Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives
Abstract
1. Introduction
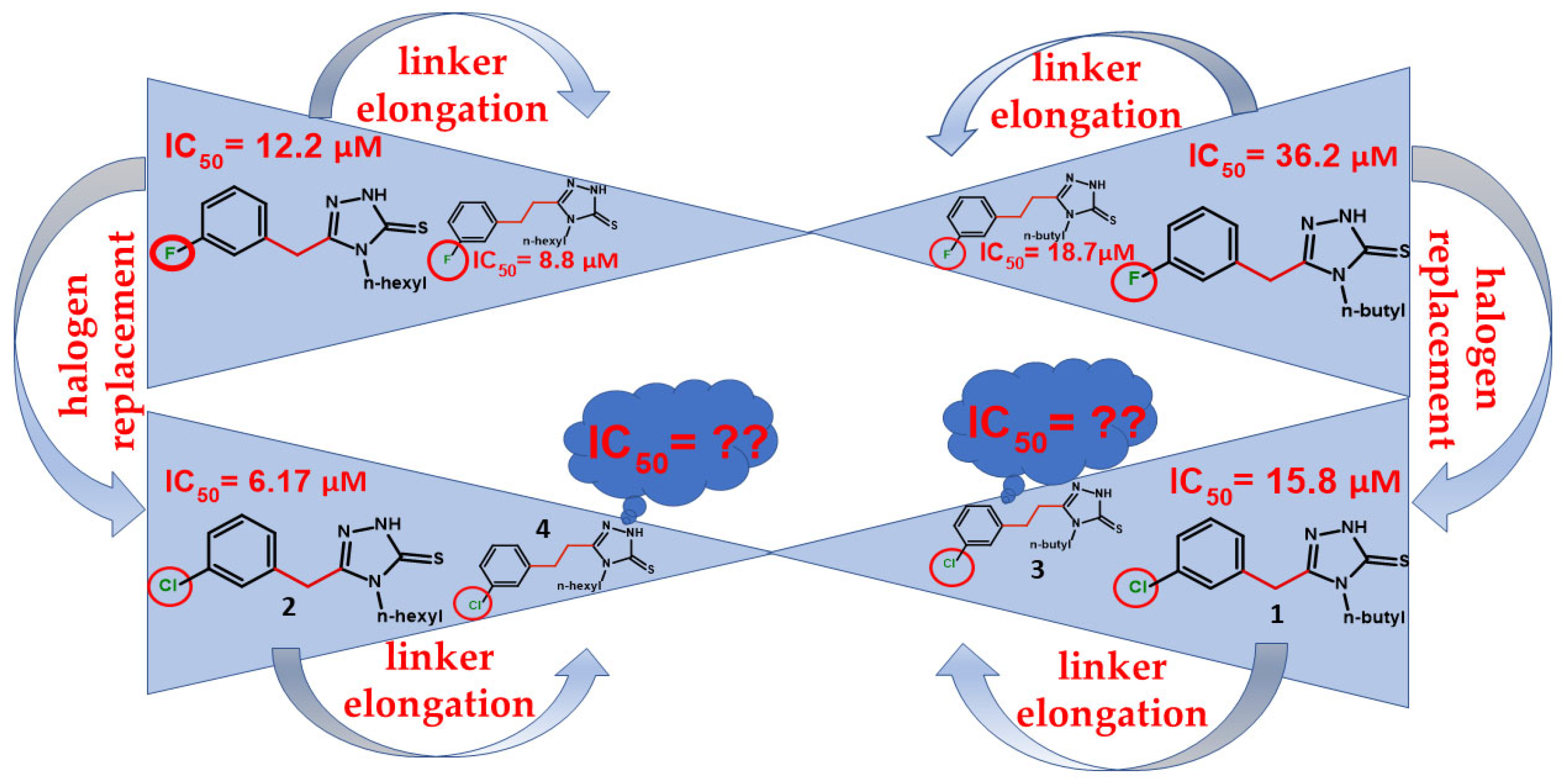
2. Results and Discussion
2.1. Molecular Docking

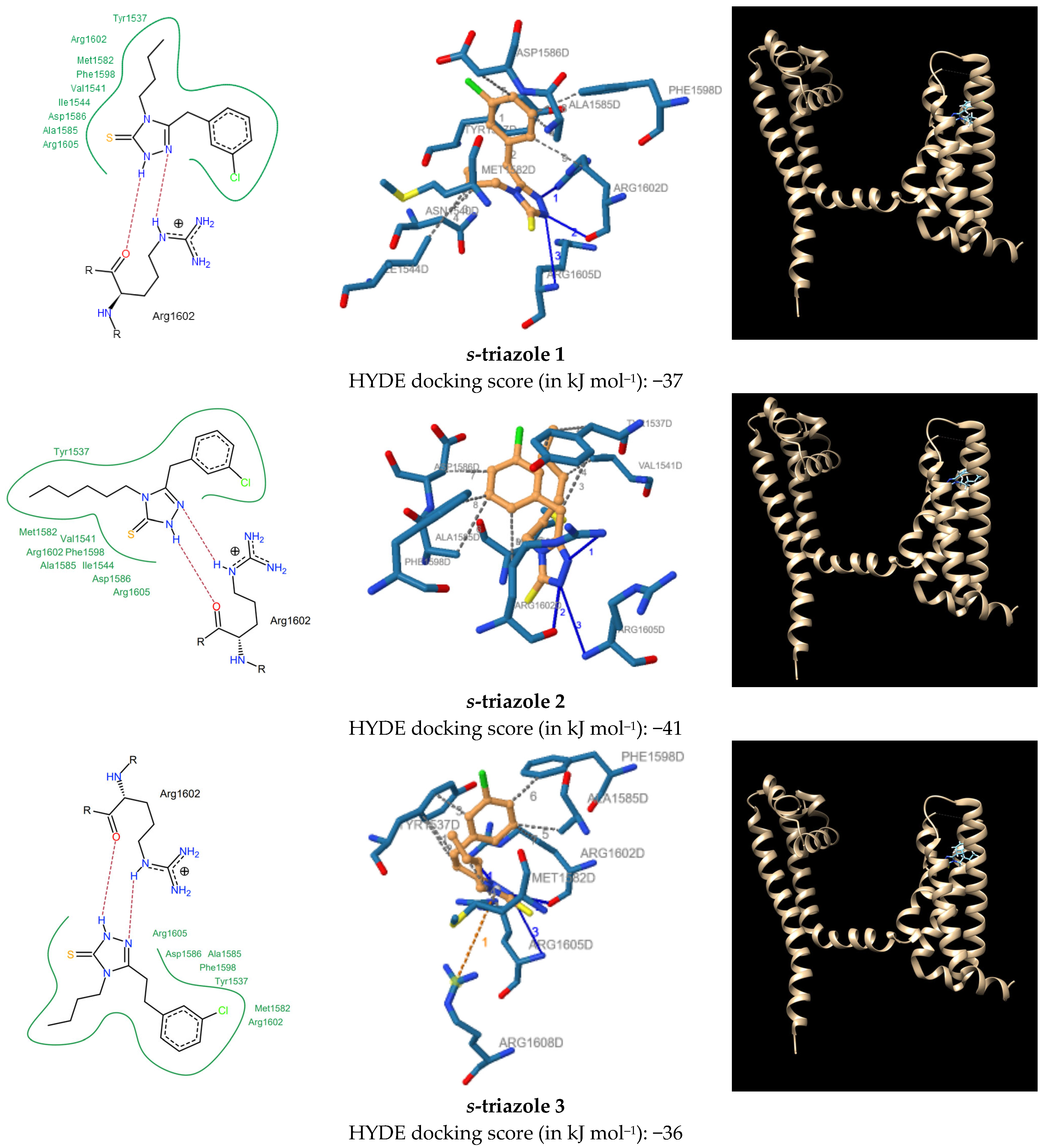
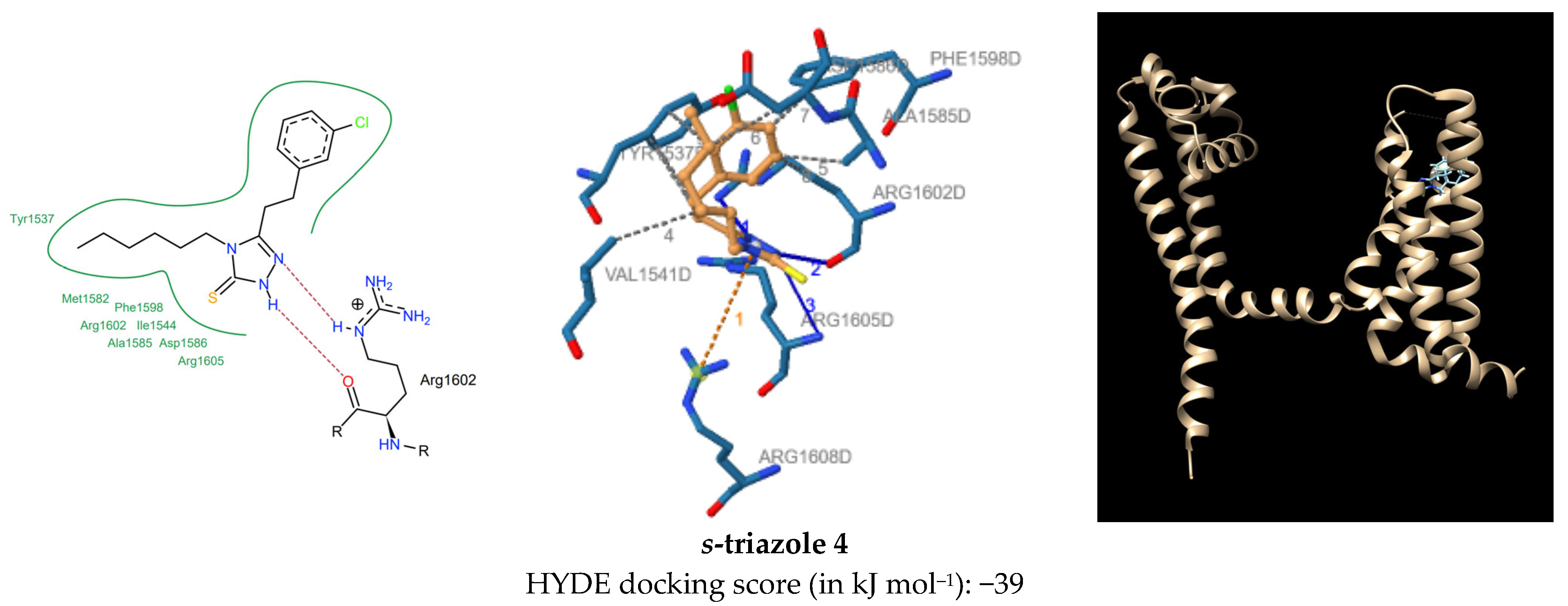
2.2. Chemistry
2.3. Radioligand Binding Assay for Na + Channel (Site 2) Using [3H]Batrachotoxin
2.4. Anticonvulsant Activity
3. Materials and Methods
3.1. Docking Methodology
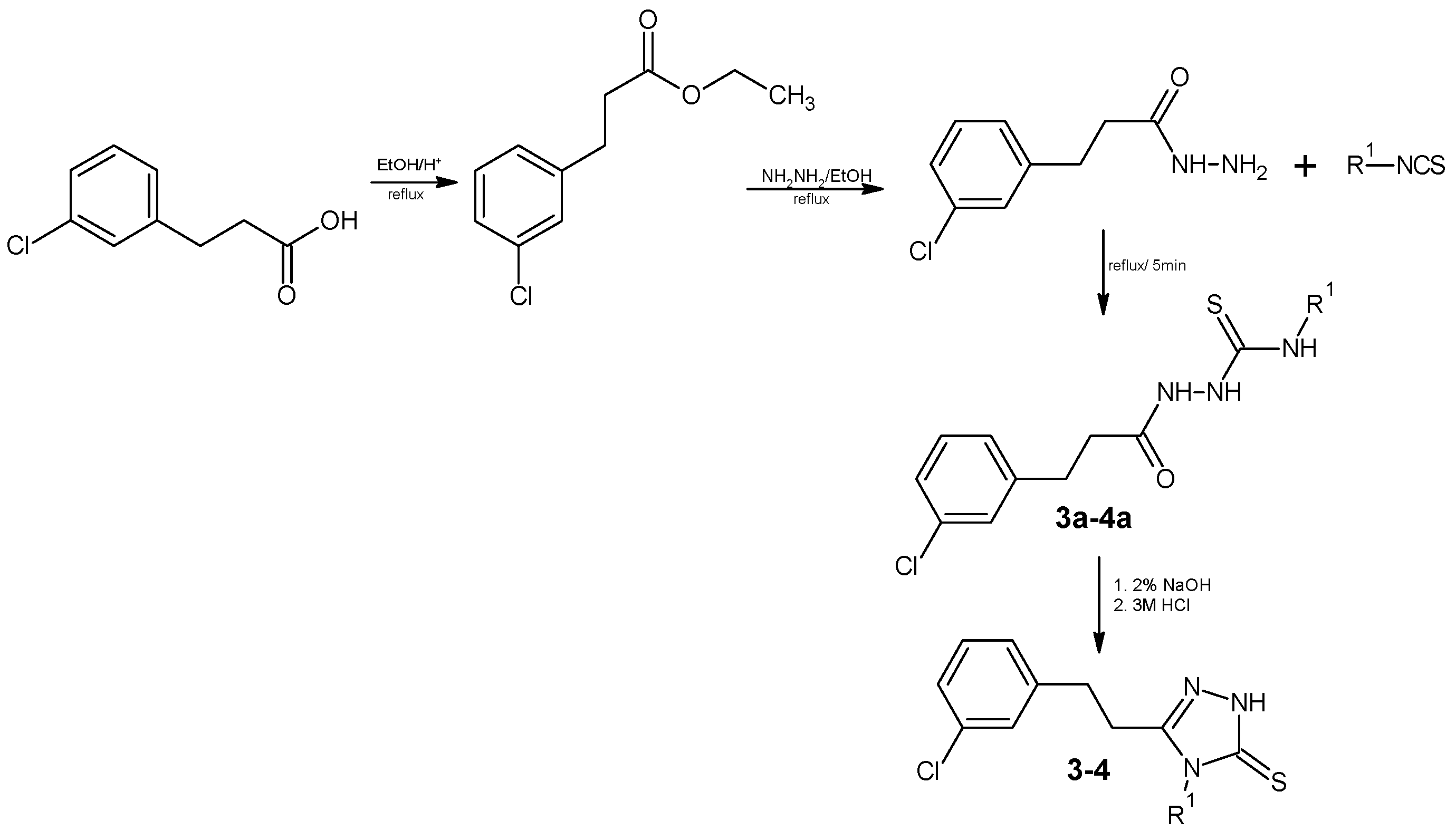
3.2. Chemistry
3.2.1. Synthesis of the Compound 3–4
4-Butyl-1-[(3-chlorophenyl)propionyl]thiosemicarbazide (3a)
1-[(3-Chlorophenyl)propionyl]-4-hexylthiosemicarbazide (4a)
4-Butyl-5-[2-(3-chlorophenyl)ethyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (3)
5-[2-(3-Chlorophenyl)ethyl]-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (4)
3.3. Radioligand Binding Assay for Na + Channel (Site 2) Using [3H]Batrachotoxin
3.4. Animal Experimentations
3.4.1. MES Test
3.4.2. Rotarod Test
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mioramalala, S.A.; Bruand, P.E.; Ratsimbasoa, A.; Rafanomezantsoa, R.M.; Raharinivo, M.M.; Vincent, C.; Preux, P.M.; Boumédiène, F.; Raharivelo, A. Effects of an educational comic book on epilepsy-related knowledge, attitudes and practices among schoolchildren in Madagascar. Epilepsy Res. 2021, 176, 106737. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E.; Carmant, L. Seizures and epilepsy: An overview for neuroscientists. Cold Spring Harb. Perspect. Med. 2015, 5, a022426. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Moshé, S.L. The evolution of the concepts of seizures and epilepsy: What’s in a name? Epilepsia Open 2020, 5, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415. [Google Scholar]
- Guery, D.; Rheims, S. Clinical management of drug resistant epilepsy: A review on current strategies. Neuropsychiatr. Dis. Treat. 2021, 17, 2229–2242. [Google Scholar] [CrossRef]
- Perucca, E. The pharmacological treatment of epilepsy: Recent advances and future perspectives. Acta Epileptol. 2021, 3, 22. [Google Scholar] [CrossRef]
- Walia, K.S.; Khan, E.A.; Ko, D.H.; Raza, S.S.; Khan, Y.N. Side Effects of Antiepileptics—A Review. Pain Pract. 2004, 4, 194–203. [Google Scholar] [CrossRef]
- Pal, R.; Singh, K.; Khan, S.A.; Chawla, P.; Kumar, B.; Akhtar, M.J. Reactive metabolites of the anticonvulsant drugs and approaches to minimize the adverse drug reaction. Eur. J. Med. Chem. 2021, 226, 113890. [Google Scholar] [CrossRef]
- Fattorusso, A.; Matricardi, S.; Mencaroni, E.; Dell’Isola, G.B.; Di Cara, G.; Striano, P.; Verrotti, A. The Pharmacoresistant Epilepsy: An Overview on Existant and New Emerging Therapies. Front. Neurol. 2021, 12, 674483. [Google Scholar] [CrossRef]
- Łuszczki, J.J.; Plech, T.; Wujec, M. Influence of 5-(3-chlorophenyl)-4-(4-methylphenyl)-2,4-dihydro-3H-1,2,4- triazole-3-thioneon the anticonvulsant action of 4 classical antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Pharmacol. Rep. 2012, 64, 970–978. [Google Scholar] [CrossRef]
- Plech, T.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Siwek, A.; Kołaczkowski, M.; Zołnierek, M.; Nowak, G. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on GABAergic system. Eur. J. Med. Chem. 2014, 86, 690–699. [Google Scholar] [CrossRef]
- Plech, T.; Luszczki, J.J.; Wujec, M.; Flieger, J.; Pizoń, M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur. J. Med. Chem. 2013, 60, 208–215. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D. An Important Potential Anti-Epileptic/Anticonvulsant Active Group: A Review of 1,2,4-Triazole Groups and Their Action. Drug Res. 2022, 72, 131–138. [Google Scholar] [CrossRef]
- Kaproń, B.; Łuszczki, J.J.; Siwek, A.; Karcz, T.; Nowak, G.; Zagaja, M.; Andres-Mach, M.; Stasiłowicz, A.; Cielecka-Piontek, J.; Kocki, J.; et al. Preclinical evaluation of 1,2,4-triazole-based compounds targeting voltage-gated sodium channels (VGSCs) as promising anticonvulsant drug candidates. Bioorg. Chem. 2020, 94, 103355. [Google Scholar] [CrossRef]
- Kaproń, B.; Czarnomysy, R.; Wysokiński, M.; Andrys, R.; Musilek, K.; Angeli, A.; Supuran, C.T.; Plech, T. 1,2,4-Triazole-based anticonvulsant agents with additional ROS scavenging activity are effective in a model of pharmacoresistant epilepsy. J. Enzyme Inhib. Med. Chem. 2020, 35, 993–1002. [Google Scholar] [CrossRef]
- Makuch-Kocka, A.; Andres-Mach, M.; Zagaja, M.; Śmiech, A.; Pizoń, M.; Flieger, J.; Cielecka-Piontek, J.; Plech, T. Effect of Chronic Administration of Anticonvulsant Drug Candidate—On Living Organisms. Int. J. Mol. Sci. 2021, 22, 3358. [Google Scholar] [CrossRef]
- Kaproń, B.; Łuszczki, J.; Paneth, A.; Wujec, M.; Siwek, A.; Karcz, T.; Mordyl, B.; Głuch-Lutwin, M.; Gryboś, A.; Nowak, G.; et al. Molecular mechanism of action and safety of 5-(3-chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione-a novel anticonvulsant drug candidate. Int. J. Med. Sci. 2017, 14, 741–749. [Google Scholar] [CrossRef]
- Kaproń, B.; Łuszczki, J.J.; Płazińska, A.; Siwek, A.; Karcz, T.; Gryboś, A.; Nowak, G.; Makuch-Kocka, A.; Walczak, K.; Langner, E.; et al. Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in-vivo, in-vitro, and in-silico studies. Eur. J. Pharm. Sci. 2019, 129, 42–57. [Google Scholar] [CrossRef]
- Xu, H.; Li, T.; Rohou, A.; Arthur, C.P.; Tzakoniati, F.; Wong, E.; Estevez, A.; Kugel, C.; Franke, Y.; Chen, J.; et al. Structural Basis of Nav1.7 Inhibition by a Gating-Modifier Spider Toxin. Cell 2019, 176, 702–715.e14. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, J.; Fan, X.; Wang, K.; Jin, X.; Huang, G.; Li, J.; Pan, X.; Yan, N. Structural mapping of Nav1.7 antagonists. Nat. Commun. 2023, 14, 3224. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.F.S.; Sabiá Júnior, E.F.; Tibery, D.V.; Carneiro, L.D.A.; Schwartz, E.F. Epilepsy-Related Voltage-Gated Sodium Channelopathies: A Review. Front. Pharmacol. 2020, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Pappas, C.; Dahle, E.J.; Claes, L.R.F.; Pruess, T.H.; De Jonghe, P.; Thompson, J.; Dixon, M.; Gurnett, C.; Peiffer, A.; et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet. 2009, 5, e1000649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Z.; Shen, Y.; Zhu, Y.; Du, K.; Guo, J.; Ji, Y.; Tao, J. SCN9A Epileptic Encephalopathy Mutations Display a Gain-of-function Phenotype and Distinct Sensitivity to Oxcarbazepine. Neurosci. Bull. 2020, 36, 11–24. [Google Scholar] [CrossRef]
- Ahuja, S.; Mukund, S.; Deng, L.; Khakh, K.; Chang, E.; Ho, H.; Shriver, S.; Young, C.; Lin, S.; Johnson, J.P.; et al. Structural basis of Nav1.7 inhibition by an isoform-selective small-molecule antagonist. Science 2015, 350, aac5464. [Google Scholar] [CrossRef]
- Yang, N.; George, A.L.; Horn, R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 1996, 16, 113–122. [Google Scholar] [CrossRef]
- McCormack, K.; Santos, S.; Chapman, M.L.; Krafte, D.S.; Marron, B.E.; West, C.W.; Krambis, M.J.; Antonio, B.M.; Zellmer, S.G.; Printzenhoff, D.; et al. Voltage sensor interaction site for selective small molecule inhibitors of voltage-gated sodium channels. Proc. Natl. Acad. Sci. USA 2013, 110, E2724–E2732. [Google Scholar] [CrossRef]
- Willow, M.; Catterall, W.A. Inhibition of binding of [3H]batrachotoxinin A 20-alpha-benzoate to sodium channels by the anticonvulsant drugs diphenylhydantoin and carbamazepine. Mol. Pharmacol. 1982, 22, 627–635. [Google Scholar]
- Castel-Branco, M.M.; Alves, G.L.; Figueiredo, I.V.; Falcão, A.C.; Caramona, M.M. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 101–106. [Google Scholar] [CrossRef]
- Zagaja, M.; Szewczyk, A.; Szala-Rycaj, J.; Raszewski, G.; Chrościńska-Krawczyk, M.; Abram, M.; Kamiński, K.; Andres-Mach, M. C-11, a new antiepileptic drug candidate: Evaluation of the physicochemical properties and impact on the protective action of selected antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Molecules 2021, 26, 3144. [Google Scholar] [CrossRef]
- Drabik, M.; Głuszak, M.; Wróblewska-Łuczka, P.; Plewa, Z.; Jankiewicz, M.; Kozińska, J.; Florek-Łuszczki, M.; Plech, T.; Łuszczki, J.J. Anticonvulsant Effectiveness and Neurotoxicity Profile of 4-butyl-5-[(4-chloro-2-methylphenoxy)methyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (TPL-16) in Mice. Neurochem. Res. 2021, 46, 396–410. [Google Scholar] [CrossRef]
- Schneider, N.; Lange, G.; Hindle, S.; Klein, R.; Rarey, M. A consistent description of hydrogen bond and dehydration energies in protein-ligand complexes: Methods behind the HYDE scoring function. J. Comput. Aided. Mol. Des. 2013, 27, 15–29. [Google Scholar] [CrossRef]
- Paneth, A.; Płonka, W.; Paneth, P. Assessment of nonnucleoside inhibitors binding to HIV-1 reverse transcriptase using HYDE scoring. Pharmaceuticals 2019, 12, 64. [Google Scholar] [CrossRef]
- García-Fernández, E.; Koch, G.; Wagner, R.M.; Fekete, A.; Stengel, S.T.; Schneider, J.; Mielich-Süss, B.; Geibel, S.; Markert, S.M.; Stigloher, C.; et al. Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171, 1354–1367. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Callaway, J.K.; Castillo-Melendez, M.; Giardina, S.F.; Krstew, E.K.; Beart, P.M.; Jarrott, B. Sodium channel blocking activity of AM-36 and sipatrigine (BW619C89): In vitro and in vivo evidence. Neuropharmacology 2004, 47, 146–155. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Andres, M.M.; Czuczwar, P.; Cioczek-Czuczwar, A.; Wojcik-Cwikla, J.; Ratnaraj, N.; Patsalos, P.N.; Czuczwar, S.J. Levetiracetam selectively potentiates the acute neurotoxic effects of topiramate and carbamazepine in the rotarod test in mice. Eur. Neuropsychopharmacol. 2005, 15, 609–616. [Google Scholar] [CrossRef]
| s-Triazole | Hydrophobic Interactions and Distance ······ | H-Bond Interactions — | H-A Distance | D-A Distance | pi-Cation Interactions ······ |
|---|---|---|---|---|---|
| 1 | Tyr1537 (3.06 Å, 3.86 Å), Asn1540 (3.44 Å), Ile1544 (3.81 Å), Met1582 (3.45 Å), Ala1585 (3.25 Å), Asp1586 (3.85 Å), Phe1598 (2.97 Å), Arg1602 (3.49 Å) | Arg1602 Arg1602 Arg1605 | 3.01 Å 1.81 Å 3.44 Å | 3.63 Å 2.71 Å 4.02 Å | — |
| 2 | Tyr1537 (3.68 Å, 2.90 Å, 3.65 Å), Val1541 (3.31 Å), Met1582 (3.60 Å), Ala1585 (3.44 Å), Asp1586 (3.54 Å), Phe1598 (3.73 Å), Arg1602 (3.92 Å) | Arg1602 Arg1602 Arg1605 | 2.90 1.82 3.49 | 3.53 2.69 4.09 | — |
| 3 | Tyr1537 (3.39 Å, 3.51 Å, 3.02 Å), Met1582 (3.85 Å), Ala1585 (3.11 Å), Phe1598 (2.81 Å), Arg1602 (3.14 Å) | Arg1602 Arg1602 Arg1605 | 2.68 1.94 3.54 | 3.40 2.90 4.07 | Arg1608 (5.83 Å) |
| 4 | Tyr1537 (3.60 Å, 3.66 Å, 3.19 Å), Val1541 (3.99 Å), Ala1585 (3.12 Å), Asp1586 (3.91 Å), Phe1598 (3.15 Å), Arg1602 (3.36 Å) | Arg1602 Arg1602 Arg1605 | 2.71 1.88 3.46 | 3.44 2.85 3.99 | Arg1608 (5.73 Å) |
| Compound Number | Compound Structures | Na+ Channel (Site 2) Affinity IC50 [µM] ± SEM | Dose—Response Binding Curves |
|---|---|---|---|
| 3 | 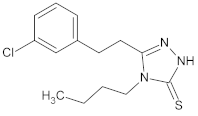 | 11.9 ± 0.4 | 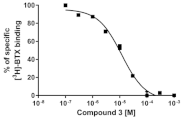 |
| 4 | 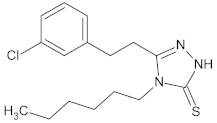 | 18.9 ± 0.3 | 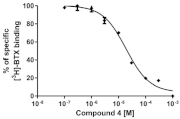 |
| Veratridine | 17.8 ± 0.9 | 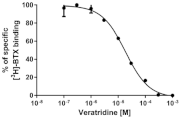 | |
| Carbamazepine | 131 1 |
| Compound Number | Compound | Anticonvulsant Activity | Neurotoxicity | ||
|---|---|---|---|---|---|
| Pretreatment Time (min) | ED50 (mg/kg) ± SEM | TD50 (mg/kg) ± SEM | PI (TD50/ED50) | ||
| 3 | 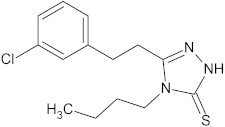 | 15 | 138.7 ± 31.4 | 329.5 ± 30.0 | 2.4 |
| 30 | 96.6 ± 14.8 | 329.5 ± 30.0 | 3.4 | ||
| 60 | 148.3 ± 17.1 | 337.7 ± 26.3 | 2.3 | ||
| 120 | 194.9 ± 18.9 | 325.9 ± 23.1 | 1.7 | ||
| 4 | 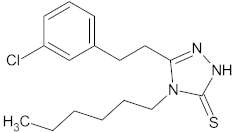 | 15 | 115.4 ± 7.6 | 710.5 ± 47.4 | 6.2 |
| 30 | 129.0 ± 21.1 | 685.4 ± 49.0 | 5.3 | ||
| 60 | 214.9 ± 21.1 | 698.4 ± 46.2 | 3.2 | ||
| 120 | 254.8 ± 22.5 | 478.9 ± 49.6 | 1.9 | ||
| Valproate 1 | 15 | 189.0 ± 17.3 | 363.3 ± 14.2 | 1.9 | |
| 30 | 216.9 ± 9.4 | 372.9 ± 16.9 | 1.7 | ||
| 60 | 218.4 ± 18.9 | 417.3 ± 9.5 | 1.9 | ||
| 120 | 246.6 ± 21.4 | 512.3 ± 30.2 | 2.1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paruch, K.; Kaproń, B.; Łuszczki, J.J.; Paneth, A.; Plech, T. Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives. Molecules 2023, 28, 5287. https://doi.org/10.3390/molecules28135287
Paruch K, Kaproń B, Łuszczki JJ, Paneth A, Plech T. Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives. Molecules. 2023; 28(13):5287. https://doi.org/10.3390/molecules28135287
Chicago/Turabian StyleParuch, Kinga, Barbara Kaproń, Jarogniew J. Łuszczki, Agata Paneth, and Tomasz Plech. 2023. "Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives" Molecules 28, no. 13: 5287. https://doi.org/10.3390/molecules28135287
APA StyleParuch, K., Kaproń, B., Łuszczki, J. J., Paneth, A., & Plech, T. (2023). Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives. Molecules, 28(13), 5287. https://doi.org/10.3390/molecules28135287






