Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors
Abstract
1. Introduction
1.1. Psychostimulant Use Disorder (PSUD)
1.2. Psychostimulant-Induced Sex-Dependent Behavioral Effects
1.3. Psychostimulant-Induced Sex-Dependent Neurochemical Effects
1.3.1. Sex Differences at Baseline
1.3.2. Sex-Dependent Response to Psychostimulant Administration
2. Therapeutic Options
3. Modafinil (MOD)
4. MOD Analogs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNODC. World Drug Report 2022; United Nations Publications: New York, NY, USA, 2022. [Google Scholar]
- Kariisa, M.; Scholl, L.; Wilson, N.; Seth, P.; Hoots, B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential—United States, 2003–2017. Morb. Mortal. Wkly. Rep. 2019, 68, 388. [Google Scholar] [CrossRef]
- Beery, A.K. Inclusion of females does not increase variability in rodent research studies. Curr. Opin. Behav. Sci. 2018, 23, 143–149. [Google Scholar] [CrossRef]
- Woitowich, N.C.; Beery, A.; Woodruff, T. A 10-year follow-up study of sex inclusion in the biological sciences. Elife 2020, 9, e56344. [Google Scholar] [CrossRef]
- Nicolas, C.; Zlebnik, N.E.; Farokhnia, M.; Leggio, L.; Ikemoto, S.; Shaham, Y. Sex differences in opioid and psychostimulant craving and relapse: A critical review. Pharmacol. Rev. 2022, 74, 119–140. [Google Scholar] [CrossRef]
- Quigley, J.A.; Logsdon, M.K.; Turner, C.A.; Gonzalez, I.L.; Leonardo, N.; Becker, J.B. Sex differences in vulnerability to addiction. Neuropharmacology 2021, 187, 108491. [Google Scholar] [CrossRef]
- Kokane, S.S.; Perrotti, L.I. Sex differences and the role of estradiol in mesolimbic reward circuits and vulnerability to cocaine and opiate addiction. Front. Behav. Neurosci. 2020, 14, 74. [Google Scholar] [CrossRef]
- Wise, R.A.; Rompre, P.-P. Brain dopamine and reward. Annu. Rev. Psychol. 1989, 40, 191–225. [Google Scholar] [CrossRef]
- Jaber, M.; Robinson, S.W.; Missale, C.; Caron, M.G. Dopamine receptors and brain function. Neuropharmacology 1996, 35, 1503–1519. [Google Scholar] [CrossRef]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Williams, O.O.; Coppolino, M.; George, S.R.; Perreault, M.L. Sex differences in dopamine receptors and relevance to neuropsychiatric disorders. Brain Sci. 2021, 11, 1199. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Regulation of the vesicular monoamine transporter-2: A novel mechanism for cocaine and other psychostimulants. J. Pharmacol. Exp. Ther. 2001, 296, 762–767. [Google Scholar]
- Brown, J.M.; Hanson, G.R.; Fleckenstein, A.E. Cocaine-induced increases in vesicular dopamine uptake: Role of dopamine receptors. J. Pharmacol. Exp. Ther. 2001, 298, 1150–1153. [Google Scholar]
- Venton, B.J.; Seipel, A.T.; Phillips, P.E.; Wetsel, W.C.; Gitler, D.; Greengard, P.; Augustine, G.J.; Wightman, R.M. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 2006, 26, 3206–3209. [Google Scholar] [CrossRef]
- Kile, B.M.; Guillot, T.S.; Venton, B.J.; Wetsel, W.C.; Augustine, G.J.; Wightman, R.M. Synapsins differentially control dopamine and serotonin release. J. Neurosci. 2010, 30, 9762–9770. [Google Scholar] [CrossRef]
- Hoffman, A.F.; Spivak, C.E.; Lupica, C.R. Enhanced dopamine release by dopamine transport inhibitors described by a restricted diffusion model and fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2016, 7, 700–709. [Google Scholar] [CrossRef]
- Keighron, J.D.; Bonaventura, J.; Li, Y.; Yang, J.-W.; DeMarco, E.M.; Hersey, M.; Cao, J.; Sandtner, W.; Michaelides, M.; Sitte, H.H.; et al. Interactions of calmodulin kinase II with the dopamine transporter facilitate cocaine-induced enhancement of evoked dopamine release. Transl. Psychiatry 2023, 13, 202. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J. Neurosci. 1997, 17, 8491–8497. [Google Scholar] [CrossRef]
- Robinson, T.E.; Kolb, B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 1999, 11, 1598–1604. [Google Scholar] [CrossRef]
- Van Etten, M.L.; Anthony, J.C. Male-female differences in transitions from first drug opportunity to first use: Searching for subgroup variation by age, race, region, and urban status. J. Women’s Health Gend.-Based Med. 2001, 10, 797–804. [Google Scholar] [CrossRef]
- Griffin, M.L.; Weiss, R.D.; Mirin, S.M.; Lange, U. A Comparison of Male and Female Cocaine Abusers. Arch. Gen. Psychiatry 1989, 46, 122–126. [Google Scholar] [CrossRef]
- Brady, K.T.; Randall, C.L. Gender Differences in Substance Use Disorders. Psychiatr. Clin. N. Am. 1999, 22, 241–252. [Google Scholar] [CrossRef]
- Lynch, W.J.; Roth, M.E.; Carroll, M.E. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology 2002, 164, 121–137. [Google Scholar] [CrossRef]
- Chen, K.; Kandel, D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002, 68, 65–85. [Google Scholar] [CrossRef]
- Greenfield, S.F.; Back, S.E.; Lawson, K.; Brady, K.T. Substance abuse in women. Psychiatr. Clin. 2010, 33, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Sofuoglu, M.; Dudish-Poulsen, S.; Nelson, D.; Pentel, P.R.; Hatsukami, D.K. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp. Clin. Psychopharmacol. 1999, 7, 274–283. [Google Scholar] [CrossRef]
- Kennedy, A.P.; Epstein, D.H.; Phillips, K.A.; Preston, K.L. Sex differences in cocaine/heroin users: Drug-use triggers and craving in daily life. Drug Alcohol Depend. 2013, 132, 29–37. [Google Scholar] [CrossRef]
- Elman, I.; Karlsgodt, K.H.; Gastfriend, D.R. Gender Differences in Cocaine Craving among Non-Treatment-Seeking Individuals with Cocaine Dependence. Am. J. Drug Alcohol Abus. 2001, 27, 193–202. [Google Scholar] [CrossRef]
- Moran-Santa Maria, M.M.; McRae-Clark, A.; Baker, N.L.; Ramakrishnan, V.; Brady, K.T. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology 2014, 231, 4157–4165. [Google Scholar] [CrossRef]
- Dos Anjos Rosario, B.; de Fatima SantanaNazare, M.; de Souza, D.V.; Le Sueur-Maluf, L.; Estadella, D.; Ribeiro, D.A.; de Barros Viana, M. The influence of sex and reproductive cycle on cocaine-induced behavioral and neurobiological alterations: A review. Exp. Brain Res. 2022, 240, 3107–3140. [Google Scholar] [CrossRef]
- Roth, M.E.; Carroll, M.E. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol. Biochem. Behav. 2004, 78, 199–207. [Google Scholar] [CrossRef]
- Sahakian, B.J.; Robbins, T.W.; Morgan, M.J.; Iversen, S.D. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain Res. 1975, 84, 195–205. [Google Scholar] [CrossRef]
- Glick, S.D.; Hinds, P.A.; Shapiro, R.M. Cocaine-Induced Rotation: Sex-Dependent Differences Between Left- and Right-Sided Rats. Science 1983, 221, 775–777. [Google Scholar] [CrossRef]
- Kerstetter, K.A.; Ballis, M.A.; Duffin-Lutgen, S.; Carr, A.E.; Behrens, A.M.; Kippin, T.E. Sex Differences in Selecting Between Food and Cocaine Reinforcement are Mediated by Estrogen. Neuropsychopharmacology 2012, 37, 2605–2614. [Google Scholar] [CrossRef]
- Becker, J.B. Sex differences in addiction. Dialogues Clin. Neurosci. 2016, 18, 395–402. [Google Scholar] [CrossRef]
- Becker, J.B.; Arnold, A.P.; Berkley, K.J.; Blaustein, J.D.; Eckel, L.A.; Hampson, E.; Herman, J.P.; Marts, S.; Sadee, W.; Steiner, M. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005, 146, 1650–1673. [Google Scholar] [CrossRef]
- Lebron-Milad, K.; Milad, M.R. Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biol. Mood Anxiety Disord. 2012, 2, 10330322. [Google Scholar] [CrossRef]
- Febo, M.; González-Rodríguez, L.A.; Capó-Ramos, D.E.; González-Segarra, N.Y.; Segarra, A.C. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J. Neurochem. 2003, 86, 405–412. [Google Scholar] [CrossRef]
- Segarra, A.C.; Torres-Díaz, Y.M.; Silva, R.D.; Puig-Ramos, A.; Menéndez-Delmestre, R.; Rivera-Bermúdez, J.G.; Amadeo, W.; Agosto-Rivera, J.L. Estrogen receptors mediate estradiol’s effect on sensitization and CPP to cocaine in female rats: Role of contextual cues. Horm. Behav. 2014, 65, 77–87. [Google Scholar] [CrossRef]
- Feltenstein, M.W.; Henderson, A.R.; See, R.E. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: Sex differences and the role of the estrous cycle. Psychopharmacology 2011, 216, 53–62. [Google Scholar] [CrossRef]
- Knouse, M.C.; Briand, L.A. Behavioral sex differences in cocaine and opioid use disorders: The role of gonadal hormones. Neurosci. Biobehav. Rev. 2021, 128, 358–366. [Google Scholar] [CrossRef]
- Robbins, S.J.; Ehrman, R.N.; Childress, A.R.; O’Brien, C.P. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999, 53, 223–230. [Google Scholar] [CrossRef]
- Kosten, T.A.; Gawin, F.H.; Kosten, T.R.; Rounsaville, B.J. Gender differences in cocaine use and treatment response. J. Subst. Abus. Treat. 1993, 10, 63–66. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Weiss, R.; Griffin, M.; Mirin, S.M.; Teoh, S.K.; Mello, N.K.; Lex, B.W. Some special considerations for treatment of drug abuse and dependence in women. NIDA Res. Monogr. 1991, 106, 313–327. [Google Scholar]
- Gallop, R.J.; Crits-Christoph, P.; Ten Have, T.R.; Barber, J.P.; Frank, A.; Griffin, M.L.; Thase, M.E. Differential transitions between cocaine use and abstinence for men and women. J. Consult. Clin. Psychol. 2007, 75, 95–103. [Google Scholar] [CrossRef]
- Fuchs, R.A.; Evans, K.A.; Mehta, R.H.; Case, J.M.; See, R.E. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 2005, 179, 662–672. [Google Scholar] [CrossRef]
- Anker, J.J.; Carroll, M.E. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010, 107, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Nelson, S.E.; Carroll, M.E. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp. Clin. Psychopharmacol. 2008, 16, 165–177. [Google Scholar] [CrossRef]
- Becker, J.B.; Koob, G.F. Sex differences in animal models: Focus on addiction. Pharmacol. Rev. 2016, 68, 242–263. [Google Scholar] [CrossRef]
- Bobzean, S.A.; DeNobrega, A.K.; Perrotti, L.I. Sex differences in the neurobiology of drug addiction. Exp. Neurol. 2014, 259, 64–74. [Google Scholar] [CrossRef]
- Kritzer, M.F.; Creutz, L.M. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J. Neurosci. 2008, 28, 9525–9535. [Google Scholar] [CrossRef]
- McArthur, S.; McHale, E.; Gillies, G.E. The size and distribution of midbrain dopaminergic populations are permanently altered by perinatal glucocorticoid exposure in a sex-region-and time-specific manner. Neuropsychopharmacology 2007, 32, 1462–1476. [Google Scholar] [CrossRef]
- Locklear, M.N.; Michaelos, M.; Collins, W.F.; Kritzer, M.F. Gonadectomy but not biological sex affects burst-firing in dopamine neurons of the ventral tegmental area and in prefrontal cortical neurons projecting to the ventral tegmentum in adult rats. Eur. J. Neurosci. 2017, 45, 106–120. [Google Scholar] [CrossRef]
- Rincón-Cortés, M.; Grace, A.A. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: Modulation by ketamine. Int. J. Neuropsychopharmacol. 2017, 20, 823–832. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, S.; Yang, C.; Jin, G.; Zhen, X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology 2008, 199, 625–635. [Google Scholar] [CrossRef]
- Shanley, M.R.; Miura, Y.; Guevara, C.A.; Onoichenco, A.; Kore, R.; Ustundag, E.; Darwish, R.; Renzoni, L.; Urbaez, A.; Blicker, E. Estrous cycle mediates midbrain neuron excitability altering social behavior upon stress. J. Neurosci. 2023, 43, 736–748. [Google Scholar] [CrossRef]
- Castner, S.A.; Xiao, L.; Becker, J.B. Sex differences in striatal dopamine: In vivo microdialysis and behavioral studies. Brain Res. 1993, 610, 127–134. [Google Scholar] [CrossRef]
- Walker, Q.; Rooney, M.; Wightman, R.; Kuhn, C. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 1999, 95, 1061–1070. [Google Scholar] [CrossRef]
- Yu, L.; Liao, P.-C. Sexual differences and estrous cycle in methamphetamine-induced dopamine and serotonin depletions in the striatum of mice. J. Neural Transm. 2000, 107, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Becker, J.B. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: Effects of estrous cycle and gonadectomy. Neurosci. Lett. 1994, 180, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Brundage, J.N.; Mason, C.P.; Wadsworth, H.A.; Finuf, C.S.; Nelson, J.J.; Ronström, P.J.W.; Jones, S.R.; Siciliano, C.A.; Steffensen, S.C.; Yorgason, J.T. Regional and sex differences in spontaneous striatal dopamine transmission. J. Neurochem. 2022, 160, 598–612. [Google Scholar] [CrossRef]
- Calipari, E.S.; Juarez, B.; Morel, C.; Walker, D.M.; Cahill, M.E.; Ribeiro, E.; Roman-Ortiz, C.; Ramakrishnan, C.; Deisseroth, K.; Han, M.-H. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017, 8, 13877. [Google Scholar] [CrossRef] [PubMed]
- Vandegrift, B.J.; You, C.; Satta, R.; Brodie, M.S.; Lasek, A.W. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE 2017, 12, e0187698. [Google Scholar] [CrossRef]
- Yoest, K.E.; Cummings, J.A.; Becker, J.B. Oestradiol influences on dopamine release from the nucleus accumbens shell: Sex differences and the role of selective oestradiol receptor subtypes. Br. J. Pharmacol. 2019, 176, 4136–4148. [Google Scholar] [CrossRef]
- Becker, J.B.; Ramirez, V. Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinology 1981, 32, 168–173. [Google Scholar] [CrossRef]
- Di Paolo, T.; Rouillard, C.; Bédard, P. 17β-estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur. J. Pharmacol. 1985, 117, 197–203. [Google Scholar] [CrossRef]
- Thompson, T.L. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Res. 1999, 834, 164–167. [Google Scholar] [CrossRef]
- Pitts, E.G.; Stowe, T.A.; Christensen, B.A.; Ferris, M.J. Comparing dopamine release, uptake, and D2 autoreceptor function across the ventromedial to dorsolateral striatum in adolescent and adult male and female rats. Neuropharmacology 2020, 175, 108163. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Wong, D.F.; Oswald, L.M.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W. Sex differences in striatal dopamine release in healthy adults. Biol. Psychiatry 2006, 59, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Trick, L.; Butler, K.; Chukwueke, C.; Di Ciano, P.; Ibrahim, C.; Rubin-Kahana, D.S.; Boileau, I.; Le Foll, B. Abnormalities of Neurotransmission in Drug Addiction. In PET and SPECT in Psychiatry; Springer: Cham, Switzerland, 2021; pp. 653–712. [Google Scholar]
- Andersen, S.L.; Rutstein, M.; Benzo, J.M.; Hostetter, J.C.; Teicher, M.H. Sex differences in dopamine receptor overproduction and elimination. Neuroreport 1997, 8, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Hasbi, A.; Nguyen, T.; Rahal, H.; Manduca, J.D.; Miksys, S.; Tyndale, R.F.; Madras, B.K.; Perreault, M.L.; George, S.R. Sex difference in dopamine D1–D2 receptor complex expression and signaling affects depression-and anxiety-like behaviors. Biol. Sex Differ. 2020, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Hruska, R.E.; Silbergeld, E.K. Estrogen treatment enhances dopamine receptor sensitivity in the rat striatum. Eur. J. Pharmacol. 1980, 61, 397–400. [Google Scholar] [CrossRef]
- Lévesque, D.; Di Paolo, T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17β-estradiol. Neurosci. Lett. 1988, 88, 113–118. [Google Scholar] [CrossRef]
- Campi, K.L.; Greenberg, G.D.; Kapoor, A.; Ziegler, T.E.; Trainor, B.C. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology 2014, 77, 208–216. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Beraki, S.; Scott, L.; Aperia, A.; Forssberg, H. Sex differences in the motor inhibitory and stimulatory role of dopamine D1 receptors in rats. Eur. J. Pharmacol. 2002, 445, 97–104. [Google Scholar] [CrossRef]
- Bazzett, T.J.; Becker, J.B. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994, 637, 163–172. [Google Scholar] [CrossRef]
- Lévesque, D.; Di Paolo, T. Chronic estradiol treatment increases ovariectomized rat striatal D-1 dopamine receptors. Life Sci. 1989, 45, 1813–1820. [Google Scholar] [CrossRef]
- Walker, Q.D.; Ray, R.; Kuhn, C.M. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology 2006, 31, 1193–1202. [Google Scholar] [CrossRef]
- Zhou, W.; Cunningham, K.A.; Thomas, M.L. Estrogen regulation of gene expression in the brain: A possible mechanism altering the response to psychostimulants in female rats. Mol. Brain Res. 2002, 100, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kaasinen, V.; Någren, K.; Hietala, J.; Farde, L.; Rinne, J.O. Sex differences in extrastriatal dopamine D2-like receptors in the human brain. Am. J. Psychiatry 2001, 158, 308–311. [Google Scholar] [CrossRef]
- Wong, D.F.; Wagner, H.N., Jr.; Dannals, R.F.; Links, J.M.; Frost, J.J.; Ravert, H.T.; Wilson, A.A.; Rosenbaum, A.E.; Gjedde, A.; Douglass, K.H. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science 1984, 226, 1393–1396. [Google Scholar] [CrossRef]
- Chavez, C.; Hollaus, M.; Scarr, E.; Pavey, G.; Gogos, A.; van den Buuse, M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: An autoradiography study. Brain Res. 2010, 1321, 51–59. [Google Scholar] [CrossRef]
- Bossé, R.; Rivest, R.; Di Paolo, T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Mol. Brain Res. 1997, 46, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Best, S.E.; Sarrel, P.M.; Malison, R.T.; Laruelle, M.; Zoghbi, S.S.; Baldwin, R.M.; Seibyl, J.P.; Innis, R.B.; Van Dyck, C.H. Striatal dopamine transporter availability with [123 I] β-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology 2005, 183, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Kuikka, J.T.; Tiihonen, J.; Karhu, J.; Bergström, K.A.; Räsänen, P. Fractal analysis of striatal dopamine re-uptake sites. Eur. J. Nucl. Med. 1997, 24, 1085–1090. [Google Scholar] [PubMed]
- Lavalaye, J.; Booij, J.; Reneman, L.; Habraken, J.B.; van Royen, E.A. Effect of age and gender on dopamine transporter imaging with [123 I] FP-CIT SPET in healthy volunteers. Eur. J. Nucl. Med. 2000, 27, 867–869. [Google Scholar] [CrossRef]
- Di Chiara, G.; Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA 1988, 85, 5274–5278. [Google Scholar] [CrossRef]
- Weiss, F.; Paulus, M.P.; Lorang, M.T.; Koob, G.F. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: Effects of acute and repeated administration. J. Neurosci. 1992, 12, 4372–4380. [Google Scholar] [CrossRef]
- Pontieri, F.E.; Tanda, G.; Di Chiara, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.A.; Jagannathan, L.; Jackson, L.R.; Becker, J.B. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: Neurochemistry and behavior. Drug Alcohol Depend. 2014, 135, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse 1990, 5, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Beer, M.E.; Robinson, T.E. Striatal dopamine release stimulated by amphetamine or potassium: Influence of ovarian hormones and the light-dark cycle. Brain Res. 1984, 311, 157–160. [Google Scholar] [CrossRef]
- Peris, J.; Decambre, N.; Coleman-Hardee, M.L.; Simpkins, J.W. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H] dopamine release. Brain Res. 1991, 566, 255–264. [Google Scholar] [CrossRef]
- Lewis, C.; Dluzen, D.E. Testosterone enhances dopamine depletion by methamphetamine in male, but not female, mice. Neurosci. Lett. 2008, 448, 130–133. [Google Scholar] [CrossRef]
- Martinez, D.; Narendran, R.; Foltin, R.W.; Slifstein, M.; Hwang, D.-R.; Broft, A.; Huang, Y.; Cooper, T.B.; Fischman, M.W.; Kleber, H.D. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am. J. Psychiatry 2007, 164, 622–629. [Google Scholar] [CrossRef]
- Martinez, D.; Carpenter, K.M.; Liu, F.; Slifstein, M.; Broft, A.; Friedman, A.C.; Kumar, D.; Van Heertum, R.; Kleber, H.D.; Nunes, E. Imaging dopamine transmission in cocaine dependence: Link between neurochemistry and response to treatment. Am. J. Psychiatry 2011, 168, 634–641. [Google Scholar] [CrossRef]
- Volkow, N.; Wang, G.-J.; Fowler, J.; Logan, J.; Gatley, S.; Hitzemann, R.; Chen, A.; Dewey, S.; Pappas, N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 1997, 386, 830–833. [Google Scholar] [CrossRef]
- Clare, K.; Pan, C.; Kim, G.; Park, K.; Zhao, J.; Volkow, N.D.; Lin, Z.; Du, C. Cocaine reduces the neuronal population while upregulating dopamine D2-receptor-expressing neurons in brain reward regions: Sex-effects. Front. Pharmacol. 2021, 12, 624127. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Ferris, M.J.; Jones, S.R. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J. Neurochem. 2014, 128, 224–232. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J. Neurosci. 1993, 13, 266–275. [Google Scholar] [CrossRef]
- Wilson, J.M.; Shannak, K.; Kish, S.J.; Levey, A.I.; Bergeron, C.; Deck, J.; Kalasinsky, K.; Ang, L.; Peretti, F.; Adams, V.I. Striatal dopamine, dopamine transporter, and vesicular monoamine transporter in chronic cocaine users. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1996, 40, 428–439. [Google Scholar] [CrossRef]
- Wilson, J.M.; Kalasinsky, K.S.; Levey, A.I.; Bergeron, C.; Reiber, G.; Anthony, R.M.; Schmunk, G.A.; Shannak, K.; Haycock, J.W.; Kish, S.J. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat. Med. 1996, 2, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Ballard, M.E.; Dean, A.C.; Mandelkern, M.A.; London, E.D. Striatal dopamine D2/D3 receptor availability is associated with executive function in healthy controls but not methamphetamine users. PLoS ONE 2015, 10, e0143510. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; London, E.D.; Poldrack, R.A.; Farahi, J.; Nacca, A.; Monterosso, J.R.; Mumford, J.A.; Bokarius, A.V.; Dahlbom, M.; Mukherjee, J. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci. 2009, 29, 14734–14740. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Fowler, J.S.; Wolf, A.P.; Schlyer, D.; Shiue, C.-Y.; Alpert, R.; Dewey, S.L.; Logan, J.; Bendriem, B.; Christman, D. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am. J. Psychiatry 1990, 147, 719–724. [Google Scholar]
- Worhunsky, P.D.; Matuskey, D.; Gallezot, J.-D.; Gaiser, E.C.; Nabulsi, N.; Angarita, G.A.; Calhoun, V.D.; Malison, R.T.; Potenza, M.N.; Carson, R.E. Regional and source-based patterns of [11C]-(+)-PHNO binding potential reveal concurrent alterations in dopamine D2 and D3 receptor availability in cocaine-use disorder. Neuroimage 2017, 148, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Martinez, D.; Broft, A.; Foltin, R.W.; Slifstein, M.; Hwang, D.-R.; Huang, Y.; Perez, A.; Frankel, W.G.; Cooper, T.; Kleber, H.D. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology 2004, 29, 1190–1202. [Google Scholar] [CrossRef]
- Narendran, R.; Martinez, D.; Mason, N.S.; Lopresti, B.J.; Himes, M.L.; Chen, C.M.; May, M.A.; Price, J.C.; Mathis, C.A.; Frankle, W.G. Imaging of dopamine D2/3 agonist binding in cocaine dependence: A [11C] NPA positron emission tomography study. Synapse 2011, 65, 1344–1349. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.J.; Hitzemann, R.; Logan, J.; Schlyer, D.J.; Dewey, S.L.; Wolf, A.P. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993, 14, 169–177. [Google Scholar] [CrossRef]
- Volkow, N.D.; Chang, L.; Wang, G.-J.; Fowler, J.S.; Ding, Y.-S.; Sedler, M.; Logan, J.; Franceschi, D.; Gatley, J.; Hitzemann, R. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am. J. Psychiatry 2001, 158, 2015–2021. [Google Scholar] [CrossRef]
- Okita, K.; Ghahremani, D.G.; Payer, D.E.; Robertson, C.L.; Mandelkern, M.A.; London, E.D. Relationship of alexithymia ratings to dopamine D2-type receptors in anterior cingulate and insula of healthy control subjects but not methamphetamine-dependent individuals. Int. J. Neuropsychopharmacol. 2016, 19, pyv129. [Google Scholar] [CrossRef]
- Martinez, D.; Slifstein, M.; Narendran, R.; Foltin, R.W.; Broft, A.; Hwang, D.-R.; Perez, A.; Abi-Dargham, A.; Fischman, M.W.; Kleber, H.D. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology 2009, 34, 1774–1782. [Google Scholar] [CrossRef]
- Matuskey, D.; Gallezot, J.-D.; Pittman, B.; Williams, W.; Wanyiri, J.; Gaiser, E.; Lee, D.E.; Hannestad, J.; Lim, K.; Zheng, M.-Q. Dopamine D3 receptor alterations in cocaine-dependent humans imaged with [11C](+) PHNO. Drug Alcohol Depend. 2014, 139, 100–105. [Google Scholar] [CrossRef]
- Payer, D.E.; Behzadi, A.; Kish, S.J.; Houle, S.; Wilson, A.A.; Rusjan, P.M.; Tong, J.; Selby, P.; George, T.P.; McCluskey, T. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: A positron emission tomography study with [11C]-(+)-PHNO. Neuropsychopharmacology 2014, 39, 311–318. [Google Scholar] [CrossRef]
- Boileau, I.; Payer, D.; Houle, S.; Behzadi, A.; Rusjan, P.M.; Tong, J.; Wilkins, D.; Selby, P.; George, T.P.; Zack, M. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: A positron emission tomography study. J. Neurosci. 2012, 32, 1353–1359. [Google Scholar] [CrossRef]
- McGinnis, M.M.; Siciliano, C.A.; Jones, S.R. Dopamine D3 autoreceptor inhibition enhances cocaine potency at the dopamine transporter. J. Neurochem. 2016, 138, 821–829. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Newman, A.H. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann. N. Y. Acad. Sci. 2010, 1187, 4–34. [Google Scholar] [CrossRef]
- Newman, A.H.; Xi, Z.-X.; Heidbreder, C. Current perspectives on selective dopamine D3 receptor antagonists/partial agonists as pharmacotherapeutics for opioid and psychostimulant use disorders. In Therapeutic Applications of Dopamine D3 Receptor Function; Springer: Cham, Switzerland, 2022; pp. 157–201. [Google Scholar]
- Lynch, W.J.; Kiraly, D.D.; Caldarone, B.J.; Picciotto, M.R.; Taylor, J.R. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology 2007, 191, 263–271. [Google Scholar] [CrossRef]
- Nazarian, A.; Sun, W.-L.; Zhou, L.; Kemen, L.M.; Jenab, S.; Quinones-Jenab, V. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology 2009, 203, 641–650. [Google Scholar] [CrossRef]
- Schmitt, K.C.; Reith, M.E. Regulation of the dopamine transporter: Aspects relevant to psychostimulant drugs of abuse. Ann. N. Y. Acad. Sci. 2010, 1187, 316–340. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zheng, X.; Shang, L.; Zhan, C.G.; Zheng, F. Gender differences in cocaine-induced hyperactivity and dopamine transporter trafficking to the plasma membrane. Addict. Biol. 2022, 27, e13236. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Fordahl, S.C.; Jones, S.R. Cocaine self-administration produces long-lasting alterations in dopamine transporter responses to cocaine. J. Neurosci. 2016, 36, 7807–7816. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, C.A.; Saha, K.; Calipari, E.S.; Fordahl, S.C.; Chen, R.; Khoshbouei, H.; Jones, S.R. Amphetamine reverses escalated cocaine intake via restoration of dopamine transporter conformation. J. Neurosci. 2018, 38, 484–497. [Google Scholar] [CrossRef]
- Siciliano, C.A.; Ferris, M.J.; Jones, S.R. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur. J. Neurosci. 2015, 42, 2091–2096. [Google Scholar] [CrossRef]
- Siciliano, C.A.; Calipari, E.S.; Ferris, M.J.; Jones, S.R. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem. Neurosci. 2015, 6, 27–36. [Google Scholar] [CrossRef]
- Siciliano, C.A.; Mauterer, M.I.; Fordahl, S.C.; Jones, S.R. Modulation of striatal dopamine dynamics by cocaine self-administration and amphetamine treatment in female rats. Eur. J. Neurosci. 2019, 50, 2740–2749. [Google Scholar] [CrossRef]
- Malison, R.T.; Best, S.E.; Van Dyck, C.H.; McCance, E.F.; Wallace, E.A.; Laruelle, M.; Baldwin, R.; Seibyl, J.P.; Price, L.H.; Kosten, T.R. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] β-CIT SPECT. Am. J. Psychiatry 1998, 155, 832–834. [Google Scholar]
- Crits-Christoph, P.; Newberg, A.; Wintering, N.; Ploessl, K.; Gibbons, M.B.C.; Ring-Kurtz, S.; Gallop, R.; Present, J. Dopamine transporter levels in cocaine dependent subjects. Drug Alcohol Depend. 2008, 98, 70–76. [Google Scholar] [CrossRef]
- McCann, U.D.; Wong, D.F.; Yokoi, F.; Villemagne, V.; Dannals, R.F.; Ricaurte, G.A. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C] WIN-35,428. J. Neurosci. 1998, 18, 8417–8422. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Smith, L.; Fowler, J.S.; Telang, F.; Logan, J.; Tomasi, D. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. Neuroimage 2015, 121, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lv, R.; Brašić, J.R.; Han, M.; Liu, X.; Wang, Y.; Zhang, G.; Liu, C.; Li, Y.; Deng, Y. Dopamine transporter dysfunction in Han Chinese people with chronic methamphetamine dependence after a short-term abstinence. Psychiatry Res.: Neuroimaging 2014, 221, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Spronk, D.B.; van Wel, J.H.; Ramaekers, J.G.; Verkes, R.J. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci. Biobehav. Rev. 2013, 37, 1838–1859. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gainetdinov, R.R.; Wetsel, W.C.; Jones, S.R.; Bohn, L.M.; Miller, G.W.; Wang, Y.-M.; Caron, M.G. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat. Neurosci. 2000, 3, 465–471. [Google Scholar] [CrossRef]
- Anglin, J.C.; Brooks, V.L. Tyrosine hydroxylase and norepinephrine transporter in sympathetic ganglia of female rats vary with reproductive state. Auton. Neurosci. 2003, 105, 8–15. [Google Scholar] [CrossRef]
- Mayer, F.P.; Schmid, D.; Owens, W.A.; Gould, G.G.; Apuschkin, M.; Kudlacek, O.; Salzer, I.; Boehm, S.; Chiba, P.; Williams, P.H. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology 2018, 43, 2408–2417. [Google Scholar] [CrossRef]
- Clauss, N.J.; Koek, W.; Daws, L.C. Role of Organic Cation Transporter 3 and Plasma Membrane Monoamine Transporter in the Rewarding Properties and Locomotor Sensitizing Effects of Amphetamine in Male andFemale Mice. Int. J. Mol. Sci. 2021, 22, 13420. [Google Scholar] [CrossRef]
- Beaver, J.N.; Weber, B.L.; Ford, M.T.; Anello, A.E.; Kassis, S.K.; Gilman, T.L. Uncovering Functional Contributions of PMAT (Slc29a4) to Monoamine Clearance Using Pharmacobehavioral Tools. Cells 2022, 11, 1874. [Google Scholar] [CrossRef]
- Barreto, B.R.; D’Acunzo, P.; Ungania, J.M.; Das, S.; Hashim, A.; Goulbourne, C.N.; Canals-Baker, S.; Saito, M.; Saito, M.; Sershen, H.; et al. Cocaine Modulates the Neuronal Endosomal System and Extracellular Vesicles in a Sex-Dependent Manner. Neurochem. Res. 2022, 47, 2263–2277. [Google Scholar] [CrossRef]
- Lopez, A.J.; Johnson, A.R.; Euston, T.J.; Wilson, R.; Nolan, S.O.; Brady, L.J.; Thibeault, K.C.; Kelly, S.J.; Kondev, V.; Melugin, P.; et al. Cocaine self-administration induces sex-dependent protein expression in the nucleus accumbens. Commun. Biol. 2021, 4, 883. [Google Scholar] [CrossRef] [PubMed]
- Peterson, V.L.; Richards, J.B.; Meyer, P.J.; Cabrera-Rubio, R.; Tripi, J.A.; King, C.P.; Polesskaya, O.; Baud, A.; Chitre, A.S.; Bastiaanssen, T.F. Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. EBioMedicine 2020, 55, 102769. [Google Scholar] [CrossRef]
- Fox, H.C.; Sinha, R. Sex differences in drug-related stress-system changes: Implications for treatment in substance-abusing women. Harv. Rev. Psychiatry 2009, 17, 103–119. [Google Scholar] [CrossRef]
- Smith, K.; Lacadie, C.M.; Milivojevic, V.; Fogelman, N.; Sinha, R. Sex Differences in Neural Responses to Stress and Drug Cues Predicts Future Drug Use in Individuals with Substance Use Disorder. Drug Alcohol Depend. 2023, 244, 109794. [Google Scholar] [CrossRef]
- Jameson, A.N.; Siemann, J.K.; Melchior, J.; Calipari, E.S.; McMahon, D.G.; Grueter, B.A. Photoperiod Impacts Nucleus Accumbens Dopamine Dynamics. Eneuro 2023, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.K.; Wolkowicz, N.R.; De Aquino, J.P.; MacLean, R.R.; Sofuoglu, M. Cocaine Use Disorder (CUD): Current Clinical Perspectives. Subst. Abus. Rehabil. 2022, 13, 25–46. [Google Scholar] [CrossRef]
- Buchholz, J.; Saxon, A.J. Medications to treat cocaine use disorders: Current options. Curr. Opin. Psychiatry 2019, 32, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hersey, M.; Bacon, A.K.; Bailey, L.G.; Coggiano, M.A.; Newman, A.H.; Leggio, L.; Tanda, G. Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap? Front. Neurosci. 2021, 15, 656475. [Google Scholar] [CrossRef]
- McKee, S.A.; McRae-Clark, A.L. Consideration of sex and gender differences in addiction medication response. Biol. Sex Differ. 2022, 13, 34. [Google Scholar] [CrossRef]
- Schmitt, K.C.; Reith, M.E.A. The Atypical Stimulant and Nootropic Modafinil Interacts with the Dopamine Transporter in a Different Manner than Classical Cocaine-Like Inhibitors. PLoS ONE 2011, 6, e25790. [Google Scholar] [CrossRef]
- Loland, C.J.; Mereu, M.; Okunola, O.M.; Cao, J.; Prisinzano, T.E.; Mazier, S.; Kopajtic, T.; Shi, L.; Katz, J.L.; Tanda, G. R-modafinil (armodafinil): A unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol. Psychiatry 2012, 72, 405–413. [Google Scholar] [CrossRef]
- Rahbek-Clemmensen, T.; Lycas, M.D.; Erlendsson, S.; Eriksen, J.; Apuschkin, M.; Vilhardt, F.; Jørgensen, T.N.; Hansen, F.H.; Gether, U. Super-resolution microscopy reveals functional organization of dopamine transporters into cholesterol and neuronal activity-dependent nanodomains. Nat. Commun. 2017, 8, 740. [Google Scholar] [CrossRef]
- Lycas, M.D.; Ejdrup, A.L.; Sørensen, A.T.; Haahr, N.O.; Jørgensen, S.H.; Guthrie, D.A.; Støier, J.F.; Werner, C.; Newman, A.H.; Sauer, M. Nanoscopic dopamine transporter distribution and conformation are inversely regulated by excitatory drive and D2 autoreceptor activity. Cell Rep. 2022, 40, 111431. [Google Scholar] [CrossRef] [PubMed]
- Keighron, J.D.; Giancola, J.B.; Shaffer, R.J.; DeMarco, E.M.; Coggiano, M.A.; Slack, R.D.; Newman, A.H.; Tanda, G. Distinct effects of (R)-modafinil and its (R)-and (S)-fluoro-analogs on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats. Eur. J. Neurosci. 2019, 50, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Abramyan, A.M.; Stolzenberg, S.; Li, Z.; Loland, C.J.; Noe, F.; Shi, L. The Isomeric Preference of an Atypical Dopamine Transporter Inhibitor Contributes to Its Selection of the Transporter Conformation. ACS Chem. Neurosci. 2017, 8, 1735–1746. [Google Scholar] [CrossRef]
- Newman, A.H.; Ku, T.; Jordan, C.J.; Bonifazi, A.; Xi, Z.-X. New drugs, old targets: Tweaking the dopamine system to treat psychostimulant use disorders. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Hersey, M.; Hempel, B.; Xi, Z.X.; Newman, A.H. Modafinil and its structural analogs as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder. Curr. Opin. Pharmacol. 2021, 56, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Keighron, J.D.; Quarterman, J.C.; Cao, J.; DeMarco, E.M.; Coggiano, M.A.; Gleaves, A.; Slack, R.D.; Zanettini, C.; Newman, A.H.; Tanda, G. Effects of (R)-modafinil and modafinil analogues on dopamine dynamics assessed by voltammetry and microdialysis in the mouse nucleus accumbens shell. ACS Chem. Neurosci. 2019, 10, 2012–2021. [Google Scholar] [CrossRef]
- Newman, A.H.; Cao, J.; Keighron, J.D.; Jordan, C.J.; Bi, G.-H.; Liang, Y.; Abramyan, A.M.; Avelar, A.J.; Tschumi, C.W.; Beckstead, M.J. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology 2019, 44, 1435–1444. [Google Scholar] [CrossRef]
- Ferraro, L.; Fuxe, K.; Tanganelli, S.; Fernandez, M.; Rambert, F.; Antonelli, T. Amplification of cortical serotonin release: A further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology 2000, 39, 1974–1983. [Google Scholar] [CrossRef]
- de Saint Hilaire, Z.; Orosco, M.; Rouch, C.; Blanc, G.; Nicolaidis, S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: A microdialysis study in rats. Neuroreport 2001, 12, 3533–3537. [Google Scholar] [CrossRef]
- Madras, B.K.; Xie, Z.; Lin, Z.; Jassen, A.; Panas, H.; Lynch, L.; Johnson, R.; Livni, E.; Spencer, T.J.; Bonab, A.A.; et al. Modafinil Occupies Dopamine and Norepinephrine Transporters in Vivo and Modulates the Transporters and Trace Amine Activity in Vitro. J. Pharmacol. Exp. Ther. 2006, 319, 561. [Google Scholar] [CrossRef] [PubMed]
- Tanda, G.; Pontieri, F.E.; Frau, R.; Di Chiara, G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur. J. Neurosci. 1997, 9, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, H.P.; Desaiah, D.; Schuh, K.J.; Bymaster, F.P.; Kallman, M.J.; Clarke, D.O.; Durell, T.M.; Trzepacz, P.T.; Calligaro, D.O.; Nisenbaum, E.S. A review of the abuse potential assessment of atomoxetine: A nonstimulant medication for attention-deficit/hyperactivity disorder. Psychopharmacology 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Wee, S.; Woolverton, W.L. Evaluation of the reinforcing effects of atomoxetine in monkeys: Comparison to methylphenidate and desipramine. Drug Alcohol Depend. 2004, 75, 271–276. [Google Scholar] [CrossRef]
- Hersey, M.; Chen, A.Y.; Bartole, M.K.; Anand, J.; Newman, A.H.; Tanda, G. An FSCV study on the effects of targeted typical and atypical DAT inhibition on dopamine dynamics in the nucleus accumbens shell of male and female mice. ACS Chem. Neurosci. 2023, in press. [Google Scholar]
- Mereu, M.; Hiranita, T.; Jordan, C.J.; Chun, L.E.; Lopez, J.P.; Coggiano, M.A.; Quarterman, J.C.; Bi, G.H.; Keighron, J.D.; Xi, Z.X.; et al. Modafinil potentiates cocaine self-administration by a dopamine-independent mechanism: Possible involvement of gap junctions. Neuropsychopharmacology 2020, 45, 1518–1526. [Google Scholar] [CrossRef]
- Mereu, M.; Chun, L.E.; Prisinzano, T.E.; Newman, A.H.; Katz, J.L.; Tanda, G. The unique psychostimulant profile of (±)-modafinil: Investigation of behavioral and neurochemical effects in mice. Eur. J. Neurosci. 2017, 45, 167–174. [Google Scholar] [CrossRef]
- Shuman, T.; Cai, D.J.; Sage, J.R.; Anagnostaras, S.G. Interactions between modafinil and cocaine during the induction of conditioned place preference and locomotor sensitization in mice: Implications for addiction. Behav. Brain Res. 2012, 235, 105–112. [Google Scholar] [CrossRef]
- Bernardi, R.E.; Broccoli, L.; Spanagel, R.; Hansson, A.C. Sex differences in dopamine binding and modafinil conditioned place preference in mice. Drug Alcohol Depend. 2015, 155, 37–44. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Logan, J.; Alexoff, D.; Zhu, W.; Telang, F.; Wang, G.-J.; Jayne, M.; Hooker, J.M.; Wong, C.; et al. Effects of Modafinil on Dopamine and Dopamine Transporters in the Male Human Brain: Clinical Implications. JAMA 2009, 301, 1148–1154. [Google Scholar] [CrossRef]
- Kate, N.; Grover, S.; Ghormode, D. Dependence on supratherapeutic doses of modafinil: A case report. Prim. Care Companion CNS Disord. 2012, 14, 26219. [Google Scholar] [CrossRef]
- Ozturk, A.; Deveci, E. Drug Abuse of Modafinil by a Cannabis User. Bull. Clin. Psychopharmacol. 2014, 24, 405–407. [Google Scholar] [CrossRef]
- Krishnan, R.; Chary, K.V. A rare case modafinil dependence. J. Pharmacol. Pharmacother. 2015, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Mereu, M.; Bonci, A.; Newman, A.H.; Tanda, G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 2013, 229, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Rush, C.R.; Kelly, T.H.; Hays, L.R.; Wooten, A.F. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002, 67, 311–322. [Google Scholar] [CrossRef]
- Rush, C.R.; Kelly, T.H.; Hays, L.R.; Baker, R.W.; Wooten, A.F. Acute behavioral and physiological effects of modafinil in drug abusers. Behav. Pharmacol. 2002, 13, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Vosburg, S.K.; Hart, C.L.; Haney, M.; Rubin, E.; Foltin, R.W. Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend. 2010, 106, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Sangroula, D.; Motiwala, F.; Wagle, B.; Shah, V.C.; Hagi, K.; Lippmann, S. Modafinil Treatment of Cocaine Dependence: A Systematic Review and Meta-Analysis. Subst. Use Misuse 2017, 52, 1292–1306. [Google Scholar] [CrossRef]
- Cephalon, F. Approved Labeling Text for NDA 20-717/S-005 & S008. Provigil®(modafinil) Tablets [C-IV], Approved 2004.
- Spencer, T.J.; Madras, B.K.; Bonab, A.A.; Dougherty, D.D.; Clarke, A.; Mirto, T.; Martin, J.; Fischman, A.J. A positron emission tomography study examining the dopaminergic activity of armodafinil in adults using [11C]altropane and [11C]raclopride. Biol. Psychiatry 2010, 68, 964–970. [Google Scholar] [CrossRef]
- Wang, X.-F.; Bi, G.-H.; He, Y.; Yang, H.-J.; Gao, J.-T.; Okunola-Bakare, O.M.; Slack, R.D.; Gardner, E.L.; Xi, Z.-X.; Newman, A.H. R-modafinil attenuates nicotine-taking and nicotine-seeking behavior in alcohol-preferring rats. Neuropsychopharmacology 2015, 40, 1762–1771. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Bi, G.H.; Yang, H.J.; He, Y.; Xue, G.; Cao, J.; Tanda, G.; Gardner, E.L.; Newman, A.H.; Xi, Z.X. The Novel Modafinil Analog, JJC8-016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacology 2017, 42, 1871–1883. [Google Scholar] [CrossRef]
- Cao, J.; Prisinzano, T.E.; Okunola, O.M.; Kopajtic, T.; Shook, M.; Katz, J.L.; Newman, A.H. SARs at the monoamine transporters for a novel series of modafinil analogues. ACS Med. Chem. Lett. 2011, 2, 48–52. [Google Scholar] [CrossRef]
- Cao, J.; Slack, R.D.; Bakare, O.M.; Burzynski, C.; Rais, R.; Slusher, B.S.; Kopajtic, T.; Bonifazi, A.; Ellenberger, M.P.; Yano, H. Novel and high affinity 2-[(diphenylmethyl) sulfinyl] acetamide (modafinil) analogues as atypical dopamine transporter inhibitors. J. Med. Chem. 2016, 59, 10676–10691. [Google Scholar] [CrossRef]
- Slack, R.D.; Ku, T.C.; Cao, J.; Giancola, J.B.; Bonifazi, A.; Loland, C.J.; Gadiano, A.; Lam, J.; Rais, R.; Slusher, B.S.; et al. Structure-Activity Relationships for a Series of (Bis(4-fluorophenyl)methyl)sulfinyl Alkyl Alicyclic Amines at the Dopamine Transporter: Functionalizing the Terminal Nitrogen Affects Affinity, Selectivity, and Metabolic Stability. J. Med. Chem. 2020, 63, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Giancola, J.B.; Bonifazi, A.; Cao, J.; Ku, T.; Haraczy, A.J.; Lam, J.; Rais, R.; Coggiano, M.A.; Tanda, G.; Newman, A.H. Structure-activity relationships for a series of (Bis(4-fluorophenyl)methyl)sulfinylethyl-aminopiperidines and -piperidine amines at the dopamine transporter: Bioisosteric replacement of the piperazine improves metabolic stability. Eur. J. Med. Chem. 2020, 208, 112674. [Google Scholar] [CrossRef]
- Tunstall, B.J.; Ho, C.P.; Cao, J.; Vendruscolo, J.C.; Schmeichel, B.E.; Slack, R.D.; Tanda, G.; Gadiano, A.J.; Rais, R.; Slusher, B.S. Atypical dopamine transporter inhibitors attenuate compulsive-like methamphetamine self-administration in rats. Neuropharmacology 2018, 131, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, O.; Cao, J.; Lam, J.; Childers, S.R.; Rais, R.; Porrino, L.J.; Newman, A.H.; Nader, M.A. The Effects of the Dopamine Transporter Ligands JJC8-088 and JJC8-091 on Cocaine versus Food Choice in Rhesus Monkeys. J. Pharmacol. Exp. Ther. 2023, 384, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Sartori, S.B.; Murau, R.; Kopel, F.; Kalaba, P.; Dragačević, V.; Leban, J.J.; Singewald, N.; Engelmann, M.; Lubec, G. The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice. Biomolecules 2022, 12, 506. [Google Scholar] [CrossRef]
- Lubec, J.; Kalaba, P.; Hussein, A.M.; Feyissa, D.D.; Kotob, M.H.; Mahmmoud, R.R.; Wieder, O.; Garon, A.; Sagheddu, C.; Ilic, M.; et al. Reinstatement of synaptic plasticity in the aging brain through specific dopamine transporter inhibition. Mol. Psychiatry 2021, 26, 7076–7090. [Google Scholar] [CrossRef]
- Kearney, P.J.; Bolden, N.C.; Kahuno, E.; Conklin, T.L.; Martin, G.E.; Lubec, G.; Melikian, H.E. Presynaptic Gq-coupled receptors drive biphasic dopamine transporter trafficking that modulates dopamine clearance and motor function. J. Biol. Chem. 2023, 299, 102900. [Google Scholar] [CrossRef]
- Kouhnavardi, S.; Ecevitoglu, A.; Dragačević, V.; Sanna, F.; Arias-Sandoval, E.; Kalaba, P.; Kirchhofer, M.; Lubec, J.; Niello, M.; Holy, M.; et al. A Novel and Selective Dopamine Transporter Inhibitor, (S)-MK-26, Promotes Hippocampal Synaptic Plasticity and Restores Effort-Related Motivational Dysfunctions. Biomolecules 2022, 12, 881. [Google Scholar] [CrossRef] [PubMed]
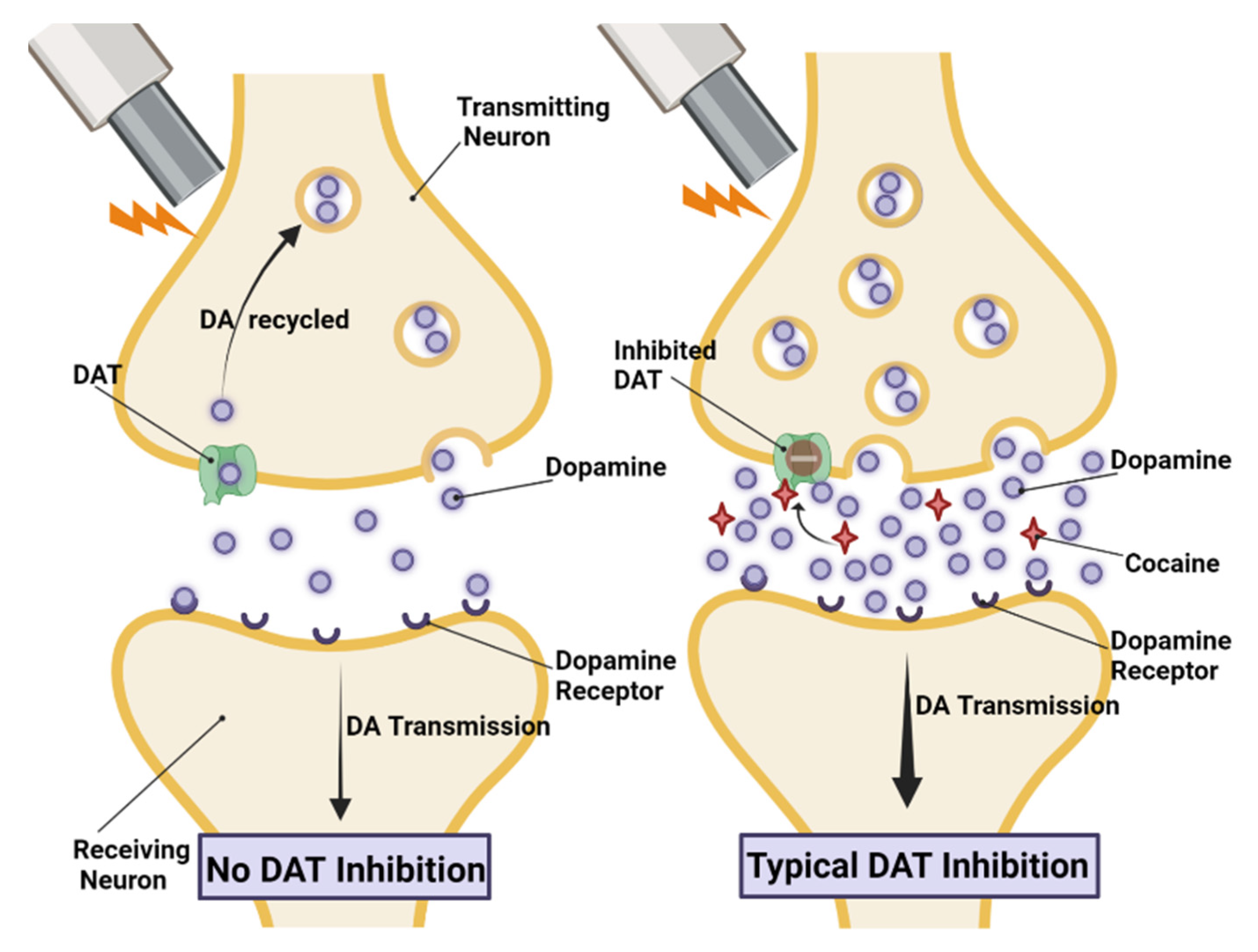
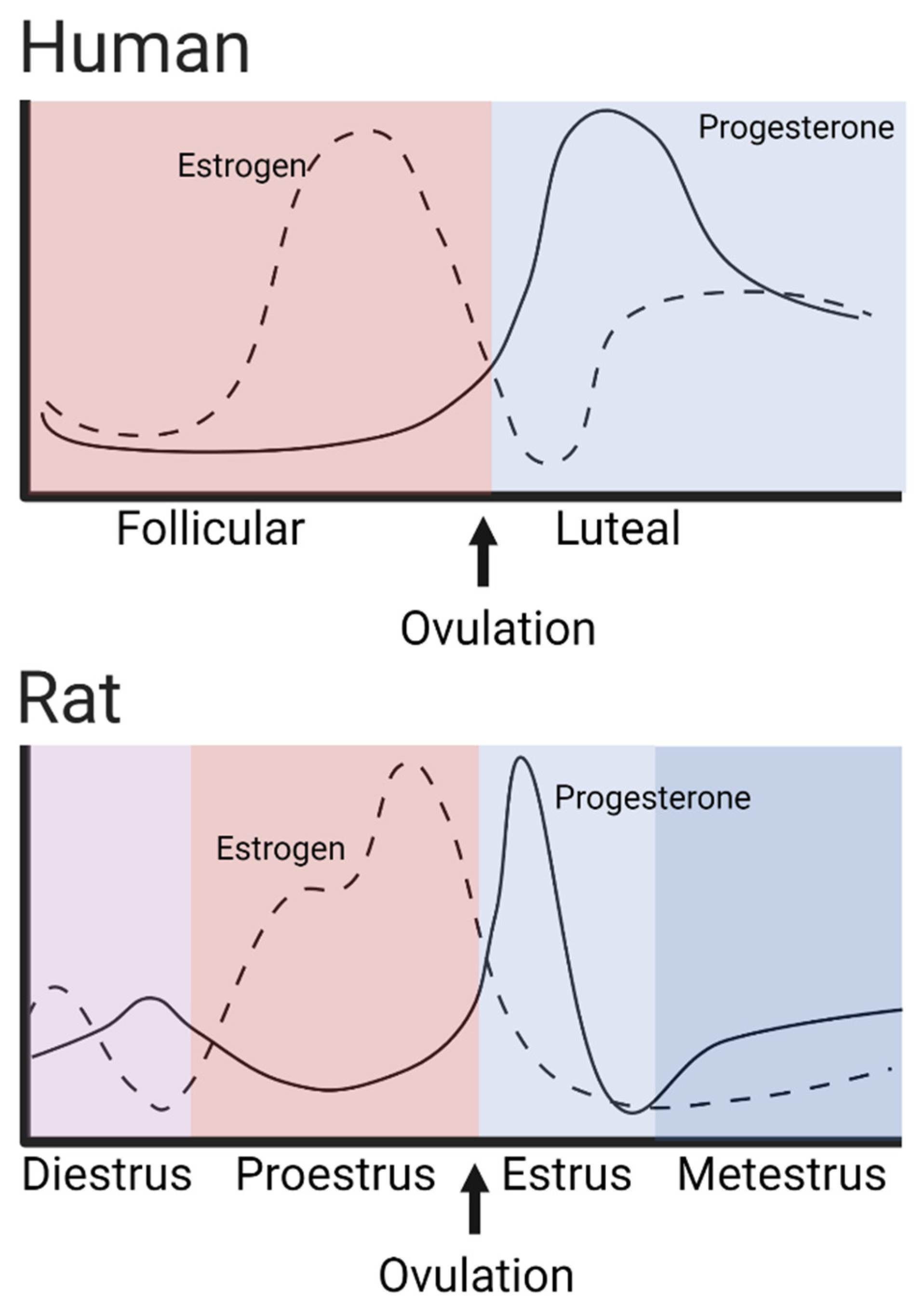
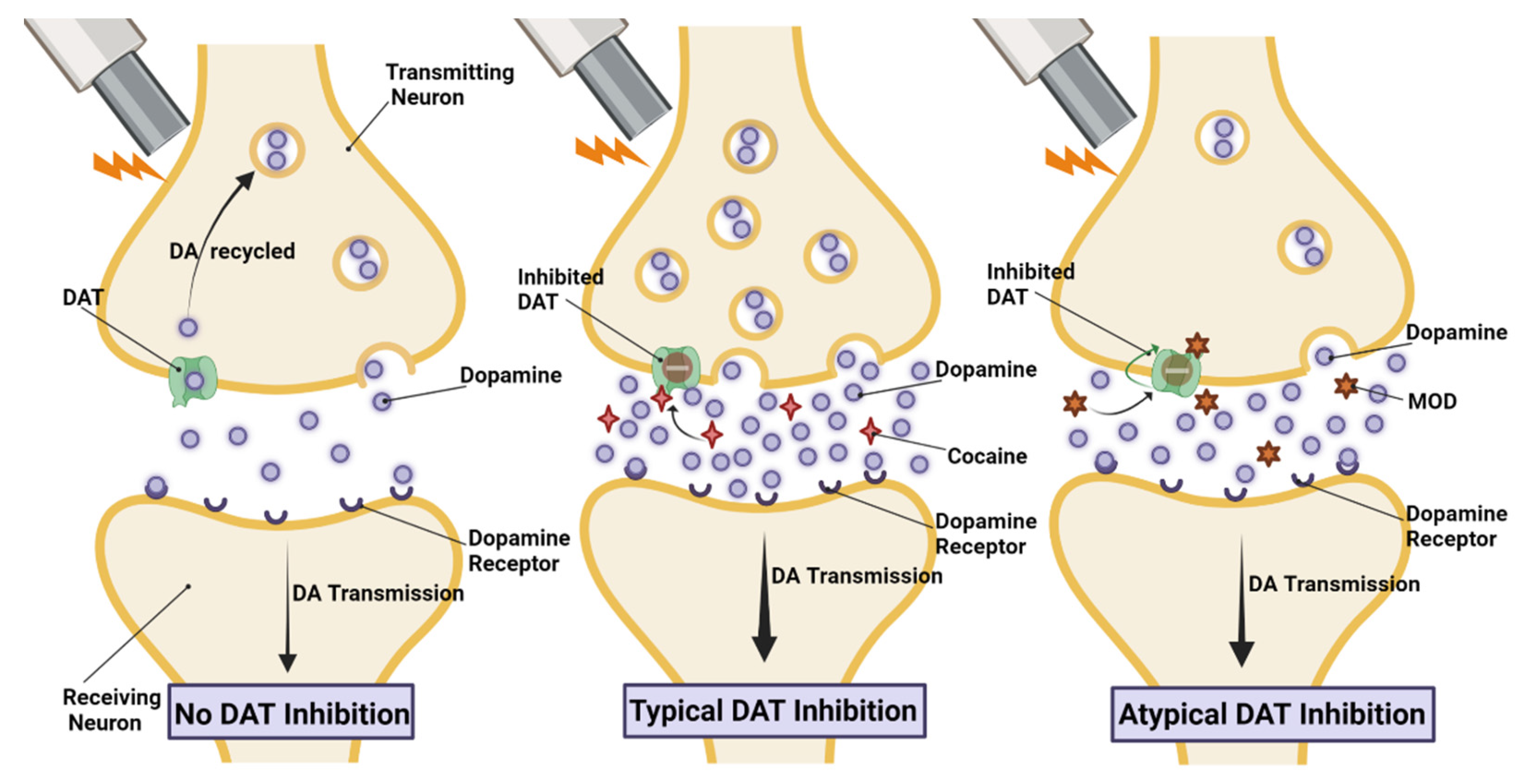
| Effect Type | Species | Sex-Dependent Finding | Reference |
|---|---|---|---|
| Extracellular DA | Rat | - Ovariectomized female rats have lower extracellular striatal DA concentrations than castrated male rats. | [60] |
| Rat | - DA extracellular levels are sensitive to changes in the female estrous cycle, and the levels of extracellular striatal DA were highest during proestrus and estrus phases of the estrous cycle. | [63] | |
| Rat | - Electrical stimulation produced significantly more DA release and extracellular concentration in female rats compared to male rats. | [61] | |
| Mice | - No significant differences on average in basal extracellular striatal DA in male and female mice. However, female mice have highest extracellular DA during the proestrus phase of the estrous cycle and lowest during diestrus in the striatum. | [62] | |
| DA neurons | Rat | - The VTA of female rats contained significantly higher numbers of tyrosine hydroxylase immunoreactive cells (DA neurons) as compared to male rats. - Total volume of the VTA was found to be larger in female rats compared to male rats. | [55] |
| Rat | - Female rats have a higher proportion of dopaminergic cells in mesocortical projections than male rats. | [54] | |
| Rat | - No sex differences were observed in DA neuron activity in the VTA at baseline. | [56] | |
| Rat | - VTA DA activity was consistent in male and female rats. | [57] | |
| Mice | - Observed significant effects of female estrous cycle on VTA DA neuron excitability. | [59] | |
| DA neurons + DA dynamics | Rat | - VTA DA neuron basal firing rates are statistically different throughout the estrous cycle (greatest in the estrus phase and least in the proestrus phase). - The inhibition of VTA DA neuron firing with acute cocaine administration was greater in the proestrus phase than estrus phase. | [58] |
| DA dynamics | Rat | - Estradiol administration increased DA turnover in ovariectomized rats. | [69] |
| Rat | - DAT density decreased in the nucleus accumbens and the striatum following ovariectomy in female rats. | [87] | |
| Rat | - DA uptake was significantly slowed following estradiol administration to ovariectomized female rats using voltammetry. | [70] | |
| Mice | - VTA DA neurons in female mice in high estradiol phases of the estrous cycle were more sensitive to excitation (via ethanol exposure) and inhibition (via DA exposure). | [66] | |
| Rat | - Estradiol enhanced cocaine-induced increases in NAS DA release in female gonadectomized rats in contrast to male gonadectomized rats. - Estradiol blunted cocaine-induced slowing of DA clearance in the NAS in male, not female rats. | [67] | |
| Rat | - Electrically evoked DA release in the nucleus accumbens core is greater in adult male rats than male adolescent rats. - Electrically evoked DA release in the striatum (NAS, nucleus accumbens core, and dorsomedial striatum) is greater in female adolescent rats than female adult rats. - DA autoreceptor regulation varies over lifespan. | [71] | |
| Mice | - Spontaneous DA dynamics signals were larger in the dorsal striatum of male compared to female mice, consistent in the nucleus accumbens core, and lower in the NAS of female versus male mice using voltammetry. | [64] | |
| DA dynamics + DA receptors | Mice | - High estradiol stages of the estrous cycle in female mice are associated with increased activity of VTA DA neurons, increased DAT phosphorylation, and decreased D2R autoreceptor activity. - Female mice in high estradiol levels of the estrous cycle show increased cocaine-conditioned place preference and cocaine-induced increases in nucleus accumbens DA electrically evoked release. | [65] |
| Rat | - Ovariectomized female rats with estradiol implants, compared to ovariectomized controls, had decreased D3R mRNA in the midbrain, increased D3R in the VTA, increased D1R in the hypothalamus, and increased D2LR in the midbrain. No changes in DAT mRNA were observed. | [83] | |
| Rat | - Ovariectomized female rats had decreased DAT density in the nucleus accumbens compared to intact controls and this effect was reversed with estradiol treatment. - Ovariectomized female rats had increased D2R density in the nucleus accumbens and caudate compared to intact controls and this effect was reversed with estradiol treatment. | [86] | |
| DA receptors | Rat | - Male rats administered estrogen exhibited increased striatal DA receptor binding sites. | [76] |
| Rat | - Estradiol administration in ovariectomized female rats increased DA turnover and conversion of high to low agonist binding sites on D2R. | [77] | |
| Rat | - Estradiol administration in ovariectomized female rats increased D1R density in the striatum. | [81] | |
| Rat | - There are sex- and region-dependent differences in D2R binding in gonadectomized rats following estradiol administration. | [80] | |
| Rat | - Female rats showed lower levels of overproduction and elimination of striatal D1R and D2R. - Adult male and female rats did not show differences in D1R and D2R densities in the striatum. - Male D1R overexpression and adult densities were higher than female rats in the nucleus accumbens. | [74] | |
| Rat | - Sexually dimorphic effects of D1R agonist (SFK-81297); males showed higher inhibitory phase effects and females showed higher stimulatory phase effects. | [79] | |
| Rat | - Cocaine-induced increases in electrically evoked striatal DA are higher in female compared to male rats. - Haloperidol (D2R ligand)-induced increases in electrically evoked striatal DA are higher in female compared to male rats. - Quinpirole (D3R/D2R agonist)-induced increases in electrically evoked striatal DA were only observed in female rats. | [82] | |
| California mouse | - At baseline, male and female mice did not show differences in D1R or D2R mRNA expression in the nucleus accumbens. | [78] | |
| Rat and Monkey | - In rats, females had lower D1R expression than males, females had higher D1R-D2R heteromer complexes, and no sex differences in D2R expression were observed in the striatum. - In monkeys, males had lower densities of D1R-D2R heteromer complexes than females in the caudate nucleus. | [75] |
| Species | Behavioral Effects | Neurochemical Effects | Reference |
|---|---|---|---|
| Modafinil (MOD) | |||
| Human | - Cocaine-like subjective effects were not produced by MOD | [179] | |
| Human | - MOD abuse potential appears limited due to its lack of cocaine-like drug effects (self-reported high) | [180] | |
| Human | - MOD inhibited DAT and increased brain DA levels | [174] | |
| Human | - Cocaine-like subjective effects were not produced by MOD | [181] | |
| In vitro/monkey | - MOD occupied brain DAT and NET measured by positron emission tomography | [165] | |
| Rats | - MOD did not sustain self-administration in cocaine-dependent rats -MOD administration potentiated cocaine self-administration | - MOD administration increased DA extracellular levels in the NAS - MOD administration did not alter cocaine-induced stimulation of NAS DA levels | [170] |
| Mice | - MOD did not increase locomotor activity - MOD induced conditioned place preference | [172] | |
| Mice | - Conditioned place preference was observed only in female mice following a low dose of MOD - No observed sex differences on locomotor activity following acute and chronic MOD administration | - Brain-region-dependent sex differences in D1R, D2R, and DAT binding availability in response to MOD administration | [173] |
| Mice | - MOD generalized with cocaine subjective effects in drug discrimination studies | - MOD administration increased DA extracellular levels in the nucleus accumbens core and shell | [171] |
| R-Modafinil (R-MOD) | |||
| Human | - R-MOD was found to significantly occupy DAT in the striatum - R-MOD increased extracellular striatal DA | [184] | |
| Rat | - R-MOD blocked nicotine self-administration | - R-MOD administration increased extracellular DA levels - R-MOD administration blunted nicotine-induced increases in NAS DA levels | [185] |
| Rat | - R-MOD administration increased locomotion - High dose of R-MOD inhibited cocaine-induced reinstatement | [186] | |
| Rat | - R-MOD administration increased extracellular DA levels, increased maximum evoked DA following electrical stimulation, and decreased rate of DA clearance in the NAS | [157] | |
| Mice | - R-MOD administration produced increases in DA efflux in the NAS | [154] | |
| Mice | - R-MOD administration increased extracellular DA levels, increased maximum evoked DA following electrical stimulation, and decreased rate of DA clearance in the NAS | [161] | |
| Mice | - R-MOD administration produced increased maximum evoked DA following electrical stimulation and decreased rate of DA clearance in the NAS | [20] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hersey, M.; Bartole, M.K.; Jones, C.S.; Newman, A.H.; Tanda, G. Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors. Molecules 2023, 28, 5270. https://doi.org/10.3390/molecules28135270
Hersey M, Bartole MK, Jones CS, Newman AH, Tanda G. Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors. Molecules. 2023; 28(13):5270. https://doi.org/10.3390/molecules28135270
Chicago/Turabian StyleHersey, Melinda, Mattingly K. Bartole, Claire S. Jones, Amy Hauck Newman, and Gianluigi Tanda. 2023. "Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors" Molecules 28, no. 13: 5270. https://doi.org/10.3390/molecules28135270
APA StyleHersey, M., Bartole, M. K., Jones, C. S., Newman, A. H., & Tanda, G. (2023). Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors. Molecules, 28(13), 5270. https://doi.org/10.3390/molecules28135270







