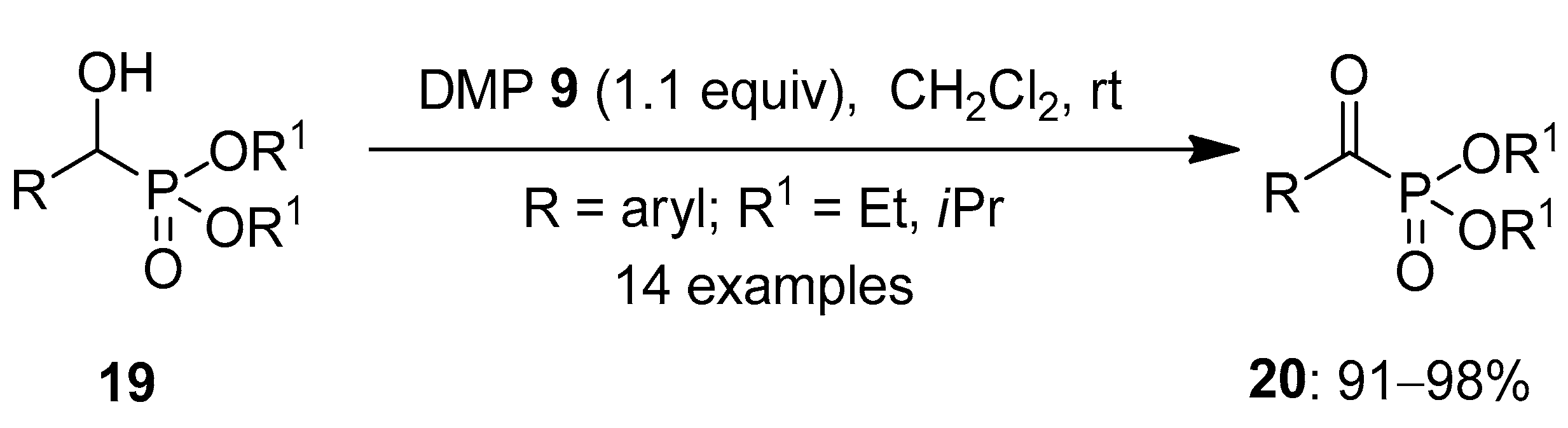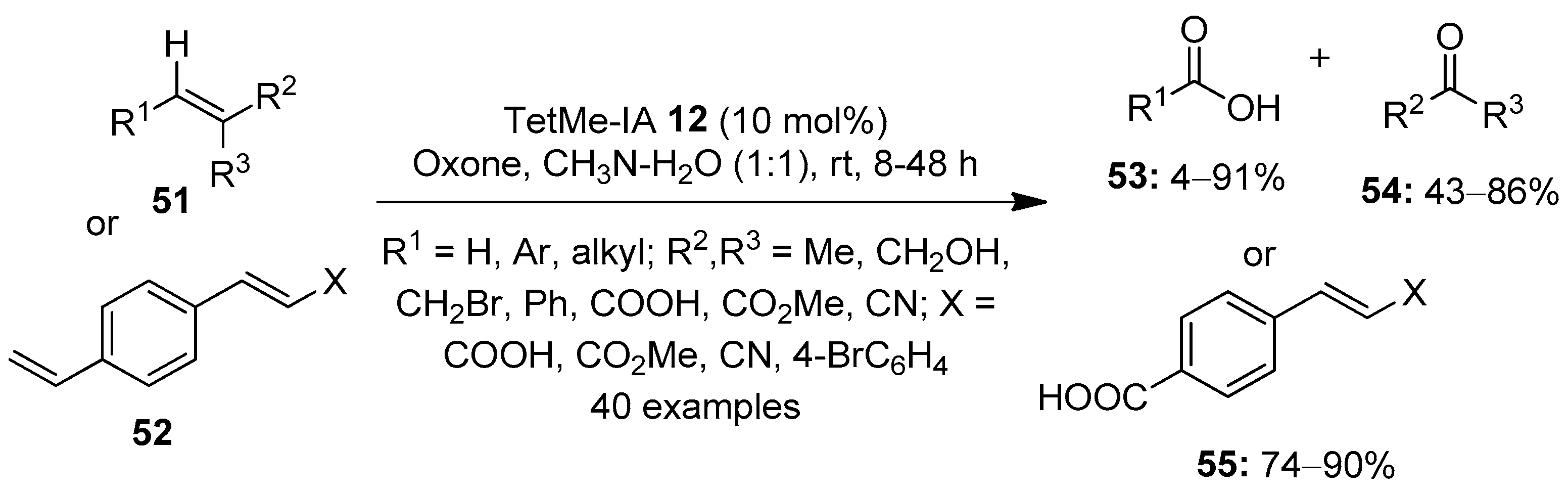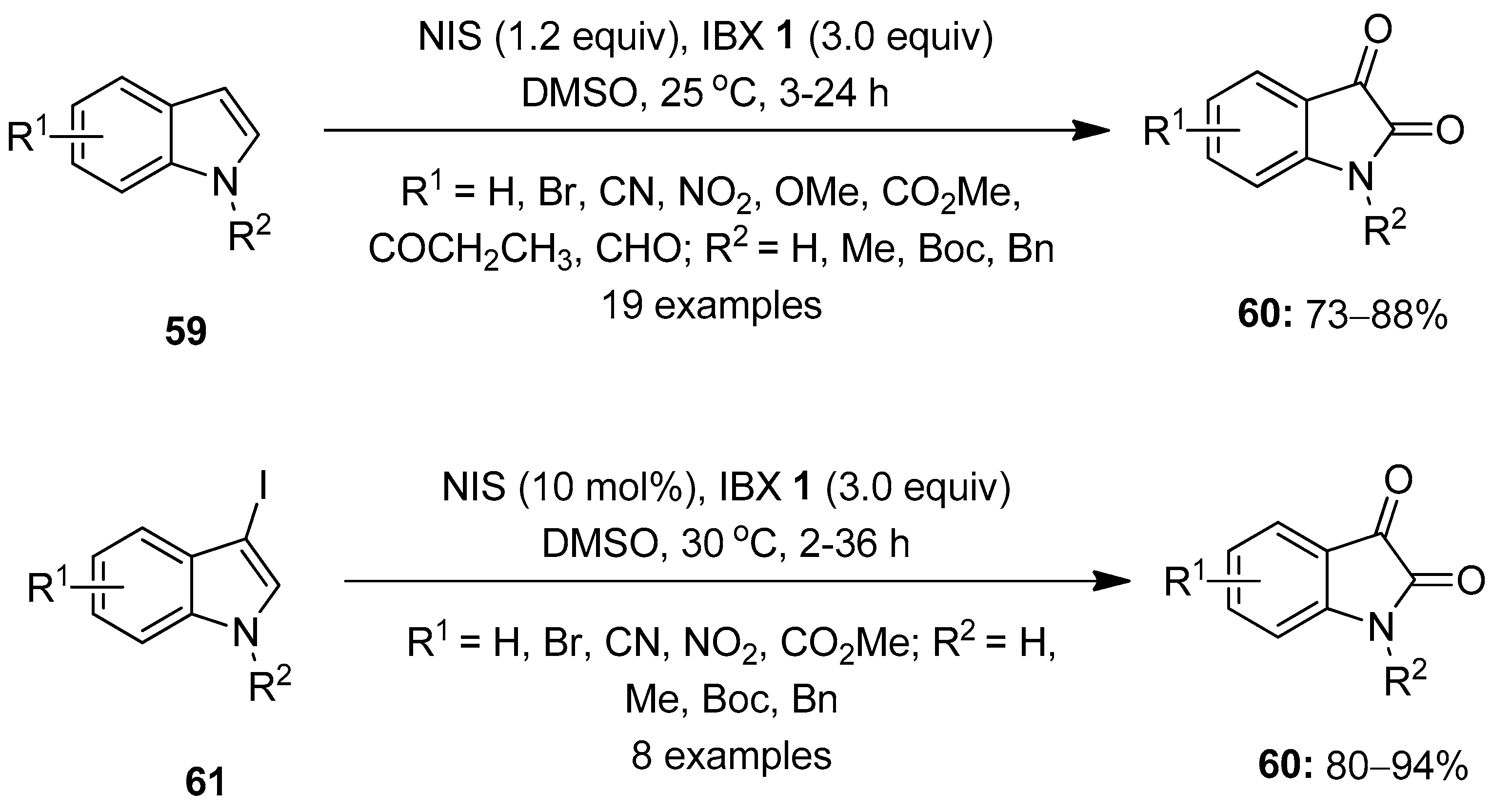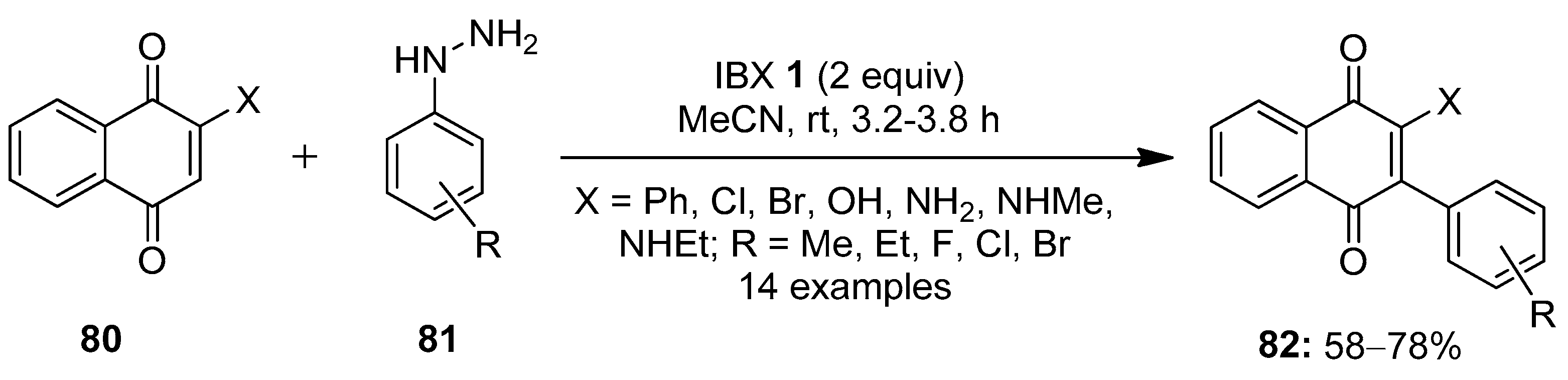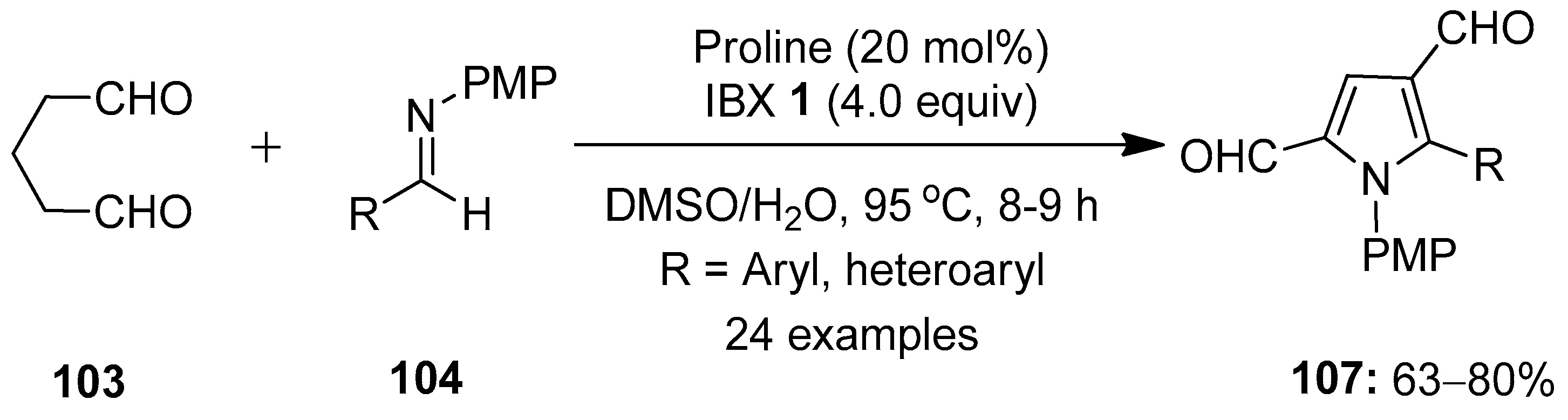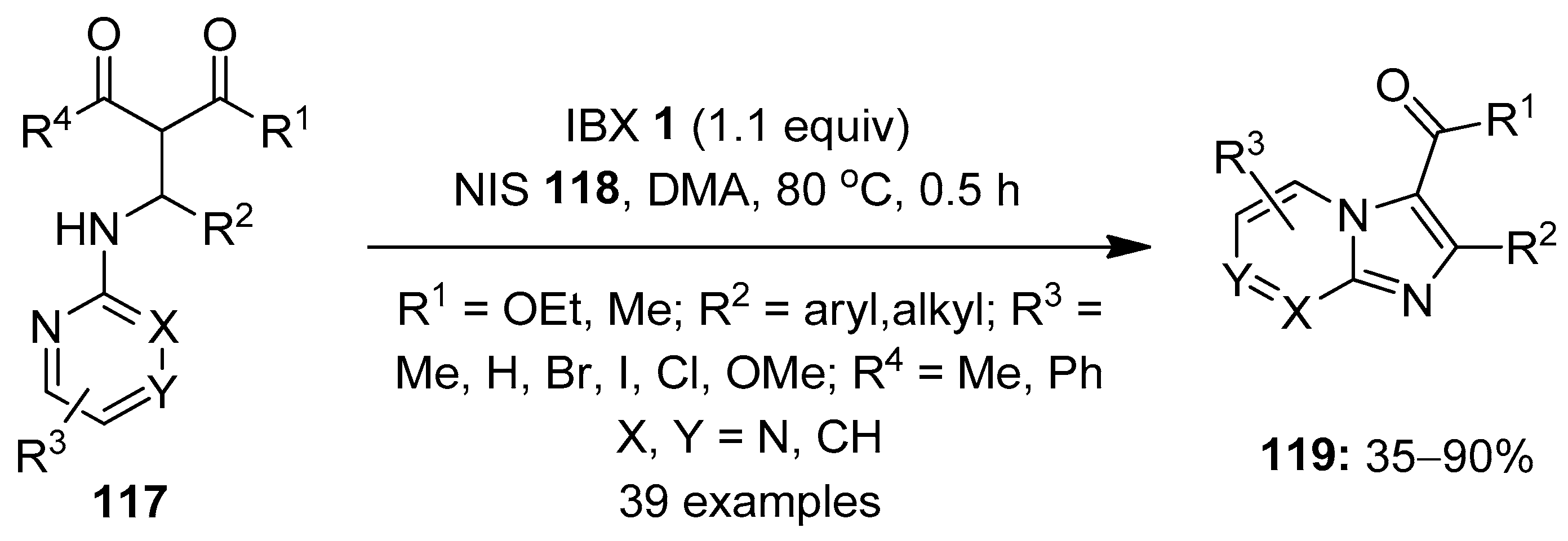Abstract
The chemistry of hypervalent iodine reagents has now become quite valuable due to the reactivity of these compounds under mild reaction conditions and their resemblance in chemical properties to transition metals. The environmentally friendly nature of these reagents makes them suitable for Green Chemistry. Reagents with a dual nature, such as iodine(III) reagents, are capable electrophiles, while iodine(V) reagents are known for their strong oxidant behavior. Various iodine(V) reagents including IBX and DMP have been used as oxidants in organic synthesis either in stoichiometric or in catalytic amounts. In this review article, we describe various oxidation reactions induced by iodine(V) reagents reported in the past decade.
1. Introduction
Hypervalent iodine reagents are environment friendly tools for the construction of simple and complex organic molecules [1,2,3,4,5,6,7,8]. These reagents are potential oxidants due to their excellent oxidizing and electrophilic properties [9,10,11,12,13]. The unique characteristics attributed to these reagents are non-toxicity, easy handling, high reactivity and stability, combined with good site selectivity and broad applicability in several synthetic transformations [14,15,16]. Therefore, hypervalent iodine reagents are of paramount importance to organic chemistry for the development of new asymmetric and non-asymmetric reactions. In particular, hypervalent iodine compounds are the reagents of choice for oxidation reactions [17,18,19,20], cyclizations [21,22,23], rearrangements [24,25,26], a-functionalization of carbonyl compounds [27,28], atom-transfer reactions [29] and alkene difunctionalizations reactions [30,31]. Recently, the application of these reagents has been successfully expanded to organocatalysis [32,33,34,35,36,37], C−H bond functionalization [38], stereoselective synthesis [39,40] and photochemical reactions [41,42].
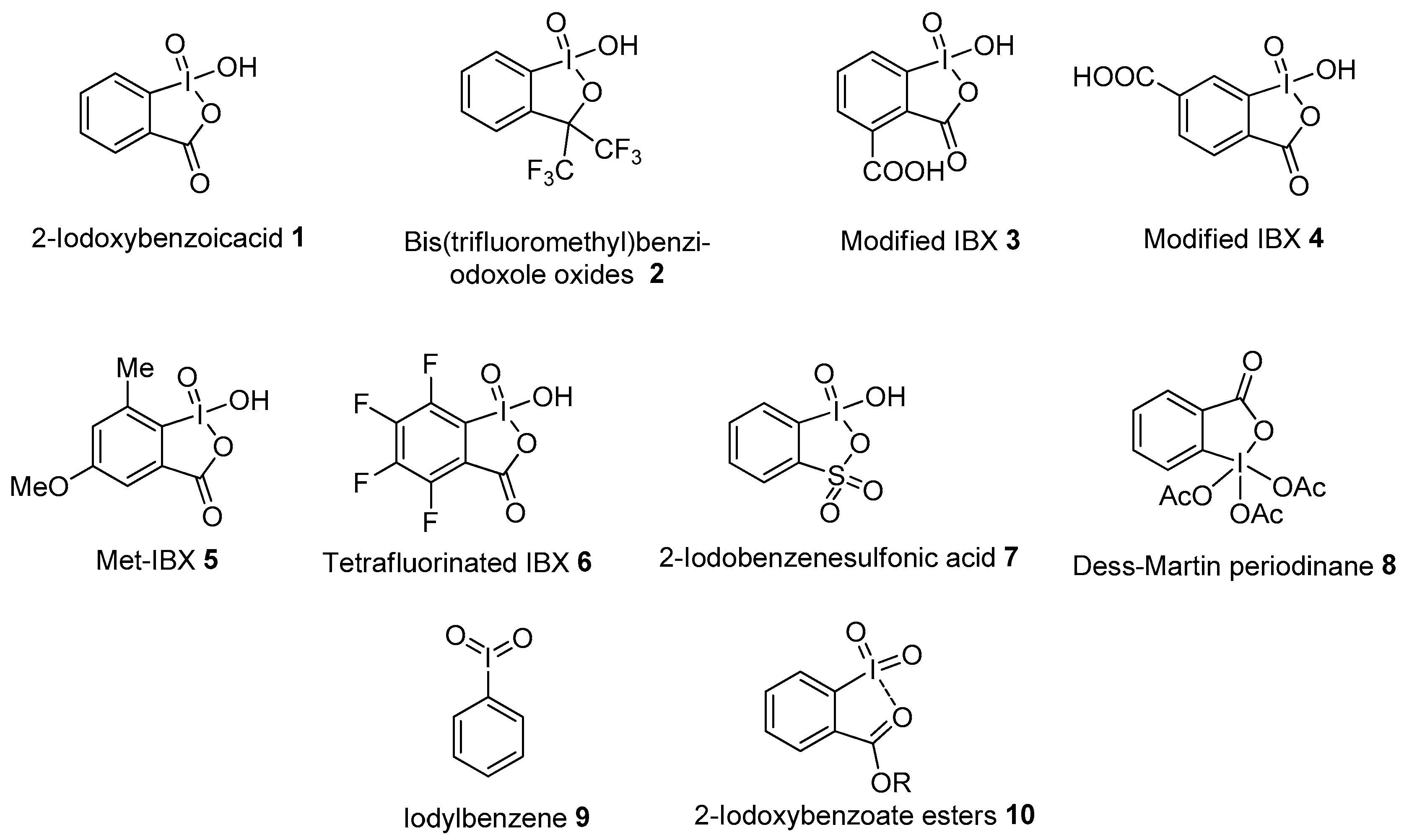
In recent years, the chemistry of hypervalent iodine(V) compounds has witnessed considerable growth in comparison to that of trivalent iodine reagents, as reviewed by Zhdankin in 2006 [43] and 2011 [44]. Some examples of common hypervalent iodine(V) reagents are presented in Figure 1. The most versatile hypervalent iodine(V) reagent is o-iodoxybenzoic acid (IBX 1), first synthesized by Hartmann and Meyer in 1893 [45]. Later, Mullins’s research group synthesized IBX 1 through the oxidation of 2-iodobenzoic acid using potassium bromate under acidic conditions [46]. However, the presence of bromate impurities imparted an explosive nature to IBX 1 under excessive heating conditions. In addition, the practical use of IBX 1 as a potential oxidant was overlooked for many years due to its poor solubility in most organic solvents except DMSO. Nevertheless, IBX has received renewed attention after the pioneering work by Santagostino et al. regarding its improved synthesis t from 2-iodobenzoic acid in the presence of oxone in an aqueous medium [47]. Since then, IBX 1 has become the main representative of hypervalent iodine chemistry owing to its unique reactivity and excellent oxidizing properties. The numerous IBX-mediated chemical transformations include the oxidation of alcohols to carbonyl compounds, the oxidation of amines, the oxidation of benzylic carbon and the oxidation of phenols [43,44]. IBX-mediated oxidative cyclization reactions giving access to diverse heterocycles have also been well explored over the years [48].
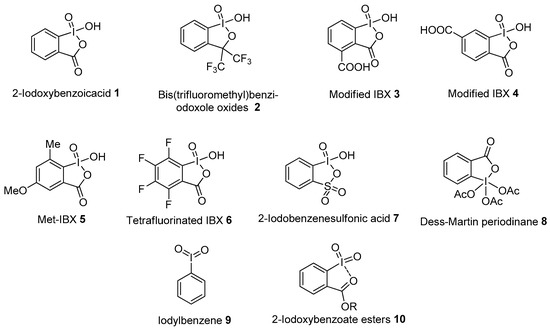
Figure 1.
Hypervalent iodine(V) reagents 1−10.
In order to solve solubility issues, several analogs of IBX were prepared by functionalizing its aromatic core. Dess and Martin synthesized the stable, non-explosive bis(trifluoromethyl)benziodoxole oxide 2 having good solubility in many organic solvents [49]. Later, the water-soluble modified IBX (mIBX) 3 and 4 were prepared from terephthalic acid by Thottumkara and Vinod for the oxidation of benzylic and allylic alcohols [50,51]. Furthermore, Moorthy and co-workers designed and synthesized the ortho-methyl-substituted IBX (Me-IBX, 5) that oxidizes alcohols in common organic solvents [52]. Then, Wirth and co-workers introduced a novel tetrafluorinated IBX analogue (FIBX 6), which has higher solubility and reactivity than IBX 1 [53]. Zhdankin’s group prepared 2-iodobenzenesulfonic acid (IBS 7) from 2-iodobenzenesulfonic acid using Oxone in aqueous solution [54]. This thia-IBX 7 was eventually used by Ishihara and co-workers for the oxidation of alcohols [55]. Another interesting iodine(V) reagent is Dess–Martin periodinane (DMP 8), mainly used for the oxidation of primary alcohols to aldehydes and secondary alcohols to ketones [56,57]. Among acyclic iodine(V) reagents, iodylbenzene 9 is the most explored and is well suited for the oxidation of phenols, sulfides and alcohols [43,44]. Recently, Motlagh and Zakavi synthesized, characterized and studied the oxidizing strength of iodylbenzene nanofibers for the oxidation of 1,5-dihydroxynaphthalene to juglone [58]. Besides this, pseudocyclic iodine(V) compounds 10 are also important oxidants having the characteristic of establishing intramolecular secondary I---O bonding interactions between the iodine center and the oxygen atom in the ortho substituent [59,60]. The present review article summarizes the recent advances in oxidative transformation reactions such as the oxidation of alcohols, amines, amides, aromatic compounds and oxidative cyclizations using hypervalent iodine(V) reagents. Moreover, recent developments achieved in the design of catalytic systems based on in situ generated hypervalent iodine(V) reagents from corresponding iodoarenes will be discussed in great detail.
2. Oxidation of Alcohols
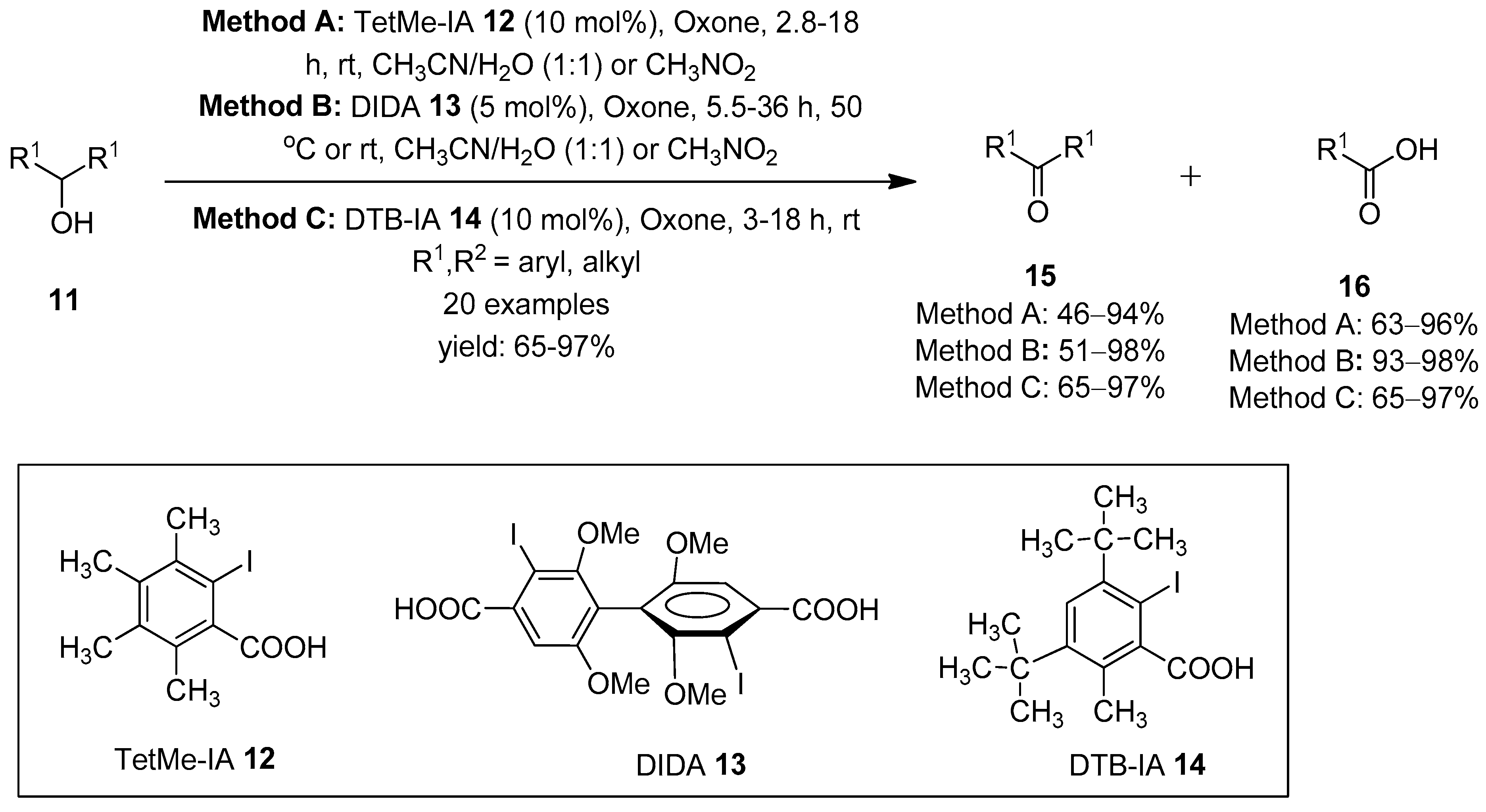
Carbonyl compounds (aldehydes, ketones, carboxylic acids, esters, amides, lactones, etc., are versatile building blocks in organic chemistry [61,62]. The oxidation of alcohols to the corresponding carbonyl compounds have been well explored using hypervalent iodine(V) reagents as stoichiometric oxidants [43,44]. The explosive nature and low solubility of IBX in organic solvents stimulated researchers to develop catalytic routes involving the in situ generation of hypervalent iodine(V) species from organoiodo compounds in the presence of a suitable co-oxidant. Within this context, in 2005, Thottumkara et al. successfully achieved the catalytic oxidations of alcohols by generating iodine(V) species in situ from o-iodobenzoic acid in the presence of Oxone as an oxidant in the solution state [63]. Later in 2009, Ishihara et al. employed o-iodobenzenesulfonate (IBS) 7 as a catalyst to produce iodine(V) species for the oxidation of alcohols to the corresponding carbonyl compounds in good yields [55]. Furthermore, Moorthy’s research group accomplished significant achievements in the catalytic oxidation of alcohols using different iodoarenes as precatalysts. Initially, they employed 3,4,5,6-tetramethyl-2-iodobenzoic acid (TetMe-IA) 12 as an iodo-acid precursor for the in situ generation of reactive TetMe-IBX that facilitated the oxidation of alcohols 11 to carbonyl compounds 15 at room temperature (Scheme 1) [64]. Notably, primary alcohols 11 were oxidized to carboxylic acids 16 through the oxidation of initially formed aldehydes by Oxone. Further catalytic oxidation of a variety of diols to the corresponding lactones was achieved using TetMe-IA 12 as a precursor of TetMe-IBX [64].
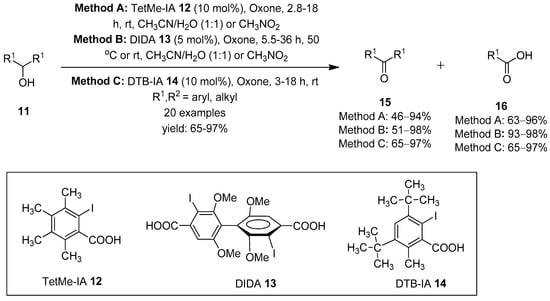
Scheme 1.
Oxidation of alcohols 11 to carbonyl compounds 15 and 16 using iodo-acids 12–14 as precatalysts in the presence of Oxone.
Later, the same group generated the Bis-IBX catalyst in situ from twisted 3,3′-diiodo-2,2′,6,6′-tetramethoxybiphenyl-4,4′-dicarboxylic acid (DIDA) 13 for the catalytic oxidation of alcohols 11 [65]. Furthermore, Mishra and Moorthy recently designed and synthesized a catalyst, 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA) 14, for the in situ generation of IBX 1 using Oxone as a terminal oxidant (Scheme 1) [66]. The highly reactive DTB-IA 14 was then used to oxidize a variety of primary, secondary, aliphatic and aromatic alcohols 11 in solid state under ball-milling conditions.
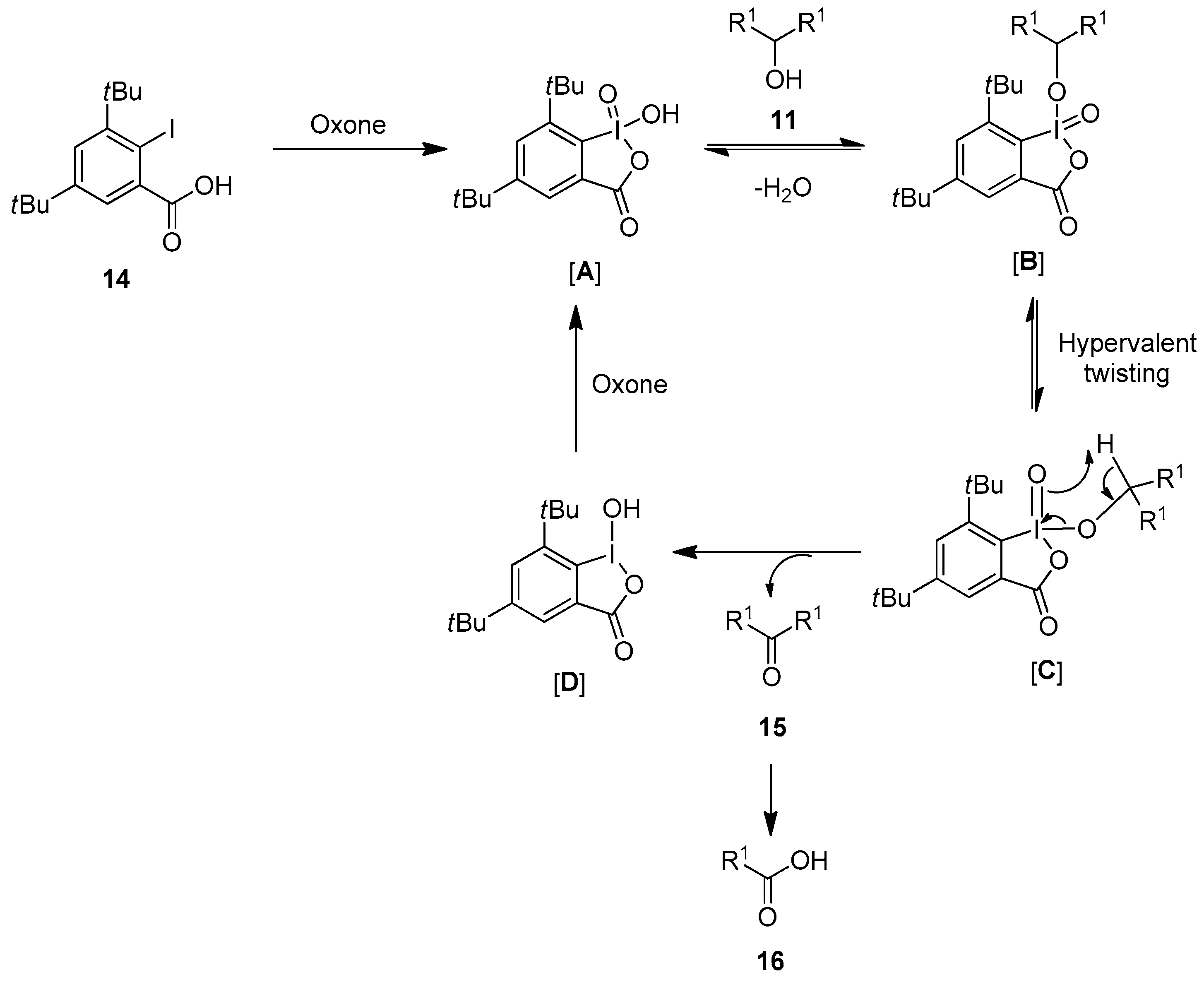
The reaction mechanism for the oxidation of alcohols 11 to carbonyl compounds 15 using the precatalysts 12–14 in the presence of Oxone is depicted in Scheme 2. The reaction proceeds with the in situ oxidation of iodarene 14 to the iodine(V) intermediate [A], which reacts with the alcoholic substrate 11 to form another intermediate [B]. Furthermore, the intermediate [B] undergoes a process called hypervalent iodine twisting and produces the intermediate [C]. Finally, the intermediate [C] undergoes a reductive elimination and yields the final product along with the formation of the iodine(III) intermediate [D]. Furthermore, the iodine(III) intermediate [D] oxidizes to an active iodine(V) species [A] in the presence of Oxone to continue the catalytic cycle. Notably, final product 15 could be further converted into to carboxylic acids 16 through the oxidation of initially formed aldehydes by Oxone.
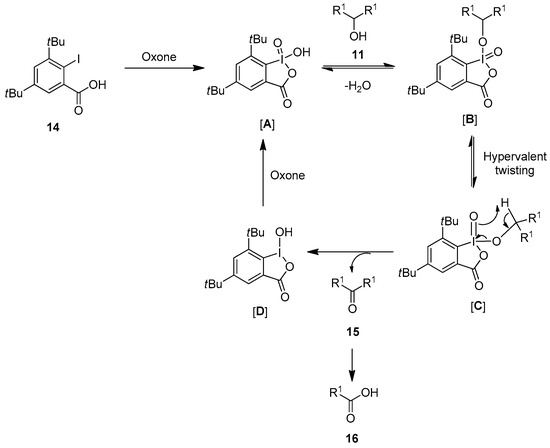
Scheme 2.
Catalytic cycle for the oxidation of alcohols 11 to carbonyl compounds 15 using the iodo-acids 14 as precatalysts in the presence of Oxone.
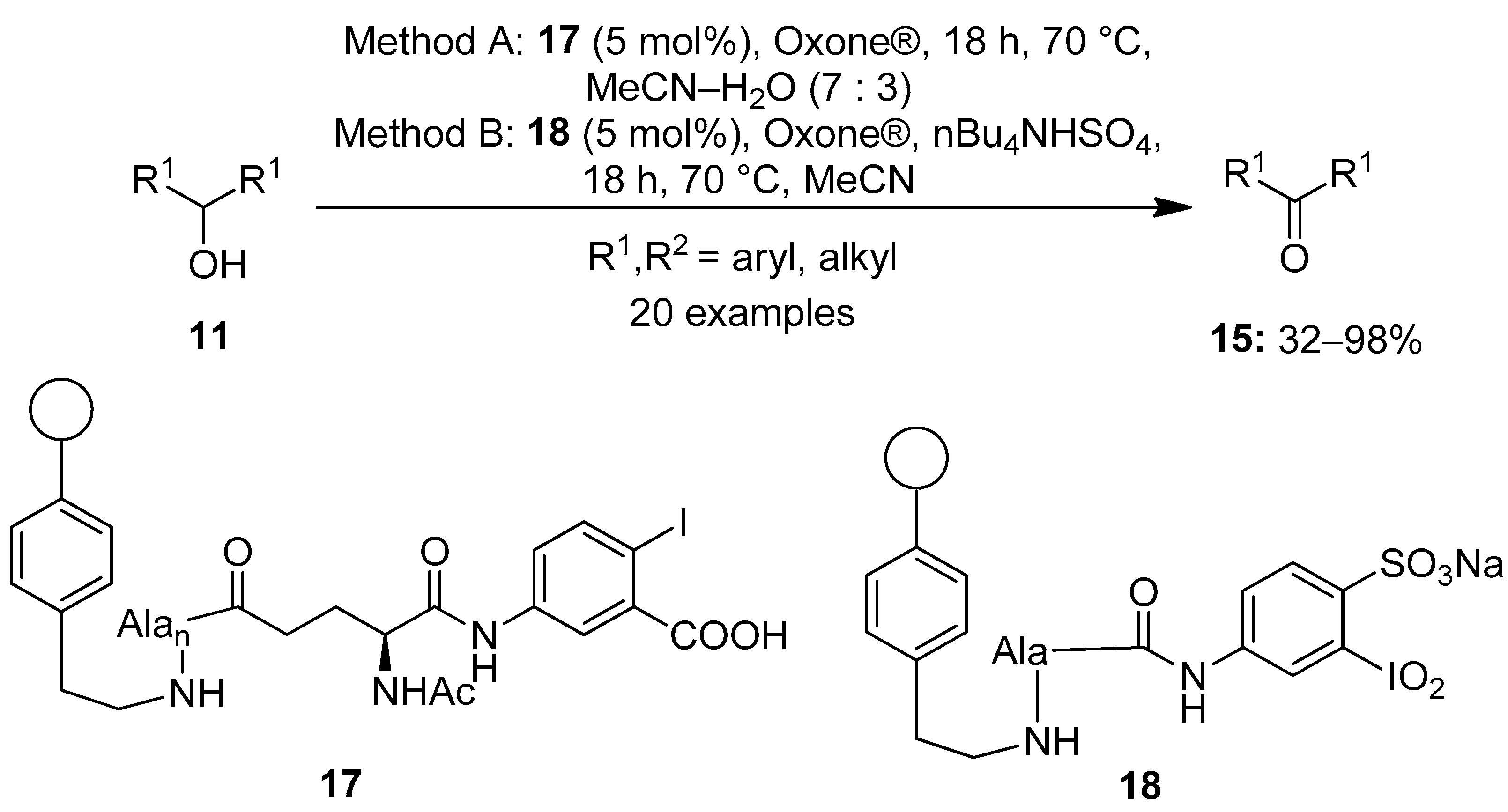
Meanwhile, Ballaschk and Kirsch performed the oxidation of secondary alcohols 11 to produce ketones 15 using the inexpensive and recyclable solid-supported hypervalent iodine catalysts 17 and 18 in the presence of stoichiometric amounts of Oxone® (Scheme 3) [67]. In this, the hypervalent iodine precursor was connected by stable amide bonds to the aminoethyl polystyrene resin. The catalysts were easily regenerated by simple filtration, and the activity lasted for five rounds. Both IBX-derived (Method A) and IBS-derived (Method B) catalytic systems yielded a variety of structurally diverse carbonyl compounds 15 in good to excellent yields. nBu4NHSO4 was used as phase transfer catalyst in non-aqueous conditions (method B). Notably, the IBS-derived catalyst 18 was found to be more active and provided higher product yields compared to 17. Secondary alcohols which were sterically hindered provided better yields when method B was used.
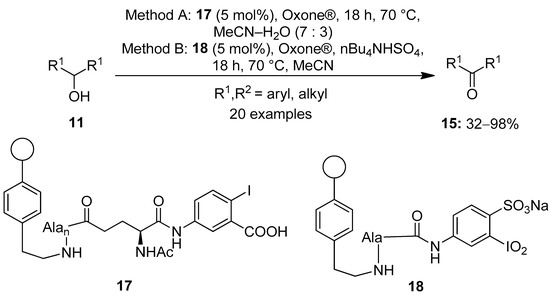
Scheme 3.
Oxidation of alcohols 11 to carbonyl compounds 15 using solid-supported iodoarenes 17 and 18 in the presence of a terminal oxidant.
In 2021, Kupwade et al. worked towards synthesizing α-ketophosphonates 20 by oxidizing α-hydroxyphosphonates 19 in the absence of metal catalysts (Scheme 4) [68]. Usually, o-iodoxybezoic acid 1 (IBX) is used for the oxidation of alcohols. However, it was found to be inefficient for the oxidation of such compounds. Later, IBX 1 in combination with benzyltriphenylphosphonium peroxymonosulfate (BTPP) in the ratio of 1:3 was used, and this resulted in excellent yields. The major limitation for this technique was the high molecular weight, cost and reflux conditions of BTPP. Hence, Dess–Martin periodinane (DMP) 8 and 19 in the ratio of 1:1 were stirred together for a very short time, resulting in the desired products 20 under ambient conditions. The reagent when tested with several α-hydroxyphosphonates 19 substituted with electron-donating and -withdrawing groups furnished 20 in 91–98% yield. Notably, α-ketophosphonates exhibits interesting biological activities [69,70] and are versatile molecules in organic synthesis [71].
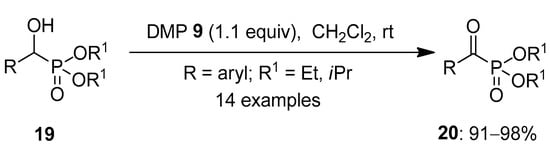
Scheme 4.
Oxidation of α-hydroxyphosphonates 19 to α-ketophosphonates 20 using DMP 8 as an oxidant.
3. Oxidation of Amines
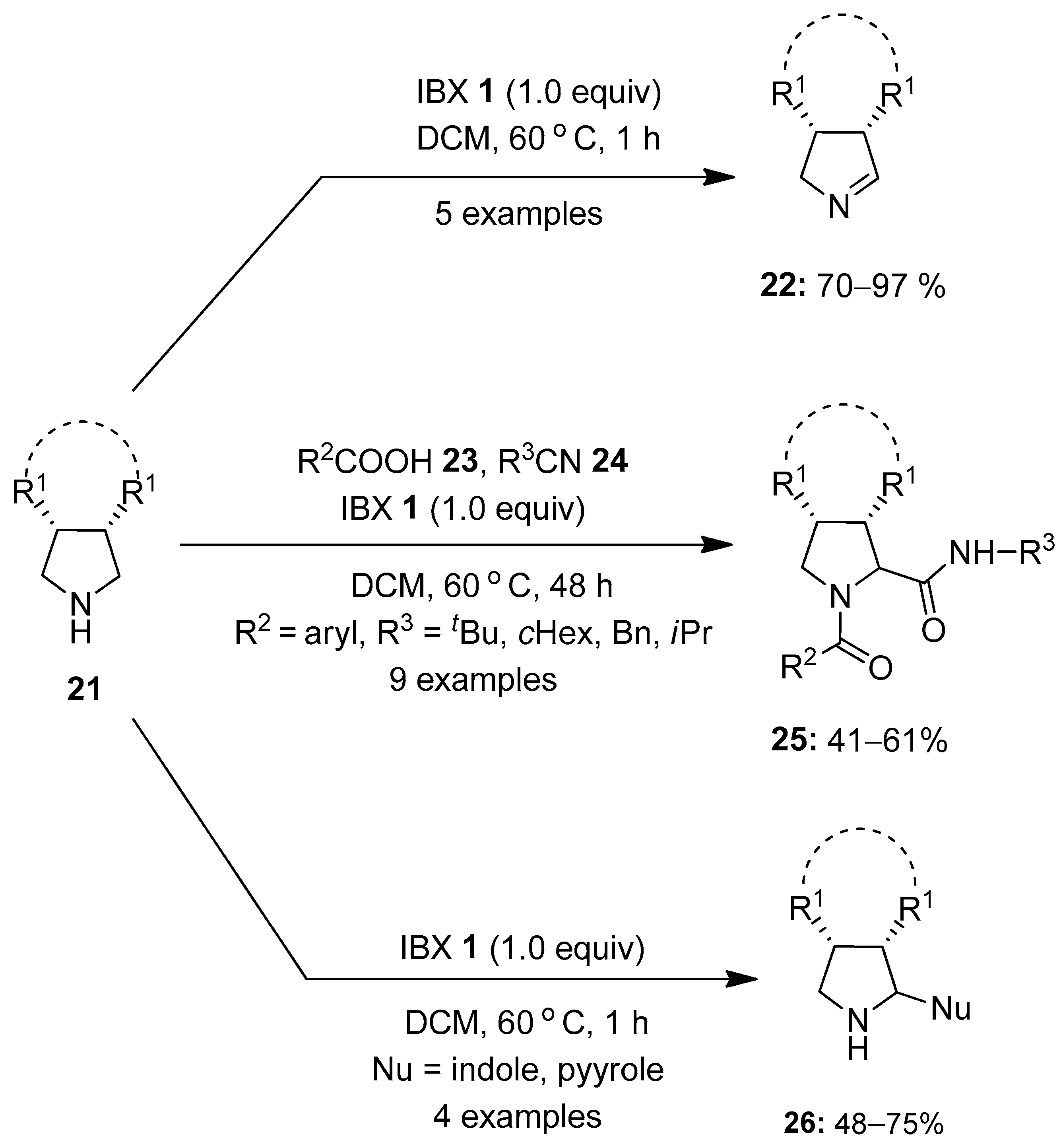
The oxidation of amines using hypervalent iodine reagents has attracted great attention in recent years [72,73,74]. Recent accomplishments achieved in this area using iodine (V) reagents are reported in this section. In 2015, Orru and co-workers for the first time reported the oxidation of unactivated amines 21 to the corresponding imines 22 using IBX 1 as an oxidant (Scheme 5) [75]. Delightedly, a number of aliphatic meso-pyrrolidines 21 were oxidized selectively by IBX 1 to furnish bi- and tricyclic 1-pyrrolines 22 in 70–97% yield. Furthermore, a one-pot Ugi-type three-component reaction between meso-pyrrolidines 21, carboxylic acids 23 and isocyanides 24 produced dipeptides 25 as a single diastereoisomer in moderate to good yields (41–61%). The molecular diversity of in situ-generated 1-pyrrolines 22 was further explored through the oxidative aza-Friedel–Crafts reaction of meso-pyrrolidines 21 with pyrrole and indoles, providing 2-substituted pyrrolidines 26 in useful yields.
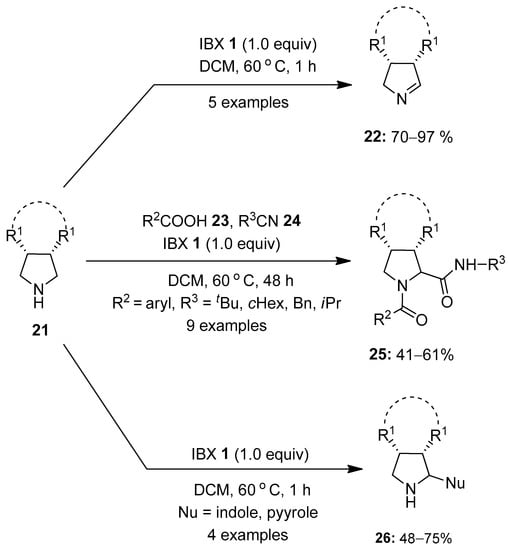
Scheme 5.
Oxidation of unactivated amines 21 to imines 22 and 2-substituted pyrrolidines 25/26 using IBX 1 as an oxidant.
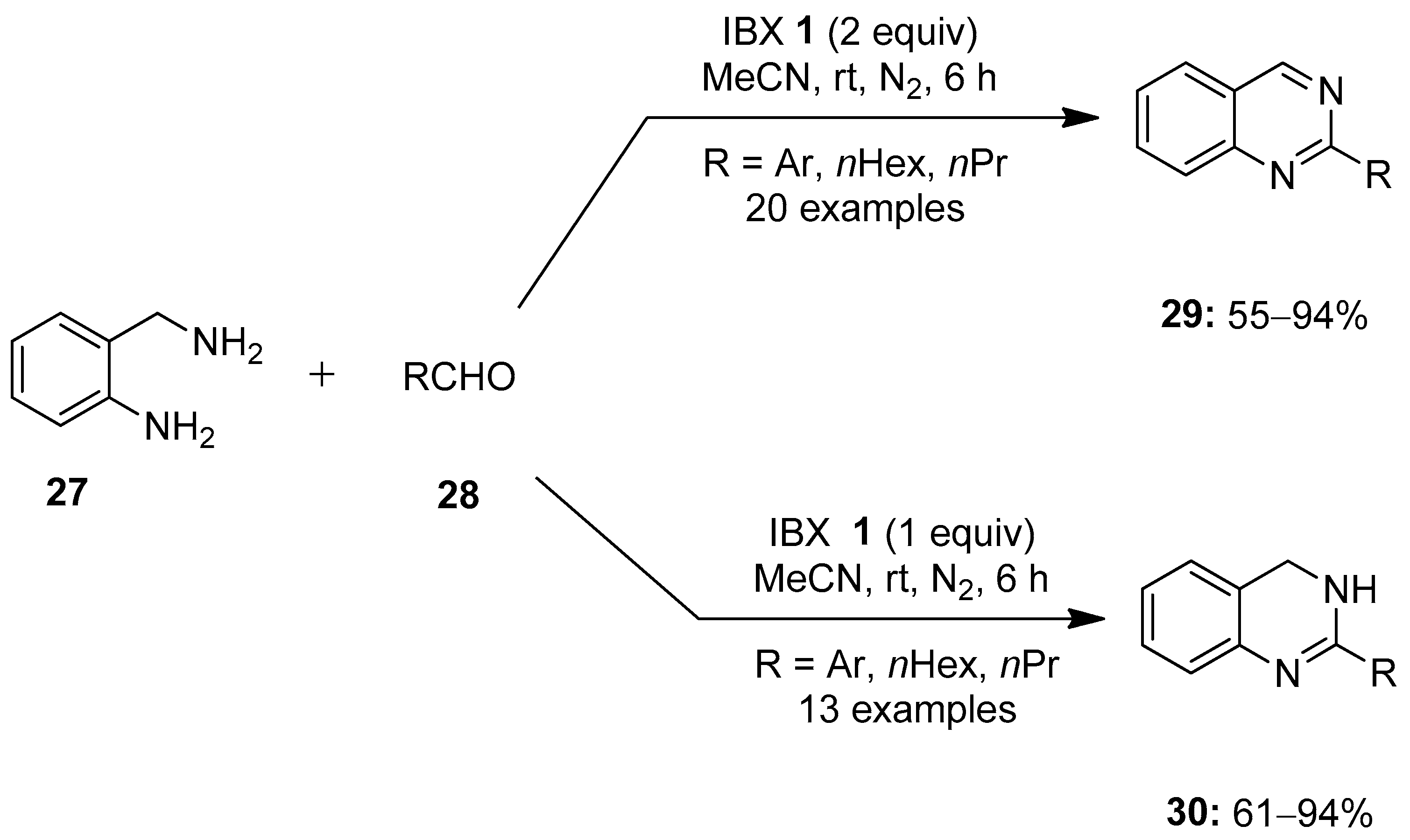
In 2016, Hati and Sen reported a facile method for the synthesis of functionalized quinazolines 29 and 3,4-dihydroquinazolines 30 via an IBX-mediated tandem reaction of o-aminobenzylamine 27 with aldehydes 28 (Scheme 6) [76]. Notably, the reaction with two equivalents of IBX 1 yielded quinazolines 29, while one equivalent of IBX 1 provided dihydroquinazolines 30. This strategy was found effective for a number of aryl, heteroaryl and alkyl aldehydes and also tolerated both electron-donating and -withdrawing functional groups.
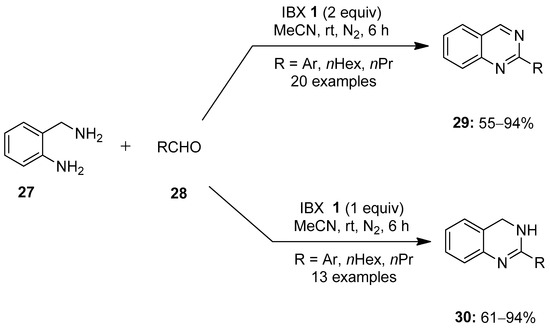
Scheme 6.
Synthesis of quinazolines 29 and 3,4-dihydroquinazolines 30 from o-aminobenzylamine 27 with aldehydes 28 using IBX 1 as an oxidant.
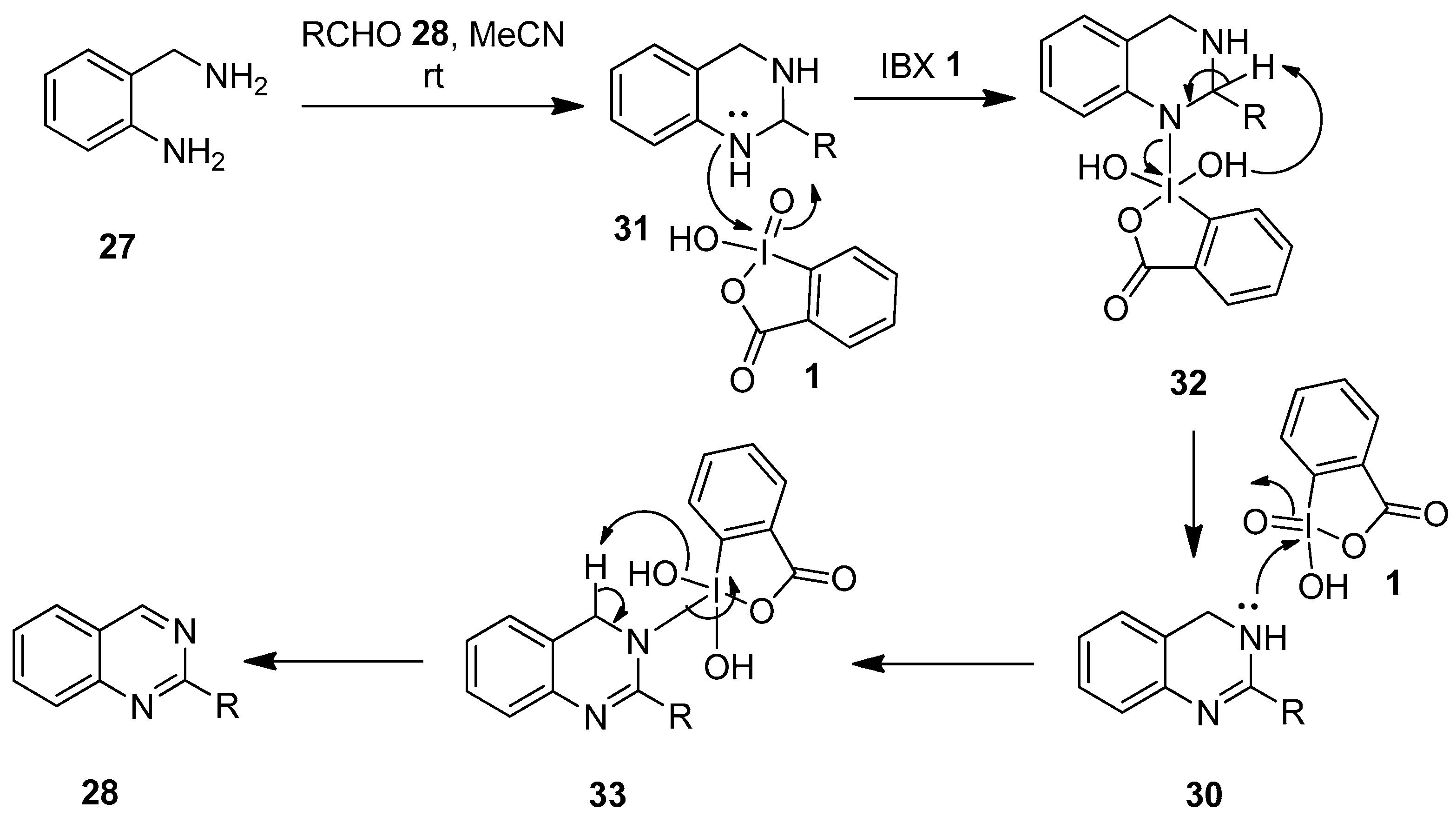
A plausible mechanism envisioned for the synthesis of quinazolines 29 and 3,4-dihydroquinazolines 30 is depicted in Scheme 7 [76]. Initially, o-aminobenzylamine 27 reacts with aldehydes 28 to form the tetrahydroquinazoline intermediate 31, which attacks the electrophilic iodine center of IBX 1 to produce intermediate 32. Subsequently, the reduction of 32 generates the dihydroquinazoline 30. Finally, the IBX-mediated oxidation of 30 through intermediate 33 yields the desired quinazoline 28.
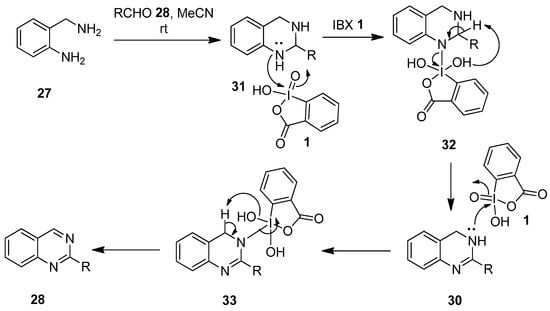
Scheme 7.
Plausible mechanism for the synthesis of quinazolines 28 and 3,4-dihydroquinazolines 30 using IBX 1 as an oxidant.
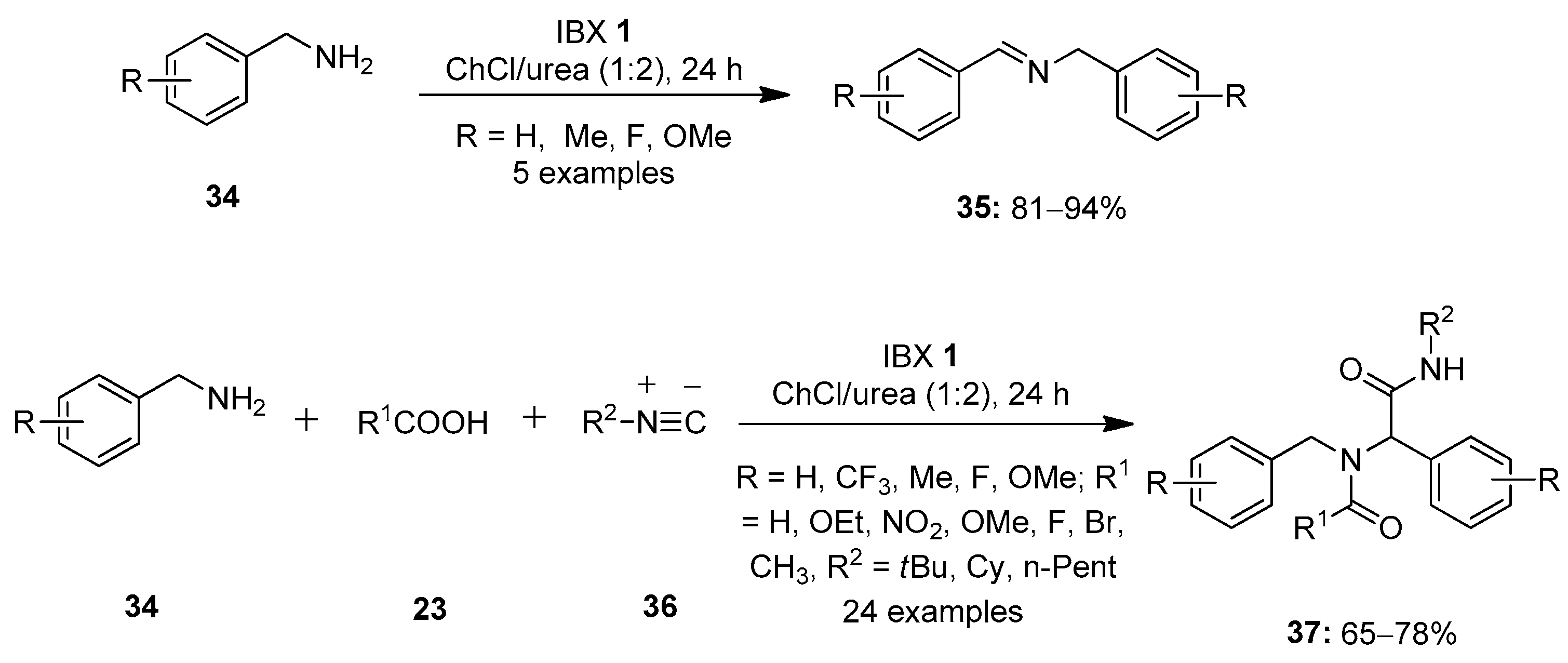
In 2019, Singh et al. reported a methodology to selectively oxidize the primary amines 34 to the corresponding imines 35 using IBX 1 as an oxidant (Scheme 8) [77]. It was found that for oxidative coupling, IBX 1 and DMP 8 were highly selective. Due to the high solubility of IBX 1 in the deep eutectic solvent choline chloride/urea (ChCl/urea), this solvent is used as solvent system for this reaction. A number of electron-rich and electron-deficient amines were readily converted into secondary imines in good yields. Notably, IBX 1 and the solvent could be recovered and reused up to five times without loss of much activity. Further, a one-pot three-component Ugi reaction involving the condensation of diverse carboxylic acids 23 and primary amines 34 was carried out to form the imine intermediate 35 followed by a reaction with an isocyanide 36 to yield bis(amide)s 37 in high yields.
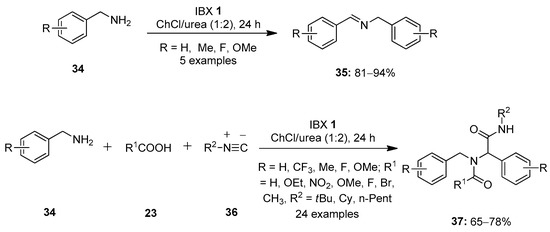
Scheme 8.
Oxidation of primary amines 34 to imines 35 using IBX 1 as an oxidant.
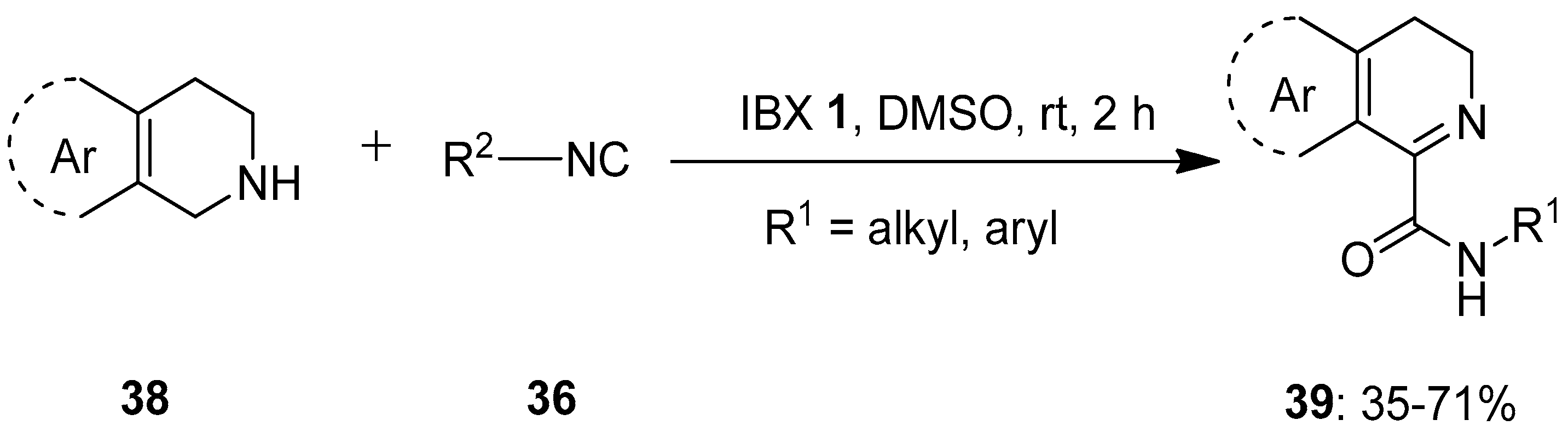
In 2019, Ambule et al. reported a pioneering work on the IBX-mediated oxidative addition of isocyanides 36 to the cyclic amines 38 such as tryptolines and 1,2,3,4-tetrahydroisoquinolines to obtain imino-carboxamides 39 under metal-free conditions (Scheme 9) [78]. The dual role of IBX 1 as an oxidant and as a Lewis acid to activate an imine facilitates the isocyanide addition in this transformation. A variety of aliphatic and aromatic isocyanides 36 reacted well with 38 to afford products 39 in good to moderate yields. However, the reactions with unactivated secondary amines such as pyrrolidine, piperidine, epoxyisoindoline and indoline were sluggish due to the formation of a complex mixture of products. Furthermore, this method was successfully employed for the gram-scale preparation of two alkaloids, alangiobussine (63%) and alangiobussinine (45%).
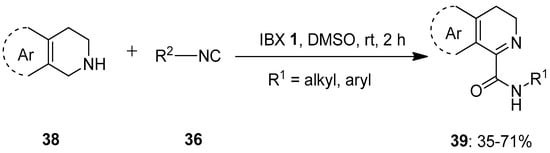
Scheme 9.
Oxidative addition of isocyanides 36 to the cyclic amines 38 to yield imino-carboxamides 39 using IBX 1 as an oxidant.
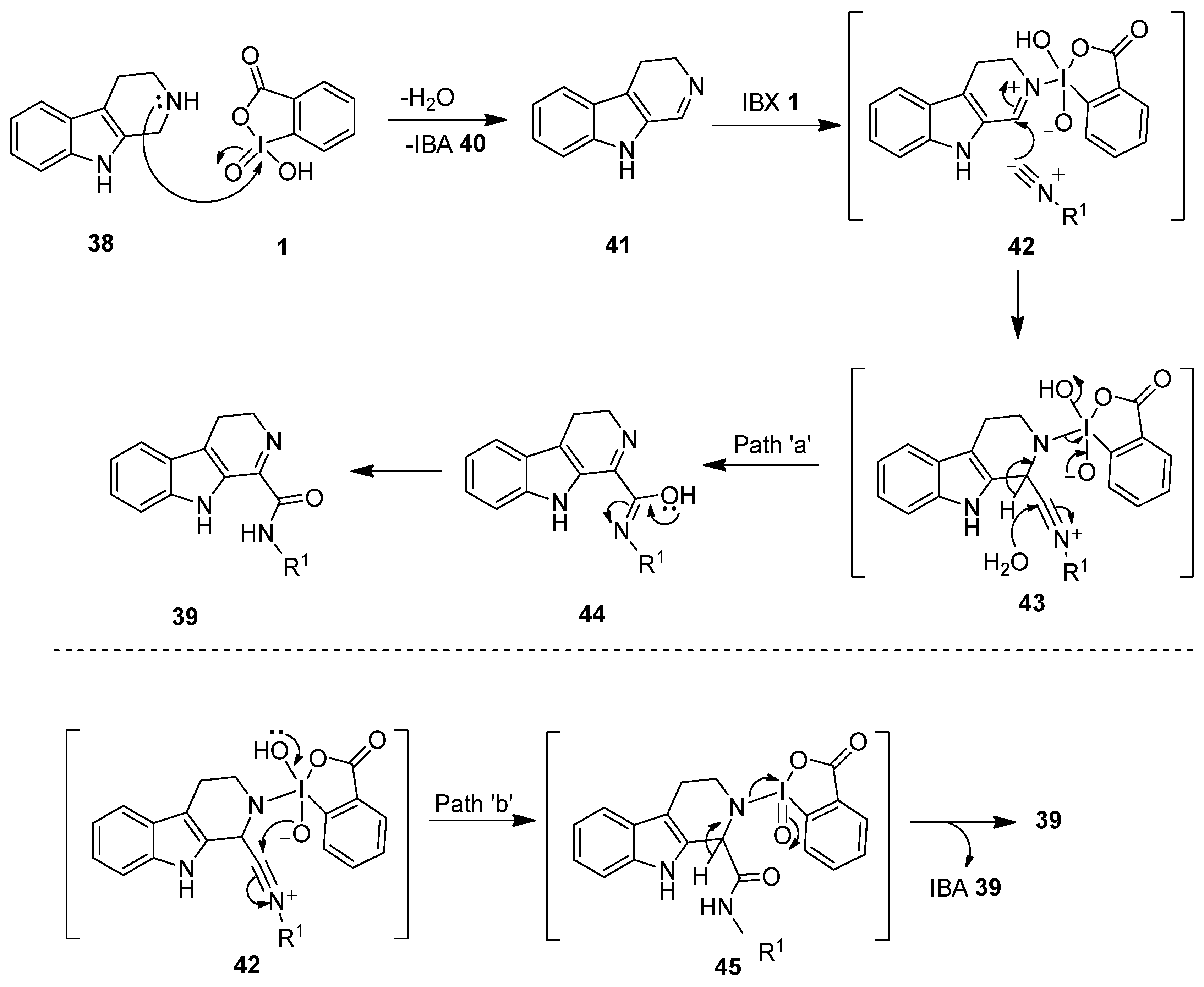
A proposed mechanistic pathway for this reaction is displayed in Scheme 10 [78]. Initially, IBX oxidizes tryptoline 38 to 3,4-dihydro-β-carboline 41, followed by the imine activation with another molecule of IBX 1 or IBA 40 to yield the intermediate 42. Notably, the activation of the imine by IBX 1 facilitates the isocyanide 36 addition to the intermediate 42 to form the nitrilium ion 43. Product 39 could be obtained from the intermediate 43 via two routes (paths a and b). In path ‘a’, the addition of water to the intermediate 43 yields product 39 via the intermediate 44 (Path a). In path ‘b’, the intermediate 43 undergoes an intramolecular hydroxyl transfer to form 45, which provides product 39 upon loss of IBA 40.
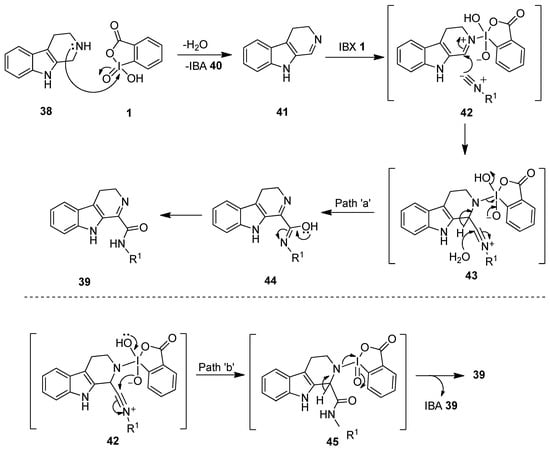
Scheme 10.
The proposed mechanism for the synthesis of imino-carboxamides 39 using IBX 1 as an oxidant.
4. Oxidative Cleavage of Amides
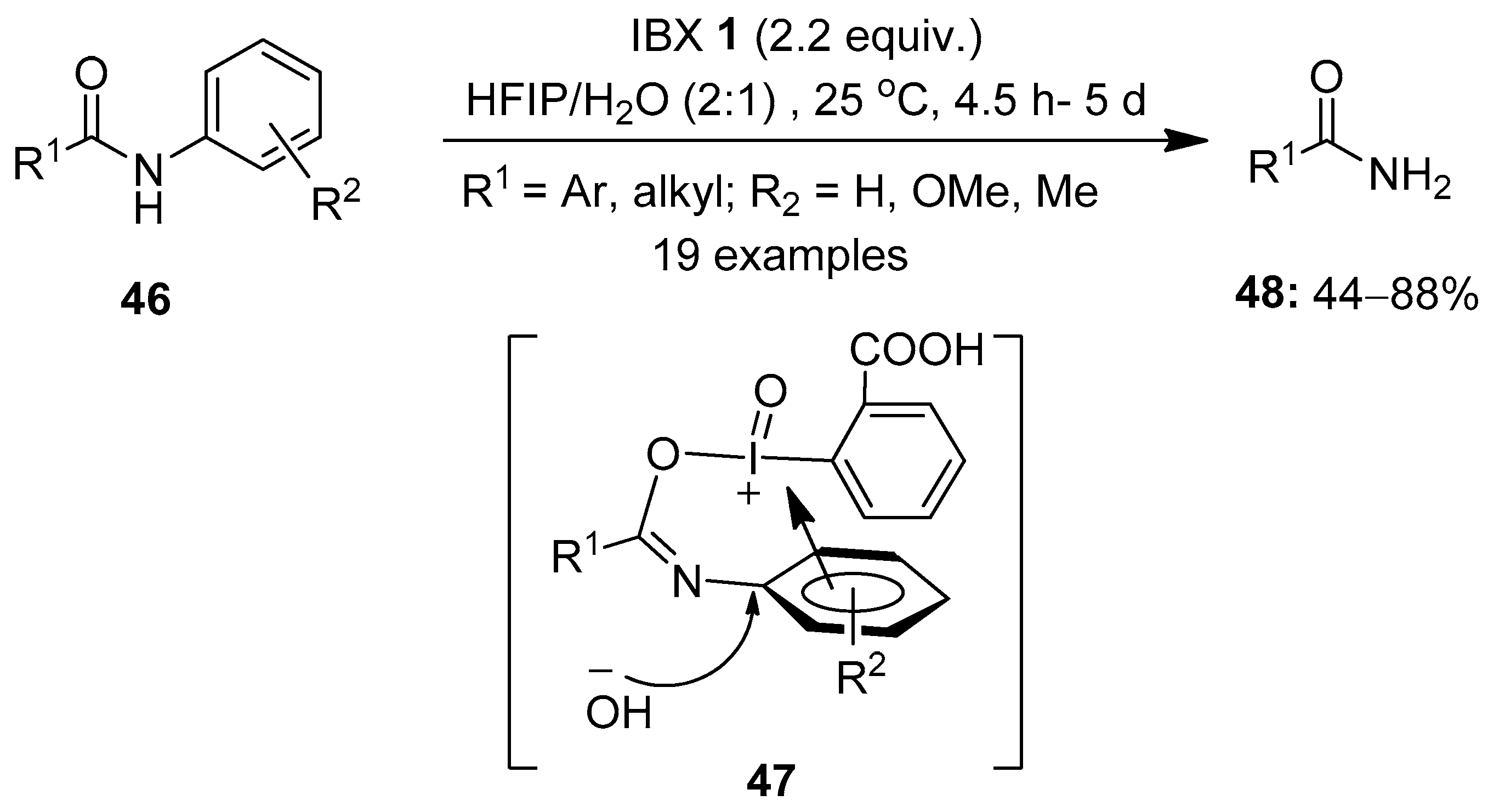
Another interesting area of great interest is the oxidation of amides using hypervalent iodine(V) reagents. In 2018, Zhang et al. demonstrated an excellent method for the oxidative cleavage of inert aryl C−N bonds in N-aryl amides 46 to yield primary amides 48 using IBX 1 as an oxidant (Scheme 11) [79]. Among the different solvent systems screened, HFIP/H2O was found to be very efficient for these reactions. The plausible mechanism involves the interaction of IBX with substrates 46 to form the annular π-complex 47, which is subsequently attacked by the hydroxy group obtained from H2O to provide the primary amide 48 via a regioselective cleavage of the C(aryl)−N bond. Notably, substrates with electron-donating groups yielded products in good yields, whereas those with electron-withdrawing groups failed to provide the desired products. The key aspect of this method is that IBX enables the selective cleavage of the C(aryl)−N bond in N-aryl amides, keeping the C(carbonyl)−N bond untouched. Furthermore, this novel strategy was extended to a number of α-mono- and α,α-disubstituted β-ketoamides 46 to yield the anticipated amides 48 in useful yields.
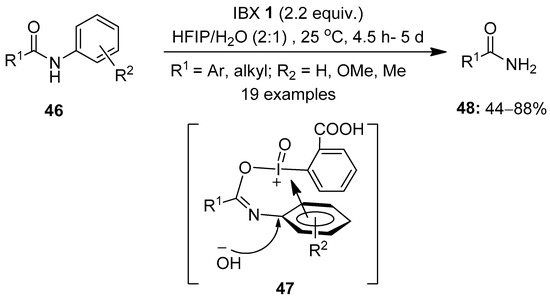
Scheme 11.
Oxidative cleavage of aryl C−N bonds in N-aryl amides 46 to yield primary amides 48 using IBX 1 as an oxidant.
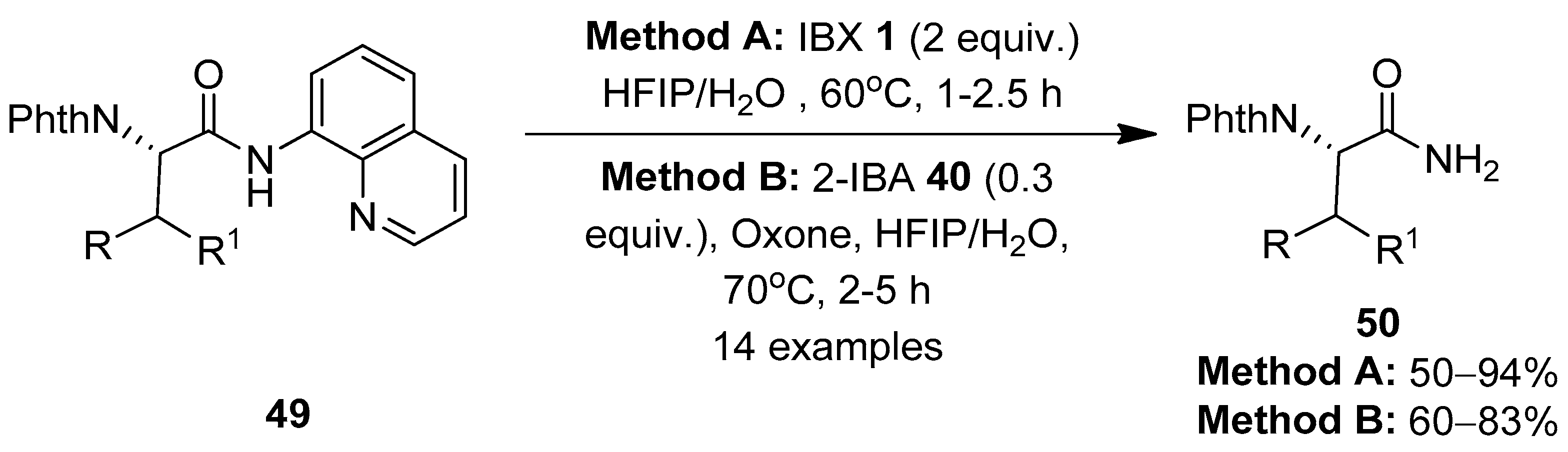
The same group reported a chemoselective method for the oxidative cleavage of 8-aminoquinoline (AQ) in N-quinolyl carboxamides 49 and the removal of the AQ group using IBX 1 as a stoichiometric oxidant (Method A) (Scheme 12) [80]. The reaction scope was evaluated with a variety of AQ-coupled substrates, and the corresponding primary amide products 50 were obtained in moderate to good yields. The mixture of HFIP and H2O solvents in a 1:1 ratio was critical for obtaining high yields of products. An additional catalytic system (Method B) comprising 2-iodobenzoic acid 40 (0.3 equiv.) and Oxone (a mixture of 2KHSO5·KHSO4·K2SO4) as a co-oxidant successfully furnished products 50 in comparable amounts to those obtained with method A. Notably, the reactions exhibited excellent chemoselectivity towards the C-terminal N-quinolyl carboxamide, without affecting the internal alkyl amide groups. Finally, the resulting primary amides 50 were easily converted into carboxylic acids by treating with tert-butyl nitrite in AcOH.
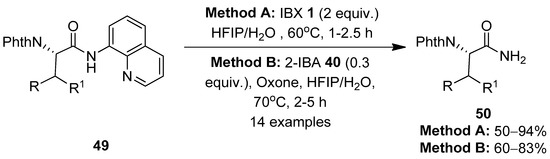
Scheme 12.
Oxidative cleavage of 8-aminoquinoline in N-quinolyl carboxamides 49 to yield primary amides 50 using IBX 1 as an oxidant.
5. Oxidation of Alkenes
The selective oxidation of alkenes to more polar compounds using hypervalent iodine reagents is yet another interesting area of research. In 2014, Moorthy’s research group demonstrated the oxidative cleavage of alkenes 51 or 52 into ketones 54/carboxylic acids 53 using a catalytic amount of TetMe-IA 12 in the presence of Oxone (Scheme 13) [81]. Mechanistically, the reaction proceeds via the initial dihydroxylation of alkenes followed by oxidative cleavage by the in situ generated TetMe-IBX to aldehydes, which undergo a rapid oxidation with Oxone to produce the corresponding acids 53. The reaction was carried out with a variety of terminal and internal alkenes, and the desired products were obtained in respectable yields. Notably, for substrates containing two double bonds, chemoselective cleavage of electron-rich alkenes was observed.
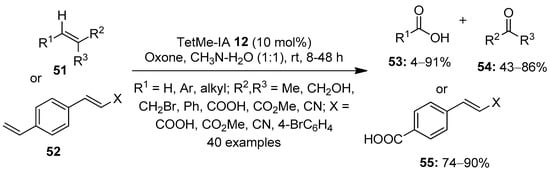
Scheme 13.
Oxidative cleavage of alkenes 51 or 52 into ketones 54/carboxylic acids 53 using TetMe-IA 12 as a precatalyst in the presence of Oxone.
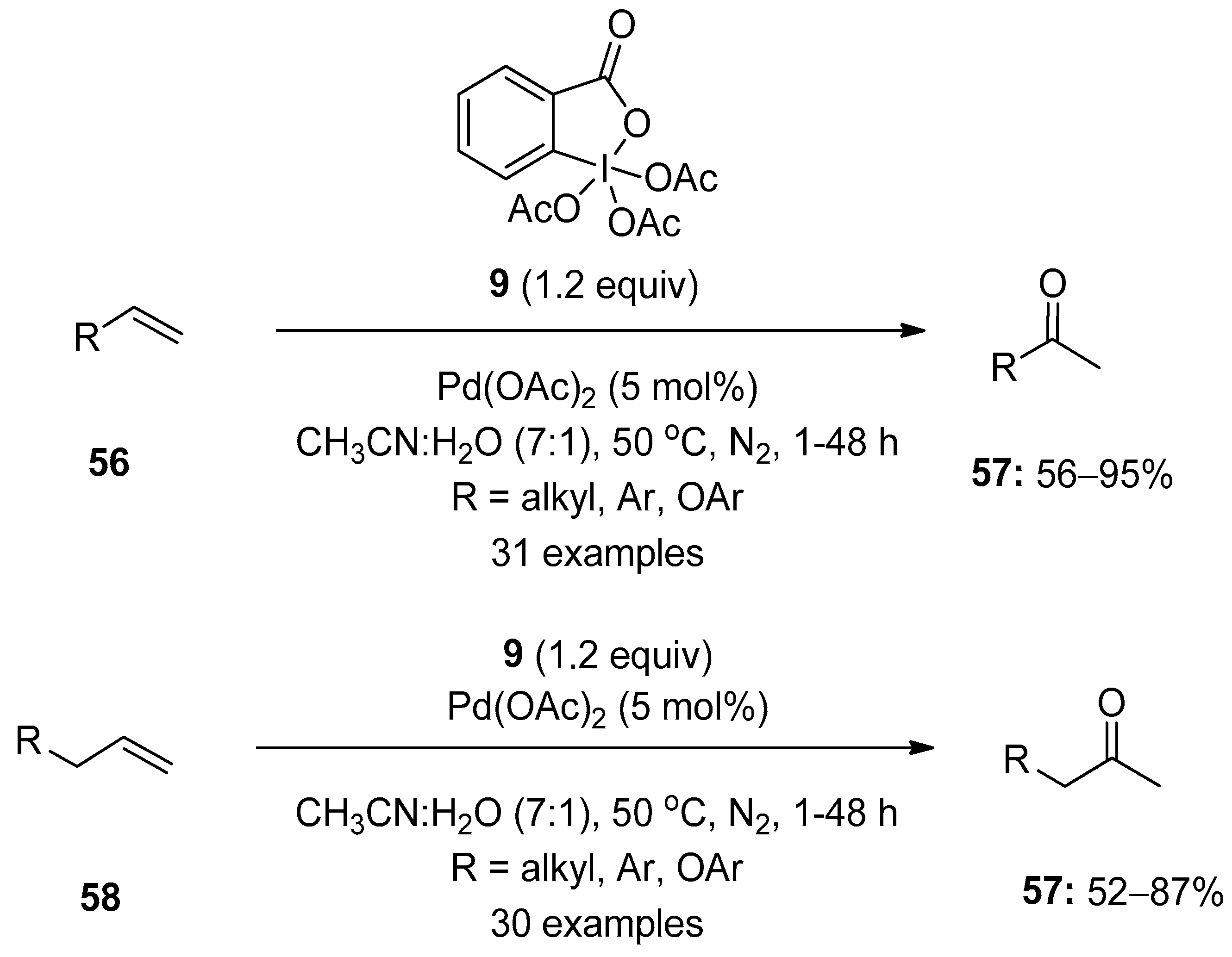
Chaudhari and Fernandes reported the palladium-catalyzed Wacker-type oxidation of terminal alkenes 56 using Dess–Martin periodinane (DMP) 8 as an oxidant (Scheme 14) [82]. This operationally simple method enabled the synthesis of diverse methyl ketones 57 in good yields with complete Markonikov selectivity. Additionally, allylic or homoallylic compounds 58 were oxidized to methyl ketones 57 under similar conditions. The key features of this reaction are its broad substrates scope, excellent functional group tolerance and high yields.
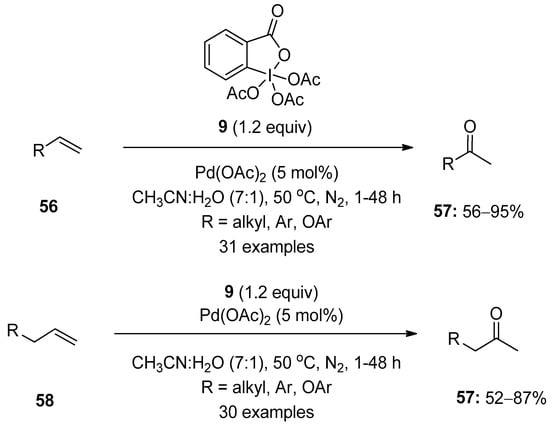
Scheme 14.
Pd(II)-catalyzed Wacker-type oxidation of alkenes 56 and 58 to ketones 57 using DMP 8 as an oxidant.
6. Oxidation of Aromatic Compounds
The hypervalent iodine-mediated oxidation of aromatic compounds has been well studied by several researchers. In 2006, Moorthy and co-workers reported a one-pot oxidation of stilbene derivatives to the corresponding benzils with NIS/IBX in DMSO [83]. In continuation, Moorthy’s research group demonstrated a method for the direct oxidation of indoles 59 to isatins 60 using the NIS/IBX 1 reagent in DMSO at room temperature (Scheme 15) [84]. The reactions of a variety of substituted indoles 59 proceeded smoothly under the optimized conditions, providing isatins 60 in good yields. Notably, the reaction proceeds through the formation of the intermediary 3-iodoindole 61, which is oxidized by IBX 1 to produce isatins 60. Furthermore, 3-iodoindoles 61 were synthesized independently by reacting indoles 59 with NIS and efficiently converted into isatins 60 with IBX 1. A similar method was developed for the synthesis of isatins by Kirsch and others using a NaI/IBX-SO3K reagent mixture [85].
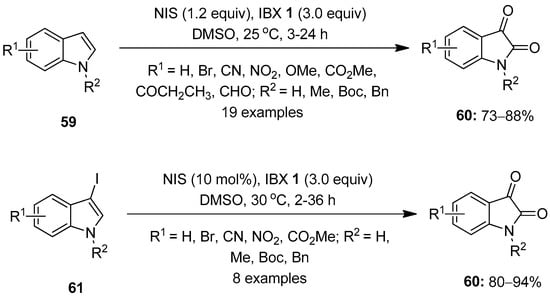
Scheme 15.
Oxidation of indoles 59 and 3-iodoindoles 61 to isatins 60 using IBX 1 as an oxidant.
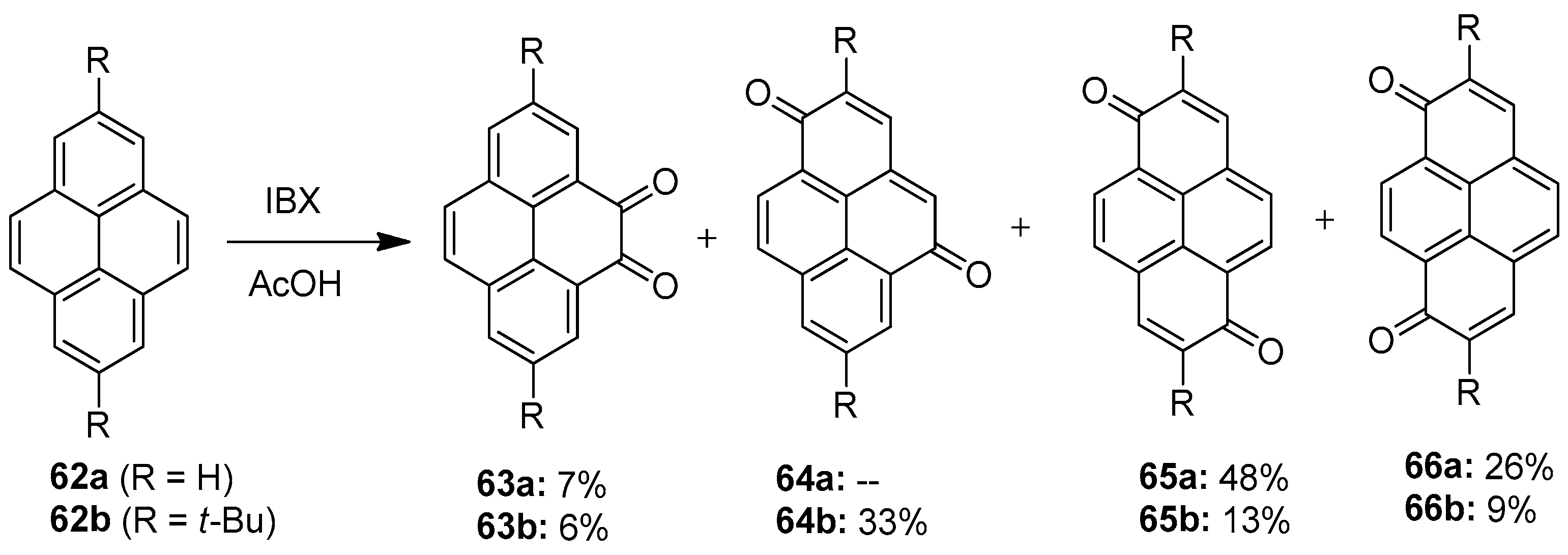
The oxidation of the K-region (4,5,9,10 position) in pyrene 62 is found to be very difficult, as most of the oxidants produce very less yields (Scheme 16). El-Assaad et al. reported a new method for this reaction, using an hypervalent iodine compound, in 2020 [86]. They performed the oxidation of pyrene 62 by employing IBX 1 as an oxidizing agent in acetic acid. A mixture of diones 63–66 was obtained, and pseudo-para-diones were found to be the major product (diones 65a and 64b).
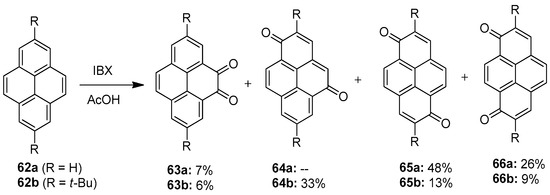
Scheme 16.
Oxidation of pyrenes 62 to diones 64–66 using IBX 1 as an oxidant.
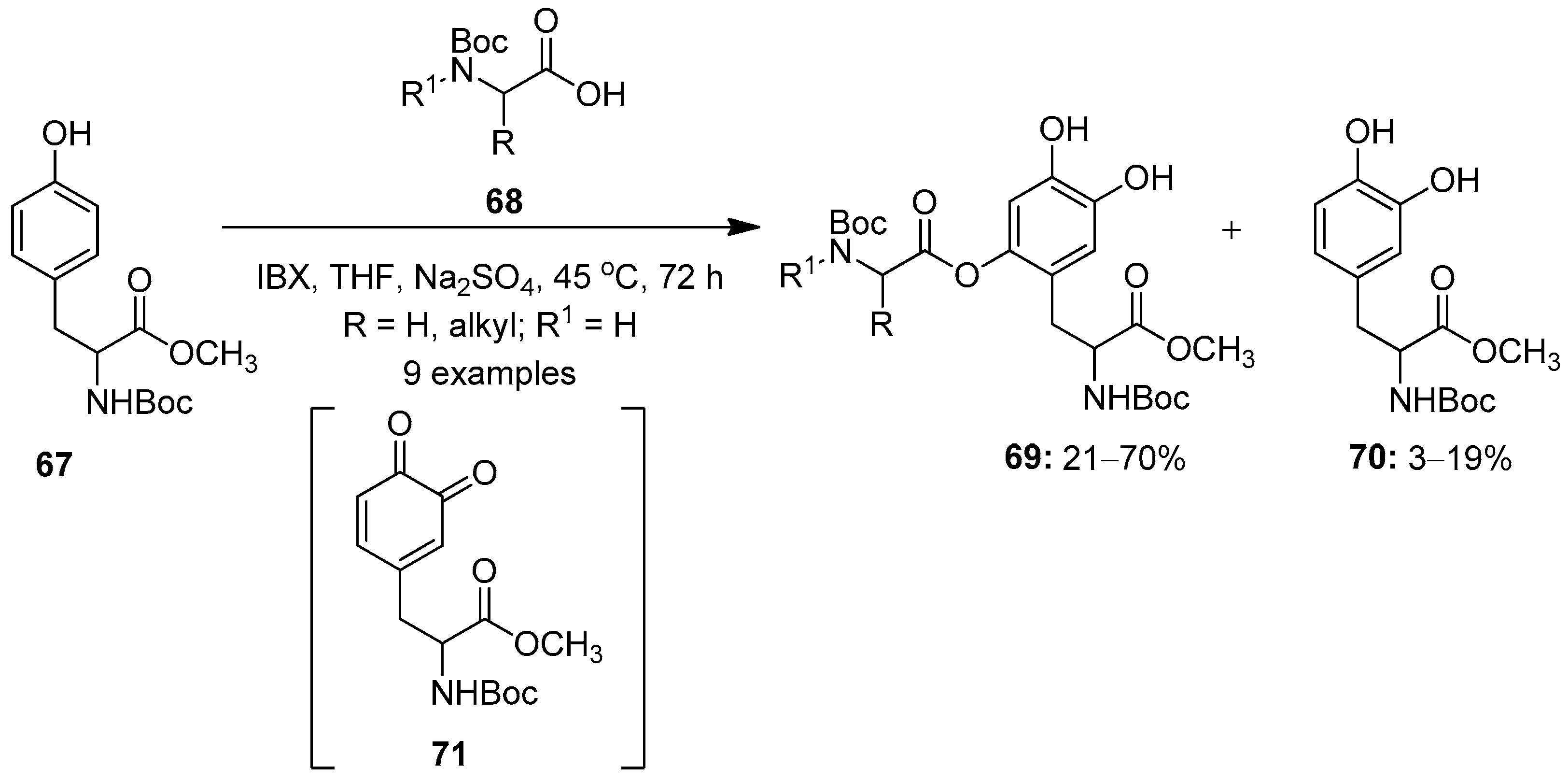
Meanwhile, Saladino’s research group synthesized DOPA peptidomimetics 69 by the aromatic oxidative functionalization of the tyrosine molecule 67 with IBX 1 (Scheme 17) [87]. The reactions proceeds through the oxidation of tyrosine by IBX 1 to form the DOPA quinone intermediate 71, followed by a Michael-like nucleophilic addition of nitrogen-protected amino acids 68 to yield new L-DOPA-peptidomimetics 69 along with the formation of the side product 70. A further oxidative functionalization of tyrosine 67 with O-protected α-amino acids was achieved under similar conditions.
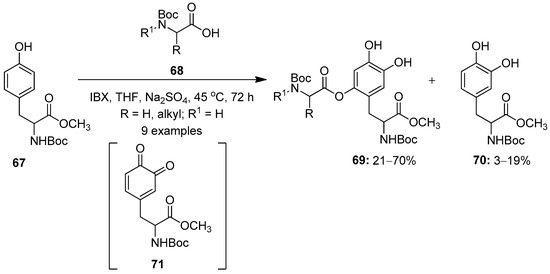
Scheme 17.
Oxidative functionalization of tyrosine 67 to L-DOPA-peptidomimetics 69 using IBX 1 as an oxidant.
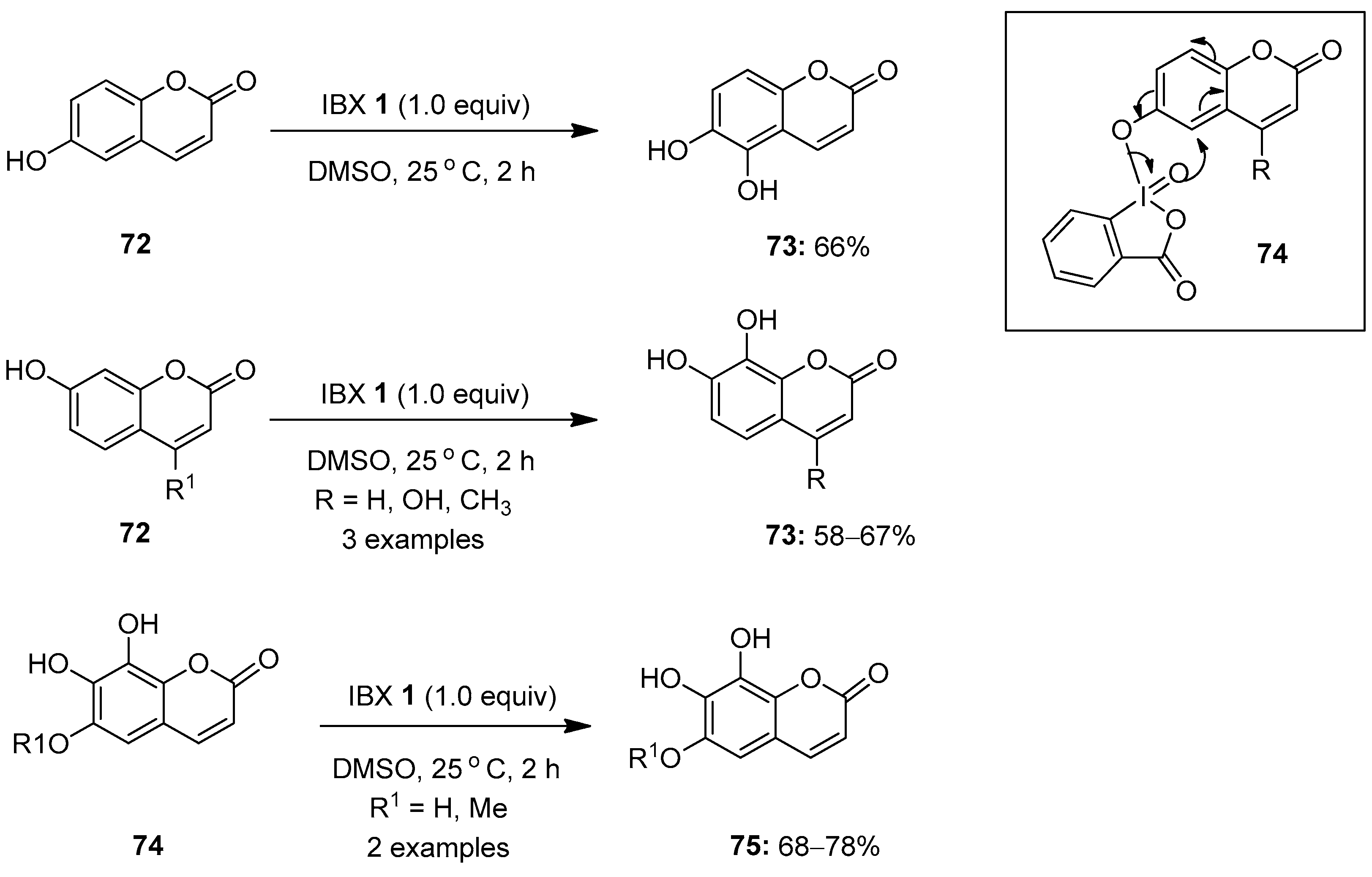
In continuation, Nencioni, Saladino and co-workers eventually reported the IBX-mediated oxidation of coumarins 72 in DMSO for the regioselective synthesis of catechols 73 (Scheme 18) [88]. Additionally, the synthesis of pyrogallol derivatives 75 was achieved through the oxidation of fraxetin and esculetin 74 under similar conditions, in good yields. Notably, the regioselectivity observed in this transformation is due to the intramolecular delivery of the oxygen atom from the λ5-iodanyl intermediate 74 to the ortho-position of the phenolic moiety. Moreover, the oxidation of coumarins was also achieved by replacing IBX 1 with polystyrene-supported IBX in the presence of water as a solvent. Finally, the synthesized coumarin derivatives were tested for antioxidant and antiviral activities, and the corresponding pyrogallols 75 were found to be the most active compounds.
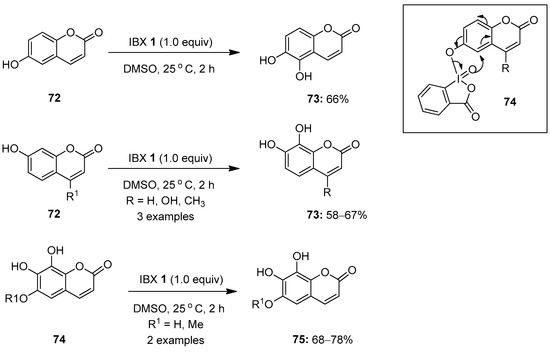
Scheme 18.
Oxidation of coumarins 72 and 74 to catechols 73 and pyrogallol derivatives 75 using IBX 1 as an oxidant.
7. C−H Functionalization Reactions
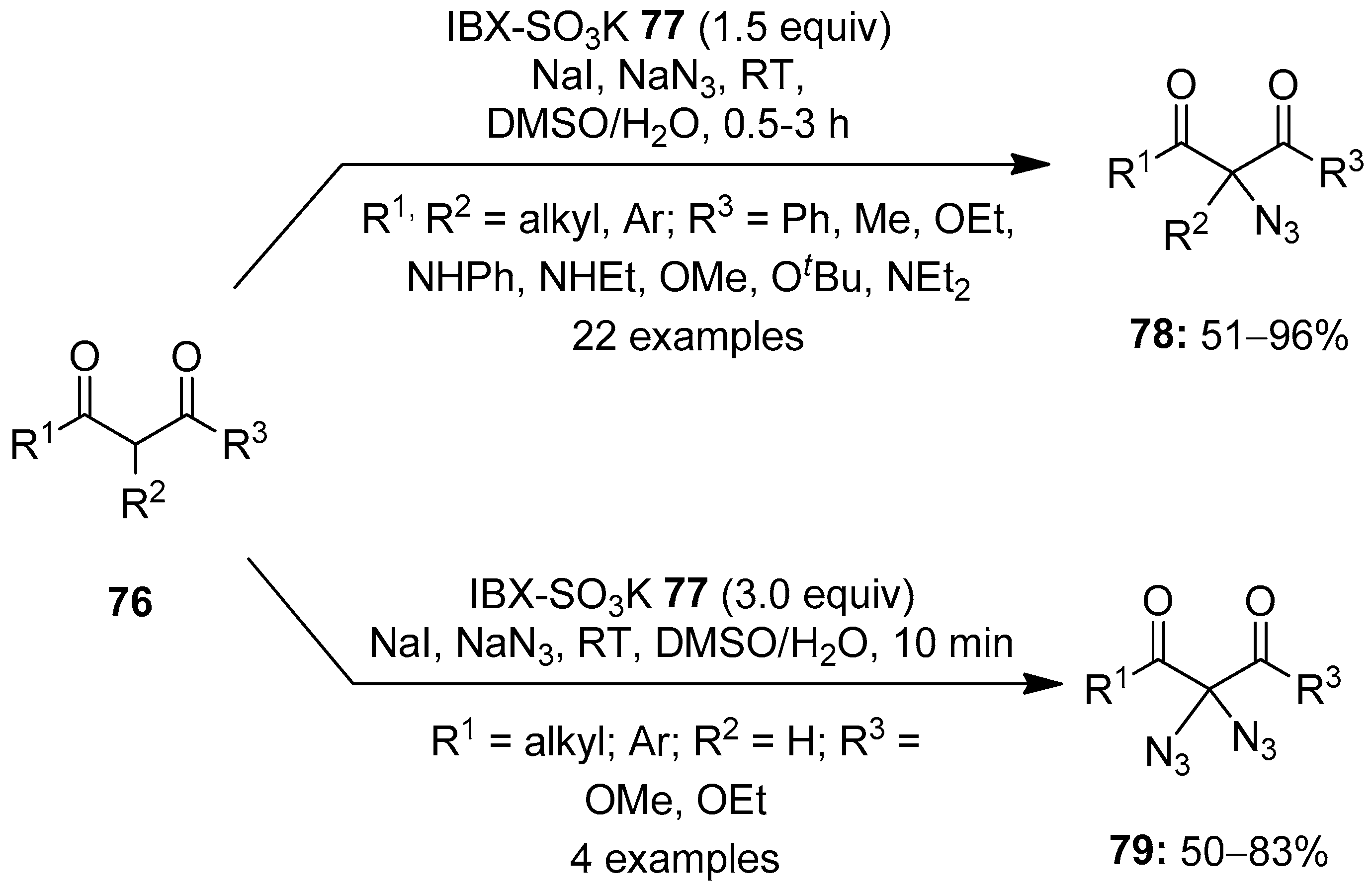
The C−H functionalization of organic compounds has emerged as a powerful tool to access biologically and pharmaceutically important molecules. The use of hypervalent iodine(V) reagents in C−H functionalization reactions is well studied, and the recent advancements in this area will be discussed in this section. In 2012, Klahn and others reported an operationally simple method for the azidation of 1,3-dicarbonyl compounds 76 using NaN3 as an azide source (Scheme 19) [89]. The reaction proceeds in the presence of 2-iodoxybenzoic acid (IBX)-SO3K 77/NaI as an oxidant. The present azidation protocol exhibited a broad substrates scope and tolerated a multitude of functional groups. Furthermore, 1,3-dicarbonyl compounds 76 with no substituent at the 2 position (R2 = H) smoothly underwent a novel double azidation reaction to furnish 2,2-bisazido-1,3-dicarbonyl compounds 79 in good yields under slightly modified conditions. Moreover, the azidation of two natural products, β-estradiol and strychnine, was achieved under these conditions in useful yields.
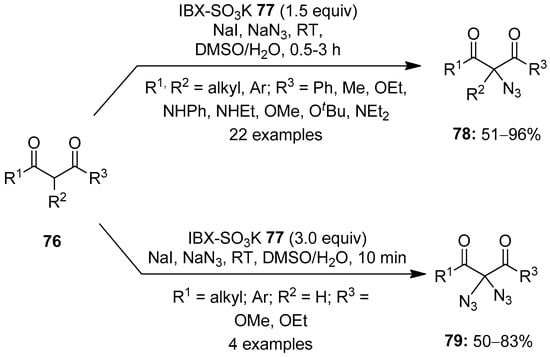
Scheme 19.
Azidation of 1,3-dicarbonyl compounds 76 to the azide products 78 and 79 using iodine(V) reagent 77 in combination with NaI.
In 2014, Akamanchi and co-workers reported an excellent method for the arylation of naphthoquinones 80 with arylhydrazines 81 using IBX 1 as an oxidizing agent (Scheme 20) [90]. The combination of arylhydrazines 81 and IBX 1 facilitates the in situ generation of aryl free radicals, which act as the aryl source. The reactions went smoothly with a number of substituted naphthoquinones 80 and arylhydrazine derivatives 81. Electronically diverse arylated naphthoquinones 82 were isolated in moderate to good yields under mild conditions. The synthetic utility of arylated naphthoquinones 82 was demonstrated through the short and high-yielding synthesis of benzocarbazoledione, an important antitumor–antibiotic precursor. Previously, the same group reported the N-arylation of aromatic amines using a combination of arylhydrazines and IBX [91].
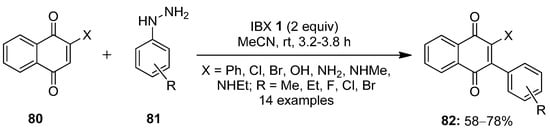
Scheme 20.
C−H arylation of naphthoquinones 80 using arylhydrazines 81 as an aryl source and IBX 1 as an oxidant.
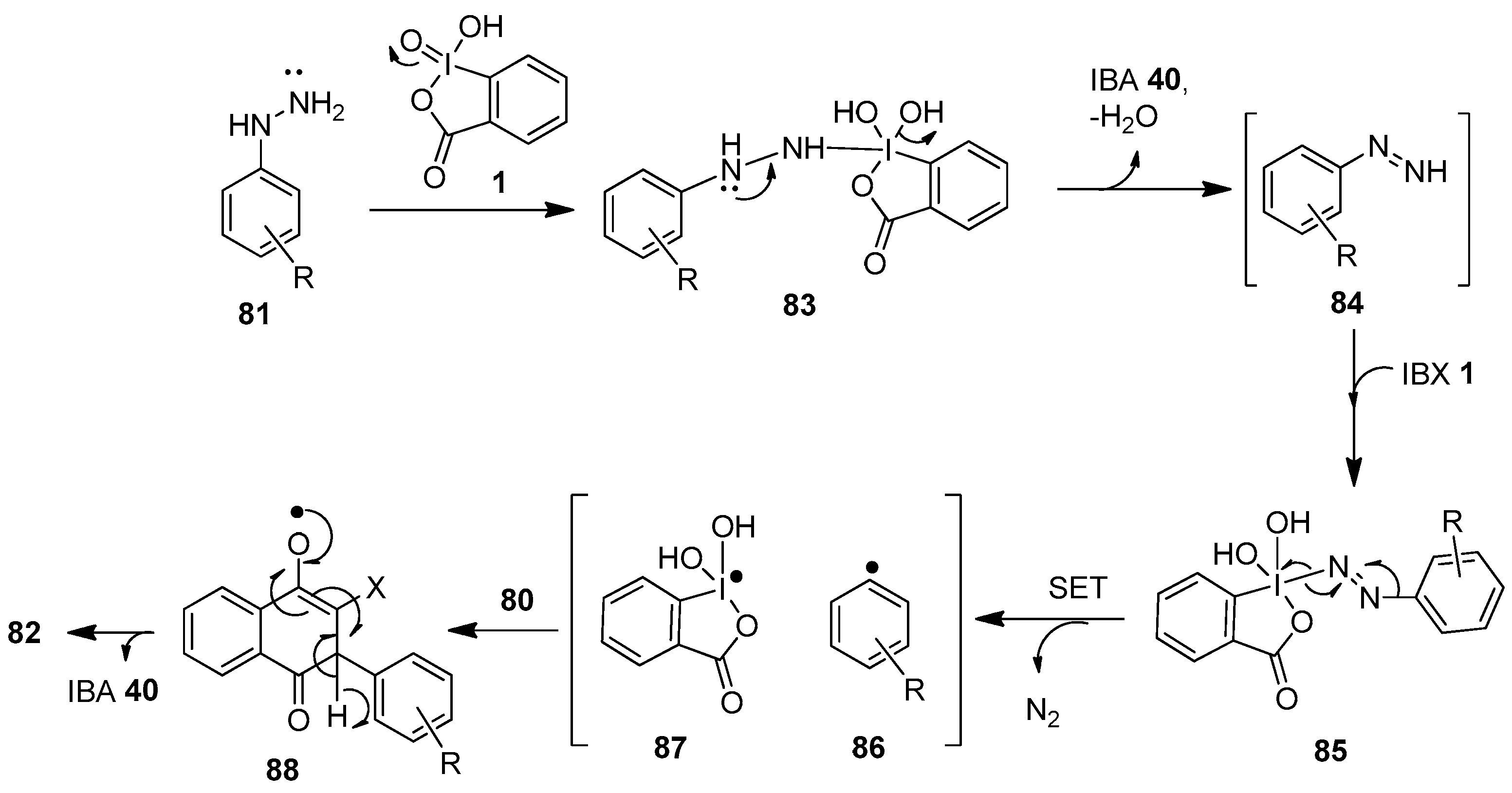
A postulated radical-mediated mechanism for the C−H arylation of naphthoquinones 80 is displayed in Scheme 21 [90]. In the beginning, IBX 1 oxidizes arylhydrazine 81 to generate the intermediate 83, which loses a water molecule to produce phenyldiazine 84 and IBA 40. Then, the nucleophilic phenyldiazine 84 attacks another IBX 1 molecule to form the intermediate 85, which later undergoes oxidative cleavage to yield the phenyl radical 86 and the species 87 through single-electron transfer (SET). Finally, the phenyl radical 86 attracts the electrophilic C-2 or C-3 positions of naphthoquinone 80 to form the intermediate 88, which provides the arylated product 82 with the release of IBA 40 and water.
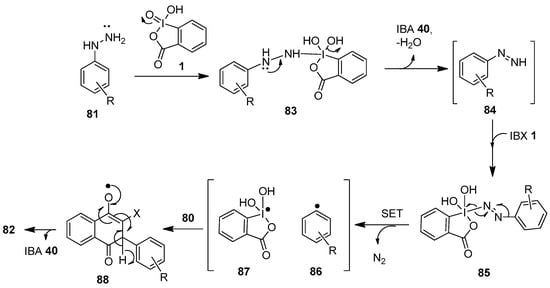
Scheme 21.
A proposed mechanism for the IBX-mediated C−H arylation of naphthoquinones 80 using arylhydrazines 81 as an aryl source.
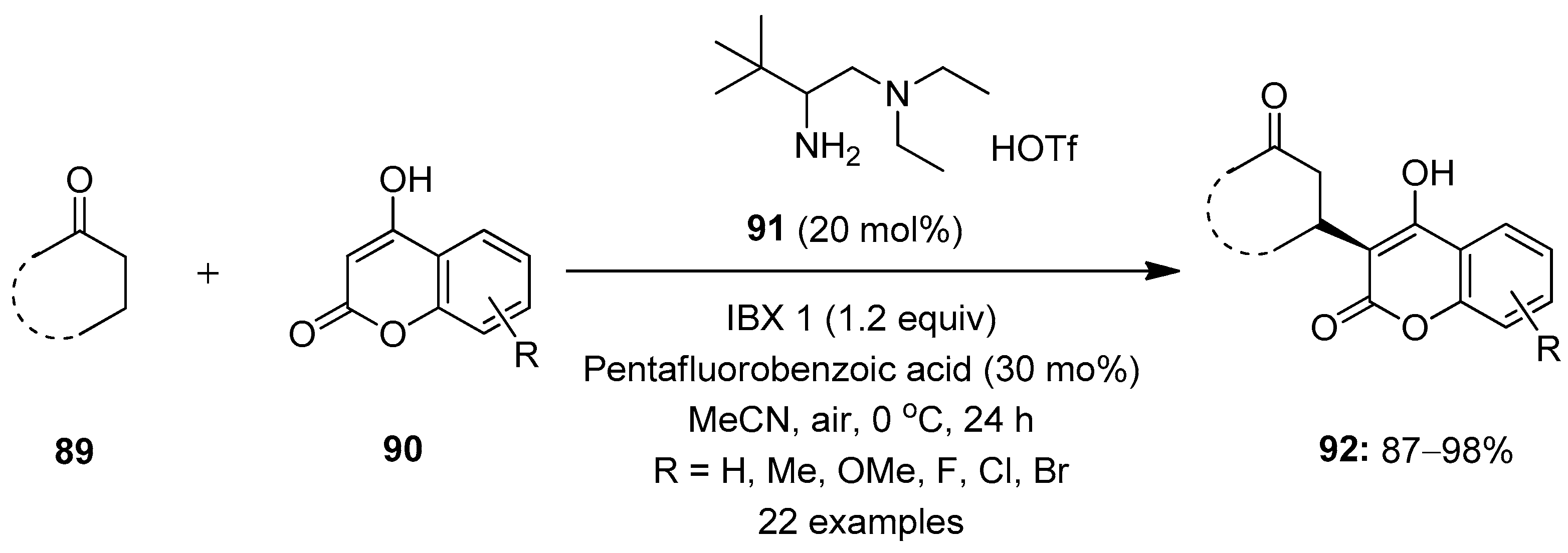
Zhu et al. reported a method involving the enantioselective β-C−H functionalization of simple ketones 89 with coumarins in the presence of the chiral primary amine 91 as a catalyst under mild oxidizing conditions, with IBX 1 as an oxidant (Scheme 22) [92]. The reaction was carried out at 0 °C in the presence of acetonitrile as a solvent. The weak acid-like additive pentafluorobenzoic acid was necessary for high reactivity and enantioselectivity. Using these conditions, cyclic and acyclic ketones 89 smoothly underwent reactions, furnishing chiral ketones 92 with β-stereocenters. A number of electron-rich and -deficient coumarins 90 as nucleophiles tolerated the reaction and produced good yields. However, the β-C−H functionalization of cyclopentanone failed, and no product formation was observed under these conditions.
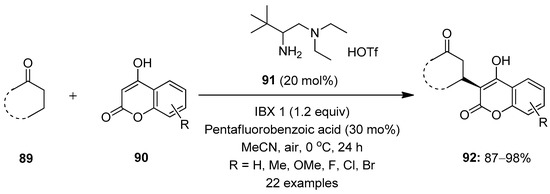
Scheme 22.
Enantioselective β-C−H functionalization of simple ketones 89 using IBX 1 as an oxidant.
8. Oxidative Cyclization Reactions
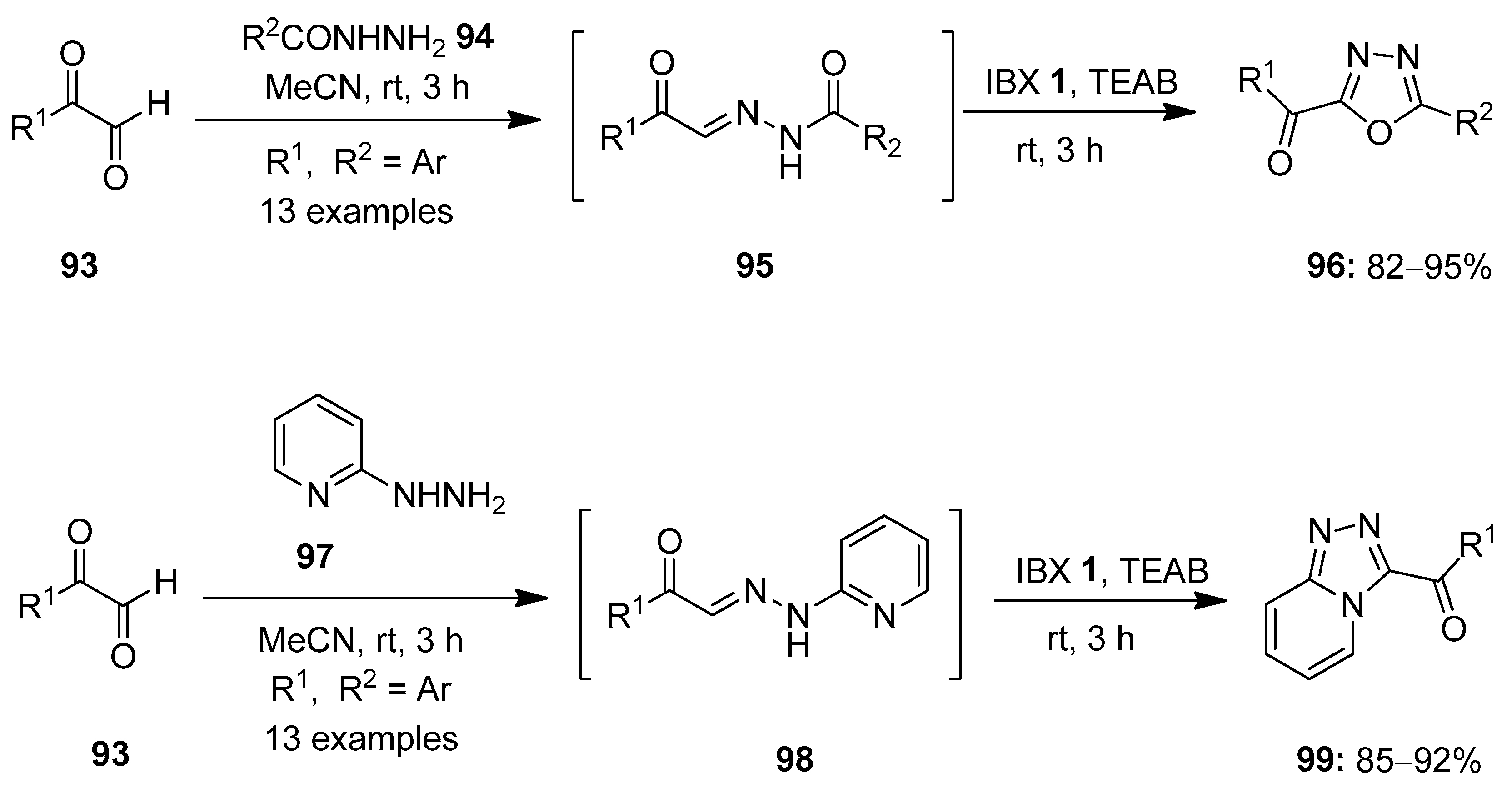
Hypervalent iodine(V) reagents have been widely used in oxidative cyclization reactions owing to their excellent electrophilic character. Several heterocycles including benzimidazoles, benzoxazoles, 1,3,4-oxadiazoles, imidazoles, imidazo-pyridines, thiazoles, thiazolines, etc., were synthesized using IBX 1 as an oxidant [93,94,95,96,97]. In this section of the review, we will discuss recent work conducted on the oxidative cyclization reactions mediated by iodine(V) reagents. In 2014, Kumar and co-workers prepared α-keto-1,3,4-oxadiazoles 96 via IBX-mediated oxidative cyclization of hydrazide-hydrazones 95 generated in situ from arylgyloxals 93 and hydrazides 94 (Scheme 23) [98]. The use of tetraethylammonium bromide (TEAB) as an additive was necessary to activate IBX 1. The key features associated with this method were high yield, mild conditions, gram -cale synthesis, short reaction times and broad functional group tolerance. Furthermore, α-keto-1,2,4-triazolo [4,3-a]pyridines 99 were synthesized from arylglyoxals 93 and 2-hydrazinopyridines 97 under the same reaction conditions.
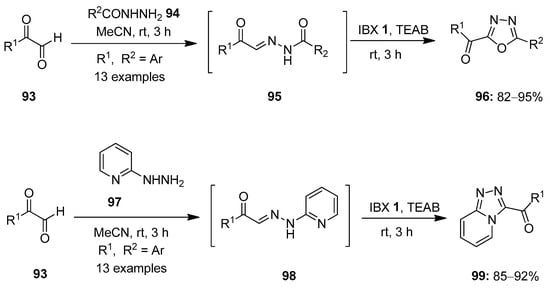
Scheme 23.
Oxidative cyclization of hydrazide-hydrazones 95 to α-keto-1,3,4-oxadiazoles 96 and α-keto-1,2,4-triazolo [4,3-a]pyridines 99 using IBX 1 as an oxidant.
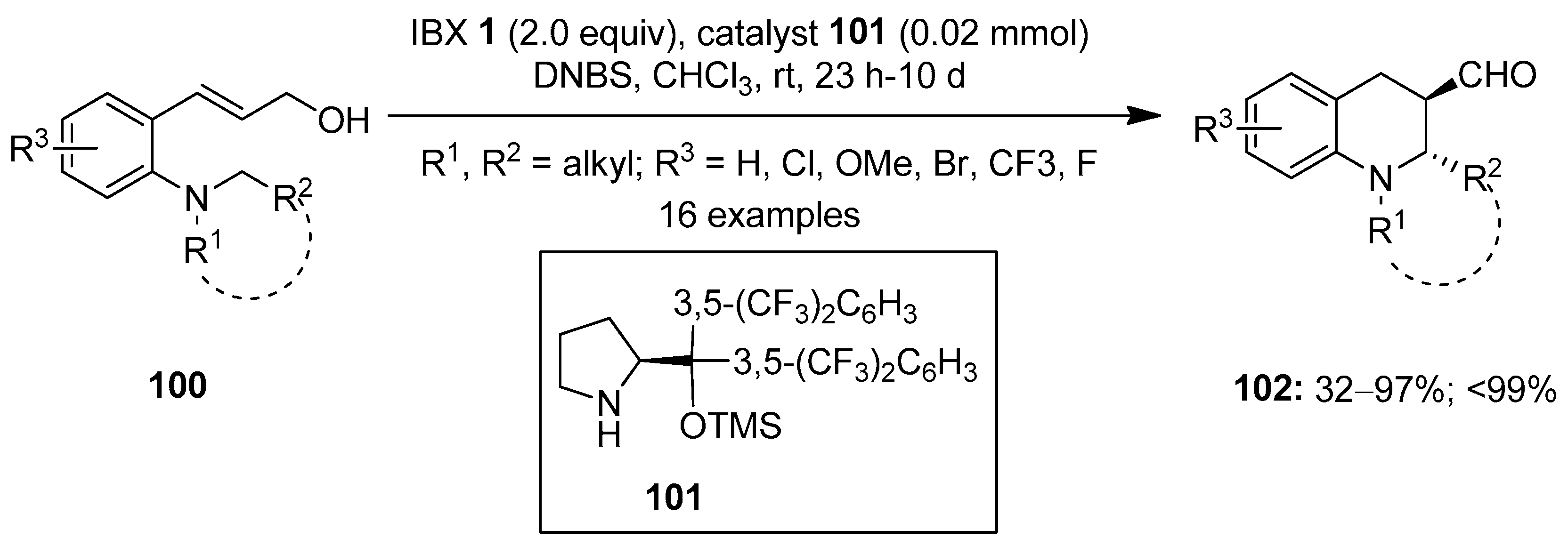
In 2015, Kim and co-workers described the synthesis of chiral tetrahydroquinolines 102 via IBX-mediated enantioselective intramolecular oxidative coupling of 3-arylprop-2-en-1-ols 100 using 2,4-dinitrobenzensulfonic acid (DNBS) 101 as a catalyst (Scheme 24) [99]. Both electron-withdrawing and electron-donating substituents were tolerated in 100, and the corresponding products 102 were isolated with excellent enantioselectivity and up to 99% ee. The reaction was proposed to proceed via the oxidation of 100 using IBX 1 followed by 1,5-hydride transfer/ring closure to yield the desired products 102.
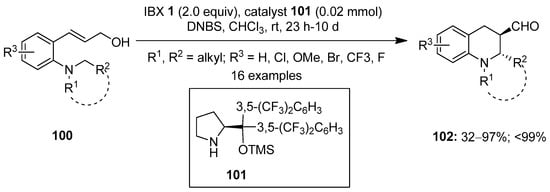
Scheme 24.
Oxidative coupling of 3-arylprop-2-en-1-ols 100 to yield chiral tetrahydroquinolines 102 using IBX 1 as an oxidant.
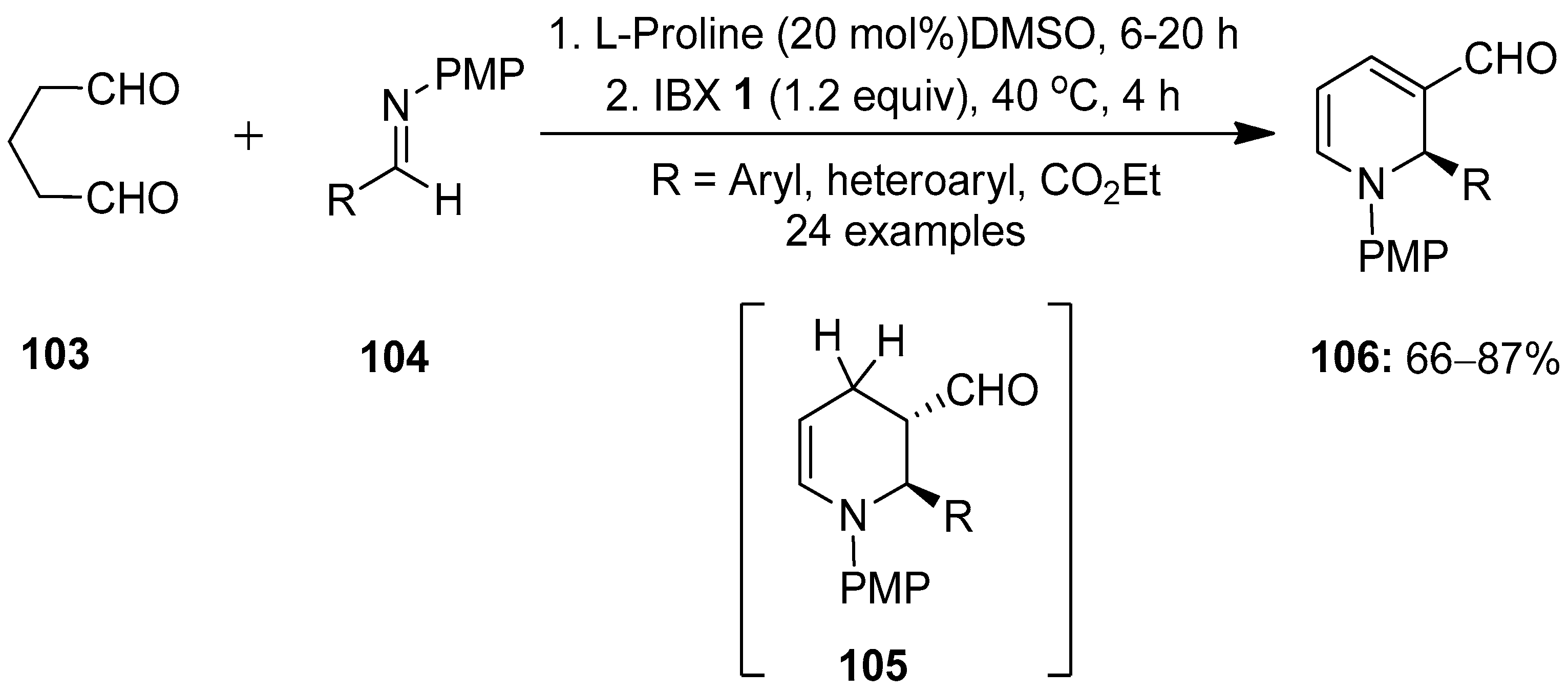
In continuation, Kumar and co-workers reported the enantioselective proline-catalyzed synthesis of N-PMP-1,2-dihydropyridines (DHPs) 106 via a one-pot [4 + 2] cycloaddition reaction (Scheme 25) [100]. This chemistry, involving the L-proline-catalyzed direct Mannich reaction/cyclization between glutaraldehyde 103 and aldimines 104 generating tetrahydropyridines 105 in situ, followed by IBX-mediated oxidation, led to the synthesis of DHPs 106. The practical utility of this method was demonstrated through the gram-scale synthesis of N-PMP-1,2-DHPs 106 and the rapid synthesis of a fused chiral tetrahydroquinoline-based skeleton.
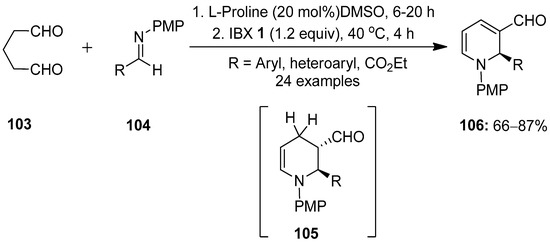
Scheme 25.
IBX-mediated synthesis of N-PMP-1,2-dihydropyridines (DHPs) 106 via the proline-catalyzed [4 + 2] cycloaddition of glutaraldehyde 103 with aldimines 104.
The same group developed an interesting approach for the preparation of pyrrole-2,4-dialdehydes by treating glutaraldehyde 103 with N-(4-methoxyphenyl)aldimines 104 in a one-pot process (Scheme 26) [101]. This pseudo-[3 + 2]-annulation reaction proceeds via a proline-catalyzed Mannich reaction/cyclization followed by an IBX-induced oxidative rearrangement to provide the final product 107. A number of aldimines 104 decorated with electron-deficient substituents such as NO2, CN, CF3, F, Cl and Br worked well under optimized reaction conditions. Additionally, heteroaromatic aldehydes-based imines 104 furnished the desired pyrrole-2,4-dialdehydes 107 in good yields. Moreover, the practical utility of this method was examined through the gram-scale synthesis of 107, the chemoselective functionalization of aldehyde groups at C2 and the synthesis of the medicinally important pyrrolo [3,2-c]quinoline scaffolds.
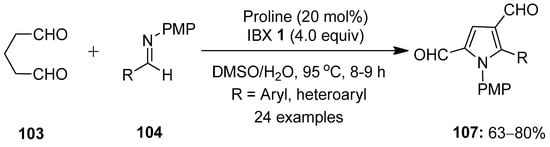
Scheme 26.
IBX-mediated synthesis of pyrrole-2,4-dialdehydes 107 by reacting glutaraldehyde 103 with N-(4-methoxyphenyl)aldimines 104 using proline as a catalyst.
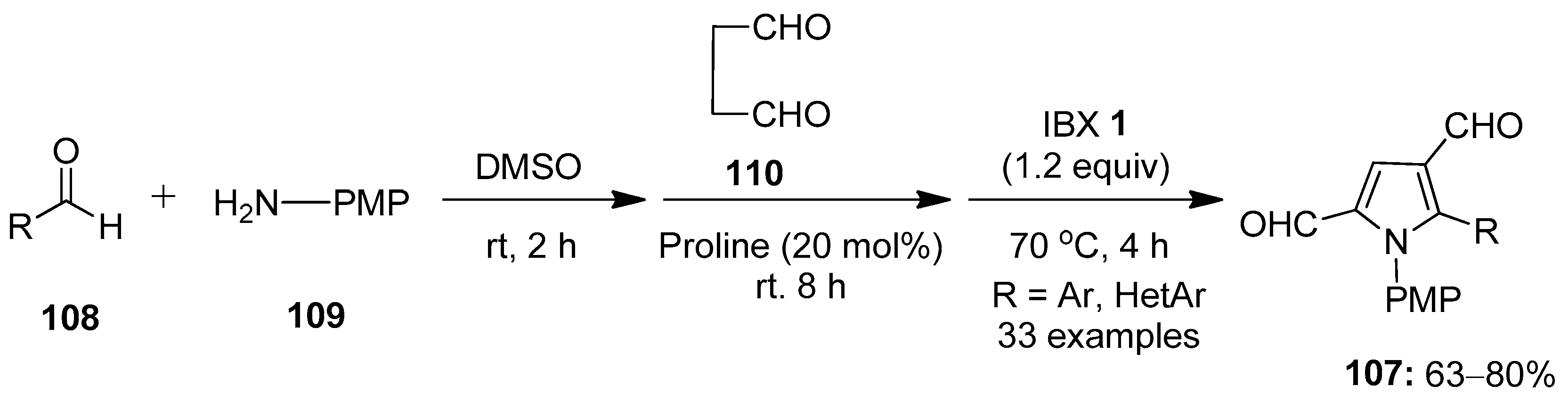
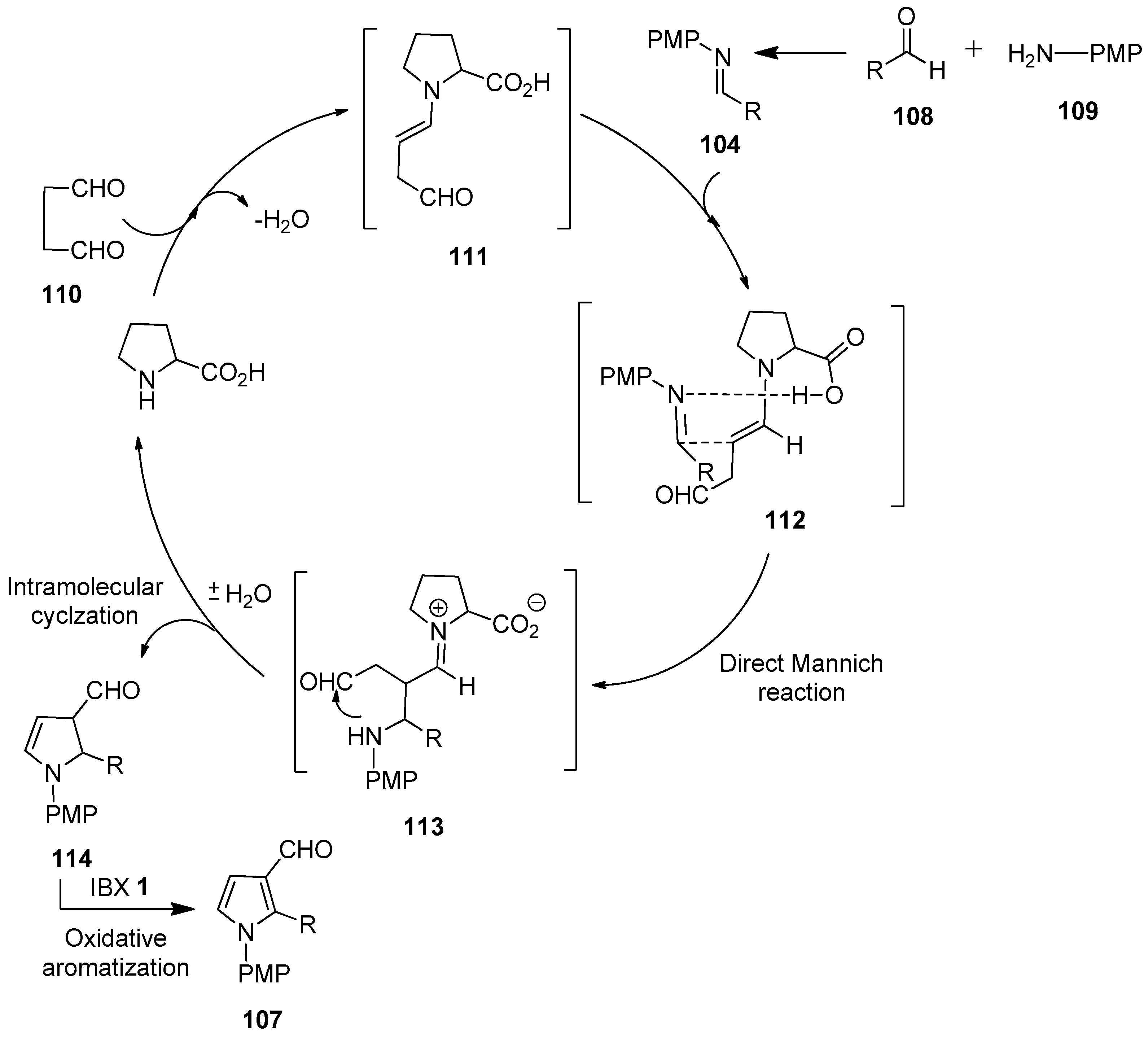
In continuation, Kumar’s group demonstrated the one-pot multicomponent synthesis of N-arylpyrrole-3-carbaldehydes 107 via the in situ formation of aldimines 104 from aldehydes 108 and aromatic amines 109, followed by sequential Mannich reaction–cyclization with succinaldehyde 110 and final IBX-mediated oxidative aromatization (Scheme 27) [102]. The scope of the reaction was examined with a variety of in situ generated aryl/hetero-aryl imines 104 to provide the corresponding products 107 in good yields.
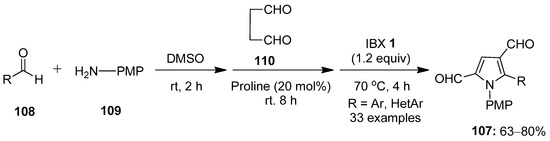
Scheme 27.
IBX-mediated synthesis of N-arylpyrrole-3-carbaldehydes 107 from in situ generated aldimines 104 and succinaldehyde 110 using proline as a catalyst.
A stepwise mechanism proposed for the one-pot synthesis of N-arylpyrrole-3-carbaldehydes 107 is depicted in Scheme 28 [102]. Initially, the reaction of succinaldehyde 110 with the proline catalyst generates enamine 111, which reacts with the in situ generated NPMP-imine 104 via a direct Mannich reaction to form the Mannich product 113. Then, the intermediate 113 undergoes intramolecular cyclization to furnish dihydropyrrole 114, along with the regeneration of the catalyst. Finally, the IBX-mediated oxidation of the cyclic enamine intermediate 114 affords pyrrole-3-carboxaldehyde 107.
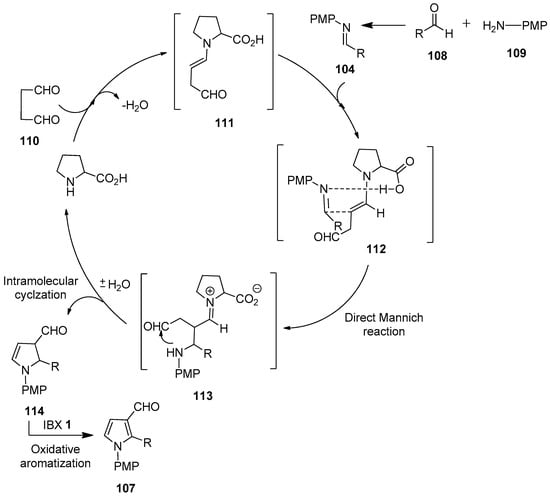
Scheme 28.
Proposed reaction mechanism for the IBX-mediated one-pot synthesis of N-arylpyrrole-3-carbaldehydes 107 using proline as a catalyst.
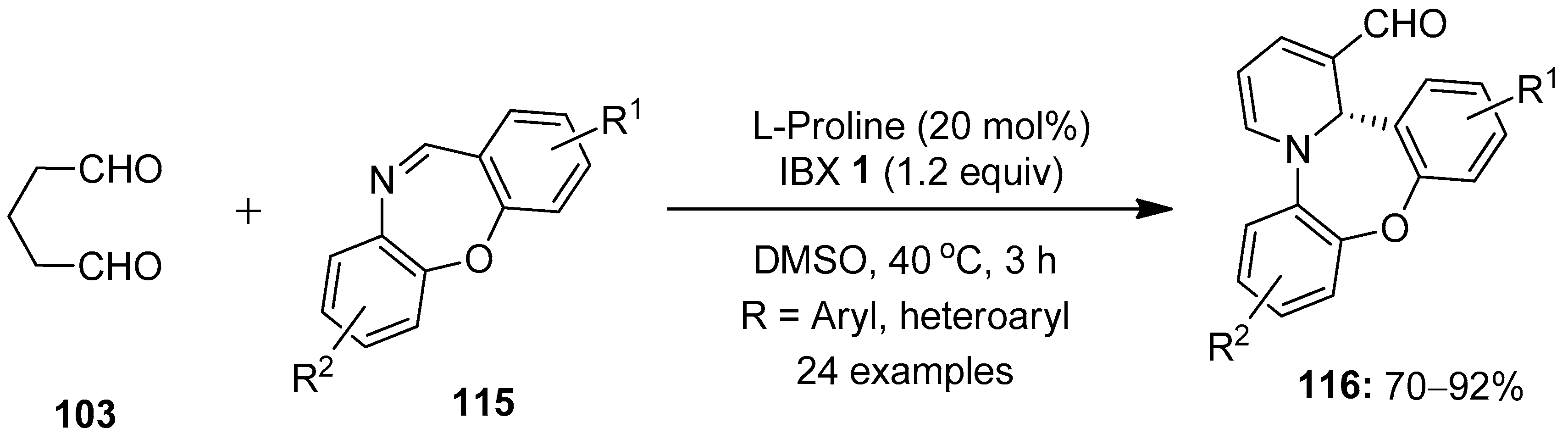
Dibenzo[b,f][1,4]oxazepine (DBO) derivatives are privileged scaffolds in organic chemistry, owing to their interesting medicinal and biological properties [103,104]. In this respect, Kumar’s group developed the synthesis of 1,4-oxazepines-fused 1,2-dihydropyridines (DHPs) 116 via a proline-catalyzed [4 + 2] annulation between glutaraldehyde 103 and cyclic imines 115 (Scheme 29) [105]. The reaction scope was explored with a variety of substituted dibenzoxazepine imines 115, and the resulting products 116 were isolated in high yields (70–92%) with excellent enantioselectivity (up to >99:1 er). However, oxazepine-imines with o-CF3 substitution failed to provide the desired products, possibly due to steric hindrance caused by the CF3 group.
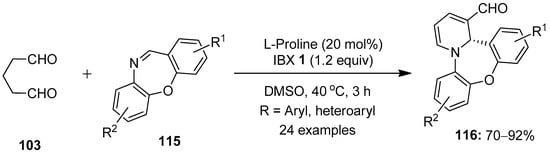
Scheme 29.
IBX-mediated synthesis of 1,4-oxazepines-fused 1,2-dihydropyridines 116 from glutaraldehyde 103 and cyclic imines 115.
In 2019, Makra et al. developed the hypervalent iodine-mediated intramolecular oxidative annulation of Mannich precursors 117 towards the synthesis of imidazo [1,2-a]-fused heterobicyclic scaffolds 119 via a C–H functionalization/C–N bond formation strategy (Scheme 30) [106]. Among the tested oxidants, IBX 1 provided the highest product yield. A variety of Mannich precursors were treated with IBX 1 (1.1 equiv.) in the presence of NIS 118 (1.5 equiv.) as an additive in DMA to yield functionally diverse imidazo [1,2-a]-pyridine, -pyrimidine and -pyrazine scaffolds. Gratifyingly, the one-pot synthesis of the selected compounds 119 was achieved by reacting β-keto esters with primary aromatic amines and aldehydes in the presence of phosphotungstic acid (PTA) and IBX/NIS, providing overall yields up to 25%. The synthesized imidazo [1,2-a]pyridine motif (IPY) is a key structural unit in various bioactive compounds [107,108].
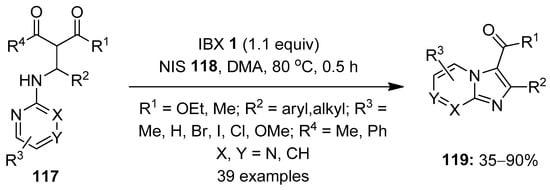
Scheme 30.
Oxidative cyclization of the Mannich precursors 117 to imidazo [1,2-a]-fused heterobicyclic scaffolds 119 using IBX 1 as an oxidant.
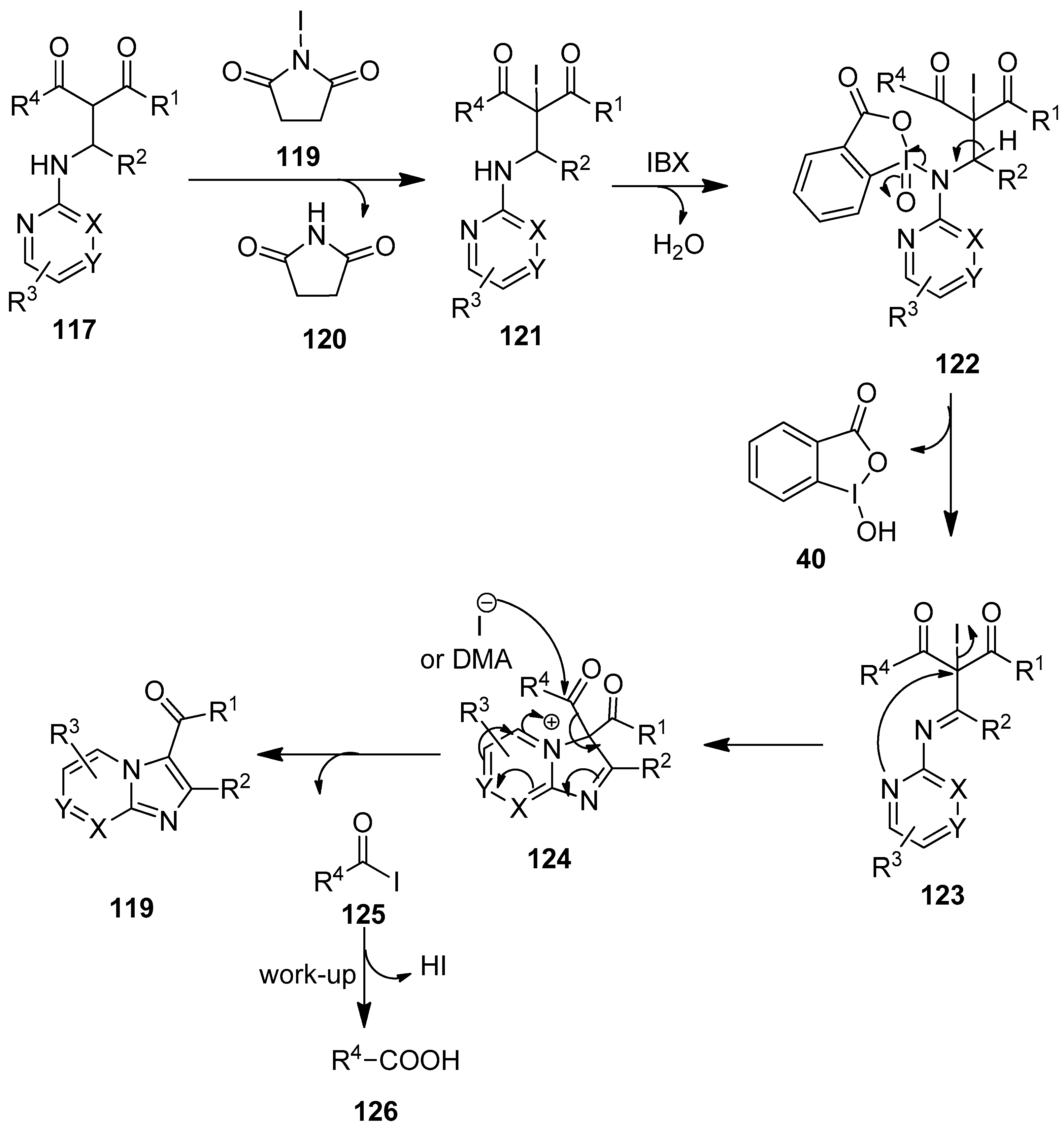
A plausible mechanism for the oxidative annulation reaction of 117 is depicted in Scheme 31 [106]. The reaction begins with the α-halogenation of the Mannich precursor 117 with NIS 118 to afford the iodo intermediate 121, followed by subsequent NH-oxidation with IBX 1, yielding the intermediate 123. Then the intermediate 123 cyclizes intramolecularly through the formation of a new C–N bond to produce the corresponding intermediate 124. Finally, stabilization with the retro-Claisen–Schmidt reaction leads to the desired product 119.
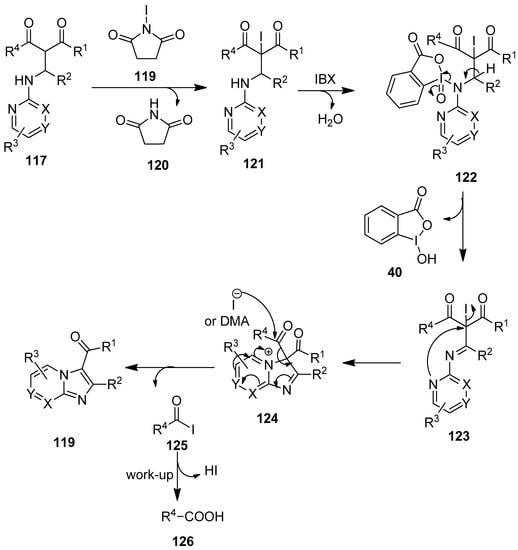
Scheme 31.
The plausible mechanism for the oxidative cyclization of the Mannich precursors 117 using IBX 1 as an oxidant.
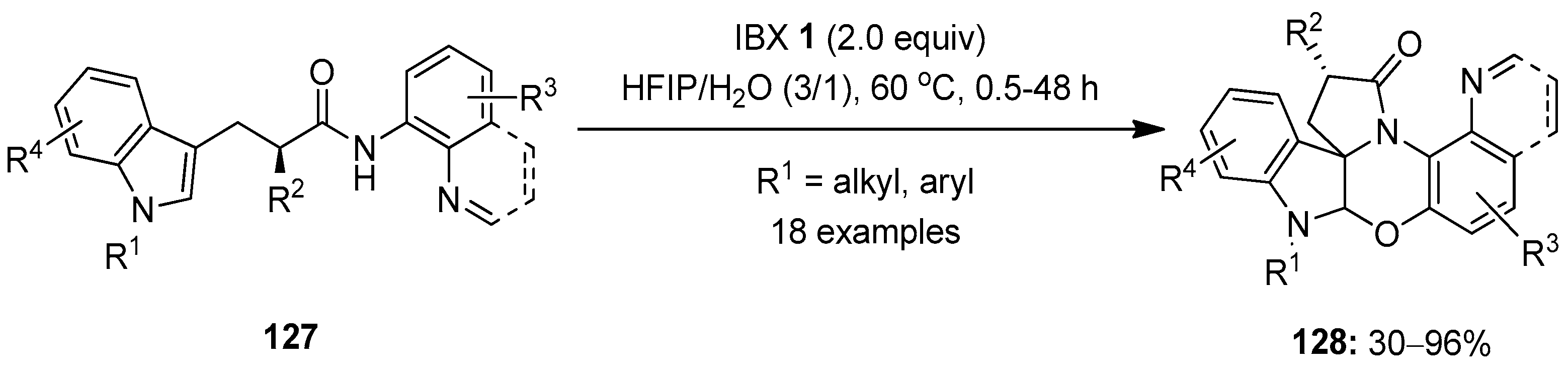
In 2020, Zhang et al. demonstrated the IBX-mediated tandem oxidation–cyclization of tryptophan analogs 127 with N-arylamide side chains, producing a library of polycyclic spiroindolines 128 under mild conditions (Scheme 32) [109]. A number of N-protected tryptophan derivatives 127 worked well, and the anticipated oxazine-bearing complex polycyclicindolines 128 were synthesized in 30–96% yields. However, the N-unprotected tryptophan analog 127 (R1 = H) failed to yield the desired product. The key feature of this tandem cyclization reaction is the creation of multiple stereocenters, including a quaternary stereocenter, in a single step.
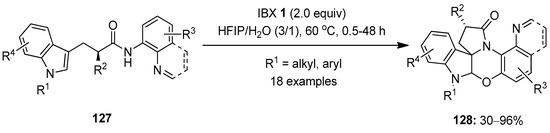
Scheme 32.
Tandem oxidation–cyclization of tryptophan analogs 127 to yield polycyclic spiroindolines 128 using IBX 1 as an oxidant.
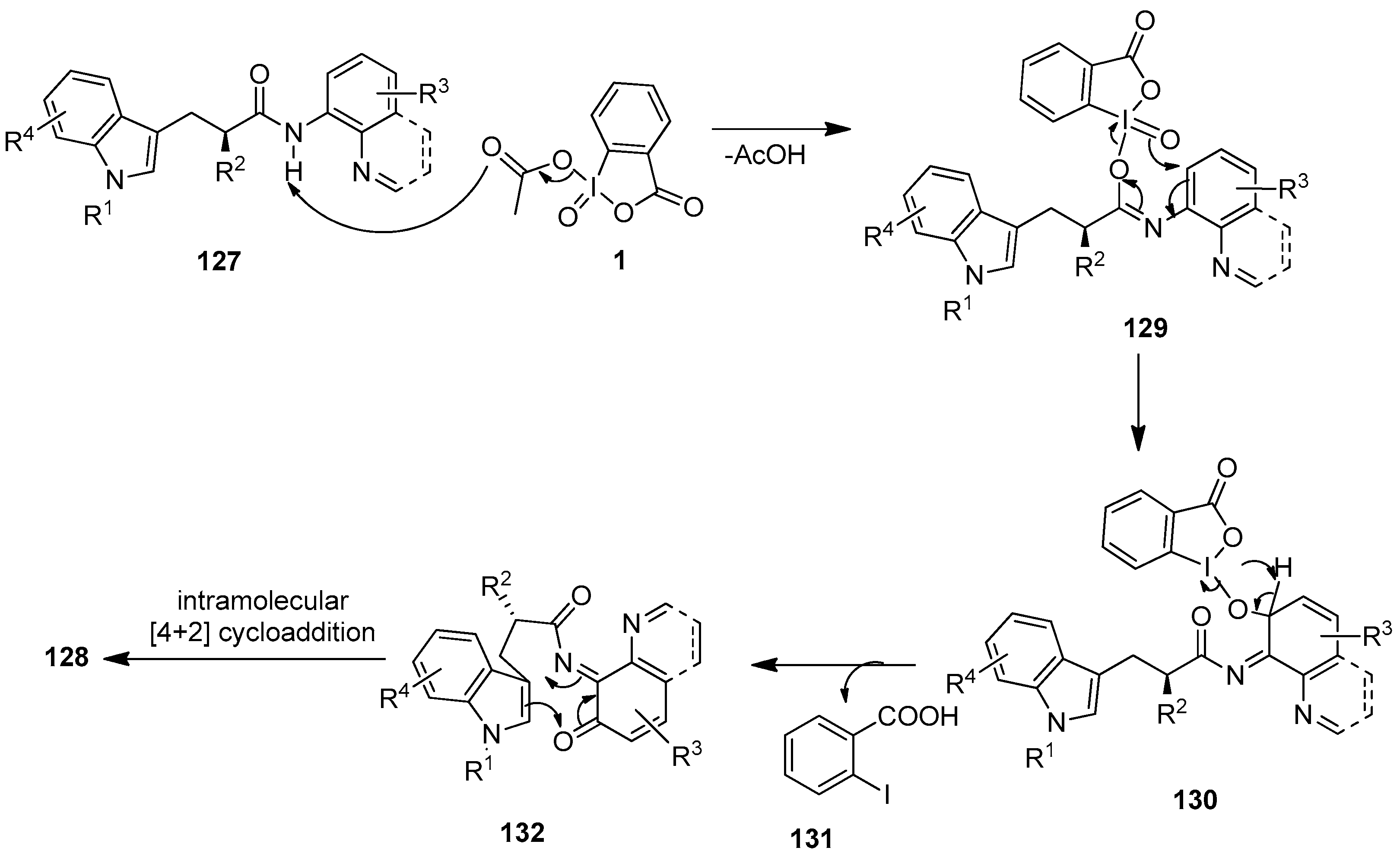
The proposed mechanism for the IBX-mediated spiro-fused cyclization of tryptophan analogs 127 is shown in Scheme 33 [109]. The reaction initiates with the attack of an amide O atom on to the iodine center of IBX 1 to produce the iodoimidate intermediate 129 with the release of one AcOH molecule. Then, the nucleophilic oxo group on the iodine center of 129 intramolecularly attacks the ortho-position of the aminoquinoline (AQ) group, triggering the dearomatization of the aniline ring, followed by the cleavage of the O−I bond to yield the intermediate 130. Deprotonation and subsequent cleavage of the O−I bond of 130 generates the o-iminoquinone intermediate 132 along with 2-iodobenzoic acid 131 as a by-product. Finally, o-imidoquinone 132 undergoes an intramolecular [4 + 2] cycloaddition to furnish polycyclic 128.
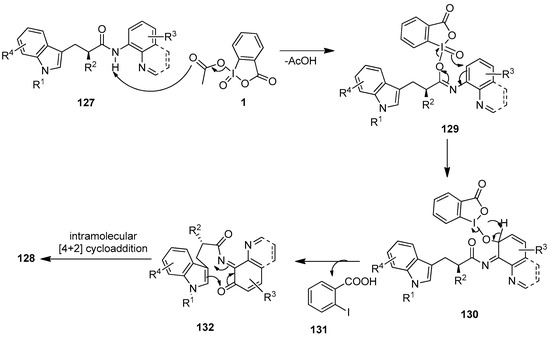
Scheme 33.
The proposed mechanism for the tandem oxidation–cyclization of tryptophan analogs 127 using IBX 1 as an oxidant.
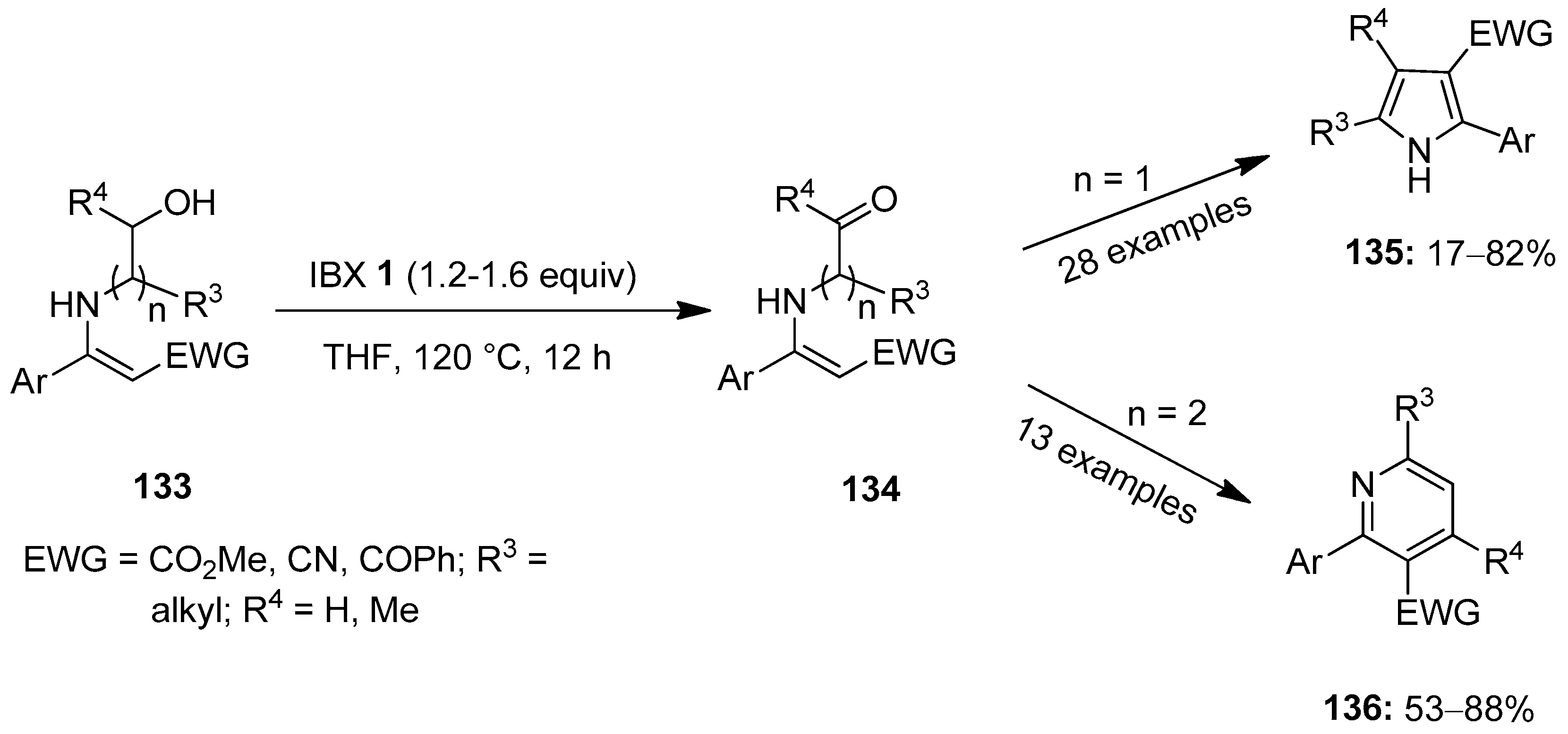
In the same year, Gao et al. presented an important method for the synthesis of 2,3-disubstituted pyrroles 135 through the IBX-mediated oxidative cyclization of N-hydroxyethyl enamines 133 (n = 1) via the intermediate 134 (Scheme 34) [110]. Phenyl enaminoesters substituted with methoxy, fluoro, chloro and bromo groups provided the desired pyrrole 135 in good yields. Likewise, substrates with electron-withdrawing groups such as CO2Me, -CO2Et, -CN and -COPh, were well tolerated under these conditions. Further exchanging the N-substituted moiety with N-hydroxypropyls (n = 2) yielded 2,3-disubstituted pyridines 136 in moderate to good yields.
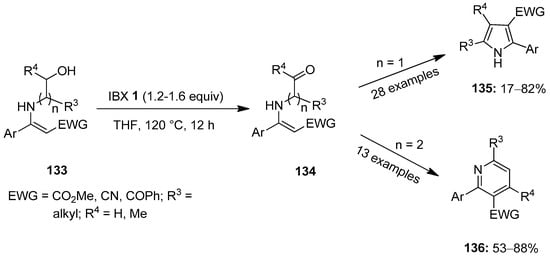
Scheme 34.
Oxidative cyclization of N-hydroxyethyl enamines 133 to yield 2,3-disubstituted pyrroles 135 and pyridines 136 using IBX 1 as an oxidant.
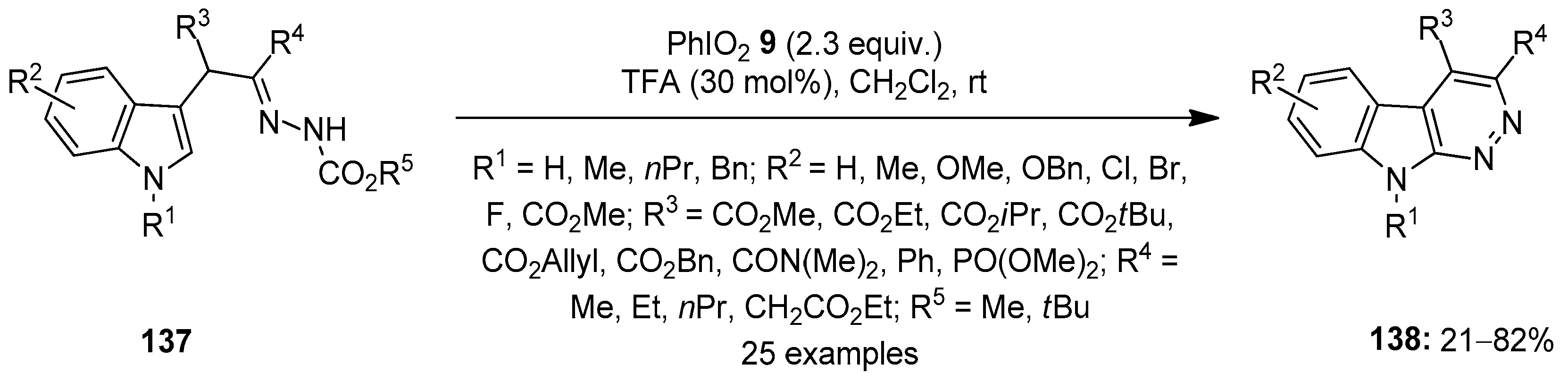
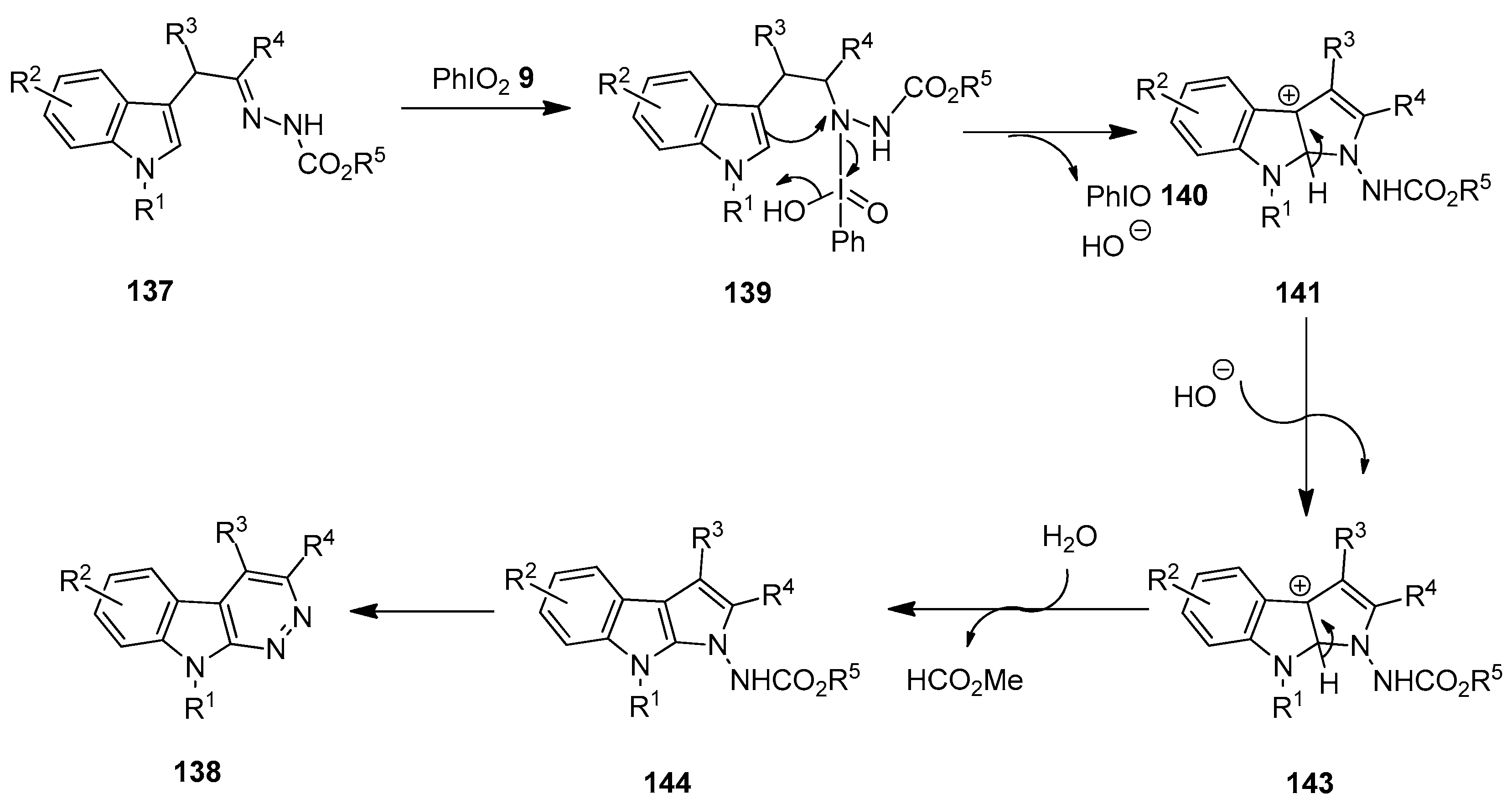
In 2021, Favi and others developed an unprecedented method to access polysubstituted indolefused pyridazines 138 via the intramolecular oxidative cyclization of α-indolylhydrazones 137 using iodylbenzene (PhIO2) 9 as an oxidant (Scheme 35) [111]. The addition of TFA (20 mol%) was essential for the smooth proceeding of the reaction. The substrate scope of the cycloamination reaction was investigated with an array of α-indolylhydrazones 137, and the anticipated azacarbolines 138 were obtained in good to excellent yields.
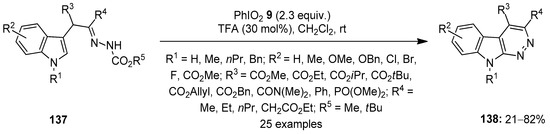
Scheme 35.
Oxidative cyclization of α-indolylhydrazones 137 to yield azacarbolines 138 using PhIO2 9 as an oxidant.
A proposed mechanistic pathway for the C(sp2)−H/N−H dehydrogenative coupling reaction of α-indolylhydrazones 137 is depicted in Scheme 36 [111]. The reaction begins with the oxidation of 137 by PhIO2 9 to form the N-iodo intermediate 139 following CH/NH tautomerization. Subsequently, the intramolecular electrophilic cyclization of indole at C-2 with activated nitrogen generates the intermediate 141 with the release of PhIO 140 and HO−. The further deprotonation and aromatization lead to the key intermediate, pyrrolo [2,3-b]indole 143. Finally, the hydrolysis of the intermediate 143 followed by ring expansion and oxidative aromatization affords the expected azacarboline product 138.
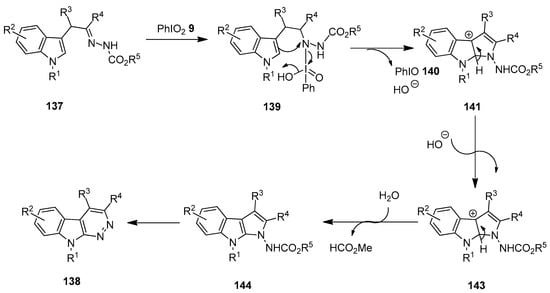
Scheme 36.
Tentative mechanism for the oxidative cyclization of α-indolylhydrazones 137 using PhIO2 9 as an oxidant.
9. Miscellaneous Reactions
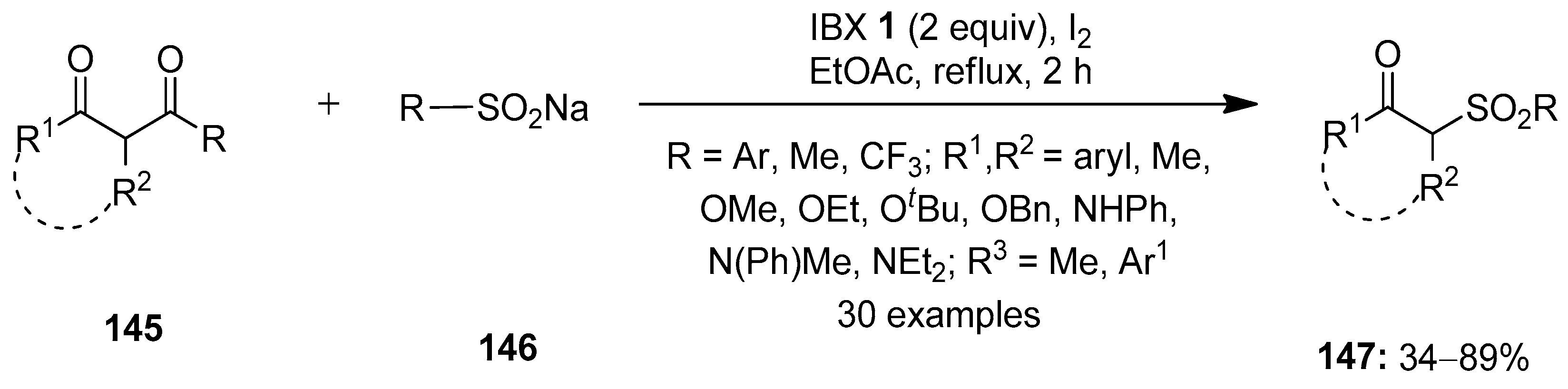
In 2016, Kuhakarn disclosed the deacylative sulfonylation of 1,3-dicarbonyl compounds 145 with sodium sulfinates 146 by employing IBX 1 and a catalytic amount of iodine (Scheme 37) [112]. This led to the one-pot synthesis of β-carbonyl sulfones 147 in good yields with a broad substrates scope. Notably, benzoylacetone derivatives 145 with electron-donating groups (Me, tBu, OMe) provided higher product yields compared to derivatives with electron-attracting groups (Cl and NO2). The reactions with acetylacetone, β-keto esters and β-keto amides as substrates yielded the corresponding products in low to moderate yields. The same group previously reported the synthesis of β-keto sulfones by reacting alkenes with sodium arenesulfinates in the presence of IBX–iodine [113].
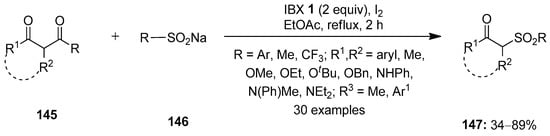
Scheme 37.
IBX–I2-mediated synthesis of β-carbonyl sulfones via the deacylative sulfonylation of 1,3-dicarbonyl compounds.
10. Conclusions
This review summarized the recent developments in oxidative transformation reactions using hypervalent iodine(V) reagents. Hypervalent iodine compounds have emerged as versatile, non-toxic and environment friendly oxidants in organic synthesis. Although the chemistry of trivalent iodine reagents is well developed, the synthetic application of organoiodine(V) reagents has seen considerable growth only in recent times. Various synthetic transformations such as oxidation of alcohols, oxidation of amines, oxidation of amides, oxidation of aromatic compounds, oxidation of alkenes and oxidative cyclizations have been achieved using iodine(V) reagents. In particular, 2-iodoxybenzoic acid (IBX) and Dess–Martin periodinane (DMP) have received great attention owing to their mild oxidizing properties, high chemoselectivity and broad applicability. Moreover, significant work has been accomplished for the development of new catalytic systems based on in situ generated hypervalent iodine(V) reagents through the oxidation of organoiodine compounds. Addressing the solubility issues of IBX and designing new catalytic systems involving the in situ generation of hypervalent iodine(V) species represent an intriguing area of future investigation. In addition, the development of novel recyclable polymer-supported hypervalent iodine(V) reagents is a topic of great interest from a future perspective.
Author Contributions
S.E.S. reviewed the literature and compiled the different oxidation reactions including C-H functionalization. S.J. devoted efforts to the compilation of oxidative cyclizations and miscellaneous reactions. The editing of the review manuscript was carried out by F.V.S. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
S.E.S. acknowledges the School of Chemical Sciences, Goa University, Taleigao Plateau, Goa, India, for providing infrastructure. F.V.S. and S.J. acknowledge the support of VIT Chennai, Chennai. F.V.S. acknowledges support from CSIR New Delhi, India (Grant Number: 02/(0330)/17-EMR-II). T.D. acknowledges support from the Ritsumeikan Global Innovation Research Organization (R-GIRO) project.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Wirth, T. (Ed.) Hypervalent Iodine Chemistry Modern Developments in Organic Synthesis; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhdankin, V.V. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; Wiley: Chichester, UK, 2013; Volume I. [Google Scholar]
- Singh, F.V. Thomas Wirth, in Comprehensive Organic Synthesis II; Knochel, P., Molander, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; p. 880. [Google Scholar]
- Singh, F.V.; Wirth, T. Science of Synthesis: Catalytic Oxidation in Organic Synthesis; Muñiz, K., Ed.; Thieme: Stuttgart, Germany, 2017; p. 29. [Google Scholar]
- Singh, F.V.; Wirth, T. The Chemistry of Hypervalent Halogen Compounds; Olofsson, B., Ilan, M., Zvi, R., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2018; p. 809. [Google Scholar]
- Wang, Z. Innovation of hypervalent(III) iodine in the synthesis of natural products. New J. Chem. 2021, 45, 509–516. [Google Scholar] [CrossRef]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, F.V.; Takenaga, N.; Dohi, T. Asymmetric Direct/Stepwise Dearomatization Reactions Involving Hypervalent Iodine Reagents. Chem. Asian J. 2022, 17, e202101115. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, F.D.; Dave, L.; David, N.; Kaur, K.; Medard, M.; Mowdawalla, C. Hypervalent iodine reactions utilized in carbon–carbon bond formations. Org. Biomol. Chem. 2019, 17, 7822–7848. [Google Scholar] [CrossRef] [PubMed]
- Shetgaonkar, S.E.; Singh, F.V. Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions. Front. Chem. 2020, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Shetgaonkar, S.E.; Mamgain, R.; Kikushima, K.; Dohi, T.; Singh, F.V. Palladium-Catalyzed Organic Reactions Involving Hypervalent Iodine Reagents. Molecules 2022, 27, 3900. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-G.; Hu, Z.-N.; Jia, M.-C.; Du, F.-H.; Zhang, C. Recent Advances and the Prospect of Hypervalent Iodine Chemistry. Synlett 2021, 32, 1289–1296. [Google Scholar] [CrossRef]
- Kokila, S.; Kole, P.B.; Mamgain, R.; Singh, F.V. Iodine(III)-Based Hypervalent Iodine Electrophiles in Organic Synthesis. Curr. Org. Chem. 2022, 26, 1917–1934. [Google Scholar] [CrossRef]
- Shetgaonkar, S.E.; Raju, A.; China, H.; Takenaga, N.; Dohi, T.; Singh, F.V. Non-Palladium-Catalyzed Oxidative Coupling Reactions Using Hypervalent Iodine Reagents. Front. Chem. 2022, 10, 909250. [Google Scholar] [CrossRef]
- Maiti, S.; Alam, M.T.; Bal, A.; Mal, P. Nitrenium Ions from Amine-Iodine(III) Combinations. Adv. Synth. Catal. 2019, 361, 4401–4425. [Google Scholar] [CrossRef]
- Zhdankin, V.V. Hypervalent iodine compounds: Reagents of the future. Arkivoc 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Selenium-Catalyzed Regioselective Cyclization of Unsaturated Carboxylic Acids Using Hypervalent Iodine Oxidants. Org. Lett. 2011, 13, 6504–6507. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, M.; Ishihara, K. Hypervalent iodine-mediated oxidation of alcohols. Chem. Commun. 2009, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Mangaonkar, S.; Kole, P.B. Ultrasound-assisted rapid synthesis of β-cyanoepoxides using hypervalent iodine reagents. Synth. Commun. 2018, 48, 2169–2176. [Google Scholar] [CrossRef]
- Mangaonkar, S.R.; Kole, P.B.; Singh, F.V. Oxidation of Organosulfides to Organosulfones with Trifluoromethyl 3-Oxo-1λ3,2-benziodoxole-1(3H)-carboxylate as an Oxidant. Synlett 2018, 29, 199–202. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Hypervalent Iodine(III) Mediated Cyclization of ortho-Stillbenes into Benzofurans. Synthesis 2012, 44, 1171–1177. [Google Scholar] [CrossRef]
- Singh, F.V.; Kole, P.B.; Mangaonkar, S.R.; Shetgaonkar, S.E. Synthesis of spirocyclic scaffolds using hypervalent iodine reagent. Beilstein J. Org. Chem. 2018, 14, 1778–1805. [Google Scholar] [CrossRef]
- Shetgaonkar, S.E.; Singh, F.V. Hypervalent iodine-mediated synthesis and late-stage functionalization of heterocycles. Arkivoc 2020, 2020, 86–161. [Google Scholar] [CrossRef]
- Singh, F.V.; Rehbein, J.; Wirth, T. Facile Oxidative Rearrangements Using Hypervalent Iodine Reagents. ChemistryOpen 2012, 1, 245–250. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Oxidative rearrangements with hypervalent iodine reagents. Synthesis 2013, 45, 2499–2511. [Google Scholar] [CrossRef]
- Shetgaonkar, S.E.; Krishnan, M.; Singh, F.V. Hypervalent Iodine Reagents for Oxidative Rearrangements. Mini Rev. Org. Chem. 2021, 18, 138. [Google Scholar] [CrossRef]
- Merritt, E.A.; Olofsson, B. α-Functionalization of carbonyl compounds using hypervalent iodine reagents. Synthesis 2011, 4, 517–538. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Hao, S.-H.; Wang, Z.-L.; Chen, C. Hypervalent iodine: A powerful electrophile for asymmetric α-functionalization of carbonyl compounds. Org. Biomol. Chem. 2014, 12, 4278–4289. [Google Scholar] [CrossRef]
- Li, Y.; Hari, D.P.; Vita, M.V.; Waser, J. Cyclic hypervalent iodine reagents for atom-transfer reactions: Beyond trifluoromethylation. Angew. Chem. Int. Ed. 2016, 55, 4436–4454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, P.; Liu, G. Recent advances in hypervalent iodine(III)-catalyzed functionalization of alkenes. Beilstein J. Org. Chem. 2018, 14, 1813–1825. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.; Hong, K.B. Alkene Difunctionalization Using Hypervalent Iodine Reagents: Progress and Developments in the Past Ten Years. Molecules 2019, 24, 2634. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Wirth, T. Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions. Chem. Asian J. 2014, 9, 950–971. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.V.; Mangaonkar, S.R. Hypervalent Iodine(III)-Catalyzed Synthesis of 2-Arylbenzofurans. Synthesis 2018, 50, 4940–4948. [Google Scholar] [CrossRef]
- Mangaonkar, S.R.; Singh, F.V. Hypervalent Iodine(III)-Catalyzed Epoxidation of β-Cyanostyrenes. Synthesis 2019, 51, 4473–4486. [Google Scholar] [CrossRef]
- Mangaonkar, S.R.; Shetgaonkar, S.E.; Vernekar, A.; Singh, F.V. Ultrasonic-Assisted Hypervalent Iodine-Catalyzed Cyclization of (E)-2-Hydroxystilbenes to Benzofurans Using Iodobenzene as Pre-catalyst. ChemistrySelect 2020, 5, 10754–10758. [Google Scholar] [CrossRef]
- Dohi, T.; Kita, Y. Iodine Catalysis in Organic Synthesis; Ishihara, K., Muñiz, K., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2022; p. 211. [Google Scholar]
- Singh, F.V.; Shetgaonkar, S.E.; Krishnan, M.; Wirth, T. Progress in organocatalysis with hypervalent iodine catalysts. Chem. Soc. Rev. 2022, 51, 8102–8139. [Google Scholar] [CrossRef] [PubMed]
- Antonchick, A.P.; Narayan, R.; Manna, S. Hypervalent Iodine(III) in Direct Carbon–Hydrogen Bond Functionalization. Synlett 2015, 26, 1785–1803. [Google Scholar] [CrossRef]
- Parra, A. Chiral Hypervalent Iodines: Active Players in Asymmetric Synthesis. Chem. Rev. 2019, 119, 12033–12088. [Google Scholar] [CrossRef] [PubMed]
- Shetgaonkar, S.E.; Singh, F.V. Catalytic stereoselective synthesis involving hypervalent iodine-based chiral auxiliaries. Org. Biomol. Chem. 2023, 21, 4163–4180. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Hypervalent iodine chemistry and light: Photochemical reactions involving hypervalent iodine Chemistry. Arkivoc 2021, 2021, 12–47. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Yang, T. Recent Synthetic Applications of the Hypervalent Iodine(III) Reagents in Visible-Light-Induced Photoredox Catalysis. Front. Chem. 2020, 8, 551159. [Google Scholar] [CrossRef]
- Ladziata, U.; Zhdankin, V.V. Hypervalent iodine(V) reagents in organic synthesis. Arkivoc 2006, 2006, 26–58. [Google Scholar] [CrossRef]
- Zhdankin, V.V. Organoiodine(V) Reagents in Organic Synthesis. J. Org. Chem. 2011, 76, 1185–1197. [Google Scholar] [CrossRef]
- Hartman, C.; Mayer, V. Ueber Jodobenzoësäure. Chem. Ber. 1893, 26, 1727–1732. [Google Scholar] [CrossRef]
- Boeckman, R.K.; Shao, P.; Mullins, J. The Dess-Martin Periodinane: 1,1,1-Triacetoxy-1,1-Dihydro-1,2-Benziodoxol-3(1H)-One. J. Org. Synth. 2000, 77, 141. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Nageswar, Y.V.D.; Ramesh, K.; Rakhi, K. IBX-Mediated Organic Transformations in Heterocyclic Chemistry-A Decade Update. Front. Chem. 2022, 10, 841751. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Vinod, T.K. Synthesis and oxidation reactions of a user- and eco-friendly hypervalent iodine reagent. Tetrahedron Lett. 2002, 43, 569–572. [Google Scholar] [CrossRef]
- Kommreddy, A.; Bowsher, M.S.; Gunna, M.R.; Botha, K.; Vinod, T.K. Expedient synthesis and solvent dependent oxidation behavior of a water-soluble IBX derivative. Tetrahedron Lett. 2008, 49, 4378–4382. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Singhal, N.; Senapati, K. Modified o-methyl-substituted IBX: Room temperature oxidation of alcohols and sulfides in common organic solvents. Tetrahedron Lett. 2008, 49, 80–84. [Google Scholar] [CrossRef]
- Richardson, R.D.; Zayed, J.M.; Altermann, S.; Smith, D.; Wirth, T. Tetrafluoro-IBA and-IBX: Hypervalent Iodine Reagents. Angew. Chem. Int. Ed. 2007, 46, 6529–6532. [Google Scholar] [CrossRef]
- Koposov, A.Y.; Litvinov, D.N.; Zhdankin, V.V.; Ferguson, M.J.; McDonald, R.; Tykwinski, R.R. Preparation and Reductive Decomposition of 2-Iodoxybenzenesulfonic Acid. X-ray Crystal Structure of 1-Hydroxy-1H-1,2,3-benziodoxathiole 3,3-Dioxide. Eur. J. Org. Chem. 2006, 2006, 4791–4795. [Google Scholar] [CrossRef]
- Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone. J. Am. Chem. Soc. 2009, 131, 251–262. [Google Scholar] [CrossRef]
- Parlow, J.J.; Case, B.L.; South, M.S. High-throughput purification of solution-phase periodinane mediated oxidation reactions utilizing a novel thiosulfate resin. Tetrahedron 1999, 55, 6785–6796. [Google Scholar] [CrossRef]
- De Munari, S.; Frigerio, M.; Santagostino, M. Hypervalent Iodine Oxidants: Structure and Kinetics of the Reactive Intermediates in the Oxidation of Alcohols and 1,2-Diols by o-Iodoxybenzoic Acid (IBX) and Dess−Martin Periodinane. A Comparative 1H-NMR Study. J. Org. Chem. 1996, 61, 9272–9279. [Google Scholar] [CrossRef]
- Motlagh, R.J.; Zakavi, S. Synthesis, characterization and oxidizing strength of a nano-structured hypervalent iodine(V) compound: Iodylbenzene nanofibers. New J. Chem. 2018, 42, 19137–19143. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Litvinov, D.N.; Koposov, A.Y.; Luu, T.; Ferguson, M.J.; McDonald, R.; Tykwinski, R.R. Preparation and structure of 2-iodoxybenzoate esters: Soluble and stable periodinane oxidizing reagents. Chem. Commun. 2004, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Zhdankin, V.V.; Koposov, A.Y.; Litvinov, D.N.; Ferguson, M.J.; McDonald, R.; Luu, T.; Tykwinski, R.R. Esters of 2-Iodoxybenzoic Acid: Hypervalent Iodine Oxidizing Reagents with a Pseudobenziodoxole Structure. J. Org. Chem. 2005, 70, 6484–6491. [Google Scholar] [CrossRef]
- Tojo, G.; Fernandez, M. Oxidation of Primary Alcohols to Carboxylic Acids; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Liang, S.; Xu, K.; Zeng, C.-C.; Tian, H.-Y.; Sun, B.-G. Recent Advances in the Electrochemical α-C–H Bond Functionalization of Carbonyl Compounds. Adv. Synth. Catal. 2018, 360, 4266–4292. [Google Scholar] [CrossRef]
- Thottumkara, P.; Bowsherand, M.S.; Vinod, T.K. In Situ Generation of o-Iodoxybenzoic Acid (IBX) and the Catalytic Use of It in Oxidation Reactions in the Presence of Oxone as a Co-oxidant. Org. Lett. 2005, 7, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Jhulki, S.; Moorthy, J.N. Catalytic and Chemoselective Oxidation of Activated Alcohols and Direct Conversion of Diols to Lactones with in Situ-Generated Bis-IBX Catalyst. Eur. J. Org. Chem. 2013, 2013, 2445–2452. [Google Scholar] [CrossRef]
- Jhulki, S.; Seth, S.; Mondal, M.; Moorthy, J.N. Facile Organocatalytic Domino Oxidation of Diols to Lactones by In Situ-Generated TetMe-IBX. Tetrahedron 2014, 70, 2286–2293. [Google Scholar] [CrossRef]
- Mishra, A.K.; Moorthy, J.N. Mechanochemical catalytic oxidations in the solid state with in situ-generated modified IBX from 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA)/Oxone. Org. Chem. Front. 2017, 4, 343–349. [Google Scholar] [CrossRef]
- Ballaschk, F.; Kirsch, S.F. Oxidation of secondary alcohols using solid supported hypervalent iodine catalysts. Green Chem. 2019, 21, 5896–5903. [Google Scholar] [CrossRef]
- Kupwade, R.V.; Mitragotri, S.D.; Kulkarni, M.A.; Desai, U.V.; Wadagaonkar, P.P. Highly efficient and extremely simple protocol for the oxidation α-hydroxyphosphonates to α-ketophosphonates using Dess-Martin periodinane. Arkivoc 2020, part iv, 50–58. [Google Scholar] [CrossRef]
- Kaushik, P.; Applegate, G.A.; Malik, G.; Berkowitz, D.B. Combining a Clostridial Enzyme Exhibiting Unusual Active Site Plasticity with a Remarkably Facile Sigmatropic Rearrangement: Rapid, Stereocontrolled Entry into Densely Functionalized Fluorinated Phosphonates for Chemical Biology. J. Am. Chem. Soc. 2015, 137, 3600–3609. [Google Scholar] [CrossRef]
- Kaushik, P.; Xiang, F.; Masato, K.; Berkowitz, D.B. Rapid Entry into Biologically Relevant α,α-Difluoroalkylphosphonates Bearing Allyl Protection–Deblocking under Ru(II)/(IV)-Catalysis. Org. Lett. 2019, 21, 9846–9851. [Google Scholar] [CrossRef]
- Engel, R. Phosphonates as analogues of natural phosphates. Chem. Rev. 1977, 77, 349–367. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Mathison, C.J.N.; Montagnon, T. New Reactions of IBX: Oxidation of Nitrogen- and Sulfur-Containing Substrates to Afford Useful Synthetic Intermediates. Angew. Chem. Int. Ed. 2003, 42, 4077–4082. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Mathison, C.J.N.; Montagnon, T. o-Iodoxybenzoic Acid (IBX) as a Viable Reagent in the Manipulation of Nitrogen- and Sulfur-Containing Substrates: Scope, Generality, and Mechanism of IBX-Mediated Amine Oxidations and Dithiane Deprotections. J. Am. Chem. Soc. 2004, 126, 5192–5201. [Google Scholar] [CrossRef]
- Murthy, S.N.; Nageswar, Y.V.D. o-Iodoxybenzoic acid (IBX): A versatile reagent for the synthesis of N-substituted pyrroles mediated by β-cyclodextrin in water. Tetrahedron Lett. 2011, 52, 4481–4484. [Google Scholar] [CrossRef]
- de Graaff, C.; Bensch, L.; van Lint, M.J.; Ruijter, E.; Orru, R.V.A. IBX-Mediated Oxidation of Unactivated Cyclic Amines: Application in Highly Diastereoselective Oxidative Ugi-type and aza-Friedel-Crafts Reactions. Org. Biomol. Chem. 2015, 13, 10108–10112. [Google Scholar] [CrossRef]
- Hati, S.; Sen, S. Synthesis of Quinazolines and Dihydroquinazolines: O-Iodoxybenzoic Acid Mediated Tandem Reaction of o-Aminobenzylamine with Aldehydes. Synthesis 2016, 48, 1389–1398. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, A.; Mithu, V.S.; Sharma, S. Metal-Free Organocatalytic Oxidative Ugi Reaction Promoted by Hypervalent Iodine. J. Org. Chem. 2017, 82, 5285–5293. [Google Scholar] [CrossRef]
- Ambule, M.D.; Tripathi, S.; Ghoshal, A.M.; Srivastava, A.K. IBX-mediated oxidative addition of isocyanides to cyclic secondary amines: Total syntheses of alangiobussine and alangiobussinine. Chem. Commun. 2019, 55, 10872–10875. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, D.; Wan, Y.; Zhang, G.; Bi, J.; Liu, Q.; Liu, T.; Shi, L. Selective Cleavage of Inert Aryl C−N Bonds in N-Aryl Amides. J. Org. Chem. 2018, 83, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Song, M.; Wan, Y.; Zheng, D.; Zhang, G.; Chen, G. Selective Removal of Aminoquinoline Auxiliary by IBX Oxidation. J. Org. Chem. 2019, 84, 12792–12799. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Parida, K.N. Oxidative Cleavage of Olefins by In Situ-Generated Catalytic 3,4,5,6-Tetramethyl-2-iodoxybenzoic Acid/Oxone. J. Org. Chem. 2014, 79, 11431–11439. [Google Scholar] [CrossRef]
- Chaudhari, D.A.; Fernandes, R.A. Hypervalent iodine as a terminal oxidant in Wacker-type oxidation of terminal olefins to methyl ketones. J. Org. Chem. 2016, 81, 2113–2121. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Singhal, N.; Senapati, K. Oxidations with IBX: Benzyl halides to carbonyl compounds, and the one-pot conversion of olefins to 1,2-diketones. Tetrahedron Lett. 2006, 47, 1757–1761. [Google Scholar] [CrossRef]
- Chandra, A.; Yadav, N.R.; Moorthy, J.N. Facile synthesis of isatins by direct oxidation of indoles and 3-iodoindoles using NIS/IBX. Tetrahedron 2019, 75, 2169–2174. [Google Scholar] [CrossRef]
- Bredenkamp, A.; Mohr, F.; Kirsch, S.F. Synthesis of Isatins through Direct Oxidation of Indoles with IBX-SO3K/NaI. Synthesis 2015, 47, 1937–1943. [Google Scholar] [CrossRef]
- El-Assaad, T.; Parida, K.N.; Cesario, M.F.; McGrath, D.V. Sterically Driven Metal-Free Oxidation of 2,7-di-tert-butylpyrene. Green Chem. 2020, 22, 5966–5971. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Pieri, C.; Botta, G.; Arabuli, L.; Mosesso, P.; Cinelli, S.; Schinoppia, A.; Saladino, R. Synthesis and antioxidant activity of DOPA peptidomimetics by a novel IBX mediated aromatic oxidative functionalization. RSC Adv. 2015, 5, 60354–60364. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Botta, L.; Capecchi, E.; Celestino, I.; Checconi, P.; Palamara, A.T.; Nencioni, L.; Saladino, R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-influenza Activities. J. Nat. Prod. 2017, 80, 3247–3254. [Google Scholar] [CrossRef]
- Harschneck, T.; Hummel, S.; Kirsch, S.F.; Klahn, P. Practical Azidation of 1,3-Dicarbonyls. Chem. Eur. J. 2012, 18, 1187–1193. [Google Scholar] [CrossRef]
- Patil, P.; Nimonkar, A.; Akamanchi, K.G. Aryl-Free Radical-Mediated Oxidative Arylation of Naphthoquinones Using o-Iodoxybenzoic Acid and Phenylhydrazines and Its Application toward the Synthesis of Benzocarbazoledione. J. Org. Chem. 2014, 79, 2331–2336. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Huddar, S.N.; Akamanchi, K.G. o-Iodoxybenzoic Acid Mediated N-Arylation of Aromatic Amines by Using Arylhydrazines as the Arylating Counterpart. Eur. J. Org. Chem. 2013, 30, 6779–6783. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Luo, S. Catalytic Asymmetric β-C−H Functionalizations of Ketones via Enamine Oxidation. Org. Lett. 2018, 20, 1672–1675. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, J.N.; Neogi, I. IBX-mediated one-pot synthesis of benzimidazoles from primary alcohols and arylmethyl bromides. Tetrahedron Lett. 2011, 52, 3868–3871. [Google Scholar] [CrossRef]
- Wagh, Y.S.; Tiwari, N.J.; Bhanage, B.M. Metal-free synthesis of 2-aminobenzoxazoles using hypervalent iodine reagent. Tetrahedron Lett. 2013, 54, 1290–1293. [Google Scholar] [CrossRef]
- Prabhu, G.; Sureshbabu, V.V. Hypervalent iodine(V) mediated mild and convenient synthesis of substituted 2-amino-1,3,4-oxadiazoles. Tetrahedron Lett. 2012, 53, 4232–4234. [Google Scholar] [CrossRef]
- Chaudhari, P.S.; Pathare, S.P.; Akamanchi, K.G. o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. J. Org. Chem. 2012, 77, 3716–3723. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, T.J.; Kabeshov, M.A.; Rathi, A.H.; Smith, I.E.D. Direct preparation of thiazoles, imidazoles, imidazopyridines and thiazolidines from alkenes. Org. Biomol. Chem. 2012, 10, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Pilania, M.; Arun, V.; Mishra, B. A Facile and Expeditious One-Pot Synthesis of α-Keto-1,3,4-oxadiazoles. Synlett 2014, 25, 1137–1141. [Google Scholar] [CrossRef]
- Kim, M.H.; Jeong, H.J.; Kim, D.Y. IBX-Mediated Oxidation and Internal Redox Reaction Cascade: Asymmetric One-Pot Synthesis of Ring-fused Tetrahydroquinolines. Bull. Korean Chem. Soc. 2015, 36, 1155–1160. [Google Scholar] [CrossRef]
- Ramaraju, P.; Mir, N.A.; Singh, D.; Gupta, V.K.; Kant, R.; Kumar, I. Enantioselective Synthesis of N-PMP-1,2-dihydropyridines via Formal [4 + 2] Cycloaddition between Aqueous Glutaraldehyde and Imines. Org. Lett. 2015, 17, 5582–5585. [Google Scholar] [CrossRef] [PubMed]
- Ramaraju, P.; Mir, N.A.; Singh, D.; Sharma, P.; Kant, R.; Kumar, I. An Unprecedented Pseudo-[3 + 2] Annulation between N-(4-Methoxyphenyl)aldimines and Aqueous Glutaraldehyde: Direct Synthesis of Pyrrole-2,4-dialdehydes. Eur. J. Org. Chem. 2017, 2017, 3461–3465. [Google Scholar] [CrossRef]
- Singh, A.; Mir, N.A.; Choudhary, S.; Singh, D.; Sharma, P.; Kant, R.; Kumar, I. One-pot sequential multicomponent reaction between in situ generated aldimines and succinaldehyde: Facile synthesis of substituted pyrrole-3-carbaldehydes and applications towards medicinally important fused heterocycles. RSC Adv. 2018, 8, 15448–15458. [Google Scholar] [CrossRef] [PubMed]
- Zaware, N.; Ohlmeyer, M. Recent advances in dibenzo[b, f][1,4] oxazepine synthesis. Heterocycl. Commun. 2014, 20, 251–256. [Google Scholar] [CrossRef]
- Dols, P.P.M.A.; Folmer, B.J.B.; Hamersma, H.; Kuil, C.W.; Lucas, H.; Ollero, L.; Rewinkel, J.B.M.; Hermkens, P.H.H. SAR study of 2,3,4,14b-tetrahydro-1H-dibenzo[b,f]pyrido[1,2-d][1,4]oxazepines as progesterone receptor agonists. Bioorg. Med. Chem. Lett. 2008, 18, 1461–1467. [Google Scholar] [CrossRef]
- Choudhary, S.; Pawar, A.P.; Yadav, J.; Sharma, D.K.; Kant, R.; Kumar, I. One-Pot Synthesis of Chiral Tetracyclic Dibenzo[b,f][1,4]oxazepine-Fused 1,2-Dihydropyridines (DHPs) under Metal-Free Conditions. J. Org. Chem. 2018, 83, 9231–9239. [Google Scholar] [CrossRef]
- Makra, Z.; Puskás, L.G.; Kanizsai, I. A convenient approach for the preparation of imidazo[1,2-a]-fused bicyclic frameworks via IBX/NIS promoted oxidative annulation. Org. Biomol. Chem. 2019, 17, 9001–9007. [Google Scholar] [CrossRef]
- Belanger, D.B.; Curran, P.J.; Hruza, A.; Voigt, J.; Meng, Z.; Mandal, A.K.; Siddiqui, M.A.; Basso, A.D.; Gray, K. Discovery of imidazo[1,2-a]pyrazine-based Aurora kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 5170. [Google Scholar] [CrossRef]
- Meng, W.; Brigance, R.P.; Chao, H.J.; Fura, A.; Harrity, T.; Marcinkeviciene, J.; O’Connor, S.P.; Tamura, J.K.; Xie, D.; Zhang, Y.; et al. Discovery of 6-(Aminomethyl)-5-(2,4-dichlorophenyl)-7-methylimidazo[1,2-a]pyrimidine-2-carboxamides as Potent, Selective Dipeptidyl Peptidase-4 (DPP4) Inhibitors. J. Med. Chem. 2010, 53, 5620. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Li, G.; Li, X.; Zheng, D.; Zhao, X.; Miao, H.; Zhang, G.; Liu, L. Synthesis of polycyclic spiro-fused indolines via IBX-mediated cascade cyclization. Chin. Chem. Lett. 2021, 32, 1423–1426. [Google Scholar] [CrossRef]
- Gao, P.; Chen, H.-J.; Bai, Z.-J.; Zhao, M.-N.; Yang, D.; Wang, J.; Wang, N.; Du, L.; Guan, Z.-H. IBX-Promoted Oxidative Cyclization of N-Hydroxyalkyl Enamines: A Metal-free Approach towards 2,3-Disubstituted Pyrroles and Pyridines. J. Org. Chem. 2020, 85, 7939–7951. [Google Scholar] [CrossRef] [PubMed]
- Corrieri, M.; Crescentini, L.D.; Mantellini, F.; Mari, G.; Santeusanio, S.; Favi, G. Synthesis of Azacarbolines via PhIO2-Promoted Intramolecular Oxidative Cyclization of α-Indolylhydrazones. J. Org. Chem. 2021, 86, 17918–17929. [Google Scholar] [CrossRef] [PubMed]
- Katrun, P.; Songsichan, T.; Soorukram, D.; Pohmakotr, M.; Reutrakul, V.; Kuhakarn, C. o-Iodoxybenzoic Acid (IBX)–Iodine Mediated One-Pot Deacylative Sulfonylation of 1,3-Dicarbonyl Compounds: A Synthesis of β-Carbonyl Sulfones. Synthesis 2016, 48, 1109–1121. [Google Scholar] [CrossRef]
- Samakkanad, N.; Katrun, P.; Techajaroonjit, T.; Hlekhlai, S.; Pohmakotr, M.; Reutrakul, V.; Jaipetch, T.; Soorukram, D.; Kuhakarn, C. IBX/I2-Mediated Reaction of Sodium Arenesulfinates with Alkenes: Facile Synthesis of β-Keto Sulfones. Synthesis 2012, 44, 1693–1699. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).