fibers mat for simultaneous detection of multi-class drug residues in various animal-original foods. Food Control 2022, 132, 108532. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Hou, C.; Zhang, T.; Chen, M.; Meng, H.; Su, M.; Xu, H.; Hua, Z.; Wang, Y.; et al. Automatic analytical approach for the determination of 12 illicit drugs and nicotine metabolites in wastewater using on-line SPE-UHPLC-MS/MS. J. Pharm. Anal. 2021, 11, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Wang, L.; Feng, Y.; Liu, M.; Li, Q.; Wang, G.; Zhang, L.; Kang, W.; Cheng, B.; Liu, Y. Co-based and Cu-based MOFs modified separators to strengthen the kinetics of redox reaction and inhibit lithium-dendrite for long-life lithium-sulfur batteries. Chem. Eng. J. 2020, 388, 124241. [Google Scholar] [CrossRef]
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Microporous Mesoporous Mater. 2018, 270, 258–264. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Yan, M.; Wang, Q.; Xu, H.; Wang, X.; Zhou, H.; Hao, Y.; Wang, M. Fabrication of iron oxide@MOF-808 as a sorbent for magnetic solid phase extraction of benzoylurea insecticides in tea beverages and juice samples. J. Chromatogr. A 2020, 1615, 460766. [Google Scholar] [CrossRef] [PubMed]
- Uflyand, I.E.; Zhinzhilo, V.A.; Nikolaevskaya, V.O.; Kharisov, B.I.; González, C.M.; Kharissova, O.V. Recent strategies to improve MOF performance in solid phase extrac-tion of organic dyes. Microchem. J. 2021, 168, 106387. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, L.; Zhang, Y.; Zhang, Q.; Hong, Y.; Shen, W.; Wang, Y.; Zhu, J. Insight into the liquid adsorption of tobacco specific nitrosamines on ZIF-8. Microporous Mesoporous Mater. 2022, 333, 111730. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, C.; Zhang, Z.; Xiang, L.; Yue, S.; Shen, Z.; Li, J. Structure engineering of Zn-ZIF adsorbents for efficient and highly-selective phosphate removal from wastewater: Roles of surface mesopore and defect. Appl. Surf. Sci. 2022, 586, 152814. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.; Liu, L.; Meng, Q.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 382–390. [Google Scholar] [CrossRef]
- Stypulkowska, K.; Blazewicz, A.; Brudzikowska, A.; Grzeskiewicz, M.W.; Sarna, K.; Fijalek, Z. Development of high performance liquid chromatography methods with charged aerosol detection for the determination of lincomycin, spectinomycin and its impurities in pharmaceutical products. J. Pharm. Biomed. Anal. 2015, 112, 8–14. [Google Scholar] [CrossRef]
- Ren, X.; He, T.; Wang, J.; Wang, L.; Wang, Y.; Liu, X.; Dong, Y.; Ma, J.; Jia, J.; Song, R.; et al. UV spectroscopy and HPLC combined with chemometrics for rapid discrimination and quantification of Curcumae Rhizoma from three botanical origins. J. Pharm. Biomed. Anal. 2021, 202, 114145. [Google Scholar] [CrossRef]
- Wong, J.W.; Zhang, K.; Tech, K.; Hayward, D.G.; Makovi, C.M.; Krynitsky, A.J.; Schenck, F.J.; Banerjee, K.; Dasgupta, S.; Brown, D. Multiresidue pesticide analysis in fresh produce by capillary gas chromatography-mass spectrometry/selective ion monitoring (GC-MS/SIM) and -tandem mass spectrometry (GC-MS/MS). J. Agric. Food Chem. 2010, 58, 5868–5883. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Zhang, K.; Tech, K.; Hayward, D.G.; Krynitsky, A.J.; Cassias, I.; Schenck, F.J.; Banerjee, K.; Dasgupta, S.; Brown, D. Multiresidue pesticide analysis of ginseng powders using acetonitrile- or acetone-based extraction, solid-phase extraction cleanup, and gas chromatography-mass spectrometry/selective ion monitoring (GC-MS/SIM) or -tandem mass spectrometry (GC-MS/MS). J. Agric. Food Chem. 2010, 58, 5884–5896. [Google Scholar] [CrossRef] [PubMed]
- McCullum, C.; Tchounwou, P.; Ding, L.S.; Liao, X.; Liu, Y.M. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for HPLC-MS/MS analysis. J. Agric. Food Chem. 2014, 62, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Li, Q.; Maimaiti, T.; Lan, S.; Ouyang, P.; Ouyang, B.; Wu, X.; Yang, S.T. Toxicity and photosynthetic inhibition of metal-organic framework MOF-199 to pea seedlings. J. Hazard. Mater. 2021, 409, 124521. [Google Scholar] [CrossRef] [PubMed]
- Homayoonnia, S.; Zeinali, S. Design and fabrication of capacitive nanosensor based on MOF nanoparticles as sensing layer for VOCs detection. Sens. Actuators B Chem. 2016, 237, 776–786. [Google Scholar] [CrossRef]
- Niu, H.; Liu, P.; Qin, F.; Liu, X.; Akinay, Y. PEDOT coated Cu-BTC metal-organic frameworks decorated with Fe3O4 nanoparticles and their enhanced electromagnetic wave absorption. Mater. Chem. Phys. 2020, 253, 123458. [Google Scholar] [CrossRef]
- Liu, X.M.; Rather, S.-u.; Li, Q.; Lueking, A.; Zhao, Y.; Li, J. Hydrogenation of CuBTC Framework with the Introduction of a PtC Hydrogen Spillover Catalyst. J. Phys. Chem. C 2012, 116, 3477–3485. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic synthesis of the microporous metal–organic framework Cu3(BTC)2 at ambient temperature and pressure: An efficient and environmentally friendly method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Chen, Y.C.; Andrew Lin, K.Y.; Chen, K.F.; Jiang, X.Y.; Lin, C.H. In vitro renal toxicity evaluation of copper-based metal-organic framework HKUST-1 on human embryonic kidney cells. Environ. Pollut. 2021, 273, 116528. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST-1 by “coordination modulation method”. Microporous Mesoporous Mater. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Chung, H.-K.; Kim, W.-H.; Park, J.; Cho, J.; Jeong, T.-Y.; Park, P.-K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Liu, C.H.; Chuang, Y.H.; Li, H.; Boyd, S.A.; Teppen, B.J.; Gonzalez, J.M.; Johnston, C.T.; Lehmann, J.; Zhang, W. Long-term sorption of lincomycin to biochars: The intertwined roles of pore diffusion and dissolved organic carbon. Water Res. 2019, 161, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; El-Ghanam, A.M.; Mohamed, R.H.A.; Saad, S.R. Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110199. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Li, F.F.; Cui, W.R.; Jiang, W.; Zhang, C.R.; Liang, R.P.; Qiu, J.D. Stable sp(2) carbon-conjugated covalent organic framework for detection and efficient adsorption of uranium from radioactive wastewater. J. Hazard. Mater. 2020, 392, 122333. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Huang, L.; Lin, Z.; Cai, Z. Core-Shell Structured Magnetic Covalent Organic Framework Nanocomposites for Triclosan and Triclocarban Adsorption. ACS Appl. Mater. Interfaces 2019, 11, 22492–22500. [Google Scholar] [CrossRef] [PubMed]
- Alamgir; Talha, K.; Wang, B.; Liu, J.-H.; Ullah, R.; Feng, F.; Yu, J.; Chen, S.; Li, J.-R. Effective adsorption of metronidazole antibiotic from water with a stable Zr(IV)-MOFs: Insights from DFT, kinetics and thermodynamics studies. J. Environ. Chem. Eng. 2020, 8, 103642. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of aqueous pharmaceuticals by magnetically functionalized Zr-MOFs: Adsorption Kinetics, Isotherms, and regeneration. J. Colloid Interface Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef]
- Gbylik-Sikorska, M.; Lebkowska-Wieruszewska, B.; Gajda, A.; Nowacka-Kozak, E.; Lisowski, A.; Posyniak, A. Transfer of enrofloxacin, ciprofloxacin, and lincomycin into eggshells and residue depletion in egg components after multiple oral administration to laying hens. Poult. Sci. 2021, 100, 101341. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, K.; Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Ding, S. Development of a microsphere-based fluorescence immunochromatographic assay for monitoring lincomycin in milk, honey, beef, and swine urine. J. Agric. Food Chem. 2014, 62, 12061–12066. [Google Scholar] [CrossRef] [PubMed]

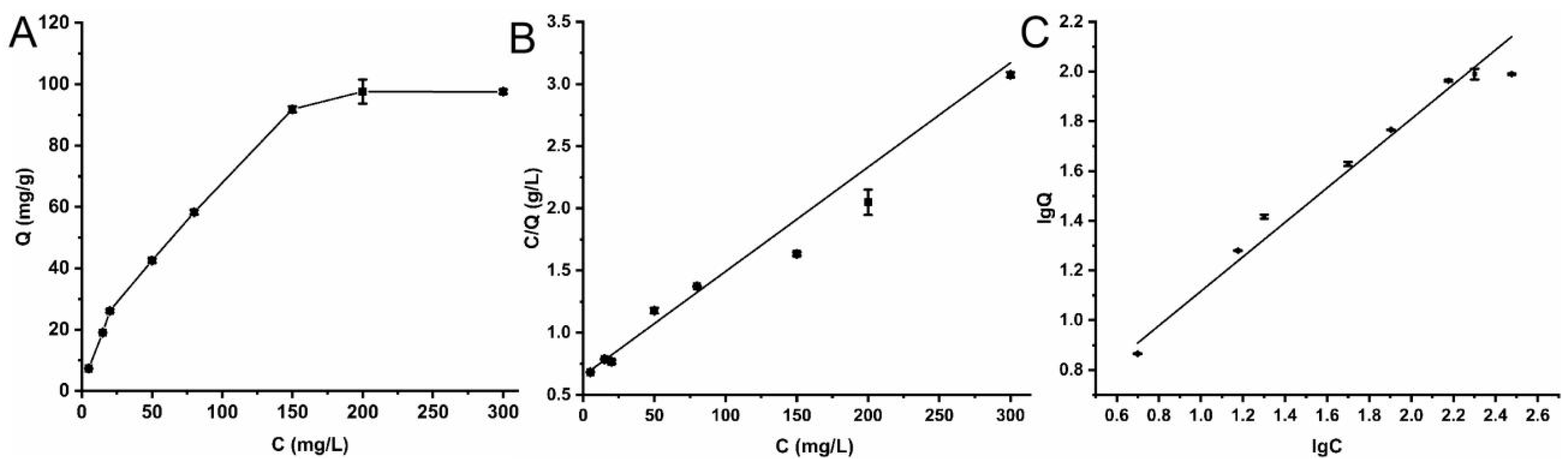
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| b (L·mg−1) | Q (mg·g−1) | R2 | Kf/(mg·g−1·(L·mg−1)1/n) | n | R2 | |
| Lincomycin | 0.012 | 131.41 | 0.98 | 4.256 | 1.55 | 0.97 |
| The Pseudo First Order | The Pseudo Second Order | |||||
|---|---|---|---|---|---|---|
| k1/min−1 | q/(mg·g−1) | R2 | k2/(g·mg−1·min−1) | q/(mg·g−1) | R2 | |
| Lincomycin | 0.00329 | 112.974 | 0.96 | 0.00051 | 68.2128 | 0.99 |
| Simple | Spiked (μg/L) | Recovery/% | RSD/% |
|---|---|---|---|
| Milk A | 100 | 92.3 | 0.83 |
| 500 | 96.9 | 0.25 | |
| 1000 | 97.0 | 1.81 | |
| Milk B | 100 | 92.3 | 1.16 |
| 500 | 96.7 | 1.29 | |
| 1000 | 96.9 | 0.89 | |
| Milk C | 100 | 93.1 | 1.49 |
| 500 | 97.1 | 1.96 | |
| 1000 | 97.2 | 0.97 |
| Method | Sample | Adsorbent | LOD | Recovery (%) | Ref. |
|---|---|---|---|---|---|
| LC-MS/MS | eggs | NO | 0.5 μg/kg | 86.00–111.00 | [40] |
| ASE-SPE-GC–MS/MS | poultry muscles and pork | diatomaceous earth | 4.6 μg/kg | 79.70–94.20 | [4] |
| PPE-LC-MS/MS | human blood | NO | 0.2 ng/mL | 72.70–84.13 | [2] |
| FMIA | Milk, Honey, Beef, and Swine Urine | NO | 0.69 ng/mL | 73.92–120.50 | [41] |
| SPE-HPLC-MS/MS | Milk | Cu-MOFs | 0.13 ng/mL | 92.30–97.20 | This work |
| Time/min | Mobile Phase A/% | Mobile Phase B/% | Flow Rate/(mL·min−1) |
|---|---|---|---|
| Initial | 65 | 35 | 0.300 |
| 1.00 | 65 | 35 | 0.300 |
| 1.20 | 100 | 0 | 0.300 |
| 1.50 | 0 | 100 | 0.300 |
| 2.00 | 65 | 35 | 0.300 |
| 4.00 | 65 | 35 | 0.300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wu, J.; Bai, J.; Wu, J.; Wu, J. Determination of Lincomycin in Milk Using Cu-Based Metal-Organic Framework Adsorbent and Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2023, 28, 5307. https://doi.org/10.3390/molecules28145307
Li H, Wu J, Bai J, Wu J, Wu J. Determination of Lincomycin in Milk Using Cu-Based Metal-Organic Framework Adsorbent and Liquid Chromatography-Tandem Mass Spectrometry. Molecules. 2023; 28(14):5307. https://doi.org/10.3390/molecules28145307
Chicago/Turabian StyleLi, Hanle, Jinhai Wu, Jialei Bai, Jianhu Wu, and Jin Wu. 2023. "Determination of Lincomycin in Milk Using Cu-Based Metal-Organic Framework Adsorbent and Liquid Chromatography-Tandem Mass Spectrometry" Molecules 28, no. 14: 5307. https://doi.org/10.3390/molecules28145307
APA StyleLi, H., Wu, J., Bai, J., Wu, J., & Wu, J. (2023). Determination of Lincomycin in Milk Using Cu-Based Metal-Organic Framework Adsorbent and Liquid Chromatography-Tandem Mass Spectrometry. Molecules, 28(14), 5307. https://doi.org/10.3390/molecules28145307





